Mesenchymal stem cells: An efficient cell therapy for tendon repair (Review)
- Authors:
- Published online on: June 30, 2023 https://doi.org/10.3892/ijmm.2023.5273
- Article Number: 70
-
Copyright: © Jiang et al. This is an open access article distributed under the terms of Creative Commons Attribution License.
Abstract
1. Introduction
The tendon is composed of longitudinally arranged collagen fibers and a scattered distribution of spindle-shaped tendon cells. Its primary function is to transmit the force generated by contraction of the muscles and to drive the movement of the bones (1). It is hypothesized that when the tendon is overused for a long time, bears a large load or is stretched repeatedly, many pathological changes will occur in the tendon including cell and extracellular matrix (ECM) lesions, increased proteoglycan and damage to the collagen structure (2), which leads to tendon injury. In addition, other factors may also lead to tendon injury, including age, incorrectly performed exercise, previous injury, weight and medication (3). In recent years (4), injured athletes have accounted for the majority of injured people. Numerous athletes suffer from chronic tendinopathy due to overwork, especially those who play basketball, football and volleyball, as well as those who perform in the high jump. The injured areas include the Achilles and patellar tendon, rotator cuff and the tendons around the elbow joint, all of which result in inconvenience to the lives of those affected.
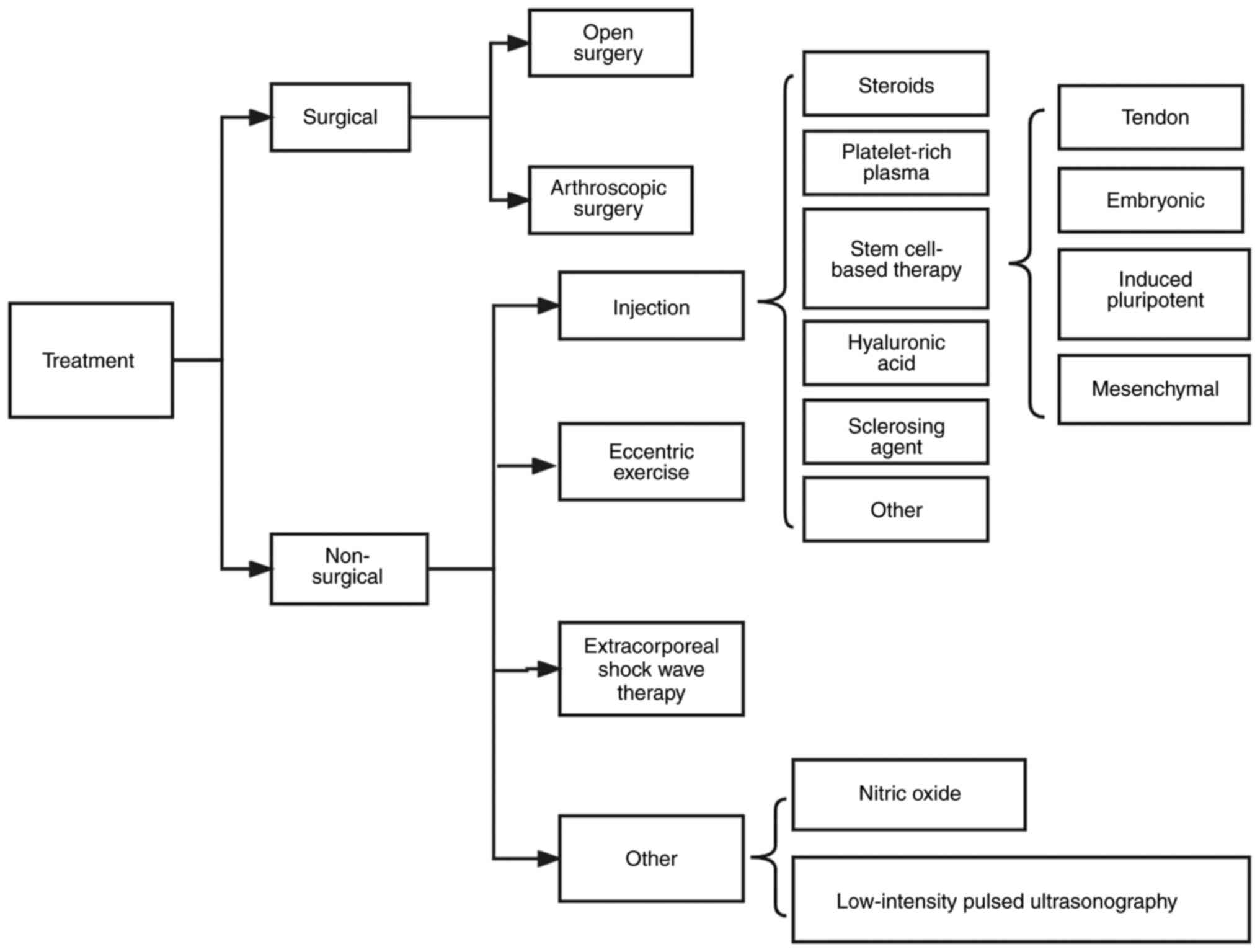
Many effective treatment methods (Fig. 1) have been employed in a number of clinical practices. Patients with tendon injury initially engage in conservative treatment, including eccentric training, shock wave therapy and injection therapy. However, in 10% of patients, conservative treatment has no significant effect (5). For example, patients with partial tears of the supraspinatus tendon and shoulder pain lasting >3 months were treated with mesenchymal stem cell (MSC) injections at the lesion site. However, final treatment effect in terms of pain, shoulder function and tear size was smaller and less significant than the clinical effect of MSC injection compared with the control treatment (6). However, there is potential of MSCs in tissue regeneration. Therefore, surgical treatment, including open and arthroscopic surgery, is required for the small percentage of patients who do not do well with conservative treatment. Although the aforementioned treatment approaches have achieved good results in the treatment of tendon injury, they still cannot fully restore the composition, structure, and mechanical properties of the injured tendon (5).
In recent years, studies have shown that MSCs are a promising treatment method. Many clinical trials have demonstrated that MSCs have good therapeutic effects (7-9). Firstly, MSCs differentiate into targeted cell types, and under specific induction conditions in vivo or in vitro, they can differentiate into tendon cells to stimulate tendon tissue regeneration. Secondly, they have a paracrine effect and can secrete cytokines and growth factors into neighboring cells, thereby promoting vascularization and cell proliferation in damaged tissue and helping to repair the damaged area (10). MSCs also have immunomodulatory properties and decrease the inflammatory response of damaged tissue (11). Additionally, MSCs have a wide range of sources; they can be isolated and prepared from bone marrow, adipose tissue, placenta, umbilical cord and other tissues. Overall, MSCs have numerous advantages over other conservative treatments such as NSAIDs, low level lasers, including strong multiplication capability, safety, economy and efficiency. The present review summarized the mechanisms, progress, and challenges of MSCs in the treatment of tendon injury based on published literature and provides support for future clinical practice and research.
Search strategy
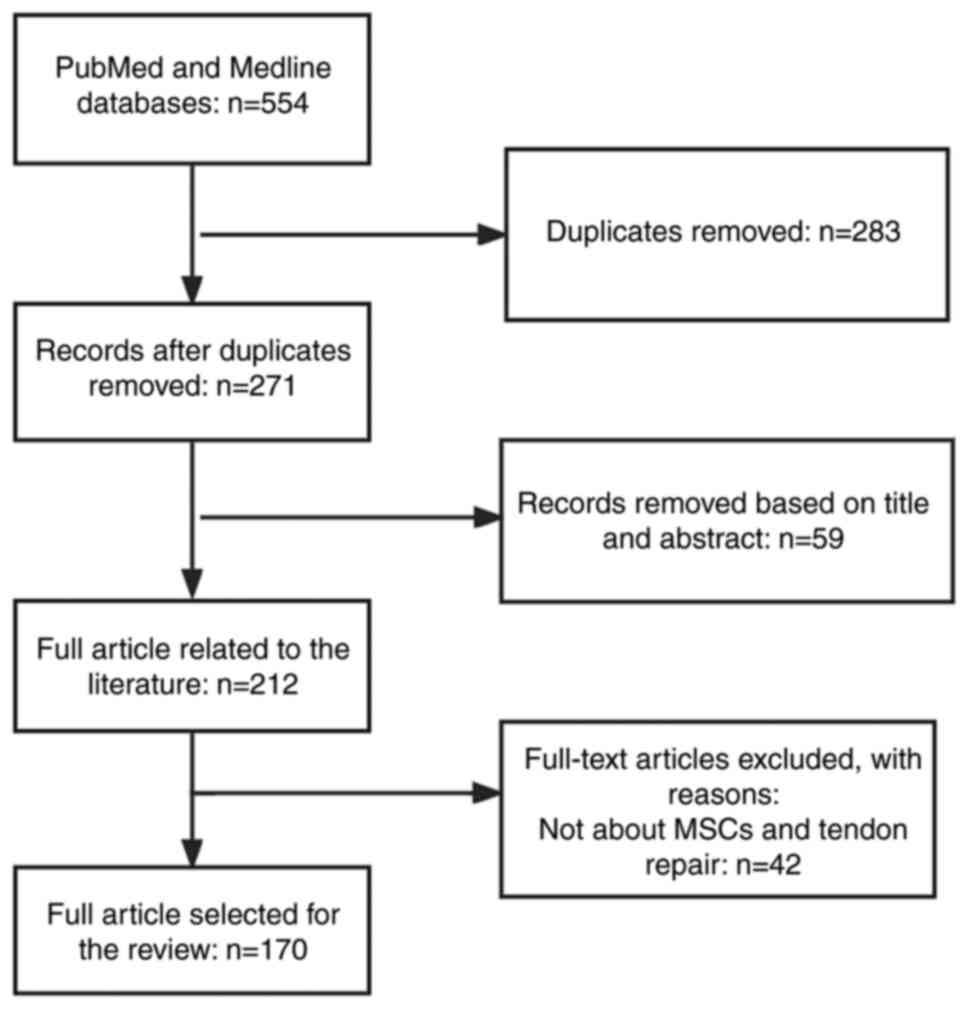
References cited in the manuscript were retrieved from PubMed (pubmed.ncbi.nlm.nih.gov/), a database of papers on biomedical sciences. Some literature was also retrieved from the MEDLINE database (https://www.webofscience.com/wos/medline/basic-search). The keywords we applied in the search were: mesenchymal stem cells, tendon injury, exosomes, tendon repair, function. The Boolean algorithms we applied were: ('mesenchymal stem cells' or 'exosomes') and ('tendon injury' or 'tendon healing' or 'tendon repair'). The time frame of the searched literature was from 2000 to 2023. The inclusion and exclusion criteria for the literature were that articles were included if their topic was related to MSCs and tendon repair, and if the article was a review or an experimental paper. The search process is presented in Fig. 2.
2. MSCs and other cell therapy in tendon repair
Cell-based tissue regeneration therapies are attractive and well-explored therapeutic approaches, especially in the application of tendon repair (12,13). The most discussed cell-based therapies include MSCs (from sources such as bone marrow, adipose, umbilical cord) and tendon, embryonic and induced pluripotent SCs (iPSCs) (14-17). Tables I and II summarize the properties of MSCs and other cellular therapies in tendon repair and their characteristics.
MSCs are widely available, relatively simple to obtain and can be injected directly or following processing, purification and amplification. Several studies have shown that MSCs in the tendon are actively involved in the tendon repair process (15,18). They migrate to the injury site following tendon injury and secrete growth factors and other soluble cytokines that induce cell proliferation and regulate signaling, in addition to enhancing the tendon-forming properties of tendon stem/progenitor cells (TSPCs), thereby promoting tendon repair (19). Studies have showed that the efficiency of differentiation of MSCs into tendon cells could be better improved by injecting growth factors such as bone morphogenetic protein-12 (BMP-12), growth/differentiation factor-5 (GDF-5) and TGF-β compared to treatment with MSCs alone (20,21). There are numerous studies on the use of exogenous MSCs of different sources in tendon repair following injury through intravenous or topical wound injection, bioengineered scaffolds and gels (22-24). For example, Smith et al (25) injected bone marrow MSCs (BMSCs) into racehorses with tendon injury; after 6 months of treatment, the tendons of racehorses showed enhanced biomechanics, morphology and normalization of extracellular matrix (ECM) component of the tendon (25). In particular, adipose-derived MSCs (ADSCs) show advantages over other sources of BMSC in terms of decreased donor morbidity and avoiding ethical concerns. Therefore, it has a high value in the treatment of tendon injuries (26).
The tendon also contains a small population of resident cells that maintain homeostasis of tendon growth and repair (27). Similar to other SCs, these TSPCs have the capacity to undergo self-renewal and multidirectional differentiation. Numerous studies have exploited this feature to promote the self-proliferation of TSPCs and induce differentiation to tendon cells by injecting growth factors such as TGF-β and basic fibroblast growth factor (bFGF) in vivo or creating hypoxic states in vitro. Subsequently, TSPCs upregulate IL-10 and TIMP-3 through the JNK/STAT signaling pathway, thus playing a regulatory role in inflammation and tendon remodeling (16,28). However, alterations in the tendon microenvironment following injury may lead to misdifferentiation of TSPCs to chondrocytes, osteoblasts and adipocytes, resulting in failure of tendon healing (29). There are many triggers that lead to misdifferentiation, including age-associated cellular aging, mechanical stretch stimulation >8% and some inflammatory factors such as prostaglandin E2 (PGE2) (30,31). Understanding the factors that induce TSPC (mis)differentiation may facilitate use of TSPCs in the treatment of tendon injuries.
Embryonic SCs (ESCs) are isolated from early embryos (before protointestinal embryonic stage) that have properties of unlimited proliferation, self-renewal and multidirectional differentiation (32). ESCs can be induced to differentiate into almost all cell types, both in vitro and in vivo. Therefore, they have potential in regenerative medicine (17). It has been shown that human ESCs can be induced to differentiate into tendon-like cells by the addition of exogenous BMP-12, GDF-7 and BMP-13 (33). It has also been shown that tendon injury sites treated with ESCs recover better and collagen fibers can be restored to a more normal linear fiber pattern compared to other cellular therapies (33). However, there are concerns regarding the use of ESCs. First, ESC isolation destroys the embryo, which may be considered a violation of bioethics. Despite the potential use of ESCs in both basic research and clinical applications, research on ESCs and their applications is prohibited in some countries, such as the United States and some European countries where religious organizations are prevalent (14). ESCs can theoretically be induced into various types of somatic cells for tissue regeneration. Therefore, there is a risk of teratoma formation following application of ESCs for treatment (34). Teratomas consist of three embryonic germ layers, which are due to residual undifferentiated cells in the transplanted population. Therefore, it is necessary to remove residual undifferentiated stem cells from ESCs before application (35). Similar to ESCs, iPSCs) can be prepared from the patient's own somatic cells, thus avoiding immune rejection (36). Compared with ESCs, iPSCs can be obtained from more convenient sources, such as fibroblasts and hepatocytes, and do not involve ethical concerns. In horses, the application of iPSCs promotes tendon tissue regeneration and significantly decreases the frequency of re-injury (37). However, since iPSCs have the ability of multidirectional differentiation, there is also a risk of teratoma formation (38). In addition, the time and cost required to prepare iPSCs may prevent them from becoming a therapeutic option.
3. Structure, composition and mechanical properties of tendons
Tendons are dense tissues that connect muscle and bones. Their unique composition and structure give them appropriate mechanical properties (39). Therefore, understanding of the association between the composition, structure and mechanical properties of normal tendons can help to prevent tendon injury and select the most appropriate method for treatment.
In terms of ultrastructure, tendons are hierarchical structures with a regular arrangement of collagen fibers (40). This hierarchical structure provides ideal load-bearing and tensile force transmission properties (41). The smallest structural unit of the tendon is the fibril, with a diameter of 20-500 nm, consisting of rod-shaped collagen molecules (42). Electron microscopy in the absence of load shows fibrils become 'crimped'. This is thought to be due to a non-linear change in the strain-stress curve caused by small tensile forces at low strains. Studies have shown that this change can be effective for cushioning and shock absorption in tendons (43,44). The fibrils are cross-linked to form a stable structure, referred to as a collagen fiber. Multiple collagen fibers reassemble to form the tendon fascicle, which is the largest structural unit of the tendon. The tendon fascicle is a tubular-like structure 150-500 µm in diameter, aligned parallel to the long axis of the tendon. Each fascicle is surrounded by connective tissue called the endotenon, thus forming a complete tendon structural unit (45). The tendon is covered with a layer of connective tissue attached to the endotenon called the epitenon. The epitenon effectively reduces friction between the tendon and adjacent tissue (46). In addition, there are nerves and blood and lymphatic vessels on the endotenon and epitenon, which serve a key role in development of the tendon. A layer of loose connective tissue, called paratenon, surrounds the tendon away from the joint area. During joint movement, the paratenon facilitates the smooth gliding of the tendon and completion of the movement (47). Normal tendons consist of collagen, water, proteoglycans, glycoproteins, cells and other components (48). Type I collagen is the primary component of tendons and is also the main element in connective tissue responsible for transmitting force (49). Type III collagen is less abundant in normal tendons (50). Due to its rapid cross-linking properties, type III collagen increases rapidly following tendon injury, allowing for rapid repair in the injured area and tendon healing (51). In addition to containing high amounts of type I and type III collagen, collagen fibers expressed at lower levels, including types V, VI, XI, XII and XIV, serve key roles in regulating the biological properties of fibers in terms of diameter, number and density (52-54). Tendons also contain a large amount of water; studies have shown that the higher the water content, the lower the stiffness of tendons (55,56).
In addition to collagen, non-collagenous glycoproteins, proteoglycans, elastin, and other components of the ECM also play important roles in the growth and development of tendons (57). Non-collagenous glycoproteins mediate signaling between TSPCs and muscle, thereby promoting maturation of the tendon-muscle junction and maintaining tendon stability (58). Proteoglycan is an important component of extracellular matrix (59); it mainly regulates the diameter of linear and lateral fibers during the later stage of tendon development and cooperates with growth factors to regulate cell proliferation, thus promoting collagen production (60,61). Glycoproteins are part of the ECM; cartilage oligomeric matrix protein (COMP) is the most abundant glycoprotein in tendons. The amount of COMP is positively associated with tensile stress and stiffness (62). Tenascin-C is glycoprotein expressed at low levels that is primarily present in locations where the tendon is subjected to higher load (63). Tenascin-C maintains mechanical properties of the ECM by interacting with collagen fibers (64). Different numbers of elastic fibers are found between tendon fascicles and around tenocytes; multiple elastic fibers surround groups of tenocytes and travel longitudinally along tendon, whereas fibers present between fascicles form a loose mesh-like organization oriented in the transverse direction (65); these play important roles in maintaining tendon strength and conferring elasticity to tissue such as ligaments, aorta and skin (66). Thus, elastin fibers may contribute to recovery of fibers to their original form after stretching (46).
Tendon cells are primarily divided into two categories. Tendon cells present between collagen fibers, known as tenocytes, are the main cells in the tendon tissue. These produce ECM components such as collagen, fibronectin and proteoglycans, and therefore serve an important role in maintaining tendon homeostasis (67). Furthermore, tenocytes increase expression levels of the junctional and stress fiber components when exposed to tensile load. Thus, in tendinopathy, loading with an appropriate tensile load promotes recovery of mechanical properties of the damaged tendon (68). Another group of cells located in the interfascicular matrix (IFM) is called interfascicular cells (69). Interfascicular cells are round in shape and are more densely distributed compared with tenocytes. They are also more metabolically active because of faster protein turnover in the IFM (47). Marr showed that CD146+ cells are interfascicular cells (70). Following injury to a rat Achilles tendon, CD146+ cells migrate toward the injury on days 4 and 7 and to fill the wound on day 21 after the injury (70). Studies have demonstrated that CD146+ cells promote cell proliferation through mTORC2 signaling and thus serve a role in tendon repair (71,72). CD146+ cells also bind Laminin α4 and may play a role in the resolution of inflammation but the exact mechanism of action is currently unknown (73). The presence of interfascicular cells may be key for maintaining tendon function but their exact mechanism of action requires further study. In addition to the aforementioned cells, other cells are present in tendons, such as chondrocytes, synovial cells and tendon SCs (46). Among them, the recently identified tendon SCs have good ability to maintain homeostasis of tendons and promote repair of tendinopathy (74). Tendon SCs have the ability to self-renew and differentiate into tendon cells (75). Their differentiation into tendon cells is promoted at low levels of mechanical stretch (4%) and produces collagen, thereby promoting remodeling of the tendon ECM. However, at high mechanical stretch (8%), tendon SCs are induced to differentiate into non-tendon cells, such as adipocytes, chondrocytes and osteocytes, resulting in histopathological features of lipid deposition, proteoglycan accumulation and calcification in the tendon, thus leading to the development of tendinopathy (76).
Because of the unique layered structure and composition, tendons have characteristic biomechanical properties such as high mechanical strength and viscoelasticity (46). The unique mechanical properties of tendons are reflected in a stress-strain curve composed of four regions. In the initial area when the tendon is stretched <2%, the curled fiber is straightened. When the degree of stretch is 2-4%, it is referred to as the linear area. As the tendon is stretched, the stiffness of fibers increases rapidly and they distort in a linear manner. The slope of this region is called the Young's modulus of the tendon and is used to express the stiffness of the tendon. When a tendon is stretched >4%, microscopic tears occur in the fibers. Tendons undergo significant tissue damage when subjected to >8% strain and continued stretching leads to tendon rupture (46). Tendons have several other mechanical properties, including non-linearity, viscoelasticity, and heterogeneity (77). Viscoelasticity may result from the interaction between collagen, water and proteoglycan (78). Viscoelasticity is important for load transfer in tendons. Tendons are more prone to deformation at low strain rates and are less effective in transferring loads. At high strain rates, tendons are less prone to deformation with higher stiffness and are more effective at transmitting larger loads (79). Thus, the unique mechanical properties of tendons are key to their function of carrying and transmitting loads. The high levels and regular arrangement of collagen create high tensile strength required to provide efficient load transfer. However, it is not clear how small changes in the structure and composition of the tendon lead to changes in mechanical properties.
4. Sources of MSC in preclinical studies
MSCs are considered to be 'medicinal cell factories' that are capable of secreting a range of bioactive molecules, either in the form of soluble biofactors or through MSC-exosomes (Exos), which have functions in immunomodulation, anti-apoptotic activity, and promoting synthesis of ECM components, such as collagen (80). MSCs have been used in regenerative medicine since their discovery in the late 1960s (80). MSCs are isolated from a number of tissues, including adipose, muscle, tendon, synovial sac, dental pulp, skin, lung, placenta and umbilical cord (11). Furthermore, MSCs self-renew and differentiate to produce specialized cell types such as chondrocytes, muscle cells, and skin cells (82). MSCs derived from BM and adipose are most frequently used in the treatments of tendon injury as they exhibit self-renewal, multidirectional differentiation, and paracrine functions. And they can also lead to tendon healing and functional improvement through minimally invasive treatment approaches (83). The effect of MSCs from different sources in tendon healing is summarized in Table III.
MSCs were originally isolated from BM (84). BMSCs are usually obtained from the iliac crest by minimally invasive puncture and isolated by density centrifugation (85). Chong et al (86) identified two roles of BMSCs in tendon healing. BMSCs secrete growth factors and promote tendon healing by differentiating into tenocytes and participating in collagen synthesis and remodeling. However, activity of alkaline phosphatase is increased after treatment of tendon injury using BMSCs, which leads to the formation of ectopic bone (84,87) and impedes tendon healing.
In recent years, more attention has turned to ADSCs because they are easier to isolate compared to other sources of MSCs. And because BMSCs need to be collected using a trocar to drill through the iliac crest, while ADSCs can be collected using only minimally invasive liposuction techniques, this is easier and less painful for the patient. In addition, over time, the donor site providing BMSCs is prone to pain and stiffness, whereas ADSCs have a much lower incidence (15,88,89). Specifically, adipose tissue is easily aspirated from abdominal subcutaneous adipose; collected tissue is passed through specific systems including forming, granulating, cutting, purification, centrifugation, nitrification, absorption of antimicrobial or antitoxin arrangements, cleansing, partition, and lyophilization to obtain ADSCs (90,91). Furthermore, ADSCs can be obtained from autologous or allogeneic sources, but ADSCs isolated from autologous adipose tissue are the best candidates for the treatment of tendon injuries because they do not induce immune rejection after application in injured tendons (92). Studies have detected transcription factors associated with hypoxia, such as hypoxia-inducible factor-1 (HIF-1), in models of ruptured Achilles tendon that show ectopic ossification; therefore, hypoxia may be associated with formation of cartilage in tendons (93,94). Adding ADSCs in the early stage of tendon healing can reverse or prevent hypoxia by inhibiting inflammation and promoting formation of new blood vessels to inhibit occurrence of heterotopic ossification (95). Consequently, ADSCs have advantages over BMSCs in the treatment of tendon injury. In addition, compared with MSCs from other sources, ADSCs enhance tenogenic properties of tendon resident cells, increase the ratio of collagen I/III and promote repair of ECM (15). These characteristics make ADSCs promising in tendon healing.
5. Mechanisms of MSCs in tendon repair
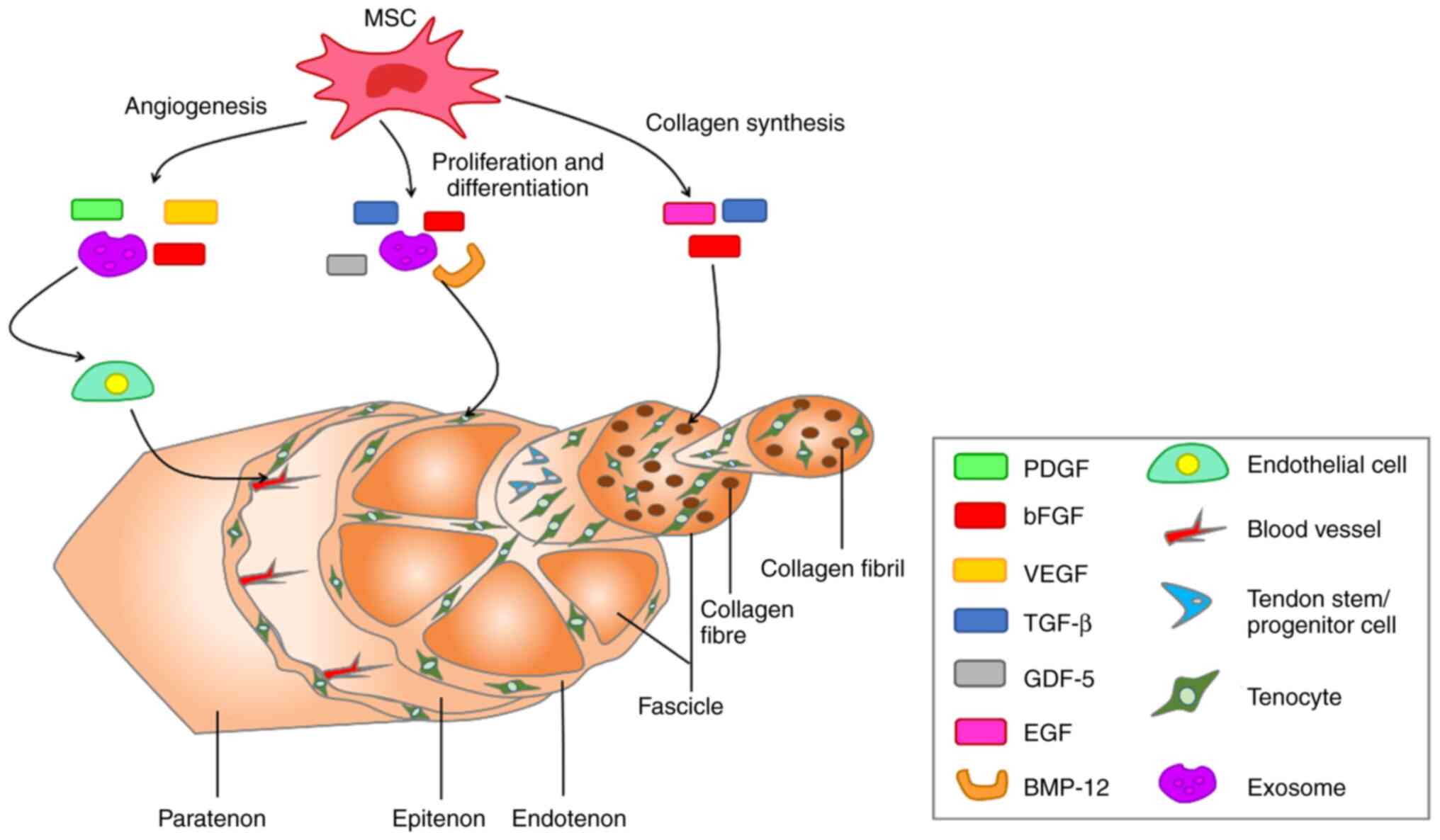
MSCs secrete biologically active soluble factors (cytokines, growth factors, chemokines, MSC-Exos) to accelerate healing of tendons (96,97). MSC-Exos are extracellular vesicles (EVs) containing complex RNAs and proteins that target cells via endocytosis, membrane fusion or receptor-ligand interactions and are key paracrine factors for MSCs. MSC-Exos serve important roles in immune regulation, apoptosis and tissue regeneration in numerous types of disease such as osteoarthritis (98). In recent years, there have been numerous studies on the use of MSC-Exos in tendon repair (99-101). MSC-Exos serve roles in tendon repair by regulating biological factors and activating signaling pathways (102). For example, MSC-Exos inhibit the inflammatory response, primarily by promoting AMPK signaling (103). The angiogenesis phase, which is important for tendon healing, also involves MSC-Exos, which secrete growth factors such as vascular endothelial growth factor (VEGF), which is associated with angiogenesis (104). In addition, MSC-Exos play a key role in subsequent cell proliferation and collagen synthesis. MSC-Exos promote proliferation and differentiation of tenocytes by activating the SMAD signaling pathway and increasing the ratio of type I/III collagen genes, thereby promoting type I collagen synthesis (105,106). Other growth factors secreted by MSCs that serve important roles in tendon repair include insulin-like growth factor-I, TGF-β, platelet-derived growth factor (PDGF) and bFGF. These growth factors promote tendon repair by participating in intercellular messaging, as well as signaling pathways during the three phases of tendon healing: inflammation, proliferation, and remodeling (10,107). The biologically active soluble factors secreted by MSCs and their effects on the molecular structure of the tendon are presented in Fig. 3. In addition, mechanical stimulation induces MSCs to differentiate into tenocytes, thereby accelerating the repair of tendon tissue (108,109). For example, in Zhu's (110) experiments, a mechanical stretching force of 10% was applied to MSCs and stimulated for 6 h with 30 cycles per minute. However, in Kasper's (111) experiments, he applied a mechanical load of 10 kPa to MSCs at a frequency of 1 Hz for 72 h of stimulation.
MSCs decrease the inflammatory response
After tendon injury, the injury site may show signs of pain, exudation, redness and dysfunction (112). Although studies have shown that tendon injury is a degenerative condition caused by excessive use of tendons and does not involve inflammatory cells, there is mounting evidence that inflammatory factors serve an important role following tendon injury (113-115). Tendon healing involves three phase: Inflammatory, proliferative and remodeling phase (116). The inflammatory phase removes necrotic cells and creates a temporary ECM to prepare for proliferation and differentiation of new tenocytes in the subsequent repair process. The immune system begins to recruit immune cells, such as macrophages and mast cells. It also starts to secrete cytokines such as IL-1β, IL-4 thus stimulating cell proliferation and tissue remodeling. These inflammatory responses are directed by type I (pro-inflammatory) and type II (anti-inflammatory) immune regimens (117). S100A9, an alarmin that can form calprotectin (CP) heterodimers with S100A8, is mainly produced by keratinocytes and innate immune cells. In the type I immune response, S100A8 and S100A9 serve as alarm elements that are released into the extracellular environment by necrotic cells or activated immune cells (118). This leads to enhanced recruitment of immune cells [T helper (Th)1 T, neutrophils, M1-type macrophages] and promotes release of pro-inflammatory factors such as tumor necrosis factor-α (TNF-α), IFN-γ, IL-1β and inducible nitric oxide synthase (iNOS) from tendon cells (119). Subsequently, downstream inflammatory signaling pathways such as NF-κB and NLRP3 are activated, regulating inflammatory gene expression and transcription (120,121). The presence of pro-inflammatory factors breaks down ECM and promotes new ECM deposition (122). To prevent the excessive pro-inflammatory type I immune response, the body activates the type II immune anti-inflammatory response by secreting IL-4 or IL-33 from damaged cells (123). The release of IL-33 triggers downstream responses from macrophages, T regulatory cells (Tregs) and other intrinsic immune cells (124). Tregs can produce IL-10, which acts as a key anti-inflammatory factor to resolve inflammation caused by the type I immune response. IL-4 also promotes conversion of naive CD4 T cells and macrophages to Th2 cells and M2-type macrophages, thus exerting anti-inflammatory effects (125).
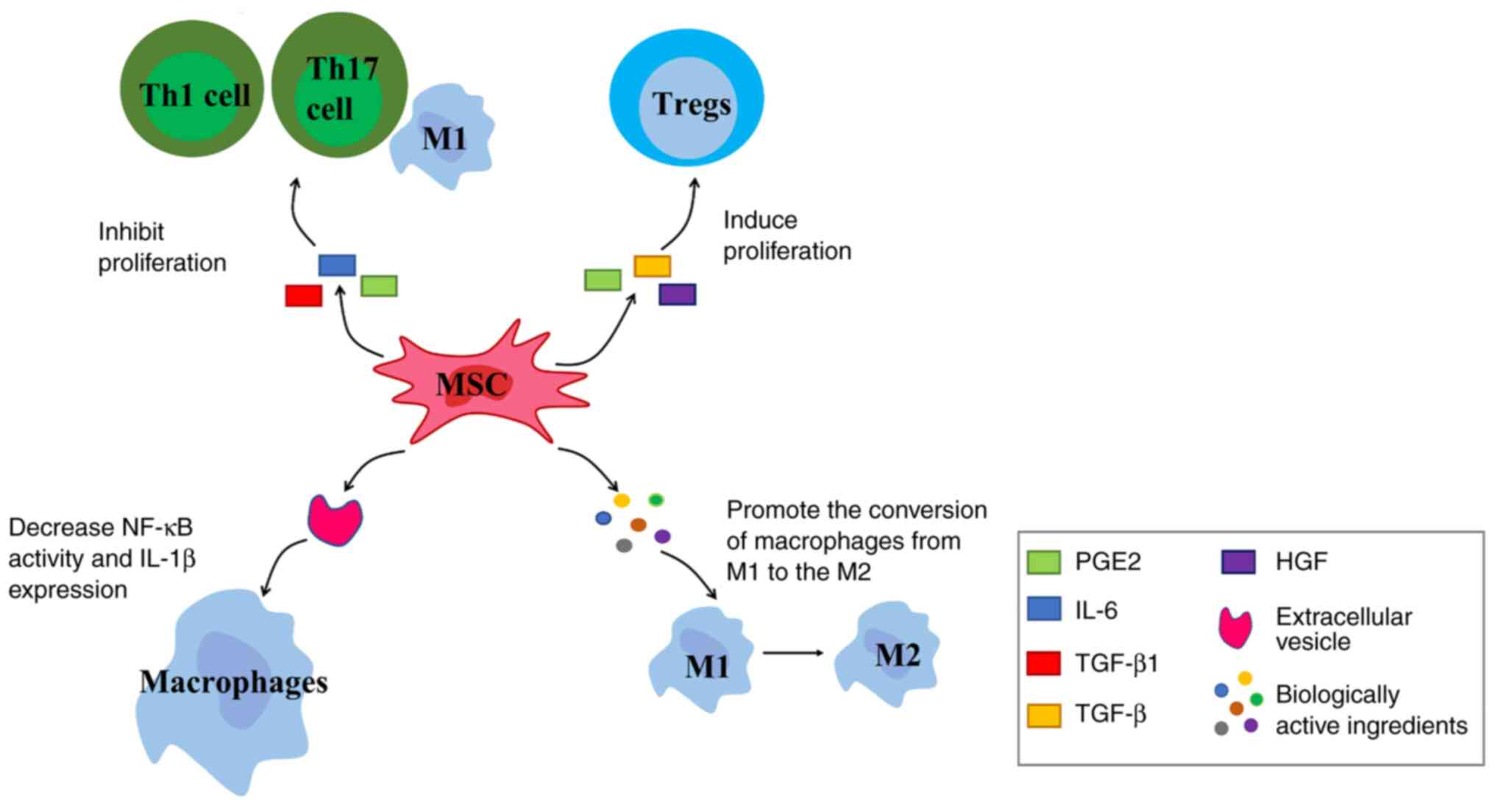
Chemokine receptors are expressed by MSCs; MSCs detect inflammation signals through chemokine receptors and migrate to them. Under stimulation of pro-inflammatory factors, MSCs exert immunomodulatory effects by secreting immunomodulatory mediators such as chemokines, cytokines and growth factors (Fig. 4). For example, TGF-β1, IL-6 and PGE2 inhibit proliferation of Th1 and Th17 cells, M1-type macrophages and other pro-inflammatory cells (126). MSCs also express and secrete soluble factors such as PGE2, TGF-β and hepatocyte growth factor. These factors induce proliferation of Tregs, thereby controlling inflammation (127).
EVs secreted by MSCs (MSC-EVs) play an important role in the anti-inflammatory response. The inflammatory response at the injury site stimulates MSCs to secrete EVs. MSC-EVs target macrophages and decrease NF-κB activity and IL-1β expression, thereby decreasing inflammation in the early stage of tendon repair (128). IL-1 is a key inflammatory cytokine (129). It plays an important role in degrading ECM, inhibiting tendon cell marker expression and inducing pain. Following tendon injury, inflammatory factors such as IL-1 and TNF-α are released by inflammatory cells such as neutrophils and macrophages during the exogenous healing phase (130). IL-1β downregulates gene expression of early growth response gene 1 and type I and III collagen while upregulating expression of MMP1, 3, 8, and 13 (131). MMPs mediate catabolism of collagen, leading to sustained tissue degradation (132). In addition, IL-1β downregulates expression of tendon cell markers scleraxis (SCX) and tenomodulin (TNMD), which leads to a decrease in ultimate tensile strength and elastic modulus of the repaired tendon (133). In damaged tissues, PGE2 acts to promote vasodilation and elicit a pain hypersensitivity response. IL-1β accelerates the conversion from PGH2 to PGE2 and causes an increase in pain by enhancing expression of prostaglandin E synthase (134). This suggests that IL-1 serves a key role in the development of the inflammatory response. After IL-1 and TNF-α are released, they bind to toll-like receptor 4 (TLR4) on the cell membrane (135). The polymerization of TLR4 enables signal transduction into cells; there is a toll/IL-1 receptor region in the cell membrane of TLR4 that binds to the carboxy terminus of myeloid differentiation primary response gene 88 (MyD88) (136). The amino-terminal death domain of MyD88 binds to the amino-terminal death domain of IL-1 receptor-associated kinase (IRAK). This promotes the phosphorylation of IRAK and acquisition of free IRAK1, 2 and 4, which in turn activates TNF-α receptor-associated factor 6 (TRAF-6). TRAF-6 binds to NF-κB kinase and phosphorylates the β subunit (IKKβ), thereby activating the IκB kinase (IKK) complex (137). IKK induces IκB phosphorylation at residues Ser32 and Ser36 of IκBα and residues Ser19 and Ser23 of IκBβ via the 26S proteasome (138). IκB is an inhibitory protein of NF-κB, which binds to NF-κB dimer to inhibit its activity. IκB is degraded, which results in entry of p50-p65 complex into the nucleus to initiate expression of downstream genes regulated by NF-κB (139,140). NF-κB serves as a powerful pro-inflammatory signaling pathway that drives the production of pro-inflammatory cytokines, including IL-1, IL-6, C-C motif chemokine ligand 2 and TNF-α. These inflammatory factors reactivate NF-κB activity so there is often a persistent inflammatory response during tendon healing (141). The persistent inflammatory environment has a negative impact on tendon healing and leads to tissue adhesion during the collagen remodeling phase (142). MSCs-Exos can downregulate phosphorylated (p-)P65 by secreting microRNA (miR)-23a-3p (143). While NF-κB is a typical heterodimer, its common structure is a complex composed of proteins P65 and P50; MSCs-Exos directly inhibits NF-κB activity by downregulating activity of p-P65 (144). In addition, Shen et al (128) showed that ADSC EVs inhibit NF-κB activity in an indirect manner by inhibiting IL-1β secretion via macrophages, thereby blocking IL-1β-induced activation of the NF-κB signaling pathway.
MSC-Exos have been shown to reduce inflammatory cell infiltration (129). MSC-Exos may act by promoting the AMPK signaling pathway. β-catenin is a protein that accelerates the inflammatory response and is an effector of Wnt signaling. MSC-Exos maintain a stable metabolic environment in tendon cells by promoting AMPK signaling while inhibiting the Wnt/β-catenin signaling pathway, thereby decreasing the inflammatory response and promoting tendon healing (145). In injured tendon tissue, inflammatory cell infiltration is dominated by macrophages, which are primarily divided into phenotypes M1 and M2 (8). M1 macrophages have pro-inflammatory effects. Together with leukocytes and other cells, they secrete pro-inflammatory factors such as IL-1β and TNF-α. They promote the inflammatory response, while the increase in IL-1β leads to production of MMPs and decreased type I collagen, which in turn leads to the breakdown of ECM (95). M2 macrophages have anti-inflammatory effects. MSC-Exos secrete miR-23a-3p and target IFN regulatory factor 1, which has been reported to be a key regulator of inflammation and M1 macrophage polarization (143). Markers of M1 macrophages (iNOS, IL-6, IL-1β and TNF-α) are significantly decreased after treatment with MSC-Exos, while markers of M2 macrophages (CD163, IL-10, TGF-β and Arginase 1) are increased. These results suggest that miR-23a-3p secreted by MSC-Exos mediates macrophage polarization (143). During inflammation, released cytokines promote expression of iNOS or indoleamine 2,3-dioxygenase (IDO) in MSCs. IDO regulates immune activity by inducing monocytes to differentiate into M2 macrophages, thereby decreasing inflammation (126). Additionally, M2 macrophages inhibit inflammation via immunosuppressive cytokines, such as IL-10 and IL-4, secreted by MSCs (146), which promote the proliferation of tenocytes and tissue repair and guide remodeling of the ECM (119). However, while MSCs exert anti-inflammatory effects, they also promotes the infiltration of monocytes, macrophages and neutrophils to the inflammation site in a chemokine-dependent manner, thereby promoting progression of inflammation (126). Although this effect seems to be contradictory, it is key to maintain the balance between pro- and anti-inflammatory responses. A study used MSCs-EVs to culture M2 macrophages in vitro to generate EV-educated macrophages (EEMs) (147). The injection of EEMs into the injured site tendon accelerates healing and significantly improves tendon function and regeneration. Additionally, the anti-inflammation effect is greater compared with direct injection of MSCs or MSCs-EVs. This provides a potential novel approach for the treatment of tendon injury because EEMs are terminally differentiated and do not proliferate or differentiate into undesirable cell types. In addition, IL-10 and IL-6 are expressed at high levels in EEMs, while IL-12 and TNF-α are expressed at low levels (147). This serves a key role in controlling inflammation during tendon healing. The proliferation phase only begins when necrotic tissue and waste products are removed from the injured site in the inflammatory phase (148).
MSCs promote angiogenesis
Tendons have a specific structure with lower metabolic demands compared with other tissue and therefore have lower cellular and vascular content (149). However, tendons are surrounded by a rich network of blood vessel. Degenerative lesions of tendons tend to occur in areas with decreased vascularity and decreased blood supply can lead to hypoxia (150). Hypoxia is one of the most common environmental stresses experienced by cells and serves an important role in the early stages of tendinopathy (151). Following tendon injury, decreased tissue perfusion and increased energy demands lead to a lack of oxygen and nutrients in local tissue, which in turn creates a hypoxic environment (152). In tenocytes, hypoxia induces expression of key cytokines and pro-inflammatory molecules, including platelet-derived growth factor, IL-6, IL-8 and platelet-activating factor, which may disrupt the balance of ECM repair (153). HIF-1 is a heterodimer composed of subunits HIF-1α and HIF-1β. HIF-1α is ubiquitous in cells and plays an important role in intracellular hypoxia response (154). During hypoxia, the activity of hydroxylase such as Prolyl Hydroxylase (PHD) and factor-inhibiting hydroxylase, is inhibited, which activates the NF-κB signaling pathway (155). At the same time, the activated NF-κB pathway leads to upregulation of HIF mRNA levels, which further promotes the activation of signaling. HIF-1 can induce expression of NF-κB target genes, including cyclooxygenase-2, TNF-α, IL-6 and macrophage phagophageal inflammatory protein-2, which leads to the continued development of inflammation (155). Thus, decreased vascularity in injured tendons and a hypoxic state induce an inflammatory response in tendon cells and lead to decreased synthesis of collagen matrix. Moreover, the recovery process takes longer in less vascularized tissue, with a greater likelihood of re-injury occurring before full recovery (156). Therefore, it is key to promote neovascularization in tendon repair.
MSCs secrete VEGF to enhance the proliferation and differentiation of vascular endothelial cells, thereby directly promoting angiogenesis (157-159). Studies have identified numerous cytokines that promote blood vessel formation, such as VEGF, PDGF and bFGF; VEGF has been proven to act on vascular endothelial cells (160,161). Angiogenesis is caused by the degradation of the basement membrane by MMPs. In the early stages of tendon healing, high levels of VEGF are secreted after injection of MSCs and its receptors are highly expressed (162). VEGF stimulates the expression of MMPs, accelerates degradation of the basement membrane and ECM components and initiates migration of endothelial cells. Subsequently, it responds to locally produced factors such as PDGF and bFGF, promoting development of capillary structure and forming an anastomosis with other blood vessels (163,164). VEGF may be considered the most effect mitogen promoting vascular growth. After VEGF secreted by MSCs binds to receptors VEGFR 2 and vascular endothelial growth factor receptor 1 (Flt-1) on endothelial cells with high affinity, it directly stimulates proliferation of vascular endothelial cells, inducing their migration and leading to formation of new blood vessels. At the same time, it can increase the permeability of capillaries (165). The ability to drive angiogenesis is significantly enhanced after culturing MSCs under hypoxic conditions (166,167). This is because hypoxic conditions inhibit cellular senescence, increase cell proliferation and enhance the differentiation potential of MSCs. Thus, the biological activity of MSCs is significantly increased in hypoxic environments, while hypoxia activates the angiogenic pathway by regulating the paracrine function of MSCs, leading to enhanced secretion of growth factors, including VEGF and IL-6 (168,169).
Studies have also shown that MSC-Exos deliver biologically active molecules, including microRNAs (miRs), to endothelial cells and mediate angiogenesis (129,170). miR-30b serves a key role in MSC-mediated angiogenesis. Delta-like protein 4 (DLL4), an miR-30 family target, is responsible for regulating blood vessel growth and branching during angiogenesis (171). In addition, miR-125a is a key factor in promoting angiogenesis. In the early stages of tendon healing, MSC-Exos promote secretion of MMP2 and miR-125a, which targets endothelial cells, thereby increasing endothelial cell migration and leading to increased angiogenesis, thereby accelerating tendon healing (172). MSCs promote endothelial cell proliferation via paracrine cytokines and MSC-Exos to increase blood vessel formation, which is necessary in tendon healing. The formation of new blood vessels ensures that sufficient nutrients, such as oxygen and growth factors, are provided to the injured area (150,173).
MSCs stimulate proliferation and migration of tenocytes
Tenocytes (also known as tendon fibroblasts) are the primary cells in tendons. They produce ECM components such as collagen, fibronectin and proteoglycans; thus, tenocytes play a crucial role in maintaining the stability of the ECM (67). During the proliferative phase of tendon healing, tenocytes gradually move to the injury site and proliferate, while production of collagen and glycoprotein increases to promote tissue regeneration (77). However, the proliferation and remodeling phases of tendon healing are usually slow due to low levels of tenocytes, as well as the relatively poor blood supply due to the low vascularity in the tendon, resulting in a mismatch between the rates of production of ECM components and tendon healing, which eventually leads to incomplete recovery of mechanical properties (174). Studies have identified another cell population in tendons, TSPCs, which account for 1-4% of the total number of cells within the tendon (85,175). TSPCs differentiate into tenocytes as well as chondrogenic, osteogenic and adipogenic lineages following induction in vitro and may then form tendon, cartilage, bone and tendon-bone junction tissues in animal models (176,177). Injection of TSPCs into a rat model of Achilles tendon injury shows strong healing ability (178). TSPCs are primarily responsible for the rapid replenishment of tenocytes after tendon injury to maintain numbers of tenocytes (179). However, the limited number of TSPCs isolated from tendon tissue requires expansion in vitro; this process may lead to genetic drift, which negatively affects proliferation and differentiation into tenocytes (180). Therefore, during in vitro amplification of TSPCs, the construction of a medium suitable for the amplification and survival of TSPCs is crucial for tendon regeneration.
Therapeutic approaches have focused on expansion of endogenous TSPCs and tenocytes by MSCs, as well as secretion of growth factors that induce TSPC differentiation to promote tendon regeneration (181). MSCs promote activation of protein kinase B (Akt) and extracellular signal-regulated kinase (ERK)-1/2, which are involved in tenocyte proliferation and migration via MEK/ERK1/2 and PI3K/Akt signaling (182). In addition, MSCs-Exos can promote activation of SMAD2/3 and SMAD1/5/9 signaling pathways, which significantly increases expression of TNMD, type I collagen and SCX protein (183). SCX and Mohawk are the major tendon cell-specific transcription factors that support matrix generation, tenocyte proliferation and differentiation. Moreover, they inhibit the differentiation of non-tendinous lineages including osteogenesis, chondrogenesis, and adipogenesis, thus promoting proliferation and differentiation of TSPCs to tenocytes (184). Thus, MSCs promote tendon healing in indirect and direct manners. A number of growth factors promote differentiation of co-cultured MSCs into tenocytes, including connective tissue growth factor (CTGF), TGF-β, GDF-7 and GDF-5 (20,21,185). Among them, CTGF, a member of the CCN protein family, has satisfactory effects on tendon repair when co-cultured with ADSCs; differentiation of ADSCs to tenocytes is induced by CTGF. CTGF significantly enhances the mRNA and protein expression of SCX and TNMD in a time- and dose-dependent manner (186). The most effective dose and treatment duration of CTGF is 100 ng/ml for 14 days (187). On the other hand, CTGF can induce the self-proliferation of ADSCs. This may be mediated by the FAK/ERK1/2 signaling pathway, which is the typical pathway for cell division and proliferation (188). Research (187) has shown that treatment of ADSCs with CTGF promotes proliferation in a dose-dependent manner on days 5 and 7. CTGF induces ERK1/2 phosphorylation in 5 and FAK phosphorylation in 15 min, both of which can last for 120 min. DNA methylation is induced via the FAK/ERK1/2 signaling pathway, increasing chromatin condensation and nuclear stiffness, thereby promoting cell migration (189). Therefore, combining CTGF with ADSCs can effectively increase tendon healing and provide a molecular and cytological basis for better application of tissue engineering methods to promote tendon healing. Another growth factor, BMP-12, induces differentiation of MSCs to tenocytes; this process is mainly mediated through the SMAD1/5/8 signaling pathway (190). However, the induction of differentiation of MSCs by transgenic BMP-12 is currently controversial: Although this has been shown to be effective in animal experiments, there are difficulties in clinical application of transgenic cells in humans, including possibility of side effects and ethical issues (191). In addition to the aforementioned growth factors, epidermal growth factor (EGF), platelet-derived growth factor-BB and TGF-β3 can also effectively increase expression of tendonogenic genes such as SRY-box containing gene 9 and TNMD when co-cultured with MSCs (192).
Although BMSCs are frequently used in the treatment of tendon repair and express a number of tendon-related markers, including SCX, TNMD, proteoglycan and type I and III collagen, it is hypothesized that levels of these markers are lower in BMSCs than in TSPCs (85). Therefore, co-culture of TSPCs with BMSCs may be a good option for the treatment of tendon injuries. In vitro experiment (193) have shown that bi-directional crosstalk between TSPCs and BMSCs upregulates tendon-associated genes (including SCX and TNMD) and tendon ECM markers (such as type I collagen, decorin and tenascin) and promotes ECM deposition. Thus, co-culture serves a role in inducing cell differentiation to tenocytes (193). Furthermore, adding mechanical stimuli to the surface of the medium can affect cell density, cellular arrangement and ECM deposition. For example, this results in a significantly higher cross-sectional cell density and a 2.5-fold increase in cell alignment (194,195). Cells can be cultured on micropatterned silicone substrates and subjected to cyclic stretching to simulate the in vivo biomechanical environment during tendon healing (195). When cells in medium are exposed to intermittent cyclic strain, cell differentiation to tenocytes is induced (196). When the tensile strength is increased to 4 and 8%, MSCs cultured in vitro exhibit a spindle-like shape and produce type I collagen (197). In summary, studying the effects of growth factors or TSPCs in combination with MSCs and mechanical stimulation may provide novel options for differentiation of MSCs to tenocytes (198).
MSCs increase synthesis of collagen
In normal tendons, the levels of type I collagen in the ECM are high, accounting for 95%, and type III collagen is expressed at lower levels (49,85). Type III collagen is weaker than type I collagen bearing mechanical loads and type I collagen plays a crucial role in the tensile strength of the tendon (199). The activity of MMPs is key in the remodeling phase of tendon repair. MMPs are collagen hydrolases that break down damaged collagen (200,201). During the collagen remodeling phase, MMP13 and MMP3 are highly expressed and their increased expression degrades type I and III collagen and proteoglycans in ECM. During the early stages of tendon healing, VEGF and its receptors are highly expressed, which stimulates the expression of MMPs (202). ADSC-Exos significantly inhibit the expression of MMP9/13 genes and indirectly increase the ratio of type I/III collagen, thus promoting collagen synthesis and tendon healing (203). In addition, BMSCs cultured under hypoxic conditions exhibit high expression of type I/III collagen α1, decorin and TNMD in the early stages of tendon repair (168). Decorin regulates the diameter of collagen fibers and works in combination with growth factors to regulate cell proliferation, thereby promoting collagen production (61). TNMD is a specific marker of tendon maturation. TNMD promotes proliferation, migration and tendon differentiation of TSPCs and prevents scar formation during early stage of tendon healing; TNMD also regulates levels of type I collagen and promotes collagen remodeling (101). Therefore, BMSCs are an effective therapeutic method to promote tendon tissue regeneration. Similarly, ADSC-Exos increase expression of TNMD, type I collagen and SCX in TSPCs by activating SMAD2/3 and SMAD1/5/9 signaling pathways, thereby promoting TSPC proliferation, migration and tendon differentiation (99). SMAD2/3 and SMAD1/5/9 are typical SMAD signaling pathways and SMAD3 is also a key transcription factor for type I collagen synthesis (204). SMAD3 activates TGF-β signaling pathway via TGF-β type I and II transmembrane receptors (205); SMAD2 and SMAD3 dissociate from the receptor after phosphorylation, form a complex with SMAD4 and translocate to the nucleus (206). SMAD3 is involved in transcription of genes associated with cell proliferation, inflammatory response and ECM formation. Therefore, TGF-β signaling is involved in regulating collagen formation, MMPs activity and tissue remodeling during tendon healing via the transcription factor SMAD3 (207).
Studies have shown that MSCs injected following tendon injury secrete growth factors, including TGF-β, bFGF and EGF (208,209). These factors accelerate ECM deposition and remodeling at the injured site and start collagen synthesis and maturation (210,211). TGF-β promotes collagen production, which increases the strength of the repaired tendon; however, when TGF-β is overexpressed, the overproduction of disordered collagen may lead to the formation of adhesions at the tendon (212). bFGF promotes cell mitosis, increases proliferation of fibroblasts and secretes type I and III collagen (213). There is a unique pattern of collagen production during tendon repair. Type III collagen increases significantly in the early stages of tendon healing, providing a 'quick fix' for the damaged site. At 6-8 weeks after injury, the tissue replaces type III collagen with type I collagen and restores its linear structure, resulting in increased collagen fiber crosslinking and tendine-like tissue formation (113,173). In co-culture experiments with ADSCs and tenocytes, ratio of ADSCs to tenocytes of 3:1 increases proliferation of tenocytes; ADSCs also differentiate into tendon cells and expression of tenascin-C and SCX increases (214). Tenascin-C regulates the number of collagen fibers as well as their growth direction and is key for maintaining the mechanical stability of the ECM (64). Likewise, SCX serves an important role in tenocyte differentiation as well as tendon development. In SCX-knockout mice, tendon development is notably disrupted (215). Certain genes, including type I collagen α1 and TNMD, are potential direct target genes of SCX in tenocytes but how SCX regulates tenocyte differentiation is unknown (216). ADSCs increase proliferation rate and gene expression in tenocytes, thereby enhancing the function of tenocytes, accelerating the turnover of ECM and increasing the proportion of normal collagen structure in tendons. The strength of the tendon is quickly restored, thereby inhibiting further degeneration of the tendon (217).
6. Conclusion
Tendon injury is common in orthopedics. After tendon injury, the tendon shows a local inflammatory response, hypocellularity, lack of collagen and blood vessels and increase levels of proteoglycans (218). The injured tendon exhibits discontinuous and disorganized tendon fibers. Since tendon is a tissue with low cellular content and poor blood supply, the tendon has a limited ability to heal (219,220). The process of tendon healing is divided into three overlapping phases: Inflammatory, proliferative and remodeling phase. During the remodeling phase, scars often form (116). Scars have different biomechanical properties compared with natural tendons, including decreased strength, increased stiffness and greater tendency to form adhesions. Reconstructed tendon tissue exhibits poorer biochemical and mechanical properties compared with uninjured tendon (221). This leads to dysfunction in the limb and makes the tendon more prone to re-rupture (11). With the development of SC research, MSCs have attracted attention (222). MSCs have high proliferation and self-renewal capacity (223). MSCs differentiate into target cells and directly promote tissue regeneration; MSCs also secrete biological factors and EVs, thus indirectly affecting tissue repair (220). MSCs can be obtained from numerous types of tissue and MSCs from different sources show different characteristics, indicating potential advantages and disadvantages of each type of MSC for specific clinical applications (224). Among sources, the most commonly used are BM and adipose tissue. ADSCs are more readily available compared with BM, yield more abundant MSCs after isolation and also decrease donor site morbidity (225). This is because BMSCs need to be collected using a trocar to drill through the iliac crest, while ADSCs can be collected using only minimally invasive liposuction techniques, this is easier and less painful for the patient. In addition, over time, the donor site providing BMSCs is prone to pain and stiffness, whereas ADSCs have a much lower incidence (88). In addition to application in tendon injury, MSCs can also be used to treat fractures, osteoarthritis and other disease (226). MSCs have shown satisfactory results in tissue engineering and regenerative medicine, not only in promoting tissue regeneration, but also in restoring the tissue to its original biomechanical function to the greatest extent possible (227).
The roles and mechanisms of MSCs may involve promoting angiogenesis, cell proliferation and differentiation and collagen formation and decreasing inflammation during tendon repair (129,228). MSCs participate in localized anti-inflammatory response in the early stage following tendon damage. Anti-inflammatory factors and MSCs-EVs are hypothesized to be intercellular messengers in immune regulation (229). They interact with various types of immune cell, including T and B lymphocytes, natural killer cells, macrophages, neutrophils and monocytes (230). MSCs promote angiogenesis mainly by releasing VEGF and Exos (157). During the remodeling and collagen production phase, in vitro experiment indicated MSCs enhance their ability to differentiate into tenocytes by co-culture with growth factors and TSPCs, thus promoting tenocyte proliferation and differentiation, as well as collagen fiber production and ECM remodeling (198,217).
The present review summarized the functions and mechanisms of MSCs in tendon repair but there are still some issues with the clinical application of MSCs that need to be addressed. To the best of our knowledge is no consensus on practical considerations regarding the source, dose, administration technique and timing of MSC usage (231). Although the commonly used sources of MSCs are adipose and BM tissue, there are still debates on the applications of both; for example, whether ectopic bone can develop after application of BMSCs for treatment and whether the application of ADSCs involves ethical issues. MSCs isolated from young living sources survive longer, secrete more Exos and have a broader differentiation capacity than MSCs isolated from older tissue (232). Therefore, MSCs isolated from embryonic sources may be promising therapeutic tools for tendon repair and regeneration (233). The current mode of administration is direct injection of MSCs at the site of injury, but a study (234) has shown that intravenous MSCs can promote better interaction with the immune system and initiate systemic anti-inflammatory effects. However, due to small sample, more research is needed on intravenous MSC administration (235). Although study have shown that MSCs show satisfactory therapeutic effects when co-cultured with growth factors or TSPCs, few studies have compared the effects of culture conditions (236,237). Therefore, randomized controlled trials are required (238). In addition, current treatments to promote tendon repair lack standardization so treatment results may differ. Therefore, future studies should investigate the effects of clinical treatment with MSCs alone to develop standardized treatment modalities, which may lead to more uniform outcomes.
In conclusion, MSCs are a promising cell therapy to promote tendon healing and understanding of the functions and mechanisms of MSCs in tendon healing can help improve its efficiency. However, further studies are required to maximize the therapeutic value of MSCs.
Availability of data and materials
Not applicable.
Authors' contributions
LJ, JL, YC, KL, LL, XW, TL and SL performed study conception and design. KL, LL and XW wrote the manuscript. LJ performed the literature review. LJ, TL and SL edited the manuscript. All authors have read and approved the final manuscript. Data authentication is not applicable.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Acknowledgments
The authors would like to thank Mr Robert Constantine, Everett Adult Learning Center, Everett, Massachusetts, United States for language editing the manuscript.
Funding
The present study was supported by 2022 Sichuan Provincial Science and Technology Plan Project (grant no. 22ZDYF3799), Luzhou Science and Technology Program Project (grant no. 2020-SYF-31), Luzhou Municipal Government-Southwest Medical University Joint Project (grant no. 2021LZXNYD-J10), Sichuan Science and Technology Program Project (grant no. 2022NSFSC0688) and Southwest Medical University Applied Basic Fundamental Research Project (grant no. 2021ZKMS050).
References
|
Andarawis-Puri N, Flatow EL and Soslowsky LJ: Tendon basic science: Development, repair, regeneration, and healing. J Orthop Res. 33:780–784. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Cook JL and Purdam C: Is compressive load a factor in the development of tendinopathy? Br J Sports Med. 46:163–168. 2012. View Article : Google Scholar | |
|
Beatty NR, Félix I, Hettler J, Moley PJ and Wyss JF: Rehabilitation and prevention of proximal hamstring tendinopathy. Curr Sports Med Rep. 16:162–171. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Silbernagel KG, Hanlon S and Sprague A: Current Clinical concepts: Conservative management of achilles tendinopathy. J Athl Train. 55:438–447. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Figueroa D, Figueroa F and Calvo R: Patellar tendinopathy: Diagnosis and treatment. J Am Acad Orthop Surg. 24:e184–e192. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Chun SW, Kim W, Lee SY, Lim CY, Kim K, Kim JG, Park CH, Hong SH, Yoo HJ and Chung SG: A randomized controlled trial of stem cell injection for tendon tear. Sci Rep. 12:8182022. View Article : Google Scholar : PubMed/NCBI | |
|
Chen Z, Jin M, He H, Dong J, Li J, Nie J, Wang Z, Xu J and Wu F: Mesenchymal stem cells and macrophages and their interactions in tendon-bone healing. J Orthop Translat. 39:63–73. 2023. View Article : Google Scholar : PubMed/NCBI | |
|
Malekpour K, Hazrati A, Zahar M, Markov A, Zekiy AO, Navashenaq JG, Roshangar L and Ahmadi M: The potential use of mesenchymal stem cells and their derived exosomes for orthopedic diseases treatment. Stem Cell Rev Rep. 18:933–951. 2022. View Article : Google Scholar | |
|
Xu Y, Zhang WX, Wang LN, Ming YQ, Li YL and Ni GX: Stem cell therapies in tendon-bone healing. World J Stem Cells. 13:753–775. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Fu X, Liu G, Halim A, Ju Y, Luo Q and Song AG: Mesenchymal stem cell migration and tissue repair. Cells. 8:7842019. View Article : Google Scholar : PubMed/NCBI | |
|
Torres-Torrillas M, Rubio M, Damia E, Cuervo B, Del Romero A, Peláez P, Chicharro D, Miguel L and Sopena JJ: Adipose-derived mesenchymal stem cells: A promising tool in the treatment of musculoskeletal diseases. Int J Mol Sci. 20:31052019. View Article : Google Scholar : PubMed/NCBI | |
|
Docheva D, Müller SA, Majewski M and Evans CH: Biologics for tendon repair. Adv Drug Deliv Rev. 84:222–239. 2015. View Article : Google Scholar : | |
|
Mirghaderi SP, Valizadeh Z, Shadman K, Lafosse T, Oryadi-Zanjani L, Yekaninejad MS and Nabian MH: Cell therapy efficacy and safety in treating tendon disorders: A systemic review of clinical studies. J Exp Orthop. 9:852022. View Article : Google Scholar : PubMed/NCBI | |
|
Lui PP: Stem cell technology for tendon regeneration: Current status, challenges, and future research directions. Stem Cells Cloning. 8:163–174. 2015.PubMed/NCBI | |
|
Migliorini F, Tingart M and Maffulli N: Progress with stem cell therapies for tendon tissue regeneration. Expert Opin Biol Ther. 20:1373–1379. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Guo D, Li H, Liu Y, Yu X, Zhang X, Chu W, She Y, Wang D and Chen G: Fibroblast growth factor-2 promotes the function of tendon-derived stem cells in Achilles tendon restoration in an Achilles tendon injury rat model. Biochem Biophys Res Commun. 521:91–97. 2020. View Article : Google Scholar | |
|
Cohen S, Leshansky L, Zussman E, Burman M, Srouji S, Livne E, Abramov N and Itskovitz-Eldor J: Repair of full-thickness tendon injury using connective tissue progenitors efficiently derived from human embryonic stem cells and fetal tissues. Tissue Eng Part A. 16:3119–3137. 2010. View Article : Google Scholar : PubMed/NCBI | |
|
Yea JH, Kim Y and Jo CH: Comparison of mesenchymal stem cells from bone marrow, umbilical cord blood, and umbilical cord tissue in regeneration of a full-thickness tendon defect in vitro and in vivo. Biochem Biophys Rep. 34:1014862023.PubMed/NCBI | |
|
Baberg F, Geyh S, Waldera-Lupa D, Stefanski A, Zilkens C, Haas R, Schroeder T and Stühler K: Secretome analysis of human bone marrow derived mesenchymal stromal cells. Biochim Biophys Acta Proteins Proteom. 1867:434–441. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Thomopoulos S, Das R, Sakiyama-Elbert S, Silva MJ, Charlton N and Gelberman RH: bFGF and PDGF-BB for tendon repair: Controlled release and biologic activity by tendon fibroblasts in vitro. Ann Biomed Eng. 38:225–234. 2010. View Article : Google Scholar : | |
|
Gelberman RH, Linderman SW, Jayaram R, Dikina AD, Sakiyama-Elbert S, Alsberg E, Thomopoulos S and Shen H: Combined administration of ASCs and BMP-12 promotes an M2 macrophage phenotype and enhances tendon healing. Clin Orthop Relat Res. 475:2318–2331. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Yea JH, Bae TS, Kim BJ, Cho YW and Jo CH: Regeneration of the rotator cuff tendon-to-bone interface using umbilical cord-derived mesenchymal stem cells and gradient extracellular matrix scaffolds from adipose tissue in a rat model. Acta Biomater. 114:104–116. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Lim WL, Chowdhury SR, Ng MH and Law JX: Physicochemical properties and biocompatibility of electrospun polycaprolactone/gelatin nanofibers. Int J Environ Res Public Health. 18:47642021. View Article : Google Scholar : PubMed/NCBI | |
|
Ciardulli MC, Marino L, Lovecchio J, Giordano E, Forsyth NR, Selleri C, Maffulli N and Porta GD: Tendon and cytokine marker expression by human bone marrow mesenchymal stem cells in a hyaluronate/poly-lactic-co-glycolic acid (PLGA)/fibrin three-dimensional (3D) scaffold. Cells. 9:12682020. View Article : Google Scholar : PubMed/NCBI | |
|
Smith RK, Werling NJ, Dakin SG, Alam R, Goodship AE and Dudhia J: Beneficial effects of autologous bone marrow-derived mesenchymal stem cells in naturally occurring tendinopathy. PLoS One. 8:e756972013. View Article : Google Scholar : PubMed/NCBI | |
|
Manning CN, Martel C, Sakiyama-Elbert SE, Silva MJ, Shah S, Gelberman RH and Thomopoulos S: Adipose-derived mesenchymal stromal cells modulate tendon fibroblast responses to macrophage-induced inflammation in vitro. Stem Cell Res Ther. 6:742015. View Article : Google Scholar : PubMed/NCBI | |
|
Tan Q, Lui PP and Lee YW: In vivo identity of tendon stem cells and the roles of stem cells in tendon healing. Stem Cells Dev. 22:3128–3140. 2013. View Article : Google Scholar : PubMed/NCBI | |
|
Li P, Xu Y, Gan Y, Song L, Zhang C, Wang L and Zhou Q: Role of the ERK1/2 signaling pathway in osteogenesis of rat tendon-derived stem cells in normoxic and hypoxic cultures. Int J Med Sci. 13:629–637. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Rui YF, Lui PP, Chan LS, Chan KM, Fu SC and Li G: Does erroneous differentiation of tendon-derived stem cells contribute to the pathogenesis of calcifying tendinopathy? Chin Med J (Engl). 124:606–610. 2011.PubMed/NCBI | |
|
Nie D, Zhou Y, Wang W, Zhang J and Wang JHC: Mechanical overloading induced-activation of mTOR signaling in tendon stem/progenitor cells contributes to tendinopathy development. Front Cell Dev Biol. 9:6878562021. View Article : Google Scholar : PubMed/NCBI | |
|
Zhang J and Wang JH: Prostaglandin E2 (PGE2) exerts biphasic effects on human tendon stem cells. PLoS One. 9:e877062014. View Article : Google Scholar : PubMed/NCBI | |
|
Bajada S, Mazakova I, Richardson JB and Ashammakhi N: Updates on stem cells and their applications in regenerative medicine. J Tissue Eng Regen Med. 2:169–183. 2008. View Article : Google Scholar : PubMed/NCBI | |
|
Dale TP, Mazher S, Webb WR, Zhou J, Maffulli N, Chen GQ, El Haj AJ and Forsyth NR: Tenogenic differentiation of human embryonic stem cells. Tissue Eng Part A. 24:361–368. 2018. View Article : Google Scholar | |
|
Blum B and Benvenisty N: The tumorigenicity of human embryonic stem cells. Adv Cancer Res. 100:133–158. 2008. View Article : Google Scholar : PubMed/NCBI | |
|
Wyles SP, Yamada S, Oommen S, Maleszewski JJ, Beraldi R, Martinez-Fernandez A, Terzic A and Nelson TJ: Inhibition of DNA topoisomerase II selectively reduces the threat of tumorigenicity following induced pluripotent stem cell-based myocardial therapy. Stem Cells Dev. 23:2274–2282. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
Aguiar C, Theoret C, Smith O, Segura M, Lemire P and Smith LC: Immune potential of allogeneic equine induced pluripotent stem cells. Equine Vet J. 47:708–714. 2015. View Article : Google Scholar | |
|
Bavin EP, Smith O, Baird AEG, Smith LC and Guest DJ: Equine induced pluripotent stem cells have a reduced tendon differentiation capacity compared to embryonic stem cells. Front Vet Sci. 2:552015. View Article : Google Scholar : PubMed/NCBI | |
|
Polo JM, Liu S, Figueroa ME, Kulalert W, Eminli S, Tan KY, Apostolou E, Stadtfeld M, Li Y, Shioda T, et al: Cell type of origin influences the molecular and functional properties of mouse induced pluripotent stem cells. Nat Biotechnol. 28:848–855. 2010. View Article : Google Scholar : PubMed/NCBI | |
|
Nourissat G, Berenbaum F and Duprez D: Tendon injury: From biology to tendon repair. Nat Rev Rheumatol. 11:223–233. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Wang JH: Mechanobiology of tendon. J Biomech. 39:1563–1582. 2006. View Article : Google Scholar | |
|
Whittaker P and Canham PB: Demonstration of quantitative fabric analysis of tendon collagen using two-dimensional polarized light microscopy. Matrix. 11:56–62. 1991. View Article : Google Scholar : PubMed/NCBI | |
|
Canty EG and Kadler KE: Procollagen trafficking, processing and fibrillogenesis. J Cell Sci. 118:1341–1353. 2005. View Article : Google Scholar : PubMed/NCBI | |
|
Herchenhan A, Kalson NS, Holmes DF, Hill P, Kadler KE and Margetts L: Tenocyte contraction induces crimp formation in tendon-like tissue. Biomech Model Mechanobiol. 11:449–459. 2012. View Article : Google Scholar | |
|
Stammers M, Niewczas IS, Segonds-Pichon A and Clark J: Mechanical stretching changes crosslinking and glycation levels in the collagen of mouse tail tendon. J Biol Chem. 295:10572–10580. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Thorpe CT, Udeze CP, Birch HL, Clegg PD and Screen HR: Specialization of tendon mechanical properties results from interfascicular differences. J R Soc Interface. 9:3108–3117. 2012. View Article : Google Scholar : PubMed/NCBI | |
|
Wang JHC, Guo Q and Li B: Tendon biomechanics and mechanobiology-a minireview of basic concepts and recent advancements. J Hand Ther. 25:133–141. 2012. View Article : Google Scholar | |
|
Thorpe CT and Screen HR: Tendon structure and composition. Adv Exp Med Biol. 920:3–10. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Zhang G, Young BB, Ezura Y, Favata M, Soslowsky LJ, Chakravarti S and Birk DE: Development of tendon structure and function: Regulation of collagen fibrillogenesis. J Musculoskelet Neuronal Interact. 5:5–21. 2005.PubMed/NCBI | |
|
Screen HRC, Berk DE, Kadler KE, Ramirez F and Young MF: Tendon functional extracellular matrix. J Orthop Res. 33:793–799. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Riechert K, Labs K, Lindenhayn K and Sinha P: Semiquantitative analysis of types I and III collagen from tendons and ligaments in a rabbit model. J Orthop Sci. 6:68–74. 2001. View Article : Google Scholar : PubMed/NCBI | |
|
Liu SH, Yang RS, al-Shaikh R and Lane JM: Collagen in tendon, ligament, and bone healing. A current review. Clin Orthop Relat Res. 265–278. 1995.PubMed/NCBI | |
|
Birk DE and Mayne R: Localization of collagen types I, III and V during tendon development. Changes in collagen types I and III are correlated with changes in fibril diameter. Eur J Cell Biol. 72:352–361. 1997.PubMed/NCBI | |
|
Wenstrup RJ, Smith SM, Florer JB, Zhang G, Beason DP, Seegmiller RE, Soslowsky LJ and Birk DE: Regulation of collagen fibril nucleation and initial fibril assembly involves coordinate interactions with collagens V and XI in developing tendon. J Biol Chem. 286:20455–20465. 2011. View Article : Google Scholar : PubMed/NCBI | |
|
Izu Y, Ansorge HL, Zhang G, Soslowsky LJ, Bonaldo P, Chu ML and Birk DE: Dysfunctional tendon collagen fibrillogenesis in collagen VI null mice. Matrix Biol. 30:53–61. 2011. View Article : Google Scholar | |
|
Taye N, Karoulias SZ and Hubmacher D: The 'other' 15-40%: The role of non-collagenous extracellular matrix proteins and minor collagens in tendon. J Orthop Res. 38:23–35. 2020. View Article : Google Scholar | |
|
Birch HL: Tendon matrix composition and turnover in relation to functional requirements. Int J Exp Pathol. 88:241–248. 2007. View Article : Google Scholar : PubMed/NCBI | |
|
Ristaniemi A, Regmi D, Mondal D, Torniainen J, Tanska P, Stenroth L, Finnilä MAJ, Töyräs J and Korhonen RK: Structure, composition and fibril-reinforced poroviscoelastic properties of bovine knee ligaments and patellar tendon. J R Soc Interface. 18:202007372021. View Article : Google Scholar : PubMed/NCBI | |
|
Schwartz AG, Lipner JH, Pasteris JD, Genin GM and Thomopoulos S: Muscle loading is necessary for the formation of a functional tendon enthesis. Bone. 55:44–51. 2013. View Article : Google Scholar : PubMed/NCBI | |
|
Thorpe CT, Birch HL, Clegg PD and Screen HRC: The role of the non-collagenous matrix in tendon function. Int J Exp Pathol. 94:248–259. 2013. View Article : Google Scholar : PubMed/NCBI | |
|
Chen S and Birk DE: The regulatory roles of small leucine-rich proteoglycans in extracellular matrix assembly. FEBS J. 280:2120–2137. 2013. View Article : Google Scholar : PubMed/NCBI | |
|
Halper J: Proteoglycans and diseases of soft tissues. Adv Exp Med Biol. 802:49–58. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
Smith RKW, Gerard M, Dowling B, Dart AJ, Birch HL and Goodship AE: Correlation of cartilage oligomeric matrix protein (COMP) levels in equine tendon with mechanical properties: A proposed role for COMP in determining function-specific mechanical characteristics of locomotor tendons. Equine Vet J Suppl. 241–244. 2002.PubMed/NCBI | |
|
Järvinen TAH, Józsa L, Kannus P, Järvinen TL, Hurme T, Kvist M, Pelto-Huikko M, Kalimo H and Järvinen M: Mechanical loading regulates the expression of tenascin-C in the myotendinous junction and tendon but does not induce de novo synthesis in the skeletal muscle. J Cell Sci. 116:857–866. 2003. View Article : Google Scholar : PubMed/NCBI | |
|
Pajala A, Melkko J, Leppilahti J, Ohtonen P, Soini Y and Risteli J: Tenascin-C and type I and III collagen expression in total Achilles tendon rupture. An immunohistochemical study. Histol Histopathol. 24:1207–1211. 2009.PubMed/NCBI | |
|
Grant TM, Thompson MS, Urban J and Yu J: Elastic fibres are broadly distributed in tendon and highly localized around tenocytes. J Anat. 222:573–579. 2013. View Article : Google Scholar : PubMed/NCBI | |
|
Thakkar D, Grant TM, Hakimi O and Carr AJ: Distribution and expression of type VI collagen and elastic fibers in human rotator cuff tendon tears. Connect Tissue Res. 55:397–402. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
Zhang J and Wang JHC: Characterization of differential properties of rabbit tendon stem cells and tenocytes. BMC Musculoskelet Disord. 11:102010. View Article : Google Scholar : PubMed/NCBI | |
|
Ralphs JR, Waggett AD and Benjamin M: Actin stress fibres and cell-cell adhesion molecules in tendons: Organisation in vivo and response to mechanical loading of tendon cells in vitro. Matrix Biol. 21:67–74. 2002. View Article : Google Scholar : PubMed/NCBI | |
|
Zhang S, Ju W and Chen X, Zhao Y, Feng L, Yin Z and Chen X: Hierarchical ultrastructure: An overview of what is known about tendons and future perspective for tendon engineering. Bioact Mater. 8:124–139. 2022. View Article : Google Scholar | |
|
Marr N, Meeson R, Kelly EF, Fang Y, Peffers MJ, Pitsillides AA, Dudhia J and Thorpe CT: CD146 Delineates an interfascicular cell sub-population in tendon that is recruited during injury through its ligand laminin-α4. Int J Mol Sci. 22:97292021. View Article : Google Scholar | |
|
Xu W, Hua H, Chiu YH, Li G, Zhi H, Yu Z, Ren F, Luo Y and Cui W: CD146 regulates growth factor-induced mTORC2 activity independent of the PI3K and mTORC1 pathways. Cell Rep. 29:1311–1322.e5. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Grol MW, Haelterman NA, Lim J, Munivez EM, Archer M, Hudson DM, Tufa SF, Keene DR, Lei K, Park D, et al: Tendon and motor phenotypes in the Crtap−/− mouse model of recessive osteogenesis imperfecta. Elife. 10:e634882021. View Article : Google Scholar | |
|
Flanagan K, Fitzgerald K, Baker J, Regnstrom K, Gardai S, Bard F, Mocci S, Seto P, You M, Larochelle C, et al: Laminin-411 is a vascular ligand for MCAM and facilitates TH17 cell entry into the CNS. PLoS One. 7:e404432012. View Article : Google Scholar : PubMed/NCBI | |
|
Wei B and Lu J: Characterization of tendon-derived stem cells and rescue tendon injury. Stem Cell Rev Rep. 17:1534–1551. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Zhou Z, Akinbiyi T, Xu L, Ramcharan M, Leong DJ, Ros SJ, Colvin AC, Schaffler MB, Majeska RJ, Flatow EL and Sun HB: Tendon-derived stem/progenitor cell aging: Defective self-renewal and altered fate. Aging Cell. 9:911–915. 2010. View Article : Google Scholar : PubMed/NCBI | |
|
Zhang J and Wang JHC: Mechanobiological response of tendon stem cells: Implications of tendon homeostasis and pathogenesis of tendinopathy. J Orthop Res. 28:639–643. 2010. View Article : Google Scholar | |
|
Voleti PB, Buckley MR and Soslowsky LJ: Tendon healing: repair and regeneration. Annu Rev Biomed Eng. 14:47–71. 2012. View Article : Google Scholar : PubMed/NCBI | |
|
Elliott DM, Robinson PS, Gimbel JA, Sarver JJ, Abboud JA, Iozzo RV and Soslowsky LJ: Effect of altered matrix proteins on quasilinear viscoelastic properties in transgenic mouse tail tendons. Ann Biomed Eng. 31:599–605. 2003. View Article : Google Scholar : PubMed/NCBI | |
|
Winnicki K, Ochała-Kłos A, Rutowicz B, Pękala PA and Tomaszewski KA: Functional anatomy, histology and biomechanics of the human Achilles tendon-A comprehensive review. Ann Anat. 229:1514612020. View Article : Google Scholar | |
|
Zhang M, Liu H, Cui Q, Han P, Yang S, Shi M, Zhang T, Zhang Z and Li Z: Tendon stem cell-derived exosomes regulate inflammation and promote the high-quality healing of injured tendon. Stem Cell Res Ther. 11:4022020. View Article : Google Scholar : PubMed/NCBI | |
|
Margiana R, Markov A, Zekiy AO, Hamza MU, Al-Dabbagh KA, Al-Zubaidi SH, Hameed NM, Ahmad I, Sivaraman R, Kzar HH, et al: Clinical application of mesenchymal stem cell in regenerative medicine: A narrative review. Stem Cell Res Ther. 13:3662022. View Article : Google Scholar : PubMed/NCBI | |
|
Chanda D, Kumar S and Ponnazhagan S: Therapeutic potential of adult bone marrow-derived mesenchymal stem cells in diseases of the skeleton. J Cell Biochem. 111:249–257. 2010. View Article : Google Scholar : PubMed/NCBI | |
|
McDougall RA, Canapp SO and Canapp DA: Ultrasonographic findings in 41 dogs treated with bone marrow aspirate concentrate and platelet-rich plasma for a supraspinatus tendinopathy: A retrospective study. Front Vet Sci. 5:982018. View Article : Google Scholar : PubMed/NCBI | |
|
Ruzzini L, Longo UG, Rizzello G and Denaro V: Stem cells and tendinopathy: State of the art from the basic science to clinic application. Muscles Ligaments Tendons J. 2:235–238. 2012. | |
|
Bi Y, Ehirchiou D, Kilts TM, Inkson CA, Embree MC, Sonoyama W, Li L, Leet AI, Seo BM, Zhang L, et al: Identification of tendon stem/progenitor cells and the role of the extracellular matrix in their niche. Nat Med. 13:1219–1227. 2007. View Article : Google Scholar : PubMed/NCBI | |
|
Chong AKS, Ang AD, Goh JCH, Hui JHP, Lim AYT, Lee EH and Lim BH: Bone marrow-derived mesenchymal stem cells influence early tendon-healing in a rabbit achilles tendon model. J Bone Joint Surg Am. 89:74–81. 2007. View Article : Google Scholar : PubMed/NCBI | |
|
Harris MT, Butler DL, Boivin GP, Florer JB, Schantz EJ and Wenstrup RJ: Mesenchymal stem cells used for rabbit tendon repair can form ectopic bone and express alkaline phosphatase activity in constructs. J Orthop Res. 22:998–1003. 2004. View Article : Google Scholar : PubMed/NCBI | |
|
Arnaud-Franco Á, Lara-Arias J, Marino-Martínez IA, Cienfuegos-Jiménez O, Barbosa-Quintana Á and Peña-Martínez VM: Effect of adipose-derived mesenchymal stem cells (ADMSCs) application in achilles-tendon injury in an animal model. Curr Issues Mol Biol. 44:5827–5838. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Peng L, Jia Z, Yin X, Zhang X, Liu Y, Chen P, Ma K and Zhou C: Comparative analysis of mesenchymal stem cells from bone marrow, cartilage, and adipose tissue. Stem Cells Dev. 17:761–773. 2008. View Article : Google Scholar : PubMed/NCBI | |
|
Chu DT, Nguyen Thi Phuong T, Tien NLB, Tran DK, Minh LB, Thanh VV, Gia Anh P, Pham VH and Thi Nga V: Adipose tissue stem cells for therapy: An update on the progress of isolation, culture, storage, and clinical application. J Clin Med. 8:9172019. View Article : Google Scholar : PubMed/NCBI | |
|
Gentile P, Calabrese C, De Angelis B, Pizzicannella J, Kothari A and Garcovich S: Impact of the different preparation methods to obtain human adipose-derived stromal vascular fraction cells (AD-SVFs) and human adipose-derived mesenchymal stem cells (AD-MSCs): Enzymatic digestion versus mechanical centrifugation. Int J Mol Sci. 20:54712019. View Article : Google Scholar : PubMed/NCBI | |
|
Itro A, Trotta MC, Miranda R, Paoletta M, De Cicco A, Lepre CC, Tarantino U, D'Amico M, Toro G and Schiavone Panni A: Why use adipose-derived mesenchymal stem cells in tendinopathic patients: A systematic review. Pharmaceutics. 14:11512022. View Article : Google Scholar : PubMed/NCBI | |
|
Lin L, Shen Q, Xue T and Yu CL: Heterotopic ossification induced by Achilles tenotomy via endochondral bone formation: Expression of bone and cartilage related genes. Bone. 46:425–431. 2010. View Article : Google Scholar | |
|
Schipani E: Posttranslational modifications of collagens as targets of hypoxia and Hif-1alpha in endochondral bone development. Ann N Y Acad Sci. 1192:317–321. 2010. View Article : Google Scholar : PubMed/NCBI | |
|
Kokubu S, Inaki R, Hoshi K and Hikita A: Adipose-derived stem cells improve tendon repair and prevent ectopic ossification in tendinopathy by inhibiting inflammation and inducing neovascularization in the early stage of tendon healing. Regen Ther. 14:103–110. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Lee SY, Kwon B, Lee K, Son YH and Chung SG: Therapeutic mechanisms of human adipose-derived mesenchymal stem cells in a rat tendon injury model. Am J Sports Med. 45:1429–1439. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Naji A, Eitoku M, Favier B, Deschaseaux F, Rouas-Freiss N and Suganuma N: Biological functions of mesenchymal stem cells and clinical implications. Cell Mol Life Sci. 76:3323–3348. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Fu G, Lu L, Pan Z, Fan A and Yin F: Adipose-derived stem cell exosomes facilitate rotator cuff repair by mediating tendon-derived stem cells. Regen Med. 16:359–372. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Liu H, Zhang M, Shi M, Zhang T, Lu W, Yang S, Cui Q and Li Z: Adipose-derived mesenchymal stromal cell-derived exosomes promote tendon healing by activating both SMAD1/5/9 and SMAD2/3. Stem Cell Res Ther. 12:3382021. View Article : Google Scholar : PubMed/NCBI | |
|
Shi Y, Kang X, Wang Y, Bian X, He G, Zhou M and Tang K: Exosomes derived from bone marrow stromal cells (BMSCs) enhance tendon-bone healing by regulating macrophage polarization. Med Sci Monit. 26:e9233282020. View Article : Google Scholar : PubMed/NCBI | |
|
Wu XD, Kang L, Tian J, Wu Y, Huang Y, Liu J, Wang H, Qiu G and Wu Z: Exosomes derived from magnetically actuated bone mesenchymal stem cells promote tendon-bone healing through the miR-21-5p/SMAD7 pathway. Mater Today Bio. 15:1003192022. View Article : Google Scholar : PubMed/NCBI | |
|
Heo JS, Choi Y and Kim HO: Adipose-derived mesenchymal stem cells promote M2 macrophage phenotype through exosomes. Stem Cells Int. 2019:79217602019. View Article : Google Scholar : PubMed/NCBI | |
|
Ragni E, Papait A, Perucca Orfei C, Silini AR, Colombini A, Viganò M, Libonati F, Parolini O and de Girolamo L: Amniotic membrane-mesenchymal stromal cells secreted factors and extracellular vesicle-miRNAs: Anti-inflammatory and regenerative features for musculoskeletal tissues. Stem Cells Transl Med. 10:1044–1062. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Hong P, Yang H, Wu Y, Li K and Tang Z: The functions and clinical application potential of exosomes derived from adipose mesenchymal stem cells: A comprehensive review. Stem Cell Res Ther. 10:2422019. View Article : Google Scholar : PubMed/NCBI | |
|
Chen SH, Chen ZY, Lin YH, Chen SH, Chou PY, Kao HK and Lin FH: Extracellular vesicles of adipose-derived stem cells promote the healing of traumatized achilles tendons. Int J Mol Sci. 22:123732021. View Article : Google Scholar : PubMed/NCBI | |
|
Racchetti G and Meldolesi J: Extracellular vesicles of mesenchymal stem cells: Therapeutic properties discovered with extraordinary success. Biomedicines. 9:6672021. View Article : Google Scholar : PubMed/NCBI | |
|
Tetta C, Consiglio AL, Bruno S, Tetta E, Gatti E, Dobreva M, Cremonesi F and Camussi G: The role of microvesicles derived from mesenchymal stem cells in tissue regeneration; a dream for tendon repair? Muscles Ligaments Tendons J. 2:212–221. 2012. | |
|
Rodas G, Soler-Rich R, Rius-Tarruella J, Alomar X, Balius R, Orozco L, Masci L and Maffulli N: Effect of autologous expanded bone marrow mesenchymal stem cells or leukocyte-poor platelet-rich plasma in chronic patellar tendinopathy (with gap > 3 mm): Preliminary outcomes after 6 months of a double-blind, randomized, prospective study. Am J Sports Med. 49:1492–1504. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Caplan AI: Review: Mesenchymal stem cells: Cell-based reconstructive therapy in orthopedics. Tissue Eng. 11:1198–1211. 2005. View Article : Google Scholar : PubMed/NCBI | |
|
Zhu Z, Gan X, Fan H and Yu H: Mechanical stretch endows mesenchymal stem cells stronger angiogenic and anti-apoptotic capacities via NFκB activation. Biochem Biophys Res Commun. 468:601–605. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Kasper G, Dankert N, Tuischer J, Hoeft M, Gaber T, Glaeser JD, Zander D, Tschirschmann M, Thompson M, Matziolis G and Duda GN: Mesenchymal stem cells regulate angiogenesis according to their mechanical environment. Stem Cells. 25:903–910. 2007. View Article : Google Scholar : PubMed/NCBI | |
|
Longo UG, Garau G, Denaro V and Maffulli N: Surgical management of tendinopathy of biceps femoris tendon in athletes. Disabil Rehabil. 30:1602–1607. 2008. View Article : Google Scholar : PubMed/NCBI | |
|
Millar NL, Murrell GAC and McInnes IB: Inflammatory mechanisms in tendinopathy-towards translation. Nat Rev Rheumatol. 13:110–122. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Dakin SG, Newton J, Martinez FO, Hedley R, Gwilym S, Jones N, Reid HAB, Wood S, Wells G, Appleton L, et al: Chronic inflammation is a feature of Achilles tendinopathy and rupture. Br J Sports Med. 52:359–367. 2018. View Article : Google Scholar | |
|
Frich LH, Fernandes LR, Schrøder HD, Hejbøl EK, Nielsen PV, Jørgensen PH, Stensballe A and Lambertsen KL: The inflammatory response of the supraspinatus muscle in rotator cuff tear conditions. J Shoulder Elbow Surg. 30:e261–e275. 2021. View Article : Google Scholar | |
|
Yang G, Rothrauff BB and Tuan RS: Tendon and ligament regeneration and repair: Clinical relevance and developmental paradigm. Birth Defects Res C Embryo Today. 99:203–222. 2013. View Article : Google Scholar : PubMed/NCBI | |
|
Arvind V and Huang AH: Reparative and maladaptive inflammation in tendon healing. Front Bioeng Biotechnol. 9:7190472021. View Article : Google Scholar : PubMed/NCBI | |
|
Crowe LAN, McLean M, Kitson SM, Melchor EG, Patommel K, Cao HM, Reilly JH, Leach WJ, Rooney BP, Spencer SJ, et al: S100A8 & S100A9: Alarmin mediated inflammation in tendinopathy. Sci Rep. 9:14632019. View Article : Google Scholar : PubMed/NCBI | |
|
Marsolais D, Côté CH and Frenette J: Neutrophils and macrophages accumulate sequentially following Achilles tendon injury. J Orthop Res. 19:1203–1209. 2001. View Article : Google Scholar | |
|
Abraham AC, Shah SA, Golman M, Song L, Li X, Kurtaliaj I, Akbar M, Millar NL, Abu-Amer Y, Galatz LM and Thomopoulos S: Targeting the NF-κB signaling pathway in chronic tendon disease. Sci Transl Med. 11:eaav43192019. View Article : Google Scholar | |
|
Thankam FG, Dilisio MF, Dietz NE and Agrawal DK: TREM-1, HMGB1 and RAGE in the shoulder tendon: Dual mechanisms for inflammation based on the coincidence of glenohumeral arthritis. PLoS One. 11:e01654922016. View Article : Google Scholar : PubMed/NCBI | |
|
Voloshin I, Gelinas J, Maloney MD, O'Keefe RJ, Bigliani LU and Blaine TA: Proinflammatory cytokines and metalloproteases are expressed in the subacromial bursa in patients with rotator cuff disease. Arthroscopy. 21:1076.e1–1076.e9. 2005. View Article : Google Scholar : PubMed/NCBI | |
|
Eming SA, Wynn TA and Martin P: Inflammation and metabolism in tissue repair and regeneration. Science. 356:1026–1030. 2017. View Article : Google Scholar | |
|
Jin R, Xu J, Gao Q, Mao X, Yin J, Lu K, Guo Y, Zhang M and Cheng R: IL-33-induced neutrophil extracellular traps degrade fibronectin in a murine model of bronchopulmonary dysplasia. Cell Death Discov. 6:332020. View Article : Google Scholar : PubMed/NCBI | |
|
Gause WC, Wynn TA and Allen JE: Type 2 immunity and wound healing: Evolutionary refinement of adaptive immunity by helminths. Nat Rev Immunol. 13:607–614. 2013. View Article : Google Scholar : PubMed/NCBI | |
|
Shi Y and Wang Y, Li Q, Liu K, Hou J, Shao C and Wang Y: Immunoregulatory mechanisms of mesenchymal stem and stromal cells in inflammatory diseases. Nat Rev Nephrol. 14:493–507. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Selmani Z, Naji A, Zidi I, Favier B, Gaiffe E, Obert L, Borg C, Saas P, Tiberghien P, Rouas-Freiss N, et al: Human leukocyte antigen-G5 secretion by human mesenchymal stem cells is required to suppress T lymphocyte and natural killer function and to induce CD4+CD25highFOXP3+regulatory T cells. Stem Cells. 26:212–222. 2008. View Article : Google Scholar | |
|
Shen H, Yoneda S, Abu-Amer Y, Guilak F and Gelberman RH: Stem cell-derived extracellular vesicles attenuate the early inflammatory response after tendon injury and repair. J Orthop Res. 38:117–127. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Huang Y, He B, Wang L, Yuan B, Shu H, Zhang F and Sun L: Bone marrow mesenchymal stem cell-derived exosomes promote rotator cuff tendon-bone healing by promoting angiogenesis and regulating M1 macrophages in rats. Stem Cell Res Ther. 11:4962020. View Article : Google Scholar : PubMed/NCBI | |
|
Challoumas D, Biddle M and Millar NL: Recent advances in tendinopathy. Fac Rev. 9:162020. View Article : Google Scholar | |
|
Zhang K, Asai S, Yu B and Enomoto-Iwamoto M: IL-1β irreversibly inhibits tenogenic differentiation and alters metabolism in injured tendon-derived progenitor cells in vitro. Biochem Biophys Res Commun. 463:667–672. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Tohyama H, Yasuda K, Uchida H and Nishihira J: The responses of extrinsic fibroblasts infiltrating the devitalised patellar tendon to IL-1beta are different from those of normal tendon fibroblasts. J Bone Joint Surg Br. 89:1261–1267. 2007. View Article : Google Scholar : PubMed/NCBI | |
|
Guerquin MJ, Charvet B, Nourissat G, Havis E, Ronsin O, Bonnin MA, Ruggiu M, Olivera-Martinez I, Robert N, Lu Y, et al: Transcription factor EGR1 directs tendon differentiation and promotes tendon repair. J Clin Invest. 123:3564–3576. 2013. View Article : Google Scholar : PubMed/NCBI | |
|
Yang G, Im HJ and Wang JHC: Repetitive mechanical stretching modulates IL-1beta induced COX-2, MMP-1 expression, and PGE2 production in human patellar tendon fibroblasts. Gene. 363:166–172. 2005. View Article : Google Scholar : PubMed/NCBI | |
|
Lawrence T: The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb Perspect Biol. 1:a0016512009. View Article : Google Scholar | |
|
Gohda J, Matsumura T and Inoue JI: Cutting edge: TNFR-associated factor (TRAF) 6 is essential for MyD88-dependent pathway but not toll/IL-1 receptor domain-containing adaptor-inducing IFN-beta (TRIF)-dependent pathway in TLR signaling. J Immunol. 173:2913–2917. 2004. View Article : Google Scholar : PubMed/NCBI | |
|
Striz I, Brabcova E, Kolesar L and Sekerkova A: Cytokine networking of innate immunity cells: A potential target of therapy. Clin Sci (Lond). 126:593–612. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
Schottelius AJ, Mayo MW, Sartor RB and Baldwin AS Jr: Interleukin-10 signaling blocks inhibitor of kappaB kinase activity and nuclear factor kappaB DNA binding. J Biol Chem. 274:31868–31874. 1999. View Article : Google Scholar : PubMed/NCBI | |
|
Karin M and Delhase M: The I kappa B kinase (IKK) and NF-kappa B: Key elements of proinflammatory signalling. Semin Immunol. 12:85–98. 2000. View Article : Google Scholar : PubMed/NCBI | |
|
Oeckinghaus A and Ghosh S: The NF-kappaB family of transcription factors and its regulation. Cold Spring Harb Perspect Biol. 1:a0000342009. View Article : Google Scholar | |
|
Xie F, Teng L, Xu J, Lu J, Zhang C, Yang L, Ma X and Zhao M: Interleukin-10 modified bone marrow mesenchymal stem cells prevent hypertrophic scar formation by inhibiting inflammation. Pharmazie. 75:571–575. 2020.PubMed/NCBI | |
|
Best KT, Nichols AEC, Knapp E, Hammert WC, Ketonis C, Jonason JH, Awad HA and Loiselle AE: NF-κB activation persists into the remodeling phase of tendon healing and promotes myofibroblast survival. Sci Signal. 13:eabb72092020. View Article : Google Scholar | |
|
Li Z, Li Q, Tong K, Zhu J, Wang H, Chen B and Chen L: BMSC-derived exosomes promote tendon-bone healing after anterior cruciate ligament reconstruction by regulating M1/M2 macrophage polarization in rats. Stem Cell Res Ther. 13:2952022. View Article : Google Scholar : PubMed/NCBI | |
|
Gumina S, Natalizi S, Melaragni F, Leopizzi M, Carbone S, Postacchini F, Milani A and Della Rocca C: The possible role of the transcription factor nuclear factor-κB on evolution of rotator cuff tear and on mechanisms of cuff tendon healing. J Shoulder Elbow Surg. 22:673–680. 2013. View Article : Google Scholar | |
|
Kirkham AM, Bailey AJM, Tieu A, Maganti HB, Montroy J, Shorr R, Campbell TM, Fergusson DA, Lalu MM, Elmoazzen H and Allan DS: MSC-derived extracellular vesicles in preclinical animal models of bone injury: A systematic review and meta-analysis. Stem Cell Rev Rep. 18:1054–1066. 2022. View Article : Google Scholar | |
|
Tardito S, Martinelli G, Soldano S, Paolino S, Pacini G, Patane M, Alessandri E, Smith V and Cutolo M: Macrophage M1/M2 polarization and rheumatoid arthritis: A systematic review. Autoimmun Rev. 18:1023972019. View Article : Google Scholar : PubMed/NCBI | |
|
Chamberlain CS, Clements AEB, Kink JA, Choi U, Baer GS, Halanski MA, Hematti P and Vanderby R: Extracellular vesicle-educated macrophages promote early achilles tendon healing. Stem Cells. 37:652–662. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Andia I, Sanchez M and Maffulli N: Tendon healing and platelet-rich plasma therapies. Expert Opin Biol Ther. 10:1415–1426. 2010. View Article : Google Scholar : PubMed/NCBI | |
|
Durgam S and Stewart M: Cellular and molecular factors influencing tendon repair. Tissue Eng Part B Rev. 23:307–317. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Pufe T, Petersen W, Tillmann B and Mentlein R: The angiogenic peptide vascular endothelial growth factor is expressed in foetal and ruptured tendons. Virchows Arch. 439:579–585. 2001. View Article : Google Scholar : PubMed/NCBI | |
|
Kannus P: Etiology and pathophysiology of chronic tendon disorders in sports. Scand J Med Sci Sports. 7:78–85. 1997. View Article : Google Scholar : PubMed/NCBI | |
|
Taylor CT: Interdependent roles for hypoxia inducible factor and nuclear factor-kappaB in hypoxic inflammation. J Physiol. 586:4055–4059. 2008. View Article : Google Scholar : PubMed/NCBI | |
|
Berse B, Hunt JA, Diegel RJ, Morganelli P, Yeo K, Brown F and Fava RA: Hypoxia augments cytokine (transforming growth factor-beta (TGF-beta) and IL-1)-induced vascular endothelial growth factor secretion by human synovial fibroblasts. Clin Exp Immunol. 115:176–182. 1999. View Article : Google Scholar : PubMed/NCBI | |
|
Semenza GL: Hydroxylation of HIF-1: Oxygen sensing at the molecular level. Physiology (Bethesda). 19:176–182. 2004.PubMed/NCBI | |
|
Cummins EP and Taylor CT: Hypoxia-responsive transcription factors. Pflugers Arch. 450:363–371. 2005. View Article : Google Scholar : PubMed/NCBI | |
|
Wezenbeek E, Willems T, Mahieu N, De Muynck M, Vanden Bossche L, Steyaert A, De Clercq D and Witvrouw E: The role of the vascular and structural response to activity in the development of achilles tendinopathy: A prospective study. Am J Sports Med. 46:947–954. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Kaigler D, Krebsbach PH, Polverini PJ and Mooney DJ: Role of vascular endothelial growth factor in bone marrow stromal cell modulation of endothelial cells. Tissue Eng. 9:95–103. 2003. View Article : Google Scholar : PubMed/NCBI | |
|
Furumatsu T, Shen ZN, Kawai A, Nishida K, Manabe H, Oohashi T, Inoue H and Ninomiya Y: Vascular endothelial growth factor principally acts as the main angiogenic factor in the early stage of human osteoblastogenesis. J Biochem. 133:633–639. 2003. View Article : Google Scholar : PubMed/NCBI | |
|
Al-Khaldi A, Eliopoulos N, Martineau D, Lejeune L, Lachapelle K and Galipeau J: Postnatal bone marrow stromal cells elicit a potent VEGF-dependent neoangiogenic response in vivo. Gene Ther. 10:621–629. 2003. View Article : Google Scholar : PubMed/NCBI | |
|
Yancopoulos GD, Davis S, Gale NW, Rudge JS, Wiegand SJ and Holash J: Vascular-specific growth factors and blood vessel formation. Nature. 407:242–248. 2000. View Article : Google Scholar : PubMed/NCBI | |
|
Rauniyar K, Jha SK and Jeltsch M: Biology of vascular endothelial growth factor C in the morphogenesis of lymphatic vessels. Front Bioeng Biotechnol. 6:72018. View Article : Google Scholar : PubMed/NCBI | |
|
Boyer MI, Watson JT, Lou J, Manske PR, Gelberman RH and Cai SR: Quantitative variation in vascular endothelial growth factor mRNA expression during early flexor tendon healing: An investigation in a canine model. J Orthop Res. 19:869–872. 2001. View Article : Google Scholar : PubMed/NCBI | |
|
Conway EM, Collen D and Carmeliet P: Molecular mechanisms of blood vessel growth. Cardiovasc Res. 49:507–521. 2001. View Article : Google Scholar : PubMed/NCBI | |
|
Karamysheva AF: Mechanisms of angiogenesis. Biochemistry (Mosc). 73:751–762. 2008. View Article : Google Scholar : PubMed/NCBI | |
|
Ferrara N: Molecular and biological properties of vascular endothelial growth factor. J Mol Med (Berl). 77:527–543. 1999. View Article : Google Scholar : PubMed/NCBI | |
|
Ge L, Xun C, Li W, Jin S, Liu Z, Zhuo Y, Duan D, Hu Z, Chen P and Lu M: Extracellular vesicles derived from hypoxia-preconditioned olfactory mucosa mesenchymal stem cells enhance angiogenesis via miR-612. J Nanobiotechnology. 19:3802021. View Article : Google Scholar : PubMed/NCBI | |
|
Hou J, Zhong T, Guo T, Miao C, Zhou C, Long H, Wu H, Zheng S, Wang L and Wang T: Apelin promotes mesenchymal stem cells survival and vascularization under hypoxic-ischemic condition in vitro involving the upregulation of vascular endothelial growth factor. Exp Mol Pathol. 102:203–209. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Chen G, Zhang W, Zhang K, Wang S, Gao Y, Gu J, He L, Li W, Zhang C, Zhang W, et al: Hypoxia-induced mesenchymal stem cells exhibit stronger tenogenic differentiation capacities and promote patellar tendon repair in rabbits. Stem Cells Int. 2020:88226092020. View Article : Google Scholar : PubMed/NCBI | |
|
Haque N, Rahman MT, Abu Kasim NH and Alabsi AM: Hypoxic culture conditions as a solution for mesenchymal stem cell based regenerative therapy. ScientificWorldJournal. 2013:6329722013. View Article : Google Scholar : PubMed/NCBI | |
|
Qu Q, Wang L, Bing W, Bi Y, Zhang C, Jing X and Liu L: miRNA-126-3p carried by human umbilical cord mesenchymal stem cell enhances endothelial function through exosome-mediated mechanisms in vitro and attenuates vein graft neointimal formation in vivo. Stem Cell Res Ther. 11:4642020. View Article : Google Scholar : PubMed/NCBI | |
|
Gong M, Yu B, Wang J, Wang Y, Liu M, Paul C, Millard RW, Xiao DS, Ashraf M and Xu M: Mesenchymal stem cells release exosomes that transfer miRNAs to endothelial cells and promote angiogenesis. Oncotarget. 8:45200–45212. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Liang X, Zhang L, Wang S, Han Q and Zhao RC: Exosomes secreted by mesenchymal stem cells promote endothelial cell angiogenesis by transferring miR-125a. J Cell Sci. 129:2182–2189. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Wu F, Nerlich M and Docheva D: Tendon injuries: Basic science and new repair proposals. EFORT Open Rev. 2:332–342. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Thomopoulos S, Parks WC, Rifkin DB and Derwin KA: Mechanisms of tendon injury and repair. J Orthop Res. 33:832–839. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Walia B and Huang AH: Tendon stem progenitor cells: Understanding the biology to inform therapeutic strategies for tendon repair. J Orthop Res. 37:1270–1280. 2019. View Article : Google Scholar : | |
|
Lovati AB, Corradetti B, Lange Consiglio A, Recordati C, Bonacina E, Bizzaro D and Cremonesi F: Characterization and differentiation of equine tendon-derived progenitor cells. J Biol Regul Homeost Agents. 25(2 Suppl): S75–S84. 2011.PubMed/NCBI | |
|
Qin S, Wang W, Liu Z, Hua X, Fu S, Dong F, Li A, Liu Z, Wang P, Dai L, et al: Fibrochondrogenic differentiation potential of tendon-derived stem/progenitor cells from human patellar tendon. J Orthop Translat. 22:101–108. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Al-Ani MKH, Xu K, Sun Y, Pan L, Xu Z and Yang L: Study of bone marrow mesenchymal and tendon-derived stem cells transplantation on the regenerating effect of achilles tendon ruptures in rats. Stem Cells Int. 2015:9841462015. View Article : Google Scholar : PubMed/NCBI | |
|
Leong DJ and Sun HB: Mesenchymal stem cells in tendon repair and regeneration: Basic understanding and translational challenges. Ann N Y Acad Sci. 1383:88–96. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Vermeulen S, Vasilevich A, Tsiapalis D, Roumans N, Vroemen P, Beijer NRM, Dede Eren A, Zeugolis D and de Boer J: Identification of topographical architectures supporting the phenotype of rat tenocytes. Acta Biomater. 83:277–290. 2019. View Article : Google Scholar | |
|
Lee CH, Cook JL, Mendelson A, Moioli EK, Yao H and Mao JJ: Regeneration of the articular surface of the rabbit synovial joint by cell homing: A proof of concept study. Lancet. 376:440–448. 2010. View Article : Google Scholar : PubMed/NCBI | |
|
Sun Y, Liu WZ, Liu T, Feng X, Yang N and Zhou HF: Signaling pathway of MAPK/ERK in cell proliferation, differentiation, migration, senescence and apoptosis. J Recept Signal Transduct Res. 35:600–604. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Seuntjens E, Umans L, Zwijsen A, Sampaolesi M, Verfaillie CM and Huylebroeck D: Transforming growth factor type beta and Smad family signaling in stem cell function. Cytokine Growth Factor Rev. 20:449–458. 2009. View Article : Google Scholar : PubMed/NCBI | |
|
Liu H, Zhang C, Zhu S, Lu P, Zhu T, Gong X, Zhang Z, Hu J, Yin Z, Heng BC, et al: Mohawk promotes the tenogenesis of mesenchymal stem cells through activation of the TGFβ signaling pathway. Stem Cells. 33:443–455. 2015. View Article : Google Scholar | |
|
Kim HM, Galatz LM, Das R, Havlioglu N, Rothermich SY and Thomopoulos S: The role of transforming growth factor beta isoforms in tendon-to-bone healing. Connect Tissue Res. 52:87–98. 2011. View Article : Google Scholar | |
|
Lee CH, Shah B, Moioli EK and Mao JJ: CTGF directs fibroblast differentiation from human mesenchymal stem/stromal cells and defines connective tissue healing in a rodent injury model. J Clin Invest. 125:39922015. View Article : Google Scholar : PubMed/NCBI | |
|
Li X, Pongkitwitoon S, Lu H, Lee C, Gelberman R and Thomopoulos S: CTGF induces tenogenic differentiation and proliferation of adipose-derived stromal cells. J Orthop Res. 37:574–582. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Lee CH, Lee FY, Tarafder S, Kao K, Jun Y, Yang G and Mao JJ: Harnessing endogenous stem/progenitor cells for tendon regeneration. J Clin Invest. 125:2690–2701. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Zhang B, Luo Q, Chen Z, Shi Y, Ju Y, Yang L and Song G: Increased nuclear stiffness via FAK-ERK1/2 signaling is necessary for synthetic mechano-growth factor E peptide-induced tenocyte migration. Sci Rep. 6:188092016. View Article : Google Scholar : PubMed/NCBI | |
|
Shen H, Gelberman RH, Silva MJ, Sakiyama-Elbert SE and Thomopoulos S: BMP12 induces tenogenic differentiation of adipose-derived stromal cells. PLoS One. 8:e776132013. View Article : Google Scholar : PubMed/NCBI | |
|
Violini S, Ramelli P, Pisani LF, Gorni C and Mariani P: Horse bone marrow mesenchymal stem cells express embryo stem cell markers and show the ability for tenogenic differentiation by in vitro exposure to BMP-12. BMC Cell Biol. 10:292009. View Article : Google Scholar : PubMed/NCBI | |
|
Gonçalves AI, Rodrigues MT, Lee SJ, Atala A, Yoo JJ, Reis RL and Gomes ME: Understanding the role of growth factors in modulating stem cell tenogenesis. PLoS One. 8:e837342013. View Article : Google Scholar | |
|
Wu T, Liu Y, Wang B, Sun Y, Xu J, Yuk-Wai LW, Xu L, Zhang J and Li G: The use of cocultured mesenchymal stem cells with tendon-derived stem cells as a better cell source for tendon repair. Tissue Eng Part A. 22:1229–1240. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Kuo CK, Marturano JE and Tuan RS: Novel strategies in tendon and ligament tissue engineering: Advanced biomaterials and regeneration motifs. Sports Med Arthrosc Rehabil Ther Technol. 2:202010.PubMed/NCBI | |
|
Park SA, Kim IA, Lee YJ and Shin JW, Kim CR, Kim JK, Yang YI and Shin JW: Biological responses of ligament fibroblasts and gene expression profiling on micropatterned silicone substrates subjected to mechanical stimuli. J Biosci Bioeng. 102:402–412. 2006. View Article : Google Scholar : PubMed/NCBI | |
|
Riboh J, Chong AK, Pham H, Longaker M, Jacobs C and Chang J: Optimization of flexor tendon tissue engineering with a cyclic strain bioreactor. J Hand Surg Am. 33:1388–1396. 2008. View Article : Google Scholar : PubMed/NCBI | |
|
Yang G, Crawford RC and Wang JH: Proliferation and collagen production of human patellar tendon fibroblasts in response to cyclic uniaxial stretching in serum-free conditions. J Biomech. 37:1543–1550. 2004. View Article : Google Scholar : PubMed/NCBI | |
|
Costa-Almeida R, Calejo I and Gomes ME: Mesenchymal stem cells empowering tendon regenerative therapies. Int J Mol Sci. 20:30022019. View Article : Google Scholar : PubMed/NCBI | |
|
Crovace A, Lacitignola L, Rossi G and Francioso E: Histological and immunohistochemical evaluation of autologous cultured bone marrow mesenchymal stem cells and bone marrow mononucleated cells in collagenase-induced tendinitis of equine superficial digital flexor tendon. Vet Med Int. 2010:2509782010. View Article : Google Scholar : PubMed/NCBI | |
|
Bramono DS, Richmond JC, Weitzel PP, Kaplan DL and Altman GH: Matrix metalloproteinases and their clinical applications in orthopaedics. Clin Orthop Relat Res. 272–285. 2004. View Article : Google Scholar : PubMed/NCBI | |
|
Oshiro W, Lou J, Xing X, Tu Y and Manske PR: Flexor tendon healing in the rat: A histologic and gene expression study. J Hand Surg Am. 28:814–823. 2003. View Article : Google Scholar : PubMed/NCBI | |
|
Gruber R, Kandler B, Holzmann P, Vögele-Kadletz M, Losert U, Fischer MB and Watzek G: Bone marrow stromal cells can provide a local environment that favors migration and formation of tubular structures of endothelial cells. Tissue Eng. 11:896–903. 2005. View Article : Google Scholar : PubMed/NCBI | |
|
Zhang X, Cai Z, Wu M, Huangfu X, Li J and Liu X: Adipose stem cell-derived exosomes recover impaired matrix metabolism of torn human rotator cuff tendons by maintaining tissue homeostasis. Am J Sports Med. 49:899–908. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Katzel EB, Wolenski M, Loiselle AE, Basile P, Flick LM, Langstein HN, Hilton MJ, Awad HA, Hammert WC and O'Keefe RJ: Impact of Smad3 loss of function on scarring and adhesion formation during tendon healing. J Orthop Res. 29:684–693. 2011. View Article : Google Scholar | |
|
Brown KA, Pietenpol JA and Moses HL: A tale of two proteins: Differential roles and regulation of Smad2 and Smad3 in TGF-beta signaling. J Cell Biochem. 101:9–33. 2007. View Article : Google Scholar : PubMed/NCBI | |
|
Li F, Zeng B, Chai Y, Cai P, Fan C and Cheng T: The linker region of Smad2 mediates TGF-beta-dependent ERK2-induced collagen synthesis. Biochem Biophys Res Commun. 386:289–293. 2009. View Article : Google Scholar : PubMed/NCBI | |
|
Moustakas A, Pardali K, Gaal A and Heldin CH: Mechanisms of TGF-beta signaling in regulation of cell growth and differentiation. Immunol Lett. 82:85–91. 2002. View Article : Google Scholar : PubMed/NCBI | |
|
Han Y, Yang J, Fang J, Zhou Y, Candi E, Wang J, Hua D, Shao C and Shi Y: The secretion profile of mesenchymal stem cells and potential applications in treating human diseases. Signal Transduct Target Ther. 7:922022. View Article : Google Scholar : PubMed/NCBI | |
|
Almalki SG: Adipose-derived mesenchymal stem cells and wound healing: Potential clinical applications in wound repair. Saudi Med J. 43:1075–1086. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Chen X, Song XH, Yin Z, Zou XH, Wang LL, Hu H, Cao T, Zheng M and Ouyang HW: Stepwise differentiation of human embryonic stem cells promotes tendon regeneration by secreting fetal tendon matrix and differentiation factors. Stem Cells. 27:1276–1287. 2009. View Article : Google Scholar : PubMed/NCBI | |
|
Chen X, Zou XH, Yin GL and Ouyang HW: Tendon tissue engineering with mesenchymal stem cells and biografts: An option for large tendon defects? Front Biosci (Schol Ed). 1:23–32. 2009. View Article : Google Scholar : PubMed/NCBI | |
|
Wang XT, Liu PY and Tang JB: Tendon healing in vitro: Modification of tenocytes with exogenous vascular endothelial growth factor gene increases expression of transforming growth factor beta but minimally affects expression of collagen genes. J Hand Surg Am. 30:222–229. 2005. View Article : Google Scholar : PubMed/NCBI | |
|
Wang QW, Chen ZL and Piao YJ: Mesenchymal stem cells differentiate into tenocytes by bone morphogenetic protein (BMP) 12 gene transfer. J Biosci Bioeng. 100:418–422. 2005. View Article : Google Scholar : PubMed/NCBI | |
|
Kraus A, Woon C, Raghavan S, Megerle K, Pham H and Chang J: Co-culture of human adipose-derived stem cells with tenocytes increases proliferation and induces differentiation into a tenogenic lineage. Plast Reconstr Surg. 132:754e–766e. 2013. View Article : Google Scholar : PubMed/NCBI | |
|
Sugimoto Y, Takimoto A, Akiyama H, Kist R, Scherer G, Nakamura T, Hiraki Y and Shukunami C: Scx+/Sox9+ progenitors contribute to the establishment of the junction between cartilage and tendon/ligament. Development. 140:2280–2288. 2013. View Article : Google Scholar : PubMed/NCBI | |
|
Liu H, Xu J, Lan Y, Lim HW and Jiang R: The scleraxis transcription factor directly regulates multiple distinct molecular and cellular processes during early tendon cell differentiation. Front Cell Dev Biol. 9:6543972021. View Article : Google Scholar : PubMed/NCBI | |
|
Oshita T, Tobita M, Tajima S and Mizuno H: Adipose-derived stem cells improve collagenase-induced tendinopathy in a rat model. Am J Sports Med. 44:1983–1989. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Aicale R, Tarantino D and Maffulli N: Overuse injuries in sport: A comprehensive overview. J Orthop Surg Res. 13:3092018. View Article : Google Scholar : PubMed/NCBI | |
|
Steinmann S, Pfeifer CG, Brochhausen C and Docheva D: Spectrum of tendon pathologies: Triggers, trails and end-state. Int J Mol Sci. 21:8442020. View Article : Google Scholar : PubMed/NCBI | |
|
Canapp SO Jr, Canapp DA, Ibrahim V, Carr BJ, Cox C and Barrett JG: The use of adipose-derived progenitor cells and platelet-rich plasma combination for the treatment of supraspinatus tendinopathy in 55 dogs: A retrospective study. Front Vet Sci. 3:612016. View Article : Google Scholar : PubMed/NCBI | |
|
Bruns J, Kampen J, Kahrs J and Plitz W: Achilles tendon rupture: Experimental results on spontaneous repair in a sheep-model. Knee Surg Sports Traumatol Arthrosc. 8:364–369. 2000. View Article : Google Scholar | |
|
Im GI and Kim TK: Stem cells for the regeneration of tendon and ligament: A perspective. Int J Stem Cells. 13:335–341. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Arnhold S, Elashry MI, Klymiuk MC and Wenisch S: Biological macromolecules and mesenchymal stem cells: Basic research for regenerative therapies in veterinary medicine. Int J Biol Macromol. 123:889–899. 2019. View Article : Google Scholar | |
|
Harman RM, Patel RS, Fan JC, Park JE, Rosenberg BR and Van de Walle GR: Single-cell RNA sequencing of equine mesenchymal stromal cells from primary donor-matched tissue sources reveals functional heterogeneity in immune modulation and cell motility. Stem Cell Res Ther. 11:5242020. View Article : Google Scholar : PubMed/NCBI | |
|
Gonçalves AI, Gershovich PM, Rodrigues MT, Reis RL and Gomes ME: Human adipose tissue-derived tenomodulin positive subpopulation of stem cells: A promising source of tendon progenitor cells. J Tissue Eng Regen Med. 12:762–774. 2018. View Article : Google Scholar | |
|
Dias IE, Cardoso DF, Soares CS, Barros LC, Viegas CA, Carvalho PP and Dias IR: Clinical application of mesenchymal stem cells therapy in musculoskeletal injuries in dogs-a review of the scientific literature. Open Vet J. 11:188–202. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Loebel C and Burdick JA: Engineering stem and stromal cell therapies for musculoskeletal tissue repair. Cell Stem Cell. 22:325–339. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Lui PPY: Mesenchymal stem cell-derived extracellular vesicles for the promotion of tendon repair-an update of literature. Stem Cell Rev Rep. 17:379–389. 2021. View Article : Google Scholar | |
|
Brennan MÁ, Layrolle P and Mooney DJ: Biomaterials functionalized with MSC secreted extracellular vesicles and soluble factors for tissue regeneration. Adv Funct Mater. 30:19091252020. View Article : Google Scholar : PubMed/NCBI | |
|
Peroni JF and Borjesson DL: Anti-inflammatory and immunomodulatory activities of stem cells. Vet Clin North Am Equine Pract. 27:351–362. 2011. View Article : Google Scholar : PubMed/NCBI | |
|
Shojaee A and Parham A: Strategies of tenogenic differentiation of equine stem cells for tendon repair: Current status and challenges. Stem Cell Res Ther. 10:1812019. View Article : Google Scholar : PubMed/NCBI | |
|
Gugjoo MB, Pal Amar, Makhdoomi DM and Sharma GT: Equine mesenchymal stem cells: Properties, sources, characterization, and potential therapeutic applications. J Equine Vet Sci. 72:16–27. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Iacono E, Pascucci L, Rossi B, Bazzucchi C, Lanci A, Ceccoli M and Merlo B: Ultrastructural characteristics and immune profile of equine MSCs from fetal adnexa. Reproduction. 154:509–519. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Ikehara S: A novel BMT technique for treatment of various currently intractable diseases. Best Pract Res Clin Haematol. 24:477–483. 2011. View Article : Google Scholar : PubMed/NCBI | |
|
De Becker A and Riet IV: Homing and migration of mesenchymal stromal cells: How to improve the efficacy of cell therapy? World J Stem Cells. 8:73–87. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Zhao T, Qi Y, Xiao S, Ran J, Wang J, Ghamor-Amegavi EP, Zhou X, Li H, He T, Gou Z, et al: Integration of mesenchymal stem cell sheet and bFGF-loaded fibrin gel in knitted PLGA scaffolds favorable for tendon repair. J Mater Chem B. 7:2201–2211. 2019. View Article : Google Scholar | |
|
Supokawej A, Korchunjit W and Wongtawan T: The combination of BMP12 and KY02111 enhances tendon differentiation in bone marrow-derived equine mesenchymal stromal cells (BM-eMSCs). J Equine Sci. 33:19–26. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Huang TF, Yew TL, Chiang ER, Ma HL, Hsu CY, Hsu SH, Hsu YT and Hung SC: Mesenchymal stem cells from a hypoxic culture improve and engraft Achilles tendon repair. Am J Sports Med. 41:1117–1125. 2013. View Article : Google Scholar : PubMed/NCBI | |
|
Han P, Cui Q, Yang S, Wang H, Gao P and Li Z: Tumor necrosis factor-α and transforming growth factor-β1 facilitate differentiation and proliferation of tendon-derived stem cells in vitro. Biotechnol Lett. 39:711–719. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Ouyang HW, Goh JCH and Lee EH: Use of bone marrow stromal cells for tendon graft-to-bone healing: Histological and immunohistochemical studies in a rabbit model. Am J Sports Med. 32:321–327. 2004. View Article : Google Scholar : PubMed/NCBI | |
|
Yao Z, Li J, Xiong H, Cui H, Ning J, Wang S, Ouyang X, Qian Y and Fan C: MicroRNA engineered umbilical cord stem cell-derived exosomes direct tendon regeneration by mTOR signaling. J Nanobiotechnology. 19:1692021. View Article : Google Scholar : PubMed/NCBI | |
|
Shen H, Kormpakis I, Havlioglu N, Linderman SW, Sakiyama-Elbert SE, Erickson IE, Zarembinski T, Silva MJ, Gelberman RH and Thomopoulos S: The effect of mesenchymal stromal cell sheets on the inflammatory stage of flexor tendon healing. Stem Cell Res Ther. 7:1442016. View Article : Google Scholar : PubMed/NCBI | |
|
Li J, Yao Z, Xiong H, Cui H, Wang X, Zheng W, Qian Y and Fan C: Extracellular vesicles from hydroxycamptothecin primed umbilical cord stem cells enhance anti-adhesion potential for treatment of tendon injury. Stem Cell Res Ther. 11:5002020. View Article : Google Scholar : PubMed/NCBI | |
|
Geburek F, Mundle K, Conrad S, Hellige M, Walliser U, van Schie HT, van Weeren R, Skutella T and Stadler PM: Tracking of autologous adipose tissue-derived mesenchymal stromal cells with in vivo magnetic resonance imaging and histology after intralesional treatment of artificial equine tendon lesions-a pilot study. Stem Cell Res Ther. 7:212016. View Article : Google Scholar | |
|
Yea JH, Park JK, Kim IJ, Sym G, Bae TS and Jo CH: Regeneration of a full-thickness defect of rotator cuff tendon with freshly thawed umbilical cord-derived mesenchymal stem cells in a rat model. Stem Cell Res Ther. 11:3872020. View Article : Google Scholar : PubMed/NCBI | |
|
Xue Y, Kim HJ, Lee J, Liu Y, Hoffman T, Chen Y, Zhou X, Sun W, Zhang S, Cho HJ, et al: Co-electrospun silk fibroin and gelatin methacryloyl sheet seeded with mesenchymal stem cells for tendon regeneration. Small. 18:e21077142022. View Article : Google Scholar : PubMed/NCBI | |
|
Uyar İ, Altuntaş Z, Fındık S, Yıldırım MEC, Yarar S, Aktan M and Avcı A: The effects of a combination treatment with mesenchymal stem cell and platelet-rich plasma on tendon healing: An experimental study. Turk J Med Sci. 52:237–247. 2022.PubMed/NCBI | |
|
Kang K, Geng Q, Cui L, Wu L, Zhang L, Li T, Zhang Q and Gao S: Upregulation of Runt related transcription factor 1 (RUNX1) contributes to tendon-bone healing after anterior cruciate ligament reconstruction using bone mesenchymal stem cells. J Orthop Surg Res. 17:2662022. View Article : Google Scholar : PubMed/NCBI |