Gut and reproductive tract microbiota: Insights into the pathogenesis of endometriosis (Review)
- Authors:
- Published online on: May 29, 2023 https://doi.org/10.3892/br.2023.1626
- Article Number: 43
-
Copyright: © Kobayashi . This is an open access article distributed under the terms of Creative Commons Attribution License.
Abstract
1. Introduction
Endometriosis is a common estrogen-dependent inflammatory disorder, which affects ~10% of reproductive-age women (1). Endometriosis is defined by the presence of endometrial-like benign lesions located outside of the uterus (1). This disease is a heterogeneous disease that can present in different forms, including superficial peritoneal disease, ovarian endometrioma and deep infiltrating endometriosis (2). Endometriosis has been reported as a complex and multifactorial disease, which is characterized by estrogen dominance, increased cytokine levels, and innate and adaptive immune system activation (2,3).
Endometriosis is caused by endometrial cell engraftment outside of the uterus and is characterized by periodic hemorrhage from ectopic endometriotic lesions (3). Epidemiologic studies have reported that the risk of endometriosis is associated with increased exposure to menstruation (e.g., early age at menarche, shorter menstrual cycle length, longer menstrual flow duration, long menstrual period or reduced parity) (4). Typically, modern females marry later in life, have ≤2 children, and experience much shorter breastfeeding periods, which results in more frequent menstrual cycles (5). Females today menstruate approximately eight times more often than females in hunter-gatherer societies (i.e., ~400 vs. 50 menstrual cycles in their lifetime) (6). Menstruation is considered an inflammatory response with cyclic fluctuations in female steroid hormone levels and results in the production and release of inflammatory mediators, such as cytokines and prostaglandins (7,8). A surge in inflammatory mediators causes immune cell activation and recruitment, which results in further stressor production (e.g., cytokines, chemokines, angiogenesis factors and growth factors) (8). Multiple stressors are produced by different immune cells, mainly macrophages, and the regurgitated endometrial tissue (9,10) is eventually terminated by the host's immune system and immunoregulatory mechanisms. Therefore, a higher frequency of menstruation may increase the risk of developing endometriosis.
Certain previous studies have suggested a role for microbes in the female reproductive tract in endometriosis (8,11). The female reproductive tract microbiota constitutes a defensive barrier to prevent infections, serves an important role in each of the structural, inflammatory, immune, metabolic, and endocrine systems, and may act as a key link between inflammation and immunity (12). Previous studies have suggested that the microbiota may be important for the prevention of non-communicable diseases (7,13). Furthermore, the abundance and composition of the gut and reproductive tract microbiota differ between females with and without endometriosis (8,14). Therefore, quantitative and qualitative imbalances between pathogenic bacteria and non-pathogenic environmental microbes (i.e., dysbiosis) may be involved in endometriosis pathogenesis (8). Dysfunction of the inflammatory and immune systems is a crucial aspect of endometriosis pathogenesis.
Thereby, both deleterious inflammations due to frequent menstrual cycles (an incessant attack from intrinsic stimuli) and pathogens or pathogenic microbes (an attack from extrinsic stimuli) are controlled by the host defense system. This review summarizes current knowledge about dysbiosis in endometriosis and discusses how this imbalance causes inappropriate immune responses, which can lead to the development of disease.
2. Search strategy and selection criteria
A literature search was performed to identify relevant studies. The PubMed and Google Scholar electronic databases were searched for literature published up to the 31st March 2022, combining the following keywords: ‘Endometriosis’, ‘microbiome’, ‘dysbiosis’, ‘retrograde menstruation’, ‘inflammation’ and ‘immunity’. The search strategy combined these keywords with the Boolean operators ‘and’ and ‘or’, as described in Table I. Inclusion criteria were studies which specifically focused on the microbiome in endometriosis and included the publication of peer-reviewed original articles and reference lists in review articles. The exclusion criteria were duplicated studies, non-English language publications, letters to the editor, poster presentations, and unrelated literature. The identified articles were initially assessed for eligibility and then subsequently, the full-text of the articles was assessed. The first identification phase included an electric database search, hand search (manually searching for necessary documents), and the generation of a reference list of collected articles and review articles to identify additional relevant articles (Fig. 1). Duplicates were removed during the second, screening, phase. Titles and abstracts were read to remove inappropriate papers. The final eligible phase included full-text articles assessed as eligible.
3. Results
Identification of studies
Searches of the PubMed and Google Scholar electronic databases yielded 422 literature citations (Fig. 1). After removing duplicates, 179 records were identified, of which 140 were excluded and 39 met the eligibility criteria.
Role of the microbiota in the gut and female reproductive tract
This subsection summarizes the mechanisms underlying the effect of microbiome dysbiosis with a focus on endometriosis. Mucosal barriers serve crucial roles in the defense against pathogens or pathogenic microbes (12). The gut, skin, lungs, mouth, vagina, etc., contain certain non-pathogenic commensal microbes (12). The human microbiota positively affects physical health by maintaining structural, immunological, metabolic and neurological homeostasis (12). Mammals, including humans, have co-evolved with host-resident microorganisms in a symbiotic relationship (15). Microbiota co-evolving with humans supported the development of a symbiotic relationship and accelerated immune system function (16). Furthermore, the microbiota has an essential role in the endocrine systems of the gut and reproductive systems by interacting with estrogen and other hormones via a healthy estrogen-gut axis (17,18).
Role of the gut microbiota in non-communicable disease development
The commensal microbiome sustains the host-microbiota symbiotic relationship and contributes to numerous interactions which affect human health, including the synthesis and absorption of essential nutrients to maintain energy homeostasis; maintenance of intestinal mucosal function and structural integrity; protection against pathogenic microbes or harmful agents; production of antimicrobial peptides that affect the epigenome (15); maturation, development and functions of the innate and adaptive immune system; modulation of host brain function and cognitive behavior; regulation of estrogen modulated by estrogen-metabolizing enzyme production; and dynamic modulation of the metabolome profile (19,20). The microbiota is recognized by innate immune receptors, in particular, toll-like receptors (TLRs) (21,22). Gut epithelial cell TLRs are the signaling pathways which can implement host defense mechanisms against invading microbes, and they not only eliminate pathogens via increased immunosurveillance (21), but also establish host-microbial symbiosis to maintain microorganism-induced homeostasis (22). Therefore, the microbial contamination theory or hygiene hypothesis, which claims the positive effect of exposure to non-pathogenic environmental microbes and pathogenic bacteria in early-life on human health, has attracted attention (7,8,13,23). The exposure of humans to non-pathogenic environmental microbes or pathogenic bacteria begins prenatally, and continues during parturition and postnatally (20). Generally, early-life exposures to microbial diversity may be associated with a reduced risk of developing allergies, including atopy and asthma (24). In contrast, abundant specific bacterial taxa which affect the gut microbiota composition are associated with disease susceptibility in adult life, including inflammatory bowel diseases (25,26), metabolic disorders (e.g., obesity, gestational diabetes mellitus, type II diabetes) (25), neurological diseases (e.g., autism) (26), and reproductive and endocrine disorders (e.g., pregnancy complications, adverse pregnancy outcomes, polycystic ovary syndrome) (27). For example, gut microbiome alteration increases inflammatory bowel disease susceptibility, as the relative abundance of beneficial organisms (e.g., Lachnospiraceae, Bifidobacterium species, Roseburia, Sutterella and Faecalibacterium prausnitzii) decreases whereas the relative abundance of pathogens (e.g., Proteobacteria, Fusobacteria species and Ruminococcus gnavus) increase (28). Furthermore, imbalances in the composition of the gut microbiota (i.e., dysbiosis) favor inflammation via specific inflammatory immune cell recruitment, proinflammatory cytokine production and compromised immunosurveillance (8,29). Diseases affecting the immune and inflammatory system, such as inflammatory bowel disease and metabolic disorders, are believed to be related to a decreased microbial community diversity. Similarly, a lifelong risk of non-communicable inflammatory diseases, including reproductive pathologies, may result from early-life microbial exposures (26).
Role of the reproductive tract microbiota in obstetric, gynecological, and reproductive diseases. The healthy human vagina is colonized by numerous types of microorganisms and is characterized by the beneficial microbiota, Lactobacillus. The presence of a vaginal microbiota dominated by Lactobacillus maintains an acidic vaginal environment with a pH of <4.5, which prevents the growth of pathogenic bacteria (30). This can protect the host from pathogenic microbial infections such as Gardnerella species (31) and Neisseria gonorrhoeae (32). Furthermore, the upper female reproductive tract, consisting of the uterus, fallopian tubes, ovaries and peritoneum, is not a sterile environment and demonstrates a highly diverse and unique microbiota. Therefore, the microbial community composition differs between the uterus and the vagina (33-35). Commensal microbes produce biological resources and other microbes can use valuable metabolic resources to persist in the ecosystem via resource-sharing/competition and cross-feeding, which limits the availability of resources to neighboring pathogenic bacteria and inhibits pathogenic microbial growth with symbiotic microbes (36). This indicates that pathogenic bacteria can survive and even grow with a decreased commensal microbial diversity and an abundance of microbial taxa.
Imbalances in the composition of the reproductive tract microbiota can cause certain obstetric, gynecological and reproductive diseases. For example, a decrease in the abundance of the typical Lactobacillus and an abnormal increase in opportunistic pathogenic bacterial diversity cause pathologies, such as bacterial vaginosis, which is the most common cause of vaginal inflammation and infection (i.e., vaginitis) (37,38). Bacteria associated with bacterial vaginosis, such as Gardnerella, Prevotella and Bacteroides, induce increased levels of proinflammatory cytokine and mucosal epithelial barrier disruption (8,37). Moreover, elevated levels of inflammatory cytokine confer an increased risk for multiple gynecologic diseases, including endometritis, pelvic inflammatory disease and infertility (8,37). Additionally, patients with pelvic inflammatory disease are generally at increased risk of developing endometriosis (39). Furthermore, the lack of Lactobacillus-dominated microbiota species has been reported to be associated with poor fertility treatment outcomes (i.e., poor in vitro fertilization outcomes, with low fertilization and pregnancy rates) (35). Changes in microbiota distribution from eubiosis to dysbiosis can affect human health; however, the timing (i.e., pre-natal, intra-natal, post-natal or over time to the adult life) of these changes remains unknown. Research in this field is only beginning to apply microbiota analysis in the clinical setting, and its physiological and pathological roles in human reproduction and disease remain to be fully understood (34).
The clinicaltrials.gov electronic database was searched using the keywords ‘endometriosis’ and ‘microbiome’. The electronic search yielded a total of six clinical trials, in which recruitment was completed, currently being performed, and not yet started in two, one and three studies, respectively. Currently, clinical studies in ‘Microbiome and immunologic analysis in females with endometriosis (NCT04159740)’ and ‘Establishment of the human intestinal and salivary microbiota biobank in gynecological diseases (NCT04698109)’ are currently being performed.
The role of the immune system is mediated by the microbiota in endometriosis
Immune cells, which are essential reproductive tract microenvironment components, secrete numerous cytokines and chemokines (40). Both innate [e.g., macrophages, neutrophils, dendritic cells and natural killer (NK) cells] and adaptive immune cells (e.g., T cells and B cells) contribute to regulation of tissue inflammation, immune cell recruitment and resolution, and the host defense response. Pelvic endometriosis is thought to arise from endometrial cell implantation through retrograde menstruation (2). Escherichia coli bacteria in menstrual blood and endotoxins in the peritoneal fluid are bioaccumulative contaminants which have been previously reported in females with endometriosis (41). Endometrial cell fragments that are shed during menstruation produce a damage-associated molecular pattern (DAMP) through the specialized pattern recognition receptors (e.g., TLRs) and activate inflammatory cytokine production, such as interleukin-1β (IL-1β), to trigger inflammation (9). Furthermore, DAMPs activate innate and adaptive immune cells, such as neutrophils, mast cells and Th17 cells, initiate immune cell response, and promote endometrial cell adhesion and angiogenesis (9). Particular TLR ligands (e.g., lipopolysaccharide, nucleic acids, flagellin, or zymosan of both commensal microbiota and pathogenic microbiota) are sensed by the immune system via the pattern recognition receptors (42). TLR-4-mediated nuclear factor κβ activation may be the main factor which affects endometriosis development (41). Over time, persistent immune stimuli may drive a vicious cycle of inflammation, adhesion and angiogenesis, which in turn facilitates ectopic endometrium implantation and growth (8). The immune system fights against bacterial infection but triggers an inflammatory response that damages reproductive tract tissue. The excessive inflammatory response may lead to endometriosis through host immune dysfunction. Therefore, the immune system mediated by the microbiota-gut-reproductive tract axis is thought to serve an important role in developing endometriosis.
Role of the gastrointestinal tract microbiome
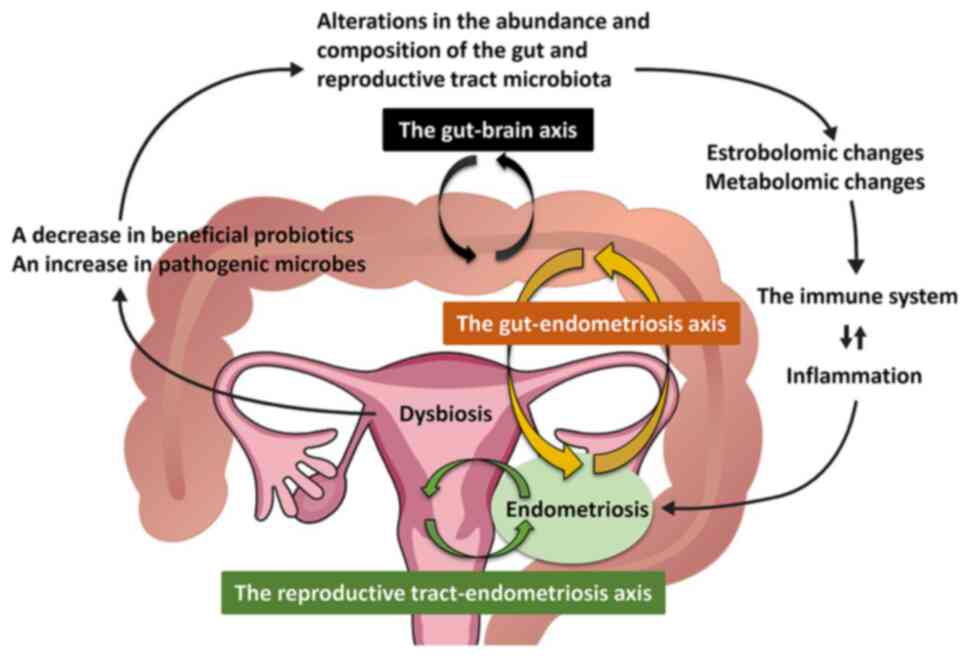
This subsection summarizes the role of the gastrointestinal tract microbiome in endometriosis (Fig. 2). Data from mice and nonhuman primates are included, as microbiome research in females with endometriosis is still in its early stages (Table II). Previous in vivo studies have reported the influence of gut microbiota on animals with endometriosis. Mouse models of endometriosis demonstrated an increased ratio of Firmicutes to Bacteroidetes and an increased number and proportion of certain gut microbes (e.g., Ruminococcaceae, Bifidobacterium and Parasutterella generae) after endometrial fragment transimplantation (43). Moreover, a study which used rhesus macaques reported lower numbers of Lactobacillus bacteria and higher Gram-negative microbial loads in the endometriosis group compared with that in the non-endometriosis group (44). Furthermore, rhesus macaque with endometriosis were reported to have an increased risk of gastrointestinal inflammation (44). This finding was consistent with clinical observations that endometriosis is associated with an increased risk of gastrointestinal disorders, such as irritable bowel syndrome (45). Gut microbiota diversity and abundance decreased in animals with endometriosis compared with healthy controls (46). Further, previous studies have reported an increased absolute number of Gardnerella, Streptococcus, Enterococci and Escherichia coli in the vagina of females with endometriosis compared with healthy females, which indicated an increased proportion of potentially pathogenic microbes and a reduced microbiome diversity (47,48). Therefore, the type and abundance of gut microbiota differ between females with and without endometriosis (49).
Experimental induction of endometriosis by fecal microbiota transplantation has been previously reported; the transplantation of fecal microbiota obtained from a surgically induced endometriosis mouse donor into the gastrointestinal tract of other endometriotic mice promoted lesion growth and associated inflammation (50). Furthermore, in vivo experiments demonstrated that broad-spectrum antibiotic treatment in mice with endometriosis reduced the endometriotic lesion size (43). The mouse model demonstrated that endometriosis-specific gut microbiota can alter disease progression by modulating inflammation (50). Non-pathogenic and pathogenic fecal bacterial contamination, followed by a process of proinflammatory cytokine- and immune-activation-mediated gut dysfunction, provides a plausible explanation of gut microbial dysbiosis in endometriosis. Furthermore, preliminary studies on the efficacy of probiotics for women with endometriosis have revealed positive effects in pain relief (8,51,52) (see Treatment of endometriosis by modulating the microbiome).
Conversely, studies with endometriosis models which used mice and rhesus macaques have reported that endometriosis induced gut microbiota alterations or dysbiosis (43,44). Previous animal studies have reported that gut microbiota composition alterations promote the development of endometriosis, and that experimental endometriosis affects the composition of the gut microbiota (43,44). However, changes in the gut microbiota in the same female patient before and after the onset of endometriosis are unknown. In contrast, other in vivo mouse studies did not report changes in the gut microbiome composition profiles (53). Therefore, a gut microbiota composition imbalance could cause endometriosis exacerbation; however, there are conflicting results that endometriosis itself may or may not change the gut microbiota.
Role of the reproductive tract microbiome
This subsection summarizes the role of the reproductive tract microbiome in endometriosis (Fig. 2). Numerous studies have analyzed the microbiome composition of the reproductive tract in females with endometriosis and reported an increased abundance of opportunistic pathogens associated with bacterial vaginosis and diminished Lactobacillus dominance along the reproductive tract (from the vagina up into the cervix, endometrium, fallopian tube and peritoneal fluid) of females with endometriosis (8,47,48,54-57). Opportunistic pathogens, including Streptococcaceae and Staphylococaceae, were reported to be enriched in the cystic fluid of females with ovarian endometrioma (54). Moreover, Alishewanella, Enterococcus, Ureaplasma and Pseudomonas are often detected in endometriotic lesions (9), whereas Atopobium is completely absent in the vagina of patients with endometriosis (48). Additionally, greater numbers of bacteria such as Gardnerella, Streptococcus, Enterococci and Escherichia coli are present in the endometrium of females with endometriosis compared with controls (14). Furthermore, an increased absolute number of bacterial pathogens, including Gardnerella, Streptococcus, Escherichia, Shigella and Ureaplasma, was reported in the cervical microbiota of females with stage III-IV endometriosis (48). Endometriosis may affect the composition of the reproductive tract microbiota, which in turn increases the risk of lower genital tract infection, endometritis, pelvic inflammatory disease and surgical site infections after hysterectomy (14). Additionally, increased numbers of Firmicutes and decreased Actinobacteria and Bacteroidetes in the cervical microbiota have been reported to increase the risk of developing endometriosis (58). Increasing disease severity (e.g., advanced stage, the coexistence of deep infiltrating endometriosis, disease burden and symptom severity) have been reported to be associated with decreasing abundance of Dialister and an increasing abundance of Lactobacillus and Streptococcus (58). Endometriosis is characterized by the loss of beneficial microorganisms (e.g., Lactobacilli) and the gain of pathogenic microorganisms. However, the specific bacteria identified as the causative microbiota remains unknown. Furthermore, evidence of the effects of the host site on the reproductive tract microbiota composition is currently scarce.
Possible mechanisms of endometriosis development by alterations of microbiota abundance and composition
This section summarizes the mechanisms by which alterations in gut and reproductive tract microbiota composition and function cause endometriosis. The growth and maintenance of endometriosis are influenced by estrogen metabolism (59). The gut microbiome is an important regulator of circulating estrogens (17). The gut microbiota is involved in regulation of estrogen levels via the β-glucuronidase enzyme, which is responsible for the deconjugation of conjugated estrogens into their active forms (18). Thus, estrogen metabolism is modulated by the estrobolome (i.e., the aggregate of genes capable of metabolizing estrogens in the gut microbiome) (17). Gut microbiome dysbiosis caused by the imbalance between the commensal and pathogenic microbiomes or reduced microbial diversity dysregulates the bidirectional crosstalk between the gut and uterus, which results in not only immune dysfunction but also altered estrogen signaling (8,60). Microbial diversity and dysbiosis reduction lower β-glucuronidase activity (17). Conversely, the estrobolome, through the increased abundance of β-glucuronidase-producing bacteria, increases circulating estrogens, thereby developing endometriosis (8,17). Investigating the number of β-glucuronidase-producing bacteria in the gut microbiome of patients with endometriosis is of great interest.
The gut microbiome communicates bidirectionally with distant tissues via the gut-brain axis (61). The gut microbiota modulates neurophysiological processes by altering immune, endocrine, reproductive and neural signaling pathways via the gut-brain axis (62). Estrogen has been reported to stimulate neural growth and differentiation both in vitro (63) and in animal studies (64). The gut-brain axis promotes the central sensitization of chronic pain (65). Therefore, endometriosis-associated pain may be affected by regulation of microglia and astrocytes via the dysbiotic gut-brain axis (65). Severe pain and infertility associated with endometriosis have been reported to be associated with decreased cervical microbial diversity and abundance (66).
Finally, changes in the microbiome abundance and composition can affect the metabolic profile, and vice versa (17). A metabolomic study in animals has reported that bile acid biosynthesis and α-linolenic acid metabolism were characteristic pathways in the feces of endometriosis mice (46). Gut dysbiosis in mice with endometriosis appears to cause fecal metabolomic alterations, but its significance in the progression of endometriosis is unclear.
Collectively, these results suggest that the gut microbiota initiates inflammation and affects the pathogenesis and progression of endometriosis via dysregulation of the estrobolome, metabolome and gut-brain axis (58,66). However, whether the composition, abundance and functional alteration of the gut microbiota causes disease progression is unknown.
Treatment of endometriosis by modulating the microbiome
This section first discusses the beneficial effect of probiotics on endometriosis. A recent review summarized dietary supplements (i.e., anti-inflammatory, anti-oxidant, anti-proliferative and immune modulators) used for treating endometriosis (67). Targeting the gut microbiota with probiotics, prebiotics and symbiotics may be beneficial dietary interventions for certain patients, including those with endometriosis, osteoporosis and obesity (67-70). Data on the oral administration of Lactobacillus and omega-3 polyunsaturated fatty acids (PUFAs) are well documented and were presented as examples. See reference (67) for the remaining drugs. The previous study reviewed the up-to-date evidence on dietary supplements used as a complementary treatment, including vitamin C, vitamin D, vitamin E, zinc, magnesium, selenium, omega 3, propolis, quercetin, curcumin, N-acetylcysteine, probiotics, resveratrol, alpha lipoic acid and epigallocatechin-3-gallate (67). Oral Lactobacillus administration reduced the growth of mouse endometriotic lesions by increasing IL-12 concentration and NK cell activity (51,71,72). Randomized, placebo-controlled trials reported that oral Lactobacillus administration ameliorated endometriosis-associated pain in females (52,73). Furthermore, Lactobacillus probiotic treatment has been reported to prevent endometriosis proliferation in rats (71). Additionally, a high intake of omega-3 PUFAs may reduce the risk of developing endometriosis by modulating inflammatory and oxidative stress (68). A mouse model of endometriosis demonstrated that a high-dose PUFA treatment inhibited lesion growth (69). Therefore, available data suggest the important role of dietary supplement-induced beneficial changes in the gut microbiota in human health and reduce the risk of inflammatory diseases, including endometriosis.
There are numerous available therapeutic approaches, such as modulating the microbiome, to treat endometriosis. Preclinical studies have evaluated the possibility of antibiotic administration, fecal microbial transplantation or administration of bacterial components for the treatment of endometriosis in addition to probiotic treatment. Chadchan et al (50) reported that antibiotic therapy with metronidazole reduced endometriotic lesion progression in mice through gut microbiota modulation. Moreover, fecal microbiota transplantation is a promising therapeutic option to target Clostridium difficile infections and holds promise in developing novel therapeutics for multiple sclerosis, autism, obesity and other systemic diseases (74). Additionally, the gut microbiota could induce inflammatory pain in endometriosis by increasing glutamate and decreasing gamma-aminobutyric acid levels through modulation of microglia, astrocytes and immune cells (65). Fecal microbiota transplantation has been reported to be a potential treatment option for endometriosis due to its beneficial effects in reducing pain despite the absence of no clinical data (74). Furthermore, a preclinical mouse model demonstrated that gut microbiota-derived short-chain fatty acids (e.g., n-butyrate) protected against endometriosis progression (75).
4. Discussion
Microbial profile abnormalities (e.g., the composition and abundance) of the gut and reproductive tract microbiome have been reported in numerous diseases, such as inflammatory bowel disease, allergies, autoimmunity, psoriasis, arthritis, reproductive disorders, cancer and endometriosis (6,7,23-28,30-37,76,77). Both animal and human studies have reported that pathogenic bacteria are enriched in the endometriosis group compared with controls (8,9,14,41-48,54-57,73). Additionally, abnormal gut and cervical microbiomes may be associated with endometriosis severity, including pain and infertility, possibly through the impact of the estrobolome and metabolome (58,76). Reproductive tract microbiota alterations were also identified in mice, nonhuman primates and females with endometriosis, which has been universally observed across species (8,43,44,50-52). Microbiota dysbiosis and endometriosis development and progression are thought to be supported by a bidirectional relationship (76); however, no evidence suggests a cause-effect relationship. Additionally, altered specific microbiota composition in endometriosis has no clear consensus. The microbiota has been reported to be a major regulator of such physiological processes, not only within the gut and reproductive tract but also at distant sites, e.g., microbiota-brain crosstalk (78). As mentioned above, microbial profiles in the gut and endometriosis and in the genital tract and endometriosis have been reported, but the crosstalk between the gut and genital tract environments remains unclear. Collectively, microbiome composition and abundance alterations may cause endometriosis and its associated symptoms, including infertility and pelvic pain, possibly through the gut-brain axis.
The microbiota is not merely composed of microorganisms, such as commensals and symbionts, and they are increasingly recognized as serving beneficial roles in human health and reproduction beyond infection (34). The immune system is unable to eliminate the microbial symbiont population once the commensal microbiomes (e.g., non-pathogenic environmental bacteria, certain commensals and probiotics) are established in the host (79). Females infected with commensals or non-pathogenic microbiota early in life maintain a symbiotic relationship, thereby avoiding excessive tissue damage, preserving immune homeostasis and suppressing the inflammatory response (7,23). However, modern females often face a rapidly changing microbial environment, with serious biodiversity loss due to industrialized Western lifestyles. Environmental changes (e.g., diet and lifestyle), which lead to reduced microorganism exposure can affect the microbiota composition and diversity. A microbiota imbalance or impairment (e.g., a combination of increased pathogenic microbes and loss of probiotics) in the gut and female reproductive tract alters immune cell profiles, disrupts normal immune function and compromises immunosurveillance, which leads to chronic states of aberrant immune activation and persistent inflammatory responses (8). This concept may be supported by epidemiological data that the prevalence of autoimmunity, allergy, inflammatory bowel disease and reproductive disease, such as endometriosis, is increasing in high-income countries (7,25,76,80). The so-called hygiene hypothesis initially focused on allergic diseases, but a reduced non-pathogenic commensal microbial diversity may cause numerous types of disease, including autoimmune diseases, through impaired immunoregulatory mechanisms (23). Microbiotas, inherited at birth, serve a role in an individual's predisposition to developing certain diseases (2). Moreover, endometriosis is generally more common in females with irritable bowel syndrome because both diseases are characterized by chronic inflammation (45,47). Interestingly, decreased NK activity (81) and increased risk of pregnancy complications (82,83) remain unchanged after surgery for endometriosis.
Microbiota dysbiosis and frequent retrograde menstruation can contribute to the development of endometriosis. Alterations of eubiosis to dysbiosis (the first event) may induce immune, metabolomic and estrobolomic disturbances, which can trigger systemic inflammation and contribute to the development and progression of endometriosis (7,8). Furthermore, frequent retrograde menstruation (the second event) causes repeated inflammation and serves a critical role in the development of endometriosis. The current retrograde menstrual theory does not provide an adequate explanation for why endometriosis occurs only in certain females while almost all females have retrograde menstruation. It can be hypothesized that only females who experience the first and second events, not just one of the two events, are more likely to develop endometriosis. The increased risk of developing endometriosis in daughters of mothers with endometriosis may be related to the effects of microbiota exposure (84). This evidence is at least partly explained by the hygiene hypothesis, which suggests that infections by microorganisms, such as commensals and symbionts, early in life prevent non-communicable inflammatory diseases (7,13).
Finally, preclinical and clinical studies have demonstrated no commonality in gut microbiota composition profiles across species. Several studies have reported that the microbial composition of the gut and reproductive tract is altered in females with endometriosis; however, its clinical relevance needs to be evaluated. Future studies will focus on the immune and molecular mechanisms which underlie the host-microbiota relationship to identify effective strategies to diagnose, manage and prevent endometriosis. In conclusion, this review provides an updated overview of the relationship between the gut and reproductive tract microbiome and endometriosis, and discusses how dysbiosis increases the risk of disease.
5. Summary and future perspective
Endometriosis has become a significant public health problem in Japan because of its higher prevalence in Asian females compared with that in Caucasian females (85). In recent years, the impact of symbiotic and pathogenic microorganisms on human health has attracted considerable attention. This review summarized the latest findings on the role of microbiomes in the study of endometriosis. The gut and reproductive tract microbiota components of females with endometriosis differ from those in healthy females, which may help identify potential biomarker candidates. Therefore, an in-depth analysis of microbiota compositions should be performed to assess the most appropriate prevention, diagnosis and treatment in patients with endometriosis. Further research is required to identify specific microbiota compositions that are altered in females with endometriosis and to explore the causal relationship.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
HK performed conception and design, acquisition of data, analysis and interpretation of data and wrote the manuscript. Data authentication is not applicable.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Author ORCID
ORCID: 0000-0002-8124-6269.
Competing interests
The author declares that they have no competing interests.
References
|
Sampson JA: Perforating hemorrhagic (chocolate) cysts of the ovary. Their importance and especially their relation to pelvic adenomas of the endometrial type (‘adenomyoma’ of the uterus, rectovaginal septum, sigmoid, etc.). Arch Surg. 3:245–323. 1921. | |
|
Amro B, Ramirez Aristondo ME, Alsuwaidi S, Almaamari B, Hakim Z, Tahlak M, Wattiez A and Koninckx PR: New understanding of diagnosis, treatment and prevention of endometriosis. Int J Environ Res Public Health. 19(6725)2022.PubMed/NCBI View Article : Google Scholar | |
|
Shafrir AL, Farland LV, Shah DK, Harris HR, Kvaskoff M, Zondervan K and Missmer SA: Risk for and consequences of endometriosis: A critical epidemiologic review. Best Pract Res Clin Obstet Gynaecol. 51:1–15. 2018.PubMed/NCBI View Article : Google Scholar | |
|
Eskenazi B and Warner ML: Epidemiology of endometriosis. Obstet Gynecol Clin North Am. 24:235–258. 1997.PubMed/NCBI View Article : Google Scholar | |
|
Emera D, Romero R and Wagner G: The evolution of menstruation: A new model for genetic assimilation: Explaining molecular origins of maternal responses to fetal invasiveness. Bioessays. 34:26–35. 2012.PubMed/NCBI View Article : Google Scholar | |
|
Mignemi G, Facchini C, Raimondo D, Montanari G, Ferrini G and Seracchioli R: A case report of nasal endometriosis in a patient affected by Behcet's disease. J Minim Invasive Gynecol. 19:514–516. 2012.PubMed/NCBI View Article : Google Scholar | |
|
Rook GA, Lowry CA and Raison CL: Microbial ‘old friends’, immunoregulation and stress resilience. Evol Med Public Health. 2013:46–64. 2013.PubMed/NCBI View Article : Google Scholar | |
|
Jiang I, Yong PJ, Allaire C and Bedaiwy MA: Intricate connections between the microbiota and endometriosis. Int J Mol Sci. 22(5644)2021.PubMed/NCBI View Article : Google Scholar | |
|
Symons LK, Miller JE, Kay VR, Marks RM, Liblik K, Koti M and Tayade C: The immunopathophysiology of endometriosis. Trends Mol Med. 24:748–762. 2018.PubMed/NCBI View Article : Google Scholar | |
|
Shigesi N, Kvaskoff M, Kirtley S, Feng Q, Fang H, Knight JC, Missmer SA, Rahmioglu N, Zondervan KT and Becker CM: The association between endometriosis and autoimmune diseases: A systematic review and meta-analysis. Hum Reprod Update. 25:486–503. 2019.PubMed/NCBI View Article : Google Scholar | |
|
Kobayashi H, Higashiura Y, Shigetomi H and Kajihara H: Pathogenesis of endometriosis: The role of initial infection and subsequent sterile inflammation (review). Mol Med Rep. 9:9–15. 2014.PubMed/NCBI View Article : Google Scholar | |
|
Agostinis C, Mangogna A, Bossi F, Ricci G, Kishore U and Bulla R: Uterine immunity and microbiota: A shifting paradigm. Front Immunol. 10(2387)2019.PubMed/NCBI View Article : Google Scholar | |
|
Ege MJ: The hygiene hypothesis in the age of the microbiome. Ann Am Thorac Soc. 14 (Suppl 5):S348–S353. 2017.PubMed/NCBI View Article : Google Scholar | |
|
Koninckx PR, Ussia A, Tahlak M, Adamyan L, Wattiez A, Martin DC and Gomel V: Infection as a potential cofactor in the genetic-epigenetic pathophysiology of endometriosis: A systematic review. Facts Views Vis Obgyn. 11:209–216. 2019.PubMed/NCBI | |
|
Hooper LV, Littman DR and Macpherson AJ: Interactions between the microbiota and the immune system. Science. 336:1268–1273. 2012.PubMed/NCBI View Article : Google Scholar | |
|
Pickard JM, Zeng MY, Caruso R and Núñez G: Gut microbiota: Role in pathogen colonization, immune responses, and inflammatory disease. Immunol Rev. 279:70–89. 2017.PubMed/NCBI View Article : Google Scholar | |
|
Baker JM, Al-Nakkash L and Herbst-Kralovetz MM: Estrogen-gut microbiome axis: Physiological and clinical implications. Maturitas. 103:45–53. 2017.PubMed/NCBI View Article : Google Scholar | |
|
Ervin SM, Li H, Lim L, Roberts LR, Liang X, Mani S and Redinbo MR: Gut microbial β-glucuronidases reactivate estrogens as components of the estrobolome that reactivate estrogens. J Biol Chem. 294:18586–18599. 2019.PubMed/NCBI View Article : Google Scholar | |
|
Laschke MW and Menger MD: The gut microbiota: A puppet master in the pathogenesis of endometriosis? Am J Obstet Gynecol. 215:68.e1–e4. 2016.PubMed/NCBI View Article : Google Scholar | |
|
Collado MC, Cernada M, Baüerl C, Vento M and Pérez-Martínez G: Microbial ecology and host-microbiota interactions during early life stages. Gut Microbes. 3:352–365. 2012.PubMed/NCBI View Article : Google Scholar | |
|
Schnupf P, Gaboriau-Routhiau V and Cerf-Bensussan N: Modulation of the gut microbiota to improve innate resistance. Curr Opin Immuno. 54:137–144. 2018.PubMed/NCBI View Article : Google Scholar | |
|
Benner M, Ferwerda G, Joosten I and van der Molen RG: How uterine microbiota might be responsible for a receptive, fertile endometrium. Hum Reprod Update. 24:393–415. 2018.PubMed/NCBI View Article : Google Scholar | |
|
Lambrecht BN and Hammad H: The immunology of the allergy epidemic and the hygiene hypothesis. Nat Immunol. 18:1076–1083. 2017.PubMed/NCBI View Article : Google Scholar | |
|
Gaffin JM, Kanchongkittiphon W and Phipatanakul W: Perinatal and early childhood environmental factors influencing allergic asthma immunopathogenesis. Int Immunopharmacol. 22:21–30. 2014.PubMed/NCBI View Article : Google Scholar | |
|
Crusell MKW, Hansen TH, Nielsen T, Allin KH, Rühlemann MC, Damm P, Vestergaard H, Rørbye C, Jørgensen NR, Christiansen OB, et al: Comparative studies of the gut microbiota in the offspring of mothers with and without gestational diabetes. Front Cell Infect Microbiol. 10(536282)2020.PubMed/NCBI View Article : Google Scholar | |
|
Jain N: The early life education of the immune system: Moms, microbes and (missed) opportunities. Gut Microbes. 12(1824564)2020.PubMed/NCBI View Article : Google Scholar | |
|
Qi X, Yun C, Pang Y and Qiao J: The impact of the gut microbiota on the reproductive and metabolic endocrine system. Gut Microbes. 13:1–21. 2021.PubMed/NCBI View Article : Google Scholar | |
|
Colquhoun C, Duncan M and Grant G: Inflammatory bowel diseases: Host-microbial-environmental interactions in dysbiosis. Diseases. 8(13)2020.PubMed/NCBI View Article : Google Scholar | |
|
Weiss GA and Hennet T: Mechanisms and consequences of intestinal dysbiosis. Cell Mol Life Sci. 74:2959–2977. 2017.PubMed/NCBI View Article : Google Scholar | |
|
Ling Z, Liu X, Luo Y, Wu X, Yuan L, Tong X, Li L and Xiang C: Associations between vaginal pathogenic community and bacterial vaginosis in Chinese reproductive-age women. PLoS One. 8(e76589)2013.PubMed/NCBI View Article : Google Scholar | |
|
He Y, Na R, Niu X, Xiao B and Yang H: Lactobacillus rhamnosus and Lactobacillus casei affect various stages of Gardnerella species biofilm formation. Front Cell Infect Microbiol. 11(568178)2021.PubMed/NCBI View Article : Google Scholar | |
|
Spurbeck RR and Arvidson CG: Inhibition of Neisseria gonorrhoeae epithelial cell interactions by vaginal Lactobacillus species. Infect Immun. 80(3742)2012. | |
|
Chen C, Song X, Wei W, Zhong H, Dai J, Lan Z, Li F, Yu X, Feng Q, Wang Z, et al: The microbiota continuum along the female reproductive tract and its relation to uterine-related diseases. Nat Commun. 8(875)2017.PubMed/NCBI View Article : Google Scholar | |
|
Peric A, Weiss J, Vulliemoz N, Baud D and Stojanov M: Bacterial colonization of the female upper genital tract. Int J Mol Sci. 20(3405)2019.PubMed/NCBI View Article : Google Scholar | |
|
Moreno I, Codoñer FM, Vilella F, Valbuena D, Martinez-Blanch JF, Jimenez-Almazán J, Alonso R, Alamá P, Remohí J, Pellicer A, et al: Evidence that the endometrial microbiota has an effect on implantation success or failure. Am J Obstet Gynecol. 215:684–703. 2016.PubMed/NCBI View Article : Google Scholar | |
|
Kallus Y, Miller JH and Libby E: Paradoxes in leaky microbial trade. Nat Commun. 8(1361)2017.PubMed/NCBI View Article : Google Scholar | |
|
Muzny CA, Łaniewski P, Schwebke JR and Herbst-Kralovetz MM: Host-vaginal microbiota interactions in the pathogenesis of bacterial vaginosis. Curr Opin Infect Dis. 33:59–65. 2020.PubMed/NCBI View Article : Google Scholar | |
|
Onderdonk AB, Delaney ML and Fichorova RN: The human microbiome during bacterial vaginosis. Clin Microbiol Rev. 29:223–238. 2016.PubMed/NCBI View Article : Google Scholar | |
|
Tai FW, Chang CYY, Chiang JH, Lin WC and Wan L: Association of pelvic inflammatory disease with risk of endometriosis: A nationwide cohort study involving 141,460 individuals. J Clin Med. 7(379)2018.PubMed/NCBI View Article : Google Scholar | |
|
Moghaddam MZ, Ansariniya H, Seifati SM, Zare F and Fesahat F: Immunopathogenesis of endometriosis: An overview of the role of innate and adaptive immune cells and their mediators. Am J Reprod Immunol. 87(e13537)2022.PubMed/NCBI View Article : Google Scholar | |
|
Khan KN, Kitajima M, Hiraki K, Yamaguchi N, Katamine S, Matsuyama T, Nakashima M, Fujishita A, Ishimaru T and Masuzaki H: Escherichia coli contamination of menstrual blood and effect of bacterial endotoxin on endometriosis. Fertil Steril. 94:2860–2863.e1-e3. 2010.PubMed/NCBI View Article : Google Scholar | |
|
Khan KN, Fujishita A, Hiraki K, Kitajima M, Nakashima M, Fushiki S and Kitawaki J: Bacterial contamination hypothesis: A new concept in endometriosis. Reprod Med Biol. 17:125–133. 2018.PubMed/NCBI View Article : Google Scholar | |
|
Yuan M, Li D, Zhang Z, Sun H, An M and Wang G: Endometriosis induces gut microbiota alterations in mice. Hum Reprod. 33:607–616. 2018.PubMed/NCBI View Article : Google Scholar | |
|
Bailey MT and Coe CL: Endometriosis is associated with an altered profile of intestinal microflora in female rhesus monkeys. Hum Reprod. 17:1704–1708. 2002.PubMed/NCBI View Article : Google Scholar | |
|
Seaman HE, Ballard KD, Wright JT and de Vries CS: Endometriosis and its coexistence with irritable bowel syndrome and pelvic inflammatory disease: Findings from a national case-control study-part 2. BJOG. 115:1392–1396. 2008.PubMed/NCBI View Article : Google Scholar | |
|
Ni Z, Sun S, Bi Y, Ding J, Cheng W, Yu J, Zhou L, Li M and Yu C: Correlation of fecal metabolomics and gut microbiota in mice with endometriosis. Am J Reprod Immunol. 84(e13307)2020.PubMed/NCBI View Article : Google Scholar | |
|
Kovács Z, Glover L, Reidy F, MacSharry J and Saldova R: Novel diagnostic options for endometriosis-based on the glycome and microbiome. J Adv Res. 33:167–181. 2021.PubMed/NCBI View Article : Google Scholar | |
|
Ata B, Yildiz S, Turkgeldi E, Brocal VP, Dinleyici EC, Moya A and Urman B: The endobiota study: Comparison of vaginal, cervical and gut microbiota between women with stage 3/4 endometriosis and healthy controls. Sci Rep. 9(2204)2019.PubMed/NCBI View Article : Google Scholar | |
|
Shan J, Ni Z, Cheng W, Zhou L, Zhai D, Sun S and Yu C: Gut microbiota imbalance and its correlations with hormone and inflammatory factors in patients with stage 3/4 endometriosis. Arch Gynecol Obstet. 304:1363–1373. 2021.PubMed/NCBI View Article : Google Scholar | |
|
Chadchan SB, Cheng M, Parnell LA, Yin Y, Schriefer A, Mysorekar IU and Kommagani R: Antibiotic therapy with metronidazole reduces endometriosis disease progression in mice: A potential role for gut microbiota. Hum Reprod. 34:1106–1116. 2019.PubMed/NCBI View Article : Google Scholar | |
|
Molina NM, Sola-Leyva A, Saez-Lara MJ, Plaza-Diaz J, Tubić-Pavlović A, Romero B, Clavero A, Mozas-Moreno J, Fontes J and Altmäe S: New opportunities for endometrial health by modifying uterine microbial composition: Present or future? Biomolecules. 10(593)2020.PubMed/NCBI View Article : Google Scholar | |
|
Itoh H, Uchida M, Sashihara T, Ji ZS, Li J, Tang Q, Ni S, Song L and Kaminogawa S: Lactobacillus gasseri OLL2809 is effective especially on the menstrual pain and dysmenorrhea in endometriosis patients: Randomized, double-blind, placebo-controlled study. Cytotechnology. 63:153–161. 2011.PubMed/NCBI View Article : Google Scholar | |
|
Hantschel J, Weis S, Schäfer KH, Menger MD, Kohl M, Egert M and Laschke MW: Effect of endometriosis on the fecal bacteriota composition of mice during the acute phase of lesion formation. PLoS One. 14(e0226835)2019.PubMed/NCBI View Article : Google Scholar | |
|
Khan KN, Fujishita A, Masumoto H, Muto H, Kitajima M, Masuzaki H and Kitawaki J: Molecular detection of intrauterine microbial colonization in women with endometriosis. Eur J Obstet Gynecol Reprod Biol. 199:69–75. 2016.PubMed/NCBI View Article : Google Scholar | |
|
Hernandes C, Silveira P, Rodrigues Sereia AF, Christoff AP, Mendes H, Valter de Oliveira LF and Podgaec S: Microbiome profile of deep endometriosis patients: Comparison of vaginal fluid, endometrium and lesion. Diagnostics (Basel). 10(163)2020.PubMed/NCBI View Article : Google Scholar | |
|
Wei W, Zhang X, Tang H, Zeng L and Wu R: Microbiota composition and distribution along the female reproductive tract of women with endometriosis. Ann Clin Microbiol Antimicrob. 19(15)2020.PubMed/NCBI View Article : Google Scholar | |
|
Dols JA, Molenaar D, van der Helm JJ, Caspers MP, de Kat Angelino-Bart A, Schuren FH, Speksnijder AG, Westerhoff HV, Richardus JH, Boon ME, et al: Molecular assessment of bacterial vaginosis by Lactobacillus abundance and species diversity. BMC Infect Dis. 16(180)2016.PubMed/NCBI View Article : Google Scholar | |
|
Chang CY, Chiang AJ, Lai MT, Yan MJ, Tseng CC, Lo LC, Wan L, Li CJ, Tsui KH, Chen CM, et al: A more diverse cervical microbiome associates with better clinical outcomes in patients with endometriosis: A pilot study. Biomedicines. 10(174)2022.PubMed/NCBI View Article : Google Scholar | |
|
Vallvé-Juanico J, Houshdaran S and Giudice LC: The endometrial immune environment of women with endometriosis. Hum Reprod Update. 25:564–591. 2019.PubMed/NCBI View Article : Google Scholar | |
|
Shi N, Li N, Duan X and Niu H: Interaction between the gut microbiome and mucosal immune system. Mil Med Res. 4(14)2017.PubMed/NCBI View Article : Google Scholar | |
|
Chadchan SB, Singh V and Kommagani R: Female reproductive dysfunctions and the gut microbiota. J Mol Endocrinol. 69:R81–R94. 2022.PubMed/NCBI View Article : Google Scholar | |
|
Collins SM, Surette M and Bercik P: The interplay between the intestinal microbiota and the brain. Nat Rev Microbiol. 10:735–742. 2012.PubMed/NCBI View Article : Google Scholar | |
|
Ferreira A and Caceres A: Estrogen-enhanced neurite growth: Evidence for a selective induction of Tau and stable microtubules. J Neurosci. 11:392–400. 1991.PubMed/NCBI View Article : Google Scholar | |
|
Toran-Allerand CD, Miranda RC, Bentham WD, Sohrabji F, Brown TJ, Hochberg RB and MacLusky NJ: Estrogen receptors colocalize with low-affinity nerve growth factor receptors in cholinergic neurons of the basal forebrain. Proc Natl Acad Sci USA. 89:4668–4672. 1992.PubMed/NCBI View Article : Google Scholar | |
|
Ustianowska K, Ustianowski Ł, Machaj F, Gorący A, Rosik J, Szostak B, Szostak J and Pawlik A: The role of the human microbiome in the pathogenesis of pain. Int J Mol Sci. 23(13267)2022.PubMed/NCBI View Article : Google Scholar | |
|
Talwar C, Singh V and Kommagani R: The gut microbiota: A double-edged sword in endometriosis†. Biol Reprod. 107:881–901. 2022.PubMed/NCBI View Article : Google Scholar | |
|
Yalçın Bahat P, Ayhan I, Üreyen Özdemir E, İnceboz Ü and Oral E: Dietary supplements for treatment of endometriosis: A review. Acta Biomed. 93(e2022159)2022.PubMed/NCBI View Article : Google Scholar | |
|
Hopeman MM, Riley JK, Frolova AI, Jiang H and Jungheim ES: Serum polyunsaturated fatty acids and endometriosis. Reprod Sci. 22:1083–1087. 2015.PubMed/NCBI View Article : Google Scholar | |
|
Attaman JA, Stanic AK, Kim M, Lynch MP, Rueda BR and Styer AK: The anti-inflammatory impact of omega-3 polyunsaturated Fatty acids during the establishment of endometriosis-like lesions. Am J Reprod Immunol. 72:392–402. 2014.PubMed/NCBI View Article : Google Scholar | |
|
Kelly OJ, Gilman JC, Kim Y and Ilich JZ: Long-chain polyunsaturated fatty acids may mutually benefit both obesity and osteoporosis. Nutr Res. 33:521–533. 2013.PubMed/NCBI View Article : Google Scholar | |
|
Uchida M and Kobayashi O: Effects of Lactobacillus gasseri OLL2809 on the induced endometriosis in rats. Biosci Biotechnol Biochem. 77:1879–1881. 2013.PubMed/NCBI View Article : Google Scholar | |
|
Itoh H, Sashihara T, Hosono A, Kaminogawa S and Uchida M: Lactobacillus gasseri OLL2809 inhibits development of ectopic endometrial cell in peritoneal cavity via activation of NK cells in a murine endometriosis model. Cytotechnology. 63:205–210. 2011.PubMed/NCBI View Article : Google Scholar | |
|
Khodaverdi S, Mohammadbeigi R, Khaledi M, Mesdaghinia L, Sharifzadeh F, Nasiripour S and Gorginzadeh M: Beneficial effects of oral Lactobacillus on pain severity in women suffering from endometriosis: A pilot placebo-controlled randomized clinical trial. Int J Fertil Steril. 13:178–183. 2019.PubMed/NCBI View Article : Google Scholar | |
|
Quaranta G, Sanguinetti M and Masucci L: Fecal microbiota transplantation: A potential tool for treatment of human female reproductive tract diseases. Front Immunol. 10(2653)2019.PubMed/NCBI View Article : Google Scholar | |
|
Chadchan SB, Popli P, Ambati CR, Tycksen E, Han SJ, Bulun SE, Putluri N, Biest SW and Kommagani R: Gut microbiota-derived short-chain fatty acids protect against the progression of endometriosis. Life Sci Alliance. 4(e202101224)2021.PubMed/NCBI View Article : Google Scholar | |
|
Salliss ME, Farland LV, Mahnert ND and Herbst-Kralovetz MM: The role of gut and genital microbiota and the estrobolome in endometriosis, infertility and chronic pelvic pain. Hum Reprod Update. 28:92–131. 2021.PubMed/NCBI View Article : Google Scholar | |
|
Cho I and Blaser MJ: The human microbiome: At the interface of health and disease. Nat Rev Genet. 13:260–270. 2012.PubMed/NCBI View Article : Google Scholar | |
|
Zhong SR, Kuang Q, Zhang F, Chen B and Zhong ZG: Functional roles of the microbiota-gut-brain axis in Alzheimer's disease: Implications of gut microbiota-targeted therapy. Transl Neurosci. 12:581–600. 2021.PubMed/NCBI View Article : Google Scholar | |
|
Zhou JZ, Way SS and Chen K: Immunology of uterine and vaginal mucosae. Trends Immunol. 39:302–314. 2018.PubMed/NCBI View Article : Google Scholar | |
|
Petraglia F, Serour GI and Chapron C: The changing prevalence of infertility. Int J Gynaecol Obstet. 123 (Suppl 2):S4–S8. 2013.PubMed/NCBI View Article : Google Scholar | |
|
Vercellini P, Sergenti G, Buggio L, Frattaruolo MP, Dridi D and Berlanda N: Advances in the medical management of bowel endometriosis. Best Pract Res Clin Obstet Gynaecol. 71:78–99. 2020.PubMed/NCBI View Article : Google Scholar | |
|
Nirgianakis K, Ma L, McKinnon B and Mueller MD: Recurrence patterns after surgery in patients with different endometriosis subtypes: A long-term hospital-based cohort study. J Clin Med. 9(496)2020.PubMed/NCBI View Article : Google Scholar | |
|
Nirgianakis K, Gasparri ML, Radan AP, Villiger A, McKinnon B, Mosimann B, Papadia A and Mueller MD: Obstetric complications after laparoscopic excision of posterior deep infiltrating endometriosis: A case-control study. Fertil Steril. 110:459–466. 2018.PubMed/NCBI View Article : Google Scholar | |
|
Dalsgaard T, Hjordt Hansen MV, Hartwell D and Lidegaard O: Reproductive prognosis in daughters of women with and without endometriosis. Hum Reprod. 28:2284–2288. 2013.PubMed/NCBI View Article : Google Scholar | |
|
Yamamoto A, Johnstone EB, Bloom MS, Huddleston HG and Fujimoto VY: A higher prevalence of endometriosis among Asian women does not contribute to poorer IVF outcomes. J Assist Reprod Genet. 34:765–774. 2017.PubMed/NCBI View Article : Google Scholar |