Influence of epigenetics and microbiota in early‑life development: A possible role for exosomes (Review)
- Authors:
- Published online on: May 13, 2024 https://doi.org/10.3892/ije.2024.22
- Article Number: 3
-
Copyright : © Mitsis et al. This is an open access article distributed under the terms of Creative Commons Attribution License [CC BY 4.0].
Abstract
1. Introduction
Research has shown that events during pregnancy and up to the first 2-3 years of life, influence biological functions, such as the immune response and shape brain structure in ways that affect well-being, health and productivity (1). Several factors that influence the developmental environment have been found to be associated with the risk of disease development during adult life. It appears that the interaction between genes and environmental factors in the prenatal to early postnatal period is critical for the development of diseases in adulthood. Until recently, the aforementioned factors were studied in a single effect-health result manner, an imprecise approach, since it does not take fully into consideration the numerous interactions between the environmental factors and genes themselves. Thus, the concept of the exposome, i.e., the totality of exposures from external and internal stimuli throughout the lifespan of an organism, was proposed as a more inclusive method of studying early-life environment-gene interactions (2). The mechanisms that consolidate the effect of these numerous stimuli are epigenetic modifications, such as DNA methylation, histone modifications and regulation by non-coding RNAs (ncRNAs), while one of the main interfaces between the external exposome and the human host is the gut microbiota (3). Extracellular vesicles (EVs), and particularly exosomes, can function as mediators of both epigenetic modifications and mechanisms that alter gut microbiota diversity (4,5). Breast milk is known to contain its own microbiota and large quantities of EVs, particularly exosomes, and plays an essential role in neonatal and infant health (6). Thus, the study of how exosomes may influence epigenetic mechanisms and microbiota in early life may further elucidate the specifics of the effects of breast milk on the health of an infant and subsequently, its adulthood.
2. Extracellular vesicles and exosomes
EVs are heterogeneous lipid bilayer-surrounded vesicles that carry bioactive molecules and are secreted by different cell types in the extracellular space (7,8). EV secretion is an ubiquitous mechanism present in all domains of life and occurs under a wide range of conditions, both physiological and pathological (Table I) (9). Prokaryotes produce EVs termed membrane vesicles. Pathogenic and non-pathogenic Gram-negative bacteria secrete outer membrane vesicles, which are 10 to 500 nm in size and originate from the outer membrane of a cell, while Gram-positive bacteria secrete membrane vesicles which are 20 to 400 nm in size and originate from the single cytoplasmic cell membrane. Bacterial EVs are involved in numerous processes, including intercellular communications, stress response and antibiotic resistance. Although research on archaea is somewhat limited, the majority of species appear to produce vesicles originating from the cytoplasmic cell membrane with an average size of 50 to 250 nm (10). These EVs appear to promote horizontal gene transfer and nutrient cycling under extreme conditions (11). In eukaryotic cells, and multicellular eukaryotic organisms such as humans, vesicles are grouped into three subtypes based on their biogenesis, size, content, release pathway and function (8). The three classical types of EVs in eukaryotes are microvesicles, which are 40 nm to 1 µm in size and emerge through direct outward budding and shedding from the plasma membrane, apoptotic bodies which are 1 to 5 µm in size and are created in the process of apoptosis, and exosomes which are 40 to 150 nm in size and originate from the endosome (12).
Exosomes, in particular, have garnered scientific attention due their potential as diagnostic and therapeutic molecules (13). These vesicles are secreted by virtually any cell type and are found in numerous biofluids including plasma, saliva, urine, semen, cerebrospinal fluid and breast milk (8). Their cargo depends on the cell of origin and consists-among others-of nucleic acids, proteins, lipids and metabolites (14). The physiological state of the cell of origin also influences exosomal cargo, with pathological conditions leading to alterations in exosome content (15). Human exosomes are mainstays of cellular communication and take part in both physiological processes, such as the immune response, tissue repair and cell programming, and pathological conditions, such as tumorigenesis, chronic inflammation and neurodegeneration (16).
3. Applications of extracellular vesicles and exosomes
EVs are poised to play a crucial role in disease diagnostics and therapeutics (17). Studies on human and mice neonates highlight the potential use of EV-derived microRNAs (miRNAs/miRs) as biomarkers for diseases common in preterm infants, such as bronchopulmonary dysplasia (BPD), necrotizing enterocolitis and hypoxic-ischemic brain damage (18). Additionally, in vitro experiments focusing on EVs secreted from cultured cancer cell lines have demonstrated that the EV content could be used as a biomarker of pediatric carcinomas (19). As regards exosomes, alterations on their miRNA cargo could potentially hint at pediatric tumors, such as neuroblastoma, hepatoblastoma and osteosarcoma (20). EVs can also be used to treat neonatal conditions that affect the brain, spine, retinas, lungs and intestines. For example, mouse pups with BPD treated with exosomes derived from human umbilical cord mesenchymal stem cells (MSCs) have been found to display less neuronal apoptosis and restored myelination (21). Experiments on newborn rats with BPD have shown that the intraperitoneal injection of MSC-derived exosomes preserves alveolarization and angiogenesis, and hinders pulmonary microvascular dysplasia, which is one of the main pathological manifestations of BPD (22).
4. Epigenetics
Epigenetics can aid in the determination of how events in early life can influence adult health. Epigenetics study the molecules and mechanisms that can extend alternative gene activity states, while the DNA sequence remains the same (23). Epigenetic mechanisms include DNA methylation, histone modifications and regulation by ncRNAs (24). These mechanisms act as the intermediate between genes and the environment, and influence physiology and disease by altering gene expression and thus regulating gene activity (25). DNA methylation consists of a covalent attachment of a methyl group at the C-5 position of cytosine, resulting in the formation of 5-methyl cytosine. This attachment mostly occurs in in cytosine-guanosine (CpG) dinucleotide sequences. The catalyzation of this process is achieved through the action of DNA methyltransferases (DNMTs), while demethylation is achieved through the action of ten-eleven translocation dioxygenases (26,27). Gene promoters often showcase regions rich in such sequences, which are termed CpG islands (28). DNA methylation functions together with histone modifications to alter chromatin structure and influence DNA accessibility to factors that regulate gene expression (29). Function-wise DNA methylation is considered a regulator of gene silencing (30). Histone modifications include the acetylation, methylation, ubiquitination, sumoylation, deimination, phosphorylation and ADP ribosylation of histone proteins. Similar to DNA methylation, histone modifications influence the availability of DNA to the transcriptional machinery. Generally, histone modifications are catalyzed by enzymes that mostly act on the N-terminal tail of histones and involve amino acids such as lysine, arginine, threonine, serine and tyrosine (31,32). These modifications are catalyzed by enzymes, such as histone acetyltransferases, histone methyltransferases, histone deacetylases (HDACs) and histone demethylases (33). The most extensively studied histone modification is histone acetylation, which usually results in a higher gene expression (31). ncRNAs are ribonucleic acid molecules that do not encode proteins. These molecules are ubiquitous in the human body and include ribosomal RNAs and transfer RNAs, plus RNAs with a regulatory role in gene expression, such as miRNAs, long ncRNAs (lncRNAs), small interfering RNAs, PIWI-interacting RNAs, small nucleolar RNAs and circular RNAs. The expression of ncRNAs is highly regulated and their expression profiles are distinct in each developmental stage and tissue (34). The majority of studies on ncRNAs have focused on the role of miRNAs and lncRNAs in different pathologies (35). miRNAs, which consist of 17 to 25 nucleotides, can recognize target messenger RNAs (mRNAs) via sequence complementarity and regulate their protein translation. The majority of miRNAs inhibit gene expression by interfering with mRNA translation. In certain cases, such as the binding of a miRNA to the promoter region of a gene, miRNAs may promote gene expression (36). lncRNAs, which consist of >200 nucleotides, appear to influence gene expression via numerous mechanisms. Several lncRNAs influence gene expression by creating scaffolds for protein-DNA complexes, other lncRNAs recruit histone modification enzymes, while another group bind neighboring genomic loci to promote gene imprinting. Additionally, lncRNAs can interact with mRNAs or act as sponges that inhibit the action of miRNAs (37).
5. Epigenetics and early-life development
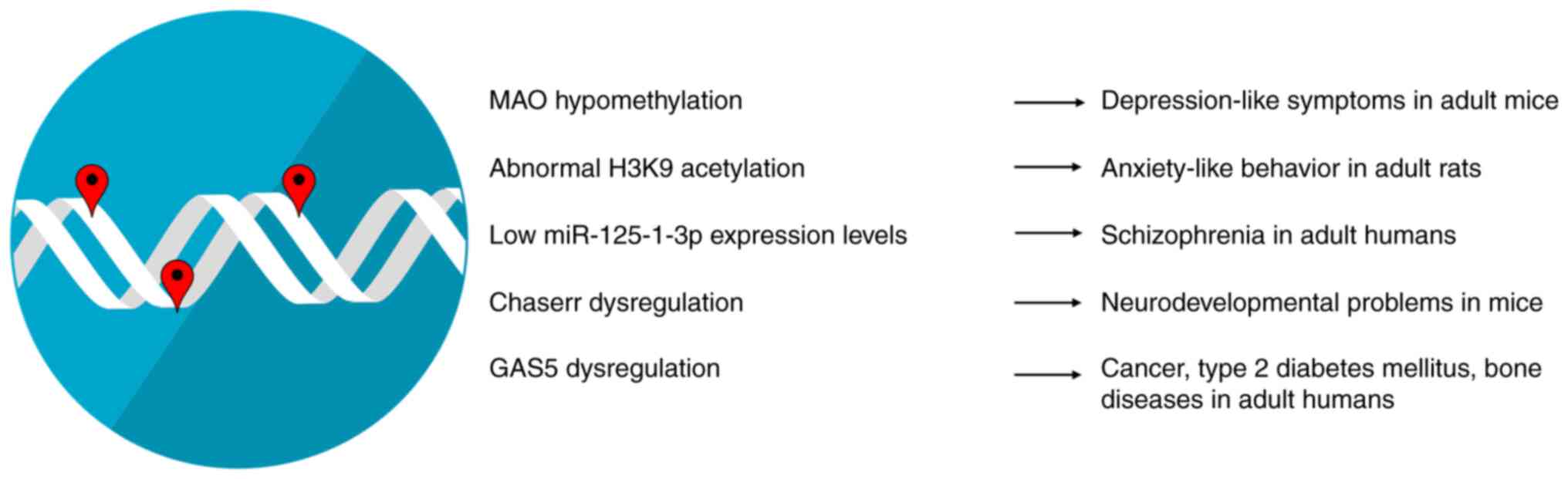
Epigenetic modifications during early-life development have a marked impact on both physiological and pathological conditions in adult life (Fig. 1). Early-life environmental factors significantly influence DNA methylation patterns across the human lifespan. The effects of DNA methylation persist during the whole human lifespan and appear stronger for heritable sites and sites with play a role in gene expression (38). Some of the environmental factors that can influence epigenetic mechanisms, such as DNA methylation in early life include dietary habits during pregnancy, exposure to metals, smoking, or alcohol consumption during the perinatal period, and in particular, early-life stress (ELS) (39,40). A prime example includes the altered methylation levels of monoamine oxidase (MAO) A gene due to early-life stress which may be associated with depression-like symptoms in adult mice. Monoamine oxidases (MAOs) are enzymes that modulate the metabolism of monoamine neurotransmitters, such as dopamine, epinephrine and serotonin. These neurotransmitters act in tandem to modulate basic emotions. Therefore, it is expected that their deregulated levels have been associated with affective disorders, such as depression, with MAO inhibitors being the first type of antidepressants developed. Patients with depression showcase MAO A gene hypomethylation, which may lead to increased MAO A activity and may result in reduced monoamine utilization (40). Epigenetic mechanisms, such as histone modifications help coordinate and fine-tune spatiotemporal gene expression, while their dysregulation has been shown to be associated with several disorders of the nervous system; indeed, abnormal histone modification levels appear to promote neuropsychiatric and neurodegenerative disorders (41). A previous study on rats found that ELS appears to lead to the abnormal acetylation of the H3K9 histone (42). Both hyperacetylation and hypoacetylation have been implicated in anxiety-like behavior in adult rats, with the conflicting results possibly arising due to methodological differences (42). miRNAs are main regulators of brain plasticity and higher brain functions. ELS appears to influence miRNA levels well into adulthood. Both human and animal studies have demonstrated that miR-125-1-3p is particularly responsive to ELS and its low expression levels are associated with schizophrenia in adulthood (43). lncRNAs have been found to be spatially and temporally restricted at certain developmental stages, thus hinting at a potential role in proper organism development. Indeed, lncRNAs, such as the CHD2 adjacent, suppressive regulatory RNA (Chaserr) appear to play an integral role in neurodevelopment in humans and has been associated with neurodevelopmental delay in mice (44,45). Nevertheless, the mechanisms through which early-life stressors influence lncRNA levels and adult health remain poorly characterized, with a study on mice showcasing that arsenic exposure during embryonic development alters the expression of the lncRNA growth arrest specific-5 in males (46). This lncRNA has been shown to be associated with several pathological conditions in adult life, such as cancer, type 2 diabetes mellitus and bone diseases (47).
6. Microbiota and early-life development
The term microbiota refers to living organisms located in a defined environment, with prime examples being the human gut, oral, skin, respiratory and female genital tract microbiota. Numerous studies on humans have highlighted the mechanisms through which microbiota influence health and disease (48,49). Specifically, human microbiota are involved in essential biological processes, such as intercellular communication and immune response, while dysbiosis, i.e., an interruption in microbiota balance, has been found to be associated with numerous pathologies (Fig. 2). Thus, it is evident that human microbiota have been hailed as potential biomarkers and means of personalized medicine (48).
The oral microbiota interacts with the host to communicate information regarding the status of immunity and metabolism via a two-way communication between the oral cavity and the systemic organs. Oral microbiota dysbiosis is associated with diseases, such as periodontal diseases, as well as systemic diseases, such as inflammatory bowel syndrome, diabetes and pregnancy complications. As regards the latter, studies on mice have suggested that oral symbiotic bacteria can colonize the placenta and influence pregnancy (50). The skin is the largest organ of the human body and carries a complex set of microorganisms, featuring bacteria, archaea, eukaryotes, fungi, viruses and phages. The skin microbiome is a main regulator of the skin immune system and dysbiosis has been associated with atopic dermatitis, acne and dandruff (51). The upper respiratory tract is colonized by bacterial, fungal and viral organisms. These respiratory microbiota are thought to hinder potential pathogens from overgrowing and spreading towards the lungs and dysbiosis has been associated with chronic respiratory diseases (52). The female genital tract microbiota play a critical role in the prevention of pathogen invasion and growth, with dysbiosis being associated with gynecological disorders and infertility (53). Gut microbiota are considered one of the most significant mediators of human health. The gut microbiota mainly consists of prokaryotic organisms and to a lesser extent, fungi and viruses (54). These organisms comprise a highly dynamic system that is influenced by both exogenous factors, such as diet or environmental alterations and endogenous factors, such as the genetic composition of the host (55). The gut microbiota, and particularly bacteria, function as a main regulator of the body homeostasis, thus influencing several processes such as metabolism, inflammation and hematopoiesis (49). It is then not surprising that gut microbiota disorders have been found to be associated with several diseases, such type 2 diabetes mellitus, atherosclerosis and even neurodegenerative disorders, such as Alzheimer's disease and Parkinson's disease (56).
Periodontal disease in mothers and the resulting dysbiosis of their oral microbiota have been linked to pregnancy complications, such as preterm birth and a low birth weight. Some of the above may be a result of the existence of Gram-negative anaerobic bacteremia, which originate from the gingival biofilm or the entrance of pro-inflammatory factors that originate from the gingival submucosa into the bloodstream (57). Microbial exposure via barrier tissues such as skin and lungs in early life help imprint a healthy immune response up to adult life in humans (58). Experiments on mice have also highlighted that the vaginal microbiota can influence offspring metabolism, immune response and even brain development (59). Research has indicated that the microbes that inhabit the human intestinal tract may influence infant development and the maturation of the immune system. It is quite possible that the risk of disease is affected by the gut microbiota during fetal development and early life, thus rendering gut microbiota development research imperative (60). Gut microbiota development during early life can be affected by numerous factors, including host genetics, gestational age, delivery mode, maternal health, antibiotics exposure, ELS, early-life environment and early-life diet (61,62). Indeed, the development of adult atherosclerosis is influenced by specific conditions during infancy, such as preterm birth, malnutrition and microbiota colonization. Malnutrition affects the gut microbiota composition and leads to an increase in the number of potentially pathogenic bacteria. This increase leads to decreased nutrient absorption and may cause epithelial damage. Such epithelial damage may promote an altered gut barrier permeability, leading to the systemic circulation of metabolic products and bacteria, which in turn results in an increased systemic inflammation, an underlying cause of atherosclerosis (62). Another instance of early-life microbiota influencing adult health is susceptibility to mood disorders. ELS in humans appears to interfere with metabolites of the glutamate pathway and promote changes in functional brain connectivity that have been found to be associated with mood disorders. These metabolites are considered to be regulated by the gut microbiota, with a prime example being 5-oxoproline (63). This metabolite is significantly increased in patients with major depressive disorder (MDD) (64). Studies on animal models have shown that mice transplanted with microbiota from MDD display high levels of 5-oxoproline, while treatment of rats with antibiotics reduces metabolite levels, thus implicating the gut microbiota in its regulation (65,66).
7. Exosomes and epigenetic regulation
As aforementioned, EVs, and specifically exosomes, can act as mediators of epigenetic mechanisms and microbiota-regulated mechanisms. The exosome-mediated transfer of epigenetic regulators is considered to be an essential mechanism of epigenetic information exchange between cells (67). In the case of DNA methylation, exosomes derived from bovine milk have been found to contain miRNAs that target DNMTs and influence the demethylation of the promoter region of metabolic regulators, such as the fat mass and obesity-associated protein (FTO). Studies on mice have suggested that FTO expression may play a pivotal role in postnatal growth and energy expenditure (68,69), while FTO loss-of-function mutations have been shown to be associated with postnatal growth retardation in humans (70). In the case of exosome-mediated histone modifications, information is somewhat limited. An in vitro study on isolated mouse cells demonstrated that exosomes derived from developing cortical neurons were enriched in HDAC2(71). HDAC2 has been implicated in the epigenetic regulation of genes that encode synaptic proteins and appears to control dendritic spine development and synapse establishment. Both in vitro and in vivo mouse studies have detected a decrease in HDAC2 levels as cortical neurons mature and acquire news synapses. The in vitro treatment of mature neurons with exosomes derived from HDAC2-rich neurons was shown to lead to an increased expression of HDAC2 in the recipient cells. These results implicate a role for exosomes in nervous system maturation via the transport of histone modifying enzymes (71). Exosomes contain a wide variety of ncRNA molecules, including miRNAs which are the most extensively studied, with lncRNAs steadily receiving an increase in scientific interest (67). Exosomal miRNAs participate in both physiological and pathological processes (72). In vitro studies on human mesenchymal stem cells (hMSCs) have indicated that hMSC-derived exosomes contribute to osteoblastic differentiation via a miRNA-dependent mechanism (73). Such studies highlight the importance of exosomal miRNAs in proper organism development. Apart from their role in cell differentiation, exosomal miRNAs are also involved in pathological conditions, such as cancer. Exosomal miRNAs take part in processes that regulate the tumor microenvironment and interfere with cancer immunity. Specifically, tumor-derived exosomes modulate cancer cell-to-cell communication via their miRNA cargo and are heavily associated with angiogenesis, tumor growth, metastasis and drug resistance (74). The majority of research on exosomal lncRNAs focuses on their severe effects on cancer, where they influence cell proliferation, angiogenesis, drug resistance and metastasis (75). In particular, several studies have focused on the interaction between cancer cells and immune cells that have infiltrated the tumor microenvironment, and how exosomal lncRNAs mediate this process. Tumor-derived exosomal lncRNAs appear to boost M2 macrophage polarization and inhibit the function of natural killer cells in vitro, thus supporting tumor progression (76). Exosomes can potentially provide a link between early-life development and cancer in adult life. Early-life exposure to adverse environmental factors lead to exosomal cargo alterations, which in turn may influence cancer risk in adults (77).
8. Extracellular vesicles and microbiota
Research on the role of EVs secreted by microbiota in host health and the effect of host exosomes on microbiota are limited and focus mainly on the gut microbiota-host interaction. EVs derived from both pathogenic and probiotic bacteria can travel short and long distances, and modulate both bacteria-bacteria and bacteria-host communications (78,79). It should be mentioned that the term probiotics refers to live bacteria with health benefits and several gut commensal bacteria display probiotic effects by boosting host health (80). Bacterial communications via EVs appears to play an essential role in the gut microenvironment and have been implicated in bacterial colonization and growth, protection from host defense peptides and bacterial population behavior. Bacteria-derived vesicles are known to play an immunomodulatory role in human physiology. Specifically, EVs derived from pathogenic bacteria display proinflammatory abilities and play a role in immune response escape, while probiotic-derived EVs are characterized by anti-inflammatory effects and promote immune tolerance (80). Microbiota-derived EVs may also play a crucial role in several processes in early-life development. A previous study on healthy suckling rats highlighted that probiotic-derived EVs promoted immune and intestinal system maturation (81). It has also been demonstrated that microbiota-derived EVs from human children exerted bone protective effects in animal models of osteoporosis, possibly suggesting an essential role in skeletal system maturation (82,83). Additionally, the amniotic fluid of pregnant women contains bacterial-derived EVs similar to those found in the maternal gut microbiota. Experiments on pregnant mice have revealed that EVs secreted from human maternal microbiota can pass to into the intra-amniotic space. These findings suggest a potential role of maternal microbiota-derived vesicles in priming the prenatal immune system for gut colonization (84). Respectively, host miRNAs can directly interact with bacterial genes in the gut, with exosomes being a possible transfer mechanism. A combination of in vitro experiments and animal studies have shown that host miRNAs can act on microbiota genes and promote or inhibit bacterial growth. Since host-derived EVs have been shown to affect bacteria in animal models of several pathologies, it is quite possible that this effect is based on miRNA transportation via exosomes (85). Studies focusing on exosomes found in human breast milk (HBM) have demonstrated that such vesicles contain ncRNAs that are associated with infant health, thus implicating maternal exosomes in proper offspring development (86).
9. Human breast milk microbiota, extracellular vesicles and exosomes
HBM is considered the optimal food source for newborn infants and promotes infant health (Fig. 3). Indeed, breast feeding has been shown to be associated with an improved infant health and immune development, while breast-fed infants display lower mortality rates than formula-fed infants (87). The HBM consists of water, nutrients such as lactose and fat, plus immune cells, hormones, miRNAs and immunomodulatory bioactive molecules (88). Additionally, HBM carries its own distinct microbiome with potentially probiotic bacteria that participate in infant gut colonization (89). Moreover, during lactation the composition of HBM microbiota alters in addition to immune and nutritional composition. It is considered that these alterations in the microbiota found in human breast milk play a role in the immune system development of the infant (90). The HBM also contains bacterial EVs, some of which originate from the gut microbiome, that promote the vertical transfer of commensal microbiota from mothers to infants (91).
HBM also contains exosomes that are secreted by mammary gland epithelium (92). These vesicles may play a key role in the beneficial effects of HBM on infant health. Several studies have highlighted that exosomes found in HBM regulate key biological processes (93-95). A number of HBM-derived exosomes carry immunomodulatory miRNAs. These exosomal miRNAs may regulate immune system development via epigenetic mechanisms (96). As a matter of fact, animal studies have identified HBM-derived exosomal miRNAs that target genes associated with allergic reaction and pathogen-host interaction (97). A prime example of an exosomal ncRNA with a known ability to epigenetically regulate infant immune system development is miR-148a-3p. This molecule appears to be the most abundant miRNA found in HBM-derived exosomes and appears to target DNMT3B, consequently hindering de novo DNA methylation and affecting infant development (98,99). Additionally, HBM-derived exosomes can cross the blood-brain barrier and can thus influence neuronal function. Several ncRNAs found in exosomes possibly act on genes implicated in brain development and function, thus acting as epigenetic regulators of proper brain development (100). Such an example is let-7a, a miRNA found in HBM-derived exosomes that influences both immune response by downregulating IL-6 secretion and brain development by regulating neural cell proliferation and differentiation (100,101). Translational studies have also shown that HBM-derived exosomes control DNMT1 expression which is involved in the upregulation of metabolism-related genes, such as insulin (102). Lastly, a study on mice by Zhou et al (103) demonstrated that exosomes found in bovine milk elicit changes in gut microbiota diversity, suggesting a potentially similar role of exosomes in humans.
10. Conclusions and future perspectives
All the aforementioned information suggests that EVs play an essential role in early-life development. Microbiota-derived EVs can influence infant gut microbiota colonization and can affect maturation via their cargo. Moreover, these types of EVs have been proposed as potential biomarkers of metabolic diseases and various pathological conditions (104). Human exosomes can, through their cargo, promote epigenetic changes that influence gene expression in infant cells or alter gut microbiota diversity. Of note, it should also be considered that the cargo found in HBM-derived exosomes is influenced by maternal lifestyle and health (95,105). For instance, maternal obesity is negatively associated with the content of exosomal miR-148a and miR-30b in human breast milk. These miRNAs are involved in glucose metabolism and adipogenesis and appear to influence infant body composition (106). Hence, studying HBM-derived exosomes could potentially provide a new direct link between maternal health and infant development, highlighting their potential role as intricate biomarkers and therapeutic agents.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
DV conceived the study. TM, EP, KD, GPC and DV wrote, drafted, revised, edited and reviewed the manuscript. All authors have read and approved the final manuscript. Data authentication is not applicable.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
GPC is the Editor in Chief of the journal, and DV is an Editor of the journal. However, they had no personal involvement in the reviewing process, or any influence in terms of adjudicating on the final decision, for this article. The other authors declare that they have no competing interests.
References
|
Richter L, Black M, Britto P, Daelmans B, Desmond C, Devercelli A, Dua T, Fink G, Heymann J, Lombardi J, et al: Early childhood development: An imperative for action and measurement at scale. BMJ Global Health. 4 (Suppl 4)(e001302)2019.PubMed/NCBI View Article : Google Scholar | |
|
Ferrante G, Fasola S, Cilluffo G, Piacentini G, Viegi G and La Grutta S: Addressing Exposome: An innovative approach to environmental determinants in pediatric respiratory health. Front Public Health. 10(871140)2022.PubMed/NCBI View Article : Google Scholar | |
|
Fenga C: Gut microbiota modulation: A tailored approach for the prevention of chronic diseases. Biomed Rep. 16(23)2022.PubMed/NCBI View Article : Google Scholar | |
|
Abeysinghe P, Turner N, Morean Garcia I, Mosaad E, Peiris HN and Mitchell MD: The role of exosomal epigenetic modifiers in cell communication and fertility of dairy cows. Int J Mol Sci. 21(9106)2020.PubMed/NCBI View Article : Google Scholar | |
|
Zhang B, Zhao J, Jiang M, Peng D, Dou X, Song Y and Shi J: The potential role of gut microbial-derived exosomes in metabolic-associated fatty liver disease: Implications for treatment. Front Immunol. 13(893617)2022.PubMed/NCBI View Article : Google Scholar | |
|
Galley JD and Besner GE: The therapeutic potential of breast milk-derived extracellular vesicles. Nutrients. 12(745)2020.PubMed/NCBI View Article : Google Scholar | |
|
Wang DR and Pan J: Extracellular vesicles: Emerged as a promising strategy for regenerative medicine. World J Stem Cells. 15:165–181. 2023.PubMed/NCBI View Article : Google Scholar | |
|
Doyle LM and Wang MZ: Overview of extracellular vesicles, their origin, composition, purpose, and methods for exosome isolation and analysis. Cells. 8(727)2019.PubMed/NCBI View Article : Google Scholar | |
|
Kumar MA, Baba SK, Sadida HQ, Marzooqi SA, Jerobin J, Altemani FH, Algehainy N, Alanazi MA, Abou-Samra AB, Kumar R, et al: Extracellular vesicles as tools and targets in therapy for diseases. Signal Transduct Target Ther. 9(27)2024.PubMed/NCBI View Article : Google Scholar | |
|
Mobarak H, Javid F, Narmi MT, Mardi N, Sadeghsoltani F, Khanicheragh P, Narimani S, Mahdipour M, Sokullu E, Valioglu F and Rahbarghazi R: Prokaryotic microvesicles Ortholog of eukaryotic extracellular vesicles in biomedical fields. Cell Commun Signal. 22(80)2024.PubMed/NCBI View Article : Google Scholar | |
|
Liu J, Cvirkaite-Krupovic V, Commere PH, Yang Y, Zhou F, Forterre P, Shen Y and Krupovic M: Archaeal extracellular vesicles are produced in an ESCRT-dependent manner and promote gene transfer and nutrient cycling in extreme environments. ISME J. 15:2892–2905. 2021.PubMed/NCBI View Article : Google Scholar | |
|
Sheta M, Taha EA, Lu Y and Eguchi T: Extracellular vesicles: New classification and tumor immunosuppression. Biology (Basel). 12(110)2023.PubMed/NCBI View Article : Google Scholar | |
|
Zhang Y, Bi J, Huang J, Tang Y, Du S and Li P: Exosome: A review of its classification, isolation techniques, storage, diagnostic and targeted therapy applications. Int J Nanomedicine. 15:6917–6934. 2020.PubMed/NCBI View Article : Google Scholar | |
|
Duréndez-Sáez E, Calabuig-Fariñas S, Torres-Martínez S, Moreno-Manuel A, Herreros-Pomares A, Escorihuela E, Mosqueda M, Gallach S, Guijarro R, Serna E, et al: Analysis of exosomal cargo provides accurate clinical, histologic and mutational information in non-small cell lung cancer. Cancers (Basel). 14(3216)2022.PubMed/NCBI View Article : Google Scholar | |
|
Dimik M, Abeysinghe P, Logan J and Mitchell M: The exosome: A review of current therapeutic roles and capabilities in human reproduction. Drug Deliv Transl Res. 13:473–502. 2023.PubMed/NCBI View Article : Google Scholar | |
|
Dilsiz N: Hallmarks of exosomes. Future Sci OA. 8(FSO764)2022.PubMed/NCBI View Article : Google Scholar | |
|
Zhang Y, Dou Y, Liu Y, Di M, Bian H, Sun X and Yang Q: Advances in therapeutic applications of extracellular vesicles. Int J Nanomedicine. 18:3285–3307. 2023.PubMed/NCBI View Article : Google Scholar | |
|
Schiller EA, Cohen K, Lin X, El-Khawam R and Hanna N: Extracellular Vesicle-microRNAs as diagnostic biomarkers in preterm neonates. Int J Mol Sci. 24(2622)2023.PubMed/NCBI View Article : Google Scholar | |
|
Lak NSM, van der Kooi EJ, Enciso-Martinez A, Lozano-Andrés E, Otto C, Wauben MHM and Tytgat GAM: Extracellular vesicles: A new source of biomarkers in pediatric solid tumors? A systematic review. Front Oncol. 12(887210)2022.PubMed/NCBI View Article : Google Scholar | |
|
Galardi A, Colletti M, Di Paolo V, Vitullo P, Antonetti L, Russo I and Di Giannatale A: Exosomal MiRNAs in pediatric cancers. Int J Mol Sci. 20(4600)2019.PubMed/NCBI View Article : Google Scholar | |
|
Chaubey S, Thueson S, Ponnalagu D, Alam MA, Gheorghe CP, Aghai Z, Singh H and Bhandari V: Early gestational mesenchymal stem cell secretome attenuates experimental bronchopulmonary dysplasia in part via exosome-associated factor TSG-6. Stem Cell Res Ther. 9(173)2018.PubMed/NCBI View Article : Google Scholar | |
|
Braun RK, Chetty C, Balasubramaniam V, Centanni R, Haraldsdottir K, Hematti P and Eldridge MW: Intraperitoneal injection of MSC-derived exosomes prevent experimental bronchopulmonary dysplasia. Biochem Biophys Res Commun. 503:2653–2658. 2018.PubMed/NCBI View Article : Google Scholar | |
|
Cavalli G and Heard E: Advances in epigenetics link genetics to the environment and disease. Nature. 571:489–499. 2019.PubMed/NCBI View Article : Google Scholar | |
|
Bertogliat MJ, Morris-Blanco KC and Vemuganti R: Epigenetic mechanisms of neurodegenerative diseases and acute brain injury. Neurochem Int. 133(104642)2020.PubMed/NCBI View Article : Google Scholar | |
|
Liang M: Epigenetic mechanisms and hypertension. Hypertension. 72:1244–1254. 2018.PubMed/NCBI View Article : Google Scholar | |
|
Nasrullah Hussain A, Ahmed S, Rasool M and Shah AJ: DNA methylation across the tree of life, from micro to macro-organism. Bioengineered. 13:1666–1685. 2022.PubMed/NCBI View Article : Google Scholar | |
|
Shi J, Xu J, Chen YE, Li JS, Cui Y, Shen L, Li JJ and Li W: The concurrence of DNA methylation and demethylation is associated with transcription regulation. Nat Commun. 12(5285)2021.PubMed/NCBI View Article : Google Scholar | |
|
Uddin MG and Fandy TE: DNA methylation inhibitors: Retrospective and perspective view. Adv Cancer Res. 152:205–223. 2021.PubMed/NCBI View Article : Google Scholar | |
|
Sallustio F, Gesualdo L and Gallone A: New findings showing how DNA methylation influences diseases. World J Biol Chem. 10:1–6. 2019.PubMed/NCBI View Article : Google Scholar | |
|
Tompkins JD: Discovering DNA methylation, the history and future of the writing on DNA. J Hist Biol. 55:865–887. 2022.PubMed/NCBI View Article : Google Scholar | |
|
Alaskhar Alhamwe B, Khalaila R, Wolf J, von Bülow V, Harb H, Alhamdan F, Hii CS, Prescott SL, Ferrante A, Renz H, et al: Histone modifications and their role in epigenetics of atopy and allergic diseases. Allergy Asthma Clin Immunol. 14(39)2018.PubMed/NCBI View Article : Google Scholar | |
|
Lee HT, Oh S, Ro DH, Yoo H and Kwon YW: The Key Role of DNA Methylation and histone acetylation in epigenetics of atherosclerosis. J Lipid Atheroscler. 9:419–434. 2020.PubMed/NCBI View Article : Google Scholar | |
|
Liu R, Wu J, Guo H, Yao W, Li S, Lu Y, Jia Y, Liang X, Tang J and Zhang H: Post-translational modifications of histones: Mechanisms, biological functions, and therapeutic targets. MedComm (2020). 4(e292)2023.PubMed/NCBI View Article : Google Scholar | |
|
Zhang SF, Gao J and Liu CM: The role of non-coding RNAs in neurodevelopmental disorders. Front Genet. 10(1033)2019.PubMed/NCBI View Article : Google Scholar | |
|
Ratti M, Lampis A, Ghidini M, Salati M, Mirchev MB, Valeri N and Hahne JC: MicroRNAs (miRNAs) and Long Non-Coding RNAs (lncRNAs) as new tools for cancer therapy: First steps from bench to bedside. Target Oncol. 15:261–278. 2020.PubMed/NCBI View Article : Google Scholar | |
|
Jorge AL, Pereira ER, Oliveira CS, Ferreira EDS, Menon ETN, Diniz SN and Pezuk JA: MicroRNAs: Understanding their role in gene expression and cancer. Einstein (Sao Paulo). 19(eRB5996)2021.PubMed/NCBI View Article : Google Scholar | |
|
Borkiewicz L, Kalafut J, Dudziak K, Przybyszewska-Podstawka A and Telejko I: Decoding LncRNAs. Cancers (Basel). 13(2643)2021.PubMed/NCBI View Article : Google Scholar | |
|
Li S, Ye Z, Mather KA, Nguyen TL, Dite GS, Armstrong NJ, Wong EM, Thalamuthu A, Giles GG, Craig JM, et al: Early life affects late-life health through determining DNA methylation across the lifespan: A twin study. EBioMedicine. 77(103927)2022.PubMed/NCBI View Article : Google Scholar | |
|
Schrott R, Song A and Ladd-Acosta C: Epigenetics as a biomarker for early-life environmental exposure. Curr Environ Health Rep. 9:604–624. 2022.PubMed/NCBI View Article : Google Scholar | |
|
Xu Q, Jiang M, Gu S, Wang F and Yuan B: Early life stress induced DNA methylation of monoamine oxidases leads to depressive-like behavior. Front Cell Dev Biol. 8(582247)2020.PubMed/NCBI View Article : Google Scholar | |
|
Park J, Lee K, Kim K and Yi SJ: The role of histone modifications: From neurodevelopment to neurodiseases. Signal Transduct Target Ther. 7(217)2022.PubMed/NCBI View Article : Google Scholar | |
|
Guan L, Shi X, Tang Y, Yan Y, Chen L, Chen Y, Gao G, Lin C and Chen A: Contribution of amygdala histone acetylation in early life stress-induced visceral hypersensitivity and emotional comorbidity. Front Neurosci. 16(843396)2022.PubMed/NCBI View Article : Google Scholar | |
|
Allen L and Dwivedi Y: MicroRNA mediators of early life stress vulnerability to depression and suicidal behavior. Mol Psychiatry. 25:308–320. 2020.PubMed/NCBI View Article : Google Scholar | |
|
Tsagakis I, Douka K, Birds I and Aspden JL: Long non-coding RNAs in development and disease: Conservation to mechanisms. J Pathol. 250:480–495. 2020.PubMed/NCBI View Article : Google Scholar | |
|
Rom A, Melamed L, Gil N, Goldrich MJ, Kadir R, Golan M, Biton I, Perry RB and Ulitsky I: Regulation of CHD2 expression by the Chaserr long noncoding RNA gene is essential for viability. Nat Commun. 10(5092)2019.PubMed/NCBI View Article : Google Scholar | |
|
Caldwell KK, Hafez A, Solomon E, Cunningham M and Allan AM: Arsenic exposure during embryonic development alters the expression of the long noncoding RNA growth arrest specific-5 (Gas5) in a sex-dependent manner. Neurotoxicol Teratol. 66:102–112. 2018.PubMed/NCBI View Article : Google Scholar | |
|
Zhou Z, Chen J, Huang Y, Liu D, Chen S and Qin S: Long Noncoding RNA GAS5: A new factor involved in bone diseases. Front Cell Dev Biol. 9(807419)2022.PubMed/NCBI View Article : Google Scholar | |
|
Hou K, Wu ZX, Chen XY, Wang JQ, Zhang D, Xiao C, Zhu D, Koya JB, Wei L, Li J and Chen ZS: Microbiota in health and diseases. Signal Transduct Target Ther. 7(135)2022.PubMed/NCBI View Article : Google Scholar | |
|
Afzaal M, Saeed F, Shah YA, Hussain M, Rabail R, Socol CT, Hassoun A, Pateiro M, Lorenzo JM, Rusu AV and Aadil RM: Human gut microbiota in health and disease: Unveiling the relationship. Front Microbiol. 13(999001)2022.PubMed/NCBI View Article : Google Scholar | |
|
Peng X, Cheng L, You Y, Tang C, Ren B, Li Y, Xu X and Zhou X: Oral microbiota in human systematic diseases. Int J Oral Sci. 14(14)2022.PubMed/NCBI View Article : Google Scholar | |
|
Skowron K, Bauza-Kaszewska J, Kraszewska Z, Wiktorczyk-Kapischke N, Grudlewska-Buda K, Kwiecińska-Piróg J, Wałecka-Zacharska E, Radtke L and Gospodarek-Komkowska E: Human skin microbiome: Impact of intrinsic and extrinsic factors on skin microbiota. Microorganisms. 9(543)2021.PubMed/NCBI View Article : Google Scholar | |
|
Man WH, de Steenhuijsen Piters WA and Bogaert D: The microbiota of the respiratory tract: Gatekeeper to respiratory health. Nat Rev Microbiol. 15:259–270. 2017.PubMed/NCBI View Article : Google Scholar | |
|
Cocomazzi G, De Stefani S, Del Pup L, Palini S, Buccheri M, Primiterra M, Sciannamè N, Faioli R, Maglione A, Baldini GM, et al: The impact of the female genital microbiota on the outcome of assisted reproduction treatments. Microorganisms. 11(1443)2023.PubMed/NCBI View Article : Google Scholar | |
|
Al Bander Z, Nitert MD, Mousa A and Naderpoor N: The gut microbiota and inflammation: An overview. Int J Environ Res Public Health. 17(7618)2020.PubMed/NCBI View Article : Google Scholar | |
|
Ferraris C, Elli M and Tagliabue A: Gut microbiota for health: How can diet maintain a healthy Gut Microbiota? Nutrients. 12(3596)2020.PubMed/NCBI View Article : Google Scholar | |
|
Chen Y, Zhou J and Wang L: Role and mechanism of gut microbiota in human disease. Front Cell Infect Microbiol. 11(625913)2021.PubMed/NCBI View Article : Google Scholar | |
|
Russo M, Calevo MG, D'Alessandro G, Tantari M, Migliorati M, Piccardo I, Perucchin PP and Arioni C: Influence of maternal oral microbiome on newborn oral microbiome in healthy pregnancies. Ital J Pediatr. 49(140)2023.PubMed/NCBI View Article : Google Scholar | |
|
Dhariwala MO and Scharschmidt TC: Baby's skin bacteria: First impressions are long-lasting. Trends Immunol. 42:1088–1099. 2021.PubMed/NCBI View Article : Google Scholar | |
|
Jašarević E, Hill EM, Kane PJ, Rutt L, Gyles T, Folts L, Rock KD, Howard CD, Morrison KE, Ravel J and Bale TL: The composition of human vaginal microbiota transferred at birth affects offspring health in a mouse model. Nat Commun. 12(6289)2021.PubMed/NCBI View Article : Google Scholar | |
|
Zhuang L, Chen H, Zhang S, Zhuang J, Li Q and Feng Z: Intestinal microbiota in early life and its implications on childhood health. Genomics Proteomics Bioinformatics. 17:13–25. 2019.PubMed/NCBI View Article : Google Scholar | |
|
Niu J, Xu L, Qian Y, Sun Z, Yu D, Huang J, Zhou X, Wang Y, Zhang T, Ren R, et al: Evolution of the gut microbiome in early childhood: A cross-sectional study of Chinese children. Front Microbiol. 11(439)2020.PubMed/NCBI View Article : Google Scholar | |
|
Sarkar A, Yoo JY, Valeria Ozorio Dutra S, Morgan KH and Groer M: The Association between early-life gut microbiota and long-term health and diseases. J Clin Med. 10(459)2021.PubMed/NCBI View Article : Google Scholar | |
|
Coley EJL, Mayer EA, Osadchiy V, Chen Z, Subramanyam V, Zhang Y, Hsiao EY, Gao K, Bhatt R, Dong T, et al: Early life adversity predicts brain-gut alterations associated with increased stress and mood. Neurobiol Stress. 15(100348)2021.PubMed/NCBI View Article : Google Scholar | |
|
Erabi H, Okada G, Shibasaki C, Setoyama D, Kang D, Takamura M, Yoshino A, Fuchikami M, Kurata A, Kato TA, et al: Kynurenic acid is a potential overlapped biomarker between diagnosis and treatment response for depression from metabolome analysis. Sci Rep. 10(16822)2020.PubMed/NCBI View Article : Google Scholar | |
|
Li B, Guo K, Zeng L, Zeng B, Huo R, Luo Y, Wang H, Dong M, Zheng P, Zhou C, et al: Metabolite identification in fecal microbiota transplantation mouse livers and combined proteomics with chronic unpredictive mild stress mouse livers. Transl Psychiatry. 8(34)2018.PubMed/NCBI View Article : Google Scholar | |
|
Behr C, Kamp H, Fabian E, Krennrich G, Mellert W, Peter E, Strauss V, Walk T, Rietjens IMCM and van Ravenzwaay B: Gut microbiome-related metabolic changes in plasma of antibiotic-treated rats. Arch Toxicol. 91:3439–3454. 2017.PubMed/NCBI View Article : Google Scholar | |
|
Padmasekar M, Savai R, Seeger W and Pullamsetti SS: Exposomes to exosomes: Exosomes as tools to study epigenetic adaptive mechanisms in high-altitude humans. Int J Environ Res Public Health. 18(8280)2021.PubMed/NCBI View Article : Google Scholar | |
|
Fischer J, Koch L, Emmerling C, Vierkotten J, Peters T, Brüning JC and Rüther U: Inactivation of the Fto gene protects from obesity. Nature. 458:894–898. 2009.PubMed/NCBI View Article : Google Scholar | |
|
Sachse G, Church C, Stewart M, Cater H, Teboul L, Cox RD and Ashcroft FM: FTO demethylase activity is essential for normal bone growth and bone mineralization in mice. Biochim Biophys Acta Mol Basis Dis. 1864:843–850. 2018.PubMed/NCBI View Article : Google Scholar | |
|
Melnik BC and Schmitz G: Milk's role as an epigenetic regulator in health and disease. Diseases. 5(12)2017.PubMed/NCBI View Article : Google Scholar | |
|
Zhang L, Lin TV, Yuan Q, Sadoul R, Lam TT and Bordey A: Small extracellular vesicles control dendritic spine development through regulation of HDAC2 signaling. J Neurosci. 41:3799–3807. 2021.PubMed/NCBI View Article : Google Scholar | |
|
Schwarzenbach H and Gahan PB: MicroRNA shuttle from cell-to-cell by exosomes and its impact in cancer. Noncoding RNA. 5(28)2019.PubMed/NCBI View Article : Google Scholar | |
|
Shirazi S, Huang CC, Kang M, Lu Y, Ravindran S and Cooper LF: The importance of cellular and exosomal miRNAs in mesenchymal stem cell osteoblastic differentiation. Sci Rep. 11(5953)2021.PubMed/NCBI View Article : Google Scholar | |
|
Li C, Zhou T, Chen J, Li R, Chen H, Luo S, Chen D, Cai C and Li W: The role of Exosomal miRNAs in cancer. J Transl Med. 20(6)2022.PubMed/NCBI View Article : Google Scholar | |
|
Wang Y, Zhang M and Zhou F: Biological functions and clinical applications of exosomal long non-coding RNAs in cancer. J Cell Mol Med. 24:11656–11666. 2020.PubMed/NCBI View Article : Google Scholar | |
|
Zhang W, Yan Y, Peng J, Thakur A, Bai N, Yang K and Xu Z: Decoding roles of exosomal lncRNAs in tumor-immune regulation and therapeutic potential. Cancers (Basel). 15(286)2022.PubMed/NCBI View Article : Google Scholar | |
|
Yang Q, Diamond MP and Al-Hendy A: Early life adverse environmental exposures increase the risk of uterine fibroid development: role of epigenetic regulation. Front Pharmacol. 7(40)2016.PubMed/NCBI View Article : Google Scholar | |
|
Díez-Sainz E, Milagro FI, Riezu-Boj JI and Lorente-Cebrián S: Effects of gut microbiota-derived extracellular vesicles on obesity and diabetes and their potential modulation through diet. J Physiol Biochem. 78:485–499. 2022.PubMed/NCBI View Article : Google Scholar | |
|
Liang X, Dai N, Sheng K, Lu H, Wang J, Chen L and Wang Y: Gut bacterial extracellular vesicles: Important players in regulating intestinal microenvironment. Gut Microbes. 14(2134689)2022.PubMed/NCBI View Article : Google Scholar | |
|
Macia L, Nanan R, Hosseini-Beheshti E and Grau GE: Host- and Microbiota-derived extracellular vesicles, immune function, and disease development. Int J Mol Sci. 21(107)2019.PubMed/NCBI View Article : Google Scholar | |
|
Martínez-Ruiz S, Sáez-Fuertes L, Casanova-Crespo S, Rodríguez-Lagunas MJ, Pérez-Cano FJ, Badia J and Baldoma L: Microbiota-Derived extracellular vesicles promote immunity and intestinal maturation in suckling rats. Nutrients. 15(4701)2023.PubMed/NCBI View Article : Google Scholar | |
|
Liu H, Zhang Q, Wang S, Weng W, Jing Y and Su J: Bacterial extracellular vesicles as bioactive nanocarriers for drug delivery: Advances and perspectives. Bioact Mater. 14:169–181. 2021.PubMed/NCBI View Article : Google Scholar | |
|
Liu JH, Chen CY, Liu ZZ, Luo ZW, Rao SS, Jin L, Wan TF, Yue T, Tan YJ, Yin H, et al: Extracellular vesicles from child gut microbiota enter into bone to preserve bone mass and strength. Adv Sci (Weinh). 8(2004831)2021.PubMed/NCBI View Article : Google Scholar | |
|
Kaisanlahti A, Turunen J, Byts N, Samoylenko A, Bart G, Virtanen N, Tejesvi MV, Zhyvolozhnyi A, Sarfraz S and Kumpula S: , et al: Maternal microbiota communicates with the fetus through microbiota-derived extracellular vesicles. Microbiome. 11(249)2023.PubMed/NCBI View Article : Google Scholar | |
|
Du X, Ley R and Buck AH: MicroRNAs and extracellular vesicles in the gut: New host modulators of the microbiome? Microlife. 2(uqab010)2021.PubMed/NCBI View Article : Google Scholar | |
|
Feng X, Chen X, Zheng X, Zhu H, Qi Q, Liu S, Zhang H and Che J: Latest trend of milk derived exosomes: Cargos, functions, and applications. Front Nutr. 8(747294)2021.PubMed/NCBI View Article : Google Scholar | |
|
Lyons KE, Ryan CA, Dempsey EM, Ross RP and Stanton C: Breast milk, a source of beneficial microbes and associated benefits for infant health. Nutrients. 12(1039)2020.PubMed/NCBI View Article : Google Scholar | |
|
Duale A, Singh P and Al Khodor S: Breast milk: A meal worth having. Front Nutr. 8(800927)2022.PubMed/NCBI View Article : Google Scholar | |
|
Yi DY and Kim SY: Human breast milk composition and function in human health: From nutritional components to microbiome and MicroRNAs. Nutrients. 13(3094)2021.PubMed/NCBI View Article : Google Scholar | |
|
Banić M, Butorac K, Čuljak N, Leboš Pavunc A, Novak J, Bellich B, Kazazić S, Kazazić S, Cescutti P, Šušković J, et al: The human milk microbiota produces potential therapeutic biomolecules and shapes the intestinal microbiota of infants. Int J Mol Sci. 23(14382)2022.PubMed/NCBI View Article : Google Scholar | |
|
Notarbartolo V, Giuffrè M, Montante C, Corsello G and Carta M: Composition of human breast milk microbiota and its role in children's health. Pediatr Gastroenterol Hepatol Nutr. 25:194–210. 2022.PubMed/NCBI View Article : Google Scholar | |
|
Kim KU, Kim WH, Jeong CH, Yi DY and Min H: More than Nutrition: Therapeutic potential of breast milk-derived exosomes in cancer. Int J Mol Sci. 21(7327)2020.PubMed/NCBI View Article : Google Scholar | |
|
Shah J, Sims B and Martin C: Therapeutic potential of human breast milk derived exosomes. J Nanopart Res. 24(260)2022. | |
|
Admyre C, Johansson SM, Qazi KR, Filén JJ, Lahesmaa R, Norman M, Neve EP, Scheynius A and Gabrielsson S: Exosomes with immune modulatory features are present in human breast milk1. J Immunol. 179:1969–1978. 2007.PubMed/NCBI View Article : Google Scholar | |
|
de la Torre Gomez C, Goreham RV, Bech Serra JJ, Nann T and Kussmann M: ‘Exosomics’-A review of biophysics, biology and biochemistry of exosomes with a focus on human breast milk. Front Genet. 9(92)2018.PubMed/NCBI View Article : Google Scholar | |
|
Mirza AH, Kaur S, Nielsen LB, Størling J, Yarani R, Roursgaard M, Mathiesen ER, Damm P, Svare J, Mortensen HB and Pociot F: Breast milk-derived extracellular vesicles enriched in exosomes from mothers with type 1 diabetes contain aberrant levels of microRNAs. Front Immunol. 10(2543)2019.PubMed/NCBI View Article : Google Scholar | |
|
Kim KU, Han K, Kim J, Kwon DH, Ji YW, Yi DY and Min H: The protective role of exosome-derived MicroRNAs and proteins from human breast milk against infectious agents. Metabolites. 13(635)2023.PubMed/NCBI View Article : Google Scholar | |
|
Chiurazzi M, Cozzolino M, Reinelt T, Nguyen TD, Elke Chie S, Natalucci G and Miletta MC: Human milk and brain development in infants. Reprod Med. 2:107–117. 2021. | |
|
Guo MM, Zhang K and Zhang JH: Human breast milk-derived exosomal miR-148a-3p protects against necrotizing enterocolitis by regulating p53 and Sirtuin 1. Inflammation. 45:1254–1268. 2022.PubMed/NCBI View Article : Google Scholar | |
|
Gialeli G, Panagopoulou O, Liosis G and Siahanidou T: Potential epigenetic effects of human milk on infants' neurodevelopment. Nutrients. 15(3614)2023.PubMed/NCBI View Article : Google Scholar | |
|
Cintio M, Polacchini G, Scarsella E, Montanari T, Stefanon B and Colitti M: MicroRNA Milk Exosomes: From cellular regulator to genomic marker. Animals (Basel). 10(1126)2020.PubMed/NCBI View Article : Google Scholar | |
|
Melnik BC, Stremmel W, Weiskirchen R, John SM and Schmitz G: Exosome-Derived MicroRNAs of human milk and their effects on infant health and development. Biomolecules. 11(851)2021.PubMed/NCBI View Article : Google Scholar | |
|
Zhou F, Paz HA, Sadri M, Cui J, Kachman SD, Fernando SC and Zempleni J: Dietary bovine milk exosomes elicit changes in bacterial communities in C57BL/6 mice. Am J Physiol Gastrointest Liver Physiol. 317:G618–G624. 2019.PubMed/NCBI View Article : Google Scholar | |
|
Turunen J, Tejesvi MV, Suokas M, Virtanen N, Paalanne N, Kaisanlahti A, Reunanen J and Tapiainen T: Bacterial extracellular vesicles in the microbiome of first-pass meconium in newborn infants. Pediatr Res. 93:887–896. 2023.PubMed/NCBI View Article : Google Scholar | |
|
Holzhausen EA, Kupsco A, Chalifour BN, Patterson WB, Schmidt KA, Mokhtari P, Baccarelli AA, Goran MI and Alderete TL: Influence of technical and maternal-infant factors on the measurement and expression of extracellular miRNA in human milk. Front Immunol. 14(1151870)2023.PubMed/NCBI View Article : Google Scholar | |
|
Shah KB, Chernausek SD, Garman LD, Pezant NP, Plows JF, Kharoud HK, Demerath EW and Fields DA: Human milk exosomal MicroRNA: Associations with maternal overweight/obesity and infant body composition at 1 month of life. Nutrients. 13(1091)2021.PubMed/NCBI View Article : Google Scholar |