Early diagnosis of cerebral vasospasm associated with cerebral ischemia following subarachnoid hemorrhage: Evaluation of computed tomography perfusion and transcranial doppler as accurate methods
- Authors:
- Published online on: November 2, 2022 https://doi.org/10.3892/mi.2022.59
- Article Number: 34
-
Copyright: © Tsolaki et al. This is an open access article distributed under the terms of Creative Commons Attribution License.
Abstract
Introduction
Cerebral vasospasm (CV) or delayed cerebral ischemia (DCI) is the main complication and cause of morbidity following surgery in individuals that have a subarachnoid hemorrhage (SAH). Unfortunately, however, 16-65% of these patients, despite the use of different therapeutic procedures, consequently develop ischemia (1-4). A variety of strategies are employed to enhance cerebral blood flow (CBF) in patients with SAH who develop CV. Intra-aortic balloon counterpulsation, hyperdynamic therapy, intra-arterial and intrathecal drug infusion, as well as novel experimental techniques, such as endovascular treatment and different drugs or their combinations, may be helpful if treatment are commenced at an early stage. However, if a CV does not appear or treatment begins at a late stage, cerebral ischemia (CI) may occur even with optimal management (5).
Identifying CV (clinically or with angiography) at an early stage in SAH can be difficult and challenging (1,2). Although 40-70% of patients exhibit the substantiation of arterial narrowing (on a Doppler ultrasound or an angiography), only 20 to 30% present with DCI (1,2). Intra-arterial digital subtraction angiography (DSA) significantly performs as a screening device in patients whose symptoms and clinical findings are consistent with focal CI (6). However, when assessing the global vasculature using DSA, the detailed delineation of the calf vessels may not be obtained when numerous stenoses are present and constitute an invasive method. Computed tomography (CT) can reveal the amount of irreversibly damaged tissue (the ischemic core) (7-9), and it indicates that reperfusion therapy may not be necessary or may even be harmful when the ischemic core is large or the perfusion is damaged to a great extent (10,11). By contrast, CT perfusion (CTP) is able to identify damage which is not recognized by other methods and may be beneficial for the assessment of CI related to SAH (12). CTP can be used in daily practice, or it can be used as a separate diagnostic tool without the need for magnetic resonance imaging data to predict the outcomes of patients with SAH (13,14). In addition, available data on its association with other non-invasive techniques, such as transcranial Doppler (TCD) ultrasound are not sufficient (1,15,16).
In this respect, the present study was performed with an aim to evaluate the utility of the CTP procedure in the daily practice of a tertiary hospital for the early recognition of symptomatic vasospasm in preventing permanent neurological deterioration and to assess the diagnostic performance of this technique in the clinical outcomes of patients with SAH (traumatic and aneurysmal).
Patients and methods
Study design and inclusion criteria
The present retrospective study included patients with spontaneous/aneurysmal or traumatic SAH i) who presented with Glasgow Coma Scale (GCS) scores of 15/15 to 4/15; and ii) whose CT scan demonstrated high-grade SAH according to neuroimaging damage degree (Fisher-IV), in a very early stage (48 h after the onset) of SAH, as well as patients presenting with traumatic brain injury (TBI) without SAH as controls, between July, 2018 and January, 2020 at the University Hospital of Larisa (Larisa, Greece). Patients with TBI without SAH were selected as controls, as TBI may be associated with the pathophysiology of ischemic post-traumatic events. The Institutional Review Board (IRB) of University of Thessaly, Greece/The School of Medicine/School of Health Sciences approved the study (IRB no. 2492/19-01-2015). The study was in line with the Declaration of Helsinki in 1995 (as revised in Edinburgh 2000). Written informed was obtained from all patients.
As the present study was retrospective, groups of patients whose parameters (such as blood pressure and intra- and extracranial artery stenosis), were the same and without any marked differences were included.
Exclusion criteria
Patients with factors predisposed to CI apart from vasospasm (i.e., those with atrial fibrillation or other arrhythmias, diabetes mellitus, hyperlipidemia and hypotension) or with factors predisposed to bleeding (anticoagulant agents and severe liver impairment) were excluded. All participants were followed up for 30 days or until they were discharged from the hospital. Outcomes were evaluated at 30 days using a CT scan and a full neurological examination was performed, together with GCS assessment. The clinical outcome was categorized according to the presence of neurological or radiological evidence as normal, adverse (headache, change of consciousness, or others), or mortality.
Treatments
During the 3rd to 6th day after SAH, CTP was performed in all the participants to identify a quantifiable index of CI after CV given that angiographically detectable cerebral artery constriction is most commonly present at 3-10 days after the onset. Cerebral blood volume (CBV) and cerebral blood flow (CBF) values were documented and assessed after receiving two contiguous 10-mm slices placed at the anatomical point of the basal ganglia with similar angulation as for native CT. A bolus of 50 ml of nonionic contrast medium (Imeron 400, Bracco Imaging Deutschland GmbH) accompanied by 30 ml of saline was then infused using a power injector at a flow rate of 4 ml/sec. Subsequently, 40 images were captured at each slice level at a rate of two images per second (120 kV, 110 mAs, 512x512 matrix). CTP color maps were assessed qualitatively using a visual grading scale, and CTP parameters were established utilizing software platforms (Perfusion CT, Siemens). A positive visual measurement was recorded for side-to-side discrepancies or obvious bilateral anomalies, which could suggest a decline in CBF, CBV, and mean transit time (MTT), which were related to the central volume principle: CBF=CBV/MTT (1,17). CBV was determined in milliliters of blood per 100 gm of brain and was established as the volume of blood flow for a certain amount of brain tissue (1,18). MTT was determined as the average time needed for blood to move through a certain volume of brain and was calculated in seconds (1).
Moreover, 15-20 min before CTP, TCD via a trans-temporal window was carried out to illustrate and assess flow velocities in the anterior cerebral artery (ACA), middle cerebral artery (MCA), posterior cerebral artery (PCA) and posterior communicating artery. Peak systolic velocity (PSV, in cm/sec), which is the maximum flow velocity value during systole, was calculated at the peak of the waveform. End-diastolic velocity (EDV, in cm/sec) was determined at the end of diastole, traditionally at the lowest point before the beginning of a new waveform, which is between 20 and 50% of the PSV value (1,19). The Lindegaard ratio, defined as MCA mean CBF velocity divided by extracranial internal carotid artery mean CBF velocity, was utilized as an indicator of systemic hemodynamic alterations. The mean flow velocity (in cm/sec) was calculated as the average of the edge frequency across a cardiac cycle, which was calculated as EDV plus one-third of the variance between PSV and EDV [MFV (cm/sec)=(PSV + 2EDV/3)] (1,19). A sonographic CV was defined as an MFV of >140 cm/sec in the MCA, ACA, and/or PCA or >90 cm/sec in the basilar artery. Pulsatility index (PI)=PSV-EDV/MFV was utilized as an index for intracranial pressure elevation (1,19).
Statistical analysis
The normality of the distribution of variables was determined using the Shapiro-Wilk test. The data were non-parametric and were compared using the Mann-Whitney U test. To identify predictors of adverse events (adverse ischemic events), statistically significant factors were subsequently examined using Cox proportional hazards multivariate regression analysis. Receiver operating characteristic (ROC) analysis was presented to reveal the implementation of TCD or CTP indicators in recognition of unfavorable outcomes. A P-value of <0.05 was characterized as statistically significant. Statistical analysis was performed with the use of the Statistical Package for the Social Sciences (SPSS 11; SPSS, Inc.).
Results
A total of 34 patients were enrolled in the present study (Table I). Of these, 15 patients were males (44.1%), and the median age was 50.00 years (interquartile range, 31 years). Of these patients, 15 patients (44.1%) had traumatic SAH following TBI, 11 patients (33.3%) had aneurysmal SAH and 8 patients (23.6%) presented with no SAH after TBI as controls. The outcomes and baseline characteristics of the study participants are presented in Table I. Overall, 12 patients (35.2%), who experienced some clinical symptoms, such as a decline in the level of consciousness or a worsening headache, were administered hypervolemic hypertensive and intra-arterial calcium channel therapy after early the identification of symptomatic vasospasm with CTP findings and were presented with permanent neurological deficits as detected using a CT scan after 1 month of follow-up.
The TCD and CTP data are presented in Tables II and III, respectively. In addition, in the 12 patients who presented with permanent neurological deficits, CTP revealed reduced CBF and prolonged MTT. TCD also revealed elevated PSVs and PIs in the same patients, apart from patient no. 4, whose right ACA PI was normal (=0.9). In addition, TCD revealed that the right ACA PI of patient no. 21 was normal (=1) with PSV=87 cm/sec and MFV=69 cm/sec, the right PCA PI of patient no. 25 was 1.2 with PSV=58 cm/sec and MFV=35 cm/sec, and the left MCA PI of patient no. 30 was normal (=0.8) with PSV=89 cm/sec and MFV=87 cm/sec.
Diagnostics
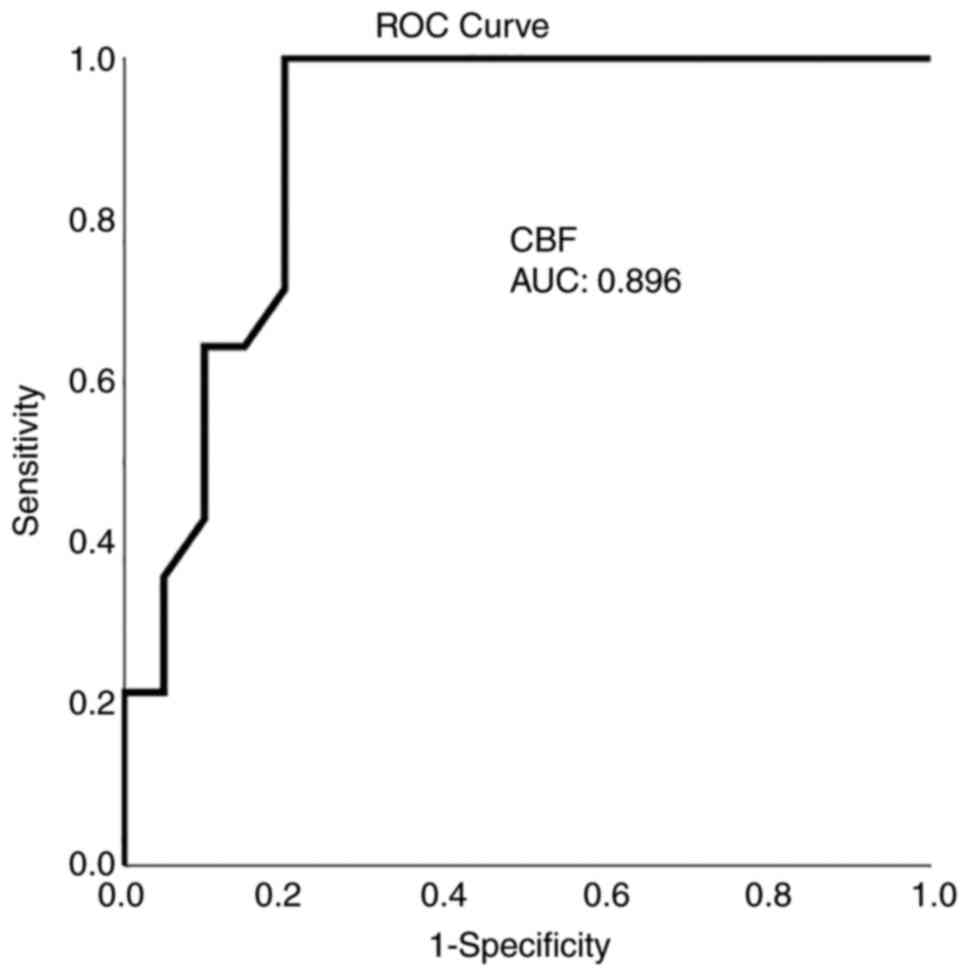
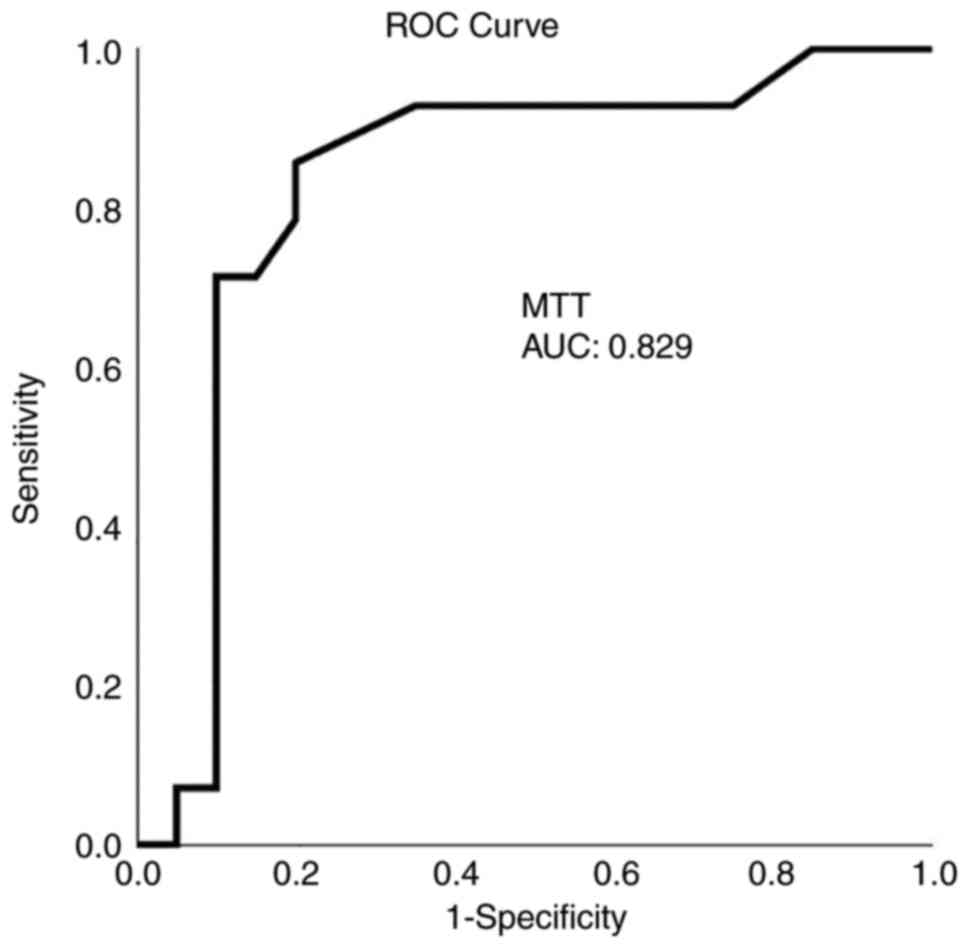
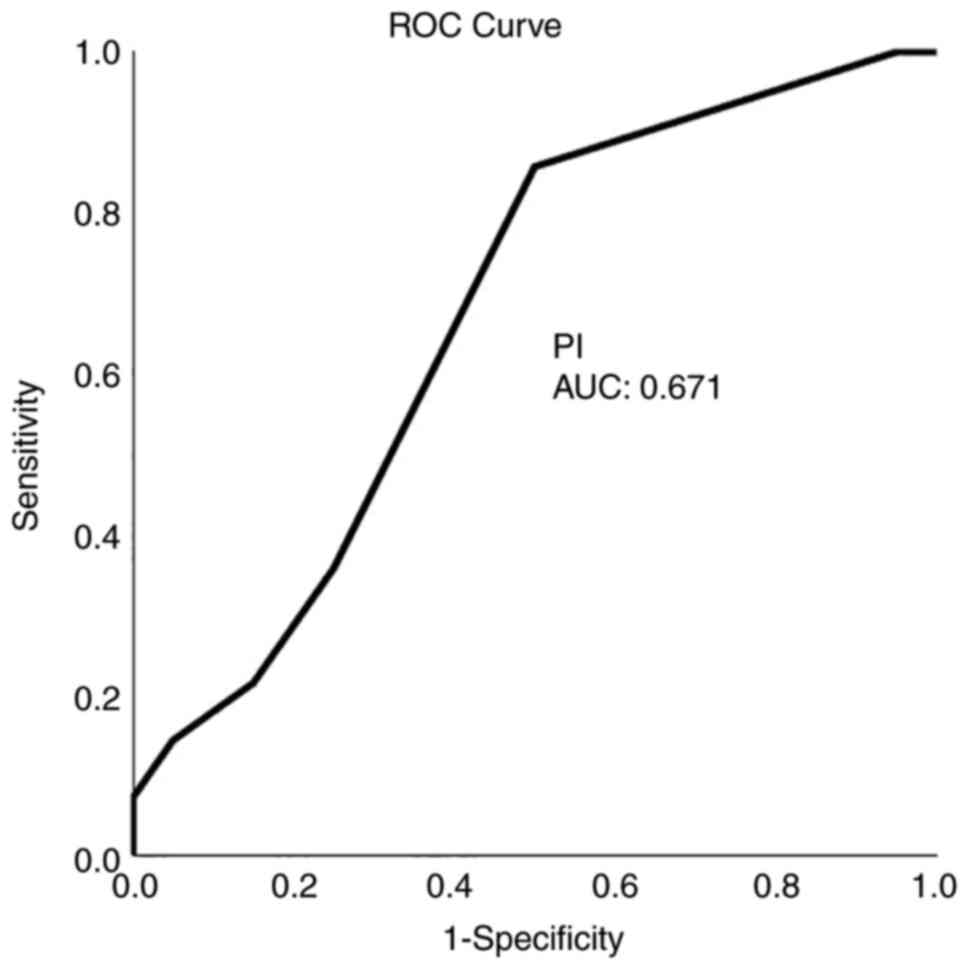
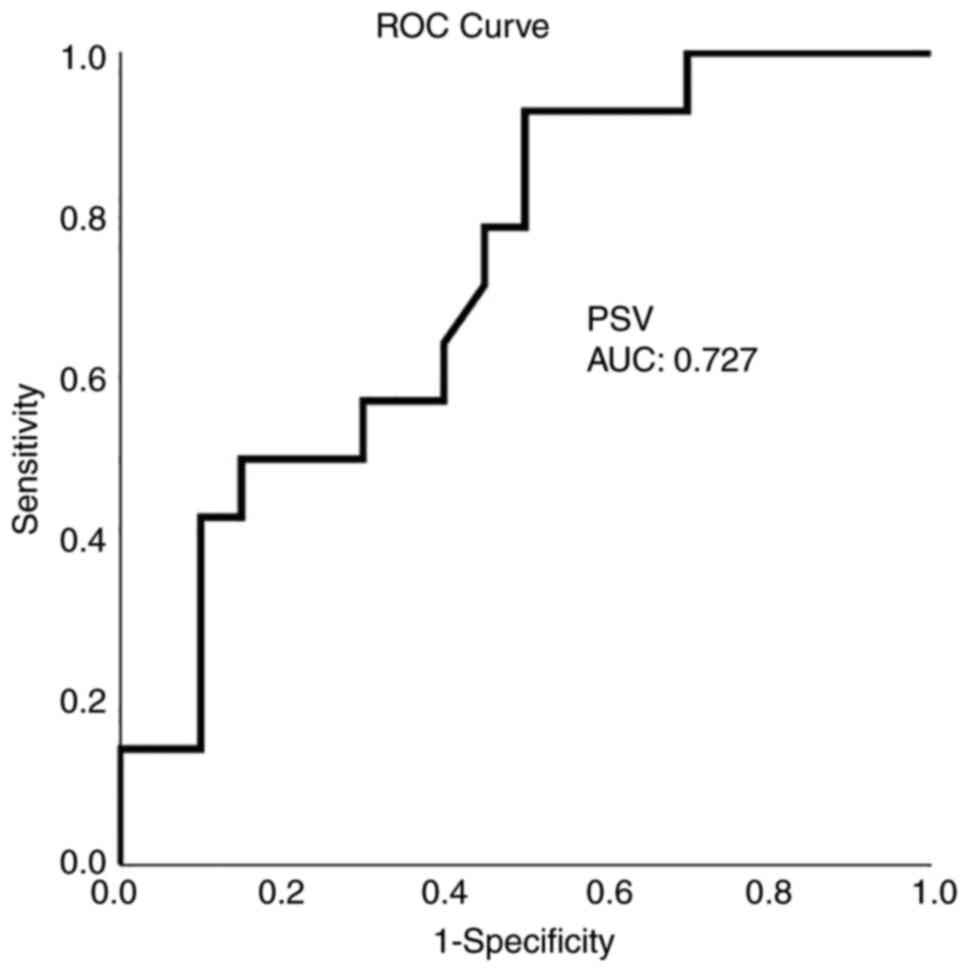
ROC analysis demonstrated that, among the CTP and TCD variables, a CBF value <21.5 ml x 100 g-1 x min-1 with 92.9% sensitivity and 80% specificity and an MTT value >3.7 sec with 86% sensitivity and 80% specificity presented the optimal performance to diagnose adverse ischemic events at 1 month, as evaluated by an area-under-the-curve standard error [AUC(SE)] of 0.89(0.06), P=0.005 (Table IV and Fig. 1, Fig. 2, Fig. 3 and Fig. 4).
Univariate analysis performed with the use of the Mann-Whitney U test revealed that there was a statistically significant difference in the mean values of PSV, CBF, CBV and MTT between participants who developed adverse ischemic events and between those who did not develop adverse ischemic events (P<0.05, Table V).
Cox proportion hazards multivariate regression analysis (Table VI) revealed that CBF was an independent factor in detecting an ischemic events in 1 month (P=0.003).
Discussion
The results of the present study suggest that CTP is obtainable in everyday practice, and it may provide valuable details about SAH-associated CV. Certainly, CTP, and mainly CBF and MTT, can help to recognize patients who develop delayed unfavorable ischemic incidents and are more precise than TCD at this point. Thus, the CTP constitutes a very hopeful diagnostic instrument for managing SAH.
DSA is the gold standard for identifying CV and different cerebrovascular entities (1,20,21). However, the invasiveness of the procedure and the radiation exposure require careful patient selection and preclude widespread use (15). In addition, a number of invasive procedures, such as intracerebral CBF-dimension and microdialysis, have been developed due to their significance in detecting CV in patients after SAH (15). Those methods eschew the hazards of setting and continuing monitoring with intracerebral probes, such as infection, and they are only accurate if the calculation is performed in an area of the brain that will eventually be affected by decreased perfusion (1,15). Furthermore, MR perfusion, CTP, positron emission tomography, Xenon CT and single-photon emission CT all permit tomographic CBF evaluation. However, CTP is currently the most commonly used and studied imaging technique (22). A range of cut-off values associated with DCI has been mentioned, counting an MTT value >5.0-6.4 sec or a local CBF value <25-40 ml x 100 g-1 x min-1 (1,23).
The present study detected two separate major types of patients depending on their CTP and TCD data. The first profile was related to the clinical outcomes without ischemic damage in the CT scan at these locations, whereas the second one was related to unfavorable clinical outcomes with ischemic damage in the CT scan. In the first profile, the majority of patients had CTP that indicated no vasospasm or mild vasospasm according to the CBF and MTT values; this was observed in patient nos. 2 and 5. In patient no. 2, CTP revealed only delayed MTT from 7.3 to 10 sec and TCD revealed elevated PIs (0.9 in both right and left ACAs), indicating severe vasospasm. In patient no. 5, CTP demonstrated a decrease in CBF with delayed MTT, and TCD demonstrated elevated PIs in the right and left MCAs (1.4 and 1.3, respectively) and the left PCA (1.6), indicating mild vasospasm. On the other hand, 12 patients with the second profile, had CTP that illustrated substantial vasospasm with clinically and radiologically evident infarct lesions (Table I); however, PI values in TCD had a range between normal and 1.3, which is in agreement mainly with mild or no vasospasm (i.e., patient nos. 1, 3, 4, 21, 25 and 30). For patient no. 1, CTP demonstrated a decreased CBF value (in the right and left frontal, as well as left parietal areas), normal CBV values, and delayed MTT (7.4 to 10.5 sec), and TCD revealed a mean PI value of 1.5. In patient no. 3, CTP revealed decreased CBF and CBV values in the right frontal and parietal areas and delayed MTT mostly in the left part (7.4 to 10.9 sec), and TCD revealed a mean PI value of 1.2. In patient no. 4, CTP revealed reduced CBF and delayed MTT in the left from 7.4 to 8.7 sec, and TCD revealed a mean PI value of 1.1 (Tables II and III).
These findings indicate that CTP indices (CBF and MTT) presented a better dispersion in ROC analysis in comparison with TCD values (Table IV). CBF represents the blood flow in the brain, and any decrease in its value is strongly connected with the incidence and severity of vasospasm, which constitutes an important predictor variable of clinical outcome as well. CTP parameters (CBF and MTT) appear to be associated quite well with CI, although focal flow reduction can also be presented as a result of brain damage, retraction, or perihematomal brain impairment (1,5). On the contrary, TCD provides considerable information about the flow changes in an arterial segment and, based on the spectral models in a range of nearby intracranial arteries, it can identify CV in the MCA (24) and may possibly help as a comparatively simple screening technique of the vasospasm; however, some authors were reluctant to report any association between TCD and angiographic evidence in patients with CV (1,25,26). Additionally, TCD ultrasonography encounters both anatomical and technical limitations and is an operator-dependent technique (27).
CTP has been applied for the assessment of CI with sensitivity between 74.1 and 84% and specificity between 79 and 93.0% (28,29). In the present study, all regions with decreased CBF or prolonged MTT were associated with delayed ischemic unfavorable incidents, i.e., CBF [AUC (SE)] of 0.89(0.06), P=0.005, and MTT [AUC(SE)] of 0.82(0.07), P=0.001. This finding is in agreement with previous reports stating that a CBF reduction may suggest permanent ischemic lesions with a CBF threshold <25 ml x 100 g-1 x min-1. In addition, when a CBF decrease is associated with a CBV value <2 ml x 100 g-1 or with an increased MTT value >145%, core ischemia or penumbra can be identified (29). In the present study, a CBF threshold of 21.5 ml x 100 g-1 x min-1 and an MTT value >3.7 sec with 80 sensitivity and 100% specificity for CBF and 86% sensitivity and 80% specificity for MTT, which were calculated after an aneurysmal or traumatic SAH and they were similar to the literature (29), detected an unfavorable ischemic incident at 1 month. Previous studies have reported that a reduced CBF with normal or raised CBV may also indicate hemodynamic hypo-perfusion without real infarction damage in reaction to the autoregulation (1,30).
MTT alone may indicate mild to moderate vasospasm, whereas a MTT delay related to CBF and/or CBV changes may predict severe vasospasm (30). In the present study, all participants who obtained the aforementioned triad had late unfavorable ischemic events; however, no patients had MTT prolongation without any other CTP abnormalities, and therefore, the value of MTT as a stand-alone indicator could not be confirmed. Furthermore, in the present study, multivariable analysis revealed that MTT prolongation was not an independent factor for the diagnosis of ischemia at 1 month (P=0.614) and was only associated with CBF abnormalities that were related to clinical and CT indications of ischemia.
It is worth mentioning that imaging technology in healthcare facilities has become more widely available worlwide over time. Furthermore, the amount to which technology availability has changed varies across different areas with some areas expand and contract through time, whereas others expand and contract faster (31). As regards SAH, consecutive TCD measurements and the calculation of the hemispheric index are currently routinely used in several neurointensive care units. A regards the CTP, it requires the transportation of the patient out of the intensive care unit, limiting the clinical relevance of this method for monitoring (32).
A strong point of the present study is that it evaluated the utility of the CTP procedure in the daily practice of a tertiary hospital for the early recognition of symptomatic vasospasm in preventing permanent neurological deterioration. The present study also assessed the diagnostic performance of this technique in the clinical outcomes, not only of patients with aneurysmal, but and traumatic SAH in the first 24 h. This expansion allows for the better evaluation and understanding of the complex vasospasm entity.
The present study has certain limitations. It was a small, single-center study. In addition, factors such as the ethnic origin and the quality of different CT instruments vary between studies. Therefore, firm conclusions regarding the role of CTP in the management of SAH cannot be reached. However, the findings presented herein may serve as a basis for future more extensive multi-centered clinical studies with more homogeneous samples formed by aneurysmal SAH, along with comparing CPT to angiogram studies and assessing whether CBF predicts the response to vasospasm treatment.
In conclusion, the data of the present study demonstrated that the combination of CTP and TCD was instrumental in diagnosing ischemic events. In patients with CV, TCD alone was unable to achieve a good association with angiographic results. On the other hand, CTP demonstrated better precision, and mainly, CBF was a considerable index that could identify the extent of CI precociously in patients experiencing SAH; thus, it may allow intervention to prevent permanent neurological deterioration, either through the accentuation of hypervolemic hypertensive therapy, intra-arterial calcium channel administration, or intracranial angioplasty. MTT prolongation was not an independent factor, and only its relation with CBF abnormalities was associated with clinical and CT evidence of ischemia.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
GF, AAF and VEG conceptualized the study. VEG, AAF, PP, PS, NT, VT, NM and KT made substantial contributions to data analysis and interpretation, and wrote and prepared the draft of the manuscript. DAS, VEG and GF analyzed the data and provided critical revisions. VEG and GF confirm the authenticity of all the data. All authors contributed to manuscript revision and have read and approved the final version of the manuscript.
Ethics approval and consent to participate
The Institutional Review Board (IRB) of University of Thessaly, Greece/The School of Medicine/School of Health Sciences approved the study (IRB no. 2492/19-01-2015). The study was in line with the Declaration of Helsinki in 1995 (as revised in Edinburgh 2000). Written informed was obtained from the patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
|
Fotakopoulos G, Makris D, Kotlia P, Kapsalaki E, Papanikolaou J, Georgiadis I, Zakynthinos E and Fountas K: The value of computed tomography perfusion & transcranial doppler in early diagnosis of cerebral vasospasm in aneurysmal & traumatic subarachnoid hemorrhage. Future Sci OA. 4(FSO313)2018.PubMed/NCBI View Article : Google Scholar | |
|
Ko NU, Rajendran P, Kim H, Rutkowski M, Pawlikowska L, Kwok PY, Higashida RT, Lawton MT, Smith WS, Zaroff JG and Young WL: Endothelial nitric oxide synthase polymorphism (-786T->C) and increased risk of angiographic vasospasm after aneurysmal subarachnoid hemorrhage. Stroke. 39:1103–1108. 2008.PubMed/NCBI View Article : Google Scholar | |
|
Dumont AS, Dumont RJ, Chow MM, Lin CL, Calisaneller T, Ley KF, Kassell NF and Lee KS: Cerebral vasospasm after subarachnoid hemorrhage: Putative role of inflammation. Neurosurgery. 53:123–133. 2003.PubMed/NCBI View Article : Google Scholar | |
|
Starke RM, Kim GH, Komotar RJ, Hickman ZL, Black EM, Rosales MB, Kellner CP, Hahn DK, Otten ML, Edwards J, et al: Endothelial nitric oxide synthase gene single-nucleotide polymorphism predicts cerebral vasospasm after aneurysmal subarachnoid hemorrhage. J Cereb Blood Flow Metab. 28:1204–1211. 2008.PubMed/NCBI View Article : Google Scholar | |
|
Francoeur CL and Mayer SA: Management of delayed cerebral ischemia after subarachnoid hemorrhage. Crit Care. 20(277)2016.PubMed/NCBI View Article : Google Scholar | |
|
Foley WD, Smith DF, Milde MW, Lawson TL, Towne JB and Bandyk DF: Intravenous DSA examination of patients with suspected cerebral ischemia. Radiology. 151:651–659. 1984.PubMed/NCBI View Article : Google Scholar | |
|
Muir KW, Baird-Gunning J, Walker L, Baird T, McCormick M and Coutts SB: Can the ischemic penumbra be identified on noncontrast CT of acute stroke? Stroke. 38:2485–2490. 2007.PubMed/NCBI View Article : Google Scholar | |
|
Murphy BD, Fox AJ, Lee DH, Sahlas DJ, Black SE, Hogan MJ, Coutts SB, Demchuk AM, Goyal M, Aviv RI, et al: Identification of penumbra and infarct in acute ischemic stroke using computed tomography perfusion-derived blood flow and blood volume measurements. Stroke. 37:1771–1777. 2006.PubMed/NCBI View Article : Google Scholar | |
|
Wintermark M and Warach SJ: STIR and Virtual International Stroke Trials Archive (VISTA)-Imaging Investigators. Acute stroke imaging research roadmap II and international survey of acute stroke imaging capabilities: We need your help! AJNR Am J Neuroradiol. 34(1671)2013.PubMed/NCBI View Article : Google Scholar | |
|
Campbell BC, Christensen S, Butcher KS, Gordon I, Parsons MW, Desmond PM, Barber PA, Levi CR, Bladin CF, De Silva DA, et al: Regional very low cerebral blood volume predicts hemorrhagic transformation better than diffusion-weighted imaging volume and thresholded apparent diffusion coefficient in acute ischemic stroke. Stroke. 41:82–88. 2010.PubMed/NCBI View Article : Google Scholar | |
|
Yassi N, Parsons MW, Christensen S, Sharma G, Bivard A, Donnan GA, Levi CR, Desmond PM, Davis SM and Campbell BC: Prediction of poststroke hemorrhagic transformation using computed tomography perfusion. Stroke. 44:3039–3043. 2013.PubMed/NCBI View Article : Google Scholar | |
|
Provenzale JM, Shah K, Patel U and McCrory DC: Systematic review of CT and MR perfusion imaging for assessment of acute cerebrovascular disease. AJNR Am J Neuroradiol. 29:1476–1482. 2008.PubMed/NCBI View Article : Google Scholar | |
|
Derex L, Nighoghossian N, Hermier M, Adeleine P, Berthezène Y, Philippeau F, Honnorat J, Froment JC and Trouillas P: Influence of pretreatment MRI parameters on clinical outcome, recanalization and infarct size in 49 stroke patients treated by intravenous tissue plasminogen activator. J Neurol Sci. 225:3–9. 2004.PubMed/NCBI View Article : Google Scholar | |
|
Parsons MW, Barber PA, Chalk J, Darby DG, Rose S, Desmond PM, Gerraty RP, Tress BM, Wright PM, Donnan GA and Davis SM: Diffusion- and perfusion-weighted MRI response to thrombolysis in stroke. Ann Neurol. 51:28–37. 2002.PubMed/NCBI View Article : Google Scholar | |
|
Kunze E, Pham M, Raslan F, Stetter C, Lee JY, Solymosi L, Ernestus RI, Vince GH and Westermaier T: Value of perfusion CT, transcranial doppler sonography, and neurological examination to detect delayed vasospasm after aneurysmal subarachnoid hemorrhage. Radiol Res Pract. 2012(231206)2012.PubMed/NCBI View Article : Google Scholar | |
|
Westermaier T, Pham M, Stetter C, Willner N, Solymosi L, Ernestus RI, Vince GH and Kunze E: Value of transcranial doppler, perfusion-CT and neurological evaluation to forecast secondary ischemia after aneurysmal SAH. Neurocrit Care. 20:406–412. 2014.PubMed/NCBI View Article : Google Scholar | |
|
Meier P and Zierler KL: On the theory of the indicator-dilution method for measurement of blood flow and volume. J Appl Physiol. 6:731–744. 1954.PubMed/NCBI View Article : Google Scholar | |
|
Konstas AA, Goldmakher GV, Lee TY and Lev MH: Theoretic basis and technical implementations of CT perfusion in acute ischemic stroke, part 1: Theoretic basis. AJNR Am J Neuroradiol. 30:662–668. 2009.PubMed/NCBI View Article : Google Scholar | |
|
Lysakowski C, Walder B, Costanza MC and Tramèr MR: Transcranial doppler versus angiography in patients with vasospasm due to a ruptured cerebral aneurysm: A systematic review. Stroke. 32:2292–2298. 2001.PubMed/NCBI View Article : Google Scholar | |
|
Chaudhary SR, Ko N, Dillon WP, Yu MB, Liu S, Criqui GI, Higashida RT, Smith WS and Wintermark M: Prospective evaluation of multidetector-row CT angiography for the diagnosis of vasospasm following subarachnoid hemorrhage: A comparison with digital subtraction angiography. Cerebrovasc Dis. 25:144–150. 2008.PubMed/NCBI View Article : Google Scholar | |
|
Yao GE, Li Q, Jiang XJ, Liu J, Li JL, Zhang LL, Li LL, Zhang J and Xie P: Vasospasm after subarachnoid hemorrhage: A 3D rotational angiography study. Acta Neurochir Suppl. 110:221–225. 2011.PubMed/NCBI View Article : Google Scholar | |
|
Cremers CH, van der Schaaf IC, Wensink E, Greving JP, Rinkel GJ, Velthuis BK and Vergouwen MD: CT perfusion and delayed cerebral ischemia in aneurysmal subarachnoid hemorrhage: A systematic review and meta-analysis. J Cereb Blood Flow Metab. 34:200–207. 2014.PubMed/NCBI View Article : Google Scholar | |
|
Sanelli PC, Ugorec I, Johnson CE, Tan J, Segal AZ, Fink M, Heier LA, Tsiouris AJ, Comunale JP, John M, et al: Using quantitative CT perfusion for evaluation of delayed cerebral ischemia following aneurysmal subarachnoid hemorrhage. AJNR Am J Neuroradiol. 32:2047–2053. 2011.PubMed/NCBI View Article : Google Scholar | |
|
Fontanella M, Valfrè W, Benech F, Carlino C, Garbossa D, Ferrio M, Perez R, Berardino M, Bradac G and Ducati A: Vasospasm after SAH due to aneurysm rupture of the anterior circle of Willis: Value of TCD monitoring. Neurol Res. 30:256–261. 2008.PubMed/NCBI View Article : Google Scholar | |
|
Bornstein NM and Norris JW: Transcranial doppler sonography is at present of limited clinical value. Arch Neurol. 51:1057–1059. 1994.PubMed/NCBI View Article : Google Scholar | |
|
Steinmeier R, Laumer R, Bondár I, Priem R and Fahlbusch R: Cerebral hemodynamics in subarachnoid hemorrhage evaluated by transcranial doppler sonography. Part 2. Pulsatility indices: Normal reference values and characteristics in subarachnoid hemorrhage. Neurosurgery. 33:10–18. 1993.PubMed/NCBI View Article : Google Scholar | |
|
Washington CW and Zipfel GJ: Participants in the International Multi-disciplinary Consensus Conference on the Critical Care Management of Subarachnoid Hemorrhage. Detection and monitoring of vasospasm and delayed cerebral ischemia: A review and assessment of the literature. Neurocrit Care. 15:312–317. 2011.PubMed/NCBI View Article : Google Scholar | |
|
Dankbaar JW, de Rooij NK, Velthuis BK, Frijns CJ, Rinkel GJ and van der Schaaf IC: Diagnosing delayed cerebral ischemia with different CT modalities in patients with subarachnoid hemorrhage with clinical deterioration. Stroke. 40:3493–3498. 2009.PubMed/NCBI View Article : Google Scholar | |
|
Greenberg ED, Gold R, Reichman M, John M, Ivanidze J, Edwards AM, Johnson CE, Comunale JP and Sanelli P: Diagnostic accuracy of CT angiography and CT perfusion for cerebral vasospasm: A meta-analysis. AJNR Am J Neuroradiol. 31:1853–1860. 2010.PubMed/NCBI View Article : Google Scholar | |
|
Wintermark M, Flanders AE, Velthuis B, Meuli R, van Leeuwen M, Goldsher D, Pineda C, Serena J, van der Schaaf I, Waaijer A, et al: Perfusion-CT assessment of infarct core and penumbra: Receiver operating characteristic curve analysis in 130 patients suspected of acute hemispheric stroke. Stroke. 37:979–985. 2006.PubMed/NCBI View Article : Google Scholar | |
|
Baker L, Birnbaum H, Geppert J, Mishol D and Moyneur E: The relationship between technology availability and health care spending. Health Aff (Millwood) Suppl Web Exclusives: W3-537-551, 2003. | |
|
Springborg JB, Frederiksen HJ, Eskesen V and Olsen NV: Trends in monitoring patients with aneurysmal subarachnoid haemorrhage. Br J Anaesth. 94:259–270. 2005.PubMed/NCBI View Article : Google Scholar |