Coffee extracts and caffeine upregulate the expression of the immune checkpoint factors, PD‑1 and PD‑L1
- Authors:
- Published online on: April 19, 2024 https://doi.org/10.3892/ijfn.2024.36
- Article Number: 2
-
Copyright : © Yoshida et al. This is an open access article distributed under the terms of Creative Commons Attribution License [CC BY 4.0].
Abstract
Introduction
To date, the consumption of coffee has been suggested to prevent malignant tumors. Epidemiological studies have reported that the consumption of coffee is associated with a reduced the risk of certain types of cancer, such as hepatocellular carcinoma and colorectal cancers (1-4). In addition, in vivo studies using animals and in vitro studies using cultured cells also indicate the suppressive effects of coffee on malignant tumors (5-10). Chlorogenic acid, a polyphenol contained in coffee, possesses antioxidant functions and it is considered to contribute to the antitumor effects; however, the molecular mechanism underlying the antitumor effects of coffee have not yet been fully elucidated.
The immune checkpoint was discovered as a novel immune system (11-13), which is induced by the binding between programmed death-1 (PD-1) and programmed death-ligand 1 (PD-L1) (14-16). The immune checkpoint suppresses the excess immune response to prevent auto-immune disease, but functions as a mechanism of carcinogenesis. In normal cells, immune surveillance eliminates newborn tumor cells. On the other hand, malignant tumor cells acquire a means with which escape from immune surveillance by the expression of the immune checkpoint factor, PD-L1, on their cell surface (17-19). PD-L1 suppresses the immune response by binding to PD-1, which is expressed on immune cells. The antibody agents, nivolumab (brand name, Opdivo) (20-22) and pembrolizumab (brand name, Keytruda) (23,24), which inhibit the PD-1 and PD-L1 interaction exhibit a high efficacy in the treatment of malignant tumors. Thus, immune checkpoint inhibitors have been approved for the treatment of melanoma, lung cancer, gastric cancer, renal cell carcinoma, Hodgkin lymphoma, head and neck cancer, liver cancer, B cell lymphoma, breast cancer and colorectal cancer. by the Food and Drug Administration (FDA) (25,26). Tasuku Honjo and James P. Allison were awarded the Novel prize in physiology or medicine in 2018 for their discovery of the immune checkpoint (https://www.nobelprize.org/prizes/medicine/2018/summary/). The immune checkpoint is an attractive research field, however, the regulatory mechanisms of PD-1 and PD-L1 expression remain poorly understood.
Recently, some food components have been reported to modulate immune checkpoint factors (27). Lycopene, a type of carotenoid, downregulates the signaling and expression of PD-L1 in tongue squamous cell carcinoma cells (28) and flavonoids, such as quercetin and apigenin also decrease PD-1 expression in a number of types of malignant tumors (29-32). On the other hand, vitamin D upregulates PD-L1 expression through vitamin D response elements of the gene in epithelial and myeloid cells (33). These findings indicate that food factors contribute to controlling immune responses for onsets of inflammation and malignant tumors through regulation of immune checkpoint factors.
The present study analyzed the effects of coffee on the immune checkpoint in an aim to elucidate the molecular mechanisms underlying the preventive effects coffee on malignant tumors.
Materials and methods
Cells and cell culture
The chronic myelogenous leukemia cell line, K562 (cat. no. RCB-0027), was obtained from the RIKEN BioResource Center and maintained in RPMI-1640 medium (FUJIFILM Wako Pure Chemical, Osaka, Japan) supplemented with 10% fatal bovine serum (FBS; Sigma-Aldrich; Merck KGaA). The cervical cancer cell line, HeLa (cat. no. RCB0007), was also obtained from the RIKEN BioResource Center. The liver cancer cell line, HepG2, (cat. no. HB-8065) and the colon cancer cell line, SW480, (cat. no. CCL-228) were obtained from the American Type Culture Collection (ATCC). These cells were maintained in DMEM supplemented with 10% FBS and 2 mM-glutamine (Gibco; Thermo Fisher Scientific, Inc.). These cells were maintained at 37˚C in humidified air with 5% CO2.
Generation of plasmids
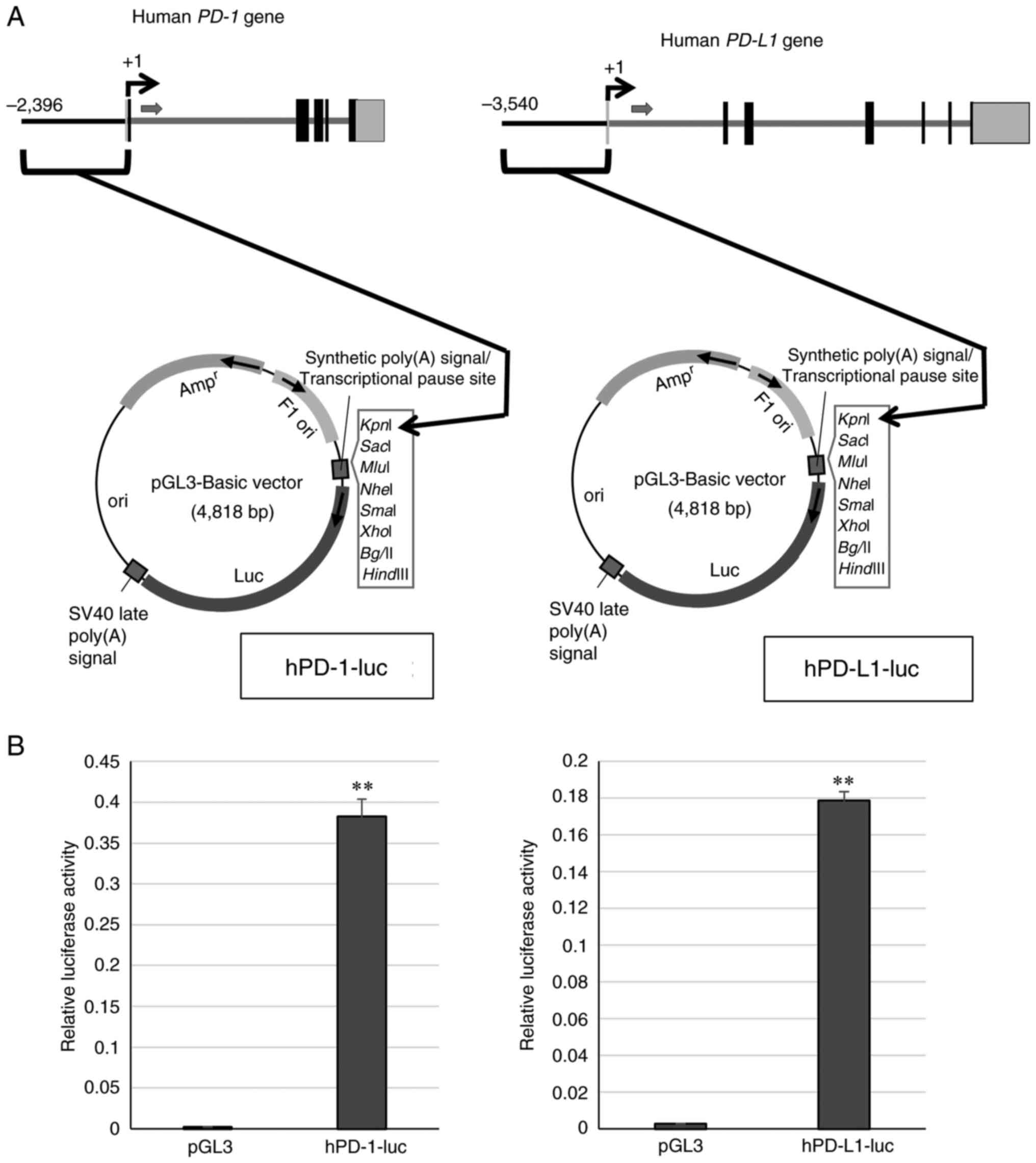
Transcriptional regulation regions, promoters on the 5'-flanking region of the human PD-1 or PD-L1 gene were obtained from human genomic DNA (Promega Corporation) by PCR using PrimeStar GXL DNA polymerase (Takara Bio, Inc.) and inserted into a luciferase reporter plasmid pGL3 (Promega Corporation) at the KpnI sites. The plasmids, hPD-1-luc and hPD-L1-1uc, were generated. DNA sequences were analyzed using a Genetic analyzer 3500 (Thermo Fisher Scientific, Inc.).
Reagents
Coffee extracts were generated by the dissolution of instant coffee powder Nescafe Excella (Nestle) with sterilized water. Caffeine, chlorogenic acid and caffeic acid were purchased from Nacalai Tesque, Inc. Quinic acid was purchased from Alfa Aesar; Thermo Fisher Scientific, Inc. These reagents were dissolved in dimethyl sulfoxide (DMSO) (FUJIFILM Wako Pure Chemical). Caffeine was used at a final concentration 1, 2 or 4 mM and the other reagents were used at final a concentration 10 µM as reoffered to in previous studies (34,35).
Transfection of plasmids
The plasmids were transfected into K562 cells by electroporation with Nucleofector2b (Program T-016) (Lonza Group, Ltd.). Cells and plasmids were applied into 100 µl buffer of the AMAXA Cell Line Nucleofector Kit V (Lonza Group, Ltd.) in a cuvette equipped with aluminium electrodes of the kit and immediately pulsed at room temperature. Following electroporation, the cells were recovered with RPMI-1640 with 10% FBS. Subsequently, 24 h following incubation at 37˚C, the cells were treated with the reagents mentioned above for 24 h, followed by analyses. In the HeLa, HepG2 and SW480 cells, the plasmids were transfected by lipofection with Lipofectamine 2000® (Thermo Fisher Scientific, Inc.). Plasmid hPD-1-luc or hPD-L1-luc (1.25 µg) which were generated by the insertion of the promoter region into pGL3 (Promega Corporation) and phRL-TK (Promega Corporation) (0.0625 µg) in 25 µl of Opti-MEM I (Life Technologies; Thermo Fisher Scientific, Inc.) and 0.5 µl Lipofectamine 2000® in 25 µl Opti-MEM I were incubated for 5 min at room temperature. These solutions were then mixed, incubated for 20 min at room temperature and added to the cells supplemented in 250 µl RPMI-1640 with 10% FBS. At 24 h following transfection, the cells were treated with the reagents mentioned above for 24 h and collected for use in the experiments.
Luciferase assay
The cells were transfected by electroporation [Nucleofector2b (Lonza Group, Ltd.)] for the K562 cells and by lipofection [Lipofectamine 2000® (Thermo Fisher Scientific, Inc.)] for the HeLa, HepG2 and SW480 cells. The cells transfected with the plasmids, hPD-1-luc or hPD-L1-luc, which were generated by the insertion of the promoter region derived from the human PD-1 or human PD-L1 gene into pGL3 (Promega Corporation) and phRL-TK (Promega Corporation) were incubated at 37˚C for 24 h. The cells were then treated with the reagents mentioned above for 24 h, harvested and lysed with a 1X concentration of Passive lysis buffer (Promega Corporation) and cell lysate were used for luciferase assay with Dual Luciferase Reporter Assay System (Promega Corporation) and a luminometer (Lumat LB9507, Berthold Detection Systems). The Renilla reniformis luciferase plasmid phRL-TK was also transfected with the reporter plasmids to standardize the transfection rate.
Reverse transcription-quantitative qPCR (RT-qPCR)
Total RNA was prepared from the cultured cells using Isogen II (Nippon Gene Co., Ltd.). Complementary DNA (cDNA) was generated from total RNA using oligo (dT) (Thermo Fisher Scientific, Inc.), dNTP mix (Thermo Fisher Scientific, Inc.) and superscript IV (Thermo Fisher Scientific, Inc.). RT-qPCR was performed with specific primers for human PD-1 and human PD-L1 synthesized by Eurofins Genomics, THUNDERBIRD™ SYBR qPCR mix (Toyobo Life Science), generated cDNA and the StepOnePlus real-time PCR system (Thermo Fisher Scientific, Inc.). The thermocycling conditions were as follows: 95˚C for 10 sec, followed by 40 cycles of 95˚C for 15 sec and 60˚C for 1 min, then 95˚C for 15 sec, 60˚C for 1 min and 95˚C for 15 sec. The quantification method used was the ΔΔCq method (36). The primer sequences were the following: Human PD-1 forward, 5'-GACAGCGGCACCTACCTCTGTG and reverse, 5'-GACCCAGACTAGCAGCACCAGG; human PD-L1 forward, 5'-GGAGATTAGATCCTGAGGAAAACCA and reverse, 5'-AACGGAAGATGAATGTCAGTGCTA; and human actin forward, 5'-GCTGTGCTACGTCGCCCTG and reverse, 5'-GGAGGAGCTGGAAGCAGCC.
Cell proliferation assay
The number of viable cells was evaluated using a Cell Counting Kit-8 (CCK-8; Dojindo Molecular Technologies, Inc.). The cells were incubated with the CCK-8 reagent at 37˚C, for 4 h. The absorbance (450 nm) was determined using SpectraMax iD3 (Molecular Devices, LLC). The percentage growth was determined relative to the control (sterilized water for the coffee extracts and DMSO for caffeine).
Cell cycle analysis
The cultured cells were harvested and suspended in propidium iodide (PI) (Nacalai Tesque, Inc.) solution (PBS containing 10 µg/ml PI, 0.1% Triton X-100) for 5 min at room temperature. Cell cycle and cell death were analyzed by flow cytometry using a FACS Canto II flow cytometer (BD Biosciences) and BD FACS Diva software v6.1.3 (BD Biosciences). The sub-G1 population was estimated as dead cells.
Analysis of apoptosis
For the analysis of cell apoptosis, the cells were counterstained using the Annexin V-FITC Apoptosis Detection Kit (Nacalai Tesque, Inc.) for 15 min at room temperature, and were analyzed using a BD Accuri C6 Flow Cytometer (BD Biosciences) and BD Accuri C6 Plus Software (BD Biosciences).
Analysis of cell surface expression
The cells were collected following treatment with caffeine at 4 mM and incubated with anti-PD-1 antibody labeled FITC (cat. no. 11-9969-42, Invitrogen; Thermo Fisher Scientific, Inc.) or anti-PD-L1 antibody labeled PE (cat. no. 12-5983-42, Invitrogen; Thermo Fisher Scientific, Inc.) for 1 h on ice. The analysis was performed using flow cytometry with a FACS Canto II flow cytometer (BD Biosciences) and BD FACS Diva software v6.1.3 (BD Biosciences).
Giemsa staining
K562 cells were attached to a glass slide using a cytospin (cytospin 3, Shandon; Thermo Fisher Scientific). The glass slide was first stained with May-Grunwald's eosine-methylene blue solution (Merck KGaA) for 3 min at room temperature and then stained with Giemsa's azur eosine methylene blue solution (Merck KGaA) for 30 min at room temperature. The slide glass was washed with water. Cell morphology was observed under a microscope (Eclipse E800 optical microscope, Nikon Corporation).
Immunostaining
All cells were treated with 4 mM caffeine for 24 h. The HepG2 and SW480 cells were inoculated in a 12-well plate with cover glasses at the bottom of the plate prior to caffeine treatment. The K562 cells were attached to glass slides using a cytospin after caffeine treatment. The cells were fixed with 100% methanol for 2 min at room temperature, washed with PBS and blocked with PBS with 2% of FBS for 30 min at room temperature. Anit-PD-1 rabbit monoclonal antibody (cat. no. 86163, Cell Signaling Technology, Inc.) or anit-PD-L1 rabbit monoclonal antibody (cat. no. 13684, Cell Signaling Technology, Inc.) was reacted with cells at a 1/200 dilution for 1 h at room temperature. The cells were then reacted with AlexaFluor 488 goat anti-rabbit IgG (cat. no. A11008, Life Technologies; Thermo Fisher Scientific, Inc.) at a 1/500 dilution for 1 h at room temperature. The nuclei were then stained using Prolong Gold antifade reagent with DAPI (P36931, Invitrogen; Thermo Fisher Scientific, Inc.). Immunostaining was observed with a BZ-X710 microscope (Keyence Corporation) at a magnification x400 (scale bar, 20 µm). The mean of fluorescence intensity per cell was measured using ImageJ software (https://imagej.net/ij/).
Statistical analysis
Data are presented as the mean of three results ± standard deviation (SD). To confirm the reproducibility, we performed the experiments more than three times. Data were statistically analyzed using a two-tailed Student's t-test with Microsoft Excel (Microsoft Corporation) for comparisons among two groups, or one-way ANOVA followed by Tukey's post hoc test with GraphPad Prism Version 8.4.3 (Dotmatics) for comparisons among multiple groups. A value of P<0.05 was considered to indicate a statistically significant difference.
Results
Coffee extracts enhance the transcriptional activity of human PD-1 and PD-L1
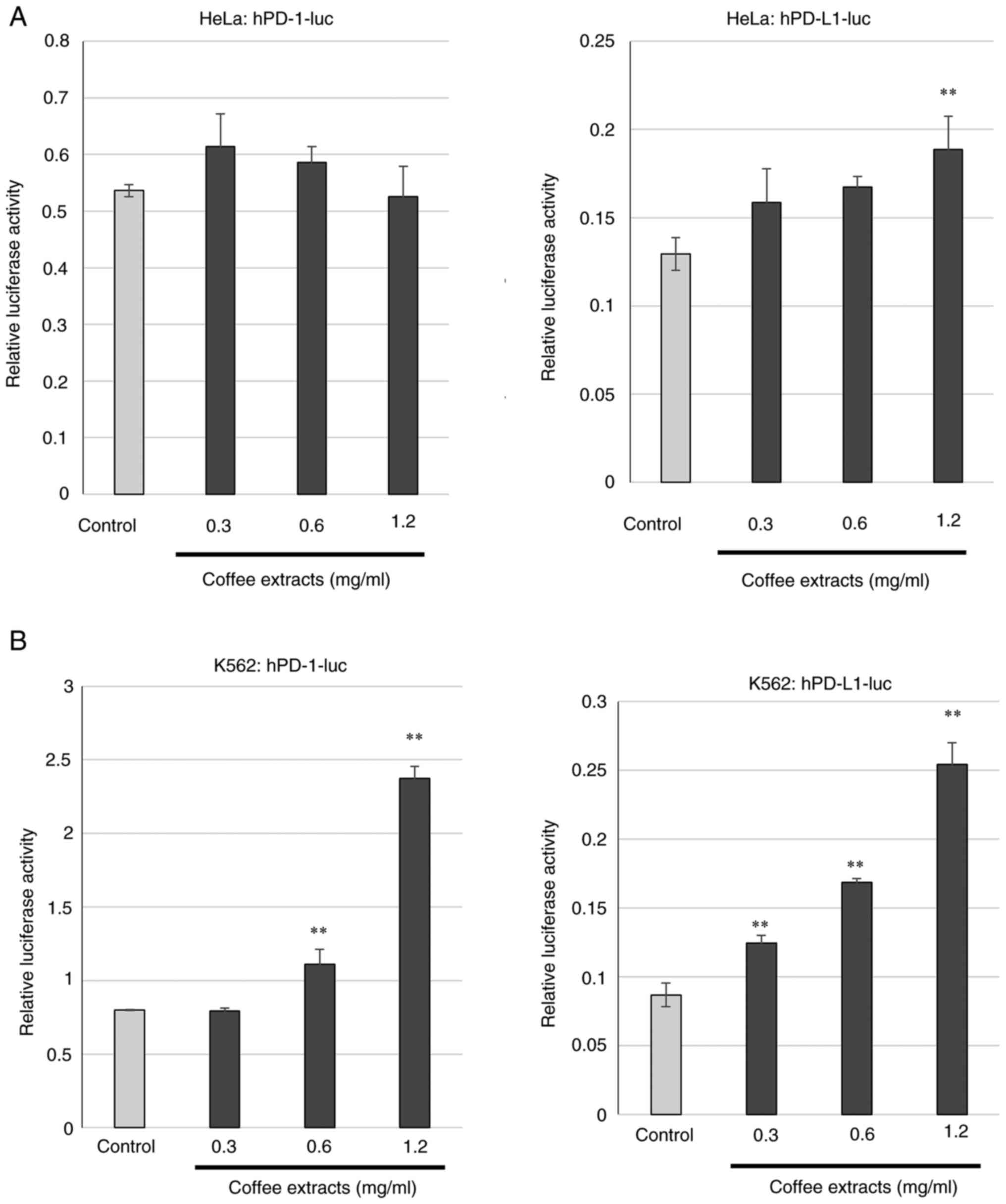
To examine whether coffee affects the expression of the immune checkpoint factors, PD-1 and PD-L1, first, the cloning of the PD-1 and PD-L1 promoter regions was performed. A part of human genomic DNA upstream transcription-start site of human PD-1 or human PD-L1 was amplified by PCR and ligated to the luciferase assay vector pGL3, and hPD-1-luc and hPD-L1-luc were generated (Fig. 1A). To confirm that the cloned regions functioned as a promoter, hPD-1-luc and hPD-L1-luc were transfected into HeLa cells and luciferase assay was performed. The values of luciferase assay for hPD-1-luc and hPD-L1-luc were compared to those of the empty vector, pGL3 (Fig. 1B). The luciferase activities of hPD-1-luc and hPD-L1-luc were markedly increased. The results indicated that the cloned regions functions as promoters of the PD-1 and PD-L1 genes. Subsequently, the transcriptional regulations of PD-1 and PD-L1 by coffee extracts were examined. The coffee extracts were generated by instant coffee powder dissolved in sterilized water. The concentrations of the coffee extracts used in the present study were as referred to in a previous study (6). The HeLa cells transfected with hPD-1-luc or hPD-L1-luc were cultured in medium containing coffee extracts and luciferase assay was performed with the cell lysate. No changes in transcriptional activity were not observed in the cells transfected with hPD-1-luc; however, the coffee extracts increased the promoter activity of hPD-L1-luc-transfected cells compared to the control (Fig. 2A). The same experiment was then performed using the K562 cells. The transcriptional activities of PD-1 and PD-L1 were markedly increased by the coffee extracts in a concentration-dependent manner (Fig. 2B).
Caffeine activates the transcription of human PD-1 and PD-L1
Subsequently, the components contained in coffee were examined. Caffeine, chlorogenic acid, caffeic acid and quinic acid were used. The concentrations used in the present study were as referred to in previous studies (34,35). Luciferase assay was performed with these components in HeLa cells; however, no change was observed (Fig. 3A). When the luciferase assay was performed in K562 cells, caffeine but not the other components increased the PD-1 and PD-L1 transcriptional activities (Fig. 3B). The enhancements of the PD-1 and PD-L1 transcriptional activities by caffeine occurred in a concentration-dependent manner (Fig. 4A). It was then examined whether endogenous mRNA levels were affected by caffeine using RT-qPCR. Treatment with caffeine increased the mRNA expression levels of PD-1 and PD-L1 (Fig. 4B). Moreover, the cell surface expression of PD-1 and PD-L1 was examined at the protein level, following treatment with caffeine. As shown in Fig. 4C, caffeine increased PD-1 and PD-L1 protein expression on the cell surface.
Effects of coffee extract and caffeine on cell cycle and cell death
The present study then used flow cytometry to reveal whether coffee extracts and caffeine affect the cell cycle and death of K562 cells. When the cells were treated with PI solution, Triton X-100 permeabilized the nucleus and nucleic DNA was stained by PI. Thus, DNA contents were measured. When the cells died, DNA became fragments and the sub-G1 population, which had a lower DNA content than the G1 population, then appeared. Sub-G1 cells were estimated as dead cells. The sub-G1 population of K562 cells treated with the coffee extracts increased in a concentration-dependent manner (Fig. 5A). Treatment with caffeine also increased the sub-G1 cell population, although the effect was less prominent than that of the coffee extracts (Fig. 5B). In addition, the G2/M cell population was slightly increased following treatment with caffeine. These data indicate that coffee extracts and caffeine induce the death of malignant tumor cells.
Subsequently, cell proliferation was examined using CCK-8 assay. The coffee extracts and caffeine inhibited cell proliferation in a concentration-dependent manner (Fig. 5C). The induction of apoptosis was analyzed using Annexin V/PI staining (Figs. 5D and S1). Coffee extracts and caffeine increased the number of Annexin V-positive cells, indicating that apoptosis was induced by treatment with coffee extracts and caffeine. The cell morphologies of the cells treated with the coffee extracts or caffeine were observed (Fig. S2). Neither coffee extracts nor caffeine induced cell differentiation, such as megakaryocytes, a change which was previously reported in 12-O-tetradecanoyl phorbol 13-acetate-treated K562 cells (37). In the cells treated with the coffee extracts, a number of cells exhibited shrunken shapes and nuclear fragmentation, indicating the induction of apoptosis. The present study examined whether the overexpression of PD-1 and PD-L1 induced cell death; however, a high level of PD-1 and PD-L1 expression did not induce cell death (Fig. S3).
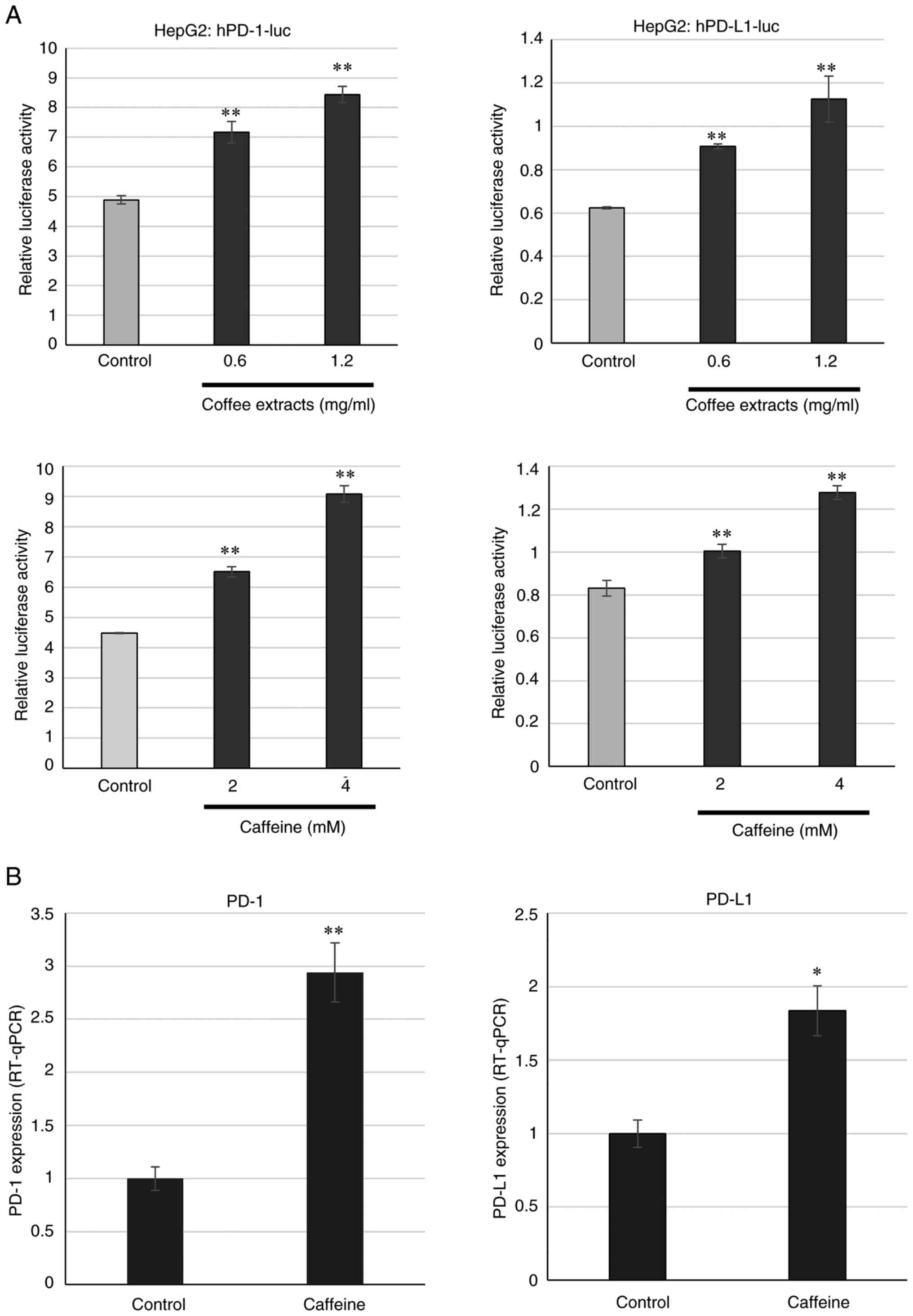
Coffee extracts and caffeine activate the transcription of human PD-1 and PD-L1 in liver cancer and colon cancer cells
The present study demonstrated that PD-1 and PD-L1 expression were increased at a transcriptional level in chronic myeloid leukemia K562 cells. Subsequently, the present study examined whether this activation also occurs in cell lines derived from other tissues. Liver cancer HepG2 cells and colon cancer SW480 cells were used. As shown in Fig. 6A, coffee extracts and caffeine activated both the PD-1 and PD-L1 promoters in a concentration-dependent manner in HepG2 cells. Moreover, treatment with caffeine increased the PD-1 and PD-L1 mRNA levels in HepG2 cells (Fig. 6B). In addition, the promoter activities of PD-1 and PD-L1 increased by the coffee extracts and caffeine in colon cancer SW480 cells (Fig. 7A). PD-1 and PD-L1 mRNA expression also increased following treatment with caffeine in SW480 cells (Fig. 7B). In addition, immunostaining revealed the increase in both PD-1 and PD-L1 protein levels in HepG2, SW480 and K562 (Figs. S4 and S5). These results indicate that coffee extracts and caffeine regulate PD-1 and PD-L1 transcription not only K562 cells, but also in HepG2 and SW480 cells.
Discussion
The present study examined the effects of coffee extracts on the transcriptional regulation of PD-1 and PD-L1 in an aim to reveal the association between coffee and the immune checkpoint. These coffee extracts enhanced the transcriptional activity of PD-1 and PD-L1 in K562 cells. Moreover, when the components contained in coffee were analyzed, caffeine increased the transcriptional activity of PD-1 and PD-L1, suggesting that the effect of coffee was exerted via caffeine. This transactivation occurred in liver cancer HepG2 and colon cancer SW480 cells, as well as in a leukemia cell line. These results indicate that the upregulation in the expression of PD-1 and PD-L1 is induced through a common signal transduction mechanism possessed by various tissues. On the other hand, both promoters were not activated by caffeine and the PD-1 promoter was also not activated by coffee extracts in cervical cancer HeLa cells. This discrepancy may have occurred due to the tissue specificity of the cervix or human papilloma virus infection. However, in order to reveal the tissue specificity, it is necessary to perform analyses using other cell lines. It has been reported that caffeine inhibits ataxia telangiectasia mutated (ATM) and ATM and Rad3-related (ATR), which are kinases acting on the DNA damage response (34). The inhibition of ATM and ATR may mediate PD-1 and PD-L1 expression levels regulated by coffee and caffeine. In addition, as caffeine has a structure similar to that adenosine, it can bind to adenosine receptor (38,39). Coffee and caffeine may regulate PD-1 and PD-L1 expression levels through these signals; however, the present study did not analyze the effects of ATM/ATR and adenosine receptor on PD-1 and PD-L1 expression. It has been reported that caffeine inhibits phosphodiesterases, leading to an increase in cytosolic cAMP (40,41). Thus, PD-1 and PD-L1 expression may be also considered to be regulated by cAMP signaling downstream of caffeine treatment. It has been reported that cAMP increases PD-L1 transcription and protein expression in diffuse large B-cell lymphoma cells (42).
The present study also found that coffee extracts and caffeine induced the death of K562 cells. The cell death-inducing effect of caffeine was weak, suggesting that coffee also induces cell death via a route other than caffeine. Caffeine induced G2/M phase arrest, although the coffee extract had no such effect. This is considered to be due to other components in coffee more strongly inducing cell death, rather than G2/M phase arrest. It was found that coffee extracts and caffeine induced cell death, and increased the expression of PD-1 and PD-L1. The results revealed that the overexpression of PD-1 and PD-L1 did not induce cell death. These results indicate that cell death and the increase in PD-1 and PD-L1 expression occurred in parallel following treatment with the coffee extracts and caffeine. The present study did not examine the effects of coffee and caffeine on cell invasion and migration. Epidemiological studies have demonstrated that the consumption of coffee decreased the risk of developing myelodysplastic syndrome in non-smoking individuals (43). Therefore, coffee and caffeine are suggested to be used for prevention and treatment of leukemia, including chronic myelogenous leukemia analyzed in the present study. On the other hand, the upregulation of PD-1 and PD-L1 expression will protect tumor cells, since PD-L1 suppresses the immune response by binding to PD-1, which is expressed on immune cells. Thus, anti-PD-1 and anti-PD-L1 antibodies are used in clinical practice against various types of malignant tumors. Moreover, cancer not only escapes the body's regulatory mechanisms, including the immune system, but also gains the ability to affect local and systemic homeostasis (44). The immunosuppressive effects of PD-1 and PD-L1 expression cause the abnormal release of immune mediators. Therefore, as PD-1 and PD-L1 exert immune suppressive effects in malignant tumors, the results of the present study suggest that the inhibitor of the immune checkpoint may synergistically enhance the preventive effects of coffee on myeloid disease and malignant tumors. However, the results presented herein are limited to in vitro experiments and thus need to be further validated in vivo in future studies.
Supplementary Material
K562 cells were treated with caffeine and cultured for 24 h. Apoptosis induction was examined by Annexin V/PI staining. A bar graph is shown on the right. The data are presented as the mean of three results ± standard deviation. *P<0.05 and **P<0.01, vs. the control.
K562 cells were treated with 1.2 mg/ml coffee extracts or 4 mM caffeine for 24 h and observed by Giemsa staining. Water: sterile water.
Human PD-1/pcDNA3.1 or human PD-L1/pcDNA3.1 were transfected into K562 cells by electroporation. At 24 h following transfection, (A) the cell surface expression of PD-1 or PD-L1 was examined and (B) cell cycle analysis was performed. PD-1, programed death-1; PD-L1, programed death-ligand 1.
Immunostaining of PD-1 in Hep G2, SW480 and K562 cells. The relative value was graphed against the control. Bar graphs were shown on the right. The data are presented as the mean of three results ± standard deviation. *P<0.05 and **P<0.01, vs. the control. PD-1, programed death-1; PD-L1, programed death-ligand 1.
Immunostaining of PD-L1 in Hep G2, SW480 and K562 cells was performed. The length of yellow bar is 20 μm. The relative value was graphed against the control and shown on the right. The data are presented as the mean of three results ± standard deviation. *P<0.05, vs. the control. PD-1, programed death-1; PD-L1, programed death-ligand 1.
Acknowledgements
The authors would like to thank Ms. E. Nishimoto for providing technical support with the experiments.
Funding
Funding: The present study was supported by a research fund from the All Japan Coffee Association for 2021.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
TY performed the experiments and wrote the manuscript. KY, MH and KT performed the experiments and edited the manuscript, TY, KY, MH and KT confirm the authenticity of all the raw data. All authors have read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
Tatsushi Yoshida is supported by a research fund from the All Japan Coffee Association.
References
|
Inoue M, Yoshimi I, Sobue T and Tsugane S: JPHC Study Group. Influence of coffee drinking on subsequent risk of hepatocellular carcinoma: A prospective study in Japan. J Natl Cancer Inst. 97:293–300. 2005.PubMed/NCBI View Article : Google Scholar | |
|
Kashino I, Akter S, Mizoue T, Sawada N, Kotemori A, Matsuo K, Oze I, Ito H, Naito M, Nakayama T, et al: Coffee drinking and colorectal cancer and its subsites: A pooled analysis of 8 cohort studies in Japan. Int J Cancer. 143:307–316. 2018.PubMed/NCBI View Article : Google Scholar | |
|
Emile SH, Barsom SH, Garoufalia Z and Wexner SD: Does drinking coffee reduce the risk of colorectal cancer? A qualitative umbrella review of systematic reviews. Tech Coloproctol. 27:961–968. 2023.PubMed/NCBI View Article : Google Scholar | |
|
Alicandro G, Tavani A and La Vecchia C: Coffee and cancer risk: A summary overview. Eur J Cancer Prev. 26:424–432. 2017.PubMed/NCBI View Article : Google Scholar | |
|
Miura Y, Furuse T and Yagasaki K: Inhibitory effect of serum from rats administered with coffee on the proliferation and invasion of rat ascites hepatoma cells. Cytotechnology. 25:221–225. 1997.PubMed/NCBI View Article : Google Scholar | |
|
Miura Y, Ono K, Okauchi R and Yagasaki K: Inhibitory effect of coffee on hepatoma proliferation and invasion in culture and on tumor growth, metastasis and abnormal lipoprotein profiles in hepatoma-bearing rats. J Nutr Sci Vitaminol (Tokyo). 50:38–44. 2004.PubMed/NCBI View Article : Google Scholar | |
|
Villota H, Santa-González GA, Uribe D, Henao IC, Arroyave-Ospina JC, Barrera-Causil CJ and Pedroza-Díaz J: Modulatory effect of chlorogenic acid and coffee extracts on Wnt/β-catenin pathway in colorectal cancer cells. Nutrients. 14(4880)2022.PubMed/NCBI View Article : Google Scholar | |
|
Vélez-Vargas LC, Santa-González GA, Uribe D, Henao-Castañeda IC and Pedroza-Díaz J: In vitro and in silico study on the impact of chlorogenic acid in colorectal cancer cells: Proliferation, apoptosis, and interaction with β-catenin and LRP6. Pharmaceuticals (Basel). 16(276)2023.PubMed/NCBI View Article : Google Scholar | |
|
Murai T and Matsuda S: The chemopreventive effects of chlorogenic acids, phenolic compounds in coffee, against inflammation, cancer, and neurological diseases. Molecules. 28(2381)2023.PubMed/NCBI View Article : Google Scholar | |
|
Gupta A, Atanasov AG, Li Y, Kumar N and Bishayee A: Chlorogenic acid for cancer prevention and therapy: Current status on efficacy and mechanisms of action. Pharmacol Res. 186(106505)2022.PubMed/NCBI View Article : Google Scholar | |
|
Francisco LM, Sage PT and Sharpe AH: The PD-1 pathway in tolerance and autoimmunity. Immunol Rev. 236:219–242. 2010.PubMed/NCBI View Article : Google Scholar | |
|
Fife BT and Bluestone JA: Control of peripheral T-cell tolerance and autoimmunity via the CTLA-4 and PD-1 pathways. Immunol Rev. 224:166–182. 2008.PubMed/NCBI View Article : Google Scholar | |
|
Gianchecchi E, Delfino DV and Fierabracci A: Recent insights into the role of the PD-1/PD-L1 pathway in immunological tolerance and autoimmunity. Autoimmun Rev. 12:1091–1100. 2013.PubMed/NCBI View Article : Google Scholar | |
|
Ishida Y, Agata Y, Shibahara K and Honjo T: Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J. 11:3887–3895. 1992.PubMed/NCBI View Article : Google Scholar | |
|
Freeman GJ, Long AJ, Iwai Y, Bourque K, Chernova T, Nishimura H, Fitz LJ, Malenkovich N, Okazaki T, Byrne MC, et al: Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 192:1027–1034. 2000.PubMed/NCBI View Article : Google Scholar | |
|
Okazaki T and Honjo T: PD-1 and PD-1 ligands: From discovery to clinical application. Int Immunol. 19:813–824. 2007.PubMed/NCBI View Article : Google Scholar | |
|
Homet Moreno B and Ribas A: Anti-programmed cell death protein-1/ligand-1 therapy in different cancers. Br J Cancer. 112:1421–1427. 2015.PubMed/NCBI View Article : Google Scholar | |
|
Pardoll DM: The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 12:252–264. 2012.PubMed/NCBI View Article : Google Scholar | |
|
Sadreddini S, Baradaran B, Aghebati-Maleki A, Sadreddini S, Shanehbandi D, Fotouhi A and Aghebati-Maleki L: Immune checkpoint blockade opens a new way to cancer immunotherapy. J Cell Physiol. 234:8541–8549. 2019.PubMed/NCBI View Article : Google Scholar | |
|
Ansell SM, Lesokhin AM, Borrello I, Halwani A, Scott EC, Gutierrez M, Schuster SJ, Millenson MM, Cattry D, Freeman GJ, et al: PD-1 blockade with nivolumab in relapsed or refractory Hodgkin's lymphoma. N Engl J Med. 372:311–319. 2015.PubMed/NCBI View Article : Google Scholar | |
|
Wolchok JD, Kluger H, Callahan MK, Postow MA, Rizvi NA, Lesokhin AM, Segal NH, Ariyan CE, Gordon RA, Reed K, et al: Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med. 369:122–133. 2013.PubMed/NCBI View Article : Google Scholar | |
|
Kang YK, Boku N, Satoh T, Ryu MH, Chao Y, Kato K, Chung HC, Chen JS, Muro K, Kang WK, et al: Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 390:2461–2471. 2017.PubMed/NCBI View Article : Google Scholar | |
|
Garon EB, Rizvi NA, Hui R, Leighl N, Balmanoukian AS, Eder JP, Patnaik A, Aggarwal C, Gubens M, Horn L, et al: Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med. 372:2018–2028. 2015.PubMed/NCBI View Article : Google Scholar | |
|
Hamid O, Robert C, Daud A, Hodi FS, Hwu WJ, Kefford R, Wolchok JD, Hersey P, Joseph RW, Weber JS, et al: Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med. 369:134–144. 2013.PubMed/NCBI View Article : Google Scholar | |
|
Chang E, Pelosof L, Lemery S, Gong Y, Goldberg KB, Farrell AT, Keegan P, Veeraraghavan J, Wei G, Blumenthal GM, et al: Systematic review of PD-1/PD-L1 inhibitors in oncology: From personalized medicine to public health. Oncologist. 26:e1786–e1799. 2021.PubMed/NCBI View Article : Google Scholar | |
|
Twomey JD and Zhang B: Cancer Immunotherapy update: FDA-approved checkpoint inhibitors and companion diagnostics. AAPS J. 23(39)2021.PubMed/NCBI View Article : Google Scholar | |
|
Zhang Q, Yang C, Gao X, Dong J and Zhong C: Phytochemicals in regulating PD-1/PD-L1 and immune checkpoint blockade therapy. Phytother Res. 38:776–796. 2024.PubMed/NCBI View Article : Google Scholar | |
|
Peng M, Fan S, Li J, Zhou X, Liao Q, Tang F and Liu W: Programmed death-ligand 1 signaling and expression are reversible by lycopene via PI3K/AKT and Raf/MEK/ERK pathways in tongue squamous cell carcinoma. Genes Nutr. 17(3)2022.PubMed/NCBI View Article : Google Scholar | |
|
Li L, Zhang M, Liu T, Li J, Sun S, Chen J, Liu Z, Zhang Z and Zhang L: Quercetin-ferrum nanoparticles enhance photothermal therapy by modulating the tumor immunosuppressive microenvironment. Acta Biomater. 154:454–466. 2022.PubMed/NCBI View Article : Google Scholar | |
|
Jing D, Wu W, Chen X, Xiao H, Zhang Z, Chen F, Zhang Z, Liu J, Shao Z and Pu F: Quercetin encapsulated in folic acid-modified liposomes is therapeutic against osteosarcoma by non-covalent binding to the JH2 domain of JAK2 via the JAK2-STAT3-PDL1. Pharmacol Res. 182(106287)2022.PubMed/NCBI View Article : Google Scholar | |
|
Jiang ZB, Wang WJ, Xu C, Xie YJ, Wang XR, Zhang YZ, Huang JM, Huang M, Xie C, Liu P, et al: Luteolin and its derivative apigenin suppress the inducible PD-L1 expression to improve anti-tumor immunity in KRAS-mutant lung cancer. Cancer Lett. 515:36–48. 2021.PubMed/NCBI View Article : Google Scholar | |
|
Coombs MRP, Harrison ME and Hoskin DW: Apigenin inhibits the inducible expression of programmed death ligand 1 by human and mouse mammary carcinoma cells. Cancer Lett. 380:424–433. 2016.PubMed/NCBI View Article : Google Scholar | |
|
Dimitrov V, Bouttier M, Boukhaled G, Salehi-Tabar R, Avramescu RG, Memari B, Hasaj B, Lukacs GL, Krawczyk CM and White JH: Hormonal vitamin D up-regulates tissue-specific PD-L1 and PD-L2 surface glycoprotein expression in humans but not mice. J Biol Chem. 292:20657–20668. 2017.PubMed/NCBI View Article : Google Scholar | |
|
Yagasaki K, Miura Y, Okauchi R and Furuse T: Inhibitory effects of chlorogenic acid and its related compounds on the invasion of hepatoma cells in culture. Cytotechnology. 33:229–235. 2000.PubMed/NCBI View Article : Google Scholar | |
|
Sarkaria JN, Busby EC, Tibbetts RS, Roos P, Taya Y, Karnitz LM and Abraham RT: Inhibition of ATM and ATR kinase activities by the radiosensitizing agent, caffeine. Cancer Res. 59:4375–4382. 1999.PubMed/NCBI | |
|
Livak KJ and Schmittgen TD: Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.PubMed/NCBI View Article : Google Scholar | |
|
Yoshida T, Yamasaki K, Tadagaki K, Kuwahara Y, Matsumoto A, Sofovic AE, Kondo N, Sakai T and Okuda T: Tumor necrosis factor-related apoptosis-inducing ligand is a novel transcriptional target of runt-related transcription factor 1. Int J Oncol. 60(6)2022.PubMed/NCBI View Article : Google Scholar | |
|
Dranoff JA: Coffee, adenosine, and the liver. Purinergic Signal. 20:21–28. 2024.PubMed/NCBI View Article : Google Scholar | |
|
Song X, Kirtipal N, Lee S, Malý P and Bharadwaj S: Current therapeutic targets and multifaceted physiological impacts of caffeine. Phytother Res. 37:5558–5598. 2023.PubMed/NCBI View Article : Google Scholar | |
|
Montoya GA, Bakuradze T, Eirich M, Erk T, Baum M, Habermeyer M, Eisenbrand G and Richling E: Modulation of 3',5'-cyclic AMP homeostasis in human platelets by coffee and individual coffee constituents. Br J Nutr. 112:1427–1437. 2014.PubMed/NCBI View Article : Google Scholar | |
|
Dixon R, Hwang S, Britton F, Sanders K and Ward S: Inhibitory effect of caffeine on pacemaker activity in the oviduct is mediated by cAMP-regulated conductances. Br J Pharmacol. 163:745–754. 2011.PubMed/NCBI View Article : Google Scholar | |
|
Sasi B, Ethiraj P, Myers J, Lin AP, Jiang S, Qiu Z, Holder KN and Aguiar RCT: Regulation of PD-L1 expression is a novel facet of cyclic-AMP-mediated immunosuppression. Leukemia. 35:1990–2001. 2021.PubMed/NCBI View Article : Google Scholar | |
|
Ugai T, Matsuo K, Sawada N, Iwasaki M, Yamaji T, Shimazu T, Goto A, Inoue M, Kanda Y, Tsugane S, et al: Coffee and green tea consumption and subsequent risk of acute myeloid leukemia and myelodysplastic syndromes in Japan. Int J Cancer. 142:1130–1138. 2018.PubMed/NCBI View Article : Google Scholar | |
|
Slominski RM, Raman C, Chen JY and Slominski AT: How cancer hijacks the body's homeostasis through the neuroendocrine system. Trends Neurosci. 46:263–275. 2023.PubMed/NCBI View Article : Google Scholar |