Sulforaphane suppresses the growth of EGFR‑overexpressing MDA‑MB‑468 triple‑negative breast cancer cells in vivo and in vitro
- Authors:
- Published online on: March 23, 2022 https://doi.org/10.3892/ijfn.2022.26
- Article Number: 3
-
Copyright: © Yasunaga et al. This is an open access article distributed under the terms of Creative Commons Attribution License.
Abstract
Introduction
Cancer is an increasing global concern, and breast cancer remains the most frequently diagnosed cancer affecting females, accounting for >20% of all cancer cases in women worldwide (1). Breast cancer is categorized based on cellular markers that reflect the available targeted therapies. Triple-negative breast cancer (TNBC) is a subtype of breast cancer characterized by the suppressed expression levels of the estrogen receptor (ER), progesterone receptor (PR) and human epidermal growth factor receptor 2 (HER2). TNBC accounts for ~10-20% of breast cancer cases and is a highly aggressive disease with frequent early relapses and a very poor overall survival rate (2). Therefore, in addition to its prevention, the development of novel treatment options for this type of breast cancer is crucial due to the limited number of available treatments.
Previous epidemiological studies have suggested that diets rich in cruciferous vegetables, such as broccoli, cabbage and kale reduce the risk of developing a number of common types of cancer, including breast cancer (3,4). Sulforaphane (SFN) is an isothiocyanate derivative generated by the hydrolytic conversion of glucoraphanin, a sulfur-containing compound found in cruciferous vegetables (5). Recently, SFN was shown to be effective in preventing breast cancer at different stages of carcinogenesis by increasing the levels of antioxidants and phase II detoxifying enzymes via the activation of the nuclear factor erythroid 2-related factor 2 (6,7). In addition to its chemopreventive effects, SFN has been found to exert anti-proliferative effects on various human breast cancer cell lines that are representative of a wide range of breast cancer phenotypes by inducing apoptosis, cell cycle arrest and exhibiting anti-angiogenic capacity (6-8). Therefore, SFN may have the potential to prevent and treat all subtypes of breast cancer.
Molecular aberrations in the epidermal growth factor receptor (EGFR)/phosphoinositide 3-kinase (PI3K)/protein kinase B (Akt)/mechanistic target of rapamycin (mTOR) pathway are well-known pathognomonic abnormalities in breast cancer across various subtypes and are commonly observed in TNBC (9). A subset of TNBC (~18%) is known to express EGFR and is associated with a poor prognosis (10). This signaling pathway is also activated in TNBC cells by the stimulation with non-receptor tyrosine kinases, such as the Src oncoprotein (11), which in turn triggers PI3K activation, followed by the phosphorylation of Akt and mTOR. Moreover, it has been demonstrated that the loss of phosphatase and tensin homolog (PTEN), a tumor suppressor gene that inhibits cell proliferation by inhibiting the PI3K signaling pathway, is a frequent event that occurs in half of TNBC cases (12), and is associated with aggressive behavior and a poor prognosis in patients with TNBC (13). Thus, it is conceivable that the oncogenic activation of the PI3K/Akt/mTOR pathway may be induced in TNBC cells, either by the overexpression/activation of various upstream tyrosine kinases, activating mutations of the PI3K catalytic subunit α, or the loss of function of PTEN. Currently, clinical drugs targeting PI3K/Akt/mTOR signaling have not yet been successfully developed. It has been shown that SFN inhibits the Akt/mTOR pathway, resulting in the decreased survival of phenotypically different breast cancer cells (14). Therefore, SFN may be potentially useful in the treatment of patients with TNBC; however, its precise inhibitory mechanisms remain poorly understood in human TNBC cells presenting an overactivated signaling pathway downstream of EGFR.
Thus, the present study investigated the cellular and molecular mechanisms of the growth-inhibitory activity of SFN against the MDA-MB-468 TNBC cell line exhibiting the activation of the PI3K/Akt/mTOR signaling pathway due to high levels of EGFR expression with the concomitant deletion of PTEN (15,16). In addition, the in vivo activity of SFN was examined using a mouse xenograft model in order to determine the potential clinical application of SFN in the prevention and treatment of this type of breast cancer. To the best of our knowledge, the present study is the first to demonstrate the in vivo antitumor activity of SFN against MDA-MB-468 TNBC cells overexpressing EGFR with the co-deletion of PTEN.
Materials and methods
Cell culture and chemicals
The present study was performed using the MDA-MB-468 TNBC cells purchased from the American Type Culture Collection (HTB-132) supplied by Summit Pharmaceuticals International Co. The origins of the cell line and its hormone receptor and HER2 status have been previously described (17). The MDA-MB-468 cells lack PTEN repressors (16) and possess high EGFR levels (15). This cell line was cultured in RPMI-1640 medium (FUJIFILM Wako Pure Chemical Corporation) supplemented with 10% fetal bovine serum (FBS; Cosmo Bio Co., Ltd.), 100 IU/ml penicillin and 100 µg/ml streptomycin (Thermo Fisher Scientific, Inc.) in a humidified atmosphere of 95% air and 5% CO2 at 37˚C. SFN for use in the in vitro experiments was purchased from Sigma-Aldrich; Merck KGaA and stored at -20˚C. A 225 mM stock solution was prepared by dissolving the original SFN with dimethyl sulfoxide (DMSO; Sigma-Aldrich; Merck KGaA) and diluted with RPMI-1640 immediately prior to experimental use. The final concentration of DMSO for all experiments and treatments (including vehicle controls, where no SFN was added) was maintained at ≤0.002%. These concentrations of DMSO were confirmed to be non-cytotoxic for at least 72 h of consecutive treatment (data not shown).
Determination of growth inhibition
The anti-proliferative effects of SFN on the growth of MDA-MB-468 cells were assessed using the Cell Counting Kit-8 (CCK-8; Dōjindo Laboratories, Inc.) according to the manufacturer's protocol. Briefly, 2,000 cells/100 µl suspension were seeded into each well of a 96-well plate (Corning Inc.). Following 24 h of incubation, 100 µl SFN at various concentrations (0-4.5 µM) was added and cells were further cultured for up to 72 h. The culture medium was then replaced with CCK-8 solution, and incubated for 2 h at 37˚C. The relative number of viable cells was determined by comparing the absorbance (490 nm) of the treated cells with the corresponding absorbance of vehicle-treated cells taken as 100%, using Infinite 200 Pro, Tecan Trading AG. The IC50 value was defined as the concentration at which cell viability was inhibited by 50%.
Cell cycle analysis and measurement of apoptosis
At different time points (0, 24, 48 h ) following treatment with 2 µM SFN (an approximate IC50 concentration), floating and trypsinized adherent cells were combined, fixed in 70% ethanol for 2 h at 4˚C and stored at 4˚C prior to use in cell cycle analysis. Following the removal of ethanol by centrifugation at 500 x g for 5 min in 4˚C, the cells were washed with PBS and stained with a solution containing RNase A (10 µ1/ml) and propidium iodide (PI; 50 µg/ml; Sigma-Aldrich; Merck KGaA) for 30 min at room temperature. Cell cycle analyses were performed on a Gallios flow cytometer with Kaluza ver. 1.2 software (Beckman Coulter, Inc.). Each cell cycle phase was classified based on each histogram, and the percentages were calculated. The extent of apoptosis was determined by measuring the sub G0/G1 population detected by flow cytometry in the same manner as described above.
Western blot analysis of signaling proteins involved in cell growth and apoptosis
Following treatment with 2 µM SFN, the cells were washed with ice-cold PBS and scraped into 0.5 ml lysis buffer (Bio-Rad Laboratories, Inc.). Then protein concentration was determined using the Bradford method. Proteins (10 µg/lane) were resolved by 4-15% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE; Bio-Rad Laboratories, Inc.) and electrotransferred onto a polyvinylidene difluoride membrane (GE Healthcare; Cytiva). Non-specific binding sites were blocked by incubating the membranes in blocking buffer (Nacalai Tesque, Inc.) at room temperature for 30 min. The membranes were then incubated overnight at 4˚C with primary antibodies against Akt (cat. no. 9272; 1:200), phosphorylated (p-)Akt (Ser473; cat. no. 4058; 1:200), mTOR (cat. no. 2983; 1:2,000), p-mTOR (Ser2448; cat. no. 2971; 1:2,000), B-cell lymphoma (Bcl)-2 (cat. no. 2876; 1:1,000), Bcl-2-associated X (Bax; cat. no. 2772; 1:1,000) or β-actin (cat. no. 4967; 1:500) (all from Cell Signaling Technology, Inc.). The membranes were hybridized with horseradish peroxidase-conjugated secondary antibody (cat. no. 7074; 1:1,000, Cell Signaling Technology, Inc.) for 1 h at room temperature. Immunoblots were developed using enhanced chemiluminescence system (GE Healthcare; Cytiva) and quantified using a Fusion Fx Imaging System (Vilber). The density ratios are shown at the bottom of the bands (in graphs) as a relative ratio vs. the untreated control.
Apoptosis was assessed by poly (ADP-ribose) polymerase (PARP) cleavage detected using western blot analysis with PARP antibody (cat. no. 9542; 1:1,000, Cell Signaling Technology, Inc.) using the aforementioned conditions. PARP is a substrate for certain caspases that are activated during the early stages of apoptosis. These proteases cleave PARP to fragments of ~89 and 24 kDa. The detection of the 89 kDa PARP fragment with anti-PARP serves as an early marker of apoptosis.
In vivo tumor xenograft model
All animal procedures were performed in accordance with the protocols approved by the Institutional Animal Care and Use Committee of the Nakamura Gakuen University (Approval no. 2018-1). Athymic nude mice (BALB/cAJcl-nu/nu) were obtained from CLEA Japan, Inc. and housed at the Nakamura-Gakuen Animal Center under the following conditions: A temperature of 24˚C, 40% humidity and a 12-h-light/-dark cycle, with free access to food and water.
A xenograft model of human TNBC was established by the subcutaneous dorsal flank injections of MDA-MB-468 cells (~2.5x106) into 8 female nude mice (4 weeks of age). At 1 week prior to implantation, the 8 female nude mice (4 weeks of age) were divided into two groups, each consisting of 4 mice. A 100 µl SFN (LKT Laboratories, Inc.) solution or PBS (vehicle control) were orally administered daily using polyurethane tubes (FCR&Bio Co., Ltd.) from 1 week prior to the inoculation of the tumor cells to the end of the experiment. The vehicle control group was treated with 100 µl PBS, and the SFN group with 100 µl of 1 mM SFN solution prepared by dissolving SFN with PBS immediately prior to administration. This oral concentration of SFN (1 mM) was arbitrarily determined by preliminary experiments (data not shown). Initially, 6 µM SFN were applied daily by referring to two previous in vivo experiments that used 5.6-6.0 µM SFN from LKT Laboratories, Inc. (18,19). The concentration of SFN administered daily was gradually increased until effects on tumor growth were observed, and finally found that 1 mM SFN was a sufficient dose for attenuating tumor growth (17.7 µg SFN/mouse/day). Food intake and body weight were monitored during the experiment. Tumor size was measured every week using calipers, and tumor volume was calculated using the following formula: Tumor volume (mm3)=[length (mm)]x[width (mm)] 2x0.52. At the end of the study period (7 weeks following tumor cell implantation), the mice were anesthetized using an intraperitoneal injection of pentobarbital (75 mg/kg) followed by euthanasia via exsanguination, and the tumors were removed, weighed and processed for pathological analysis.
Hematoxylin and eosin (H&E) staining
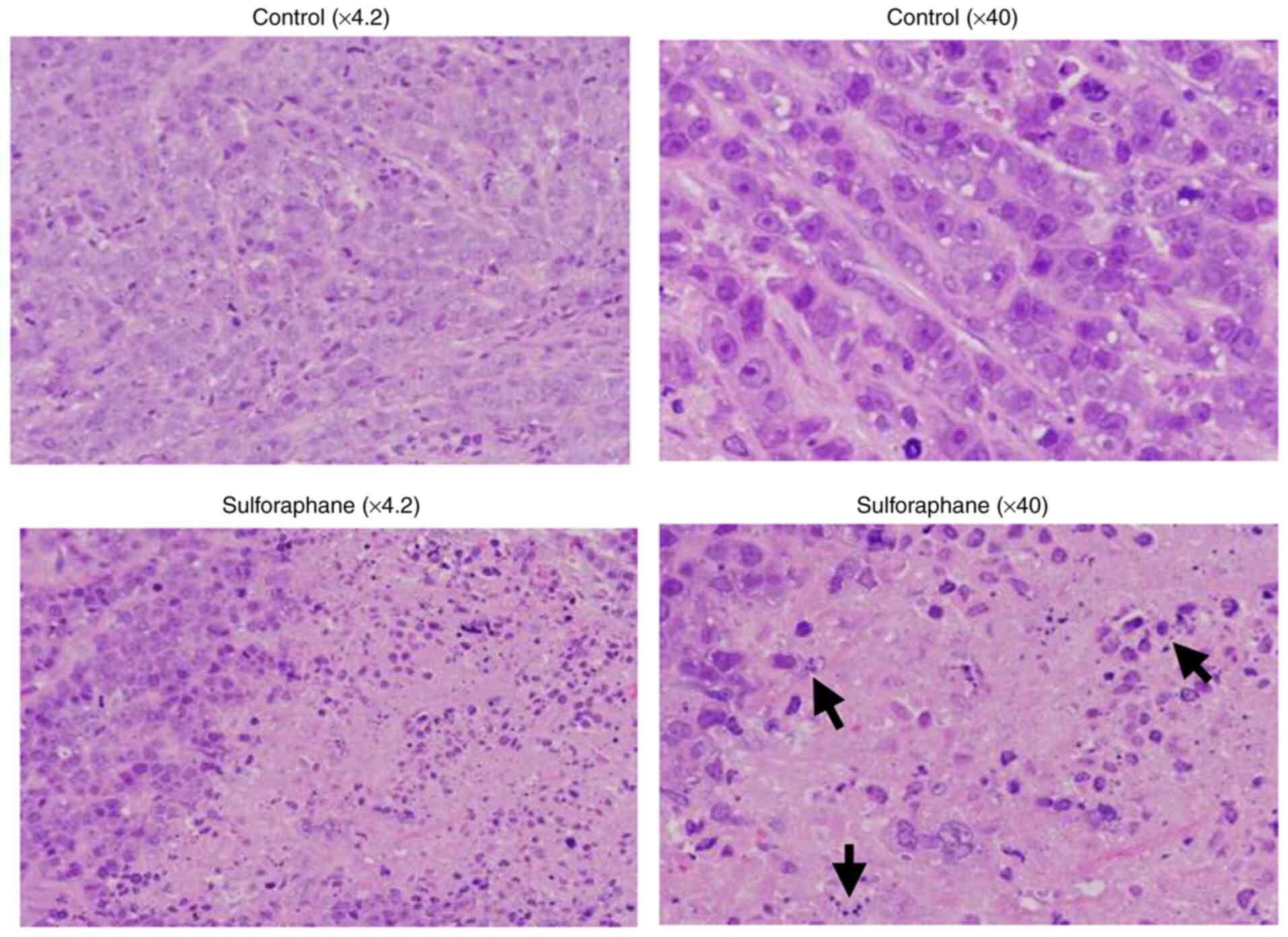
The tumors were fixed in 10% buffered formalin solution at least for 24 h at room temperature until paraffin embedding. Subsequently, the paraffin-embedded tissue blocks were cut into 3-µm-thick sections. The sections were then stained with H&E (FUJI FILM Wako Pure Chemical Corporation) each for 10 min at room temperature. Histopathological images were obtained using an Olympus FSX100 all-in-one inverted microscope (Olympus Corporation).
Statistical analyses
Statistical analyses were performed using statistical software (IBM SPSS Statistics version 25). Data from at least three independent experiments performed in triplicate are presented as the mean ± standard deviation (SD). ANOVA was used to compare changes over time in cytotoxicity experiments After having tested for normality, ANOVA was used for parametric data, and the Mann-Whitney U test for non-parametric data. Comparisons among multiple groups were first performed using one-way ANOVA. If the results revealed significant differences, comparisons were performed using Dunnett's t-test. The Mann-Whitney U test was used to compare two groups, the SF-treated group and the non-treated group, in animal experiments. Statistical tests were two-tailed, and a P-value <0.05 was considered to indicate a statistically significant difference.
Results
Effects of SFN on cell proliferation and survival
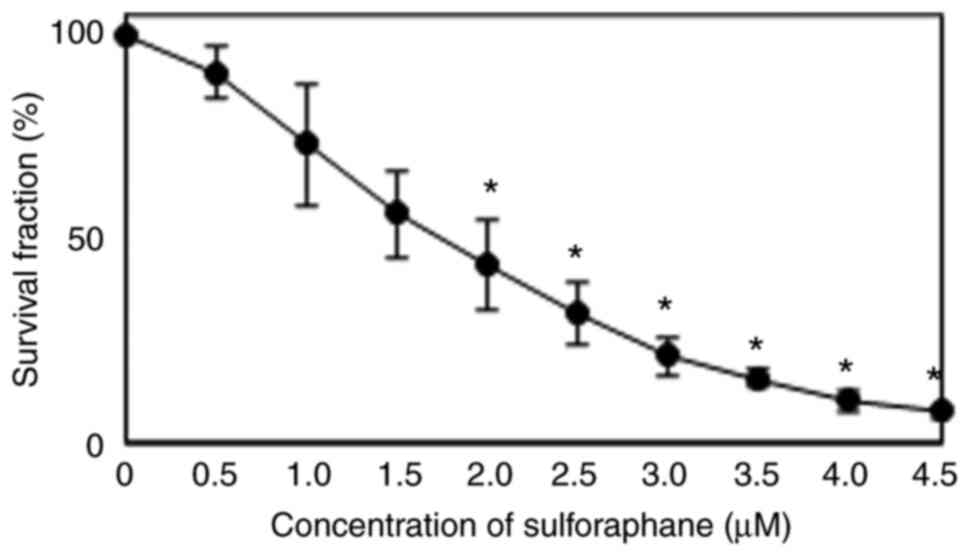
To determine the effects of SFN on cell growth and survival, MDA-MB-468 cells were treated with various concentrations of SFN for 72 h. As shown in Fig. 1, SFN exhibited concentration-dependent antitumor activity against the MDA-MB-468 cells. The 50% inhibitory concentration (IC50) was 1.8±0.4 µM following 72 h of exposure.
Time-course analysis of the effects of SFN on cell cycle progression and apoptosis
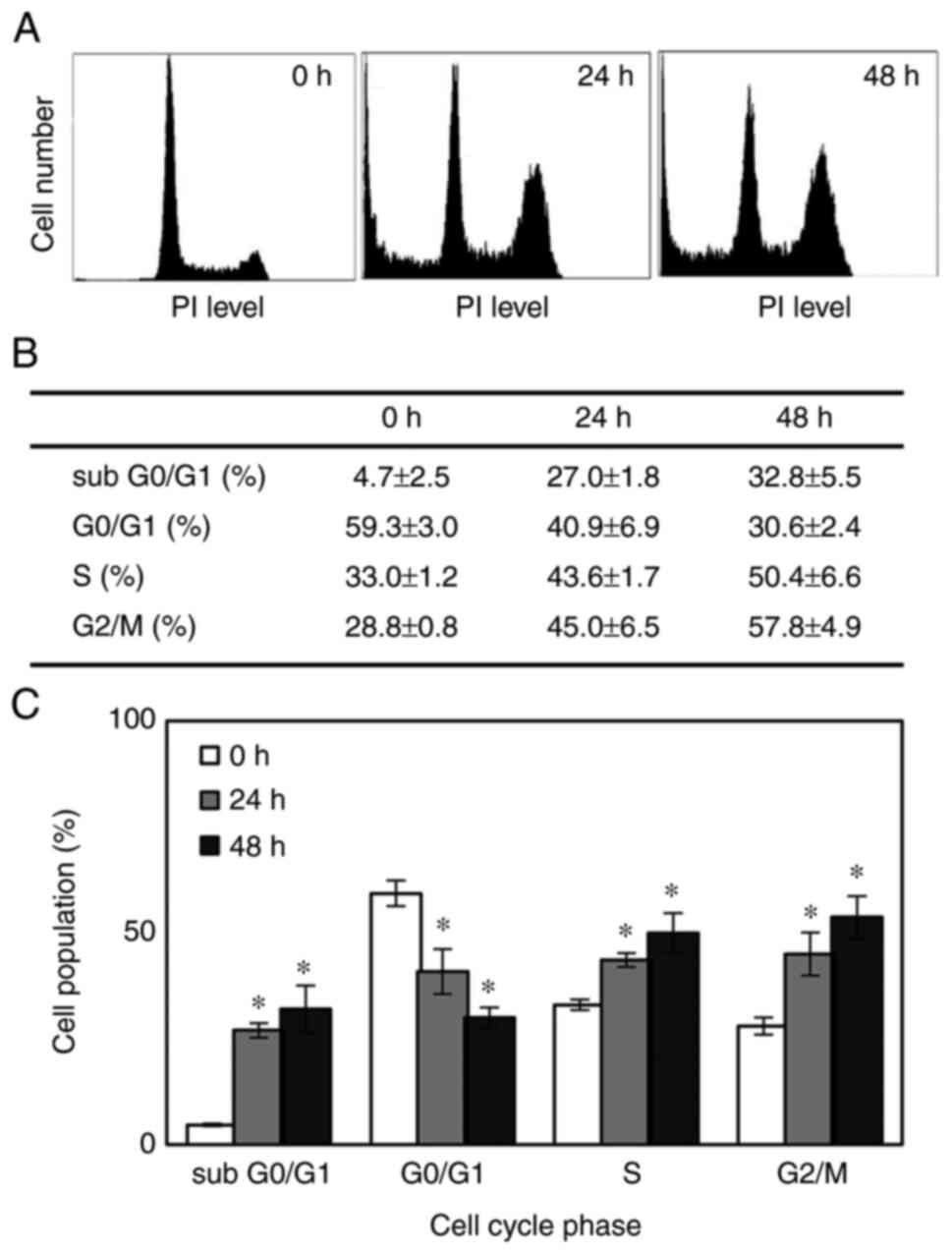
To examine whether the inhibitory effects observed in the cytotoxicity assays reflect the arrest or delay of cell cycle progression or apoptotic cell death, the cells were treated with 2 µM SFN, and cell cycle progression and apoptosis were evaluated by fluorescence-activated cell sorting (FACS) analysis. Representative cell cycle distributions following consecutive treatment with SFN at the indicated time points are shown in Fig. 2A. When the MDA-MB-468 cells were treated with 2 µM SFN, the proportion of cells in the S and G2/M phases significantly increased from 33 and 28.8% at the beginning of the treatment to 43.6 and 45.0%, respectively, with a corresponding decrease in the number of cells in the G0/G1 phase following 24 h of exposure. The percentage of cells in each cell cycle phase was not significantly altered after 48 h consecutive exposure compared with the 24-h time point (Fig. 2B and C). Therefore, SFN arrested the cell cycle at the S phase and more predominantly at the G2/M phase.
The sub G0/G1 cell population, which represents apoptotic cells, abruptly increased from 4.7 to 27.0 and 32.8% following 24 and 48 h of exposure, respectively (Fig. 2B and C), increasing by almost 7-fold following 48 h of exposure. Furthermore, the cleavage of PARP, which serves as an early marker of apoptosis, was demonstrated at 48 h post-treatment (Fig. 3A). The dissociation between the appearance of the initiation of the apoptotic cellular event (cell population at the sub G0/G1 phase) and the cleavage of PARP may be explained by the difference in their detection times. These data indicated that the observed SFN-induced growth decline appeared to be due to the combined effects of the progressive expansion of the apoptotic cell population and the S/G2/M arrest of the cell cycle.
Effects of SFN on the expression of pro- and anti-apoptotic proteins
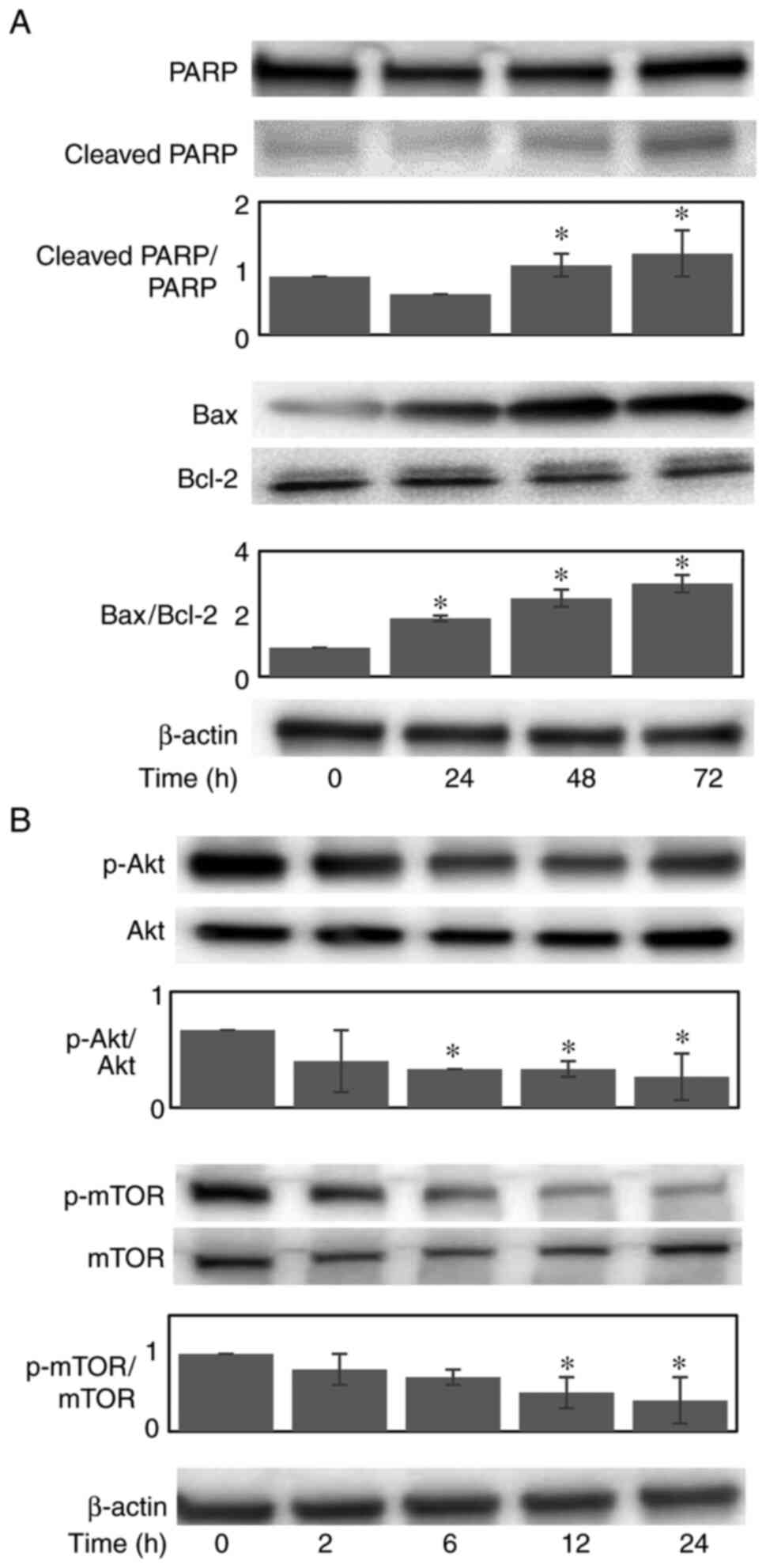
To clarify the apoptotic mechanisms induced by SFN, the protein expression levels of anti-apoptotic Bcl-2 and pro-apoptotic Bax were examined (Fig. 3A). Upon treatment with 2 µM SFN, the expression of Bax was increased in a time-dependent manner, whereas the protein expression of Bcl-2 remained unaltered. Thus, the Bax/Bcl-2 ratio increased up to 3.2-fold following 72 h of consecutive treatment. These results suggest that the increased expression of Bax plays a causative role in SFN-induced apoptosis.
Effects of SFN on the activation of Akt/mTOR signaling molecules
Since the activation of PI3K/Akt/mTOR, a major signaling pathway involved in cell proliferation and survival, is considered to be activated in MDA-MB-468 TNBC cells due to their biological features, including the overexpression of EGFR and the co-deletion of PTEN (15,16), the present study examined the effects of SFN on the expression and activation (phosphorylation) of these proteins. Upon treatment with 2 µM SFN, the phosphorylation of Akt and mTOR was substantially inhibited in a time-dependent manner (Fig. 3B). These data thus indicated that the SFN-induced reduction in cell proliferation and survival appeared to be mediated by the inactivation of Akt and mTOR.
Effects of SFN on MDA-MB-468 cells xenotransplanted into nude mice
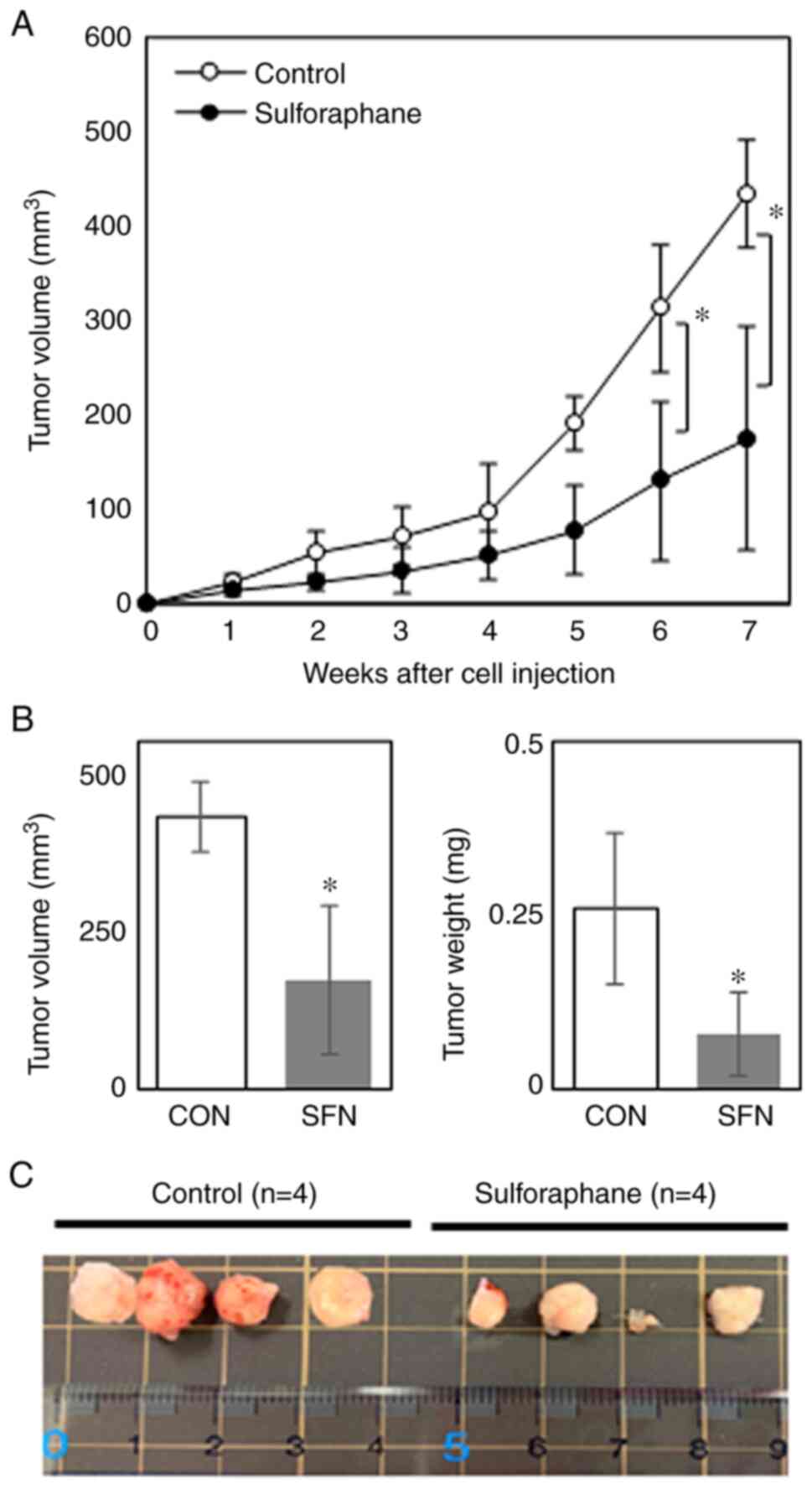
An in vivo experiment was conducted using nude mice xenotransplanted with MDA-MB-468 cells to determine whether the inhibitory effects of SFN on tumor development are observed when administered orally to mice. Although the tumors grew rapidly at ~5 weeks following cell inoculation in control mice, the oral administration of SFN significantly suppressed tumor growth from 5 weeks following inoculation (Fig. 4A), reducing the tumor size and tumor weight by ~60 and 70%, respectively, compared with the untreated control mice at 7 weeks following cell inoculation (Fig. 4B). Images of the xenograft tumors excised from four individual mice in each group at the end of experiment are depicted in Fig. 4C. Although individual tumors varied in size, the tumor sizes appeared to be evidently smaller in the SFN-treated group compared with the control group. Representative images of tumor tissue sections stained with H&E are presented in Fig. 5. The tumor specimens from SFN-treated mice exhibited a degenerated tumor cell appearance with pyknotic nuclei, resembling apoptotic cells (Fig. 5). Furthermore, no significant differences were observed in body weight and food consumption between the two groups (Table I), suggesting that SFN did not exert any or minimal adverse effects at the oral concentration used in the present study. These data thus indicated that SFN substantially inhibited TNBC tumor growth in vivo without exerting any apparent toxic effects.
Discussion
SFN is widely recognized as a promising chemopreventive agent with effects against numerous types of human cancers through a variety of mechanisms (20). Moreover, SFN has been reported to potentially prevent breast cancer development and recurrence (7). In the present study, it was found that SFN inhibited the proliferation of MDA-MB-468 TNBC cells with an IC50 value of ~2 µM following 72 h of exposure. This IC50 value appears to be quite low compared with those of various phytochemicals tested in the authors' laboratory for TNBC (21-24), indicating that SFN is a promising candidate for preventing TNBC among various phytochemicals.
Moreover, the present in vivo experiment using nude mice xenotransplanted with MDA-MB-468 cells revealed that the per os administration of SFN evidently attenuated tumor growth over a period of 7 weeks, reducing the tumor size by 60% compared with the control. Adverse effects to major organs were considered negligible as there were no significant differences in body weight gain and the consumption of food between the two groups, although this is indirect evidence of the adverse effects. More precisely, the examinations of hematological and biochemical toxicities are required in nude mice. A similar result has been reported using MDA-MB-231 TNBC xenografts (25); however, to the best of our knowledge, the present study is the first to demonstrate the in vivo antitumor activity of SFN against MDA-MB-468 cells. Moreover, SFN has been reported to synergistically enhance the efficacy of several anticancer drugs, including cisplatin (26), doxorubicin (25,27) and paclitaxel (28) in various types of human cancer cells. Therefore, co-treatment with chemotherapeutic agents and SFN may reduce the administered doses, thereby alleviating the adverse effects of anticancer agents. Furthermore, it has been reported that human subjects who ingested 100 g of broccoli daily as a soup exhibited a peak plasma concentration of ~2-7 µM SFN metabolites, including free SFN (29). Thus, it is conceivable that consuming a diet rich in cruciferous vegetables, such as broccoli sprouts, may reduce the risk and development of TNBC and may potentially be useful for the treatment of TNBC.
Cell cycle checkpoints are crucial for controlling the mechanisms that ensure the proper execution of cell cycle events. SFN has been shown to modulate cell cycle progression in several cellular models, such as prostate, colon, breast and bladder cancers, arresting cells in the G1 (30,31) or the G2/M phase (32-34), depending on the cell type, the treatment concentration and the duration of exposure (35). In the present study, SFN inhibited the proliferation of MDA-MB-468 TNBC cells by inducing S/G2/M cell cycle arrest, causing a blockade of cell cycle entry into mitosis, as previously shown in different TNBC cell lines, including MDA-MB-231(36). Furthermore, the sub G0/G1 cell population, which represents apoptotic cells, increased, followed by the cleavage of PARP, which serves as a marker of cells undergoing apoptosis. Therefore, these data indicate that the inhibitory effects of SFN observed in cytotoxicity assays reflect the combination of SFN-induced S/G2/M cell cycle arrest and apoptotic cell death.
Although SFN has been found to induce the apoptosis of a variety of breast cancer cells, the mechanisms through which SFN induces apoptosis varies between different cells. The present study demonstrated that SFN upregulated the protein expression of pro-apoptotic Bax, which has been shown to induce apoptosis by promoting the release of cytochrome c, as a result of its translocation from the cytosol to the mitochondria (37). SFN has also been reported to induce the downregulation of anti-apoptotic Bcl-2 protein in several breast cancer cell lines (36). A previous study using MDA-MB-468 cells reported that Bcl-2 levels decreased in a concentration-dependent manner from 5 µM (36). In the present study, however, Bcl-2 expression was not altered. This may be due to the concentration that we used for the experiment in which the effect of SFN on apoptosis signaling proteins was evaluated at a concentration of 2 µM. Nonetheless, the resultant increase in the Bax/Bcl-2 ratio may play an important role in SFN-induced apoptosis in MDA-MB-468 cells at such a low level of SFN.
Studies on the mechanisms underlying the anticancer activities of SFN have indicated that its regulatory effects on the tumor cell cycle, apoptosis and angiogenesis are mediated by the modulation of the related signaling pathways (6-8). MDA-MB-468 TNBC cells are devoid of PTEN, which antagonizes the activity of PI3K, and exhibit high levels of EGFR (15,16), which functions upstream of PI3K. Akt plays a critical role in controlling survival by directly phosphorylating mTOR at Ser2448(38), leading to an increase in downstream molecules (39). Upon treatment of the MDA-MB-468 cells with SFN, Akt activity was inhibited with a simultaneous decrease in mTOR activity, indicating that SFN substantially inhibited the PI3K/Akt/mTOR pathway even in cells with the overactivation of the downstream pathway caused by the overexpression of EGFR and co-deletion of PTEN. Since EGFR overexpression and loss of PTEN are frequently occurring events in TNBC cases, and are associated with aggressive behavior and a poor prognosis of patients with TNBC (10,12,13), SFN appears to be a promising target drug acting against survival signaling downstream of PI3K in these patients. Moreover, such activities of SFN appear to be crucial as mitogenic and anti-apoptotic potentials are driven by the activation of intracellular signaling molecules upstream of PI3K in almost all cancer cells.
Despite recent advances in breast cancer treatment, breast cancer recurrence is a major problem and the principal cause of breast cancer-related deaths. Emerging evidence suggests the existence of cancer stem cells, a population of cells capable of self-renewal and initiating tumor growth, which might be responsible for breast cancer recurrence (40). Recently, SFN has gained immense attention due to its wide safety profile and ability to target heterogeneous populations of cancer cells, including cancer stem cells. Accordingly, SFN has been shown to reduce the tumor volume in TNBC stem-like cells (MDA-MB-231-Luc-D3H1 cell line) administered daily by intraperitoneal injection (41). Moreover, Burnett et al (42) reported that the intraperitoneal injection of SFN enhanced the anticancer activity of taxanes against TNBC by killing cancer stem cells. In the present study, the oral administration of SFN inhibited the growth of xenotransplanted MDA-MB-468 TNBC tumors consisting of a population of in vivo selected and thus highly tumorigenic cells resembling cancer stem cells. The major difference between the two aforementioned previous studies and the present study is the design of the administration route of SFN. The present experimental design mimics the ordinary method of SFN uptake included in dietary vegetables. Therefore, absorption routes via either the intestine or peritoneal membrane may greatly affect the pharmacokinetics of SFN administered via either route. To the best of our knowledge, the present study is also the first to demonstrate the in vivo antitumor activity of SFN against the MDA-MB-468 TNBC cell line exhibiting the overactivation of the signaling pathway downstream of EGFR due to EGFR overexpression and the deletion of PTEN, as opposed to SUM149 cells, which possess tumor suppressor BRCA1 mutation (43).
In conclusion, the data of the present study suggest that SFN may prove to be potentially useful, not only for the prevention and treatment, but also for the reduction of the recurrence of TNBC.
Acknowledgements
The authors would like to thank Mrs. Takako Higuchi, Graduate School of Health and Nutritional Sciences, Nakamura Gakuen University for providing technical assistance.
Funding
Funding: The present study was supported by the Japan Society for the Promotion of Science (JSPS) KAKENHI (Grants-in-Aid for Scientific Research; grant nos. 15K00864 and 26750059). The present study was also supported by the 2019 Cancer Research Fund of the Fukuoka Foundation for Sound Health.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
AY, MO and SN designed the study. AY and MO conducted the research. AY, MO, and MT analyzed the data. MO and SN wrote the manuscript. All authors had the primary responsibility for the final content, and read and approved the final manuscript. All authors confirmed the authenticity of the raw data.
Ethics approval and consent to participate
All animal procedures were performed in accordance with the protocols approved by the Institutional Animal Care and Use Committee of the Nakamura Gakuen University (no. 2018-1).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
|
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.PubMed/NCBI View Article : Google Scholar | |
|
Kumar P and Aggarwal R: An overview of triple-negative breast cancer. Arch Gynecol Obstet. 293:247–269. 2016.PubMed/NCBI View Article : Google Scholar | |
|
Liu X and Lv K: Cruciferous vegetables intake is inversely associated with risk of breast cancer: A meta-analysis. Breast. 22:309–313. 2013.PubMed/NCBI View Article : Google Scholar | |
|
Higdon JV, Delage B, Williams DE and Dashwood RH: Cruciferous vegetables and human cancer risk: Epidemiologic evidence and mechanistic basis. Pharmacol Res. 55:224–236. 2007.PubMed/NCBI View Article : Google Scholar | |
|
Fahey JW, Wehage SL, Holtzclaw WD, Kensler TW, Egner PA, Shapiro TA and Talalay P: Protection of humans by plant glucosinolates: Efficiency of conversion of glucosinolates to isothiocyanates by the gastrointestinal microflora. Cancer Prev Res (Phila). 5:603–611. 2012.PubMed/NCBI View Article : Google Scholar | |
|
Jabbarzadeh Kaboli P, Afzalipour Khoshkbejari M, Mohammadi M, Abiri A, Mokhtarian R, Vazifemand R, Amanollahi S, Yazdi Sani S, Li M, Zhao Y, et al: Targets and mechanisms of sulforaphane derivatives obtained from cruciferous plants with special focus on breast cancer-contradictory effects and future perspectives. Biomed Pharmacother. 121(109635)2020.PubMed/NCBI View Article : Google Scholar | |
|
Kuran D, Pogorzelska A and Wiktorska K: Breast cancer prevention-is there a future for sulforaphane and its analogs? Nutrients. 12(1559)2020.PubMed/NCBI View Article : Google Scholar | |
|
Clarke JD, Dashwood RH and Ho E: Multi-targeted prevention of cancer by sulforaphane. Cancer Lett. 269:291–304. 2008.PubMed/NCBI View Article : Google Scholar | |
|
Costa RLB, Han HS and Gradishar WJ: Targeting the PI3K/AKT/mTOR pathway in triple-negative breast cancer: A review. Breast Cancer Res Treat. 169:397–406. 2018.PubMed/NCBI View Article : Google Scholar | |
|
Rimawi MF, Shetty PB, Weiss HL, Schiff R, Osborne CK, Chamness GC and Elledge RM: Epidermal growth factor receptor expression in breast cancer association with biologic phenotype and clinical outcomes. Cancer. 116:1234–1242. 2010.PubMed/NCBI View Article : Google Scholar | |
|
Lou L, Yu Z, Wang Y, Wang S and Zhao Y: c-Src inhibitor selectively inhibits triple-negative breast cancer overexpressed Vimentin in vitro and in vivo. Cancer Sci. 109:1648–1659. 2018.PubMed/NCBI View Article : Google Scholar | |
|
Dean SJ, Perks CM, Holly JM, Bhoo-Pathy N, Looi LM, Mohammed NA, Mun KS, Teo SH, Koobotse MO, Yip CH and Rhodes A: Loss of PTEN expression is associated with IGFBP2 expression, younger age, and late stage in triple-negative breast cancer. Am J Clin Pathol. 141:323–333. 2014.PubMed/NCBI View Article : Google Scholar | |
|
Beg S, Siraj AK, Prabhakaran S, Jehan Z, Ajarim D, Al-Dayel F, Tulbah A and Al-Kuraya KS: Loss of PTEN expression is associated with aggressive behavior and poor prognosis in Middle Eastern triple-negative breast cancer. Breast Cancer Res Treat. 151:541–553. 2015.PubMed/NCBI View Article : Google Scholar | |
|
Pawlik A, Wiczk A, Kaczynska A, Antosiewicz J and Herman-Antosiewicz A: Sulforaphane inhibits growth of phenotypically different breast cancer cells. Eur J Nutr. 52:1949–1958. 2013.PubMed/NCBI View Article : Google Scholar | |
|
Liu T, Yacoub R, Taliaferro-Smith LD, Sun SY, Graham TR, Dolan R, Lobo C, Tighiouart M, Yang L, Adams A and O'Regan RM: Combinatorial effects of lapatinib and rapamycin in triple-negative breast cancer cells. Mol Cancer Ther. 10:1460–1469. 2011.PubMed/NCBI View Article : Google Scholar | |
|
Lu Y, Lin YZ, LaPushin R, Cuevas B, Fang X, Yu SX, Davies MA, Khan H, Furui T, Mao M, et al: The PTEN/MMAC1/TEP tumor suppressor gene decreases cell growth and induces apoptosis and anoikis in breast cancer cells. Oncogene. 18:7034–7045. 1999.PubMed/NCBI View Article : Google Scholar | |
|
Neve RM, Chin K, Fridlyand J, Yeh J, Baehner FL, Fevr T, Clark L, Bayani N, Coppe JP, Tong F, et al: A collection of breast cancer cell lines for the study of functionally distinct cancer subtypes. Cancer Cell. 10:515–527. 2006.PubMed/NCBI View Article : Google Scholar | |
|
Singh SV, Warin R, Xiao D, Powolny AA, Stan SD, Arlotti JA, Zeng Y, Hahm ER, Marynowski SW, Bommareddy A, et al: Sulforaphane inhibits prostate carcinogenesis and pulmonary metastasis in TRAMP mice in association with increased cytotoxicity of natural killer cells. Cancer Res. 69:2117–2125. 2009.PubMed/NCBI View Article : Google Scholar | |
|
Singh AV, Xiao D, Lew KL, Dhir R and Singh SV: Sulforaphane induces caspase-mediated apoptosis in cultured PC-3 human prostate cancer cells and retards growth of PC-3 xenografts in vivo. Carcinogenesis. 25:83–90. 2004.PubMed/NCBI View Article : Google Scholar | |
|
Jiang X, Liu Y, Ma L, Ji R, Qu Y, Xin Y and Lv G: Chemopreventive activity of sulforaphane. Drug Des Devel Ther. 12:2905–2913. 2018.PubMed/NCBI View Article : Google Scholar | |
|
Takeshima M, Ono M, Higuchi T, Chen C, Hara T and Nakano S: Anti-proliferative and apoptosis-inducing activity of lycopene against three subtypes of human breast cancer cell lines. Cancer Sci. 105:252–257. 2014.PubMed/NCBI View Article : Google Scholar | |
|
Wakimoto R, Ono M, Takeshima M, Higuchi T and Nakano S: Differential anticancer activity of pterostilbene against three subtypes of human breast cancer cells. Anticancer Res. 37:6153–6159. 2017.PubMed/NCBI View Article : Google Scholar | |
|
Ono M, Takeshima M, Nishi A, Higuchi T and Nakano S: Genistein suppresses v-Src-driven proliferative activity by arresting the cell-cycle at G2/M through increasing p21 level in Src-activated human gallbladder carcinoma cells. Nutr Cancer. 73:1471–1479. 2021.PubMed/NCBI View Article : Google Scholar | |
|
Chen C, Ono M, Takeshima M and Nakano S: Antiproliferative and apoptosis-inducing activity of nobiletin against three subtypes of human breast cancer cell lines. Anticancer Res. 34:1785–1792. 2014.PubMed/NCBI | |
|
Yang F, Wang F, Liu Y, Wang S, Li X, Huang Y, Xia Y and Cao C: Sulforaphane induces autophagy by inhibition of HDAC6-mediated PTEN activation in triple negative breast cancer cells. Life Sci. 213:149–157. 2018.PubMed/NCBI View Article : Google Scholar | |
|
Gong TT, Liu XD, Zhan ZP and Wu QJ: Sulforaphane enhances the cisplatin sensitivity through regulating DNA repair and accumulation of intracellular cisplatin in ovarian cancer cells. Exp Cell Res. 393(112061)2020.PubMed/NCBI View Article : Google Scholar | |
|
Bose C, Awasthi S, Sharma R, Beneš H, Hauer-Jensen M, Boerma M and Singh SP: Sulforaphane potentiates anticancer effects of doxorubicin and attenuates its cardiotoxicity in a breast cancer model. PLoS One. 13(e0193918)2018.PubMed/NCBI View Article : Google Scholar | |
|
Kim SH, Park HJ and Moon DO: Sulforaphane sensitizes human breast cancer cells to paclitaxel-induced apoptosis by downregulating the NF-κB signaling pathway. Oncol Lett. 13:4427–4432. 2017.PubMed/NCBI View Article : Google Scholar | |
|
Gasper AV, Al-Janobi A, Smith JA, Bacon JR, Fortun P, Atherton C, Taylor MA, Hawkey CJ, Barrett DA and Mithen RF: Glutathione S-transferase M1 polymorphism and metabolism of sulforaphane from standard and high-glucosinolate broccoli. Am J Clin Nutr. 82:1283–1291. 2005.PubMed/NCBI View Article : Google Scholar | |
|
Chiao JW, Chung FL, Kancherla R, Ahmed T, Mittelman A and Conaway CC: Sulforaphane and its metabolite mediate growth arrest and apoptosis in human prostate cancer cells. Int J Oncol. 20:631–636. 2002.PubMed/NCBI View Article : Google Scholar | |
|
Shan Y, Sun C, Zhao X, Wu K, Cassidy A and Bao Y: Effect of sulforaphane on cell growth, G(0)/G(1) phase cell progression and apoptosis in human bladder cancer T24 cells. Int J Oncol. 29:883–888. 2006.PubMed/NCBI | |
|
Tang L and Zhang Y: Dietary isothiocyanates inhibit the growth of human bladder carcinoma cells. J Nutr. 134:2004–2010. 2004.PubMed/NCBI View Article : Google Scholar | |
|
Jackson SJ and Singletary KW: Sulforaphane inhibits human MCF-7 mammary cancer cell mitotic progression and tubulin polymerization. J Nutr. 134:2229–2236. 2004.PubMed/NCBI View Article : Google Scholar | |
|
Parnaud G, Li P, Cassar G, Rouimi P, Tulliez J, Combaret L and Gamet-Payrastre L: Mechanism of sulforaphane-induced cell cycle arrest and apoptosis in human colon cancer cells. Nutr Cancer. 48:198–206. 2004.PubMed/NCBI View Article : Google Scholar | |
|
Lenzi M, Fimognari C and Hrelia P: Sulforaphane as a promising molecule for fighting cancer. Cancer Treat Res. 159:207–223. 2014.PubMed/NCBI View Article : Google Scholar | |
|
Pledgie-Tracy A, Sobolewski MD and Davidson NE: Sulforaphane induces cell type-specific apoptosis in human breast cancer cell lines. Mol Cancer Ther. 6:1013–1021. 2007.PubMed/NCBI View Article : Google Scholar | |
|
Dewson G and Kluck RM: Mechanisms by which Bak and Bax permeabilise mitochondria during apoptosis. J Cell Sci. 122:2801–2808. 2009.PubMed/NCBI View Article : Google Scholar | |
|
Chiang GG and Abraham RT: Phosphorylation of mammalian target of rapamycin (mTOR) at Ser-2448 is mediated by p70S6 kinase. J Biol Chem. 280:25485–25490. 2005.PubMed/NCBI View Article : Google Scholar | |
|
Nitulescu GM, Van De Venter M, Nitulescu G, Ungurianu A, Juzenas P, Peng Q, Olaru OT, Grădinaru D, Tsatsakis A, Tsoukalas D, et al: The Akt pathway in oncology therapy and beyond (review). Int J Oncol. 53:2319–2331. 2018.PubMed/NCBI View Article : Google Scholar | |
|
Butti R, Gunasekaran VP, Kumar TVS, Banerjee P and Kundu GC: Breast cancer stem cells: Biology and therapeutic implications. Int J Biochem Cell Biol. 107:38–52. 2019.PubMed/NCBI View Article : Google Scholar | |
|
Castro NP, Rangel MC, Merchant AS, MacKinnon G, Cuttitta F, Salomon DS and Kim YS: Sulforaphane suppresses the growth of triple-negative breast cancer stem-like cells in vitro and in vivo. Cancer Prev Res (Phila). 12:147–158. 2019.PubMed/NCBI View Article : Google Scholar | |
|
Burnett JP, Lim G, Li Y, Shah RB, Lim R, Paholak HJ, McDermott SP, Sun L, Tsume Y, Bai S, et al: Sulforaphane enhances the anticancer activity of taxanes against triple negative breast cancer by killing cancer stem cells. Cancer Lett. 394:52–64. 2017.PubMed/NCBI View Article : Google Scholar | |
|
Elstrodt F, Hollestelle A, Nagel JH, Gorin M, Wasielewski M, van den Ouweland A, Merajver SD, Ethier SP and Schutte M: BRCA1 mutation analysis of 41 human breast cancer cell lines reveals three new deleterious mutants. Cancer Res. 66:41–45. 2006.PubMed/NCBI View Article : Google Scholar |