The impact of diet upon mitochondrial physiology (Review)
- Authors:
- Published online on: September 16, 2022 https://doi.org/10.3892/ijmm.2022.5191
- Article Number: 135
-
Copyright: © Kyriazis et al. This is an open access article distributed under the terms of Creative Commons Attribution License.
Abstract
1. Mitochondrial physiology
Mitochondrial biogenesis
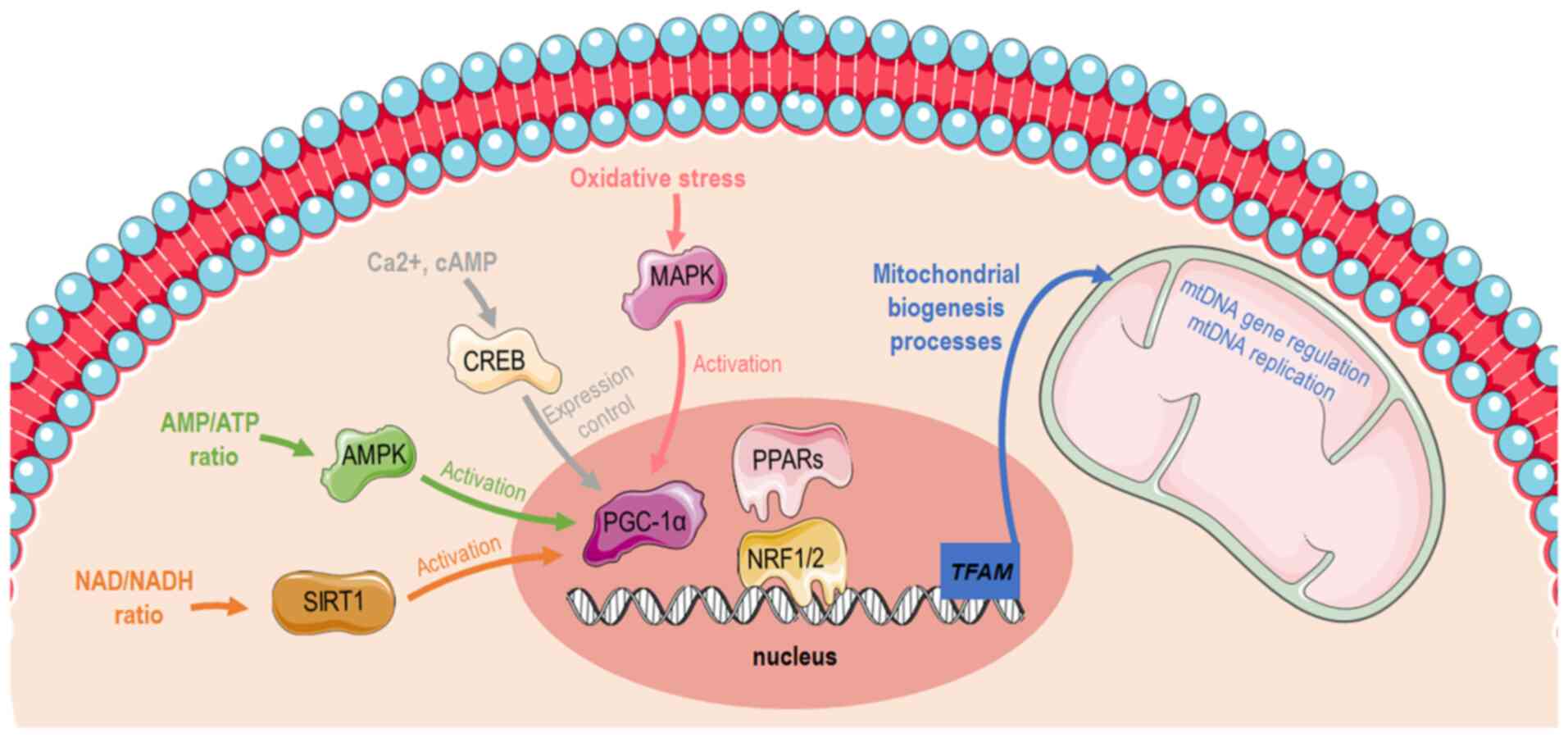
The biogenesis of mitochondria refers to the process through which mitochondria grow in number and/or size. This is mediated by physiological stimuli, including physical exercise, dietary restrictions and temperature (1). Mitochondrial biogenesis (Fig. 1) is transcriptionally controlled through the activation of peroxisome proliferator-activated receptor-gamma coactivator 1α (PGC-1α) (2). PGC-1α has specific tissue distribution and is mainly located in tissues with high energy requirements or high oxidative activity, such as the heart, skeletal muscle, liver and white or brown adipose tissue, suggesting that it is closely related to the energy metabolism of the body (3). The Ppargc1a gene structure contains a well-conserved binding site for a cAMP response element-binding protein (CREB) that allows activated CREB to bind and promote PGC-1α expression (4,5). Furthermore, PGC-1α can be activated by reduced adenosine triphosphate (ATP)/adenosine monophosphate (AMP) levels mediated by AMP-activated protein kinase (AMPK) that functions as a cellular energy sensor (1). Thus, activated PGC-1α leads to the consecutive stimulation of several transcription factors, including nuclear respiratory factors (NRFs)1 and 2, that promote the expression of nuclear genes that are responsible for controlling transcriptionally the majority of the subunits of mitochondrial complexes (6,7) and peroxisome proliferator-activated receptors (PPARs) (8). Furthermore, PGC-1α can promote oxidative phosphorylation (OXPHOS) gene expression, which encodes proteins that constitute the electron transport chain (ETC) and are responsible for ATP synthesis. Last but not least, PGC-1α cooperates with PPARα to regulate the expression of mitochondrial fatty acid oxidation (FAO) enzymes and transport proteins, enabling increases in FAO pathway activity in coordination with mitochondrial biogenesis (9). The aforementioned findings indicate that PGC-1a masters cellular mechanisms related to substrate utilization (fatty acids, glucose) and their intra-mitochondrial oxidation to produce energy for cellular demands (10,11).
There is an ample amount of available literature on the molecular pathways that induce mitochondrial biogenesis and function during conditions of high energy demands, such as exercise. One bout of acute exercise in the muscle is sufficient to initiate transcriptional signaling toward mitochondrial biogenesis. More elaborately, exercise increases intracellular calcium levels, allowing the calcium/calmodulin-dependent protein kinase IV-dependent phosphorylation and the subsequent activation of CREB (12,13). As previously described, PGC-1α is induced in the skeletal muscle by AMPK when ATP levels are low (10,14). In the liver, the hormonal and nutritional regulation of hepatic gluconeogenesis occurs mainly through the modulation of the transcriptional coactivator PGC-1α. During glucose deprivation, cells sensitize the need for additional substrates and thus stimulate the gluconeogenesis program and mitochondrial biogenesis through CREB and PGC-1a in order to supply mitochondria with new glucose molecules and build more available 'factories' in order to generate sufficient amounts of energy. Therefore, gluconeogenesis and mitochondrial biogenesis are tightly coupled to allow hepatocytes to adapt in low available glucose levels. Sirtuin (SIRT)1 protein expression is induced in the liver through a nutrient-signaling response mediated by pyruvate kinase. SIRT1 then activates forkhead box protein O1 (FOXO1) (15) and PGC-1α through their deacetylation (16). Subsequently, FOXO1 and PGC1a co-orchestrate the gluconeogenesis program, enabling organism energetic stability through glucose utilization (15).
Mitochondrial dynamics: Fusion-fission
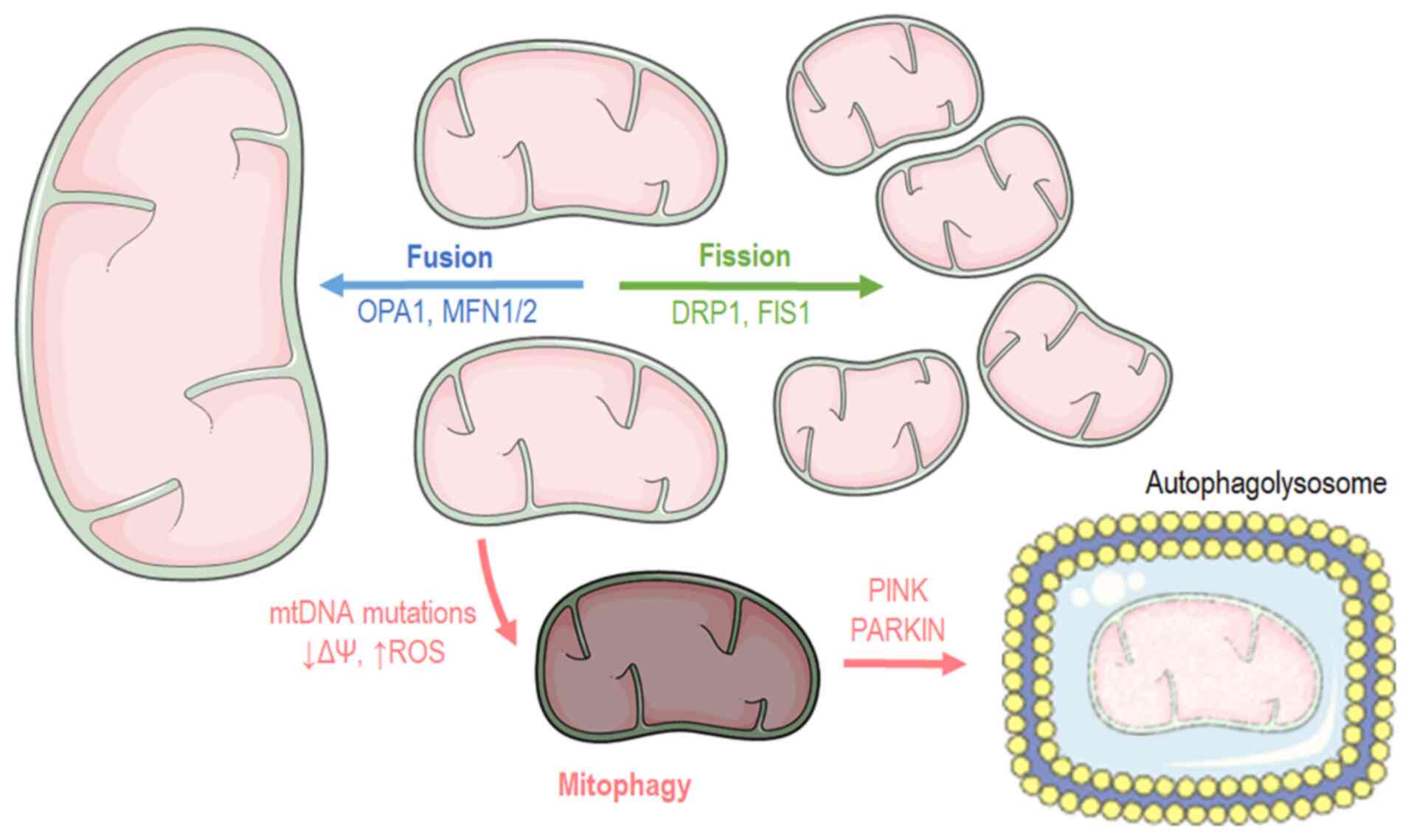
Cells have distinct mechanisms depending on their cycle status or their state to maintain mitochondrial morphology and proper function. Mitochondria carry out two processes, fusion and fission (Fig. 2), which are of paramount importance for self-controlling organelle quality (17,18). Mitochondrial fusion occurs when nearby mitochondria merge, while mitochondrial fission takes place when a mitochondrion needs to break apart into two separate organelles. Mitochondrial fusion occurs during the early stages of the S and G1 phases (19), ensuring mainly three main functions: Respiration, ATP production and intramitochondrial material exchange (20). At the G-S1 cellular phase, mitochondria shape a giant, hyper-fused network characterized by higher ATP production capacity (19). Additionally, it has been found that fusion contributes to mitochondrial repair and elongation while the substrates of the fused mitochondrial are optimally exploited for respiration. At the structural level, mitochondrial fusion is accomplished when both the inner mitochondrial membrane (IMM) and outer mitochondrial membrane (OMM) of the fused organelles merge, respectively (20), while at a molecular level, three different proteins are responsible for the structural fusion: In the OMM, both mitofusins 1 and 2 (MFN1 and MFN2), while in the IMM, optic atrophy 1 (OPA1) (18).
In contrast to fusion, the mitochondrial fission process usually occurs during the S, G2 and M phases due to the need for the even separation of organelles that will be present in offspring cells (21,22). Fission is beneficial when mitochondria are dysfunctional. Mitochondria divide and stimulate the authophagy pathway (mitophagy) (23). Briefly, mitochondrial fission begins when the endoplasmic reticulum (ER) is recruited and stimulates mtDNA replication. Fission is orchestrated by the dynamin-related protein 1 (DRP1), which is a GTPase that is recruited in the mitochondrial surface and anchored by complexes that are constructed from different mitochondrial proteins, such as mitochondrial fission 1 (FIS1), mitochondrial fission factor and mitochondrial dynamics protein 49 and 51 (MiD49 and MiD51) (18).
Mitophagy
Macroautophagy is a genetically programmed and conserved catabolic process (24) in which cytosol portions and/or complete organelles are engulfed by double-membrane structures known as autophagosomes, that fuse with lysosomes in order to form single membrane structures known as autophagolysosomes (25). Mitophagy is the macroautophagy process through which the mitochondria are driven towards degradation (Fig. 2) (26,27). Mitophagy also constitutes a mitochondrial quality control mechanism, preventing mitochondrial dysfunction, a hallmark of cellular aging (28). Several autophagy-related genes (ATGs) regulate autophagy or autophagy-related process, transcriptional control that is evolutionary preserved since 30 ATGs have also been described in yeasts (29,30). The recognition of dysfunctional mitochondria is mediated by the p62 and PARKIN proteins. PARKIN is an ubiquitin ligase that translocates into the mitochondria and tags them in order to become processed for degradation. Another protein, the mitochondrial receptor NIX, binds the cytoskeleton related protein, such as like gamma-aminobutyric acid type A receptor-associated protein (GABARAP) and microtubule-associated protein 1 light chain 3 (LC3, Atg8) (31), driving mitochondria towards apoptosis since it regulates PARKIN translocation into mitochondria (32). On the onset of Parkin-mediated mitophagy, PARKIN interacts with PTEN-induced kinase 1 (PINK1) in order to finalize dysfunctional mitochondria labeling (33). Subsequently, ATG9a and the ULK1/2 can depolarize mitochondria, a biochemical manifestation that is responsible for the recruitment of additional downstream autophagy-related proteins (ATG) apart from LC3. Finally, LC3 recruitment drives mitochondria into the autophagosome for decomposition (34). Another molecular pathway that stimulates mitophagy includes the transcriptional factor, FoxO3, which regulates autophagy through Bcl-2 inter-acting protein 3 (BNIP3) (35). BNIP3 belongs to the BH3-only proteins of the Bcl-2 family and can induce cellular apoptosis and mitophagy (36). Mitophagy pathways are closely aligned with those of mitochondrial dynamics. As such, mitochondrial fission appears to be the first step and a pre-requisite of the mitophagy process (37).
Mitochondrial function
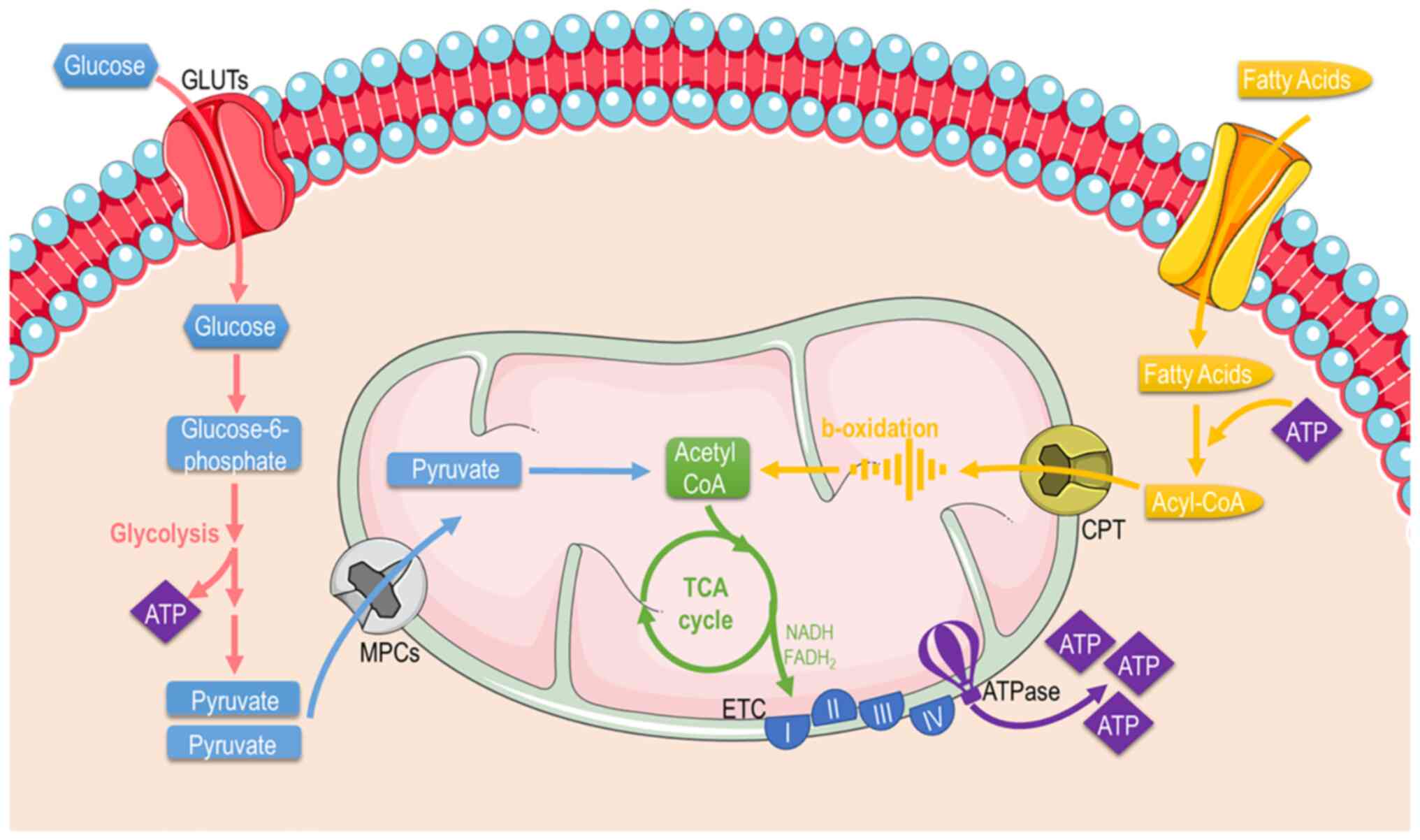
Mitochondria are mainly considered the cellular 'powerplants' with their foremost function to supply cells with energy through producing the energetic 'coin' ATP. Apart from energy metabolism, the mitochondria contribute to different aspects of cellular biology, such as signaling, differentiation, cell cycle, growth and cellular death (38). Thereafter, mitochondrial and cellular metabolism and are tightly coupled. Mitochondria possess specific metabolic lines to process different substrates and generate ATP (Fig. 3). These include pathways that pyruvate (derived from glucose or lactate), fatty acids, and amino acids are eventually oxidized to gear protons onto NADH and FADH2. NADH and FADH2 carry these electrons to the ETC, forming an electrochemical gradient that drives ATP-synthetase to produce ATP through oxidative phosphorylation.
Fuel metabolism
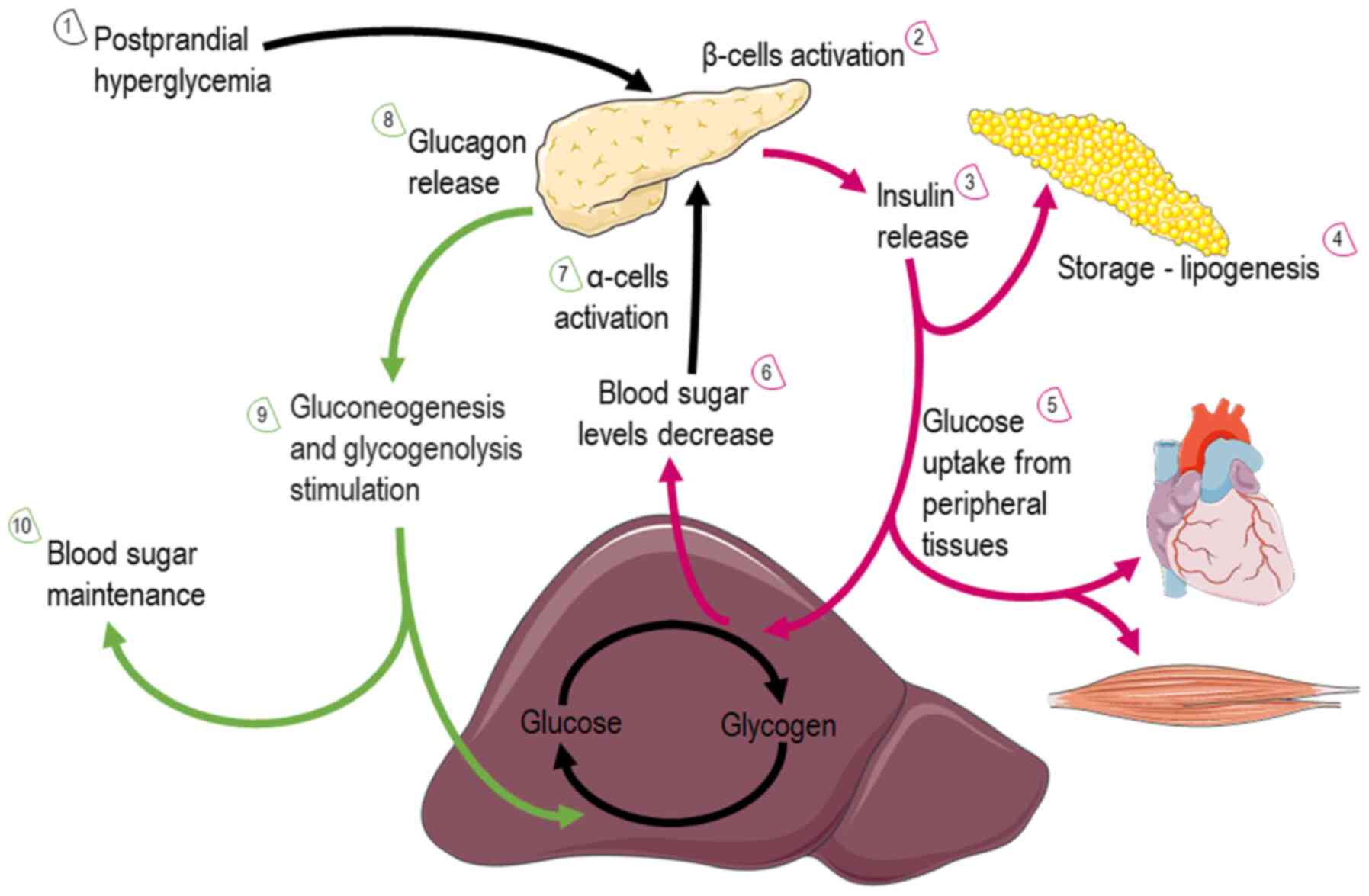
Glucose: Glucose is almost continuously cellular available through facilitated diffusion conducted by several isotypes of glucose transporters present in different cell types (39). Glucose levels are elevated in the circulation after feeding or through hepatic gluconeogenesis (Fig. 4); hepatic release during fasting, allows tissues to uptake glucose and generate pyruvate through glycolysis, producing an initially low number of ATP molecules. Subsequently, mitochondria can utilize glucose through the form of pyruvate in order to produce ATP. Depending on nutrient and oxygen availability, glucose is converted into pyruvate or lactate through pyruvate and lactate dehydrogenase, respectively. Under physiological conditions, pyruvate is transported across the double-mitochondrial membrane via mitochondrial pyruvate carrier (MPC) (40). Oxygen deprivation mitigates oxidative phosphorylation, and net energy is produced through anaerobic glycolysis and lactate generation as the end-product. Lactate can be converted back to pyruvate and glucose in hepatocytes through a different type of lactate dehydrogenase during oxygen abundancy, resulting though a net negative balance of ATP (41). Therefore, the Cori cycle cannot be maintained relentlessly. On the other hand, the inhibition of MPC proteins that block pyruvate shuttling inside the mitochondria, forces the mitochondria into a metabolic reprogram and to depend mainly on fatty acids and glutamine. The glutaminolysis pathway allows glutamine to be oxidized in the mitochondria and stimulates the Krebs cycle, producing a-ketoglutarate or pyruvate via malic enzymes (42). Surprisingly, at high glucose levels, cells lose their ability to utilize the excessive pyruvate that is available, leading to cellular glucotoxicity through the activation of polyol pathway, protein kinase C, increased advanced glycation end-products, and hexosamine pathway flux (43). Thereafter, although the hypothesis that excessive glucose facilitates higher energy production, the mitochondria are actually vulnerable in toxic intermediates that glucotoxicity is generating, resulting into mitophagy and cellular death (44). This indicate that glucose homeostasis is crucial for the proper functioning of the mitochondria and cells.
Fatty acids (FAs): FAs are the main metabolic substrates for the mitochondria of cardiac and skeletal muscle in order to suffice their energy demands (45). FAs derive from the white adipose tissue in the form of albumin-bound FAs or via the lipoprotein lipase-dependent degradation of very-low-density lipoprotein (46). FA intracellular uptake is facilitated through different carriers and proteins, such as fatty acid transporter protein 1, plasma membrane-associated fatty acid-binding protein, and long-chain fatty acid transporter and fatty acid translocase CD36 (FAT/CD36). Subsequently, FAs enter the mitochondria or peroxisomes (they process long-chain fatty acids and branched-chain fatty acids) through CPT1 and ABCD1 and are catabolized (46) through β- and α-oxidation, supplying the mitochondria with fuel substrates (47). The β-oxidation rate and the levels of its main product, acetyl-CoA, dictate the energy cellular demand, since the lack of ATP to cover increased cellular needs results in the enhanced tricarboxylic acid (TCA) cycle activity OXPHOS. Similarly, NADH and acetyl-CoA decrease leads to β-oxidation flux stimulation (48). On the other hand, the level of CPT1 activity significantly determines the β-oxidation rate in cardiac or skeletal muscle (49). As aforementioned, the end-products of β-oxidation are acetyl-CoA, which enters the TCA cycle, and NADH and FADH2, that are required for the proper flow of electrons in the ETC, providing the necessary gradient for F-ATPase to produce ATP energy molecular coins (50).
2. Diets
High-fat diet (HFD)
A HFD was the mainstream diet followed as a dietary habit in the 1980s, even though an introductory study that focused on its effects on health was assessed back in 1958 from Ancel Keys and is termed the Seven Countries Study (51), indicating that this dietary habit had already been incriminated. HFD is referred to a diet in which at least 30-35% of the amount of total calories are derived from both unsaturated and saturated fats (52) (https://health.gov/our-work/food-nutrition/previous-dietary-guidelines/1980). In addition to the popular processed foods, numerous other foods have a high fat content, including but not limited to animal fat, chocolate, butter and oily fish. Commonly higher in fat content, the majority of processed foods are easier to obtain as they are normally more economical considering socioeconomical factors, such as a lower family income. A number of dishes among different cultures and ethnicities, such as fried foods or 'soul food' contain ingredients with a high fat content, such as oils, butters and fats to increase flavor and appeal. A HFD is not a common everyday diet for humans, but rather an experimental protocol with which to create a disease model in animals and mimic the metabolic adaptations that this creates in cellular physiology (53,54). More factors, such genetic and environmental factors can contribute to obesity, generating a more perplexed field that warrants further attention.
HFD leads to obesity and is responsible for the induction of insulin resistance, which is one of the most critical pathophysiological manifestations (55). This pathophysiology is emerging since the capacity of non-adipose tissues for lipid storage is met, and additionally, lipids cause lipotoxicity that affects cellular function and cell fate. In white adipose tissue, excessive energy intake causes tissue hypertrophy and the hyperplasia of adipocytes (56). The latter manifestations stimulate lipolysis in fat cells and ultimately lead to elevated circulating levels of free fatty acids (57). As aforementioned, an increase in FA oxidation in the mitochondria is responsible for elevating lipid catabolism and energy production, through β-oxidation and Krebs cycle respectively (58). Adiponectin, leptin, acylation stimulating protein and resistin, which are hormones that are secreted by adipocytes play a critical role in regulating mitochondrial biogenesis and insulin sensitivity (59-62). During HFD consumption, adiponectin levels are decreased, leading to the diminished activation of the cellular pathways that usually stimulate. More specifically, adiponectin physiologically binds to its receptors, AdipoR1 (abundantly expressed in skeletal muscle and AdipoR2, activating AMPK, which finally leads to the stimulation of glucose uptake and FA oxidation (63). AMPK has also been implicated in the regulation of PGC-1α, the master regulator of mitochondrial biogenesis (62). More specifically, AMPK can directly interact and phosphorylate PGC-1α, thus increasing its transcriptional activity (64).
Mitochondrial biogenesis during HFD
Impaired mitochondrial biogenesis is one of the well-described pathophysiological adaptations that a HFD promotes (Table I). More specifically, in a previous study, insulin-sensitive male mice that were fed a HFD for 3 days exhibited decreased PGC-1a mRNA transcript levels in skeletal muscle (65), indicating a mechanism through which a HFD decreases the expression levels of genes that are necessary for OXPHOS and mitochondrial biogenesis. The prolonged downregulation of this molecular reprogramming can lead to mitochondrial dysfunction that is found in prediabetic conditions and insulin resistance, that eventually leads to insulin-dependent type 2 diabetes (T2D). In line with the above finding, in another study, C57Bl/6 mice treated with HFD for 3 weeks also exhibited reduced PGC1a mRNA and protein levels in skeletal muscle (65). Furthermore, as previously demonstrated 6-week-old male Sprague-Dawley rats that were fed a HFD for 28 weeks (60 kcal% fat) exhibited a reduced mtDNA copy number, suggesting impaired mitochondrial biogenesis in the myocardium (66). A reduction in mitochondrial abundance and mitochondrial dysfunction can eventually lead to both systolic or diastolic heart failure (heart failure with reduced ejection fraction and heart failure with preserved ejection fraction) and both can cause mortality if they remain undiagnosed and untreated. Following that, another study revealed that the gene expression levels of PPARGC1α were diminished in the cardiac tissue of 6-week-old Wistar rats fed a HFD (45 kcal% fat) for 10 weeks (67). Another study also linked mitochondrial abnormal function with diastolic dysfunction. However, male and female 2-month-old Wistar rats that were fed a HFD for 26 weeks had increased PGC-1α and mitochondrial transcription factor A (TFAM) protein levels in the gastrocnemius muscle (62). The same study suggested that the effects of a HFD on mitochondrial biogenesis may be sex-dependent, since male rats exhibited a greater increase in PGC-1α and TFAM levels compared to females (62). Similar findings were demonstrated in a study in which 8-week-old male C57BL/6 mice and male Wistar rats exhibited increased PGC-1α protein levels in skeletal muscle following 5 or 20 weeks of being fed a HFD (68). Furthermore, another study suggested that augmented lipid availability and decreased muscle mitochondrial fatty acid oxidative capacity due to the HFD could not generate insulin resistance and elevated intramuscular lipid abundance. Nonetheless, for a shorter period if time under a HFD (4 weeks), PGC-1α protein levels have been found to increase with no concomitant elevation in PPARGC1a mRNA levels in the skeletal muscle of male Wistar rats. This is related to the activation of PPARδ, due to increased free fatty acids that mediate a post-transcriptional increase in PGC-1α levels (69). These findings argue with the hypothesis that an impaired mitochondrial content is responsible for the development of insulin resistance.
Table ISummary table indicating the mitochondrial manifestations and the molecular targets that are affected by the high-fat diet. |
Mitochondrial function during HFD
As aforementioned, a prolonged period on a HFD affects the substrate availability that mitochondria can utilize, and thus, mitochondria need to adapt their molecular machinery to generate ATP efficiently in sufficient amounts, particularly in high-energy consuming tissues, such as the brain and myocardium. Numerous studies have tried to shed light on the effects of a HFD for different durations upon mitochondrial function in various tissues (Table I). Even though differentiated levels of substrates enter the cellular environment due to insulin resistance, master regulators of mitochondrial-substrate utilization are highly affected. More specifically, FOXO1 and PPARa transcriptionally control glucose and fatty acid utilization (61,70). Recently, KLF5, another transcriptional factor, completes a triangle that these transcriptional factors form, in order to orchestrate substrate utilization and mitochondrial function during diabetic cardiomyopathy (71). Moreover, C57Bl/6 mice fed a HFD have been shown to have OXPHOS-related mRNA transcripts followed by decreased cytochrome c protein levels (65), indicating that not only reduced mitochondrial abundance, as aforementioned, but also the decreased expression of genes related to mitochondrial function can result in diastolic and/or systolic dysfunction in insulin-resistant pathophysiological states. Similarly, a reduced mitochondrial respiration, complex I-III and citrate synthase activities have been detected in HFD-fed (60% kcal) rats (66), constituting important findings that justify an impaired cardiac function. The previous findings are also supported by ATP depletion, followed by an increase in the AMP/ATP ratio, that has been observed in the mitochondria of cardiomyocytes (66). By contrast, a study in which 8-week-old male C57BL/6 mice were fed a HFD (45% kcal) for 5 or 20 weeks, demonstrated elevated levels for all subunits from the respiratory chain in the skeletal muscle (68), most probably as a compensatory mechanism of impaired efficiency. Additionally, HFD administration for 4 weeks in male Wistar rats has been shown to increase the levels of proteins involved in the OXPHOS, such as cytochrome c oxidase (COX)I, COXIV, uncoupling protein (UCP) 3 and cytochrome b (69). In another study, the mRNA expression levels of the OXPHOS genes were shown to be significantly decreased in the myocardium of 6-week-old Wistar rats. In addition, the protein expression of NDUFB5 (complex I), one of the OXPHOS subunits in the mitochondria, was found to be lower in the HFD group (67). Even though some studies, as aforementioned, have demonstrated an increase in the OXPHOS complex, possibly as a compensatory mechanism in order to allow mitochondria to produce more energy, the HFD appears to impair mitochondrial function, affecting cellular energetics and ATP turnover.
Mitochondrial dynamics during the HFD
Mitochondrial fusion and fission are cellular processes that numerous studies have tried to shed light under the HFD (Table I). As previously noted, Chen et al (66) examined the impact of the HFD on the mitochondria of rat cardiomyocytes. Male Sprague-Dawley rats fed a HFD (60% kcal) exhibited elevated levels of mitochondrial FIS1 (66). In another study, 4-week-old healthy male C57BL/6 mice fed a HFD for 16 weeks exhibited a reduced relative expression of mitochondrial fusion protein 2 (MFN2), while the expression of mitochondrial DRP1 was detected at higher levels (72). Another previous study demonstrated that in 8-week-old male Sprague-Dawley rats fed a HFD for 6 weeks, changes in the expression levels of a protein related to mitochondrial fusion and fission were observed. More specifically, elevated levels of DRP1 and FIS1 were assessed in the HFD-treated group, while MFN2 levels remained unaltered (73). That study suggested that the HFD and low-intensity endurance training modulated mitochondrial dynamics, contributing to insulin sensitivity, whereas they failed to generate an additive effect on mitochondrial biogenesis. In line with the above, no change in the MFN1 protein levels were detected in male C57BL/6 mice (4-week-old) fed a HFD (74). However, MFN2 and OPA1 protein levels were assessed at a lower level in the HFD-fed mice (74). These findings suggest that moderate aerobic training can only mitigate insulin resistance and mitochondrial dysfunction that are induced by obesity.
Mitophagy during HFD
The protein content of total PINK1 in skeletal muscle has been detected at similar levels between groups before and after a high-fat meal in healthy non-obese, sedentary males and 10 endurance-trained male runners (75), suggesting that mitophagy is not necessary for metabolic flexibility in the healthy population. Studies concerning the effects of the HFD on mitophagy markers have also been conducted in transgenic mice that exclusively express Mito-Keima in cardiomyocytes. Mito-Keima Red is a fluorescent oligopeptide of which the excitation shifts to higher wavelength when mitochondria are placed in an acidic environment, such as lysosomes during mitophagy. Wild-type and transgenic Tg-Mito-Keima mice fed a HFD (60 kcal% fat) for 2 months, have been shown to exhibit mitophagy (elevated LC3II levels), followed by lower mitochondrial abundance (mtDNA/nuDNA) (76). Additionally, ATG7 appears to play critical role in mitophagy stimulation under a HFD since ATG7-KO mice have been shown to exhibit an alleviated phenotype after the respective treatment protocol (76). The aforementioned study suggests that mitophagy in cardiomyocytes is a quality control process during HFD consumption, and its activation can serve as a therapeutic target against HFD-induced diabetic cardiomyopathy. In another study, impaired mitophagy activity was observed in the myocardium of C57BL/6 mice fed a HFD for 24 weeks, which was driven through a reduction of PARKIN in the mitochondria that were supposed to be recruited by PINK1. Moreover, the HFD failed to alter Parkin mRNA levels, suggesting that possible independent transcriptional mechanism(s) are involved (77).
Caloric restriction (CR)
CR that has been studied since the beginning of the 20th century (78), is a nutritional approach that reduces calorie consumption without leading to malnutrition. Additionally, it is worth mentioning that during CR, adequate levels of vitamins and minerals should be maintained. CR typically involves a chronic reduction (20-30%) in energy intake from the standard calorie intake (79,80). In accordance with the current scientific literature, the term CR is often used interchangeably with dietary restriction (DR). However, CR is a partial example of DR as DR protocols include CR (81). CR has been shown to improve health and extend longevity in several studies in organisms with varying levels of complexity, ranging from yeast to humans (79,80,82-85). In contrast to starvation, the CR diet is able to lower glucose levels and increase ketone bodies levels in the plasma within normal physiological ranges (86).
Mitochondrial biogenesis during caloric restriction
Several studies have shed light on the effects of CR on mitochondrial biogenesis (Table II). Initially, CR has been studied in vitro or ex vivo to examine the effects on the molecular level and the distinctive pathways that are affected. Studies performed in both skeletal muscle C2C12 cell line or murine primary skeletal myoblasts treated under CR conditions have shown that the NAD+/NADH ratio is increased, leading to subsequent SIRT1 activation (87). That study highlights that AMPK, NAMPT and SIRT1 are necessary molecules of a functional pathway that permits cells from skeletal muscle origin to sense and react to substrate deprivation and availability. It is known that an increased SIRT1 activity triggered by elevated NAD+ levels can increase the transcriptional activity of PGC-1α (87) and FOXO1 (88), stimulating multiple pathways that need to be activated due to energy depletion and refer mainly to proliferative and metabolic processes. In a previous study, following PGC-1a and FOXO1 activation, both NRF1 and NRF2βγ transcript levels were found to be increased after CR for 24 h in HeLa cells (89). However, the same study revealed that CR treatment did not induce PPARα levels (89). Therefore, the mitochondria can adapt their bioenergetics under CR conditions and at the same time are able to stimulate mechanisms to reduce oxidative damage. Moreover, primary hepatocytes from 12-month-old male Fischer 344 rats that have received CR (40% restriction) have exhibited elevated expression levels of PGC-1α and PPARα (89). In line with the above, in vivo studies have revealed that the CR (30% restriction) diet in male C57BL/6 mice for 1, 9 and 18 months have increased PGC-1a protein levels in the skeletal muscle (90). Following that, the mRNA of several mitochondrial-relevant proteins, such as NRF1, Core 1, COXIV, Atps and COX content, have also been found to be elevated (90). Moreover, the SIRT3 (mainly expressed in the mitochondria) protein levels have been found to be increased in the skeletal muscle of C57BL/6 male mice after 12 months on the CR diet (91). SIRT3 activation is able to stimulate PGC1a through the CREB or AMPK signaling pathway (91), inducing the mitochondrial biogenesis molecular program. Of note, the CR diet in F344BNF1 male rats has been shown to result in the complete prevention of the age-related reduced levels of PGC-1α in the liver (92). Thereafter CR can be used as a therapeutic approach to alleviate age-dependent mitochondrial confinement. In agreement with this finding, PGC-1α activation has also been found in the liver of 12-24-month-old male Fischer 344 rats that have been on a CR diet for 25 months (93). Finally, the effects of CR on mitochondrial biogenesis have also been assessed in humans. In healthy and overweight participants on the CR diet for 6 months, the expression levels of SIRT1 and PPARGC1A in skeletal muscle were found to be elevated in non-obese young participants (94). The same study found that the expression of the PGC-1a target TFAM was increased, a protein that is a key activator of mitochondrial transcription and genome replication which is also used as a determinant for mitochondrial abundance (94).
Table IISummary table indicating the mitochondrial manifestations and the molecular targets that are affected during the caloric restriction diet. |
Mitochondrial function during caloric restriction
The activation of AMPK and SIRTs followed by the concomitant inhibition of mTOR downstream pathways are crucial molecular events that the CR diet is able to promote in order for cells to fine-tune oxidative metabolism, as well as the mitochondrial biogenesis and turnover (Table II). It is well established that AMPK and PGC-1α are key molecules that orchestrate these pathways (95). CR is also known to lower reactive oxygen species (ROS) production through enhanced mitochondrial aerobic metabolism and to increase the activity of antioxidant enzymes in the cardiovascular system and the skeletal muscle (96,97). In the gastrocnemius derived from 10-month-old B6C3F1 female mice fed a CR diet (40% restriction), the activities of complexes I, III, and IV were found to be diminished (98). In line with the that study, 8- to 10-month-old rats fed a CR diet for 4.5-6.5 months exhibited lower complex IV activity in the mitochondria in both skeletal and cardiac muscle (99). That study suggested that even though the CR diet can attenuate the decline in mitochondrial function that is related to cellular aging, this beneficial effect is insignificant compared with the impact that the CR diet has upon mitochondrial biogenesis. In line with that previous study, in another study, the CR diet (40% restriction) for 3 months in male and female 5-month-old Wistar rats was shown to result in a decrease at the activities of both complexes I and III (100). This adaptation to the CR diet can justify the lower mitochondrial-derived superoxide formation and the effect that the CR diet has upon age-related disorders. The above has been also confirmed by the higher complex IV efficiency in response to long-term CR administration that has been described in the skeletal muscle of F344BN rats due to an increase in high-affinity binding sites of complex IV (101). In another study, the CR (30% restriction) diet in male C57BL/6 mice for 1, 9 and 18 months did not result in any effect on citrate synthase activity (90). By contrast, the mRNA levels of several mitochondrial-associated proteins (Ppargc-1α, NRF1, Core 1, COXIV and Atps) and the COX content were increased in skeletal muscle (90). Following that, another study revealed that male Sprague-Dawley rats that fed the CR diet for 36 weeks exhibited an increase in the expression levels of genes associated with mitochondrial ATP production (six subunits of COX; COXI, II, III, IV, Va, VIII and NADH dehydrogenase) in skeletal muscle (102). The CR-related increase that has been reported, particularly in genes that encode proteins related to oxidative stress scavenging, may contribute to the impact of CR on cellular longevity. On the other hand, different levels of CR diets in male Fischer 344 rats throughout their life (10% restriction; at 3.5 months, 25% restriction; at 3.75 months, 40% restriction; at 4 months until mortality) failed to enhance or alleviate the age-related decrease in the ATP content of the mitochondria isolated from gastrocnemius muscle (103). The effect of the CR diet on mitochondrial function has also been assessed in humans. Firstly, overweight healthy males and females on the CR diet (25% restriction) for 6 months exhibited a downregulation in the levels of essential genes encoding subunits of ATP synthase, COX and NADH dehydrogenase in adipocytes (104). By contrast, in aged obese males and females (60-75 years old) that followed a CR diet, an increase in citrate synthase was observed in skeletal muscle (105). Another study revealed that healthy male and female overweight participants on a CR diet (25% restriction) for 6 months, did not exhibit an altered activity of β-hydroxyacyl-CoA dehydrogenase, citrate synthase and COXII (94). Finally, CR in overweight non-obese participants did not lead to changes in the levels of citrate synthase, β-hydroxyacyl-CoA dehydrogenase and COXII levels (94). These results indicate that the CR has a broad effect on the transcriptome in different tissues, although it does not improve mitochondrial function in overweight and obese participants.
Mitophagy during caloric restriction
The CR is one of the most potent non-genetic triggers for initiating the mitophagy process (106) (Table II). Specifically, the highest values of PINK1 and Parkin have been detected in the skeletal muscle of male 10-week-old C57BL/6 mice fed the CR diet for 18 months (107). In line with that previous study, in another study, the expression of the autophagosome formation marker, LC3/Atg8, and BNIP3 was increased, while that of PINK1 was markedly decreased in the kidneys of male 3-month-old Fischer 344 rats after 20 months of CR treatment (108). Moreover, nutrient deprivation induces mitophagy in primary hepatocytes from wild-type and GFP-LC3 transgenic male mice (109). The aforementioned findings suggest that the CR diet stimulates the mitophagy program in cells in a possible attempt to discard energy consuming and not fully functional mitochondria.
Mitochondrial dynamics during CR
As it is expected, a lack of energy intake is able to affect mitochondrial dynamics, inducing the fusion phenotype in mitochondria (Table II). A previous in vivo study using male C57BL/6 mice fed a CR diet (40% restriction) for 6 months revealed increased levels of MFN2 and OPA1 in skeletal muscle (107). A similar finding has also found in mice fed a CR diet (40% restriction) with less calories, that expressed increased levels of MFN1 and NRF1 (110). Additionally, the prolonged duration of CR was found to have no effect on mitochondrial fusion, proposing that initial energy deprivation led to a rather early control of mitochondrial fusion (107). On the contrary, 18 months of CR has been shown to result in higher expression levels of DRP1, which as aforementioned, is able to stimulate mitochondrial fission. Thereafter, distinct molecular signatures are required to orchestrate and control mitochondrial fission and fusion dynamics in mice fed a CR diet that also depend on the duration of the diet per se (107). The CR diet is also able to further induce mitochondrial fusion in the skeletal muscle of aged mice (in which mitochondrial fusion has already been induced) (111). On the contrary, the CR diet can partially inhibit the induction of mitochondrial dynamics in the glycolytic muscles of aged mice (111). This discrepancy suggests that the anti-aging effects of the CR diet in mitochondrial dynamics may be restricted to highly oxidative muscular fibers. The effects of the CR diet have also been studied in the liver. C57BL/6 mice that fed a CR diet (40% restriction) have been shown to exhibit no change in mitochondrial fusion related proteins in hepatocytes (112,113). On the contrary, the study showcased an elevation at DRP1 levels in mitochondria-enriched fractions of hepatocytes (113), indicating that a similar CR diet is able to affect mitochondrial dynamics differently between tissues. Another study revealed that the CR diet was not able to change the mitochondrial abundance in hepatocytes, but stimulated MFN2 levels (110).
The effects of the CR diet have been also studied in combination with exercise. As previously demonstrated, initially, the CR diet was able to decrease the impact of resistance training in muscle hypertrophy since rats that received both the CR diet and resistance training exhibited elevated fusion-related proteins levels (OPA1 and MFN1), while no effect was exerted on fission-regulatory proteins (FIS1 and DRP1) (114). That study suggests that CR promotes mitochondrial adaptation in the skeletal muscle, since it attenuates the effect of resistance training upon muscle hypertrophy. Finally, another study demonstrated that both CR and exercise training in obese rats was able to alleviate reduced mitochondrial fusion stimulated by HFD, since they induced MFN2 expression and reduced the phosphorylation of DPR1 protein at Ser616 (115). In this case, the CR diet appears to act synergistically with exercise against the HFD-induced mitochondrial fusion.
Ketogenic diet (KD)
The KD was first applied to a patient with epilepsy back in the 1920s (116). The KD is a diet consisting of low carbohydrates, a high-fat content, and moderate amounts of protein. Different types of the KD type have been introduced in the scientific literature, including i) the classic DK (CKD); ii) the less restrictive 'modified Atkins diet' (mAD); and iii) the 'medium-chain triglyceride' KD (MCT-KD) (117). The CKD refers to the diet in which every 4 g of fat accounts for 1 g of protein plus carbohydrates, reducing the carbohydrates (118). mAD has a different composition since every 1 g of fat corresponds to 1 g of protein plus carbohydrates (119). The ratio of MCT-KD is similar to that of CKD, although the ketone bodies are generated efficiently as the AMPA receptors are directly inhibited by the medium-chain triglycerides that are used in MCT-KD. Consequently, cellular energetics are shifted towards mitochondrial biogenesis (120).
Generally, the KD can alter the organism's metabolic state and shift its reliance from carbohydrates to FAs. This adaptation also leads to an increase in FA oxidation, gluconeogenesis and ketogenesis, with produced ketone bodies entering the blood circulation (121). Acetoacetate, β-hydroxybutyrate (β-HB) and acetone are the major forms of ketone bodies generated during the KD (117). Furthermore, the KD can stimulate numerous pathways, upregulating proteins that participate in biochemical systems related to cellular bioenergetics, such as the TCA cycle (citrate synthase, malate dehydrogenase), the OXPHOS system (CI, CII, CIII, CIV, CV and cytochrome c) and to FA oxidation [carnitine palmitoyl-transferase, medium-chain acyl-CoA dehydrogenase (MCAD), long-chain acyl-CoA dehydrogenase, very-long-chain acyl-CoA dehydrogenase, β-hydroxyacyl-CoA dehydrogenase] and ketolysis (β-hydroxybutyrate dehydrogenase) (122).
Mitochondrial function during the ketogenic diet
Generally, the KD has been reported to stimulate pathways that induce mitochondrial abundance, enhance mitochondrial performance and regulate antioxidant mechanisms (123-126) (Table III). Low-level redox signaling molecules, such electrophiles and H2O2 can stimulate adaptive pathways such as the protective transcription factor, NF E2-related factor 2 and as a result, lead to the increased production of antioxidants (e.g., glutathione) and detoxification enzymes (120). Moreover, the KD has been found to increase cytosolic and mitochondrial protein acetylation and alter protein succinylation patterns (121). More specifically, male mice fed the KD have been shown to exhibit increased mitochondrial FA oxidation in the liver, while a reduction of markers of hepatic de novo lipogenesis has been detected in comparison to mice that fed a high fat/high sucrose 'western' diet (WD) (126). Furthermore, another study revealed that the KD for 4 months increased mitochondrial activity in younger animals (127). On the contrary, the prolonged duration (14 months) of the KD was found to be required to increase mitochondrial mass and function in elder animals in comparison with mice fed the isocaloric diet (127). It was suggested that the improvement of mitochondrial function due to the KD may contribute to the improved longevity; hence, no direct link has been determined between enhanced mitochondrial function with muscle mass, strength, and muscular aging. In line with this previous study, increased mitochondrial mass was also observed in the study by Parry et al (128). More specifically, increased citrate synthase activity that corroborates with a higher mitochondria volume was found in the liver and gastrocnemius of rats fed the KD compared to rats fed standard chow. Additionally, the KD-fed rats had a higher median lifespan (128). In another study, rats fed the KD and exposed to resistance exercise training, exhibited more efficient coupling of complex II substrates in the skeletal muscle mitochondria compared with the control group that underwent the same exercise regime but received the isocaloric western diet (125). Those authors suggested that their insights may reflect unknown biochemical connections between axes involving ketone bodies or medium-chain triglycerides that modulate mitochondrial function (122). Another study revealed that in the skeletal muscles of aged male C57BL/6 mice, an increase in the levels of markers of mitochondrial content (citrate synthase, complex I and complex IV activity) was observed following long-term exposure to a KD (14 months) (129). On the contrary, in another study, the long duration of the KD in 4-month-old male rats was shown to result in lower gastrocnemius maximal citrate synthase activity and caused damage to the respiratory control in the gastrocnemius myotubes, while it did not alter mitochondial abundance and quality in the brain and liver (130). Similarly, a decrease has been documented in cytochrome c and complex IV levels in the hepatocytes of C57Bl/6 male mice that fed the KD (124). The aforementioned results suggest that the short-term use of the KD can promote mitochondrial biogenesis and/or alter mitochondrial physiology in specific tissues, with skeletal muscle being susceptible to KD-induced alterations (127). Due to contradictory findings, further investigations are required in the future to shed more light and delineate the net impact of KD on mitochondrial mass and function.
Table IIISummary table indicating the mitochondrial manifestations and the molecular targets that are affected during the ketogenic diet. |
It is known that the KD is able to shift cellular energy production reliance towards FA oxidation and not towards glycolysis. This is a consequence of the elevated levels of ketone bodies basically in the liver. Xu et al (131) reported the detrimental effect that is caused due to extensive KD or frequent deep fasting. The main manifestations reported were fibrotic lesions in the myocardium of the rats (131). This was followed by a reduction in mitochondrial biogenesis and cellular respiration, while cardiomyocyte apoptosis was found to be stimulated. At the molecular level, their study added that during the increase in ketone body β-HB levels, the acetylation of the histone Sirt7 promoter was maintained due to HDAC2 inhibition. The aforementioned molecular cascade was able to lead to the transcriptional activation of Sirt7 (131) that inhibited the expression of genes related to mitochondrial ribosome-encoding and mitochondrial biogenesis, promoting cardiomyocyte apoptosis and myocardial fibrosis (131). In a previous study, β-HB supplementation in cybrid cell lines that contain a 1.9 kb partial deletion of mtDNA (the 'FLP' deletion) derived from a patient with Kearns-Sayre syndrome (KSS) was found to increase mitochondria volume (132), while β-HB treatment improved mitochondrial morphological imperfections in brain and muscle tissue at BTBR mice (133), which present hallmarks of idiopathic autism. The authors of that study also reported that β-HB supplementation increased the diminished mitochondrial size that was induced in BTBR mice, signifying that larger fused mitochondria exhibit a higher ATP production and lower ROS levels.
Miller et al (134) investigated the changes in the mitochondria of skeletal muscle in healthy individuals who followed the KD combined with exercise training, focusing on periodized resistance training, power training and high-intensity interval training. They observed that the KD increased the mitochondrial respiratory control ratio (mitochondrial O2 consumption and membrane potential index), ATP production and the ATP/H2O2 ratio (134). The latter depicts an increase in the efficiency of energy production compared with the oxidative burst. In another study, β-HB treatment in 5-month-old male Fisher rats resulted an improvement of mitochondrial respiration followed by less H2O2 generated by the mitochondria, without affecting mitochondrial abundancy (135). A previous study conducted on mice that had manifestations of T2D and fed the KD revealed that the KD was able to reduce the mitochondrial respiratory control ratio and the ATP content (136). Notably, it has been demonstrated that homozygous mice with a missense mutation in Med30 that exhibit cardiomyopathy die soon after weaning, while they are healthy at the lactation stage (137). When weaning mice are exposed to a KD, their viability is extended after weaning, which is attributed to differences in genes involved in oxidative phosphorylation and mitochondrial integrity (137). Thus, lethal mitochondrial cardiomyopathy caused by a mutation in Med30 can be partially reversed through dietary control (137). In a different pathophysiological in vivo model, the KD was shown to reduce the progression rate in a disease mouse model (transgenic mouse line) of late-onset mitochondrial myopathy (138). More specifically, the KD diminished the amount of COX-negative muscle fibers, which is a hallmark of mitochondrial respiratory chain deficiencies. However, the mtDNA quality or quantity remained unaffected after the KD, while the study highlighted that mitochondrial biogenesis was stimulated followed by liver lipid restoration (138). The aforementioned data suggest that minor mitochondrial dysfunction can influence skeletal muscle energy metabolism by drifting towards anaerobic glycolysis. Furthermore, if lipids can enter mitochondria, then the cells reprogram and use FAs as energy substrates, boosting β-oxidation. In another study, 8-week-old male C57BL/6J mice fed D-β-hydroxybutyrate-(R)-1,3 butanediol monoester [ketone ester (KE)], replacing the carbohydrate diet content for 1 month, exhibited a higher number of mitochondria and an increase in ETC proteins levels, UCP1 and mitochondrial biogenesis-regulating proteins in the interscapular brown adipose tissue (139). An increase in mitochondrial UCP expression and activity was also observed by Sullivan et al (140) in juvenile mice that received a high-fat KD (HF-KD). In addition, ROS levels after the HF-KD were found to be decreased, and this suggested a potent neuroprotection activity of the KD (140). UCP activation is able to remove protons from mitochondrial protein, decreasing the flow of ETC. Therefore, UCP activation can indirectly diminish ROS mitochondrial production, serving as an additional axis through which the KD can function in order to promote its beneficial effect.
Mitochondrial biogenesis and mitophagy during the KD
Male C57BL/6J mice and rats fed the KD have been shown to have elevated PGC-1a and TFAM levels (141,142) in comparison with respective animals fed a standard chow diet. On the contrary, other studies have failed to document changes in the PGC-1a levels of sedentary male rodents in the gastrocnemius, the liver or brain (126,128). In addition, an increase of PPARGC1a mRNA levels has been observed in the gastrocnemius tissue of male Sprague-Dawley rats who voluntarily exercised on wheels regardless of the diet they were fed (WD or KD) (125). It can also be suggested that exercise training and the KD can correct the high-fat induced elevated mitochondrial content (122).
The majority of studies conducted on rodents have used a low-protein KD. As such, recently, Huang et al (143) investigated the possible alterations of a normal-protein KD (NPKD) upon the substrate oxidative capacity in the skeletal muscle of C57BL/6 male mice which were also introduced to an exercise training regime. The application of NPKD or exercise training alone could not produce changes in the mitochondrial content, while their combined application was found to increase both mitochondrial fission/fusion markers and PGC-1α levels. Furthermore, the NPKD/exercise training combination has been found to shift cellular metabolism towards enhanced lipid utilization, since both mitochondrial and peroxisomal lipid oxidation are stimulated (143). Even though that study suggested a mechanistic adaptation of skeletal muscle due to KD, further studies are warranted to fully elucidate the effects of a KD and/or exercise on mitochondrial biogenesis and function.
As regards brain metabolism, data obtained from experiments using male Sprague-Dawley rats have revealed that a calorie-restricted KD can improve brain metabolism via an anti-convulsant mechanism involving mitochondrial biogenesis. More elaborately, increases in transcripts that encode mitochondrial proteins, mitochondrial abundance and increases in the phosphocreatine/creatine index followed by higher glutamate levels have been found (144). It was also indicated that the calorie-restricted KD was able to promote brain metabolism and that the KD anti-convulsant mechanism affected mitochondrial biogenesis with increased energy stores of alternative substrates (144). In another study performed on mice with T2D fed the KD, mitochondrial fission was reduced, while the improvement of overall mitochondrial function was documented. More specifically, the KD was found to regulate the expression levels of mitochondrial dynamics-related proteins (OPA1 and FIS1) (136). Furthermore, in mice fed the KD, the expression levels of BNIP3 gene (mitophagy regulator gene) have been found to be increased in the liver; therefore, it was suggested that the KD may act as a mitophagy activator (145). On the contrary, in another study, mitophagy was not induced in the hepatocytes of male and female Wistar rats fed the KD (90, 5% fat) for 7 weeks (141), even though the KD increased the levels of sequesterosome-1 (p62), a scaffold protein for LC3 (141). Furthermore, another study that examined the effect of the KD (75% kcal fat) in male C57Bl/6 and BTBR mice, revealed elevated BNIP3 gene and protein expression levels in the livers of KD-fed mice, indicating the potential activation of mitophagy (145).
Mitochondrial dynamics during the KD
In a previous study, in juvenile male C57Bl/6 and BTBR mice fed the KD (75% fat) for 10-14 days, a decrease in the levels of mitochondrial proteins in the liver was observed, despite a concomitant increase in gene expression (145). Additionally, decreased levels of mtDNA were documented in the liver, indicating a decrease in mitochondrial abundancy. In the same study, BNIP3 gene and protein expression levels were found to be increased in the liver, a hallmark of activated mitophagy, while in the brain, BNIP gene expression and protein levels remained unaltered (145). Since the KD is characterized by increased β-HB levels, β-HB supplementation in myocytes from young and aged mice β-HB has been documented to be beneficial for mitochondrial repair, even though the expression of MFN2 and DRP1 is reduced by 50%, contributing to impaired fusion-fission process (146). It has been suggested that increased β-HB levels can repair mitochondria in the failing aging myocardium, an effect that is alleviated by the impaired MFN2-DRP1 axis and the reduced PARKIN levels (146). The mitochondrial translocation of DRP1, a key regulator of mitochondrial fission, has also been found to be suppressed in SH-SY-5Y cells, treated with β-HB. As a result, mitochondrial fission is inhibited during ketone bodies administration (147), suggesting that KD restores mitochondrial integrity through the mitochondrial translocation of DRP1and suppresses ER stress, exerting neuroprotective effects.
Fasting
Fasting is defined as a voluntary abstinence from food and drink for specified, recurring periods of time, ranging from 12 h to 3 weeks in humans (148). Fasting can also be considered a CR diet in periods of free access to food which are interrupted by fasting (149). Several studies that have been performed by other research groups and the authors, as well as ours indicate that the overall improvement of health through fasting involves the beneficial modulation of energy substrates, adaptive cellular stress response, signaling pathways that enhance mitochondrial health, DNA repair and autophagy (150-153). Organisms respond to different types of fasting by minimizing anabolic processes, enhancing stress resistance, recycling damaged molecules, stimulating mitochondrial biogenesis, and promoting cell survival, all of which support improvements in health and disease resistance (150,154). The energy intake, as well as the fasting duration between meals, can alter the NAD+/NADH, AMP/ATP and acetyl-CoA/CoA ratios. More specifically, during fasting, the AMP/ATP ratio is increased, leading to AMPK activation, and promoting pathways related to cellular repair and anabolic inhibition. Furthermore, acetyl coenzyme A (CoA) and NAD+ serve as cofactors for epigenetic modifiers, such as SIRTs (155). As aforementioned, SIRTs deacetylate FOXOs and PGC-1α, rendering them transcriptionally active (156). Therefore, their activation results in the expression of genes involved in stress resistance and mitochondrial biogenesis (154,157).
Mitochondrial biogenesis during fasting
Several studies have reported that fasting stimulates mitochondrial biogenesis (Table IV). In fasted C2C12 myotubes, both PGC-1a and SIRT1 deacetylation are elevated (158). Moreover, the fasting effect on the muscles of adult male northern elephant seals revealed that the phosphorylation levels of AMPK and SIRT1 mRNA ere increased, while PGC-1a expression remained unaltered (159). Furthermore, a previous study demonstrated that 6-week-old mice fasted for 14, 24 or 48 h exhibited an induction of PGC-1α in the liver (160). Similarly, the hepatic expression of PGC-1α at the mRNA level has been found to be elevated following a 24-h fastin C57BL/6 mice (161). The studies suggest that cells have a dual switch constituted by SIRT1 deacetylation and PGC-1a activation that sense substrate fluctuation and availability. The duration of fasting may have a differential effect on differentiated transcriptional and post-translational metabolic response that also can be affected by different tissue background and physiological habits that evolutionary exist. The latter may indicate the unaltered expression of PGC-1a in elephant seals that may support the energetic demands associated with the prolonged fasting in adult seals.
Table IVSummary table indicating the mitochondrial manifestations and the molecular targets that are affected during fasting. |
Importantly, mitophagy and mitochondrial function have been implicated in the pathophysiology of depression (162). Therefore, they can serve as novel therapeutic targets to alleviate the inflammation that triggers depression. Furthermore, fasting is able to promote mitochondrial biogenesis in hippocampal neuron cultures from embryonic day 17, upregulating PGC-1α levels, similar to exercise (163). This finding may provide new therapeutic interventions against neurodegenerative disorders that include synaptic degeneration since they are characterized by reduced levels of PGC-1a. Recently, a study that compared fasting with high-intensity intermittent exercise (HIIE) found increased TFAM, NRF1 and NRF2 gene expression levels in skeletal muscle, PPARGC1α levels in the liver, and elevated levels of glucose-6-phosphate in the plasma of C57BL/6 male mice (164). These observations suggest that fasting promotes mitochondrial biogenesis and enhances cellular stress adaptation in skeletal muscle.
Mitochondrial function during fasting
Real-Hohn et al (165) investigated the effects intermittent fasting (IF; every other day) in combination with a high-intensity intermittent exercise (IF/HIIE) for 8 weeks in Wistar rats (165). Their study revealed an increase in hexokinase activity in the liver, heart, and skeletal muscle, followed by an elevated skeletal muscle FoF1 activity. Additionally, they documented a molecular reprogramming in the white adipose tissue due to IF as regards UCP1, monocarboxylate transporter 1 and GLUT4 expression. However, every other day feeding alone can also promote changes in mitochondrial function. More specifically, every other day feeding can increase lipid catabolism in the muscle mitochondria of male OF-1 mice (166), preventing lipid peroxidation and muscular deterioration eventually. Furthermore, every other day feeding has also been shown to lead to stable or increased liver OXPHOS components in Ames dwarf mice compared to wild-type mice (167). These findings suggest that anabolic hormones contribute to the differential effects of strict dietary regimens, such as fasting. The ability of Ames swarf mice to adapt better via metabolism reprogramming and oxidative damage handling, indicates that more parameters should be taken into consideration before scientists bring translational outcomes to humans. In addition, IF appears to reverse some of the manifestations of the metabolic syndrome in rodents, stimulating autophagy (168,169).
Fasting is also known to affect the function of the mitochondria in adipocytes. More specifically, a 16-week isocaloric fasting in leptin-deficient ob/ob mice was shown to increase UCP1 protein levels in subcutaneous white adipose tissue (170), suggesting that fasting affects insulin and other adipokines, such as leptin in order to mitigate distinct molecular programs in the adipose tissue. In line with these findings, in another study, in 6-week-old male C57BL/6 mice subjected to fasting every other day for 6 and 30 days, an increase in UCP1 protein was also documented (171). However, in the same study, the mitochondrial content of the brown adipose tissue was found to be decreased with a concomitant decrease in UCP1 protein and mRNA levels (171). That study suggests that the effect of every other day fasting is tissue-specific, since the activation of brown adipose tissue relies mainly on β-adrenergic receptor (β-AR) signaling and that the effect of fasting on white adipose tissue is β-AR-independent. In another study, no difference was detected in markers of mitochondrial metabolism (PGC-1a, SIRT3 and MFN2) at the skeletal muscle of human subjects who fasted for 8 weeks (24-h fasting, three times per week) (149). However, a key finding of the same study was that mitochondrial ROS production in the skeletal muscle diminished due to fasting (149), an insight that has also been documented in other types of diet, such as the CR, as aforementioned.
Experiments using C2C12 myotubes mimicking fasting and feeding conditions have shown enhanced mitochondrial oxidative phosphorylation via an increase in the expression of genes implicated in mitochondrial physiology (cytochrome c, COXVa) and fatty acid utilization (MCAD, CPT-1b and PDK4) (158). In another study, 1 week of fasting was applied to 5-week-old ducklings, resulting an upregulation of the coupling efficiency in the mitochondria of the liver and skeletal muscle, followed by a reduction in oxidative phosphorylation (172). That study suggested that energy preservation was maintained through diminished mitochondrial activity, and this was counterbalanced with an elevation in mitochondrial coupling efficiency, allowing cells to conserve endogenous fuel stores during fasting periods. The above suggestion was further confirmed through an additional study in which a 6-day fasting period resulted a decrease in oxidative phosphorylation activity without affecting the inner membrane proton conductance at the mitochondria of the skeletal muscle of ducklings (173). In addition, the activity of succinate-cytochrome c reductase, which involves complex III and co-enzyme Q pool, has been shown to be significantly decreased (173). Moreover, in another study, a 24-h fasting period in 6-week-old male FVB mice resulted in an induction of the expression of enzymes of the TCA cycle and oxidative phosphorylation in the liver (174). Additionally, that study demonstrated that a short-term fasting period affected the energy production in hepatocytes, while prolonged fasting stimulated glucose production from the liver (174). Fasting for 24 and 8 h in human-derived peripheral blood mononuclear cells (PBMCs) has been shown to result in a distinct gene expression pattern, with 74% of the genes with an altered expression being similar in both time points tested. More specifically, in both time points tested, an increased expression was detected in genes related to fatty acid β-oxidation, and a reduced expression was documented in genes associated with the TCA cycle (175). That study suggests that these changes may reflect the nutrition-related metabolic alterations that fasting generates, possibly orchestrated by PPARα. Finally, a downregulation of genes associated with mitochondrial regulation has been assessed in adipose tissue derived from humans that had a time-restricted eating protocol for 8 weeks. More specifically, these genes are involved in mitochondrial biogenesis and oxidative phosphorylation pathways (MRPS35, MRPL33 and MRPL51) mitochondrial ribosome protein translocation (TOMM7 and TOMM22), and the ETC and ATP synthase (NDUFA12, NDUFS5, ATP5F1E and ATP5PD) (176). These findings support the hypothesis that time-restricted feeding can act as a preventive or therapeutic regime to exert glycemic control in humans diagnosed with T2D or pre-diabetes.
Mitochondrial dynamics and mitophagy during fasting
In a previous study, in humans who fasted for 16 h, it was found that during the fasting re-feeding transition, the mitochondrial fission in human PBMCs was induced (177). The authors of that study considered that these changes were observed due to diminished interactions between the mitochondria and ER. Likewise, the interaction of the mitochondria with the ER has been suggested to sense glucose availability in the liver. More specifically, in C57BL/6 mice that have been fasted overnight, an elevated mitochondrial volume of their hepatocytes has been documented, a hallmark of mitochondrial fusion (178). The same study also revealed that feeding promoted mitochondrial fission, since cellular 'powerhouses' were found to be smaller (178). The disruption of the dynamic adaptation of mitochondria-associated ER membranes has been incriminated by an enhanced insulin resistance. Similarly, a 12-h overnight fasting period in human subjects can promote mitochondrial fission in the skeletal muscle, which has also been associated with lipid oxidation (179). Additionally, this study indicates that MFN2, not MFN1 or OPA1, contributes more to skeletal muscle mitochondria fusion-fission processes and that lipid droplets interact with mitochondria via MFN2 rather than MFN1 (179). A 2-day fasting period for C57BL/6 mice has been shown to lead to an induction of mitochondrial autophagy with the use of Mito-Keima (180). Furthermore, in male C57BL/6 mice, BNIP3 protein expression has been shown to be markedly induced above basal levels after 6 h of fasting, with a further induction by 24 h of fasting (180). By contrast, no induction of NIX in the liver of fasted mice has been observed (181). Finally, non-significant decreases in LC3BI and BNIP3 protein levels have been documented during fasting; however, the preserved LC3II:I ratio and p62/SQSTM1 protein content suggests that autophagy/mitophagy has not been robustly activated in the skeletal muscle of human subjects after 8 h of fasting (182). The last contradictory results suggest that the induction of several molecular pathways due to fasting are early events in the adaptation of human tissues to a lack of substrates.
Other diet schemes
Mediterranean diet (MedD)
The first to define the MedD was Ancel Keys (51), as the diet pattern which included a low percentage of saturated fats and a high proportion of vegetable oils. MedD was mainly noted during the 1960s in Greece and northern Italy (183). MedD, in its traditional form, originates from civilizations inhabiting close to the Mediterranean Sea, and as a result, has a strong association with the social behaviors and lifestyles of the individuals inhabiting that region (184). Furthermore, UNESCO recognized the MedD as 'an intangible cultural heritage that is deeply rooted into its geographical origin and whose agricultural and dietary practices have a responsible interaction with the environment' (184). From a literature review conducted by Davis et al (185), it was shown that that descriptions concerning MedD are common among publications. More specifically, the major components of a MedD pattern include olive oil as the main culinary fat and the high consumption of plant-derived foods (fruit, vegetables, legumes, nuts and seeds, and whole grain cereals); the moderate intake of red wine; and the moderate consumption of seafood and dairy products, such as yogurt and cheese (185). However, dairy products, such as whole milk, butter or cream are not suggested. Furthermore, the MedD guidelines propose the moderate intake of poultry and eggs, and the low consumption of sweet desserts, red and processed meats (186). It can be drawn from the above that the MedD does not exclude specific food sources or define a caloric limit, but instead focuses more on the frequency at which each food type should be consumed (187). However, there are no clear guidelines for serving numbers or serving sizes.
The MedD has been found to reduce the progression, and in a number of cases, to prevent the development of cancer, cardiovascular disease, depression, obesity, diabetes, erectile dysfunction, asthma and cognitive decline (187). In addition, the benefits of the MedD include the reduced risk of metabolic syndrome and total mortality. The MedD is one of the most studied diets s regards to its association with cardiovascular disease (187). It is noteworthy that the Seven Countries Study revealed that farmers inhabiting the Crete region had the lowest cardiovascular mortality rate, even though they consumed some of the largest amounts of fats (51).
Numerous in vitro and in vivo studies have been conducted in order to demonstrate the effects of the MedD on mitochondrial physiology (Table V). More importantly, these studies report the effect of isolated compounds that are present in the MedD and usually they have been challenged in in vitro and in vivo models that mimic several diseases or pathophysiological conditions.
Table VSummary table indicating the mitochondrial manifestations and the molecular targets that are affected during Mediterranean, Nordic, and mixed scheme diets. |
As such, polyphenols are one of the main family of constitutes that are present in large quantities in foods and drinks of the MedD. More specifically, a flavonoid that is found in large quantities in red wine, delphinidin, is able to increase the mitochondrial activity through complex IV activity and the mitochondrial abundance. At molecular levels, delphinidin has been found to upregulate the expression of TFAM, TFB2M and NRF1 that are implicated in the mitochondrial biogenesis molecular program (188). In another study, in which endothelial cells with VEGF-induced mitochondrial dysfunction were treated with lycopene, an upregulation in the expression of genes that encode proteins related to both mitochondrial biogenesis and function, such as several COX members, PGC-1α and SIRT1 was found (189). Similarly, SIRT1 and PGC-1α have been found to be stimulated by chlorogenic acid, that is present in coffee beans and apples, in human umbilical vein endothelial cells exposed to ox-LDL. Following increased biogenesis, chlorogenic acid treatment has also been shown to alleviate elevated mitochondrial ROS levels (190). One of the most known SIRT1 activators that is used in biomedical science is resveratrol, that is found mainly in red wine and grapes. Resveratrol is able to stimulate mitochondrial function through SIRT1 and PGC-1α activation and prevent several pathophysiological conditions, such as insulin resistance and cardiac failure (191-193). Another well-studied polyphenol that is present in olive oil is hydroxytyrosol. It has been found that it can increase mitochondrial abundance via the induction of the AMPK signaling pathway, inducing PGC-1α, TFAM and NRF-1 (194). Furthermore, hydroxytyrosol is able to induce mitochondrial activity, since it has been found to elevate complex I, II and decrease complex V expression (195). Ginger extract that is also used extensively in the MedD and the Asian traditional diet, can stimulate the AMPK/PGC-1α axis in both HepG2 cells and in skeletal muscle, liver, and brown adipose tissue in mice. Moreover, mitochondrial abundance and the expression of OXPHOS-related proteins are also increased in all tissues tested (196).
Some studies have also been performed on human subjects/patients. Initially, a study that was conducted on patients that received a diet rich in polyunsaturated FAs for 2-3 weeks before they underwent cardiac surgery, revealed an upregulation of PPARγ followed by an improvement of β-oxidation and reduced a oxidative burst (197). In line with that study, treatment with docosahexaenoic acid, that can be found in fish, was also documented to decrease mitochondrial ROS and stimulate the expression of genes that are implicated in fatty acid metabolism (198). Resveratrol has also been administrated to patients who are overweight and/or with T2DM (199). It was revealed that mitochondrial function was improved since state 3 respiration was elevated, and the expression of complex IV was diminished (199). Finally, a study in which healthy, resistance-trained, male subjects received a mixture of ancient peat and apple extract demonstrated an elevation in ATP turnover, increased mitochondrial function followed by a reduction in mitochondrial ROS production (200).
In vitro studies have also aimed to document the effects of the MedD on the other aspects of mitochondrial physiology, such as mitophagy and fusion/fission. Hydroxytyrosol supplementation as aforementioned, has been found to decrease DRP1 and PARP levels, indicating a reduction of mitochondrial fission (195). In addition, ferulic acid that is present in fruits and vegetables, such as tomatoes, sweet corn and rice has been shown to improve the mitochondrial biogenesis molecular program through the induction of PGC-1a, PGC-1β and NRF1. It has also been found that it can increase all axes of dynamics and mitophagy, since it stimulates the expression of MFN1, MFN2, FIS1 and Beclin-1 (201). Finally, butyrate, that is a short-chain fatty acid, has been shown to increase mitochondrial fusion, since it is able to induce MFN1, MFN2 and OPA1 expression followed by a concomitant decrease in DRP1 and FIS1, that control mitochondrial fission (202).
Nordic diet
The Nordic diet is considered the new MedD and consists of whole, fresh, seasonal, local foods of the Scandinavian region. The Nordic diet also discourages the consumption of heavily processed foods and is rich in whole grains, berries, fruits, vegetables, fish and low-fat dairy products. Data available concerning the effects of the Nordic diet and mitochondrial function are extremely limited. A clinical trial study that was performed on the healthy Nordic diet, revealed that it altered the expression of genes related to mitochondrial function and thereby reduced ROS production in PBMCs form patients with metabolic syndrome. Apart from the involvement of genes that encode proteins of the ETC, the Nordic diet has been shown to downregulated both NRF1 and NRF2 levels, suggesting a reduction in the oxidative burst (203) (Table V). Following the hypothesis that the Nordic diet contains similar ingredients with the MedD, similar effects are expected in future studies concerning other aspects of mitochondrial physiology.
3. Clinical impact
The majority of researchers consider that the only function of the mitochondria is to generate energy for cellular demands. Hence, the mitochondria, though their functions are able to contribute to cellular metabolism, redox signaling and ion homeostasis. All these aspects are important for cellular survival and well-being. The dysregulation of their function suggests their involvement in the pathogenesis or/the progression of numerous human diseases or pathophysiologies, including, diabetes, cancer, neurodegenerative and cardiovascular diseases, sepsis, traumatic injury, inflammation, aging and, frailty and loss of elasticity in the skin. Despite the intense focus of the scientific community concerning mitochondrial physiology, one study out of every 154 studies indexed in PubMed since 1998 has studied mitochondrial function (204), the exact role and mechanisms through which the mitochondria contribute to human diseases remain elusive, opening new challenges for basic and translational biomedical science. The up-to-date information incriminates oxidative stress that is generated from the mitochondria as the main causality or a secondary aggravating effect that drive several tissue pathophysiologies.
Nutrition and dietary habits play a critical role in mitochondrial physiology, since they provide mitochondria with all necessary (or not), substrates for proper (or not) function. For that, dietary habits, such as HFD, are able to aggravate mitochondrial function and render the mitochondria a significant determinant towards the development of metabolic diseases, such as diabetes or metabolic syndrome. On the contrary, dietary interventions, such as the KD, fasting or MedD, can modulate the molecular environment of already dysfunctional mitochondria in several diseases, including cardiac failure, Alzheimer's disease, and cancer, alleviating several manifestations towards better living. It has also been well-established that fasting and MedD can contribute to longevity, since they have the ability to maintain the mitochondria in a 'fit' state. Due to the fact that diet is a daily habit, clinicians and other scientists are allowed to optimize their interventions that modulate cellular 'powerhouses' and impact human health.
4. Conclusions
The mitochondria have the flexibility to easily adapt to exogenous parameters and preserve their proper functionality in order to generate the adequate energy for cellular demands. Differentiations in substrate availability due to different dietary habits are being sensitized by the mitochondria followed by their molecular rewiring. This orchestrates the mitochondria to undergo changes in their function, the regulation of biogenesis and mitophagy, and their 'self-recycling' through fusion and fission (Fig. 5). Conclusively, a HFD impairs mitochondrial function and fusion, while it can induce mitochondrial fission. CR diets appear to reduce mitochondrial biogenesis and function but stimulate mitophagy. On the contrary, the KD appears to induce mitochondrial function and biogenesis, while contradictory results exist for mitochondrial dynamics and mitophagy. Finally, fasting and MedD appear to induce mitochondrial biogenesis and fine-tune mitochondrial function. On the contrary, fasting appears to induce mitochondrial recycle and the clearance of problematic mitochondrial units, while the MedD has the opposite effect on mitochondrial dynamics and the autophagic process.
Availability of data and materials
Not applicable.
Authors' contributions
IDK, VNSG, DAS and DK contributed to the conception and the design of the study. IDK, EV, MA, CA, ZS, FT, VNSG and DK contributed to the drafting of the manuscript, including the literature search, and to the revisions. All authors have read and approved the final manuscript. Data authentication is not applicable.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
DAS is the Editor-in-Chief for the journal, but had no personal involvement in the reviewing process, or any influence in terms of adjudicating on the final decision, for this article. The other authors declare that they have no competing interests.
Acknowledgments
Not applicable.
Funding
No funding was received.
References
|
Jornayvaz FR and Shulman GI: Regulation of mitochondrial biogenesis. Essays Biochem. 47:69–84. 2010. View Article : Google Scholar : PubMed/NCBI | |
|
Dorn GW II, Vega RB and Kelly DP: Mitochondrial biogenesis and dynamics in the developing and diseased heart. Genes Dev. 29:1981–1991. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Qiu Z, Wei Y, Song Q, Du B, Wang H, Chu Y and Hu Y: The role of myocardial mitochondrial quality control in heart failure. Front Pharmacol. 10:14042019. View Article : Google Scholar : PubMed/NCBI | |
|
Herzig S, Long F, Jhala US, Hedrick S, Quinn R, Bauer A, Rudolph D, Schutz G, Yoon C, Puigserver P, et al: CREB regulates hepatic gluconeogenesis through the coactivator PGC-1. Nature. 413:179–183. 2001. View Article : Google Scholar : PubMed/NCBI | |
|
Wu Z, Huang X, Feng Y, Handschin C, Feng Y, Gullicksen PS, Bare O, Labow M, Spiegelman B and Stevenson SC: Transducer of regulated CREB-binding proteins (TORCs) induce PGC-1alpha transcription and mitochondrial biogenesis in muscle cells. Proc Natl Acad Sci USA. 103:14379–14384. 2006. View Article : Google Scholar : PubMed/NCBI | |
|
Au HC and Scheffler IE: Promoter analysis of the human succinate dehydrogenase iron-protein gene-both nuclear respiratory factors NRF-1 and NRF-2 are required. Eur J Biochem. 251:164–174. 1998. View Article : Google Scholar : PubMed/NCBI | |
|
Scarpulla RC: Nuclear activators and coactivators in mammalian mitochondrial biogenesis. Biochim Biophys Acta. 1576:1–14. 2002. View Article : Google Scholar : PubMed/NCBI | |
|
Goffart S and Wiesner RJ: Regulation and co-ordination of nuclear gene expression during mitochondrial biogenesis. Exp Physiol. 88:33–40. 2003. View Article : Google Scholar : PubMed/NCBI | |
|
Vega RB, Huss JM and Kelly DP: The coactivator PGC-1 cooperates with peroxisome proliferator-activated receptor alpha in transcriptional control of nuclear genes encoding mitochondrial fatty acid oxidation enzymes. Mol Cell Biol. 20:1868–1876. 2000. View Article : Google Scholar : PubMed/NCBI | |
|
Puigserver P, Wu Z, Park CW, Graves R, Wright M and Spiegelman BM: A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell. 92:829–839. 1998. View Article : Google Scholar : PubMed/NCBI | |
|
Wu Z, Puigserver P, Andersson U, Zhang C, Adelmant G, Mootha V, Troy A, Cinti S, Lowell B, Scarpulla RC and Spiegelman BM: Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell. 98:115–124. 1999. View Article : Google Scholar : PubMed/NCBI | |
|
Handschin C, Rhee J, Lin J, Tarr PT and Spiegelman BM: An autoregulatory loop controls peroxisome proliferator-activated receptor gamma coactivator 1alpha expression in muscle. Proc Natl Acad Sci USA. 100:7111–7116. 2003. View Article : Google Scholar : PubMed/NCBI | |
|
Akimoto T, Sorg BS and Yan Z: Real-time imaging of peroxisome proliferator-activated receptor-gamma coactivator-1alpha promoter activity in skeletal muscles of living mice. Am J Physiol Cell Physiol. 287:C790–C796. 2004. View Article : Google Scholar : PubMed/NCBI | |
|
Zong H, Ren JM, Young LH, Pypaert M, Mu J, Birnbaum MJ and Shulman GI: AMP kinase is required for mitochondrial biogenesis in skeletal muscle in response to chronic energy deprivation. Proc Natl Acad Sci USA. 99:15983–15987. 2002. View Article : Google Scholar : PubMed/NCBI | |
|
Daitoku H, Sakamaki J and Fukamizu A: Regulation of FoxO transcription factors by acetylation and protein-protein interactions. Biochim Biophys Acta. 1813:1954–1960. 2011. View Article : Google Scholar : PubMed/NCBI | |
|
Rodgers JT, Lerin C, Haas W, Gygi SP, Spiegelman BM and Puigserver P: Nutrient control of glucose homeostasis through a complex of PGC-1alpha and SIRT1. Nature. 434:113–118. 2005. View Article : Google Scholar : PubMed/NCBI | |
|
Liu YJ, McIntyre RL, Janssens GE and Houtkooper RH: Mitochondrial fission and fusion: A dynamic role in aging and potential target for age-related disease. Mech Ageing Dev. 186:1112122020. View Article : Google Scholar : PubMed/NCBI | |
|
Tilokani L, Nagashima S, Paupe V and Prudent J: Mitochondrial dynamics: Overview of molecular mechanisms. Essays Biochem. 62:341–360. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Mitra K, Wunder C, Roysam B, Lin G and Lippincott-Schwartz J: A hyperfused mitochondrial state achieved at G1-S regulates cyclin E buildup and entry into S phase. Proc Natl Acad Sci USA. 106:11960–11965. 2009. View Article : Google Scholar : PubMed/NCBI | |
|
Sharma A, Ahmad S, Ahmad T, Ali S and Syed MA: Mitochondrial dynamics and mitophagy in lung disorders. Life Sci. 284:1198762021. View Article : Google Scholar : PubMed/NCBI | |
|
Margineantu DH, Gregory Cox W, Sundell L, Sherwood SW, Beechem JM and Capaldi RA: Cell cycle dependent morphology changes and associated mitochondrial DNA redistribution in mitochondria of human cell lines. Mitochondrion. 1:425–435. 2002. View Article : Google Scholar | |
|
Mitra K: Mitochondrial fission-fusion as an emerging key regulator of cell proliferation and differentiation. Bioessays. 35:955–964. 2013. View Article : Google Scholar : PubMed/NCBI | |
|
Amiri M and Hollenbeck PJ: Mitochondrial biogenesis in the axons of vertebrate peripheral neurons. Dev Neurobiol. 68:1348–1361. 2008. View Article : Google Scholar : PubMed/NCBI | |
|
Mizushima N: Autophagy: Process and function. Genes Dev. 21:2861–2873. 2007. View Article : Google Scholar : PubMed/NCBI | |
|
Bursch W, Ellinger A, Kienzl H, Török L, Pandey S, Sikorska M, Walker R and Hermann RS: Active cell death induced by the anti-estrogens tamoxifen and ICI 164 384 in human mammary carcinoma cells (MCF-7) in culture: The role of autophagy. Carcinogenesis. 17:1595–1607. 1996. View Article : Google Scholar : PubMed/NCBI | |
|
Yu L, Strandberg L and Lenardo MJ: The selectivity of autophagy and its role in cell death and survival. Autophagy. 4:567–573. 2008. View Article : Google Scholar : PubMed/NCBI | |
|
Petrovski G and Das DK: Does autophagy take a front seat in lifespan extension? J Cell Mol Med. 14:2543–2551. 2010. View Article : Google Scholar : PubMed/NCBI | |
|
Chen G, Kroemer G and Kepp O: Mitophagy: An emerging role in aging and age-associated diseases. Front Cell Dev Biol. 8:2002020. View Article : Google Scholar : PubMed/NCBI | |
|
Klionsky DJ, Cregg JM, Dunn WA Jr, Emr SD, Sakai Y, Sandoval IV, Sibirny A, Subramani S, Thumm M, Veenhuis M and Ohsumi Y: A unified nomenclature for yeast autophagy-related genes. Dev Cell. 5:539–545. 2003. View Article : Google Scholar : PubMed/NCBI | |
|
Nakatogawa H, Suzuki K, Kamada Y and Ohsumi Y: Dynamics and diversity in autophagy mechanisms: Lessons from yeast. Nat Rev Mol Cell Biol. 10:458–467. 2009. View Article : Google Scholar : PubMed/NCBI | |
|
Rogov VV, Suzuki H, Marinković M, Lang V, Kato R, Kawasaki M, Buljubašić M, Šprung M, Rogova N, Wakatsuki S, et al: Phosphorylation of the mitochondrial autophagy receptor Nix enhances its interaction with LC3 proteins. Sci Rep. 7:11312017. View Article : Google Scholar : PubMed/NCBI | |
|
Ding WX, Ni HM, Li M, Liao Y, Chen X, Stolz DB, Dorn GW II and Yin XM: Nix is critical to two distinct phases of mitophagy, reactive oxygen species-mediated autophagy induction and Parkin-ubiquitin-p62-mediated mitochondrial priming. J Biol Chem. 285:27879–27890. 2010. View Article : Google Scholar : PubMed/NCBI | |
|
Springer W and Kahle PJ: Regulation of PINK1-Parkin-mediated mitophagy. Autophagy. 7:266–278. 2011. View Article : Google Scholar | |
|
Itakura E, Kishi-Itakura C, Koyama-Honda I and Mizushima N: Structures containing Atg9A and the ULK1 complex independently target depolarized mitochondria at initial stages of Parkin-mediated mitophagy. J Cell Sci. 125:1488–1499. 2012.PubMed/NCBI | |
|
Chaanine AH, Kohlbrenner E, Gamb SI, Guenzel AJ, Klaus K, Fayyaz AU, Nair KS, Hajjar RJ and Redfield MM: FOXO3a regulates BNIP3 and modulates mitochondrial calcium, dynamics, and function in cardiac stress. Am J Physiol Heart Circ Physiol. 311:H1540–H1559. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Mammucari C, Milan G, Romanello V, Masiero E, Rudolf R, Del Piccolo P, Burden SJ, Di Lisi R, Sandri C, Zhao J, et al: FoxO3 controls autophagy in skeletal muscle in vivo. Cell Metab. 6:458–471. 2007. View Article : Google Scholar : PubMed/NCBI | |
|
Twig G, Elorza A, Molina AJ, Mohamed H, Wikstrom JD, Walzer G, Stiles L, Haigh SE, Katz S, Las G, et al: Fission and selective fusion govern mitochondrial segregation and elimination by autophagy. EMBO J. 27:433–446. 2008. View Article : Google Scholar : PubMed/NCBI | |
|
Tait SW and Green DR: Mitochondria and cell signalling. J Cell Sci. 125:807–815. 2012. View Article : Google Scholar : PubMed/NCBI | |
|
Navale AM and Paranjape AN: Glucose transporters: Physiological and pathological roles. Biophys Rev. 8:5–9. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
McCommis KS and Finck BN: Mitochondrial pyruvate transport: A historical perspective and future research directions. Biochem J. 466:443–454. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Glancy B, Kane DA, Kavazis AN, Goodwin ML, Willis WT and Gladden LB: Mitochondrial lactate metabolism: History and implications for exercise and disease. J Physiol. 599:863–888. 2021. View Article : Google Scholar | |
|
Yoo HC, Yu YC, Sung Y and Han JM: Glutamine reliance in cell metabolism. Exp Mol Med. 52:1496–1516. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Stefano GB, Challenger S and Kream RM: Hyperglycemia-associated alterations in cellular signaling and dysregulated mitochondrial bioenergetics in human metabolic disorders. Eur J Nutr. 55:2339–2345. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Melser S, Lavie J and Bénard G: Mitochondrial degradation and energy metabolism. Biochim Biophys Acta. 1853:2812–2821. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
King KL, Stanley WC, Rosca M, Kerner J, Hoppel CL and Febbraio M: Fatty acid oxidation in cardiac and skeletal muscle mitochondria is unaffected by deletion of CD36. Arch Biochem Biophys. 467:234–238. 2007. View Article : Google Scholar : PubMed/NCBI | |
|
Duncan RE, Ahmadian M, Jaworski K, Sarkadi-Nagy E and Sul HS: Regulation of lipolysis in adipocytes. Annu Rev Nutr. 27:79–101. 2007. View Article : Google Scholar : PubMed/NCBI | |
|
Lodhi IJ and Semenkovich CF: Peroxisomes: A nexus for lipid metabolism and cellular signaling. Cell Metab. 19:380–392. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
Fillmore N and Lopaschuk GD: Targeting mitochondrial oxidative metabolism as an approach to treat heart failure. Biochim Biophys Acta. 1833:857–865. 2013. View Article : Google Scholar | |
|
Aon MA, Bhatt N and Cortassa SC: Mitochondrial and cellular mechanisms for managing lipid excess. Front Physiol. 5:2822014. View Article : Google Scholar : PubMed/NCBI | |
|
Carley AN, Taegtmeyer H and Lewandowski ED: Matrix revisited: Mechanisms linking energy substrate metabolism to the function of the heart. Circ Res. 114:717–729. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
Keys A, Menotti A, Karvonen MJ, Aravanis C, Blackburn H, Buzina R, Djordjevic BS, Dontas AS, Fidanza F, Keys MH, et al: The diet and 15-year death rate in the seven countries study. Am J Epidemiol. 124:903–915. 1986. View Article : Google Scholar : PubMed/NCBI | |
|
Schwingshackl L and Hoffmann G: Comparison of effects of long-term low-fat vs high-fat diets on blood lipid levels in over-weight or obese patients: A systematic review and meta-analysis. J Acad Nutr Diet. 113:1640–1661. 2013. View Article : Google Scholar : PubMed/NCBI | |
|
Casper RC, Sullivan EL and Tecott L: Relevance of animal models to human eating disorders and obesity. Psychopharmacology (Berl). 199:313–329. 2008. View Article : Google Scholar | |
|
Della Vedova MC, Muñoz MD, Santillan LD, Plateo-Pignatari MG, Germanó MJ, Rinaldi Tosi ME, Garcia S, Gomez NN, Fornes MW, Gomez Mejiba SE and Ramirez DC: A mouse model of diet-induced obesity resembling most features of human metabolic syndrome. Nutr Metab Insights. 9:93–102. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Schrauwen P, Schrauwen-Hinderling V, Hoeks J and Hesselink MK: Mitochondrial dysfunction and lipotoxicity. Biochim Biophys Acta. 1801:266–271. 2010. View Article : Google Scholar | |
|
Jo J, Gavrilova O, Pack S, Jou W, Mullen S, Sumner AE, Cushman SW and Periwal V: Hypertrophy and/or hyperplasia: Dynamics of adipose tissue growth. PLoS Comput Biol. 5:e10003242009. View Article : Google Scholar : PubMed/NCBI | |
|
Yang A and Mottillo EP: Adipocyte lipolysis: From molecular mechanisms of regulation to disease and therapeutics. Biochem J. 477:985–1008. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Carracedo A, Cantley LC and Pandolfi PP: Cancer metabolism: Fatty acid oxidation in the limelight. Nat Rev Cancer. 13:227–232. 2013. View Article : Google Scholar : PubMed/NCBI | |
|
Roy C, Paglialunga S, Fisette A, Schrauwen P, Moonen-Kornips E, St-Onge J, Hesselink MK, Richard D, Joanisse DR and Cianflone K: Shift in metabolic fuel in acylation-stimulating protein-deficient mice following a high-fat diet. Am J Physiol Endocrinol Metab. 294:E1051–E1059. 2008. View Article : Google Scholar : PubMed/NCBI | |
|
Chen Z, Tao S, Li X and Yao Q: Resistin destroys mitochondrial biogenesis by inhibiting the PGC-1α/NRF1/TFAM signaling pathway. Biochem Biophys Res Commun. 504:13–18. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Blanquer-Rosselló MM, Santandreu FM, Oliver J, Roca P and Valle A: Leptin modulates mitochondrial function, dynamics and biogenesis in MCF-7 cells. J Cell Biochem. 116:2039–2048. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Yamauchi T and Kadowaki T: Physiological and pathophysiological roles of adiponectin and adiponectin receptors in the integrated regulation of metabolic and cardiovascular diseases. Int J Obes (Lond). 32(Suppl 7): S13–S18. 2008. View Article : Google Scholar | |
|
Ouchi N, Parker JL, Lugus JJ and Walsh K: Adipokines in inflammation and metabolic disease. Nat Rev Immunol. 11:85–97. 2011. View Article : Google Scholar : PubMed/NCBI | |
|
Cantó C and Auwerx J: PGC-1alpha, SIRT1 and AMPK, an energy sensing network that controls energy expenditure. Curr Opin Lipidol. 20:98–105. 2009. View Article : Google Scholar : PubMed/NCBI | |
|
Sparks LM, Xie H, Koza RA, Mynatt R, Hulver MW, Bray GA and Smith SR: A high-fat diet coordinately downregulates genes required for mitochondrial oxidative phosphorylation in skeletal muscle. Diabetes. 54:1926–1933. 2005. View Article : Google Scholar : PubMed/NCBI | |
|
Chen D, Li X, Zhang L, Zhu M and Gao L: A high-fat diet impairs mitochondrial biogenesis, mitochondrial dynamics, and the respiratory chain complex in rat myocardial tissues. J Cell Biochem. 119:96022018. View Article : Google Scholar : PubMed/NCBI | |
|
Kang KW, Kim OS, Chin JY, Kim WH, Park SH, Choi YJ, Shin JH, Jung KT, Lim DS and Lee SK: Diastolic dysfunction induced by a high-fat diet is associated with mitochondrial abnormality and adenosine triphosphate levels in rats. Endocrinol Metab (Seoul). 30:557–568. 2015. View Article : Google Scholar | |
|
Turner N, Bruce CR, Beale SM, Hoehn KL, So T, Rolph MS and Cooney GJ: Excess lipid availability increases mitochondrial fatty acid oxidative capacity in muscle: Evidence against a role for reduced fatty acid oxidation in lipid-induced insulin resistance in rodents. Diabetes. 56:2085–2092. 2007. View Article : Google Scholar : PubMed/NCBI | |
|
Hancock CR, Han DH, Chen M, Terada S, Yasuda T, Wright DC and Holloszy JO: High-fat diets cause insulin resistance despite an increase in muscle mitochondria. Proc Natl Acad Sci USA. 105:7815–7820. 2008. View Article : Google Scholar : PubMed/NCBI | |
|
Battiprolu PK, Hojayev B, Jiang N, Wang ZV, Luo X, Iglewski M, Shelton JM, Gerard RD, Rothermel BA, Gillette TG, et al: Metabolic stress-induced activation of FoxO1 triggers diabetic cardiomyopathy in mice. J Clin Invest. 122:1109–1118. 2012. View Article : Google Scholar : PubMed/NCBI | |
|
Kyriazis ID, Hoffman M, Gaignebet L, Lucchese AM, Markopoulou E, Palioura D, Wang C, Bannister TD, Christofidou-Solomidou M, Oka SI, et al: KLF5 is induced by FOXO1 and causes oxidative stress and diabetic cardiomyopathy. Circ Res. 128:335–357. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Li N, Li HP, Zhang BY, Zhang L, Shen JM and Li QY: Effect of high-fat diet on respiratory function and diaphragm fibers in mice and its mitochondrial mechanism. Zhonghua Yi Xue Za Zhi. 101:2893–2899. 2021.In Chinese. PubMed/NCBI | |
|
Kang YS, Seong D, Kim JC and Kim SH: Low-intensity exercise training additionally increases mitochondrial dynamics caused by high-fat diet (HFD) but has no additional effect on mitochondrial biogenesis in fast-twitch muscle by HFD. Int J Environ Res Public Health. 17:54612020. View Article : Google Scholar : | |
|
Heo JW, No MH, Cho J, Choi Y, Cho EJ, Park DH, Kim TW, Kim CJ, Seo DY, Han J, et al: Moderate aerobic exercise training ameliorates impairment of mitochondrial function and dynamics in skeletal muscle of high-fat diet-induced obese mice. FASEB J. 35:e213402021. View Article : Google Scholar : PubMed/NCBI | |
|
Tarpey MD, Davy KP, McMillan RP, Bowser SM, Halliday TM, Boutagy NE, Davy BM, Frisard MI and Hulver MW: Skeletal muscle autophagy and mitophagy in endurance-trained runners before and after a high-fat meal. Mol Metab. 6:1597–1609. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Tong M, Saito T, Zhai P, Oka SI, Mizushima W, Nakamura M, Ikeda S, Shirakabe A and Sadoshima J: Mitophagy is essential for maintaining cardiac function during high fat diet-induced diabetic cardiomyopathy. Circ Res. 124:1360–1371. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Shao D, Kolwicz SC Jr, Wang P, Roe ND, Villet O, Nishi K, Hsu YA, Flint GV, Caudal A, Wang W, et al: Increasing fatty acid oxidation prevents high-fat diet-induced cardiomyopathy through regulating parkin-mediated mitophagy. Circulation. 142:983–997. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Rous P: The influence of diet on transplanted and spontaneous mouse tumors. J Exp Med. 20:433–451. 1914. View Article : Google Scholar : PubMed/NCBI | |
|
Fontana L and Partridge L: Promoting health and longevity through diet: From model organisms to humans. Cell. 161:106–118. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Holloszy JO and Fontana L: Caloric restriction in humans. Exp Gerontol. 42:709–712. 2007. View Article : Google Scholar : PubMed/NCBI | |
|
Pignatti C, D'Adamo S, Stefanelli C, Flamigni F and Cetrullo S: Nutrients and pathways that regulate health span and life span. Geriatrics (Basel). 5:952020. View Article : Google Scholar | |
|
Ruetenik A and Barrientos A: Dietary restriction, mitochondrial function and aging: From yeast to humans. Biochim Biophys Acta. 1847:1434–1447. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Comfort A: Effect of delayed and resumed growth on the longevity of a fish (lebistes reticulatus, peters) in captivity. Gerontologia. 49:150–155. 1963. View Article : Google Scholar : PubMed/NCBI | |
|
Romey-Glüsing R, Li Y, Hoffmann J, von Frieling J, Knop M, Pfefferkorn R, Bruchhaus I, Fink C and Roeder T: Nutritional regimens with periodically recurring phases of dietary restriction extend lifespan in Drosophila. FASEB J. 32:1993–2003. 2018. View Article : Google Scholar | |
|
Colman RJ, Beasley TM, Kemnitz JW, Johnson SC, Weindruch R and Anderson RM: Caloric restriction reduces age-related and all-cause mortality in rhesus monkeys. Nat Commun. 5:35572014. View Article : Google Scholar : PubMed/NCBI | |
|
Seyfried TN: Ketone strong: Emerging evidence for a therapeutic role of ketone bodies in neurological and neurodegenerative diseases. J Lipid Res. 55:1815–1817. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
Fulco M, Cen Y, Zhao P, Hoffman EP, McBurney MW, Sauve AA and Sartorelli V: Glucose restriction inhibits skeletal myoblast differentiation by activating SIRT1 through AMPK-mediated regulation of Nampt. Dev Cell. 14:661–673. 2008. View Article : Google Scholar : PubMed/NCBI | |
|
Xiong S, Salazar G, Patrushev N and Alexander RW: FoxO1 mediates an autofeedback loop regulating SIRT1 expression. J Biol Chem. 286:5289–5299. 2011. View Article : Google Scholar : | |
|
López-Lluch G, Hunt N, Jones B, Zhu M, Jamieson H, Hilmer S, Cascajo MV, Allard J, Ingram DK, Navas P and de Cabo R: Calorie restriction induces mitochondrial biogenesis and bioenergetic efficiency. Proc Natl Acad Sci USA. 103:1768–1773. 2006. View Article : Google Scholar : PubMed/NCBI | |
|
Hempenstall S, Page MM, Wallen KR and Selman C: Dietary restriction increases skeletal muscle mitochondrial respiration but not mitochondrial content in C57BL/6 mice. Mech Ageing Dev. 133:37–45. 2012. View Article : Google Scholar : PubMed/NCBI | |
|
Palacios OM, Carmona JJ, Michan S, Chen KY, Manabe Y, Ward JL III, Goodyear LJ and Tong Q: Diet and exercise signals regulate SIRT3 and activate AMPK and PGC-1alpha in skeletal muscle. Aging (Albany NY). 1:771–783. 2009. View Article : Google Scholar | |
|
Picca A, Pesce V, Fracasso F, Joseph AM, Leeuwenburgh C and Lezza AM: Aging and calorie restriction oppositely affect mitochondrial biogenesis through TFAM binding at both origins of mitochondrial DNA replication in rat liver. PLoS One. 8:e746442013. View Article : Google Scholar : PubMed/NCBI | |
|
Zhu M, Miura J, Lu LX, Bernier M, DeCabo R, Lane MA, Roth GS and Ingram DK: Circulating adiponectin levels increase in rats on caloric restriction: The potential for insulin sensitization. Exp Gerontol. 39:1049–1059. 2004. View Article : Google Scholar : PubMed/NCBI | |
|
Civitarese AE, Carling S, Heilbronn LK, Hulver MH, Ukropcova B, Deutsch WA, Smith SR and Ravussin E; CALERIE Pennington Team: Calorie restriction increases muscle mitochondrial biogenesis in healthy humans. PLoS Med. 4:e762007. View Article : Google Scholar : PubMed/NCBI | |
|
Herzig S and Shaw RJ: AMPK: Guardian of metabolism and mitochondrial homeostasis. Nat Rev Mol Cell Biol. 19:121–135. 2018. View Article : Google Scholar : | |
|
López-Lluch G and Navas P: Calorie restriction as an intervention in ageing. J Physiol. 594:2043–2060. 2016. View Article : Google Scholar : | |
|
Feldman JL, Dittenhafer-Reed KE and Denu JM: Sirtuin catalysis and regulation. J Biol Chem. 287:42419–42427. 2012. View Article : Google Scholar : PubMed/NCBI | |
|
Desai VG, Weindruch R, Hart RW and Feuers RJ: Influences of age and dietary restriction on gastrocnemius electron transport system activities in mice. Arch Biochem Biophys. 333:145–151. 1996. View Article : Google Scholar : PubMed/NCBI | |
|
Hepple RT, Baker DJ, McConkey M, Murynka T and Norris R: Caloric restriction protects mitochondrial function with aging in skeletal and cardiac muscles. Rejuvenation Res. 9:219–222. 2006. View Article : Google Scholar : PubMed/NCBI | |
|
Colom B, Oliver J, Roca P and Garcia-Palmer FJ: Caloric restriction and gender modulate cardiac muscle mitochondrial H2O2 production and oxidative damage. Cardiovasc Res. 74:456–465. 2007. View Article : Google Scholar : PubMed/NCBI | |
|
Hepple RT, Baker DJ, Kaczor JJ and Krause DJ: Long-term caloric restriction abrogates the age-related decline in skeletal muscle aerobic function. FASEB J. 19:1320–1322. 2005. View Article : Google Scholar : PubMed/NCBI | |
|
Sreekumar R, Unnikrishnan J, Fu A, Nygren J, Short KR, Schimke J, Barazzoni R and Nair KS: Effects of caloric restriction on mitochondrial function and gene transcripts in rat muscle. Am J Physiol Endocrinol Metab. 283:E38–E43. 2002. View Article : Google Scholar : PubMed/NCBI | |
|
Drew B, Phaneuf S, Dirks A, Selman C, Gredilla R, Lezza A, Barja G and Leeuwenburgh C: Effects of aging and caloric restriction on mitochondrial energy production in gastrocnemius muscle and heart. Am J Physiol Regul Integr Comp Physiol. 284:R474–R480. 2003. View Article : Google Scholar | |
|
Lam YY, Ghosh S, Civitarese AE and Ravussin E: Six-month calorie restriction in overweight individuals elicits transcriptomic response in subcutaneous adipose tissue that is distinct from effects of energy deficit. J Gerontol A Biol Sci Med Sci. 71:1258–1265. 2016. View Article : Google Scholar | |
|
Menshikova EV, Ritov VB, Dube JJ, Amati F, Stefanovic-Racic M, Toledo FGS, Coen PM and Goodpaster BH: Calorie restriction-induced weight loss and exercise have differential effects on skeletal muscle mitochondria despite similar effects on insulin sensitivity. J Gerontol A Biol Sci Med Sci. 73:81–87. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Bagherniya M, Butler AE, Barreto GE and Sahebkar A: The effect of fasting or calorie restriction on autophagy induction: A review of the literature. Ageing Res Rev. 47:183–197. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Gutiérrez-Casado E, Khraiwesh H, López-Domínguez JA, Montero-Guisado J, López-Lluch G, Navas P, de Cabo R, Ramsey JJ, González-Reyes JA and Villalba JM: The impact of aging, calorie restriction and dietary fat on autophagy markers and mitochondrial ultrastructure and dynamics in mouse skeletal muscle. J Gerontol A Biol Sci Med Sci. 74:760–769. 2019. View Article : Google Scholar : | |
|
Cui J, Shi S, Sun X, Cai G, Cui S, Hong Q, Chen X and Bai XY: Mitochondrial autophagy involving renal injury and aging is modulated by caloric intake in aged rat kidneys. PLoS One. 8:e697202013. View Article : Google Scholar : PubMed/NCBI | |
|
Kim I and Lemasters JJ: Mitochondrial degradation by autophagy (mitophagy) in GFP-LC3 transgenic hepatocytes during nutrient deprivation. Am J Physiol Cell Physiol. 300:C308–C317. 2011. View Article : Google Scholar : | |
|
Rodríguez-López S, López-Bellón S, González-Reyes JA, Burón MI, de Cabo R and Villalba JM: Mitochondrial adaptations in liver and skeletal muscle to pro-longevity nutritional and genetic interventions: The crosstalk between calorie restriction and CYB5R3 overexpression in transgenic mice. Geroscience. 42:977–994. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Faitg J, Leduc-Gaudet JP, Reynaud O, Ferland G, Gaudreau P and Gouspillou G: Effects of aging and caloric restriction on fiber type composition, mitochondrial morphology and dynamics in rat oxidative and glycolytic muscles. Front Physiol. 10:4202019. View Article : Google Scholar : PubMed/NCBI | |
|
Khraiwesh H, López-Domínguez JA, Fernández del Río L, Gutierrez-Casado E, López-Lluch G, Navas P, de Cabo R, Ramsey JJ, Burón MI, Villalba JM and González-Reyes JA: Mitochondrial ultrastructure and markers of dynamics in hepatocytes from aged, calorie restricted mice fed with different dietary fats. Exp Gerontol. 56:77–88. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
Khraiwesh H, López-Domínguez JA, López-Lluch G, Navas P, de Cabo R, Ramsey JJ, Villalba JM and González-Reyes JA: Alterations of ultrastructural and fission/fusion markers in hepatocyte mitochondria from mice following calorie restriction with different dietary fats. J Gerontol A Biol Sci Med Sci. 68:1023–1034. 2013. View Article : Google Scholar : PubMed/NCBI | |
|
Kitaoka Y, Nakazato K and Ogasawara R: Combined effects of resistance training and calorie restriction on mitochondrial fusion and fission proteins in rat skeletal muscle. J Appl Physiol (1985). 121:806–810. 2016. View Article : Google Scholar | |
|
Pattanakuhar S, Sutham W, Sripetchwandee J, Minta W, Mantor D, Palee S, Pratchayasakul W, Chattipakorn N and Chattipakorn SC: Combined exercise and calorie restriction therapies restore contractile and mitochondrial functions in skeletal muscle of obese-insulin resistant rats. Nutrition. 62:74–84. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Kang HC, Lee YM, Kim HD, Lee JS and Slama A: Safe and effective use of the ketogenic diet in children with epilepsy and mitochondrial respiratory chain complex defects. Epilepsia. 48:82–88. 2007. View Article : Google Scholar : PubMed/NCBI | |
|
Qu C, Keijer J, Adjobo-Hermans MJW, van de Wal M, Schirris T, van Karnebeek C, Pan Y and Koopman WJH: The ketogenic diet as a therapeutic intervention strategy in mitochondrial disease. Int J Biochem Cell Biol. 138:1060502021. View Article : Google Scholar : PubMed/NCBI | |
|
Dhamija R, Eckert S and Wirrell E: Ketogenic diet. Can J Neurol Sci. 40:158–167. 2013. View Article : Google Scholar : PubMed/NCBI | |
|
Olgac A, İnci A, Okur İ, Biberoğlu G, Oğuz D, Ezgü FS, Kasapkara ÇS, Aktaş E and Tümer L: Beneficial effects of modified atkins diet in glycogen storage disease type IIIa. Ann Nutr Metab. 76:233–241. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Augustin K, Khabbush A, Williams S, Eaton S, Orford M, Cross JH, Heales SJR, Walker MC and Williams RSB: Mechanisms of action for the medium-chain triglyceride ketogenic diet in neurological and metabolic disorders. Lancet Neurol. 17:84–93. 2018. View Article : Google Scholar | |
|
Morris G, Maes M, Berk M, Carvalho AF and Puri BK: Nutritional ketosis as an intervention to relieve astrogliosis: Possible therapeutic applications in the treatment of neurodegenerative and neuroprogressive disorders. Eur Psychiatry. 63:e82020. View Article : Google Scholar : PubMed/NCBI | |
|
Miller VJ, Villamena FA and Volek JS: Nutritional ketosis and mitohormesis: Potential implications for mitochondrial function and human health. J Nutr Metab. 2018:51576452018. View Article : Google Scholar : PubMed/NCBI | |
|
Milder J and Patel M: Modulation of oxidative stress and mitochondrial function by the ketogenic diet. Epilepsy Res. 100:295–303. 2012. View Article : Google Scholar : | |
|
Hutfles LJ, Wilkins HM, Koppel SJ, Weidling IW, Selfridge JE, Tan E, Thyfault JP, Slawson C, Fenton AW, Zhu H and Swerdlow RH: A bioenergetics systems evaluation of ketogenic diet liver effects. Appl Physiol Nutr Metab. 42:955–962. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Hyatt HW, Kephart WC, Holland AM, Mumford P, Mobley CB, Lowery RP, Roberts MD, Wilson JM and Kavazis AN: A ketogenic diet in rodents elicits improved mitochondrial adaptations in response to resistance exercise training compared to an isocaloric western diet. Front Physiol. 7:5332016. View Article : Google Scholar : PubMed/NCBI | |
|
Kennedy AR, Pissios P, Out H, Roberson R, Xue B, Asakura K, Furukawa N, Marino FE, Liu FF, Kahn BB, et al: A high-fat, ketogenic diet induces a unique metabolic state in mice. Am J Physiol Endocrinol Metab. 292:E1724–E1739. 2007. View Article : Google Scholar : PubMed/NCBI | |
|
Wallace MA, Aguirre NW, Marcotte GR, Marshall AG, Baehr LM, Hughes DC, Hamilton KL, Roberts MN, Lopez-Dominguez JA, Miller BF, et al: The ketogenic diet preserves skeletal muscle with aging in mice. Aging Cell. 20:e133222021. View Article : Google Scholar : PubMed/NCBI | |
|
Parry HA, Kephart WC, Mumford PW, Romero MA, Mobley CB, Zhang Y, Roberts MD and Kavazis AN: Ketogenic diet increases mitochondria volume in the liver and skeletal muscle without altering oxidative stress markers in rats. Heliyon. 4:e009752018. View Article : Google Scholar : PubMed/NCBI | |
|
Zhou Z, Hagopian K, López-Domínguez JA, Kim K, Jasoliya M, Roberts MN, Cortopassi GA, Showalter MR, Roberts BS, González-Reyes JA, et al: A ketogenic diet impacts markers of mitochondrial mass in a tissue specific manner in aged mice. Aging (Albany NY). 13:7914–7930. 2021. View Article : Google Scholar | |
|
Kephart WC, Mumford PW, Mao X, Romero MA, Hyatt HW, Zhang Y, Mobley CB, Quindry JC, Young KC, Beck DT, et al: The 1-week and 8-month effects of a ketogenic diet or ketone salt supplementation on multi-organ markers of oxidative stress and mitochondrial function in rats. Nutrients. 9:10192017. View Article : Google Scholar : | |
|
Xu S, Tao H, Cao W, Cao L, Lin Y, Zhao SM, Xu W, Cao J and Zhao JY: Ketogenic diets inhibit mitochondrial biogenesis and induce cardiac fibrosis. Signal Transduct Target Ther. 6:542021. View Article : Google Scholar : PubMed/NCBI | |
|
Santra S, Gilkerson RW, Davidson M and Schon EA: Ketogenic treatment reduces deleted mitochondrial DNAs in cultured human cells. Ann Neurol. 56:662–669. 2004. View Article : Google Scholar : PubMed/NCBI | |
|
Ahn Y, Sabouny R, Villa BR, Yee NC, Mychasiuk R, Uddin GM, Rho JM and Shutt TE: Aberrant mitochondrial morphology and function in the BTBR mouse model of autism is improved by two weeks of ketogenic diet. Int J Mol Sci. 21:32662020. View Article : Google Scholar : | |
|
Miller VJ, LaFountain RA, Barnhart E, Sapper TS, Short J, Arnold WD, Hyde PN, Crabtree CD, Kackley ML, Kraemer WJ, et al: A ketogenic diet combined with exercise alters mitochondrial function in human skeletal muscle while improving metabolic health. Am J Physiol Endocrinol Metab. 319:E995–E1007. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Parker BA, Walton CM, Carr ST, Andrus JL, Cheung ECK, Duplisea MJ, Wilson EK, Draney C, Lathen DR, Kenner KB, et al: β-Hydroxybutyrate elicits favorable mitochondrial changes in skeletal muscle. Int J Mol Sci. 19:22472018. View Article : Google Scholar | |
|
Guo Y, Zhang C, Shang FF, Luo M, You Y, Zhai Q, Xia Y and Suxin L: Ketogenic diet ameliorates cardiac dysfunction via balancing mitochondrial dynamics and inhibiting apoptosis in type 2 diabetic mice. Aging Dis. 11:229–240. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Krebs P, Fan W, Chen YH, Tobita K, Downes MR, Wood MR, Sun L, Li X, Xia Y, Ding N, et al: Lethal mitochondrial cardiomyopathy in a hypomorphic Med30 mouse mutant is ameliorated by ketogenic diet. Proc Natl Acad Sci USA. 108:19678–19682. 2011. View Article : Google Scholar : PubMed/NCBI | |
|
Ahola-Erkkilä S, Carroll CJ, Peltola-Mjösund K, Tulkki V, Mattila I, Seppänen-Laakso T, Oresic M, Tyynismaa H and Suomalainen A: Ketogenic diet slows down mitochondrial myopathy progression in mice. Hum Mol Genet. 19:1974–1984. 2010. View Article : Google Scholar : PubMed/NCBI | |
|
Srivastava S, Kashiwaya Y, King MT, Baxa U, Tam J, Niu G, Chen X, Clarke K and Veech RL: Mitochondrial biogenesis and increased uncoupling protein 1 in brown adipose tissue of mice fed a ketone ester diet. FASEB J. 26:2351–2362. 2012. View Article : Google Scholar : PubMed/NCBI | |
|
Sullivan PG, Rippy NA, Dorenbos K, Concepcion RC, Agarwal AK and Rho JM: The ketogenic diet increases mitochondrial uncoupling protein levels and activity. Ann Neurol. 55:576–580. 2004. View Article : Google Scholar : PubMed/NCBI | |
|
Moore MP, Cunningham RP, Kelty TJ, Boccardi LR, Nguyen NY, Booth FW and Rector RS: Ketogenic diet in combination with voluntary exercise impacts markers of hepatic metabolism and oxidative stress in male and female Wistar rats. Appl Physiol Nutr Metab. 45:35–44. 2020. View Article : Google Scholar | |
|
Jornayvaz FR, Jurczak MJ, Lee HY, Birkenfeld AL, Frederick DW, Zhang D, Zhang XM, Samuel VT and Shulman GI: A high-fat, ketogenic diet causes hepatic insulin resistance in mice, despite increasing energy expenditure and preventing weight gain. Am J Physiol Endocrinol Metab. 299:E808–E815. 2010. View Article : Google Scholar : PubMed/NCBI | |
|
Huang TY, Linden MA, Fuller SE, Goldsmith FR, Simon J, Batdorf HM, Scott MC, Essajee NM, Brown JM and Noland RC: Combined effects of a ketogenic diet and exercise training alter mitochondrial and peroxisomal substrate oxidative capacity in skeletal muscle. Am J Physiol Endocrinol Metab. 320:E1053–E1067. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Bough KJ, Wetherington J, Hassel B, Pare JF, Gawryluk JW, Greene JG, Shaw R, Smith Y, Geiger JD and Dingledine RJ: Mitochondrial biogenesis in the anticonvulsant mechanism of the ketogenic diet. Ann Neurol. 60:223–235. 2006. View Article : Google Scholar : PubMed/NCBI | |
|
Newell C, Shutt TE, Ahn Y, Hittel DS, Khan A, Rho JM and Shearer J: Tissue specific impacts of a ketogenic diet on mitochondrial dynamics in the BTBRT+tf/j mouse. Front Physiol. 7:6542016. View Article : Google Scholar | |
|
Thai PN, Seidlmayer LK, Miller C, Ferrero M, Dorn GW II, Schaefer S, Bers DM and Dedkova EN: Mitochondrial quality control in aging and heart failure: Influence of ketone bodies and mitofusin-stabilizing peptides. Front Physiol. 10:3822019. View Article : Google Scholar : PubMed/NCBI | |
|
Guo M, Wang X, Zhao Y, Yang Q, Ding H, Dong Q, Chen X and Cui M: Ketogenic diet improves brain ischemic tolerance and inhibits NLRP3 inflammasome activation by preventing Drp1-mediated mitochondrial fission and endoplasmic reticulum stress. Front Mol Neurosci. 11:862018. View Article : Google Scholar : PubMed/NCBI | |
|
Anton SD, Moehl K, Donahoo WT, Marosi K, Lee SA, Mainous AG III, Leeuwenburgh C and Mattson MP: Flipping the metabolic switch: Understanding and applying the health benefits of fasting. Obesity (Silver Spring). 26:254–268. 2018. View Article : Google Scholar | |
|
Liu B, Hutchison AT, Thompson CH, Lange K, Wittert GA and Heilbronn LK: Effects of intermittent fasting or calorie restriction on markers of lipid metabolism in human skeletal muscle. J Clin Endocrinol Metab. 106:e1389–e1399. 2021. View Article : Google Scholar | |
|
Mattson MP, Longo VD and Harvie M: Impact of intermittent fasting on health and disease processes. Ageing Res Rev. 39:46–58. 2017. View Article : Google Scholar : | |
|
Ravanidis S, Grundler F, de Toledo FW, Dimitriou E, Tekos F, Skaperda Z, Kouretas D and Doxakis E: Fasting-mediated metabolic and toxicity reprogramming impacts circulating microRNA levels in humans. Food Chem Toxicol. 152:1121872021. View Article : Google Scholar : PubMed/NCBI | |
|
Grundler F, Mesnage R, Goutzourelas N, Tekos F, Makri S, Brack M, Kouretas D and Wilhelmi de Toledo F: Interplay between oxidative damage, the redox status, and metabolic biomarkers during long-term fasting. Food Chem Toxicol. 145:1117012020. View Article : Google Scholar : PubMed/NCBI | |
|
Wilhelmi de Toledo F, Grundler F, Goutzourelas N, Tekos F, Vassi E, Mesnage R and Kouretas D: Influence of long-term fasting on blood redox status in humans. Antioxidants (Basel). 9:4962020. View Article : Google Scholar | |
|
de Cabo R and Mattson MP: Effects of intermittent fasting on health, aging, and disease. N Engl J Med. 381:2541–2551. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Klimova N, Long A, Scafidi S and Kristian T: Interplay between NAD+ and acetyl-CoA metabolism in ischemia-induced mitochondrial pathophysiology. Biochim Biophys Acta Mol Basis Dis. 1865:2060–2067. 2019. View Article : Google Scholar | |
|
Ren Z, He H, Zuo Z, Xu Z, Wei Z and Deng J: The role of different SIRT1-mediated signaling pathways in toxic injury. Cell Mol Biol Lett. 24:362019. View Article : Google Scholar : PubMed/NCBI | |
|
Antunes F, Erustes AG, Costa AJ, Nascimento AC, Bincoletto C, Ureshino RP, Pereira GJS and Smaili SS: Autophagy and intermittent fasting: The connection for cancer therapy? Clinics (Sao Paulo). 73(Suppl 1): e814s2018. View Article : Google Scholar | |
|
Gerhart-Hines Z, Rodgers JT, Bare O, Lerin C, Kim SH, Mostoslavsky R, Alt FW, Wu Z and Puigserver P: Metabolic control of muscle mitochondrial function and fatty acid oxidation through SIRT1/PGC-1alpha. EMBO J. 26:1913–1923. 2007. View Article : Google Scholar : PubMed/NCBI | |
|
Lee D, Martinez B, Crocker DE and Ortiz RM: Fasting increases the phosphorylation of AMPK and expression of sirtuin1 in muscle of adult male northern elephant seals (Mirounga angustirostris). Physiol Rep. 5:e131142017. View Article : Google Scholar : | |
|
Rhee J, Inoue Y, Yoon JC, Puigserver P, Fan M, Gonzalez FJ and Spiegelman BM: Regulation of hepatic fasting response by PPARgamma coactivator-1alpha (PGC-1): Requirement for hepatocyte nuclear factor 4alpha in gluconeogenesis. Proc Natl Acad Sci USA. 100:4012–4017. 2003. View Article : Google Scholar : PubMed/NCBI | |
|
Zhang YK, Wu KC and Klaassen CD: Genetic activation of Nrf2 protects against fasting-induced oxidative stress in livers of mice. PLoS One. 8:e591222013. View Article : Google Scholar : PubMed/NCBI | |
|
Tripathi A, Scaini G, Barichello T, Quevedo J and Pillai A: Mitophagy in depression: Pathophysiology and treatment targets. Mitochondrion. 61:1–10. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Cheng A, Wan R, Yang JL, Kamimura N, Son TG, Ouyang X, Luo Y, Okun E and Mattson MP: Involvement of PGC-1α in the formation and maintenance of neuronal dendritic spines. Nat Commun. 3:12502012. View Article : Google Scholar | |
|
Marosi K, Moehl K, Navas-Enamorado I, Mitchell SJ, Zhang Y, Lehrmann E, Aon MA, Cortassa S, Becker KG and Mattson MP: Metabolic and molecular framework for the enhancement of endurance by intermittent food deprivation. FASEB J. 32:3844–3858. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Real-Hohn A, Navegantes C, Ramos K, Ramos-Filho D, Cahuê F, Galina A and Salerno VP: The synergism of high-intensity intermittent exercise and every-other-day intermittent fasting regimen on energy metabolism adaptations includes hexokinase activity and mitochondrial efficiency. PLoS One. 13:e02027842018. View Article : Google Scholar : PubMed/NCBI | |
|
Rodríguez-Bies E, Santa-Cruz Calvo S, Fontán-Lozano A, Peña Amaro J, Berral de la Rosa FJ, Carrión AM, Navas P and López-Lluch G: Muscle physiology changes induced by every other day feeding and endurance exercise in mice: Effects on physical performance. PLoS One. 5:e139002010. View Article : Google Scholar : PubMed/NCBI | |
|
Brown-Borg HM and Rakoczy S: Metabolic adaptations to short-term every-other-day feeding in long-living Ames dwarf mice. Exp Gerontol. 48:905–919. 2013. View Article : Google Scholar : PubMed/NCBI | |
|
Singh R, Kaushik S, Wang Y, Xiang Y, Novak I, Komatsu M, Tanaka K, Cuervo AM and Czaja MJ: Autophagy regulates lipid metabolism. Nature. 458:1131–1135. 2009. View Article : Google Scholar : PubMed/NCBI | |
|
Wan R, Ahmet I, Brown M, Cheng A, Kamimura N, Talan M and Mattson MP: Cardioprotective effect of intermittent fasting is associated with an elevation of adiponectin levels in rats. J Nutr Biochem. 21:413–417. 2010. View Article : Google Scholar : | |
|
Kim YH, Lee JH, Yeung JL, Das E, Kim RY, Jiang Y, Moon JH, Jeong H, Thakkar N, Son JE, et al: Thermogenesis-independent metabolic benefits conferred by isocaloric intermittent fasting in ob/ob mice. Sci Rep. 9:24792019. View Article : Google Scholar : PubMed/NCBI | |
|
Li G, Xie C, Lu S, Nichols RG, Tian Y, Li L, Patel D, Ma Y, Brocker CN, Yan T, et al: Intermittent fasting promotes white adipose browning and decreases obesity by shaping the gut microbiota. Cell Metab. 26:672–685.e4. 2017. View Article : Google Scholar : | |
|
Monternier PA, Teulier L, Drai J, Bourguignon A, Collin-Chavagnac D, Hervant F, Rouanet JL and Roussel D: Mitochondrial oxidative phosphorylation efficiency is upregulated during fasting in two major oxidative tissues of ducklings. Comp Biochem Physiol A Mol Integr Physiol. 212:1–8. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Roussel D, Boël M and Romestaing C: Fasting enhances mitochondrial efficiency in duckling skeletal muscle by acting on the substrate oxidation system. J Exp Biol. 221:jeb1722132018.PubMed/NCBI | |
|
Sokolović M, Sokolović A, Wehkamp D, Ver Loren van Themaat E, de Waart DR, Gilhuijs-Pederson LA, Nikolsky Y, van Kampen AH, Hakvoort TB and Lamers WH: The transcriptomic signature of fasting murine liver. BMC Genomics. 9:5282008. View Article : Google Scholar | |
|
Bouwens M, Afman LA and Müller M: Fasting induces changes in peripheral blood mononuclear cell gene expression profiles related to increases in fatty acid beta-oxidation: Functional role of peroxisome proliferator activated receptor alpha in human peripheral blood mononuclear cells. Am J Clin Nutr. 86:1515–1523. 2007. View Article : Google Scholar : PubMed/NCBI | |
|
Zhao L, Hutchison AT, Liu B, Yates CL, Teong XT, Wittert GA, Thompson CH, Nguyen L, Au J, Manoogian ENC, et al: Time-restricted eating improves glycemic control and dampens energy-consuming pathways in human adipose tissue. Nutrition. 96:1115832022. View Article : Google Scholar : PubMed/NCBI | |
|
Castro-Sepúlveda M, Morio B, Tuñón-Suárez M, Jannas-Vela S, Díaz-Castro F, Rieusset J and Zbinden-Foncea H: The fasting-feeding metabolic transition regulates mitochondrial dynamics. FASEB J. 35:e218912021. View Article : Google Scholar : PubMed/NCBI | |
|
Theurey P, Tubbs E, Vial G, Jacquemetton J, Bendridi N, Chauvin MA, Alam MR, Le Romancer M, Vidal H and Rieusset J: Mitochondria-associated endoplasmic reticulum membranes allow adaptation of mitochondrial metabolism to glucose availability in the liver. J Mol Cell Biol. 8:129–143. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Castro-Sepulveda M, Jannas-Vela S, Fernández-Verdejo R, Ávalos-Allele D, Tapia G, Villagrán C, Quezada N and Zbinden-Foncea H: Relative lipid oxidation associates directly with mitochondrial fusion phenotype and mitochondria-sarcoplasmic reticulum interactions in human skeletal muscle. Am J Physiol Endocrinol Metab. 318:E848–E855. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Shirakabe A, Fritzky L, Saito T, Zhai P, Miyamoto S, Gustafsson ÅB, Kitsis RN and Sadoshima J: Evaluating mitochondrial autophagy in the mouse heart. J Mol Cell Cardiol. 92:134–139. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Glick D, Zhang W, Beaton M, Marsboom G, Gruber M, Simon MC, Hart J, Dorn GW II, Brady MJ and Macleod KF: BNip3 regulates mitochondrial function and lipid metabolism in the liver. Mol Cell Biol. 32:2570–2584. 2012. View Article : Google Scholar : PubMed/NCBI | |
|
Islam H, Amato A, Bonafiglia JT, Rahman FA, Preobrazenski N, Ma A, Simpson CA, Quadrilatero J and Gurd BJ: Increasing whole-body energetic stress does not augment fasting-induced changes in human skeletal muscle. Pflugers Arch. 473:241–252. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Martínez-González MA and Sánchez-Villegas A: The emerging role of Mediterranean diets in cardiovascular epidemiology: Monounsaturated fats, olive oil, red wine or the whole pattern? Eur J Epidemiol. 19:9–13. 2004. View Article : Google Scholar : PubMed/NCBI | |
|
Guasch-Ferré M and Willett WC: The Mediterranean diet and health: A comprehensive overview. J Intern Med. 290:549–566. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Davis C, Bryan J, Hodgson J and Murphy K: Definition of the Mediterranean diet; a literature review. Nutrients. 7:9139–9153. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Martínez-González MA, Salas-Salvadó J, Estruch R, Corella D, Fitó M and Ros E; PREDIMED INVESTIGATORS: Benefits of the Mediterranean diet: Insights from the PREDIMED study. Prog Cardiovasc Dis. 58:50–60. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Widmer RJ, Flammer AJ, Lerman LO and Lerman A: The Mediterranean diet, its components, and cardiovascular disease. Am J Med. 128:229–238. 2015. View Article : Google Scholar | |
|
Duluc L, Jacques C, Soleti R, Andriantsitohaina R and Simard G: Delphinidin inhibits VEGF induced-mitochondrial biogenesis and Akt activation in endothelial cells. Int J Biochem Cell Biol. 53:9–14. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
Wang J, Zou Q, Suo Y, Tan X, Yuan T, Liu Z and Liu X: Lycopene ameliorates systemic inflammation-induced synaptic dysfunction via improving insulin resistance and mitochondrial dysfunction in the liver-brain axis. Food Funct. 10:2125–2137. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Tsai KL, Hung CH, Chan SH, Hsieh PL, Ou HC, Cheng YH and Chu PM: Chlorogenic acid protects against oxLDL-induced oxidative damage and mitochondrial dysfunction by modulating SIRT1 in endothelial cells. Mol Nutr Food Res. 62:e17009282018. View Article : Google Scholar : PubMed/NCBI | |
|
Lagouge M, Argmann C, Gerhart-Hines Z, Meziane H, Lerin C, Daussin F, Messadeq N, Milne J, Lambert P, Elliott P, et al: Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell. 127:1109–1122. 2006. View Article : Google Scholar : PubMed/NCBI | |
|
Kalliora C, Kyriazis ID, Oka SI, Lieu MJ, Yue Y, Area-Gomez E, Pol CJ, Tian Y, Mizushima W, Chin A, et al: Dual peroxis ome-proliferator-activated-receptor-α/γ activation inhibits SIRT1-PGC1 α axis and causes cardiac dysfunction. JCI Insight. 5:e1295562019. View Article : Google Scholar | |
|
Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A, Prabhu VV, Allard JS, Lopez-Lluch G, Lewis K, et al: Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 444:337–342. 2006. View Article : Google Scholar : PubMed/NCBI | |
|
Dong YZ, Li L, Espe M, Lu KL and Rahimnejad S: Hydroxytyrosol attenuates hepatic fat accumulation via activating mitochondrial biogenesis and autophagy through the AMPK pathway. J Agric Food Chem. 68:9377–9386. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Cao K, Xu J, Zou X, Li Y, Chen C, Zheng A, Li H, Li H, Szeto IM, Shi Y, et al: Hydroxytyrosol prevents diet-induced metabolic syndrome and attenuates mitochondrial abnormalities in obese mice. Free Radic Biol Med. 67:396–407. 2014. View Article : Google Scholar | |
|
Deng X, Zhang S, Wu J, Sun X, Shen Z, Dong J and Huang J: Promotion of mitochondrial biogenesis via activation of AMPK-PGC1α signaling pathway by ginger (Zingiber officinale Roscoe) extract, and its major active component 6-gingerol. J Food Sci. 84:2101–2111. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Anderson EJ, Thayne KA, Harris M, Shaikh SR, Darden TM, Lark DS, Williams JM, Chitwood WR, Kypson AP and Rodriguez E: Do fish oil omega-3 fatty acids enhance antioxidant capacity and mitochondrial fatty acid oxidation in human atrial myocardium via PPARγ activation? Antioxid Redox Signal. 21:1156–1163. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
Yoshino J, Smith GI, Kelly SC, Julliand S, Reeds DN and Mittendorfer B: Effect of dietary n-3 PUFA supplementation on the muscle transcriptome in older adults. Physiol Rep. 4:e127852016. View Article : Google Scholar : PubMed/NCBI | |
|
de Ligt M, Bruls YMH, Hansen J, Habets MF, Havekes B, Nascimento EBM, Moonen-Kornips E, Schaart G, Schrauwen-Hinderling VB, van Marken Lichtenbelt W and Schrauwen P: Resveratrol improves ex vivo mitochondrial function but does not affect insulin sensitivity or brown adipose tissue in first degree relatives of patients with type 2 diabetes. Mol Metab. 12:39–47. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Joy JM, Vogel RM, Moon JR, Falcone PH, Mosman MM, Pietrzkowski Z, Reyes T and Kim MP: Ancient peat and apple extracts supplementation may improve strength and power adaptations in resistance trained men. BMC Complement Altern Med. 16:2242016. View Article : Google Scholar : PubMed/NCBI | |
|
Perez-Ternero C, Werner CM, Nickel AG, Herrera MD, Motilva MJ, Böhm M, Alvarez de Sotomayor M and Laufs U: Ferulic acid, a bioactive component of rice bran, improves oxidative stress and mitochondrial biogenesis and dynamics in mice and in human mononuclear cells. J Nutr Biochem. 48:51–61. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Mollica MP, Mattace Raso G, Cavaliere G, Trinchese G, De Filippo C, Aceto S, Prisco M, Pirozzi C, Di Guida F, Lama A, et al: Butyrate regulates liver mitochondrial function, efficiency, and dynamics in insulin-resistant obese mice. Diabetes. 66:1405–1418. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Myhrstad MCW, de Mello VD, Dahlman I, Kolehmainen M, Paananen J, Rundblad A, Carlberg C, Olstad OK, Pihlajamäki J, Holven KB, et al: Healthy nordic diet modulates the expression of genes related to mitochondrial function and immune response in peripheral blood mononuclear cells from subjects with metabolic syndrome-A SYSDIET Sub-study. Mol Nutr Food Res. e18014052019.Epub ahead of print. View Article : Google Scholar : PubMed/NCBI | |
|
Brand MD, Orr AL, Perevoshchikova IV and Quinlan CL: The role of mitochondrial function and cellular bioenergetics in ageing and disease. Br J Dermatol. 169(Suppl 2): S1–S8. 2013. View Article : Google Scholar |