Kaempferol inhibits IL‑1β‑induced proliferation of rheumatoid arthritis synovial fibroblasts and the production of COX‑2, PGE2 and MMPs
- Authors:
- Published online on: August 9, 2013 https://doi.org/10.3892/ijmm.2013.1468
- Pages: 971-977
Abstract
Introduction
Rheumatoid arthritis (RA) is a chronic inflammatory systemic disease of unknown etiology characterized by chronic synovitis with subsequent articular bone and cartilage destruction (1,2). Chronic inflammation with hyperplasia of synovial lining cells, including synovial fibroblasts, are the histological characteristics of RA. When activated, rheumatoid arthritis synovial fibroblasts (RASFs) play a key role in the pathogenesis of RA synovitis through proliferation and resultant pannus formation. Inflammatory cytokines, matrix metalloproteinases (MMPs) and cyclooxygenase (COX)-2 released from RASFs are involved in the destruction of articular bone and cartilage (1,2). On the basis that interleukin (IL)-1β induces the proliferation of RASFs, resulting in the production of high levels of MMPs and prostaglandin E2 (PGE2), it has become a major target of biological therapy.
Flavonoids are natural polyphenols present in a wide variety of fruits and vegetables (3) and have a number of biological properties, such as antiviral (4), antitumor (5), antioxidant (6) and anti-inflammatory properties (7). Kaempferol (3,5,7,4′-tetrahydroxy flavone), which is found in tea, propolis and grapefruit, is one of the most common dietary flavonoids (8). It has been used as a traditional therapeutic for a number of inflammatory disorders. Previous studies have demonstrated that kaempferol reduces lipopolysaccharide-induced COX-2 levels in RAW 264.7 cells (9) and inhibits reactive oxygen species production through the inhibition of inducible nitric oxide synthase (iNOS) and tumor necrosis factor (TNF)-α protein expression in aged gingival tissues (10). Kaempferol has also exhibited anti-inflammatory effects through the inhibition of IL-4 (11), COX-2 and C-reactive protein (CRP) expression and the downregulation of nuclear factor-κB (NF-κB) in liver cells (12). Despite these anti-inflammatory effects of kaempferol and the critical role of RASFs in RA pathogenesis, to our knowledge, there are no studies to date on the effects of kaempferol on inflammatory reactions, including the production of MMPs, COX-2 and PGE2 by RASFs.
In the present study, we investigated the effects of kaempferol on the production of pro-inflammatory mediators, including MMPs, COX-2 and PGE2 produced by RASFs and the proliferation of these cells, following stimulation with IL-1β. Intracellular signaling factors were evaluated to elucidate the mechanisms behind the effects of kaempferol. We demonstrate that kaempferol inhibits the IL-1β-induced proliferation of RASFs and inflammatory reactions by inhibiting the activation of mitogen-activated protein kinases (MAPKs) and NF-κB pathways in RASFs.
Materials and methods
Reagents and antibodies
Recombinant human IL-1β was purchased from R&D Systems (Minneapolis, MN, USA) and kaempferol were obtained from Sigma-Aldrich, Inc. (St. Louis, MO, USA) and dissolved in DMSO with a concentration of 100 mM stock solution. Monoclonal antibodies (mAbs) against COX-2, MMP-1, MMP-3 and TIMP were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA). mAbs against NF-κB (p65), IkBα, ERK, p-ERK, JNK, p-JNK, p38, p-p38 and β-actin were purchased from Cell Signaling Technology (Beverly, MD, USA). Fetal bovine serum (FBS) was obtained from Gibco BRL/Life Technologies (Grand Island, NY, USA).
Isolation and culture of RASFs
Synovial tissues were obtained at the time of total knee arthroplasty from patients who fulfilled the American College of Rheumatology Criteria for RA (13), as previously described (14). Synovial tissue was digested for 2 h with 0.25% (w/v) collagenase and was then suspended in RPMI-1640 medium with 10% (v/v) FBS, 100 U/ml of penicillin and 100 μg/ml of streptomycin. The cells were incubated at 37°C in 5% CO2 for several days, after which the non-adherent cells were removed. Synovial fibroblasts from passages 3–7 were used for each experiment and were morphologically homogeneous and had the appearance of RASFs with typical fibroblastoid configuration under an inverse microscope. The purity of the cells was determined by flow cytometry using phycoerythrin (PE)-conjugated anti-Thy-1 (CD90) or anti-CD14 and fluorescein isothiocyanate (FITC)-conjugated anti-CD3 mAb (BD Pharmingen, San Diego, CA, USA). Informed consent was obtained from all patients, and the study protocol was approved by the Chonbuk National University Hospital Ethics Committee.
Cell viability analysis
Cell viability was determined by using the Cell Counting Kit-8 (CCK-8; Dojindo Laboratories, Japan) according to the manufacturer’s instructions. CCK-8 allows convenient assays using Dojindo’s tetrazolium salt, 2-(2- methoxy-4-nitrophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)- 2H-tetrazolium, monosodium salt (WST-8), which produces a water-soluble formazan dye upon bioreduction in the presence of an electron carrier, 1-methoxy phenazinium methylsulfate (PMS) (15). CCK-8 solution is added directly to the cells; no pre-mixing of the components is required. CCK-8 is a sensitive non-radioactive colorimetric assay for determining the number of viable cells in cell proliferation and cytotoxicity assays. WST-8 is bioreduced by cellular dehydrogenases to an orange formazan product that is soluble in tissue culture medium. The amount of formazan produced is directly proportional to the number of living cells. RASFs [1×105 cells/well in Dulbecco’s modified Eagle’s medium (DMEM) containing 10% (v/v) FCS in a 96-well U-bottom plate] were cultured in 200 μl medium/ well in the presence or absence of 1.0 ng/ml IL-1β and/or kaempferol (100 μM) for 2 days according to the results of a dose-dependent examination and a previous report (15), while the control RASFs were incubated in DMEM with DMSO. CCK-8 (20 μl) was added to each well of the plate and the cells were incubated for 2–3 h. The absorbance was measured at 450 nm using a microplate reader to determine the cell viability in each well.
Analysis of apoptosis
To evaluate the effects of kaempferol on the apotosis of RASFs, RASFs were incubated in DMEM for 24 h with kaempferol (100 μM), while the control RASFs were incubated in DMEM with DMSO. The cells were then trypsinized and collected for the detection of apoptosis with an Annexin V-FITC Apoptosis Detection kit according to the manufacturer’s instructions. Briefly, the cells were washed twice with cold PBS and resuspended in 500 ml of binding buffer (10 mM HEPES-NaOH pH 7.4, 140 mM NaCl, 2.5 mM CaCl2) at a concentration of 0.5×105 cells/ml. After the addition of 5 μl of Annexin V-FITC solution and propidium iodide (PI; 5 μl), the cells were incubated for 15 min at room temperature. The cells were analyzed using a flow cytometer (Beckman Coulter, Fullerton, CA, USA).
RNA isolation and semi-quantitative reverse transcription-polymerase chain reaction (RT-PCR( of COX, MMPs and TIMP
Total RNA was extracted from the cultured RASFs using TRIzol reagent (Invitrogen, Carlsbad, CA, USA) following the manufacturer’s instructions. RNA (1 μg) was reverse-transcribed using the Maxime RT Premix kit (iNtRON Biotechnology, Seoul, Korea). cDNA was amplified using the following primer sets: MMP-1 sense, 5′-GAA GGA GAT GAA GCA GCC CAG ATG T-3′ and antisense, 5′-CAG TTG TGG CCA GAA AAC AGA AGT GAA A-3′; MMP-3 sense, 5′-GAC ACC AGC ATG AAC CTT GTT-3′ and antisense, 5′-GGA ACC GAG TCA GGA CTA TG-3′; TIMP-1 sense, 5′-CCT TCT GCA ATT CCG ACC TCG TC-3′ and antisense, 5′-CGG GCA GGA TTC AGG CTA TCT GG-3′; COX-2 sense, 5′-TCC TTG CTG TTC CCA CCC ATG-3′ and antisense, 5′-CAT CAT CAG ACC AGG CAC CAG-3′; GAPDH sense, 5′-AAA TCA AGT GGG GCG ATG CT-3′ and antisense, 5′-AGC TTC CCG TTC AGC TCA GG-3′. PCR products were electrophoresed using 1% agarose gels and visualized by staining with ethidium bromide. Densitometric analysis was performed on the relative intensity of each band using the Multi Gauge software v3.0 (Fujifilm, Tokyo, Japan).
Immunoblotting
RASFs (1×106 cells) were seeded on 100-mm culture dishes and harvested in phosphate-buffered saline (PBS). After washing with PBS, cell pellets were lysed with the lysis buffer [20 mM HEPES, pH 7.2, 1% Triton X-100, 150 mM NaCl, 0.1 mM phenylmethylsulfonyl fluoride (PMSF), 1 mM EDTA and 1 μg/ml aprotinin]. Following incubation for 30 min at 4°C, cellular debris was removed by centrifugation at 100,000 × g for 30 min and the supernatants were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). To determine membrane COX-2 expression in RASFs, cell membranes were prepared from isolated RASFs as previously described (16). To determine NF-κB (p65) expression, nuclear extracts were prepared using a previously described method (14). To determine cytoplasmic IkBα expression, cytoplasmic extracts were prepared as previously described (14).
Protein concentration was determined using the Bio-Rad protein assay reagent (Bio-Rad Laboratories, Hercules, CA, USA). Samples (50 μg) were prepared with 4 vol of 0.5 M Tris buffer (pH 6.8) containing 4% SDS, 20% glycerol and 0.05% bromophenol blue at 95°C for 5 min. SDS-PAGE was performed on a 10% slab gel. Proteins were transferred onto a nitrocellulose membrane. The membrane was washed in blocking buffer (10 mM Tris-HCl pH 8.0, 150 mM NaCl, 5% fat-free milk) for 60 min at room temperature with shaking and then washed with TBST (TBS, 0.01% Tween-20). Primary antibodyies (10 μg/ml) against MMP-1, MMP-3, TIMP, COX-2, ERK, p-ERK-1/2, p-38, p-p38 MAPK, JNK, p-JNK, NF-κB (p65), IkBα and β-actin were then added followed by incubation at 4°C for 4 h. The secondary HRP-conjugated antibody was goat anti-mouse IgG (Santa Cruz Biotechnology, Inc.). Reactive proteins were detected by enhanced chemiluminescence (ECL; Amersham Life Sciences, Arlington Heights, IL, USA) using Fujifilm LAS-3000 (Fujifilm).
Assay of PGE2 production
RASFs (1×104 cells) were grown in 25 cm2 tissue-culture flasks for 48 h before treatment. After washing with PBS (pH 7.4), cells were pre-treated with IL-1β (1.0 ng/ml) or kaempferol (100 μM) at 37°C for 48 h in DMEM containing 10% (v/v) FCS in an atmosphere of 5% CO2, while the control RASFs were incubated in DMEM with DMSO. The culture supernatant described above was collected at 2 days. The level of PGE2 in the medium was determined by ELISA (R&D Systems) in accordance with the instructions of the manufacturer.
Statistical analysis
All data are expressed as the means ± SD of the results of 3 experiments with different RASFs and all data were analyzed using SPSS 12.0 software. Group mean values were compared using the Student’s t-test or ANOVA where appropriate. P-values <0.05 were considered to indicate statistically significant differences.
Results
Kaempferol inhibits the IL-1β-induced proliferation of RASFs
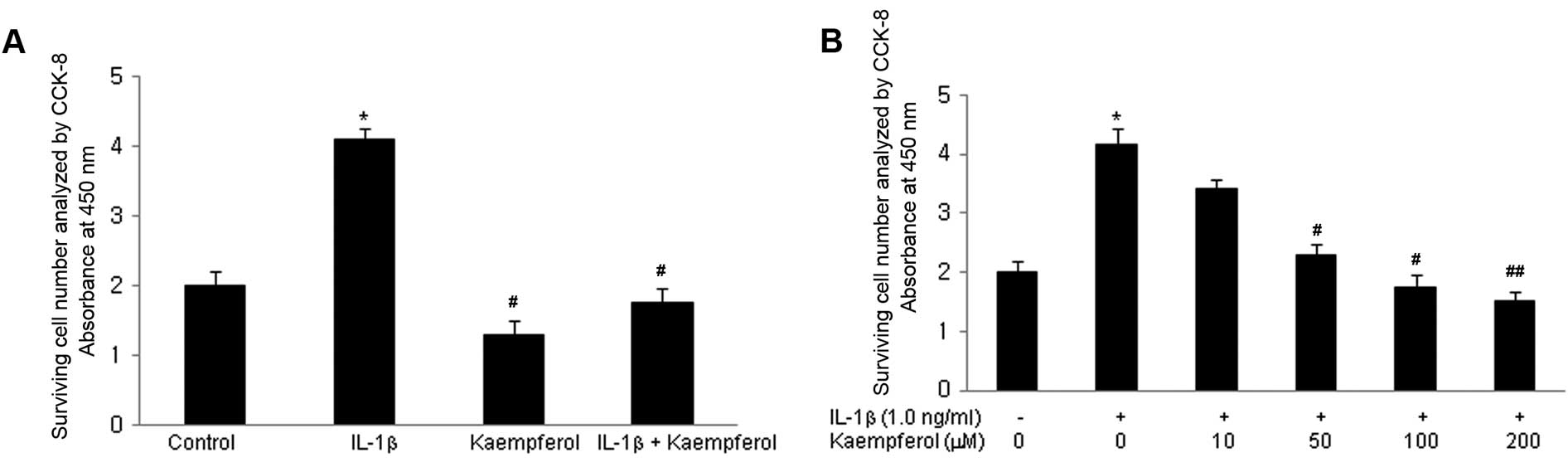
The effect of kaempferol on the growth ability of RASFs was initially evaluated. Cell proliferation was measured following treatment with IL-1β for 3 days; IL-1β is a well known potent growth-promoting factor for RASFs (17). Cell proliferation was assayed as described in Materials and methods. IL-1β increased the proliferation of RASFs in a dose-dependent manner (from 0.1 to 10 ng/ml). To examine the effects of kaempferol on the IL-1β-induced proliferation of RASFs, kaempferol (100 μM) was added to the RASF cultures with/without IL-1β (1.0 ng/ml) for 2 days and the CCK-8 assay was performed. IL-1β significantly increased the proliferation of RASFs compared with the control cells cultured in DMSO without IL-1β and kaempferol (P<0.05) (Fig. 1A). Kaempferol significantly inhibited the proliferation of RASFs treated with or without IL-1β (P<0.05). Various doses of kaempferol (10, 50, 100, 200 μM) were added to the RASF cultures with IL-1β (1.0 ng/ml) for 2 days and CCK-8 assay was performed. The inhibitory effects of kaempferol were significantly enhanced in a dose-dependant manner (Fig. 1B).
Kaempferol induces the apoptosis of RASFs
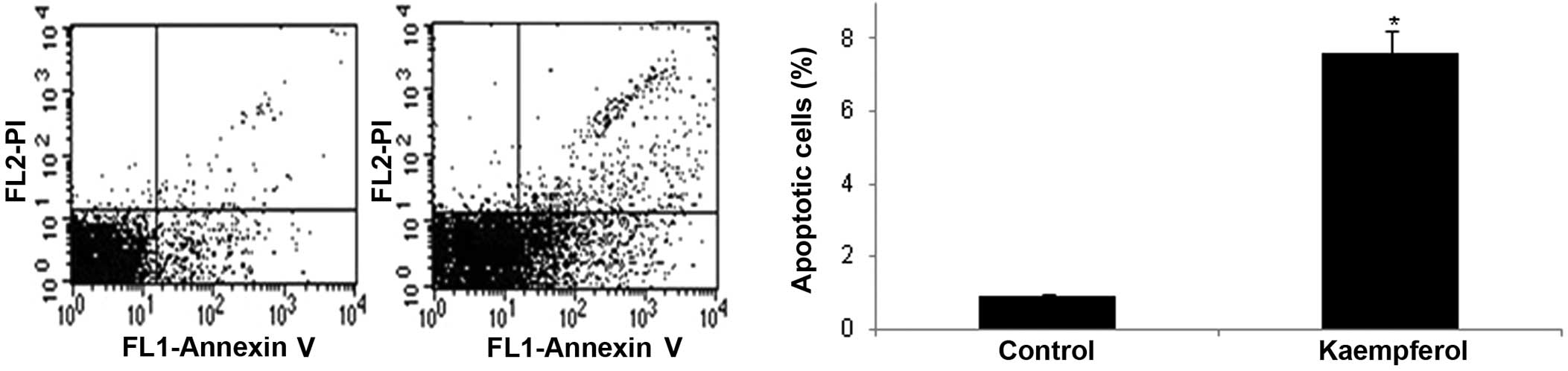
To elucidate the underlying mechanisms by which kaempferol inhibits the IL-1β-induced proliferation of RASFs, the effects of kaempferol on the apoptosis of RASFs were examined by flow cytometry and staining with Annexin V and PI. The percentage of Annexin V-positive cells was significantly increased in the RASFs treated with kaempferol compared with the cells cultured in DMEM with DMSO without kaempferol (P<0.05) (Fig. 2).
Effects of kaempferol on IL-1β-induced MMP, TIMP-1 and COX mRNA expression in RASFs
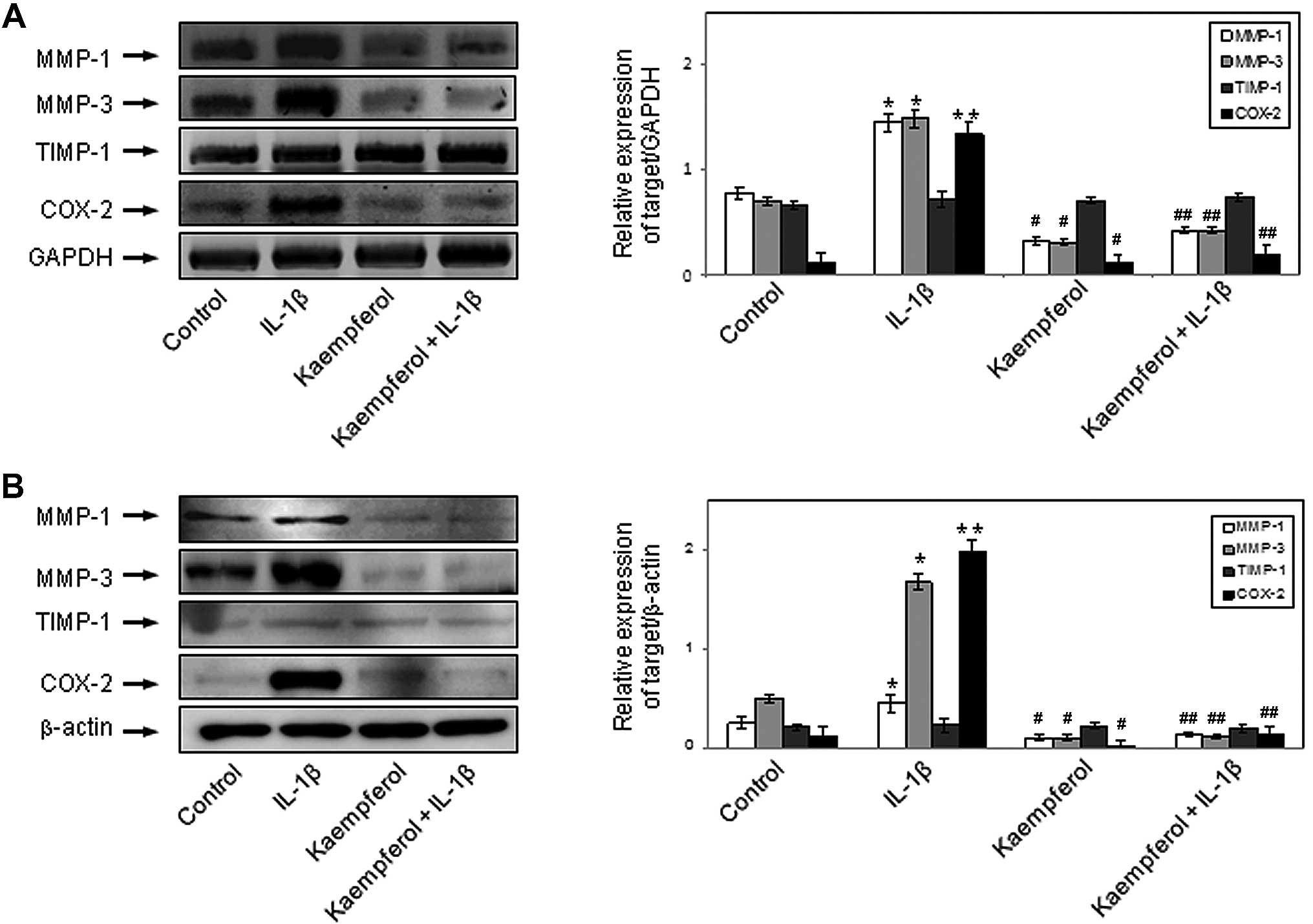
RT-PCR was performed to determine the mRNA expression of MMP-1, MMP-3 and TIMP-1 in the monocultured RASFs. RASFs were stimulated with IL-1β (1.0 ng/ml) for 48 h in the presence or absence of kaempferol (100 μM). IL-1β enhanced the mRNA expression of MMP-1 and MMP-3 in RASFs (P<0.05), but not that of TIMP-1. Kaempferol inhibited the effects of IL-1β on the mRNA expression of MMP-1 and MMP-3 (P<0.05) (Fig. 3A). IL-1β also enhanced the mRNA expression of COX-2 in RASFs (P<0.01), but not that of COX-1 (data not shown). Kaempferol inhibited the IL-1β-induced COX-2 mRNA expression (P<0.05) (Fig. 3A). Kaempferol also significantly decreased the mRNA expression of MMP-1, MMP-3 and COX-2 compared with the control cells cultured in DMSO without IL-1β and kaempferol (P<0.05).
Effects of kaempferol on IL-1β-induced MMP, TIMP-1 and COX protein expression in RASFs
To determine the protein expression of MMPs, TIMP-1 and COX in the monocultured RASFs, we performed western blot analysis. RASFs were stimulated with IL-1β (1.0 ng/ml) for 48 h in the presence or absence of kaempferol (100 μM). IL-1β enhanced the protein expression of MMP-1 and MMP-3 in RASFs (P<0.05), but not that of TIMP-1; its effects on protein expression were similar to those on mRNA expression. Kaempferol inhibited the IL-1β-induced protein expression of MMP-1 and MMP-3 (P<0.05) (Fig. 3B). IL-1β also enhanced the protein expression of COX-2 in RASFs (p < 0.01), but not that of COX-1 (data not shown). Kaempferol inhibited the IL-1β-induced protein expression of COX-2 (P<0.05) (Fig. 3B). Kaempferol also significantly decreased the protein expression of MMP-1, MMP-3 and COX-2 compared with the control cells cultured in DMSO without IL-1β and kaempferol (P<0.05).
Kaempferol inhibits IL-1β-induced PGE2 production in RASFs
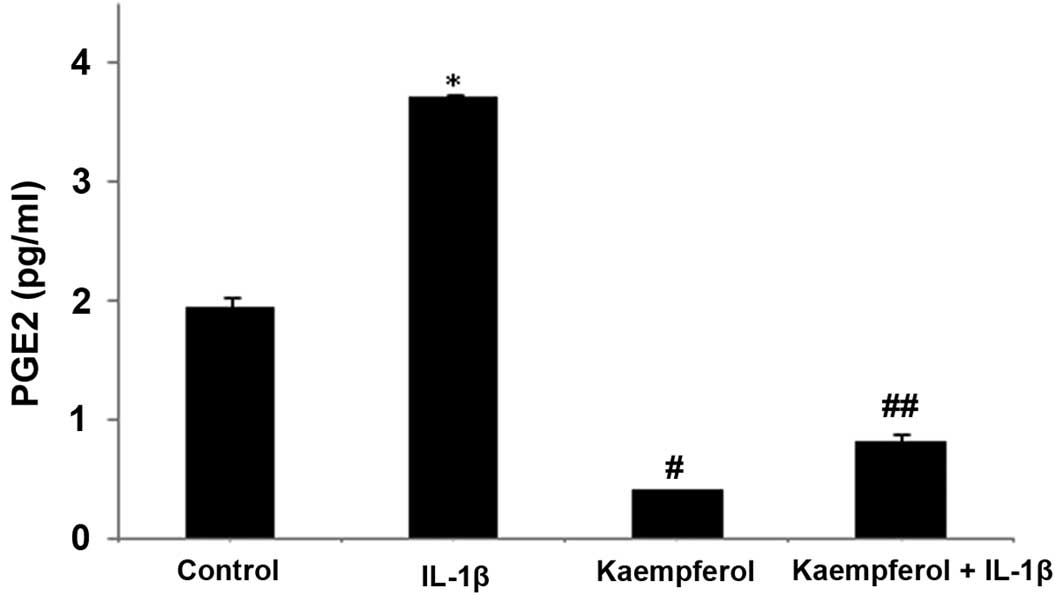
To confirm the effects of kaempferol on the IL-1β-induced production of PGE2 by RASFs, we examined the concentration of PGE2 in the culture supernatant. RASFs (1×104 cells) were grown in 25 cm2 tissue-culture flasks for 48 h before and after treatment with IL-1β (1.0 ng/ml) and/or kaempferol (100 μM). PGE2 production was increased following treatment with IL-1β (P<0.05) compared with the control cells cultured with DMSO without IL-1β, and it was significantly inhibited by treatment with kaempferol at 48 h (P<0.05) (Fig. 4); the effects of kaempferol on PGE2 were similar to those on COX-2 expression. Kaempferol also significantly decreased the production of PGE2 compared with the control cells cultured in DMSO without IL-1β and kaempferol (P<0.05) (Fig. 4).
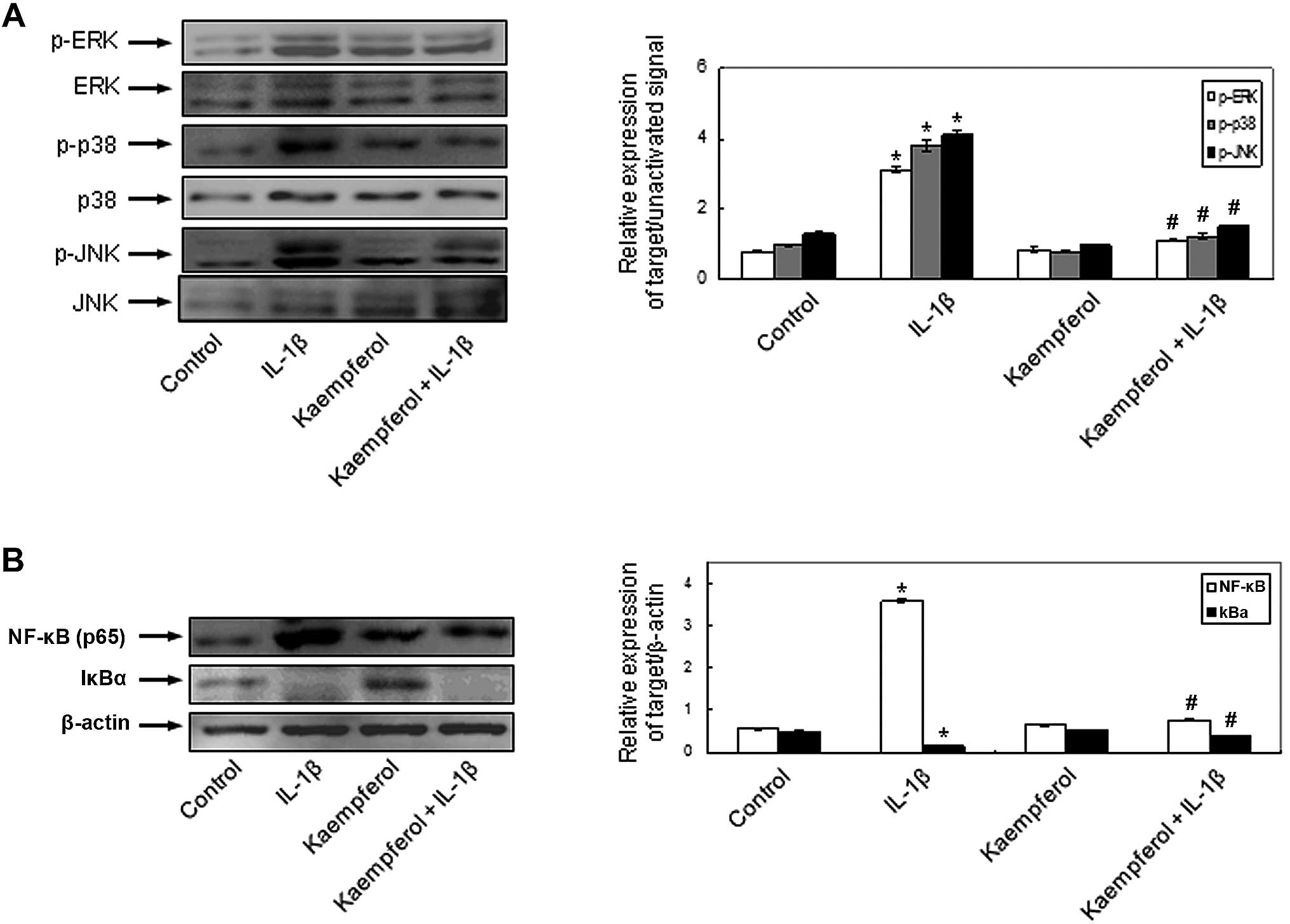
Effects of kaempferol on IL-1β-induced signaling pathways in RASFs
To demonstrate the involvement of the signal transduction pathways and to elucidate the mechanisms behind the effects of kaempferol on IL-1β-induced RASF proliferation and the production of MMPs, COX-2 and PGE2, the activation of MAPKs and NF-κB was evaluated in the RASFs. IL-1β phosphorylated intracellular MAPKs, including ERK, p-38 and JNK and kaempferol significantly inhibited the IL-1β-induced intracellular MAPK activation (Fig. 5A). The activation of NF-κB, p65 and the degradation of cytoplasmic IkBα was observed in the RASFs treated with IL-1β. The effects of IL-1β on NF-κB activation were abrogated by kaempferol (Fig. 5B). Kaempferol also inhibited the activation of the above intracellular signaling pathways compared with the control cells cultured in DMSO without IL-1β and kaempferol. These results indicate that kaempferol inhibits the IL-1β-induced proliferation of RASFs, the expression of COX-2 and the production of PGE2 by inhibiting the activation of intracellular MAPK and NF-κB pathways.
Discussion
In this study, we demonstrate that kaempferol induces the apoptosis of RASFs and inhibits the IL-1β-induced proliferation of RASFs. It inhibits the activation of the MAPK, ERK1/2, p-38 and JNK and NF-κB signaling pathways, resulting in the decreased expression of MMPs and COX-2, as well as in the decreased production of PGE2 by RASFs. These findings suggest that kaempferol may be used as a novel therapeutic agent for the management of RA by decreasing synovial inflammation.
Kaempferol, a polyphenolic flavonoid extracted from fenugreek seeds, has been shown to have strong antioxidant and anti-inflammatory properties (18). Previous studies have demonstrated that kaempferol reduces lipopolysaccharide-induced COX-2 levels in RAW 264.7 cells (9) and inhibits reactive oxygen species production through the inhibition of iNOS and TNF-α protein expression in aged gingival tissues (10). Kaempferol has also exhibited anti-inflammatory effects through the inhibition of IL-4 (11), COX-2 and CRP expression and the downregulation of NF-κB in liver cells (12). Compared with other daily dietary flavonols, kaempferol has been reported to be associated with a decreased risk of various types of cancer (19–21). However, to our knowledge, there are no reports to date on the effects of kaempferol on inflammatory reactions, including the production of MMPs, COX-2 and PGE2 by RASFs and the mechanisms involved, which play a crucial role in the pathogenesis of synovitis and articular destruction in RA.
In RA, one of the most striking features is the hyperplasia of synovial fibroblasts in the lining layer which is considered to be the main mechanism responsible for the hyperplasic growth of the RA synovium and eventually destroys articular bone and cartilage (22,23). Given that the IL-1β-induced proliferation of RASFs is closely involved in inflammatory synovitis joint destruction, the response of RASFs to IL-1β plays a crucial role in the physiopathology of RA (24). Our study demonstrated that kaempferol significantly induced the apoptosis of RASFs and inhibited the proliferation of unstimulated and IL-1β-stimulated of RASFs in a dose- and time-dependent manner.
Pro-inflammatory cytokines, including IL-1β enhance the expression of COX-2 and MMPs in human RASFs (25,26). MMPs are involved in the destruction of the extracellular matrix in articular structures and COX-2 converts free arachidonic acid into prostaglandins, including a variety of bio-active compounds [prostacyclin (PGI2), thromboxane A2 (TXA2), PGE2 and prostaglandin D2 (PGD2)]. PGE2, a pleiotropic mediator of inflammation, is involved in several pathological processes and plays a critical role in eliciting the signs and symptoms of inflammation in the joints of RA patients when produced in excess (27). We also found that kaempferol inhibits the expression of MMPs and COX-2 and PGE2 synthesis in a dose-dependent manner in both unstimulated and IL-1β-stimulated RASFs. This suggests that kaempferol may be a potent therapeutic compound for RA. However, further studies are required to investigate the overall effects of kaempferol on the pathophysiology of synovitis in in vivo systems, such as animal models of RA and collagen-induced arthritis (CIA) and to elucidate the underlying mechanisms.
NF-κB and MAPKs participate in the pathogenic mechanisms of inflammation and the destruction of joints in RA. It is known that NF-κB, JNK, p-38 and ERK are expressed in cultured RASFs and are readily activated by IL-1β and TNF-α (13,28,29). Numerous studies have demonstrated that inhibitors of MAPKs or NF-κB decrease synovial inflammation, bone destruction and cartilage damage in animal models of arthritis, including adjuvant arthritis in rats and CIA in mice (30,31). It has been described that kaempferol suppresses IL-1β-induced inflammatory responses by regulating signaling pathways, including NF-κB activation and MAPK phosphorylation in human airway epithelial cells (32). In human synovial cells, the addition of kaempferol has been shown to suppress the TNF-α-induced increase in the mRNA expression of IL-8 and monocyte chemotactic protein-1 (MCP-1) in a dose-dependent manner by inhibiting the activation of NF-κB induced by TNF-α (33). In this study, to elucidate the mechanisms behind the effects of kaempferol on IL-1β-induced RASF proliferation, the expression of COX-2 and the production of PGE2, as well as the activation of MAPKs and NF-κB were examined. Our results revealed that kaempferol inhibited the IL-1β-induced activation of NF-κB and the phosphorylation of ERK, p-38 and JNK. However, further investigations are required to elucidate the mechanisms by which kaempferol suppresses NF-κB activation, which components of NF-κB are suppressed, and which intracellular signaling factors are more specifically or directly involved in the effects of kaempferol on the proliferation of RASFs and PGE2 production.
To our knowledge, this is the first study to report that kaempferol induces the apoptosis of RASFs and inhibits the IL-1β-induced proliferation of RASFs. It also suppresses the expression of MMP-1, MMP-3 and COX-2 and the production of PGE2 by RASFs by inhibiting the IL-β-induced activation of NF-κB and the phosphorylation of the MAPK pathways, p-38, JNK and ERK. Based on our findings, kaempferol has great potential as a novel therapeutic agent and may prove useful in the treatment of inflammatory diseases, including RA. However, further studies are required to elucidate the exact mechanisms underlying the inhibitory effects of kaempferol on synovial cell proliferation and inflammatory reactions.
Acknowledgements
The present study was supported by a grant from the Korea Healthcare technology R&D Project, Ministry for Health, Welfare and Family Affairs, Korea (A084144).
References
|
Henderson B, Hardinham T, Blake S and Lewthwaite J: Experimental arthritis models in the study of the mechanisms of articular cartilage loss in rheumatoid arthritis. Agents Actions Suppl. 39:15–26. 1993.PubMed/NCBI | |
|
Han MK, Kim JS, Park BH, et al: NF-kappaB-dependent lymphocyte hyperadhesiveness to synovial fibroblasts by hypoxia and reoxygenation: potential role in rheumatoid arthritis. J Leukoc Biol. 73:525–529. 2003. View Article : Google Scholar : PubMed/NCBI | |
|
Bosetti C, Rossi M, McLaughlin JK, et al: Flavonoids and the risk of renal cell carcinoma. Cancer Epidemiol Biomarkers Prev. 16:98–101. 2007. View Article : Google Scholar : PubMed/NCBI | |
|
Guo Q, Zhao L, You Q, et al: Anti-hepatitis B virus activity of wogonin in vitro and in vivo. Antiviral Res. 74:16–24. 2007. View Article : Google Scholar : PubMed/NCBI | |
|
Cárdenas M, Marder M, Blank VC and Roquin LP: Antitumor activity of some natural flavonoids and synthetic derivatives on various human and murine cancer cell lines. Bioorg Med Chem. 14:2966–2971. 2006.PubMed/NCBI | |
|
Burda S and Oleszek W: Antioxidant and antiradical activities of flavonoids. J Agric Food Chem. 49:2774–2779. 2001. View Article : Google Scholar : PubMed/NCBI | |
|
González-Gallego J, Sánchez-Campos S and Tuñón MJ: Anti-inflammatory properties of dietary flavonoids. Nutr Hosp. 22:287–293. 2007. | |
|
Olszewska M: Separation of quercetin, sexangularetin, kaempferol and isorhamnetin for simultaneous HPLC determination of flavonoid aglycones in inflorescences, leaves and fruits of three Sorbus species. J Pharm Biomed Anal. 48:629–635. 2008. View Article : Google Scholar : PubMed/NCBI | |
|
Kim SK, Kim HJ, Choi SE, Park KH, Choi HK and Lee MW: Anti-oxidative and inhibitory activities on nitric oxide (NO) and prostaglandin E2 (COX-2) production of flavonoids from seeds of Prunus tomentosa Thunberg. Arch Pharm Res. 31:424–428. 2008. View Article : Google Scholar : PubMed/NCBI | |
|
Kim HK, Park HR, Lee JS, Chung TS, Chung HY and Chung J: Down-regulation of iNOS and TNF-alpha expression by kaempferol via NF-kappaB inactivation in aged rat gingival tissues. Biogerontology. 8:399–408. 2007. View Article : Google Scholar : PubMed/NCBI | |
|
Cortes JR, Perez-G M, Rivas MD and Zamorano J: Kaempferol inhibits IL-4-induced STAT6 activation by specifically targeting JAK3. J Immunol. 179:3881–3887. 2007. View Article : Google Scholar : PubMed/NCBI | |
|
García-Mediavilla V, Crespo I, Collado PS, Esteller A, Sánchez-Campos S, Tuñón MJ and González-Gallego J: The anti-inflammatory flavones quercetin and kaempferol cause inhibition of inducible nitric oxide synthase, cyclooxygenase-2 and reactive C-protein, and down-regulation of the nuclear factor kappaB pathway in Chang Liver cells. Eur J Pharmacol. 557:221–229. 2007. | |
|
Arnett FC, Edworthy SM, Bloch DA, et al: The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 31:315–324. 1988. View Article : Google Scholar : PubMed/NCBI | |
|
Lee HY, Jeon HS, Song EK, et al: CD40 ligation of rheumatoid synovial fibroblasts regulates RANKL-mediated osteoclastogenesis: evidence of NF-kappaB-dependent, CD40-mediated bone destruction in rheumatoid arthritis. Arthritis Rheum. 54:1747–1758. 2006. View Article : Google Scholar | |
|
Comalada M, Camuesco D, Sierra S, Ballester I, Xaus J, Gálvez J and Zarzuelo A: In vivo quercitrin anti-inflammatory effect involves release of quercetin, which inhibits inflammation through down-regulation of the NF-kappaB pathway. Eur J Immunol. 35:584–592. 2005. View Article : Google Scholar | |
|
Hodgkin PD, Yamashita LC, Coffman RL and Kehry MR: Separation of events mediating B cell proliferation and Ig production by using T cell membranes and lymphokines. J mmunol. 145:2025–2034. 1990.PubMed/NCBI | |
|
Havsteen BH: The biochemistry and medical significance of the flavonoids. Pharmacol Ther. 96:67–202. 2002. View Article : Google Scholar : PubMed/NCBI | |
|
Noh EM, Kim JS, Hur H, et al: Cordycepin inhibits IL-1beta-induced MMP-1 and MMP-3 expression in rheumatoid arthritis synovial fibroblasts. Rheumatology (Oxford). 48:45–48. 2009. View Article : Google Scholar : PubMed/NCBI | |
|
Gates MA, Tworoger SS, Hecht JL, De Vivo I, Rosner B and Hankinson SE: A prospective study of dietary flavonoid intake and incidence of epithelial ovarian cancer. Int J Cancer. 121:2225–2232. 2007. View Article : Google Scholar : PubMed/NCBI | |
|
Nöthlings U, Murphy SP, Wilkens LR, Henderson BE and Kolonel LN: Flavonols and pancreatic cancer risk: the multiethnic cohort study. Am J Epidemiol. 166:924–931. 2007.PubMed/NCBI | |
|
Garcia-Closas R, Gonzalez CA, Aqudo A and Riboli E: Intake of specific carotenoids and flavonoids and the risk of gastric cancer in Spain. Cancer Causes Control. 10:71–75. 1999. View Article : Google Scholar : PubMed/NCBI | |
|
Komatsu N and Takayanagi H: Autoimmune arthritis: the interface between the immune system and joints. Adv Immunol. 115:45–71. 2012. View Article : Google Scholar : PubMed/NCBI | |
|
Choy EH and Panayi GS: Cytokine pathways and joint inflammation in rheumatoid arthritis. N Engl J Med. 344:907–916. 2001. View Article : Google Scholar : PubMed/NCBI | |
|
Gitter BD, Labus JM, Lees SL and Scheetz ME: Characteristics of human synovial fibroblast activation by IL-1 beta and TNF alpha. Immunology. 66:196–200. 1989.PubMed/NCBI | |
|
Crofford LJ, Wilder RL, Ristimaki AP, Sano H, Remmers EF, Epps HR and Hla T: Cyclooxygenase-1 and -2 expression in rheumatoid synovial tissues. Effects of interleukin-1 beta, phorbol ester, and corticosteroids. J Clin Invest. 93:1095–1101. 1994. View Article : Google Scholar : PubMed/NCBI | |
|
Tolboom TC, Pieterman E, van der Laan WH, et al: Invasive properties of fibroblast-like synoviocytes: correlation with growth characteristics and expression of MMP-1, MMP-3, and MMP-10. Ann Rheum Dis. 61:975–980. 2002. View Article : Google Scholar : PubMed/NCBI | |
|
Martel-Pelletier J, Pelletier JP and Fahmi H: New insights into prostaglandin biology. J Rheumatol. 31:14–16. 2004.PubMed/NCBI | |
|
Verma IM, Stevenson JK, Schwartz EM, Van Antrerp D and Mitamoto S: Rel/NF-kappa B/I kappa B family: intimate tales of association and dissociation. Genes Dev. 9:2723–2735. 1995. View Article : Google Scholar : PubMed/NCBI | |
|
Han Z, Boyle DL, Chang L, et al: c-Jun N-terminal kinase is required for metalloproteinase expression and joint destruction in inflammatory arthritis. J Clin Invest. 108:73–81. 2001. View Article : Google Scholar : PubMed/NCBI | |
|
McIntyre KW, Shuster DJ, Gillooly KM, et al: A highly selective inhibitor of I kappa B kinase, BMS-345541, blocks both joint inflammation and destruction in collagen-induced arthritis in mice. Arthritis Rheum. 48:2652–2659. 2003. View Article : Google Scholar : PubMed/NCBI | |
|
Nishikawa M, Myoui A, Tomita T, Takahi K, Nampei A and Yoshikawa H: Prevention of the onset and progression of collagen-induced arthritis in rats by the potent p38 mitogen-activated protein kinase inhibitor FR167653. Arthritis Rheum. 48:2670–2681. 2003. View Article : Google Scholar : PubMed/NCBI | |
|
Kwon SH, Nam JI, Kim SH, Kim JH, Yoon JH and Kim KS: Kaempferol and quercetin, essential ingredients in Ginkgo biloba extract, inhibit interleukin-1beta-induced MUC5AC gene expression in human airway epithelial cells. Phytother Res. 23:1708–1712. 2009. View Article : Google Scholar | |
|
Kuhns DB, Priel DA and Gallin JI: Induction of human monocyte interleukin (IL)-8 by fibrinogen through the toll-like receptor pathway. Inflammation. 30:178–188. 2007. View Article : Google Scholar : PubMed/NCBI |