Focal ground glass opacity of the lung in metachronous prostate and gastric cancer: A case report
- Authors:
- Published online on: June 19, 2024 https://doi.org/10.3892/mco.2024.2752
- Article Number: 54
-
Copyright: © Wada et al. This is an open access article distributed under the terms of Creative Commons Attribution License.
Abstract
Introduction
Metastatic carcinoma in the lung generally presents with multiple nodular shadows. Metastatic carcinoma may present with ground glass opacity (GGO), usually with diffuse form, on chest computed tomography (CT). Diffuse GGO represents lymphatic and/or vascular spread of carcinoma in the lung (1). Focal GGO, which is localized or nodular form on chest CT, is uncommon in metastatic carcinoma. Since various lung diseases such as interstitial fibrosis, inflammation and primary lung adenocarcinoma present with GGO, it is difficult to reach an accurate diagnosis based solely on radiological findings. Thus, in a case with past history of carcinoma, pathological assessment of focal GGO by biopsy or partial resection is necessary to determine whether the lesion is primary or metastatic carcinoma. The assessment is also critical to decide appropriate treatment.
We herein present a case of metastatic carcinoma presenting with focal GGO. A definite diagnosis of metastatic prostate cancer was reached by CT-assisted percutaneous needle biopsy and histological and immunohistochemical examinations. We present characteristic histological and immunohistochemical features of the case. We also reviewed a literature and found cases of metastatic cancer, presenting with focal GGO (2-10), and three cases were metastatic prostate cancer. It is of note two out of three cases were associated with pulmonary tumor thrombotic microangiopathy (PTTM), which rapidly progresses and is fatal (11). The vascular pathology needs to be assessed in biopsy specimen. The histological and clinical features of PTTM, which are necessary for immediate diagnosis, are also reviewed.
Case report
A 75-year-old man, who had a medical history of metachronous prostate and gastric cancers, presented at Kashiwa Kousei General Hospital, Kashiwa, Japan in September 2023 for the follow-up examination of the cancers. He was diagnosed as prostate cancer in another hospital 12 years before. Multiple bone metastases were detected in the spine. The histology of biopsy specimen of the prostate was adenocarcinoma with Gleason score 4+4. The patient had been treated with androgen deprivation therapy (ADT) for 12 years. The patient also received pelvic irradiation. Bone metastases appeared to be stable by ADT. However, a gradual increase was observed in the level of prostate-specific antigen (PSA). Seven months before, the patient underwent subtotal gastrectomy for multiple gastric cancers, which were detected in a health examination in Kashiwa Kousei General Hospital. There were three gastric cancers in the resected stomach. The main cancer was well-differentiated adenocarcinoma with focal moderately differentiated adenocarcinoma, and carcinoma cells invaded the submucosa. Neither blood nor lymphatic invasion was observed. The two other cancers were well-differentiated adenocarcinomas that were confined to the mucosa. Lymph node metastasis was not found.
Red and white blood cell counts were 4.09x106/µl and 4.1x103/µl, respectively. His platelet count was 69x103/µl. Serum levels of carcinoembryonic antigen and CA19-9 were within normal limits. His PSA level was elevated to 7.62 ng/ml. His fibrinogen level was 283 ng/dl and fibrinogen degradation products (FDP) were below the detection level. Echo cardiogram showed no abnormalities. The patient did not have dyspnea or respiratory discomfort. Pulmonary function tests were normal.
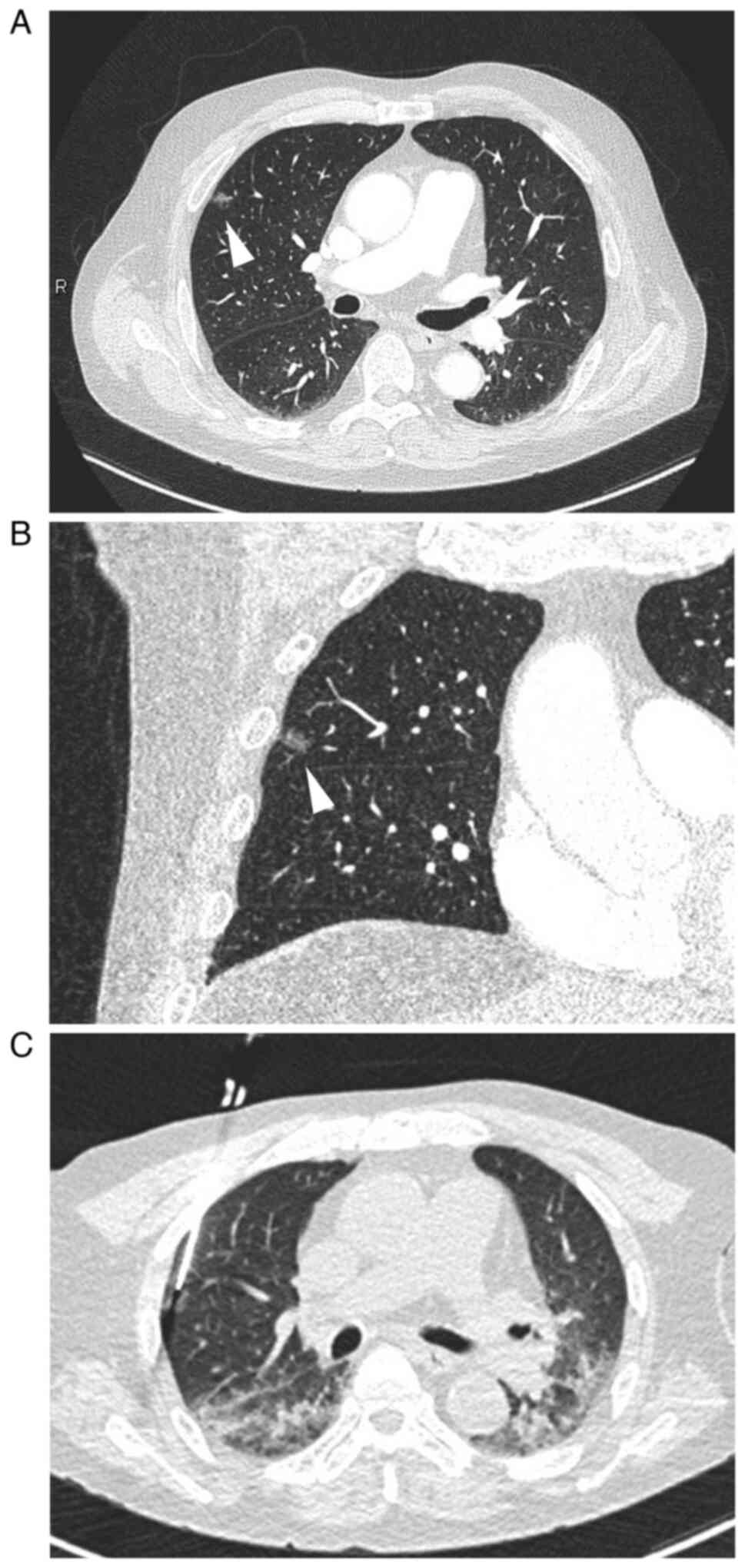
Chest CT revealed a focal GGO with a minimal solid area, measuring 11 mm in diameter, in the periphery of the right upper lobe (Fig. 1A and B). Pulmonary vessels and bronchioles were not involved. Since primary lung adenocarcinoma was suspected, CT-assisted percutaneous needle biopsy was performed (Fig. 1C).
The biopsy specimen showed the proliferation of atypical cells in fibrous tissue and the alveolar septa (Fig. 2A). In fibrous tissue, atypical cells proliferated in sheet-like, trabecular, and vague glandular patterns, suggesting that the lesion was moderately to poorly differentiated adenocarcinoma (Fig. 2B). The surrounding alveolar wall was thickened by the invasion of the poorly differentiated component of adenocarcinoma into the interstitial space (Fig. 2B). Neither the lepidic growth of carcinoma cells within the alveolar space nor invasion into the alveolar space was observed. A tumor thrombus was not found in the blood or lymphatic vessels (Fig. 2C), and there was no stenosis or re-canalization of vessels. The perivascular infiltration of macrophages and fibrocellular intimal proliferation were not present.
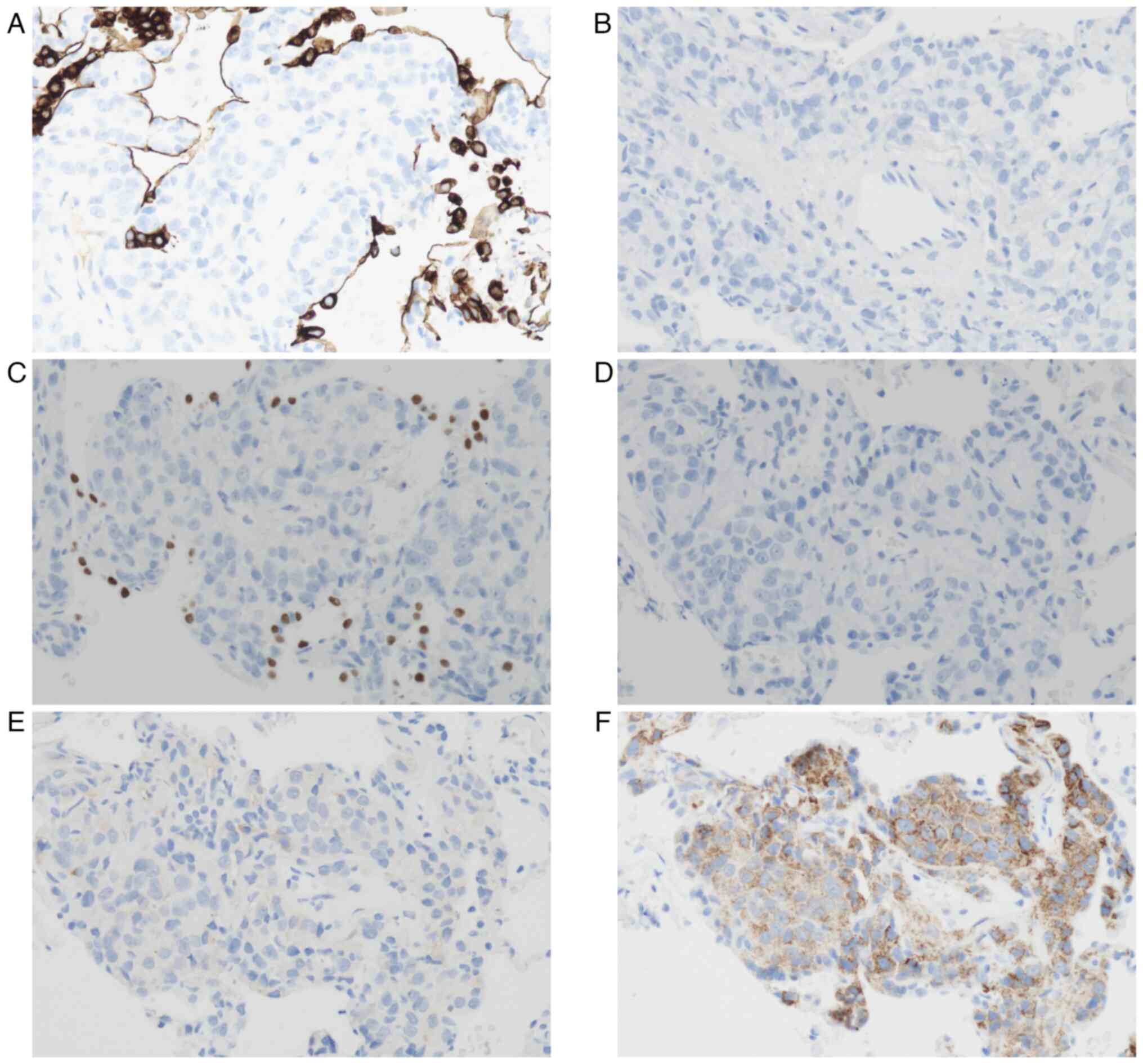
The phenotype of carcinoma cells was examined by immunohistochemistry (IHC). Carcinoma cells were negative for cytokeratin 7 (CK7) and CK20 (Fig. 3A and B) as well as thyroid transcription factor-1 (TTF1) and CDX2 (Fig. 3C and D). Therefore, primary lung adenocarcinoma and metastatic gastric cancer were considered to be unlikely. Carcinoma cells showed a faint positive reaction with PSA (Fig. 3E) and strong positive reaction with P504S (Fig. 3F). Based on these findings, the patient was diagnosed with metastatic carcinoma from prostate cancer. IHC with CK7 and TTF1 also clearly showed the subepithelial proliferation of carcinoma cells (Fig. 3A and C).
After the diagnosis, ADT was continued, and the patient is being followed up with careful observations. The condition has remained stable, and new lesions were not detected in the lungs 4 months since the diagnosis. Furthermore, there have been no abnormalities in laboratory data.
Discussion
Metastatic carcinoma and malignant neoplasms generally present as nodular shadows in the lungs; however, GGO may be encountered in cases of lung metastasis (1). Diffuse and patchy GGO are caused by diffuse thickening of the alveolar wall due to lymphangitic carcinomatosis or PTTM, and the most frequent primary site of carcinoma is the gastrointestinal tract. Focal GGO is rarely found in metastatic carcinoma and malignant neoplasms, but has been documented in cases of malignant melanoma (2-4), malignant phyllodes tumor (5), tongue cancer (6), thyroid cancer (7), and prostate cancer (8-10), including the present case (Table I). Since it was not feasible to distinguish metastatic lesions from primary lung adenocarcinoma based solely on radiological findings, a diagnosis was made by a histological examination of partially resected lung or biopsy specimens in the reported cases. In the present case, which had a previous history of metachronous prostate and gastric cancers, CT-assisted percutaneous needle biopsy was performed for a definitive diagnosis of the lesion.
In biopsy specimens, it is important to confirm whether the lesion is primary lung adenocarcinoma. A benign lesion of interstitial pneumonia and other benign lesions are also carefully differentiated. If metastatic adenocarcinoma is suspected, the primary site needs to be identified by IHC with specific markers. In the present case, carcinoma cells were negative for CK7 and TTF1, suggesting that primary lung adenocarcinoma was unlikely. Furthermore, a negative reaction for CDX2 indicated that gastric cancer was unlikely. The confirmation of metastatic prostate cancer was reached by positive reactions for PSA and P504S. The majority of prostate cancers are negative for CK7 and CK20(12). The expression of PSA may be attenuated by long-term ADT (13).
GGO is caused by various pathological conditions, such as interstitial fibrosis, inflammatory cell infiltration, thrombosis, and the lepidic growth of carcinoma cells (1). In the present case, GGO was attributed to thickening of the alveolar walls due to the extension of carcinoma cells beneath alveolar epithelial cells. This extension was evident by IHC, and proliferating carcinoma cells were covered with alveolar epithelial cells, which were positive for CK7 and TTF1 (Fig. 3A and C). The subepithelial growth of carcinoma cells is similar to reported cases of malignant melanoma (2-4) and malignant phyllodes tumor (5). Lepidic growth similar to primary lung adenocarcinoma was previously documented in a case of metastatic thyroid cancer (7), and focal GGO was caused by the invasion and destruction of alveoli and hemorrhage in a case of metastatic tongue cancer (6).
Careful attention of reported cases of metastatic prostate cancer with focal GGO is needed (Table I). Two of the three cases were associated with PTTM (9,10), while one was associated with lymphangitic carcinomatosis (8). These conditions generally present with diffuse GGO. The clinical course of PTTM is rapid and fatal; therefore, an immediate diagnosis is essential. Respiratory failure, pulmonary hypertension, an elevated D-dimer level, and thrombocytopenia indicate PTTM in cases with a previous history of carcinoma (11). PTTM may be diagnosed using biopsy specimens. The pathological features of PTTM are a tumor thrombus, fibrocellular intimal proliferation, and the recanalization of vessels. The perivascular infiltration of macrophages may also be present because the inflammatory process plays an important role in the pathogenesis of PTTM (14,15). In the present case, these histological changes were not observed in the biopsy specimen. Respiratory failure was absent and there was neither a decrease in the platelet count nor an elevation in FDP. Although the patient's condition was stable, he needs to be followed up with careful attention to laboratory data and radiological findings.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used during the present study are available from the corresponding author upon reasonable request.
Authors' contributions
RW, TY, KI, SU, NM, and HN participated in the diagnosis and treatment of the patient and wrote the first draft of this manuscript. RW and HN confirm the authenticity of all the raw data. All authors have read and approved the final manuscript.
Ethics approval and consent to participate
Ethics approval for the study was obtained from the Ethics Committee of Kashiwa Kousei General Hospital (approval no. 240025) dated February 26, 2024.
Patient consent for publication
Written informed consent was obtained from the patient to publish this case report and any accompanying images.
Competing interests
The authors declare that they have no competing interests.
References
|
Seo JB, Im JG, Goo JM, Chung MJ and Kim MY: Atypical pulmonary metastases: Spectrum of radiologic findings. Radiographics. 21:403–417. 2001.PubMed/NCBI View Article : Google Scholar | |
|
Kang MJ, Kim MA, Park CM, Lee CH, Goo JM and Lee HJ: Ground-glass nodules found in two patients with malignant melanomas: Different growth rate and different histology. Clin Imaging. 34:396–399. 2010.PubMed/NCBI View Article : Google Scholar | |
|
Okita R, Yamashita M, Nakata M, Teramoto N, Bessho A and Mogami H: Multiple ground-glass opacity in metastasis of malignant melanoma diagnosed by lung biopsy. Ann Thorac Surg. 79:e1–e2. 2005.PubMed/NCBI View Article : Google Scholar | |
|
Mizuuchi H, Suda K, Kitahara H, Shimamatsu S, Kohno M, Okamoto T and Maehara Y: Solitary pulmonary metastasis from malignant melanoma of the bulbar conjunctiva presenting as a pulmonary ground glass nodule: Report of a case. Thorac Cancer. 6:97–100. 2015.PubMed/NCBI View Article : Google Scholar | |
|
Nakamura S, Goto T, Nara S, Kawahara Y, Yashiro S, Kano S, Hosokawa Y and Kamada H: Pure ground glass opacity (GGO) on chest CT: A rare presentation of lung metastasis of malignant phyllodes tumor. Breast Cancer. 27:1187–1190. 2020.PubMed/NCBI View Article : Google Scholar | |
|
Haro A, Wakasu S, Takada K, Osoegawa A, Kamitani T, Tagawa T and Mori M: Pulmonary metastasis presenting as a ground glass opacity-like lesion with a thin-walled cavity: A case report. Int J Surg Case Rep. 60:287–290. 2019.PubMed/NCBI View Article : Google Scholar | |
|
Ryuko T, Sano Y, Kitazawa R, Otani S, Sakao N and Mori Y: Lung metastasis from thyroid carcinoma showing a pure ground-glass nodule. Ann Thorac Surg. 114:e253–e256. 2022.PubMed/NCBI View Article : Google Scholar | |
|
Lubin DJ, Holden SB, Rettig MB, Reiter RE, King CR, Lee JM, Wallace DW and Calais J: Prostate cancer pulmonary metastasis presenting as a ground-glass pulmonary nodule on 68Ga-PSMA-11 PET/CT. Clin Nucl Med. 44:e353–e356. 2019.PubMed/NCBI View Article : Google Scholar | |
|
Osaki Y, Taoka R, Miyauchi Y and Sugimoto M: Docetaxel chemotherapy temporarily improved pulmonary tumor thrombotic microangiopathy induced by prostate cancer secreting carcinoembryonic antigen and carbohydrate antigen 19-9: A case report. Urol Case Rep. 29(101098)2020.PubMed/NCBI View Article : Google Scholar | |
|
Katayama S, Takenaka T, Nakamura A, Sako S, Bessho A and Ohara N: Pulmonary tumor thrombotic microangiopathy induced by prostate cancer. Acta Med Okayama. 72:309–313. 2018.PubMed/NCBI View Article : Google Scholar | |
|
Godbole RH, Saggar R and Kamangar N: Pulmonary tumor thrombotic microangiopathy: A systematic review. Pulm Circ. 9:1–13. 2019.PubMed/NCBI View Article : Google Scholar | |
|
Dum D, Menz A, Völkel C, De Wispelaere N, Hinsch A, Gorbokon N, Lennartz M, Luebke AM, Hube-Magg C, Kluth M, et al: Cytokeratin 7 and cytokeratin 20 expression in cancer: A tissue microarray study on 15,424 cancers. Exp Mol Pathol. 126(104762)2022.PubMed/NCBI View Article : Google Scholar | |
|
Ogreid P, Berner A, Dahl O, Rettedal E and Fossa SD: Tissue prostate-specific antigen and androgen receptor immunoreactivity in prostate cancer biopsies before, during and after neo-adjuvant androgen deprivation followed by radiotherapy. Eur Urol. 36:116–122. 1999.PubMed/NCBI View Article : Google Scholar | |
|
Mantovani A, Allavena P, Sica A and Balkwill F: Cancer-related inflammation. Nature. 454:436–444. 2008.PubMed/NCBI View Article : Google Scholar | |
|
Price LC, Wells AU and Wort SJ: Pulmonary tumour thrombotic microangiopathy. Curr Opin Pulm Med. 22:421–428. 2016.PubMed/NCBI View Article : Google Scholar |