Ozone therapy in dentistry: An overview of the biological mechanisms involved (Review)
- Authors:
- Published online on: June 12, 2024 https://doi.org/10.3892/br.2024.1803
- Article Number: 115
-
Copyright: © Veneri et al. This is an open access article distributed under the terms of Creative Commons Attribution License.
Abstract
1. Introduction
Ozone (O3) is a naturally occurring compound formed from three oxygen atoms as a result of conversion by ultraviolet radiation (1). At low medical concentrations, ozone acts as a powerful oxidant with wide-spectrum antimicrobial activity and the ability to promote healing processes and reduce inflammation through protective antioxidant pathways, thus providing therapeutic benefits in the treatment of a number of diseases (2-5).
The antimicrobial action is due to ozone-induced oxidation, which causes direct and indirect damage to microbial cell structures and metabolism (6-8). Ozone is also involved in pharmacological immunomodulation as it induces mild oxidative stress, which triggers antioxidant responses through the activation of specific molecular pathways, activating anti-inflammatory mechanisms (9,10). Additionally, topical application of ozone has been reported to improve the rheological properties of blood by inducing functional and structural changes at the erythrocyte level, thereby enhancing peripheral oxygen perfusion and general metabolism (11-13).
Due to its strong oxidizing activity, ozone has been used as a disinfectant and germicidal agent for both industrial and medical purposes, with a variety of applications as a sterilizing agent for water treatment, medical equipment, dental settings and closed environments (14-18). Since 1930, ozone has also been studied for applications in dentistry (1,19). However, these studies were abandoned until the 1980s, due to the risk of inhalation toxicity and the difficulty of achieving optimal gas concentrations without dispersion (20). These issues have been addressed with modern technology and appropriate delivery and application techniques (19). A number of parenteral and topical routes are currently used to deliver ozone without toxic effects, with the exception of the inhalation route, which should be avoided due to broncho-pulmonary toxicity (11). The most widely used formulations for the oral cavity are gaseous ozone, ozonized water and ozonized oil (21).
The recommended concentration for a mixture of oxygen and ozone for medical use is between 5-50 µg ozone/1 ml oxygen (11). According to guidelines and good clinical practice recommendations in oxygen-ozone therapy published in the the World Federation of Ozone Therapy's Review on Evidence Based Ozone Therapy (20), an ozone concentration of 5-10 µg/ml is recommended for the application of ozonized products by intralesional injection or irrigation in order to obtain a therapeutic effect with no risk of toxicity, even in the event of ingestion (22,23).
Along with predominantly beneficial properties, the occurrence of some potential adverse effects must be considered, primarily in relation to toxicity upon inhalation. Excessive or prolonged exposure to gaseous ozone, which is more likely to occur in operators than in patients, may cause headaches, vomiting and irritation of the upper respiratory tract, which can manifest as a sore throat, cough, epiphora, rhinitis and bronchoconstriction (24,25). Additionally, certain forms of ozone therapy, namely those that induce systemic effects, are contraindicated in myocardial infarction, hyperthyroidism, acute alcohol intoxication, severe anemia, thrombocytopenia, active hemorrhage and pregnancy (21,26,27). However, ozone therapy is non-toxic to humans and is free of side effects when handled with care, using modern available technology in accordance with the manufacturer's instructions and current guidelines and recommendations (22).
At present, ozone therapy is implemented in certain fields of dentistry, including implantology, oral surgery, periodontology, oral medicine and management of dental caries (28-31). Ozone applications have yielded promising results in the treatment of inflammatory and immune-mediated conditions of oral soft tissues, such as oral lichen planus and aphthous stomatitis (32,33). In addition, ozone has been applied in the context of post-operative wounds and complications such as alveolar osteitis, which alleviated painful symptoms and reduced healing times (34,35). Additionally, ozone has generally contributed to a reduction of possible side effects associated with traditional treatments of these oral ulcerative conditions and surgical wounds and complications (33,34,36). Due to its antimicrobial activity, ozone has been reported to be useful in the management of oral disease of infectious etiology, such as herpetic stomatitis and oral candidiasis (37). In addition, a beneficial remineralizing effect on hard dental tissues has been reported and used to treat dental demineralization and decay (38-41). In particular, ozone application has been suggested as a possible alternative or complementary strategy to manage dental caries in a less invasive and more comfortable procedure than traditional approaches, especially in young or poorly cooperative children (31,41,42). Since dental caries is considered a global public health challenge, especially in children and the socioeconomically disadvantaged population, the implementation of effective, safe and economic interventions to prevent and treat caries should be a priority in oral health care policies (43-45).
Overall, ozone therapy may indirectly help restore functional activities compromised by disease by targeting certain biological processes as a biological response modifier (10). Although ozone therapy is now used in a number of countries, it has mostly been adopted by private health care practitioners (46). Over the past four decades, practitioners in Europe have mainly used ozone therapy empirically, potentially due to a lack of adequate knowledge of the basic principles behind optimal use (46,47). This approach has sometimes led to the inappropriate or dangerous use of ozone, which has contributed to a general misconception of its intrinsic toxicity (46,47). These issues, combined with the difficulty and cost of conducting extensive clinical trials, lack of interest by pharmaceutical companies and health authorities, as well as a lack of sponsorship, have hindered progress in this field of research, so that ozone therapy remains a poorly known and controversial complementary medical practice (47). Nevertheless, a number of studies have been carried out with promising results (2-4,29). The general mechanisms of ozone interaction with human tissues have been increasingly studied and progress has previously been made in this area of research. However, the underlying mechanisms explaining the effects of ozone on dental tissues are complex and are not yet fully understood.
The aim of the present review was to provide an overview of the currently available evidence on the mechanisms involved in ozone interaction with dental tissues underlying the beneficial effects of ozone in dentistry.
2. Literature search methods
The present review focused on the current relevant evidence on the biological mechanisms underlying the interaction of ozone with dental tissues, including periodontal structures, dental cells, enamel and dentine.
A literature search was performed using the PubMed/MEDLINE database (https://pubmed.ncbi.nlm.nih.gov) until March 22, 2024. The online literature search was performed using combined terms related to ozone and possible biological effects [‘ozone’ AND ‘antimicrobial’, ‘remineralization’, ‘immunomodulation’, ‘biostimulation’, ‘regeneration’, ‘dental cells’, ‘periodontal cells’, ‘odontoblasts’, ‘cementoblasts’, ‘osteoblasts’ AND ‘(dental OR dentistry OR oral)’] (Fig. 1). Among all records retrieved through the literature search, reviews or papers with an experimental design specifically addressing ozone-related biological mechanisms in oral tissues were identified as key references. Relevant in vitro studies, in addition to animal and human studies, published in English were considered, without restrictions on the publication date.
3. General mechanisms involved in the interaction of ozone with dental tissues
A total of 487 potentially relevant records were retrieved as a result of the literature search. The number of studies decreased to 389 after duplicate removal, which was performed using the Rayyan software algorithm (48). Handsearching was conducted by manually screening the references of the considered studies to identify possible additional relevant papers.
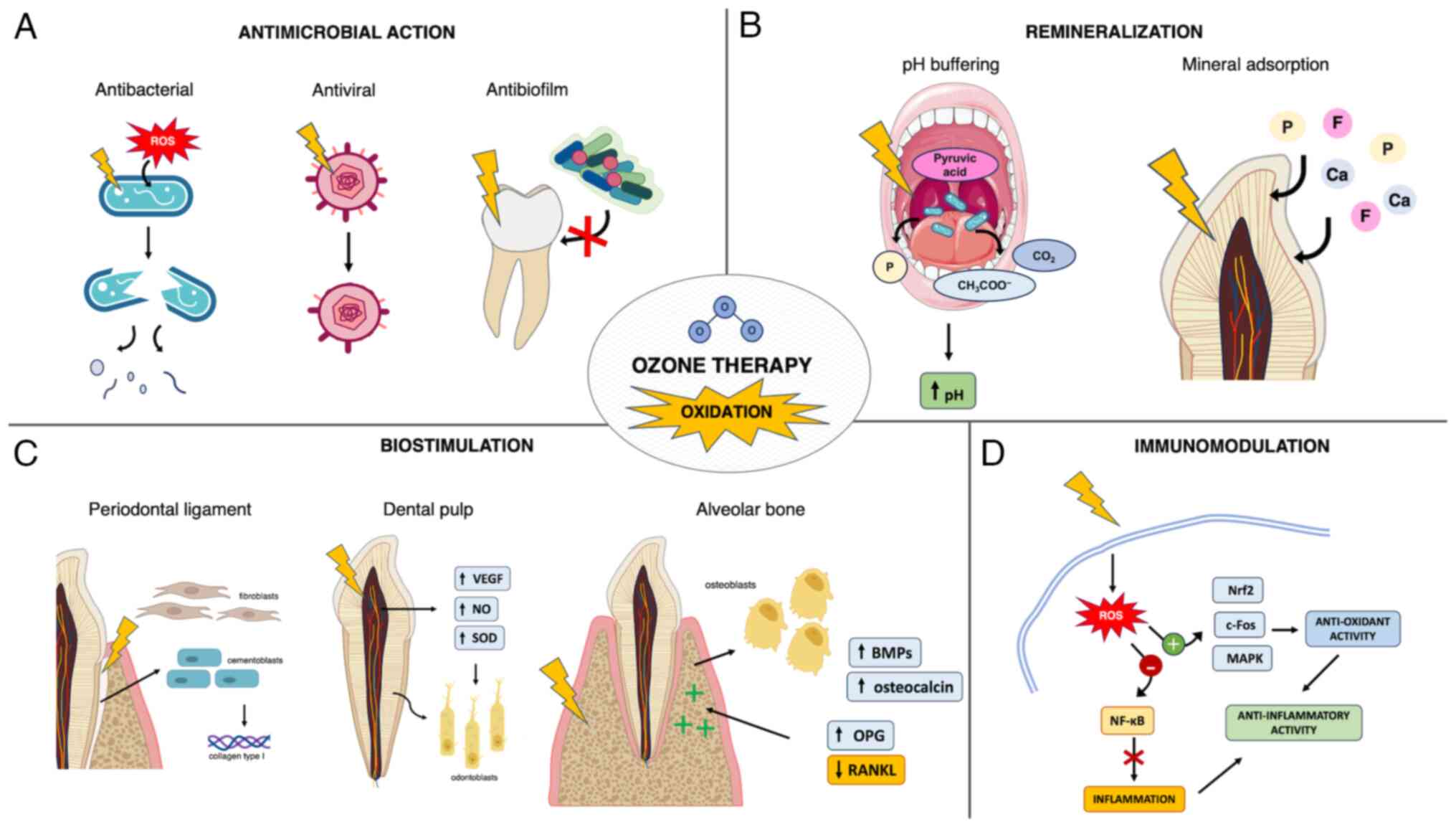
The key references considered in the present review are listed in Table I. Of these studies, 16 were in vitro studies, seven were based on animal models including one with an additional human clinical phase, one was designed as a human ex vivo study and one was a review. In the field of dentistry, the main beneficial effects of ozone could be summarized in the following domains: Antimicrobial activity, remineralization through direct interaction with hard dental tissues, immunomodulation and biostimulation of dental and periodontal cells (Fig. 2).
Table IKey references concerning ozone mechanisms considered in the present review ordered by publication date. |
4. Antimicrobial action
Ozone exhibits broad antimicrobial activity through oxidation, which causes dysfunction of the bacterial cell wall and cytoplasmic membrane, resulting in increased permeability to ozone molecules (7). Consequently, glycoproteins, glycolipids and amino acids are damaged and the enzymatic system is impaired, ultimately leading to cell lysis and death (6,8). Moreover, ozone reacts with unsaturated fatty acids in cell membranes, producing secondary reactive species such as aldehyde derivatives and lipid peroxides. These can reach the nuclei of intact cells, leading to nucleic acid breakdown that also results in the release of phosphate ions (49). The underlying mechanism by which this occurs remains largely unknown, but may explain the ozone buffering effect on biofilm fluids, which promotes a remineralizing environment (40). The bactericidal activity of ozone may be mediated by functional and structural disruption occurring at the level of the cytoplasmic membrane (50). Ozonized water (0.5-4.0 mg/l) has shown effective bactericidal properties against various strains of cariogenic bacteria and oral pathogens in vitro. Among these, however, gram-negative bacteria such as Porphyromonas endodontalis and Porphyromonas gingivalis were observed to be more sensitive to ozonized water compared with gram-positive streptococci and Candida albicans (50). Such discrepancies have been linked to differences in the structure of microorganism cell walls (51). The effect of ozone was also investigated on C. albicans and Enterococcus faecalis in infected root dentinal tubules, alone or in association with other antimicrobial agents (52). Ozonized water significantly reduced the number of C. albicans and E. faecalis, but showed no residual effect and activity against lipopolysaccharide (LPS) endotoxin (53). The authors hypothesized that these findings were related to the poor penetration of irrigants into the inner regions of dentinal tubules, allowing the remaining microorganisms to multiply over the course of the experiment (53).
The aforementioned findings were supported by a study that investigated the effects of ozone on an oral biofilm of Streptococcus mutans and Lactobacillus acidophilus, which reported no evidence of bacterial colonization on ozone-treated dentin surfaces in vitro (54). Due to the short half-life of ozone, however, it is unlikely that ozone could have interfered with the activity of the bacterial glycosyltransferase enzyme (54). This enzyme is responsible for the synthesis of polysaccharides that serve an essential role in biofilm formation and adhesion of the microorganism to tooth surfaces (55). It is more likely for ozone treatment to alter the dentin surface, making it more difficult for organisms to colonize the surface (6,54). Ozone oxidation is capable of altering organic surface components, such as collagen, and inducing the release of specific low molecular weight salivary biomolecules from macromolecular binding sites (56). This may result in ‘smoothing’ of the protein molecules, thereby affecting the wettability of the dentin surface.
Lastly, ozone has also exhibited promising virucidal effects. A recent study on severe acute respiratory syndrome coronavirus 2 reported that ozonized water rapidly caused conformational changes in the S1 subunit of the S protein (57). Based on previous studies, the authors also hypothesized that ozone could oxidize the glycoprotein on viral particles such as the viral envelope, which may contribute to virus inactivation (57,58). These mechanisms may be similarly involved in viruses commonly affecting the oral cavity, such as herpes simplex virus type 1(59). Additionally, ozone activated the immune system in immunosuppressed rats by stimulating T-cell mediated immunity and enhancing the immune response against pathogens (59,60).
5. Remineralization and interaction with dental hard tissues
With regard to the direct interaction of ozone with hard dental tissues, studies were retrieved that investigated the effects of ozone applications on physical and microstructural properties of enamel and dentin.
Celiberti et al (6) reported that gaseous ozone applications on sound enamel caused no significant changes to enamel physical properties, such as microhardness and acid resistance, without compromising etching and adhesive procedures for the placement of resinous restorative materials. The authors also reported that after initial reversible slight dehydration, ozone did not significantly change the free surface energy of the enamel. Consequently, the enamel-acid contact angle and interfacial area remained unaltered, which could explain the unchanged acid resistance. Similarly, Floare et al (24) measured an increase in enamel microhardness by dehydration, which was based on an early assessment after gaseous ozone application on demineralized enamel. At a microstructural level, the aforementioned study observed a progressive leveling of the enamel surface, opening of interprismatic canals and enamel prism pattern homogenization resulting from oxidation-induced disruption of the proteins embedded in the enamel matrix. Ozone oxidative proteolytic action can break down the organic contents of demineralized enamel and enhance diffusion of remineralizing agents. Additionally, ozone-induced oxidation of microbial pyruvic acid into acetate and CO2 has a buffering effect, which counteracts the acid environment sustaining the carious process, thus promoting surface remineralization (24,38). Furthermore, a randomized controlled clinical trial reported that gaseous ozone treatment of non-cavitated carious lesions in primary teeth produced results comparable with fluoride varnish application in terms of fluorescence values and visual inspection index, with little or no caries progression reported in both treatment groups (61). These findings suggested that in young or uncooperative patients where accidental ingestion of topical products may occur, ozone may be a valid alternative to professionally applied topical fluorides to achieve remineralization (62). Parents or caregivers may also object to the use of fluorides, as early excessive exposure to fluoride has been implicated, albeit controversially, in a number of potential adverse health effects (63-66).
Although the effect of ozone on dentin is less well studied, it has been reported that, in addition to improving diffusion of salivary ions to the surface of degraded dentin, ozone can neutralize acidic proteins produced by cariogenic bacteria. These constitute the osmotic stimuli responsible for the movement of fluids in dentin tubules that causes hypersensitivity (67,68). Moreover, promising results have been reported in preliminary studies investigating ozone management of dentine hypersensitivity (67,68). Ozone oxidation of organic matter occurs through a selective reaction with molecules containing double bonds, amines or activated organic groups (68). Additionally, the resulting ozone byproducts, such as free hydroxyl radicals, undergo a stronger oxidizing action that selectively involves the organic components of dentin (69). These direct and indirect reactions may be responsible for the preferential degradation of demineralized peritubular dentin, which results in an increase in dentinal tubule diameter (68). This, associated with the local presence of remineralizing agents, enhances their deposition in open dentinal tubules, which can lead to increased tubular occlusion rates (68). Furthermore, in vitro evidence suggested that gaseous ozone autonomously induces occlusion of dentinal tubules, similarly to erbium-doped yttrium-aluminum-garnet laser treatment. This resulted in a significant reduction in the diameter and number of open dentinal tubules, which may be due to the immediate precipitation of minerals from oxidation induced-disruption of the surface matrix (70). Such ozone-induced effects could be affected by specific experimental setup and protocols, which have not been compared in the currently available literature.
Lastly, it has been reported that ozone interferes, albeit partially, with the activation of embedded dentin metalloproteases (MMPs) more than other common antiseptic agents (71). Dentin MMPs serve an important role in the progression of dental caries, as they can degrade the collagen matrix and negatively affect the adhesive interface of restorations (71).
6. Biostimulation of dental and periodontal structures
With regard to the effect on dental cells, ozone was reported to stimulate the odontodifferentiation potential of dental pulp cells and the production of mineral nodules in vitro, possibly acting as an indirect biostimulator of tertiary dentin formation in vivo (71). Evidence of ozone enhancing dental pulp regenerative potential has been reported by additional previous studies (72-74). An in vitro study by Noguchi et al (74) reported that, while bacterial LPS toxin inhibits the formation of mineralized nodules by odontoblast cells, ozonized water exposure can restore this property by reducing inflammatory response in odontoblast cells, exhibiting a direct toxic effect against bacterial LPS in vitro. Similarly, gaseous ozone application followed by the placement of mineral trioxide aggregate (MTA) was reported to improve pulp regenerative potential by increasing odontoblast proliferation and dentin bridge formation in an in vivo animal study, when compared with the placement of MTA alone (72). The increased ability to normalize pulp microcirculation and reduce inflammation was also observed in a concurrent human clinical study in cases of accidental pulpal exposure (72). The interaction of ozone with protein-lipid complexes of cell membranes and blood plasma promotes the synthesis of biologically active compounds, enhances the activity of immunocompetent cells and improves rheology and oxygen-carrying capacity of the blood (75). Similar findings were reported by Küçük et al (73) who observed that ozonized water at different concentrations demonstrated a good biocompatibility according to cell viability and enhanced the proliferation of primary dental pulp cells in vitro. This finding may also support the potential application of ozone as an adjuvant irrigant in the field of regenerative endodontics.
Ozone has also been reported to stimulate tissue regeneration of dental pulp. Similar to healing and regeneration processes occurring in other types of tissues, the mechanisms involved in the aforementioned process include increased expression of VEGF, increased levels of nitric oxide (NO) and reduced oxidative stress due to increased activity of antioxidant enzymes such as superoxide dismutase (SOD) (76-79). NO is involved in pulp healing as a regulator of vascular homeostasis and pulp afferent sensitivity, as an indicator of cell differentiation and as a mediator of inflammatory activity (80). In particular, one of the three isoenzymes of NO synthase (NOS), neuronal NOS (nNOS), is typically present in healthy human pulp tissue, which demonstrates its involvement in pulp homeostasis (81). VEGF is responsible for the angiogenesis, activity and differentiation of odontoblasts and a slight increase in VEGF levels may be related to an increase in pulp vascularization inducing a beneficial response (81,82). A previous study reported increased VEGF and nNOS levels and decreased SOD levels in pulp tissue following a single application of gaseous ozone in the deep cavities of healthy teeth, where increased SOD activity and subsequent depletion may have occurred in response to mild oxidative stress induced by ozone (81). These findings also indicated that ozone could effectively diffuse through dentin to pulp tissue after a single application (81).
Additionally, promising effects of ozone on periodontal cells and structures have been previously reported, which may support the notion that ozone has biostimulating effects. Cementoblasts and fibroblasts in the residual periodontal ligament (PDL) adhering to the root surface of healthy avulsed teeth showed a higher proliferation rate when irrigated with ozonized water compared with saline (83). This suggested that ozone, in addition to effectively decontaminating the root surface, may also promote periodontal regeneration after the reimplantation of avulsed teeth. These findings have been supported by several studies investigating the biostimulatory potential of ozone on the periodontal structures and bone remodeling. Ozonized oil has been reported to significantly promote the production of type I collagen by human gingival fibroblasts, which was associated with the regenerative capacity of periodontal tissues (84). Furthermore, ozone, particularly ozonized water, has shown optimal biocompatibility with gingival fibroblasts, outperforming other antiseptics commonly used in oral care such as chlorhexidine digluconate (85,86).
Beneficial effects of ozone on bone metabolism have also been reported. The application of gaseous ozone was reported to improve bone metabolism and homeostasis in a zoledronate-treated rat model of osteoporosis, which showed a synergistic effect between zoledronate and ozone associated with increased bone regeneration (87). This ozone-induced biostimulatory effect was further confirmed by other previous studies that reported increased bone regeneration of calvarial defects of both healthy rats and rats with diabetes mellitus (88,89). Diabetes mellitus and hyperglycemia are associated with impaired healing processes due to activation of the NF-κB pathway, as well as increased alkaline phosphatase expression and suppressed osteocalcin, MMP-13, VEGF and glycolytic enzyme levels in osteoblasts, resulting in osteoporosis (90,91). Conversely, an increased number of osteoblastic cells and increased expression levels of osteocalcin and bone morphogenetic protein-2 (BMP-2) were observed in diabetic rat calvarial defects treated with xenografts and ozone compared with a xenograft-only group, which suggested ozone-induced improved bone formation and remodeling (89).
Similar results have been reported for periodontal disease-induced bone destruction (92,93). Untreated periodontal pockets caused by the presence of anaerobic pathogens, vascular endothelial damage, edema and increased inflammatory cell infiltration, exhibit low oxygen levels (94). HIF-1α (hypoxia-inducible factor 1α) is activated by pro-inflammatory signals in periodontal cells and gingival tissue and is the major regulatory protein that responds to hypoxia (94). Hypoxia also causes an increase in the release of receptor activator of NF-κB ligand (RANKL), which causes bone resorption and a decrease in the level of osteoprotegerin (OPG), which is responsible for bone formation (95). Furthermore, a study investigating the effects of ozone therapy on periodontal disease-induced bone destruction in rats reported a lower number of HIF-1-α positive cells in the ozone-treated group compared with both positive and negative controls, as well as significantly fewer RANKL-stained cells. Conversely, OPG levels were higher in the ozone-treated group, which suggests that ozone therapy may potentially be effective in promoting bone healing (92).
7. Immunomodulation
Knowledge of the underlying immunomodulatory mechanisms of ozone is developing and is important in a number of medical fields, including oral medicine. The pharmacological activity of medical ozone mainly depends on the ability of ozone byproducts to induce mild reactive oxygen species (ROS) signaling or mitochondrial stress that triggers an antioxidant response (9). This occurs through the activation of the Nrf2 (nuclear factor erythroid 2-related factor)-mediated system and inhibition of NF-κB pathway, which modulates immunity toward anti-inflammatory mechanisms (9). Ozone has also been used to treat myofascial pain and inflammation associated with temporomandibular disorders in rats (96). Despite a lack of improvement in nociception and inflammation that could have been due to the local infiltrative route of ozone administration and mechanically damaged muscle tissue, there was significant evidence of ozone stimulating collagen deposition and tissue repair (96). This finding was attributed to ozone-induced stimulation of fibroblast migration and increased immunohistochemical expression of platelet-derived growth factor, TGF-β and VEGF, which serve essential roles in tissue repair (96).
With specific reference to the dental field, Huth et al (10) investigated the effect of aqueous ozone on NF-κB-associated signaling both in periodontal ligament tissue from root surfaces of periodontally damaged teeth and in oral cells stimulated with TNF. It was reported that the NF-κB pro-inflammatory pathway was inhibited following incubation with ozonized medium, through ozone-mediated prevention of NF-κB inhibitor IκBα proteolysis, cytokine expression and κB-dependent transcription (10,59). Furthermore, the inhibitory effect was not directly caused by ozone, but was mediated, to varying degrees, by the formation of specific ozonized amino acids (10). Similarly, Leewananthawet et al (93) reported that ozone ultrafine bubble water induced oxidative stress through ROS production in human primary periodontal ligament fibroblasts. Consequently, certain genes and pathways involved in oxidative stress responses, such as c-Fos, Nrf2 and p38-MAPK signaling were upregulated after ozone treatment. Furthermore, the MAPK pathways have been reported to be associated with BMP-9-mediated differentiation of PDL fibroblasts to osteoblastic-like cells, possibly serving a pivotal role in regenerating mineralized dental tissues (93,97).
8. Limitations
There are certain limitations to the present review, mainly related to the available evidence. A number of the studies considered were published >10 years ago. However, a number of these studies were informative and were important sources for the purposes of the present review. Furthermore, most of the evidence reviewed was based on in vitro studies which, unlike animal models, may not be able to replicate real clinical conditions and may therefore only provide partial insights into the in vivo mechanisms of action of ozone. Finally, the limited availability and heterogeneity of the relevant literature prevented the assessment of the specific effects of different ozone formulations and protocols of application.
9. Clinical relevance and future perspectives
Future studies should focus on investigating different formulations, concentrations and application protocols of ozone in order to induce the specific desired effects. In addition, further research is warranted in the less-studied fields, such as dental cell biostimulation and pulp regeneration. These are topics of increasing interest to clinicians, therefore, in-depth research could expand clinical applications of ozone in a number of areas of dentistry, including cariology and regenerative endodontics. The development of in vitro experimental models through modern technologies could help overcome the limitations of animal studies, such as cost and ethical issues and traditional in vitro settings, which fail to replicate the biological environment. Selected optimal protocols should then be tested in well-designed randomized clinical trials. This would allow comparisons between established traditional treatments for specific oral health conditions, both in terms of clinical efficacy and patient acceptance. For example, the use of ozone for oral ulcerative lesions of various etiologies, as well as for the treatment of dental caries in young children, has shown promising results (31,32). This has provided an alternative treatment option that is less invasive compared with traditional approaches, free of side effects and with good patient compliance.
10. Conclusions
A variety of biological mechanisms acting through multiple biochemical target pathways are responsible for the therapeutic effects of ozone. These mechanisms are complex and at present not fully understood, especially with respect to specific effects on the oral and dental environment. Overall, they include antimicrobial action, immunomodulatory and biostimulatory effects and the direct modifications of hard dental tissues. Additional research in this area could provide further insights, enhance the use of ozone for broader medical purposes and dentistry applications and assist in the selection of targeted and more effective ozone treatment protocols.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by grant 'FAR UNIMORE 2023' from the University of Modena and Reggio Emilia.
Availability of data and materials
Not applicable.
Authors' contributions
FV, TF and LG conceptualized the topic of the review and methodology. FV retrieved the relevant literature. FV and TF prepared the manuscript draft. UC, MV and LG reviewed and edited the manuscript. All authors read and approved of the final version of the manuscript. Data authentication is not applicable.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
|
El Meligy OA, Elemam NM and Talaat IM: Ozone therapy in medicine and dentistry: A review of the literature. Dent J (Basel). 11(187)2023.PubMed/NCBI View Article : Google Scholar | |
|
Costa T, Linhares D, Ribeiro da Silva M and Neves N: Ozone therapy for low back pain. A systematic review. Acta Reumatol Port. 43:172–181. 2018.PubMed/NCBI | |
|
Oliveira Modena DA, de Castro Ferreira R, Froes PM and Rocha KC: Ozone therapy for dermatological conditions: A systematic review. J Clin Aesthet Dermatol. 15:65–73. 2022.PubMed/NCBI | |
|
Shang W, Wang Y, Wang G and Han D: Benefits of ozone on mortality in patients with COVID-19: A systematic review and meta-analysis. Complement Ther Med. 72(102907)2023.PubMed/NCBI View Article : Google Scholar | |
|
Song M, Zeng Q, Xiang Y, Gao L, Huang J, Huang J, Wu K and Lu J: The antibacterial effect of topical ozone on the treatment of MRSA skin infection. Mol Med Rep. 17:2449–2455. 2018.PubMed/NCBI View Article : Google Scholar | |
|
Celiberti P, Pazera P and Lussi A: The impact of ozone treatment on enamel physical properties. Am J Dent. 19:67–72. 2006.PubMed/NCBI | |
|
Foroughi M, Khiadani M, Kakhki S, Kholghi V, Naderi K and Yektay S: Effect of ozonation-based disinfection methods on the removal of antibiotic resistant bacteria and resistance genes (ARB/ARGs) in water and wastewater treatment: A systematic review. Sci Total Environ. 811(151404)2022.PubMed/NCBI View Article : Google Scholar | |
|
Santos GM, Pacheco RL, Bussadori SK, Santos E, Riera R, De Oliveira Cruz Latorraca C, Mota P, Benavent Caldas Bellotto EF and Martmbianco ALC: Effectiveness and safety of ozone therapy in dental caries treatment: Systematic review and meta-analysis. J Evid Based Dent Pract. 20(101472)2020.PubMed/NCBI View Article : Google Scholar | |
|
Chirumbolo S, Valdenassi L, Simonetti V, Bertossi D, Ricevuti G, Franzini M and Pandolfi S: Insights on the mechanisms of action of ozone in the medical therapy against COVID-19. Int Immunopharmacol. 96(107777)2021.PubMed/NCBI View Article : Google Scholar | |
|
Huth KC, Saugel B, Jakob FM, Cappello C, Quirling M, Paschos E, Ern L, Hickel R and Brand K: Effect of aqueous ozone on the NF-kappaB system. J Dent Res. 86:451–456. 2007.PubMed/NCBI View Article : Google Scholar | |
|
Bocci V: Ozone: A new medical drug. Springer, Dordrecht, The Netherlands ; Norwell, MA, 2005. | |
|
Górnicki A and Gutsze A: In vitro effects of ozone on human erythrocyte membranes: An EPR study. Acta Biochim Pol. 47:963–971. 2000.PubMed/NCBI | |
|
Tükel SS, Bilgin R and Gül S: Effects of ozone on the activity of erythrocyte membrane Na(+)-K+ ATPase. Biochem Mol Biol Int. 33:1033–1040. 1994.PubMed/NCBI | |
|
Azuma T, Usui M and Hayashi T: Inactivation of antibiotic-resistant bacteria in hospital wastewater by ozone-based advanced water treatment processes. Sci Total Environ. 906(167432)2024.PubMed/NCBI View Article : Google Scholar | |
|
Bardellini E, Amadori F, Veneri F, Conti G and Majorana A: Coronavirus disease-2019 and dental practice: A project on the use of ozonized water in the water circuit of the dental armchair. Stomatologija. 22:35–38. 2020.PubMed/NCBI | |
|
Irie MS, Dietrich L, Souza GLD, Soares PBF, Moura CCG, Silva GRD and Paranhos LR: Ozone disinfection for viruses with applications in healthcare environments: A scoping review. Braz Oral Res. 36(e006)2022.PubMed/NCBI View Article : Google Scholar | |
|
Lim S, Shi JL, Von Gunten U and McCurry DL: Ozonation of organic compounds in water and wastewater: A critical review. Water Res. 213(118053)2022.PubMed/NCBI View Article : Google Scholar | |
|
Uppal T, Khazaieli A, Snijders AM and Verma SC: Inactivation of human coronavirus by FATHHOME's dry sanitizer device: Rapid and eco-friendly ozone-based disinfection of SARS-CoV-2. Pathogens. 10(339)2021.PubMed/NCBI View Article : Google Scholar | |
|
Beretta M and Federici Canova F: A new method for deep caries treatment in primary teeth using ozone: A retrospective study. Eur J Paediatr Dent. 18:111–115. 2017.PubMed/NCBI View Article : Google Scholar | |
|
Lynch E: Ozone: The revolution in dentistry. Quintessence, Copenhagen, 2004. | |
|
Gupta S and Deepa D: Applications of ozone therapy in dentistry. J Oral Res Rev. 8:86–91. 2016. | |
|
International Scientific Committee of Ozone Therapy-ISCO3: Learning methodology instructions and perfection in ozone therapy for medical doctors, 2015. Available online at: https://isco3.org/wp-content/uploads/2015/09/ISCO3-HUM-00-01.pdf. | |
|
World Federation of Ozone Therapy: WFOT'S review on evidence based ozone therapy, 2015. Available online at: https://spozonoterapia.pt/wp-content/uploads/2016/02/WFOT-OZONE-2015-ENG.compressed.pdf. | |
|
Floare AD, Focht D, Hajdu AI, Niculescu Talpoş IC, Bălean OJ, Muntean CV, Sebeşan D, Jumanca DE and Găluşcan A: Ozone and microstructural morphological changes of tooth enamel. Rom J Morphol Embryol. 63:539–544. 2022.PubMed/NCBI View Article : Google Scholar | |
|
D'Amario M, Di Carlo M, Natale SM, Memè L, Marzo G, Matarazzo G and Capogreco M: Application of ozone therapy in paediatric dentistry. Appl Sci. 12(11100)2022. | |
|
Nogales CG, Ferrari PH, Kantorovich EO and Lage-Marques JL: Ozone therapy in medicine and dentistry. J Contemp Dent Pract. 9:75–84. 2008.PubMed/NCBI | |
|
Re K, Gandhi J, Liang R, Patel S, Joshi G, Smith NL, Reid I and Khan SA: Clinical utility of ozone therapy and hyperbaric oxygen therapy in degenerative disc disease. Med Gas Res. 13:1–6. 2023.PubMed/NCBI View Article : Google Scholar | |
|
Randi CJ, Heiderich CMC, Serrano RV, Morimoto S, de Moraes LOC, Campos L and Palma LF: Use of ozone therapy in Implant Dentistry: A systematic review. Oral Maxillofac Surg. 28:39–49. 2024.PubMed/NCBI View Article : Google Scholar | |
|
Rapone B, Ferrara E, Santacroce L, Topi S, Gnoni A, Dipalma G, Mancini A, Di Domenico M, Tartaglia GM, Scarano A and Inchingolo F: The gaseous ozone therapy as a promising antiseptic adjuvant of periodontal treatment: A randomized controlled clinical trial. Int J Environ Res Public Health. 19(985)2022.PubMed/NCBI View Article : Google Scholar | |
|
Tricarico G, Rodrigues Orlandin J, Rocchetti V, Ambrosio CE and Travagli V: A critical evaluation of the use of ozone and its derivatives in dentistry. Eur Rev Med Pharmacol Sci. 24:9071–9093. 2020.PubMed/NCBI View Article : Google Scholar | |
|
Veneri F, Filippini T, Consolo U, Vinceti M and Generali L: Ozone treatment for the management of caries in primary dentition: A systematic review of clinical studies. Dent J (Basel). 12(69)2024.PubMed/NCBI View Article : Google Scholar | |
|
Kumar T, Arora N, Puri G, Aravinda K, Dixit A and Jatti D: Efficacy of ozonized olive oil in the management of oral lesions and conditions: A clinical trial. Contemp Clin Dent. 7:51–54. 2016.PubMed/NCBI View Article : Google Scholar | |
|
Al-Omiri MK, Alhijawi M, AlZarea BK, Abul Hassan RS and Lynch E: Ozone treatment of recurrent aphthous stomatitis: A double blinded study. Sci Rep. 6(27772)2016.PubMed/NCBI View Article : Google Scholar | |
|
Torul D, Omezli MM and Avci T: Investigation of the clinical efficacy of CGF and ozone in the management of alveolar osteitis: A randomized controlled trial. Clin Oral Investig. 27:4521–4529. 2023.PubMed/NCBI View Article : Google Scholar | |
|
Kogila AV, Kishore M, Padma Rayulu K, Raju BHRK, Tyro D and Bhupathi A: A comparative study of pain and healing in post-dental extraction sockets treated with ozonated water/oil and normal saline. J Maxillofac Oral Surg. 21:1119–1125. 2022.PubMed/NCBI View Article : Google Scholar | |
|
Veneri F, Bardellini E, Amadori F, Conti G and Majorana A: Efficacy of ozonized water for the treatment of erosive oral lichen planus: A randomized controlled study. Med Oral Patol Oral Cir Bucal. 25:e675–e682. 2020.PubMed/NCBI View Article : Google Scholar | |
|
Khatri I, Moger G and Kumar NA: Evaluation of effect of topical ozone therapy on salivary Candidal carriage in oral candidiasis. Indian J Dent Res. 26:158–162. 2015.PubMed/NCBI View Article : Google Scholar | |
|
Samuel SR, Dorai S, Khatri SG and Patil ST: Effect of ozone to remineralize initial enamel caries: In situ study. Clin Oral Investig. 20:1109–1113. 2016.PubMed/NCBI View Article : Google Scholar | |
|
Jena D, Manas A, Venkateswararao CH, Salama MT, Ismail PMS and Basha SR: Comparative evaluation of efficacy of bioactive glass, tricalcium phosphate, and ozone remineralizing agents on artificial carious lesion. J Pharm Bioall Sci. 14 (Suppl 1):S959–S961. 2022.PubMed/NCBI View Article : Google Scholar | |
|
Estrela C, Chaves RM, Cardoso PC, De Je Barata T, De Souza JB, De Torres ÉM, Estrela CR, Magalhães AP and Lopes LG: Ozone gas effect on mineral content of dentin exposed to Streptococcus mutans Biofilm: An energy-dispersive x-ray evaluation. J Contemp Dent Pract. 18:265–269. 2017.PubMed/NCBI View Article : Google Scholar | |
|
Dähnhardt JE, Jaeggi T and Lussi A: Treating open carious lesions in anxious children with ozone. A prospective controlled clinical study. Am J Dent. 19:267–270. 2006.PubMed/NCBI | |
|
Luppieri V, Manfra A, Ronfani L, Chermetz M and Cadenaro M: Ozone therapy for early childhood caries (ECC) treatment: An in vivo prospective study. Appl Sci. 12(1964)2022. | |
|
Petersen PE and Kwan S: Equity, social determinants and public health programmes-the case of oral health. Community Dent Oral Epidemiol. 39:481–487. 2011.PubMed/NCBI View Article : Google Scholar | |
|
The Lancet: Big sugar and neglect by global health community fuel oral health crisis, 2019. Available online at: https://www.eurekalert.org/pub_releases/2019-07/tl-tlb071619.php. | |
|
Veneri F, Vinceti SR and Filippini T: Fluoride and caries prevention: A scoping review of public health policies. Ann Ig: Jan 17, 2024 (Epub ahead of print). | |
|
Bocci V, Borrelli E, Travagli V and Zanardi I: The ozone paradox: Ozone is a strong oxidant as well as a medical drug. Med Res Rev. 29:646–682. 2009.PubMed/NCBI View Article : Google Scholar | |
|
Bocci VA: Scientific and medical aspects of ozone therapy. State of the art. Arch Med Res. 37:425–435. 2006.PubMed/NCBI View Article : Google Scholar | |
|
Ouzzani M, Hammady H, Fedorowicz Z and Elmagarmid A: Rayyan-a web and mobile app for systematic reviews. Syst Rev. 5(210)2016.PubMed/NCBI View Article : Google Scholar | |
|
Cataldo F: DNA degradation with ozone. Int J Biol Macromol. 38:248–254. 2006.PubMed/NCBI View Article : Google Scholar | |
|
Nagayoshi M, Fukuizumi T, Kitamura C, Yano J, Terashita M and Nishihara T: Efficacy of ozone on survival and permeability of oral microorganisms. Oral Microbiol Immunol. 19:240–246. 2004.PubMed/NCBI View Article : Google Scholar | |
|
Yamayoshi T and Tatsumi N: Microbicidal effects of ozone solution on methicillin-resistant Staphylococcus aureus. Drugs Exp Clin Res. 19:59–64. 1993.PubMed/NCBI | |
|
Camacho-Alonso F, Salmerón-Lozano P and Martínez-Beneyto Y: Effects of photodynamic therapy, 2% chlorhexidine, triantibiotic mixture, propolis and ozone on root canals experimentally infected with Enterococcus faecalis: An in vitro study. Odontology. 105:338–346. 2017.PubMed/NCBI View Article : Google Scholar | |
|
Cardoso MG, De Oliveira LD, Koga-Ito CY and Jorge AOC: Effectiveness of ozonated water on Candida albicans, Enterococcus faecalis, and endotoxins in root canals. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 105:e85–e91. 2008.PubMed/NCBI View Article : Google Scholar | |
|
Knight GM, McIntyre JM, Craig GG, Mulyani and Zilm PS: The inability of Streptococcus mutans and Lactobacillus acidophilus to form a biofilm in vitro on dentine pretreated with ozone. Aust Dent J. 53:349–353. 2008.PubMed/NCBI View Article : Google Scholar | |
|
Tamesada M, Kawabata S, Fujiwara T and Hamada S: Synergistic effects of streptococcal glucosyltransferases on adhesive biofilm formation. J Dent Res. 83:874–879. 2004.PubMed/NCBI View Article : Google Scholar | |
|
Grootveld M, Silwood CJL and Lynch E: High resolution 1H NMR investigations of the oxidative consumption of salivary biomolecules by ozone: Relevance to the therapeutic applications of this agent in clinical dentistry. Biofactors. 27:5–18. 2006.PubMed/NCBI View Article : Google Scholar | |
|
Takeda Y, Jamsransuren D, Makita Y, Kaneko A, Matsuda S, Ogawa H and Oh H: Inactivation activities of ozonated water, slightly acidic electrolyzed water and ethanol against SARS-CoV-2. Molecules. 26(5465)2021.PubMed/NCBI View Article : Google Scholar | |
|
Rowen RJ and Robins H: A plausible ‘penny’ costing effective treatment for corona virus-ozone therapy. J Infect Dis Epidemiol. 6(113)2020. | |
|
Cenci A, Macchia I, La Sorsa V, Sbarigia C, Di Donna V and Pietraforte D: Mechanisms of action of ozone therapy in emerging viral diseases: Immunomodulatory effects and therapeutic advantages with reference to SARS-CoV-2. Front Microbiol. 13(871645)2022.PubMed/NCBI View Article : Google Scholar | |
|
Amin LE: Biological assessment of ozone therapy on experimental oral candidiasis in immunosuppressed rats. Biochem Biophys Rep. 15:57–60. 2018.PubMed/NCBI View Article : Google Scholar | |
|
Johansson E, Van Dijken JWV, Karlsson L and Andersson-Wenckert I: Treatment effect of ozone and fluoride varnish application on occlusal caries in primary molars: A 12-month study. Clin Oral Invest. 18:1785–1792. 2014.PubMed/NCBI View Article : Google Scholar | |
|
American Academy of Pediatric Dentistry: Fluoride therapy. In: The Reference Manual of Pediatric Dentistry. American Academy of Pediatric Dentistry, Chicago, Ill., pp352-358, 2023. | |
|
Veneri F, Vinceti M, Generali L, Giannone ME, Mazzoleni E, Birnbaum LS, Consolo U and Filippini T: Fluoride exposure and cognitive neurodevelopment: Systematic review and dose-response meta-analysis. Environ Res. 221(115239)2023.PubMed/NCBI View Article : Google Scholar | |
|
Iamandii I, De Pasquale L, Giannone ME, Veneri F, Generali L, Consolo U, Birnbaum LS, Castenmiller J, Halldorsson TI, Filippini T and Vinceti M: Does fluoride exposure affect thyroid function? A systematic review and dose-response meta-analysis. Environ Res. 242(117759)2024.PubMed/NCBI View Article : Google Scholar | |
|
Fiore G, Veneri F, Di Lorenzo RD, Generali L, Vinceti M and Filippini T: Fluoride exposure and ADHD: A systematic review of epidemiological studies. Medicina (Kaunas). 59(797)2023.PubMed/NCBI View Article : Google Scholar | |
|
Veneri F, Filippini T, Cecchini M, Vinceti M, Consolo U and Generali L: Early fluoride intake and molar incisor hypomineralisation (MIH) defects: A systematic review and dose-response meta-analysis. Acta Biomed. 95(e2024079)2024. | |
|
Lena K and Marianne K: Ozone treatment on dentin hypersensitivity surfaces-a pilot study. Open Dent J. 11:65–70. 2017.PubMed/NCBI View Article : Google Scholar | |
|
Raafat Abdelaziz R, Mosallam RS and Yousry MM: Tubular occlusion of simulated hypersensitive dentin by the combined use of ozone and desensitizing agents. Acta Odontol Scand. 69:395–400. 2011.PubMed/NCBI View Article : Google Scholar | |
|
Summerfelt ST and Hochheimer JN: Review of ozone processes and applications as an oxidizing agent in aquaculture. Prog Fish Cult. 59:94–105. 1997. | |
|
Gürsoy H, Çakar G, İpçi ŞD, Kuru B and Yilmaz S: In vitro evaluation of the effects of different treatment procedures on dentine tubules. Photomed Laser Surg. 30:695–698. 2012.PubMed/NCBI View Article : Google Scholar | |
|
Ferreira LDAQ, Anestino TA, Branco NTT, Diniz LA, Diniz MG, de Magalhães CS, Peixoto RTRDC, Moreira AN, Dias DR, Madeira MFM and Diniz IMA: Adjunctive therapies for in vitro carious lesions: Antimicrobial activity, activation of dentin metalloproteinases and effects on dental pulp cells. Photodiagnosis Photodyn Ther. 40(103168)2022.PubMed/NCBI View Article : Google Scholar | |
|
Dikopova NZH, Volkov AG, Kopecki IS, Nikolskaya IA, Margaryan EG, Budina TV, Samokhlib YA, Kondratiev SA, Paramonov YOU and Arakelyan MG: Clinical and experimental validation of the ozone therapy effectiveness in case of accidental exposure of the dental pulp. New Armen Med J. 15(77)2021. | |
|
Küçük F, Yıldırım S and Çetiner S: Cytotoxicity assessment of different doses of ozonated water on dental pulp cells. BMC Oral Health. 21(32)2021.PubMed/NCBI View Article : Google Scholar | |
|
Noguchi F, Kitamura C, Nagayoshi M, Chen KK, Terashita M and Nishihara T: Ozonated water improves lipopolysaccharide-induced responses of an odontoblast-like cell line. J Endod. 35:668–672. 2009.PubMed/NCBI View Article : Google Scholar | |
|
Seidler V, Linetskiy I, Hubálková H, Stanková H, Smucler R and Mazánek J: Ozone and its usage in general medicine and dentistry. A review article. Prague Med Rep. 109:5–13. 2008.PubMed/NCBI | |
|
Lu W, Xu W, Li J, Chen Y, Pan Y and Wu B: Effects of vascular endothelial growth factor and insulin growth factor-1 on proliferation, migration, osteogenesis and vascularization of human carious dental pulp stem cells. Mol Med Rep. 20:3924–3932. 2019.PubMed/NCBI View Article : Google Scholar | |
|
Yildirim AO, Eryilmaz M, Kaldirim U, Eyi YE, Tuncer SK, Eroğlu M, Durusu M, Topal T, Kurt B, Dilmen S, et al: Effectiveness of hyperbaric oxygen and ozone applications in tissue healing in generated soft tissue trauma model in rats: An experimental study. Ulus Travma Acil Cerrahi Derg. 20:167–175. 2014.PubMed/NCBI View Article : Google Scholar | |
|
Zhang J, Guan M, Xie C, Luo X, Zhang Q and Xue Y: Increased growth factors play a role in wound healing promoted by noninvasive oxygen-ozone therapy in diabetic patients with foot ulcers. Oxid Med Cell Longev. 2014(273475)2014.PubMed/NCBI View Article : Google Scholar | |
|
Zhang W, Zhang X, Ling J, Wei X and Jian Y: Osteo-/odontogenic differentiation of BMP2 and VEGF gene-co-transfected human stem cells from apical papilla. Mol Med Rep. 13:3747–3754. 2016.PubMed/NCBI View Article : Google Scholar | |
|
Korkmaz Y, Baumann MA, Steinritz D, Schröder H, Behrends S, Addicks K, Schneider K, Raab WHM and Bloch W: NO-cGMP signaling molecules in cells of the rat molar dentin-pulp complex. J Dent Res. 84:618–623. 2005.PubMed/NCBI View Article : Google Scholar | |
|
Krunić J, Stojanović N, Đukić L, Roganović J, Popović B, Simić I and Stojić D: Clinical antibacterial effectiveness and biocompatibility of gaseous ozone after incomplete caries removal. Clin Oral Invest. 23:785–792. 2019.PubMed/NCBI View Article : Google Scholar | |
|
Ferrara N, Gerber HP and LeCouter J: The biology of VEGF and its receptors. Nat Med. 9:669–676. 2003.PubMed/NCBI View Article : Google Scholar | |
|
Ebensberger U, Pohl Y and Filippi A: PCNA-expression of cementoblasts and fibroblasts on the root surface after extraoral rinsing for decontamination. Dent Traumatol. 18:262–266. 2002.PubMed/NCBI View Article : Google Scholar | |
|
Wang PL, Tachi Y, Masuno K, Okusa N and Imamura Y: The study of ozone ointment on human gingival fibroblasts cell proliferation ability and anti-inflammatory. J Hard Tissue Biol. 27:209–212. 2018. | |
|
Colombo M, Ceci M, Felisa E, Poggio C and Pietrocola G: Cytotoxicity evaluation of a new ozonized olive oil. Eur J Dent. 12:585–589. 2018.PubMed/NCBI View Article : Google Scholar | |
|
Huth KC, Jakob FM, Saugel B, Cappello C, Paschos E, Hollweck R, Hickel R and Brand K: Effect of ozone on oral cells compared with established antimicrobials. Eur J Oral Sci. 114:435–440. 2006.PubMed/NCBI View Article : Google Scholar | |
|
de Lima Neto TJ, Delanora LA, Sá Simon ME, Carmo Ribeiro KH, Matsumoto MA, Quírino Louzada MJ, Shibli JA, Ervolino E and Faverani LP: Ozone improved bone dynamic of female rats using zoledronate. Tissue Eng Part C Methods. 30:1–14. 2024.PubMed/NCBI View Article : Google Scholar | |
|
Vieira VSJG, da Rosa ÂR, Montagner PG, de Campos FUF, Teixeira LN, Aura JM, Joly JC, Passador-Santos F and Martinez EF: Effect of ozone therapy on the modulation of inflammation and on new bone formation in critical defects of rat calvaria filled with autogenous graft. J Stomatol Oral Maxillofac Surg. 124(101292)2023.PubMed/NCBI View Article : Google Scholar | |
|
Alpan AL, Toker H and Ozer H: Ozone therapy enhances osseous healing in rats with diabetes with calvarial defects: A morphometric and immunohistochemical study. J Periodontol. 87:982–989. 2016.PubMed/NCBI View Article : Google Scholar | |
|
Botolin S and McCabe LR: Chronic hyperglycemia modulates osteoblast gene expression through osmotic and non-osmotic pathways. J Cell Biochem. 99:411–424. 2006.PubMed/NCBI View Article : Google Scholar | |
|
Sarkar PD and Choudhury AB: Relationships between serum osteocalcin levels versus blood glucose, insulin resistance and markers of systemic inflammation in central Indian type 2 diabetic patients. Eur Rev Med Pharmacol Sci. 17:1631–1635. 2013.PubMed/NCBI | |
|
Bayer Alinca S, Sağlam E, Zengin Celik T, Hacisalihoglu P and Doğan MA: Is low level laser therapy or ozone therapy more effective for bone healing? Understanding the mechanisms of HIF-1α, RANKL and OPG. Biotech Histochem. 95:597–604. 2020.PubMed/NCBI View Article : Google Scholar | |
|
Leewananthawet A, Arakawa S, Okano T, Daitoku Kinoshita R, Ashida H, Izumi Y and Suzuki T: Ozone ultrafine bubble water induces the cellular signaling involved in oxidative stress responses in human periodontal ligament fibroblasts. Sci Technol Adv Mat. 20:589–598. 2019.PubMed/NCBI View Article : Google Scholar | |
|
Afacan B, Öztürk VÖ, Paşalı Ç, Bozkurt E, Köse T and Emingil G: Gingival crevicular fluid and salivary HIF-1α, VEGF, and TNF-α levels in periodontal health and disease. J Periodontol. 90:788–797. 2019.PubMed/NCBI View Article : Google Scholar | |
|
Yu XJ, Xiao CJ, Du YM, Liu S, Du Y and Li S: Effect of hypoxia on the expression of RANKL/OPG in human periodontal ligament cells in vitro. Int J Clin Exp Pathol. 8:12929–12935. 2015.PubMed/NCBI | |
|
de Souza KBR, Almeida Guerra LR, da Silva Guerreiro ML, Casais-E-Silva LL and Aguiar MC: Nociceptive and histomorphometric evaluation of the effects of ozone therapy on the rat masseter muscle in a carrageenan model of myofascial pain. Arch Oral Biol. 160(105893)2024.PubMed/NCBI View Article : Google Scholar | |
|
Fuchigami S, Nakamura T, Furue K, Sena K, Shinohara Y and Noguchi K: Recombinant human bone morphogenetic protein-9 potently induces osteogenic differentiation of human periodontal ligament fibroblasts. Eur J Oral Sci. 124:151–157. 2016.PubMed/NCBI View Article : Google Scholar |