Antioxidant and anti‑inflammatory effects of a mixture of propolis, red bean and tomato extracts
- Authors:
- Published online on: April 11, 2024 https://doi.org/10.3892/ijfn.2024.35
- Article Number: 1
-
Copyright : © Kang et al. This is an open access article distributed under the terms of Creative Commons Attribution License [CC BY 4.0].
Abstract
Introduction
In the modern industrial society, environmental pollutants, including particulate matter (PM) such as fine dust, have a significant impact on public health (1). PM is typically categorized into dust with a diameter of ≤10 µm and dust with a diameter of ≤2.5 µm [fine PM (PM2.5)]. It is primarily generated from industrial facilities and vehicles, and consists of organic components, such as dioxins and benzene, as well as inorganic components such as nitrates, sulfates and metal compounds (2). PM infiltrates the respiratory and circulatory systems of the human body, leading to various health issues. Particularly, PM2.5 is recognized as a key factor causing severe diseases in humans, including respiratory diseases, cardiovascular diseases and cancer (3,4). Health issues related to environmental pollution are exponentially increasing, and pollution has a lethal impact on individuals with respiratory and cardiovascular diseases, including the elderly, resulting in an increase in mortality rates (5). Therefore, there is a marked emphasis on research, not only on the mechanistic aspects of the impact of PM2.5 on human health, but also on the development of materials that can effectively control its presence (6).
Oxidative stress in the human body has been reported to play a crucial role in causing genetic mutations in cells and tissues, as well as in exerting lethal effects on cellular organelles, ultimately leading to various human diseases (7). Reactive oxygen species (ROS), a key factor contributing to oxidative stress, are generated not only during physiological conditions such as immune responses, but also due to physical and chemical environmental pollutants. Specifically, PM2.5, when inhaled through the respiratory system, has been reported to directly generate large amounts of ROS along the bloodstream, affecting various tissues in the human body and inducing oxidative stress (8,9). Since ROS are considered essential factors in inducing both acute and chronic inflammatory diseases, there is an increasing need to effectively control them. This has led to a concentration of interest not only in the field of biomedicine, but also in the health food sector, prompting numerous researchers to focus on developing natural food materials for effective ROS control (10). Consequently, research in the food industry is actively pursuing the development of natural food materials that can control ROS effectively with minimal or no side-effects (11,12). However, research on natural food materials specifically aimed at managing and improving oxidative stress and inflammation caused by PM2.5 is still insufficiently advanced.
Propolis, a natural resin collected by bees to protect their hives, contains various bioactive components, such as flavonoids, phenolic acids, esters, terpenes, amino acids and vitamins (13-15). These components exhibit potent antioxidant effects by neutralizing ROS and eliminating free radicals. Propolis is well-known for its anti-inflammatory effects, inhibiting the generation of inflammatory mediators and reducing inflammatory responses (16). Red bean (Vigna angularis), a 1-year vine plant cultivated in East Asia, has been reported to have anticancer, antioxidant, anti-inflammatory and anti-obesity effects (17). Red beans are rich in polyphenols and flavonoids, which help prevent oxidative damage and contribute to maintaining cellular health (18). Furthermore, polyphenols and flavonoid components derived from red beans have been reported to regulate inflammatory responses and contribute to the prevention and management of chronic inflammatory-related diseases (19). Additionally, tomatoes (Solanum lycopersicum) contain antioxidants, such as polyphenols, flavonoids and lycopene. These components protect cells from free radicals, reduce DNA damage and contribute to inhibiting inflammatory responses (20,21). Lycopene, in particular, provides protective effects against various health issues related to oxidative stress (22). Despite the well-known benefits of propolis, red beans and tomatoes, there is a consistent increase in consumer demand due to a growing interest in health. However, despite their efficacy, the utilization of extracts from these three sources is relatively low based on consumer preferences, resulting in a slow growth rate in demand.
Therefore, the aim of the present study was to develop a mixture of propolis, red bean and tomato extracts (PRTE) that could alleviate oxidative stress and inflammatory responses caused by PM2.5. The aim was to manufacture PRTE, verify its antioxidant abilities based on the ratios of each extract, and create a novel natural extract. In order to achieve this, optimal ratios were determined, and the antioxidant and anti-inflammatory effects were investigated using keratinocyte cells (HaCaT cells) and macrophages (RAW264.7 cells) following treatment with the developed mixture.
Materials and methods
Cells and materials
HaCaT cells (cat. no. 300493-SF) were acquired from the CLS Cell Lines Service GmbH. RAW264.7 cells (cat. no. TIB-71) were purchased from ATCC. Dulbecco's modified Eagle's medium (DMEM), fetal bovine serum (FBS), penicillin-streptomycin, RIPA buffer, and trypsin-EDTA were purchased from Thermo Fisher Scientific, Inc. The Quanti-MAX™ WST-8 cell viability assay kit, and TBST buffer was obtained from BIOMAX, Inc. 2,2'-Azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) (ABTS), potassium persulfate, 2,2-diphenyl-1-picrylhydrazyl (DPPH), Griess reagent, lipopolysaccharide (LPS), PM2.5 and goat anti-rabbit IgG HRP-conjugated antibody (cat. no. 31458) were purchased from MilliporeSigma. The prostaglandin E2 (PGE2), interleukin (IL)-1β), IL-6 and tumor necrosis factor-α (TNF-α) ELISA kits were obtained from R&D Systems, Inc. The superoxide dismutase (SOD), glutathione peroxidase (GPx) and glutathione (GSH) assay kits were purchased from Cayman Chemical Company. Inducible nitric oxide synthase (iNOS; sc-7271), cyclooxygenase-2 (COX-2; sc-514489) and β-actin (cat. no. sc-8432) antibodies, along with goat anti-mouse IgG HRP-conjugated antibody (cat. no. sc-2354), were acquired from Santa Cruz Biotechnology, Inc. The Bradford assay reagent and SDS-PAGE sample loading buffer were purchased from Bio-Rad Laboratories, Inc.
PRTE
The propolis used in the present study was provided by Unique BioTech Co., Ltd. Red beans and tomatoes were purchased from a local market, and following verification by Professor Hong-Jun Kim at the College of Oriental Medicine, Woosuk University (Wanju-gun, Korea) the samples (voucher specimen; #2023-06-07) were stored at the research laboratory of SIJ at Jeonju University (Jeonju, Korea). Red bean and tomato extracts were prepared by mixing them in a 1:20 ratio with 70% ethanol and subjecting them to vibration extraction at 161 x g for 3 days at 50˚C. The extracts were filtered once through a nylon mesh and twice through a 0.45-µm filter paper. The filtered extracts were concentrated under a rotary vacuum (A-3S; EYELA) at 50˚C and then freeze-dried to obtain powder samples. The obtained powder samples were stored at -80˚C and used in the following experiments.
The derivation of the mixture ratio of propolis, red bean and tomato
PRTE were mixed under four different conditions as follows: Mixture 1 (M1-PRTE) was prepared by combining propolis, red bean and tomato at a ratio of 1:1:1. Mixture 2 (M2-PRTE) was prepared at a ratio of 1.5:1:0.5, mixture 3 (M3-PRTE) at a ratio of 1.5:0.5:1 and mixture 4 (M4-PRTE) at a ratio of 1.2:0.9:0.9. In order to determine the optimal mixture ratio, the radical-scavenging efficacy of each mixture was evaluated, as described below. Based on these results, the optimal mixture was determined.
DPPH radical scavenging activity
The DPPH radical scavenging activity experiment was conducted with a slight modification of the method proposed in the study by Blois (23). Each extract and mixture were dissolved in distilled water. Subsequently, 100 µl of each sample solution and 100 µl of 0.3 mM DPPH solution were mixed in a 96-well plate and allowed to react at room temperature for 20 min. The absorbance was then measured at 540 nm (Sunrise™, Tecan Group, Ltd.), and the percentage difference in absorbance between the sample solution and the blank solution was calculated.
ABTS radical scavenging activity
The ABTS radical scavenging activity was measured according to the method described in the study by Re et al (24). A mixture of 7 mM ABTS and 2.45 mM potassium persulfate (K2S2O8) at a 1:1 ratio was allowed to stand for 24 h at room temperature to generate radicals. The resulting radical solution was diluted with distilled water to achieve an absorbance of 0.70±0.04 at 720 nm. Subsequently, 50 µl of each extract and mixture were mixed with 950 µl of the prepared ABTS solution and allowed to react for 30 min at 23˚C. After the reaction, 100 µl of the mixture were transferred to a 96-well plate, and the absorbance was measured at 720 nm (Sunrise™, Tecan Group, Ltd.). The percentage difference in absorbance between the sample solution and the blank solution was calculated.
Cell culture
The human-derived keratinocyte cell line (HaCaT) was obtained from CLS Cell Lines Service GmbH, and the murine macrophage cell line (RAW264.7) was acquired from ATCC. The cells were cultured in DMEM containing 10% FBS and 1% antibiotics (penicillin and streptomycin) in a humidified atmosphere at 37˚C with 5% CO2.
Cell viability
The HaCaT cells were seeded at a concentration of 2x105 cells/ml in a 96-well plate and the cells were cultured at 37˚C and 5% CO2 for 24 h. The cells were then exposed to with various concentrations of PM2.5 (0-100 µg/ml) or M2-PRTE (0-50 µg/ml). Following 24 h of incubation, the cells were cultured at 37˚C and 5% CO2, WST-8 solution (10 µl per well) was added, and after 4 h, the absorbance was measured at 450 nm (Sunrise™, Tecan Group, Ltd.) to calculate the cell viability. The RAW264.7 cells were seeded at a final concentration of 2x105 cells/ml in a 96-well plate, then cultured for 24 h at 37˚C with 5% CO2. Subsequently, they were treated with M2-PRTE at concentrations of 25 and 50 µg/ml, followed by exposed to LPS at a concentration of 1 µg/ml after 1 h. Following 24 h of incubation, the cells were cultured at 37˚C and 5% CO2, WST-8 solution (10 µl per well) was added, and after 4 h, the absorbance was measured at 450 nm (Sunrise™, Tecan Group, Ltd.) to calculate cell viability.
Measurement of SOD and GPx, and determination of the GSH content
After seeding tbe HaCaT cells in a 60-mm dish at a final concentration of 2x105 cells/ml, the cells were cultured at 37˚C and 5% CO2 for 24 h. Subsequently, the cells were treated with M2-PRTE at concentrations of 25 and 50 µg/ml. After 1 h, PM2.5 was added at a concentration of 100 µg/ml, and the cells were then cultured at 37˚C and 5% CO2 for an additional 24 h. Subsequently, the cells were washed twice with PBS and protein extraction was performed using RIPA buffer. The extracted proteins were quantified by measuring the absorbance at 595 nm using Bradford protein assay reagent, and the activities of SOD and GPx, as well as the GSH content, were measured according to the manufacturer's instructions.
Measurement of nitric oxide (NO) production
After seeding the RAW264.7 cells in a 48-well plate at a final concentration of 2x105 cells/ml, the cells were cultured in an incubator at 37˚C and 5% CO2 for 24 h. Following this, the cells were treated with M2-PRTE at concentrations of 25 and 50 µg/ml, and 1 h later, LPS was added at a concentration of 1 µg/ml. After 24 h, a mixture of 100 µl Griess reagent and 100 µl cell culture supernatant was prepared in a 96-well plate, and the absorbance was measured at 540 nm using a microplate reader (Tecan Group Ltd.) at room temperature. A standard curve was constructed using sodium nitrate, and the amount of NO production was calculated.
Western blot analysis
After seeding the RAW264.7 cells in a 60-mm dish at a final concentration of 2x105 cells/ml, the cells were cultured in an incubator at 37˚C and 5% CO2 for 24 h. Following this, the cells were treated with M2-PRTE at concentrations of 25 and 50 µg/ml. Subsequently, 1 h later, LPS was added at a concentration of 1 µg/ml, and the cells were cultured at 37˚C and 5% CO2 for 24 h. Subsequently, the cells were washed twice with PBS and protein extraction was performed using RIPA buffer. The extracted proteins were quantified by measuring the absorbance at 595 nm using Bradford protein assay reagent. The quantified proteins were separated by SDS-PAGE (7.5%) at 100 V for 1 h and transferred onto a PVDF (polyvinylidene difluoride) membrane (Bio-Rad Laboratories, Inc.). The membrane was blocked with 5% skim milk at room temperature for 1 h, followed by three washes with TBST buffer for 10 min each. Primary antibodies for iNOS (1:200), COX-2 (1:100) and β-actin (1:2,000) were then applied, and the membrane was incubated at 4˚C for 24 h. The membrane was then washed three times with TBST for 10 min each. The secondary antibody (mouse IgG HRP; 1:5,000) was applied at room temperature for 2 h, followed by three washes with TBST for 10 min each. Subsequently, images were obtained using a UV imaging system (ALLIANCE LD4; UVITEC). Protein band intensity was analyzed using ImageJ (1.53a) gel analysis software (National Institutes of Health).
Measurement of TNF-α, IL-1β and IL-6 cytokines, and PGE2 levels
After seeding the RAW264.7 cells in a 12-well plate at a final concentration of 2x105 cells/ml, the cells were cultured in an incubator at 37˚C and 5% CO2 for 24 h. Following this, the cells were treated with M2-PRTE at concentrations of 25 and 50 µg/ml. Subsequently, 1 h later, LPS was added at a concentration of 1 µg/ml. After 24 h, the supernatant was collected, and the levels of TNF-α, IL-1β, IL-6 and PGE2 were measured according to the protocol of the ELISA assay kits provided by the manufacturer.
High-performance liquid chromatography (HPLC) analysis
Solvent extracts of propolis, red bean and tomato were filtered using a 0.45-µm syringe filter and then used for HPLC analysis. HPLC analysis was performed using a Waters e2695 Alliance HPLC System (Waters Corporation) equipped with a binary pump delivery system, degasser (G1379A), autosampler (G1313A) and PDA detector (G1315B) operating at 330 nm. Separation was performed with a gradient elution (0 min-10% B, 13 min-10% B, 20 min-25% B, 24 min-30% B, 28 min-35% B, 32 min-45% B, 35 min-45% B, 40 min-50% B, 43 min-55% B, 47 min-60% B, 50 min-60% B, 55 min-10% B) and flow rate and sample consisting of 0.1% formic acid in acetonitrile and 0.1% acetic acid in distilled H2O over an Xbridge C18 column (Waters Corporation, 4.6x250 mm, 5 µm). The injection volume was fixed at 0.5 ml/min and 15 µl, respectively. The column temperature was 35˚C. Standards were identified based on retention time, and the concentrations of caffeic acid, ferulic acid, chlorogenic acid, caffeic acid phenethyl ester, isoquercetin, rutin and lycopene were calculated by comparing the peak area with that of the standard.
Statistical analysis
All experimental values are expressed as the mean ± standard deviation (mean ± SD). Statistical comparisons were performed using IBM SPSS Statistics 22 (IBM Corp.). Comparisons between different experimental groups were conducted using one-way analysis of variance (ANOVA), and post hoc multiple comparisons were carried out using Tukey's test to identify significant differences among the experimental groups. P-value <0.05 was considered to indicate a statistically significant difference.
Results and Discussion
Determination of the ratio of propolis, red bean and tomato mixture, and the measurement of the antioxidant activity
Prior to assessing the intracellular antioxidant and anti-inflammatory efficacy of PRTE, the PRTE were mixed under four conditions as follows: M1-PRTE was mixed at a 1:1:1 ratio, M2-PRTE at a ratio of 1.5:1:0.5, M3-PRTE at a ratio of 1.5:0.5:1 and M4-PRTE at a ratio of 1.2:0.9:0.9. Free radicals in an unstable state can cause damage to cells within the body, and the antioxidant efficacy of using antioxidant substances can be measured by evaluating radical scavenging ability (25). In the present study, in order to determine the optimal mixture ratio, the DPPH and ABTS radical scavenging abilities of each mixture were evaluated. As presented in Table I, among the four combinations, M2-PRTE at a ratio of 1.5:1:0.5 exhibited the most superior DPPH and ABTS radical scavenging abilities compared to the other ratios. The radical scavenging abilities (IC50) of DPPH and ABTS radicals for M2-PRTE were confirmed as 192.86±3.34 µg/ml and 554.28±4.78 µg/ml, respectively (Table I). Furthermore, when comparing the radical scavenging abilities of DPPH and ABTS with the individual extracts of propolis, red bean and tomato, M2-PRTE at a ratio of 1.5:1:0.5 exhibited enhanced radical scavenging abilities compared to the individual extracts. Based on these results, M2-PRTE was selected for confirming the antioxidant efficacy in HaCaT keratinocytes.
Antioxidant effects of M2-PRTE on PM2.5-induced oxidative stress
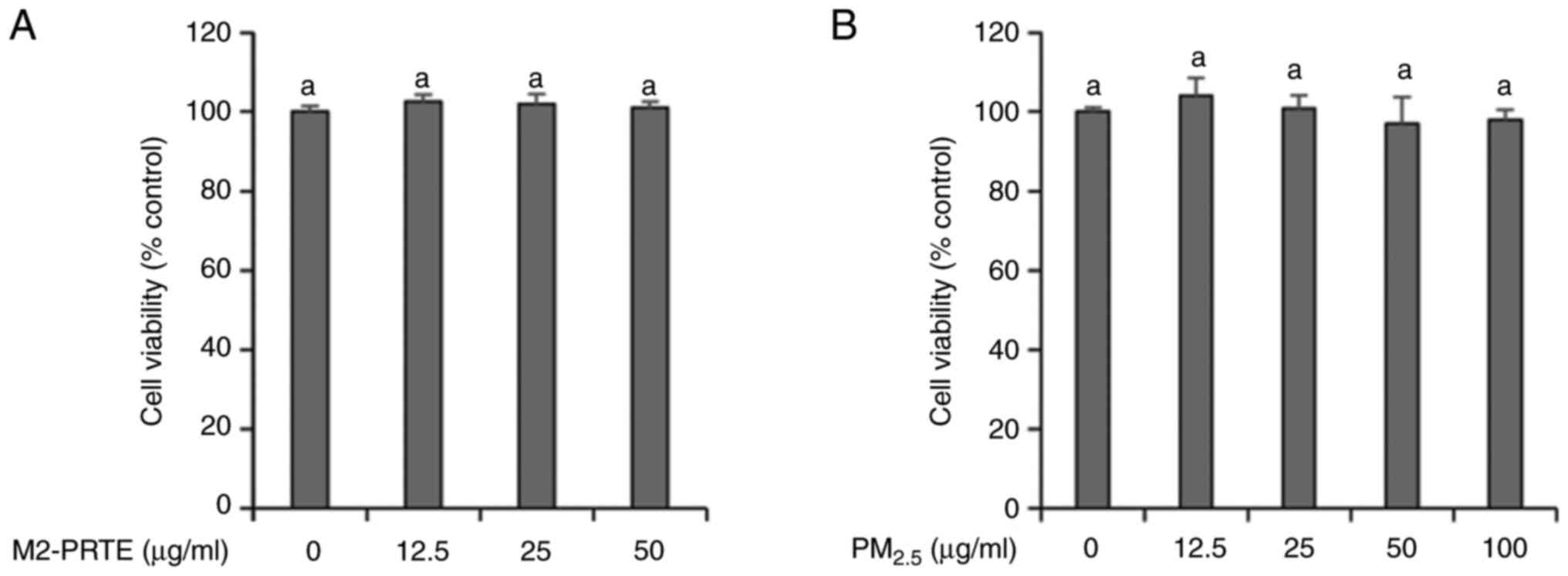
Before measuring the antioxidant effects, the cytotoxicity of PM2.5 and M2-PRTE on the human-derived keratinocyte cell line, HaCaT, was evaluated using the WST-8 assay to assess cell viability. The results revealed no cytotoxicity at all concentrations tested for both PM2.5 and M2-PRTE (Fig. 1). Based on these results, subsequent experiments were performed using the HaCaT cells with a non-cytotoxic concentration of PM2.5 at 100 µg/ml and PRTE at concentrations <50 µg/ml. To investigate the effects of M2-PRTE on the activity of antioxidant enzymes, the HaCaT cells were pre-treated with M2-PRTE (25 and 50 µg/ml) for 1 h, followed by the induction of oxidative stress with PM2.5. Subsequently, the activities of SOD and GPx, as well as the GSH content, were measured. The results revealed that exposure to PM2.5 significantly depleted the enzymatic activities of SOD and GPx, and reduced the GSH content compared to the control group (Fig. 2). However, following treatment with M2-PRTE at a concentration of 25 µg/ml, the GPx activity exhibited no significant change; however, a substantial increase was observed following treatment at a concentration of 50 µg/ml (Fig. 2A). SOD activity, which decreased in a concentration-dependent manner following exposure to PM2.5, was restored and significantly increased at a concentration of 50 µg/ml M2-PRTE (Fig. 2B). Finally, the GSH content exhibited a modest restorative effect at a concentration of 25 µg/ml M2-PRTE; notably, at a concentration of 50 µg/ml M2-PRTE, there was a marked restorative effect in the GSH content (Fig. 2C).
SOD, GPx and GSH are essential antioxidant enzymes that protect cells from oxidative stress and ROS. They significantly contribute to maintaining the health and stability of cells in unique ways (26). SOD, as an endogenous antioxidant enzyme, effectively removes reactive oxygen species such as O2-, thus playing a crucial role in protecting cells from oxidative stress (27). Furthermore, GPx collaborates with GSH to prevent cellular damage by eliminating ROS and organic peroxides (28). GSH, in turn, functions as an antioxidant responding to oxidative stress within cells. It is essential for neutralizing and detoxifying toxic substances, and is known to be involved in the proper response and protection mechanisms of cells (29). Therefore, M2-PRTE appears to be a bioactive material contributing to the restoration of antioxidant enzyme activities, such as SOD and GPx, which are depleted by oxidative stress such as PM2.5, as well as the replenishment of the antioxidant substance GSH. Hence, the superior antioxidant effects of M2-PRTE suggest its potential use as a natural antioxidant agent.
Inhibitory effects of M2-PRTE on NO production
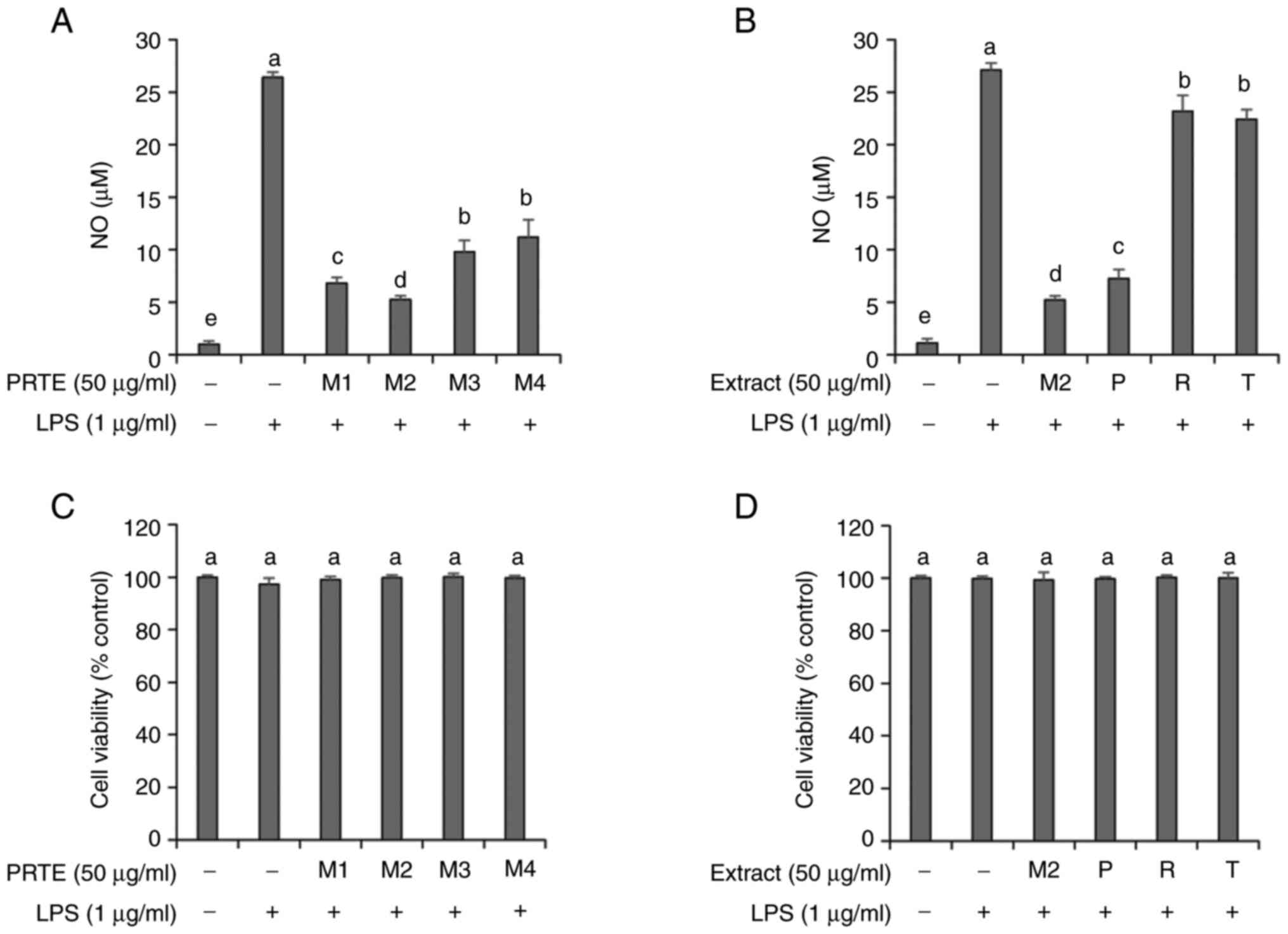
Prior to confirming whether the superior antioxidant efficacy of M2-PRTE translates to anti-inflammatory effects, the present study first evaluated the NO scavenging ability of PRTE mixtures under four conditions in RAW264.7 cells. This was performed to verify whether the anti-inflammatory efficacy of M2-PRTE aligns with its antioxidant efficacy. The cellular environment has a marked impact on the conditions the cell experiences. In the present study, the RAW 264.7 cells exhibited variable NO production rates that were associated with the number of passages. To ensure accuracy, experiments were repeated with the same number of passages (10-11) to ensure the consistency of NO production levels. The results revealed that the NO scavenging ability followed the order of M2-PRTE > M1-PRTE > M3-PRTE > M4-PRTE, and consistent with the antioxidant efficacy experiments, M2-PRTE exhibited the most superior performance (Fig. 3A). Additionally, when comparing the NO scavenging ability with the individual extracts of propolis, red bean and tomato, the ratio of M2-PRTE at 1.5:1:0.5 exhibited superior NO scavenging ability compared to the individual extracts (Fig. 3B). Subsequently, to investigate whether the observed NO scavenging ability resulted from toxicity induced by LPS and the extracts, cell toxicity was examined using WST-8. The results revealed no cytotoxicity under all conditions, confirming the absence of toxic effects (Fig. 3C and D). Based on these results, M2-PRTE was selected for further confirmation of its anti-inflammatory efficacy in RAW264.7 mouse macrophages.
The inhibitory effects of M2-PRTE on inflammatory mediators, NO and PGE2, in LPS-stimulated RAW264.7 cells were then investigated. Initially, in the LPS-stimulated RAW264.7 cells, the production of NO and PGE2 significantly increased compared to the untreated control group. However, in the cells pre-treated with M2-PRTE, a concentration-dependent and significant inhibitory effect on both NO and PGE2 production were observed (Fig. 4A and B). In acute inflammation, NO promotes vasodilation and increases blood flow to the inflammatory site, aiding in the defense against invading microorganisms (30). However, the chronic overproduction of NO can lead to tissue damage and inflammation in diseases, such as chronic lung conditions (31). At the same time, the pro-inflammatory mediator, PGE2, is involved in vasodilation, increased vascular permeability and the infiltration of immune cells into the inflammatory site. Environmental pollutants, such as fine dust can induce the excessive production of PGE2, potentially serving as a cause for chronic inflammatory diseases such as chronic bronchitis and atopic dermatitis (32). Therefore, the regulation of the excessive production of NO and PGE2 is considered a crucial therapeutic target in the management of chronic inflammatory conditions. The present study then investigated the mechanisms of action of M2-PRTE in the inhibition of NO and PGE2 production; the effects on the expression of iNOS and COX-2 proteins were examined using western blot analysis. The results revealed an increase in the protein expression of iNOS due to LPS exposure (Fig. 4C). However, following treatment with two concentrations of M2-PRTE, 25 and 50 µg/ml, the protein expression of both iNOS and COX-2 significantly decreased (Fig. 4C-E). The activation of iNOS can have negative effects on health by increasing inflammation and oxidative stress. COX-2 plays a crucial role in converting arachidonic acid to PGE2, directly participating in the inflammatory process (33). Therefore, M2-PRTE was found to inhibit the expression of iNOS and COX-2, leading to the suppression of NO and PGE2 production. Consequently, M2-PRTE may be considered as a bioactive food material that can effectively inhibit mediators causing inflammatory diseases in the human body.
Inhibitory effects of M2-PRTE on IL-1β, TNF-α and IL-6 production
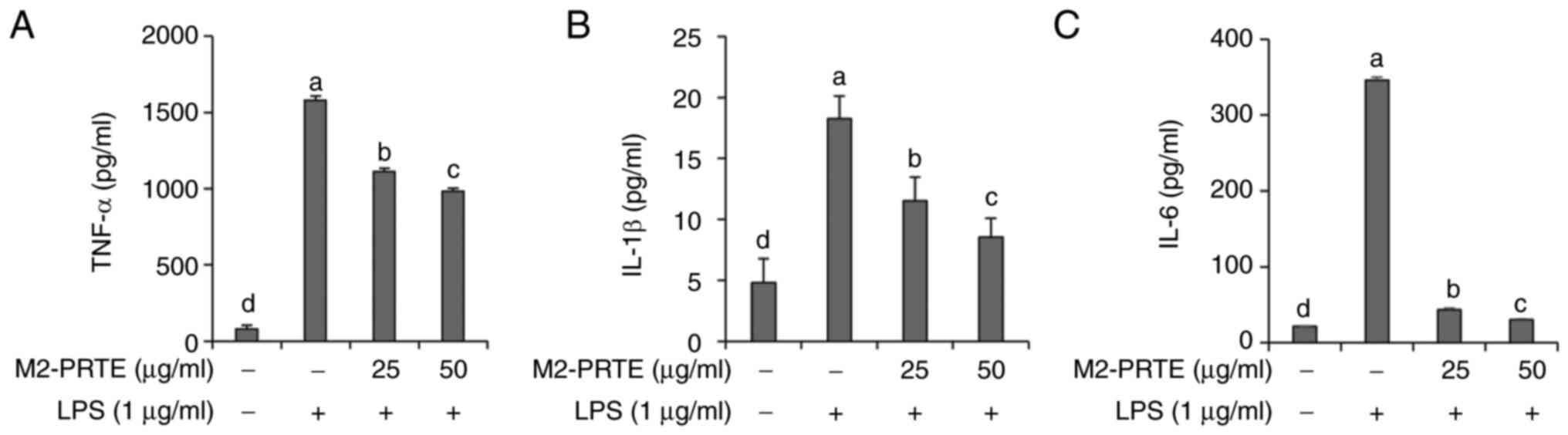
IL-1β, TNF-α and IL-6 are well-known representative pro-inflammatory cytokines that induce inflammatory responses (34). Therefore, in LPS-stimulated RAW264.7 cells, the present study investigated the inhibitory effects of M2-PRTE on the production of the pro-inflammatory cytokines, IL-1β, TNF-α and IL-6. The results revealed a significant increase in the production of IL-1β, TNF-α and IL-6 in the LPS-exposed RAW264.7 cells; however, pre-treatment with M2-PRTE exerted a concentration-dependent and significant inhibitory effect (Fig. 5). IL-1β promotes immune cell migration to the inflammatory site and triggers important responses, such as fever (35), while TNF-α increases vascular permeability at the inflammatory site, activating the movement of inflammatory cells (36). Furthermore, IL-6 functions as a key factor that activates the immune system when infection or tissue damage occurs (37). Therefore, to improve and treat inflammatory diseases, the effective regulation of pro-inflammatory cytokines, such as IL-1β, TNF-α and IL-6 is crucial, and there is a need to discover substances that can modulate these inflammatory mediators. From this perspective, M2-PRTE is considered to have the potential to effectively alleviate inflammation by controlling the production of IL-1β, TNF-α and IL-6. The present study demonstrated that the mixture of propolis, tomato and red bean provided prominent antioxidant and anti-inflammatory effects, exerting protective effects against oxidative stress and inflammation induced by PM2.5 pollution. With its ability to regulate immune function and alleviate oxidative stress, propolis functions synergistically with tomatoes (38), which are rich in lycopene, a free radical neutralizer, to maintain cellular health (39). The addition of red beans, known for their high antioxidant content, improves the protective efficacy of the mixture against oxidative damage (40). This combination not only highlights the usefulness of natural compounds in reducing health risks associated with PM2.5, but also enhances their role in alleviating oxidative stress and inflammation.
HPLC analysis
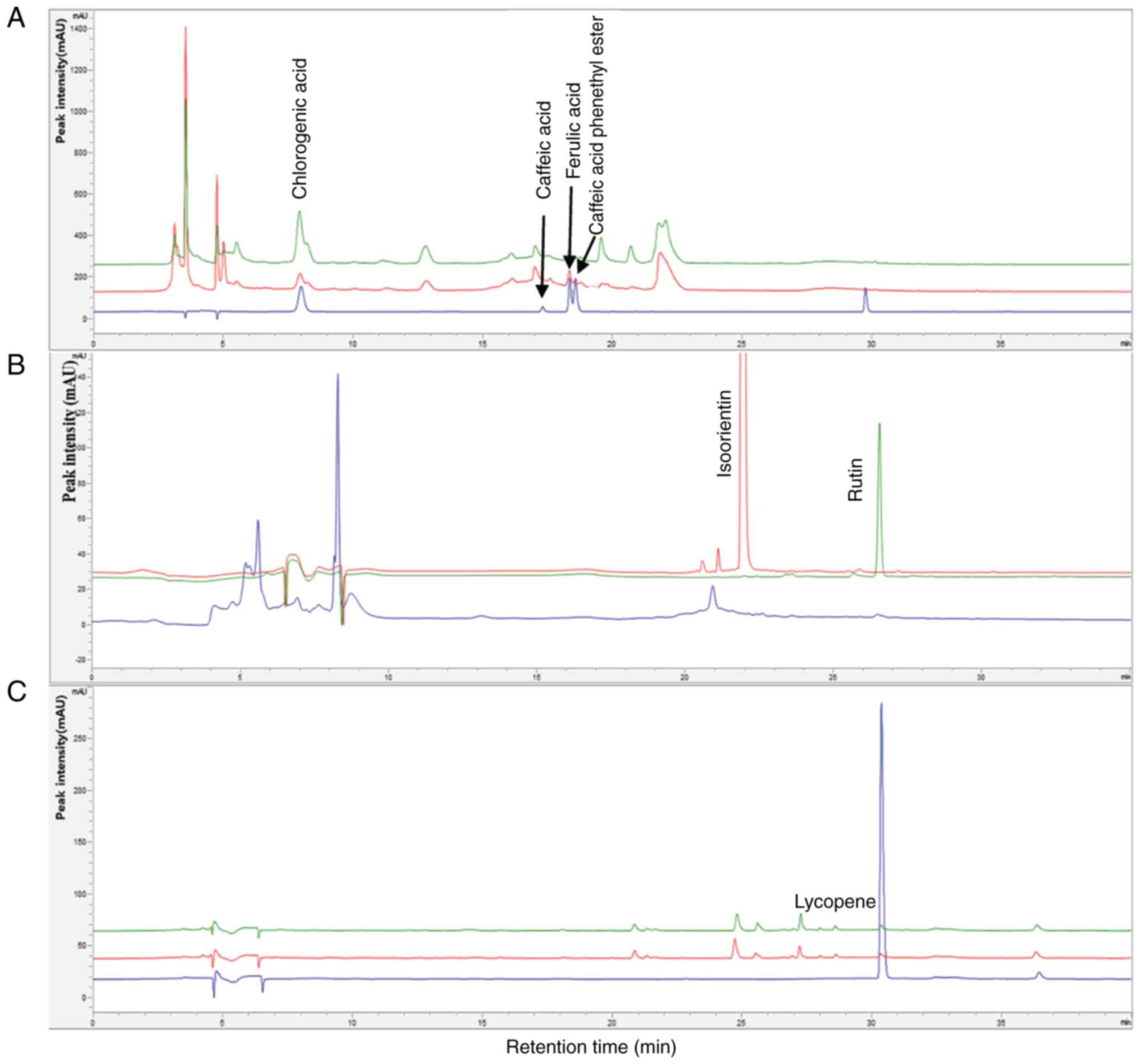
HPLC was conducted to determine the content of chemical compounds contained in the propolis, red bean and tomato extracts. The chemical compounds of the propolis extract were chlorogenic acid (42.68±0.63 µg/g), caffeic acid (17.81±0.57 µg/g), ferulic acid (0.28±1.48 µg/g), and caffeic acid phenethyl ester (18.66±1.24 µg/g), respectively (Fig. 6A). In the red bean extract, isoquercetin (23.41±0.92 µg/g) and rutin (1.38±0.58 µg/g) were detected (Fig. 6B). Moreover, lycopene (10.69±1.17 µg/g), known as a representative substance in tomato extract, was quantified (Fig. 6C). As aforementioned, as regards the anti-inflammatory and antioxidant effects of PRTE, additional verification for the chemical composition of their extracts was deemed necessary.
In conclusion, the mixture of propolis, red bean, and tomato, known as M2-PRTE, exhibited not only DPPH and ABTS radical scavenging abilities, but also demonstrated antioxidant activity by inducing the activation of antioxidant enzymes, such as SOD and GPx, as well as by increasing the intracellular GSH levels in HaCaT cells under conditions of oxidative stress induced by PM2.5. Additionally, M2-PRTE exerted inhibitory effects on the expression of iNOS and COX-2 molecules in LPS-stimulated RAW264.7 cells, leading to the suppression of NO and PGE2 production. Moreover, M2-PRTE effectively inhibited the production of pro-inflammatory cytokines, such as IL-1β, TNF-α and IL-6. Therefore, M2-PRTE is anticipated to have high potential as a functional food ingredient for alleviating oxidative stress and inhibiting inflammatory responses. However, further research is required in order to explore the efficacy and molecular mechanisms of M2-PRTE at the physiological level, and additional studies on functional components are warranted for its utilization as a health functional food ingredient.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the Collabo R&D between Industry, Academy, and Research Institute (RS-2023-00224909) funded by the Ministry of SMEs and Startups (MSS, Korea).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
ESK and BOC conceived and designed the experiments. ESK and SYJ participated in the design of the study and in the drafting of the manuscript. ESK and SYJ carried out the experiments. ESK, SYJ, MHJ, MYK, YKH, BOC and SIJ participated in acquisition, analysis and interpretation of the data. SIJ provided resources, reviewed and edited the manuscript, and supervised the study. BOC and SIJ confirm the authenticity of all the raw data. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
|
Manisalidis I, Stavropoulou E, Stavropoulos A and Bezirtzoglou E: Environmental and health impacts of air pollution: A review. Front Public Health. 8(14)2020.PubMed/NCBI View Article : Google Scholar | |
|
Adams K, Greenbaum DS, Shaikh R, van Erp AM and Russell AG: Particulate matter components, sources, and health: Systematic approaches to testing effects. J Air Waste Manag Assoc. 65:544–558. 2015.PubMed/NCBI View Article : Google Scholar | |
|
Bălă GP, Râjnoveanu RM, Tudorache E, Motișan R and Oancea C: Air pollution exposure-the (in)visible risk factor for respiratory diseases. Environ Sci Pollut Res Int. 28:19615–19628. 2021.PubMed/NCBI View Article : Google Scholar | |
|
Kim H, Kim WH, Kim YY and Park HY: Air pollution and central nervous system disease: A review of the impact of fine particulate matter on neurological disorders. Front Public Health. 8(575330)2020.PubMed/NCBI View Article : Google Scholar | |
|
Münzel T, Hahad O, Sørensen M, Lelieveld J, Duerr GD, Nieuwenhuijsen M and Daiber A: Environmental risk factors and cardiovascular diseases: A comprehensive expert review. Cardiovasc Res. 118:2880–2902. 2022.PubMed/NCBI View Article : Google Scholar | |
|
Li Z, Wen Q and Zhang R: Sources, health effects and control strategies of indoor fine particulate matter (PM2.5): A review. Sci Total Environ. 586:610–622. 2017.PubMed/NCBI View Article : Google Scholar | |
|
Giustarini D, Dalle-Donne I, Tsikas D and Rossi R: Oxidative stress and human diseases: Origin, link, measurement, mechanisms, and biomarkers. Crit Rev Clin Lab Sci. 46:241–281. 2009.PubMed/NCBI View Article : Google Scholar | |
|
Liu K, Hua S and Song L: PM2.5 exposure and asthma development: The key role of oxidative stress. Oxid Med Cell Longev. 2022(3618806)2022.PubMed/NCBI View Article : Google Scholar | |
|
Xu Z, Ding W and Deng X: PM2.5, fine particulate matter: A novel player in the epithelial-mesenchymal transition? Front Physiol. 10(1404)2019.PubMed/NCBI View Article : Google Scholar | |
|
Muchtaridi M, Az-Zahra F, Wongso H, Setyawati LU, Novitasari D and Ikram EHK: Molecular mechanism of natural food antioxidants to regulate ROS in treating cancer: A review. Antioxidants (Basel). 13(207)2024.PubMed/NCBI View Article : Google Scholar | |
|
Shin JY, Kang ES, Park JH, Cho BO and Jang SI: Anti-inflammatory effect of red ginseng marc, Artemisia scoparia, Paeonia japonica and Angelica gigas extract mixture in LPS-stimulated RAW 264.7 cells. Biomed Rep. 17(63)2022.PubMed/NCBI View Article : Google Scholar | |
|
Sasidharan S, Nishanth KS and Nair HJ: Ethanolic extract of Caesalpinia bonduc seeds triggers yeast metacaspase-dependent apoptotic pathway mediated by mitochondrial dysfunction through enhanced production of calcium and reactive oxygen species (ROS) in Candida albicans. Front Cell Infect Microbiol. 12(970688)2022.PubMed/NCBI View Article : Google Scholar | |
|
Yu M, Gouvinhas I, Rocha J and Barros AIRNA: Phytochemical and antioxidant analysis of medicinal and food plants towards bioactive food and pharmaceutical resources. Sci Rep. 11(10041)2021.PubMed/NCBI View Article : Google Scholar | |
|
Šuran J, Cepanec I, Mašek T, Radić B, Radić S, Tlak Gajger I and Vlainić J: Propolis extract and its bioactive compounds-from traditional to modern extraction technologies. Molecules. 26(2930)2021.PubMed/NCBI View Article : Google Scholar | |
|
Zullkiflee N, Taha H and Usman A: Propolis: Its role and efficacy in human health and diseases. Molecules. 27(6120)2022.PubMed/NCBI View Article : Google Scholar | |
|
Pahlavani N, Malekahmadi M, Firouzi S, Rostami D, Sedaghat A, Moghaddam AB, Ferns GA, Navashenaq JG, Reazvani R, Safarian M and Ghayour-Mobarhan M: Molecular and cellular mechanisms of the effects of Propolis in inflammation, oxidative stress and glycemic control in chronic diseases. Nutr Metab (Lond). 17(65)2020.PubMed/NCBI View Article : Google Scholar | |
|
Jiang Y, Zeng KW, David B and Massiot G: Constituents of Vigna angularis and their in vitro anti-inflammatory activity. Phytochemistry. 107:111–118. 2014.PubMed/NCBI View Article : Google Scholar | |
|
Yao Y, Cheng X, Wang S, Wang L and Ren G: Influence of altitudinal variation on the antioxidant and antidiabetic potential of azuki bean (Vigna angularis). Int J Food Sci Nutr. 63:117–124. 2012.PubMed/NCBI View Article : Google Scholar | |
|
Chu L, Zhao P, Wang K, Zhao B, Li Y, Yang K and Wan P: VaSDC1 is involved in modulation of flavonoid metabolic pathways in black and red seed coats in Adzuki Bean (Vigna angularis L.). Front Plant Sci. 12(679892)2021.PubMed/NCBI View Article : Google Scholar | |
|
Włodarczyk K, Smolińska B and Majak I: The antioxidant potential of tomato plants (Solanum lycopersicum L.) under nano-ZnO treatment. Int J Mol Sci. 24(11833)2023.PubMed/NCBI View Article : Google Scholar | |
|
Kamiloglu S, Demirci M, Selen S, Toydemir G, Boyacioglu D and Capanoglu E: Home processing of tomatoes (Solanum lycopersicum): Effects on in vitro bioaccessibility of total lycopene, phenolics, flavonoids, and antioxidant capacity. J Sci Food Agric. 94:2225–2233. 2014.PubMed/NCBI View Article : Google Scholar | |
|
Del Giudice R, Petruk G, Raiola A, Barone A, Monti DM and Rigano MM: Carotenoids in fresh and processed tomato (Solanum lycopersicum) fruits protect cells from oxidative stress injury. J Sci Food Agric. 97:1616–1623. 2017.PubMed/NCBI View Article : Google Scholar | |
|
Blois MS: Antioxidant determinations by the use of a stable free radical. Nature. 181:1199–1200. 1958. | |
|
Re R, Pellegrini N, Proteggente A, Pannala A, Yang M and Rice-Evans C: Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med. 26:1231–1237. 1999.PubMed/NCBI View Article : Google Scholar | |
|
Rahman MM, Islam MB, Biswas M and Khurshid Alam AH: In vitro antioxidant and free radical scavenging activity of different parts of Tabebuia pallida growing in Bangladesh. BMC Res Notes. 8(621)2015.PubMed/NCBI View Article : Google Scholar | |
|
Ma X, Deng D and Chen W: Inhibitors and Activators of SOD, GSH-Px, and CAT. In: Enzyme Inhibitors and Activators. Senturk M (ed). IntechOpen, Rijeka, 2017. | |
|
Zhao H, Zhang R, Yan X and Fan K: Superoxide dismutase nanozymes: An emerging star for anti-oxidation. J Mater Chem B. 9:6939–6957. 2021.PubMed/NCBI View Article : Google Scholar | |
|
Pei J, Pan X, Wei G and Hua Y: Research progress of glutathione peroxidase family (GPX) in redoxidation. Front Pharmacol. 14(1147414)2023.PubMed/NCBI View Article : Google Scholar | |
|
Al-Temimi AA, Al-Mossawi AE, Al-Hilifi SA, Korma SA, Esatbeyoglu T, Rocha JM and Agarwal V: Glutathione for food and health applications with emphasis on extraction, identification, and quantification methods: A review. Metabolites. 13(465)2023.PubMed/NCBI View Article : Google Scholar | |
|
Gewalting MT and Kojda G: Vasoprotection by nitric oxide: Mechanisms and therapeutic potential. Cardiovasc Res. 55:250–260. 2002.PubMed/NCBI View Article : Google Scholar | |
|
van der Vliet A, Eiserich JP and Cross CE: Nitric oxide: A pro-inflammatory mediator in lung disease? Respir Res. 1:67–72. 2000.PubMed/NCBI View Article : Google Scholar | |
|
Liu NM, Miyashita L, Sanak M, Barratt B and Grigg J: Prostaglandin E2 and phagocytosis of inhaled particulate matter by airway macrophages in cystic fibrosis. J Cyst Fibros. 20:673–677. 2021.PubMed/NCBI View Article : Google Scholar | |
|
Kim JB, Han AR, Park EY, Kim JY, Cho W, Lee J, Seo EK and Lee KT: Inhibition of LPS-induced iNOS, COX-2 and cytokines expression by poncirin through the NF-kappaB inactivation in RAW 264.7 macrophage cells. Biol Pharm Bull. 30:2345–2351. 2007.PubMed/NCBI View Article : Google Scholar | |
|
Ishijima T and Nakajima K: Inflammatory cytokines TNFα, IL-1β, and IL-6 are induced in endotoxin-stimulated microglia through different signaling cascades. Sci Prog. 104(368504211054985)2021.PubMed/NCBI View Article : Google Scholar | |
|
Lopez-Castejon G and Brough D: Understanding the mechanism of IL-1β secretion. Cytokine Growth Factor Rev. 22:189–195. 2011.PubMed/NCBI View Article : Google Scholar | |
|
Parameswaran N and Patial S: Tumor necrosis factor-α signaling in macrophages. Crit Rev Eukaryot Gene Expr. 20:87–103. 2010.PubMed/NCBI View Article : Google Scholar | |
|
Tanaka T, Narazaki M and Kishimoto T: IL-6 in inflammation, immunity, and disease. Cold Spring Harb Perspect Biol. 6(a016295)2014.PubMed/NCBI View Article : Google Scholar | |
|
Xu W, Lu H, Yuan Y, Deng Z, Zheng L and Li H: The antioxidant and anti-inflammatory effects of flavonoids from propolis via Nrf2 and NF-κB pathways. Foods. 11(2439)2022.PubMed/NCBI View Article : Google Scholar | |
|
Kulawik A, Cielecka-Piontek J and Zalewski P: The importance of antioxidant activity for the health-promoting effect of lycopene. Nutrients. 15(3821)2023.PubMed/NCBI View Article : Google Scholar | |
|
Chao WW, Chung YC, Shih IP, Wang HY, Chou ST and Hsu CK: Red bean extract inhibits lipopolysaccharide-induced inflammation and H2O2-Induced oxidative stress in RAW 264.7 macrophages. J Med Food. 18:724–730. 2015.PubMed/NCBI View Article : Google Scholar |