Epstein‑Barr virus infection mediated TP53 and Bcl‑2 expression in nasopharyngeal carcinoma pathogenesis
- Authors:
- Published online on: October 19, 2021 https://doi.org/10.3892/mco.2021.2422
- Article Number: 260
-
Copyright: © Vatte et al. This is an open access article distributed under the terms of Creative Commons Attribution License.
Abstract
Introduction
Head and neck squamous cell carcinomas (HNSCCs) comprise a group of cancers that evolve in the mucosal lining of the upper aerodigestive tract. HNSCCs are heterogeneous in nature (1). Nasopharyngeal carcinoma (NPC) and cancer of the larynx are the most prevalent cancers amongst HNSCC, followed by lip and oral cavity carcinomas (2). As per global cancer statistics, it was reported that 177,422 new laryngeal cancers and 129,079 new NPC cases were diagnosed globally in 2018, and they ranked the 23rd and 25th most commonly occurring cancers, respectively, with an incidence of 1.7% of all cancers (2).
In 1964, the Epstein-Barr virus (EBV) was the first human tumor virus discovered in Burkitt lymphoma tumor cells (3). Later studies found antibodies in Burkitt lymphoma patients and postnasal space carcinomas (4). De Schryver et al (5) demonstrated EBV antigen detection by immunofluorescence assay, which was followed by zur Hausen et al (6) who used the hybridization method in NPC. EBV infection is very common amongst individuals, and infects >90% of populations globally (7,8). Usually, an EBV infection is asymptomatic; however, the involvement of EBV in cancer pathogenesis was established in several epithelial and lymphoid malignancies (9).
The global burden of EBV-associated malignancies increased by 14.6% between 1990 and 2010, and East Asia accounted for almost 50% of EBV infected cancer cases during this time period (10). The incidence of EBV-associated cancers is ~1.5% of all cancers globally and represents 1.8% of all cancer-associated deaths (11-13). Amongst EBV associated cancer deaths, 92% are attributable to NPC and gastric cancer (10). The involvement of EBV in the pathogenesis of NPC may be through modification of epigenetic profiles, inducing genomic instability, evading host immunity, promoting cell survival, or contributing stem cell like properties in NPC cells and somatic mutations in apoptotic and tumor suppressor genes (14).
The tumor suppressor p53 and the anti-apoptotic protein B cell lymphoma-2 (Bcl-2) are hypothesized to play a key role in carcinogenesis, and upregulated expression of these proteins has been observed in other types of HNSCC (15). TP53 is a critical tumor suppressor that responds to various stress signals by regulating an anti-proliferative transcriptional program, including transient cell cycle arrest, cellular senescence and apoptosis (16). Despite the wide variety of TP53 network regulators and various TP53 regulated biological pathways, a clear comprehension is lacking about how and in what context TP53 exerts its diverse effects (17).
Amongst EBV-associated cancers, upregulated expression of wildtype TP53 has been observed, and this was shown to be associated with the latent EBV membrane protein 1 (LMP1). The mutual regulation between TP53 and LMP1 may play a vital role in EBV infection and latency (18). Similarly, Bcl-2, an antiapoptotic protein, is a key inhibitor of programmed cell death or apoptosis in cancer (19,20). In the pathogenesis of NPC, the upregulated expression of Bcl-2 plays an important role by regulating apoptosis (21). The present study evaluated the protein expression pattern of TP53 and Bcl-2 in association with EBV infection in a Saudi cohort with nasopharyngeal and laryngeal cancer to determine the correlation and interplay between these two proteins with EBV infection.
Materials and methods
Study cohort
Formalin fixed paraffin embedded (FFPE) tissue samples from confirmed nasopharyngeal (n=22) and laryngeal (n=11) carcinoma patients were collected from the Pathology Department, King Fahd Hospital of the University, Imam Abdulrahman Bin Faisal University (IAU), Dammam, Saudi Arabia. The median age of the patients was 51 years (age range, 18-90 years) with 22 males and 11 females forming the cohort. This study was approved by IAU's Institutional Review Board (approval no. IRB-2017-01-059). A signed informed consent form was obtained from each patient included in the study. The procedures in the present study adhered to the principles of the Declaration of Helsinki (22). All the samples were biopsy specimens. Sample size was determined based on the cancer incidence report, Saudi Arabia, 2014 using the ‘sample sizeʼ module from the Open-Source Project in Epidemiologic Computing program (openepi.com/SampleSize/SSPropor.htm). The tissue samples were collected from the patients who attended the oncology clinic at the King Fahd Hospital of the University between May 2000 and February 2015. Inclusion criteria for the collection of samples was based on the confirmation of a carcinoma of the larynx and/or nasopharynx by histopathology analysis. FFPE samples with <50% tumor tissue content were excluded. The present study included only tumor tissue obtained from the patients.
Immunohistochemistry
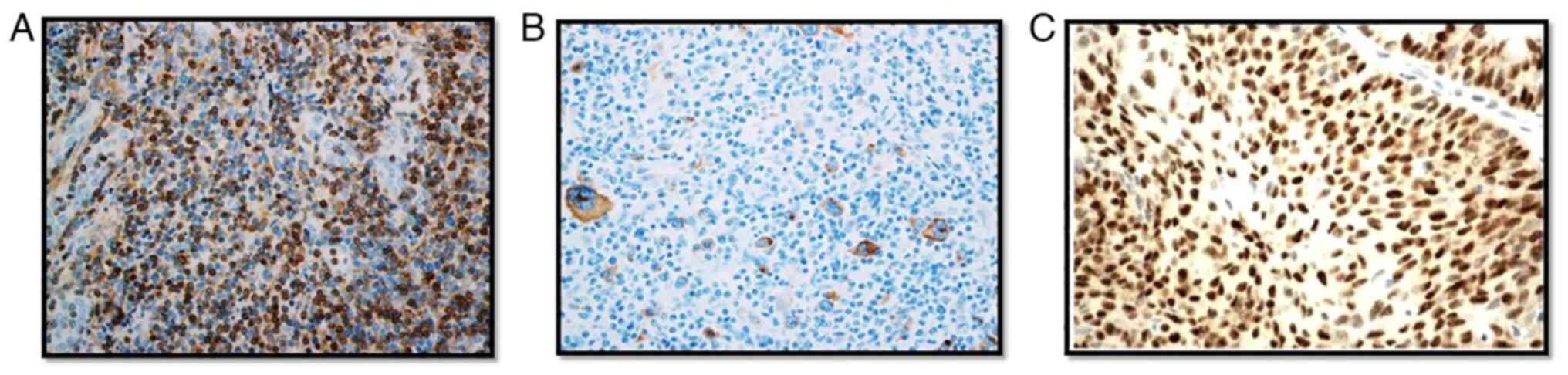
The FFPE blocks were reassessed for tumor content and diagnosis using Hematoxylin (cat. no. 6765001; Thermo Fisher Scientific, Inc.) and Eosin (cat. no. 6766008; Thermo Fisher Scientific, Inc.) staining. The tissue sections were prepared at room temperature and the total duration of Hematoxylin and Eosin staining procedure was 45 min. Immunohistochemistry was performed to detect TP53 and Bcl-2 protein expression, and EBV infection on 4-µM FFPE sections. The primary antibody for TP53 (cat. no. 760-2542; Ventana Medical Systems, Inc.; Roche Diagnostics) used in this study detected the wild and mutant form of the TP53 protein. The anti-Bcl-2 antibody (cat. no. 790-4604; Ventana Medical Systems, Inc.; Roche Diagnostics) binds to the N terminal region of human Bcl-2 protein. EBV primary antibodies bind to latent membrane protein (LMP-1) of EBV (cat. no. 760-2640; Cell Marque; Sigma-Aldrich; Merck KGaA). All of the above antibodies were used without dilution, as they were obtained in a ready to use form. The incubation temperature was 37˚C for all three antibodies and the timing of the incubation for TP53, Bcl-2 and EBV was 16, 20 and 26 min, respectively. Ultra-View Universal DAB Detection kit (Ventana Medical Systems, Inc.; Roche Diagnostics) was used to visualize the primary antibodies bound to target antigens with hydrogen peroxide substrate and 3,3'-diaminobenzidine tetrahydrochloride chromogen, which produces a brown colored precipitate (Fig. 1). BenchMark ULTRA staining instrument (Roche Diagnostics) was used for staining, and the results were interpreted qualitatively by two independent pathologists using a light microscope (magnification, x40; Leica Microsystems, Inc.). A tumor cell was considered TP53 positive if they showed nuclear staining and Bcl-2 positivity was confirmed if neoplastic cells showed cytoplasmic and/or nuclear staining. Positive and negative tissue control was used with every staining procedure (Fig. 2). The origin of the control samples for Bcl-2 and TP53 immunohistochemistry were from the tonsils and colon cancer, respectively. The patients were both male, aged 51 years old (tonsil cancer) and 48 years old (colon cancer). These samples were collected from the patients who attended the oncology clinic at the King Fahd Hospital of the University after obtaining consent. An EBV positive control slide was commercially procured (cat. no. CSE0125P; StatLab).
Statistical analysis
The clinical and histopathological data along with studied protein expression (qualitative) results were analyzed using SPSS version 20 (IBM Corp.). Distribution of proportions across category variables were analyzed using a Fisher's exact test (Table I), and for continuous variables an unpaired Student's t-test was used (Table I). The bivariate relationships between the variables were determined using a Spearman's Rank Correlation test (Tables II and III). P<0.05 was considered to indicate a statistically significant difference.
Table IClinical, pathological and protein expression parameters amongst the nasopharynx and larynx group of patients. |
Table IICorrelation between EBV, Bcl-2 or TP53 expression with clinical and histopathological parameters in the total cohort (nasopharynx and larynx groups). |
Table IIICorrelation between EBV, Bcl-2 or TP53 expression with clinical and histopathological parameters in the Nasopharynx cohort. |
Results
Sex distribution analysis revealed that in the nasopharynx and larynx groups, 59 and 81% of the patients, respectively, were males. The median age of patients with nasopharyngeal carcinoma is 49.5 years compared to 65 years in the larynx group (P=0.008). In the nasopharynx group, 68.2% had undifferentiated tumors, poorly differentiated tumors accounted for 22.7 and 9.1% were moderately differentiated tumors. In the larynx group 27.3% tumors were well differentiated, 63.6% tumors were moderately differentiated and 9.1% were poorly differentiated. The majority of the patients with NPC presented with grade 3 (86.4%) disease, whereas in laryngeal cancer cases, grade 1 and 2 tumors (91%) were predominant (P=0.0004). Similarly, 54.5% of the patients with NPC were at an advanced stage of the disease and in the larynx cancer group, the advanced stage was observed only in 27.3%. With regards to smoking, the percentage was higher in the larynx cancer group of patients (72.7%) compared to the patients with NPC (36.4%).
Prognosis was calculated based on the follow-up of the patient for 5 years. Recurrence or death due to disease was considered a poor prognostic outcome. A poor prognosis was observed in 91% of the larynx cancer patient group compared to 50% in the patients with NPC (P=0.027). EBV infection was completely absent in the larynx carcinoma patients, whereas 22.7% of patients were infected with EBV in the NPC group. A high proportion of Bcl-2 expression was seen in the patients with NPC (P=0.027) compared to the laryngeal carcinoma group. TP53 expression did not yield any significant difference between both patient groups (Table I). Bivariate association between clinical, histopathological and protein expression variables overall, also confirmed the poor prognosis in laryngeal carcinoma patients (P=0.002) and advanced tumor grade in NPC cases (P≤0.005; Table II). These results showed that EBV infection was associated with TP53 expression in the NPC cohort (P=0.009; Table III).
Discussion
EBV is currently a well-established carcinogen and an etiological factor implicated in several cancers, including cancer of epithelial and lymphoid origins, gastric cancer, NPC, and Hodgkin's and non-Hodgkin's lymphoma (10). There was a 14.6% increase in EBV associated cancers between 1990-2010, and 50% of these cases were observed in patients in East Asia (10). Approximately 1.5% of cancer cases are associated with EBV infection amongst all cancer cases globally (11-13). NPC is primarily associated with EBV, and the EBV infection frequency in NPC is varied based on ethnic and geographical differences (23). Almost all the NPC cases from the endemic regions presented with EBV infection and in non-endemic regions, NPC was negative for EBV. The EBV associated NPC prevalence is highest in Southeast Asia (24); data on the frequency of EBV in NPC is scarce in the Saudi population. The present study revealed that 22.7% of NPC cases had EBV infection. Similarly, the frequency of EBV in NPC was found to be 28% in an Iranian cohort (8), 44% from a Brazilian study (25) and 53.84% in a study from Oman (26). The relatively lower percentage of the EBV infection-associated NPC in the present study may be due to the region of study being non-endemic for EBV (27).
Identifying a single biomarker to calculate the risk and prognosis of a cancer is difficult due to tumor heterogeneity, and distinct clinical and histopathological conditions. However, a single biomarker interaction with other biomarkers in pathways such as cell signaling, apoptosis and cell differentiation may reveal the mechanism of oncogenesis. Hence, Bcl-2 and TP53 protein expression was assessed in all the cases to determine the correlation between EBV infections. The Bcl-2 family of proteins are key regulators in the apoptotic pathway (19). Amongst Bcl-2 family proteins, there were pro and anti-apoptotic regulators; the Bcl-2 protein is an anti-apoptotic member. Bcl-2 protein upregulation was observed more frequently in NPC cases compared to other types of HNSCC (28), and a similar pattern was observed in the present study; there was a lower frequency of upregulated Bcl-2 expression in patients with larynx cancer compared with patients with NPC. Bcl-2 upregulation was seen in 74.3% of NPC cases (29), similarly the present study showed that Bcl-2 upregulation was observed in 50% of NPC tumors. Bcl-2 upregulation may contribute towards tumor cell survival by inhibiting apoptosis in NPC. Thus, Bcl-2 upregulation may highlight a vital mechanism involved in NPC pathogenesis, although the exact molecular mechanism is unclear (30).
TP53 plays a vital role in cell cycle arrest and apoptosis through different mechanisms (31). The positive rate of TP53 expression in NPC tumors was varied in different studies, with reported values of 34.7% (32), 65.6% (33) and 52.2% (34). The results of the present study are in line with these previous studies with 50% of the NPC tumors exhibiting TP53 upregulation. The present data revealed that TP53 upregulation is associated with EBV infection in NPC. Similarly, several studies reported wildtype TP53 is upregulated in EBV associated cancers (18,35-37). Apoptosis is induced by TP53 upregulation. However, EBV transformed cells are sensitive to TP53-mediated apoptosis (38). This shows that the EBV positive and EBV negative tumors are distinct groups. The present study detected EBV infection by immunohistochemistry targeting the 60 kDa LMP-1 of EBV, and the results suggested that the mutual regulation between TP53 and LMP1 may play an important role in EBV associated NPC. Additional mechanistic studies need to be performed to determine TP53-mediated LMP1 stimulation. The limitation of this study is the absence of quantitative data for the immunohistochemistry experiments.
HNSCCs exhibit a variable prognosis with varying responses to standard treatment modalities, and this may be due to the significant etiological and molecular heterogeneity (39). The present data indicates that laryngeal carcinomas have a poor prognosis compared to NPC. The majority of the patients with NPC tumors presented with a higher grade tumor with a good prognosis. It has been reported that a combination of adjuvant chemotherapy and radiotherapy treatment improves prognosis and survival (40). To the best of our knowledge, this is the first pilot study to reveal the EBV associated NPC frequency using immunohistochemistry in the ethnic population. This study also attempted to divulge the poorly understood cellular mechanisms of EBV associated NPC pathogenesis by determining the correlation between EBV infection and the major tumor suppressor, TP53. Additional mechanistic studies are required to determine the TP53-mediated LMP1 stimulation of EBV in NPC. An improved understanding of the EBV carcinogenic process may aid in the development of novel therapeutics in NPC.
In conclusion, EBV infection was correlated with TP53 upregulation in patients with NPC, which suggests mutual regulation between TP53 and EBV.
Acknowledgements
The authors would like to thank Mr. Shakir Ahmed (Department of Pathology, King Fahd Hospital of the University, Imam Abdulrahman Bin Faisal University, Kingdom of Saudi Arabia) for his technical support.
Funding
This work was supported by the Deanship of Scientific Research, Imam Abdulrahman Bin Faisal University (grant nos. 2016-090-IRMC and 2016-111-Med).
Availability of data and materials
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.
Authors' contributions
CV, CC, SC and AAA conceived and designed the current study. AMA, AAL and AAH collected samples and the clinical data. CV and AAS performed the experiments. CV wrote the manuscript. AAA revised the manuscript. All authors read and approved the final manuscript. CV, CC, AMA, AAL, AAA confirm the authenticity of all the raw data.
Ethics approval and consent to participate
This study was approved by the Institutional Review Board of Imam Abdulrahman Bin Faisal University, Dammam, Saudi Arabia (approval no. IRB-2017-01-059). The procedures in the present study adhere to the principles of the Declaration of Helsinki. Written informed consent was obtained from all patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
|
Leemans CR, Snijders PJF and Brakenhoff RH: The molecular landscape of head and neck cancer. Nat Rev Cancer. 18:269–282. 2018.PubMed/NCBI View Article : Google Scholar | |
|
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 68:394–424. 2018.PubMed/NCBI View Article : Google Scholar | |
|
Epstein MA and Barr YM: Cultivation in vitro of human lymphoblasts from burkitt's malignant lymphoma. Lancet. 1:252–253. 1964.PubMed/NCBI View Article : Google Scholar | |
|
Old LJ, Boyse EA, Oettgen HF, Harven ED, Geering G, Williamson B and Clifford P: Precipitating antibody in human serum to an antigen present in cultured burkitt's lymphoma cells. Proc Natl Acad Sci USA. 56:1699–1704. 1966.PubMed/NCBI View Article : Google Scholar | |
|
de Schryver A, Friberg S Jr, Klein G, Henle W, Henle G, De-Thé G, Clifford P and Ho HC: Epstein-Barr virus-associated antibody patterns in carcinoma of the post-nasal space. Clin Exp Immunol. 5:443–459. 1969.PubMed/NCBI | |
|
zur Hausen H, Schulte-Holthausen H, Klein G, Henle W, Henle G, Clifford P and Santesson L: EBV DNA in biopsies of Burkitt tumours and anaplastic carcinomas of the nasopharynx. Nature. 228:1056–1058. 1970.PubMed/NCBI View Article : Google Scholar | |
|
Cao Y: EBV based cancer prevention and therapy in nasopharyngeal carcinoma. NPJ Precis Oncol. 1(10)2017.PubMed/NCBI View Article : Google Scholar | |
|
Shahani T, Makvandi M, Samarbafzadeh A, Teimoori A, Ranjbar N, saki N, Nikakhlagh S, Neisi N, Hosseini Z, Pourrezaei S, et al: Frequency of Epstein Barr virus type 1 among nasopharyngeal carcinomas in Iranian patients. Asian Pac J Cancer Prev. 18:327–331. 2017.PubMed/NCBI View Article : Google Scholar | |
|
Young LS and Dawson CW: Epstein-Barr virus and nasopharyngeal carcinoma. Chin J Cancer. 33:581–590. 2014. | |
|
Khan G and Hashim MJ: Global burden of deaths from Epstein-Barr virus attributable malignancies 1990-2010. Infect Agent Cancer. 9(38)2014.PubMed/NCBI View Article : Google Scholar | |
|
Varmus H and Kumar HS: Addressing the growing international challenge of cancer: A multinational perspective. Sci Transl Med. 5(175cm2)2013.PubMed/NCBI View Article : Google Scholar | |
|
Holmes D: The cancer-virus cures. Nat Med. 20:571–574. 2014.PubMed/NCBI View Article : Google Scholar | |
|
Lieberman PM: Virology. Epstein-Barr virus turns 50. Science. 343:1323–1325. 2014.PubMed/NCBI View Article : Google Scholar | |
|
Tsao SW, Tsang CM and Lo KW: Epstein-Barr virus infection and nasopharyngeal carcinoma. Philos Trans R Soc Lond B Biol Sci. 372(20160270)2017.PubMed/NCBI View Article : Google Scholar | |
|
Sahoo R, Chittibabu V, Patil G, Rao S, Thakur S, Dhondalay G, Kulkarni AJ, Banerjee A, Ajaikumar BS, Korlimarla A, et al: Relationship between molecular markers and treatment response in a retrospective cohort of Indian patients with primary carcinoma of the larynx. Oral Oncol. 45:e216–e221. 2009.PubMed/NCBI View Article : Google Scholar | |
|
Bieging KT, Mello SS and Attardi LD: Unravelling mechanisms of p53-mediated tumour suppression. Nat Rev Cancer. 14:359–370. 2014.PubMed/NCBI View Article : Google Scholar | |
|
Kastenhuber ER and Lowe SW: Putting p53 in Context. Cell. 170:1062–1078. 2017.PubMed/NCBI View Article : Google Scholar | |
|
Wang Q, Lingel A, Geiser V, Kwapnoski Z and Zhang L: Tumor suppressor p53 stimulates the expression of epstein-barr virus latent membrane protein 1. J Virol. 91:e00312–17. 2017.PubMed/NCBI View Article : Google Scholar | |
|
Chipuk JE, Moldoveanu T, Llambi F, Parsons MJ and Green DR: The BCL-2 family reunion. Mol Cell. 37:299–310. 2010.PubMed/NCBI View Article : Google Scholar | |
|
Kelly PN and Strasser A: The role of Bcl-2 and its pro-survival relatives in tumourigenesis and cancer therapy. Cell Death Differ. 18:1414–1424. 2011.PubMed/NCBI View Article : Google Scholar | |
|
Wenmei S, Yanming L, Fenping W, Hongsheng G, Lixia L, Suwen Z, Zhennan L, Rong L and Zhixiong Y: Bcl-2 regulation by miR-429 in human nasopharyngeal carcinoma in vivo. Int J Clin Exp Pathol. 9:5998–6006. 2016. | |
|
World Medical Association. World medical association declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA. 310:2191–2194. 2013.PubMed/NCBI View Article : Google Scholar | |
|
Kanno M, Narita N, Fujimoto Y, Wakisaka N, Yoshizaki T, Kodaira T, Makita C, Sato Y, Yamazaki K, Wakaoka T, et al: Third epidemiological analysis of nasopharyngeal carcinoma in the central region of Japan from 2006 to 2015. Cancers (Basel). 11(1180)2019.PubMed/NCBI View Article : Google Scholar | |
|
Ngan HL, Wang L, Lo KW and Lui VWY: Genomic landscapes of EBV-associated nasopharyngeal carcinoma vs. HPV-associated head and neck cancer. Cancers (Basel). 10(210)2018.PubMed/NCBI View Article : Google Scholar | |
|
Breda E, Catarino RJ, Azevedo I, Lobão M, Monteiro E and Medeiros R: Epstein-Barr virus detection in nasopharyngeal carcinoma: Implications in a low-risk area. Braz J Otorhinolaryngol. 76:310–315. 2010.PubMed/NCBI | |
|
Al-Shidhani SNS, Al-Sinawi S, Al-Bahri M, Al-Kindi M and Mabruk M: Determination of the expression of latent Epstein Barr virus in Omani nasopharyngeal carcinoma patients. Biomed Pharmacol J. 14:257–265. 2021. | |
|
Wu L, Li C and Pan L: Nasopharyngeal carcinoma: A review of current updates. Exp Ther Med. 15:3687–3692. 2018.PubMed/NCBI View Article : Google Scholar | |
|
Yang HJ, Cho YJ, Kim HS, Chang MS, Sung MW and Kim WH: Association of p53 and BCL-2 expression with Epstein-Barr virus infection in the cancers of head and neck. Head Neck. 23:629–636. 2001.PubMed/NCBI View Article : Google Scholar | |
|
Sarac S, Akyol MU, Kanbur B, Poyraz A, Akyol G, Yilmaz T and Sungur A: Bcl-2 and LMP1 expression in nasopharyngeal carcinomas. Am J Otolaryngol. 22:377–382. 2001.PubMed/NCBI View Article : Google Scholar | |
|
Tulalamba W and Janvilisri T: Nasopharyngeal carcinoma signaling pathway: An update on molecular biomarkers. Int J Cell Biol. 2012(594681)2012.PubMed/NCBI View Article : Google Scholar | |
|
Muller PA and Vousden KH: p53 mutations in cancer. Nat Cell Biol. 15:2–8. 2013.PubMed/NCBI View Article : Google Scholar | |
|
Tabyaoui I, Serhier Z, Sahraoui S, Sayd S, Cadi R, Bennani OM, Benider A, Zamiati S and Tahiri JN: Immunohistochemical expression of latent membrane protein 1 (LMP1) and p53 in nasopharyngeal carcinoma: Moroccan experience. Afr Health Sci. 13:710–717. 2013.PubMed/NCBI View Article : Google Scholar | |
|
Zhang P, Wu SK, Wang Y, Fan ZX, Li CR, Feng M, Xu P, Wang WD and Lang JY: p53, MDM2, eIF4E and EGFR expression in nasopharyngeal carcinoma and their correlation with clinicopathological characteristics and prognosis: A retrospective study. Oncol Lett. 9:113–118. 2015.PubMed/NCBI View Article : Google Scholar | |
|
Liu J, Liu Y, Zhang Z, Sun H, Ji X, Li B, Zhou X and Gai P: Prognostic value of the Epstein-Barr virus and tumor suppressor gene p53 gene in nasopharyngeal squamous cell carcinoma. J Cancer Res Ther. 15:426–436. 2019.PubMed/NCBI View Article : Google Scholar | |
|
Menke DM, Griesser H, Moder KG, Tefferi A, Luthra HS, Cohen MD, Colon-Otero G and Lloyd RV: Lymphomas in patients with connective tissue disease. Comparison of p53 protein expression and latent EBV infection in patients immunosuppressed and not immunosuppressed with methotrexate. Am J Clin Pathol. 113:212–218. 2000.PubMed/NCBI View Article : Google Scholar | |
|
Niemhom S, Kitazawa S, Murao S, Kunachak S and Maeda S: Co-expression of p53 and bcl-2 may correlate to the presence of epstein-barr virus genome and the expression of proliferating cell nuclear antigen in nasopharyngeal carcinoma. Cancer Lett. 160:199–208. 2000.PubMed/NCBI View Article : Google Scholar | |
|
Chou J, Lin YC, Kim J, You L, Xu Z, He B and Jablons DM: Nasopharyngeal carcinoma-review of the molecular mechanisms of tumorigenesis. Head Neck. 30:946–963. 2008.PubMed/NCBI View Article : Google Scholar | |
|
Allday MJ, Sinclair A, Parker G, Crawford DH and Farrell PJ: Epstein-Barr virus efficiently immortalizes human B cells without neutralizing the function of p53. EMBO J. 14:1382–1391. 1995.PubMed/NCBI | |
|
Belcher R, Hayes K, Fedewa S and Chen AY: Current treatment of head and neck squamous cell cancer. J Surg Oncol. 110:551–574. 2014.PubMed/NCBI View Article : Google Scholar | |
|
Perri F, Della Vittoria Scarpati G, Buonerba C, Di Lorenzo G, Longo F, Muto P, Schiavone C, Sandomenico F and Caponigro F: Combined chemo-radiotherapy in locally advanced nasopharyngeal carcinomas. World J Clin Oncol. 4:47–51. 2013.PubMed/NCBI View Article : Google Scholar |