Comparative evaluation of GSH, total protein and albumin levels in patients using smokeless tobacco with oral precancerous and cancerous lesions
- Authors:
- Published online on: February 15, 2024 https://doi.org/10.3892/mi.2024.139
- Article Number: 15
-
Copyright : © Nimbal et al. This is an open access article distributed under the terms of Creative Commons Attribution License [CC BY 4.0].
Abstract
Introduction
Smokeless tobacco (SLT) poses a significant public health concern in the Indian subcontinent, with India being widely regarded as the global epicenter of SLT usage (1). SLT, which does not involve combustion, is commonly employed intranasally or intraorally, predominantly in the form of snuffs or by chewing tobacco leaves (2). Specific varieties of SLT differ across various regions, resulting in a plethora of associated health risks. Presently, a multitude of SLT options are available, featuring diverse flavors and chewing habits, such as betel quid, khaini, mawa, pan masala plain and gutka (3). The prevalence and utilization of tobacco among young adults, specifically those aged ~30 years, are substantial. These demographics account for ~12% of global tobacco-related deaths, including the use of tobacco products and smokeless alternatives (4). Notably, the latter is considered to be a comparatively safe option for continuous cigarette smoking. It is crucial to recognize that tobacco is a significant risk factor for various chronic ailments, such as cancer, respiratory conditions and cardiovascular diseases (5).
The International Agency for Research on Cancer (IARC) has classified SLT as a group 1 carcinogen (6). Notably, tobacco-specific N-nitrosamines, namely 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone, nitrosonornicotine (NNN), N-nitrosoanatabine and N-nitrosoanabasine play a pivotal role in the production of free radicals (6). Concerning their corresponding oxidative stress actions, SLT extract is more harmful and results in oxidative tissue damage and apoptosis. Furthermore, it has been noted that the alkaline conditions present during betel nut chewing promote the generation of free radicals (7). At low or moderate levels, reactive oxygen species (ROS) and reactive nitrogen species (RNS) play crucial roles in the maturation of cellular structures and serve as tools for the host defense system. However, the excessive production of free radicals and oxidants leads to a phenomenon known as oxidative stress, which is a detrimental process capable of significantly modifying cell membranes and various structures, including proteins, lipids, lipoproteins and DNA (8). The administration of SLT at a low dose over a prolonged period of time can trigger oxidative stress, leading to detrimental effects on bodily tissues. These effects may play a role in the toxicity and carcinogenicity associated with the use of SLT (9).
Glutathione (GSH), an ubiquitous tripeptide thiol, is a vital intracellular and extracellular protective antioxidant. The intracellular and whole blood concentrations of GSH are in the millimolar range, whereas the plasma concentration is in the micromolar range accounting for ~0.4% of the total blood GSH levels (10). Of note, GSH is detected at high concentrations (5 mM) in the majority of cells. It plays a crucial role in shielding cellular macromolecules from endogenous and exogenous ROS and RNS. GSH directly scavenges diverse oxidants, such as superoxide anions, hydroxyl radicals, nitric oxide and carbon radicals (11). Studies have indicated that antioxidants exert their safeguarding effects by reducing oxidative DNA impairment and inhibiting the initiation and progression of carcinogenesis (12,13). This notion serves as a significant gauge for evaluating the mechanisms of antioxidant defense in the context of malignancy.
In a blood sample with a pH of 7.4, ~69% of nicotine exists in an ionized state, 31% remains unionized and <5% attaches to plasma proteins (14). The occurrence of hypoalbuminemia in patients with oral cancer may be attributed to the effects of free radical-mediated protein oxidation and the subsequent reduction in the protective antioxidant defense mechanism (15). The levels of oxidized proteins in plasma are noteworthy indicators of oxidative stress originating from free radicals. Protein oxidation plays a crucial role in the pathogenesis of oral cancers. Hyperproteinemia, which manifests as cachexia, is a common occurrence in oral malignancies. Therefore, serum proteins could potentially function as pivotal diagnostic and prognostic markers for oral premalignant diseases and oral malignancies. Researchers have examined the associations between enzymes, proteins and glycoproteins, and have found significant changes in the levels of protein biomarkers in blood serum (16). Additionally, SLT use leads to a significant decrease in serum albumin levels and alterations in liver enzyme levels. This is likely due to the damage and destruction of the liver tissue caused by the components of SLT, which has been proven to activate microsomal enzymes in liver cells (2). Therefore, the aim of the present study was to evaluate and compare the levels of blood GSH, total plasma protein and albumin in SLT users with precancerous and cancerous lesions, compared to non-tobacco users.
Patients and methods
The present cross-sectional observational study was conducted at the Department of Public Health Dentistry, Jawahar Medical Foundation's ACPM Dental College (Dhule, India) between January, 2022 and December, 2022. Approval was obtained from the Institutional Ethics Committee of Jawahar Medical Foundation Annasaheb Chudaman Patil Memorial Dental College Dhule (EC/NEW/INST/2022/2959/Y22/212). Written informed consent was obtained from all participants after the study protocol was explained to them. The present study was conducted in accordance with the principles of the Declaration of Helsinki and followed the Strengthening the Reporting of Observational studies (STROBE) guidelines.
Sample size calculation
The sample size was calculated using G*Power software (latest ver. 3.1.9.7; Heinrich-Heine-Universität Düsseldorf, Düsseldorf, Germany). The standard deviation was taken from a previous study, to detect the effect size of 0.5 mg/dl in of GSH in the oral cancer and control groups (17). The power of the study was 80%, with a 95% confidence interval, and type 1 error of 5%. The sample size was calculated as 60 patients per group.
Selection criteria
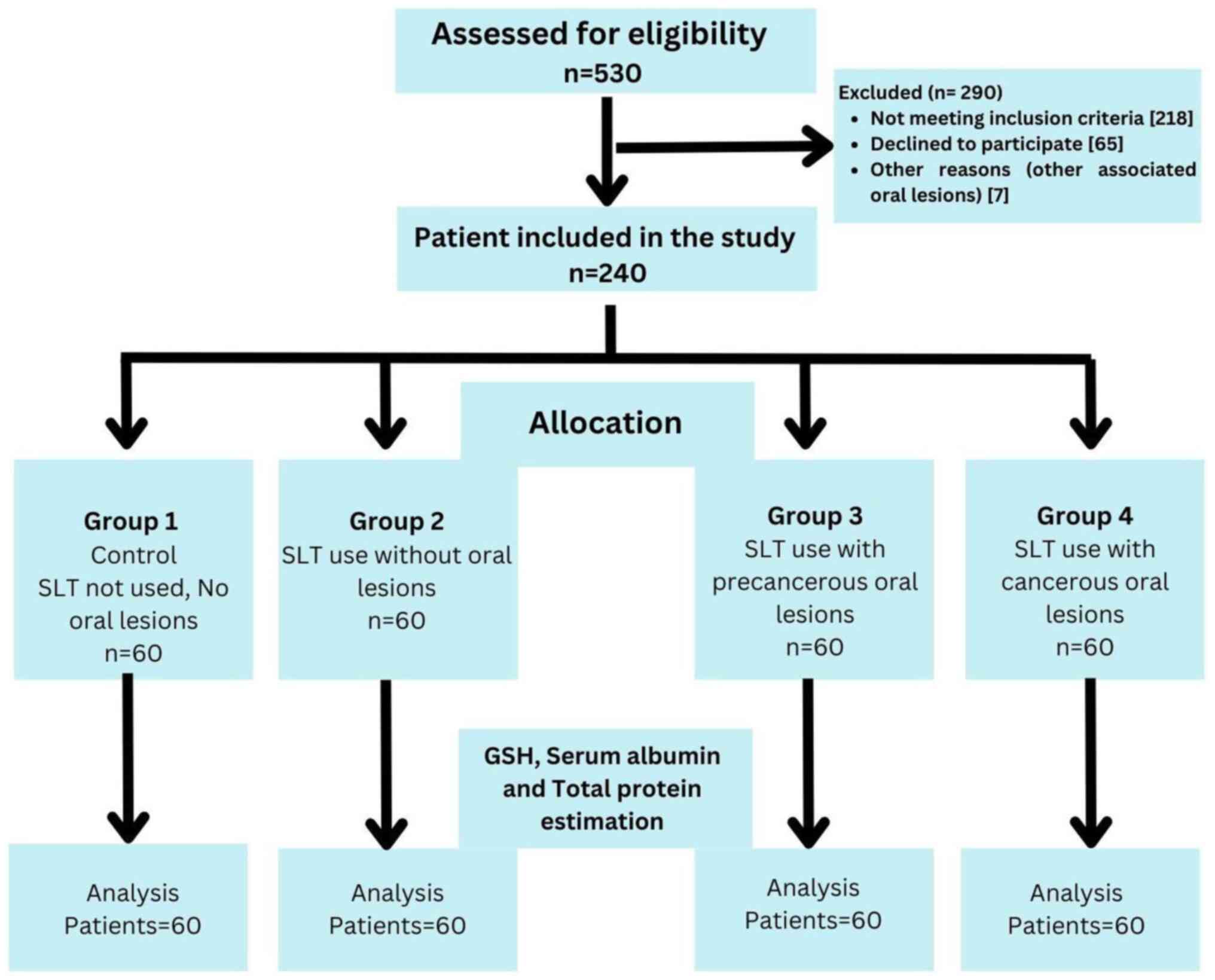
A total of 1,500 patients were screened in cancer awareness camps in the district of Dhule to select a sample of 240 patients aged 30-60 years, based on the selection criteria of the study. In total, 240 patients were divided into four groups as follows: Group 1 (control group) comprised 60 healthy individuals with no history of SLT use; group 2 comprised 60 healthy individuals with at least a 1-year history of SLT use, but without the occurrence of any oral precancerous or cancerous lesions; group 3 comprised 60 clinically and histopathologically confirmed individuals with oral precancerous lesions who had not received any prior treatment with at least a 1-year history of SLT use; and group 4 comprised 60 clinically and histopathologically confirmed individuals with oral squamous cell carcinoma without metastasis, who had not received any prior treatment with at least a year history of SLT use. Individuals >30 years of age who were willing to participate in the study and had provided written informed consent were included in the study. Individuals with any systemic disease, cancer patients undergoing treatment or radiotherapy, pregnant or lactating females, individuals using tobacco smoking, those taking any medications for >3 months, those with a previous history of malignancy or a history of antioxidant medication, and those taking corticosteroids over the past 6 months were excluded from the study. A flow diagram of the study groups and selection process is presented in Fig. 1.
Procedure
The participants were instructed to fast overnight. The following day, between 7 and 9 a.m., a total of 5 ml blood was drawn from the mid-cubital vein with the necessary aseptic precautions in a 5-ml disposable syringe and transferred to sterile tubes containing ethylenediaminetetraacetic acid (EDTA) to prevent coagulation. The blood was centrifuged at 1,007 x g for 7 min and at a temperature of 8 to 12˚C in the centrifuge. The plasma and buffy coat were separated, and plasma was used for the estimation of glutathione, whereas serum was used for the estimation of total protein and albumin. GSH, total protein and albumin levels were estimated by a trained pathologist (the author BA) at the Pathology Laboratory of ACPM Medical College, Dhule, India. Routine blood investigations were performed in all patients.
Estimation of GSH
The GSH levels were estimated using the method described in the study by Beutler et al (18), which is a simple and accurate method for determining GSH levels in blood. This method is based on the development of a relatively stable yellow color produced by the reaction of Ellman's reagent [5,5'-dithiobis-(2-nitrobenzoic acid) or DTNB reagent (MerckMillipore)] with GSH to form TNB chromophore, which has a maximum absorbance at 412 nm. The rate of TNB formation, measured at 412 nm, was proportional to the concentration of GSH in the sample. The rate of change in absorbance (∆A412 nm/min) was linear for the convenience and consistency of the measurement, and was linearly proportional to the total concentration of GSH. The optical density of the solution was measured at 412 nm using a spectrophotometer (Manti Lab Solutions), and the value of GSH was computed as mg/g Hb (18).
Estimation of serum protein levels
The serum protein levels were estimated using the Biuret method (19). Biuret reagent (MerckMillipore) comprised of sodium hydroxide and hydrated copper sulfate, together with potassium sodium tartrate, the latter of which was added to chelate and thus stabilized the cupric ions. The reaction of the cupric ions with the nitrogen atoms involved in peptide bonds led to displacement of the peptide hydrogen atoms under alkaline conditions. A tri- or tetra-dentate chelation with the peptide nitrogen produced a characteristic violet color. The intensity of the color, which had a maximum absorption at 540 nm using a spectrophotometer (Manti Lab Solutions), was proportional to the protein concentration.
Estimation of serum albumin levels
The serum albumin levels were estimated using the spectrophotometric method described by Rodkey (20). In this method, the serum was diluted with a solution of bromocresol green (MerckMillipore) at a sufficient concentration to allow an essentially linear change in absorbance with the albumin concentration. Measurements were made at 615 nm with spectrophotometer (Manti Lab Solutions), where the absorbance of hemoglobin or bilirubin did not interfere.
Statistical analysis
Data were analyzed using SPSS software version 22 (IBM Corp.). The Shapiro-Wilk test was used to assess the normal distribution of the data. As the data were normally distributed, parametric tests, such as one-way analysis of variance (ANOVA), followed by post hoc analysis with Tukey's test, were used to assess the differences between the groups. For ordinal data, the Chi-squared test and Fisher's exact test were used. Regression analysis was used to assess the associations between variables, and receiver operating curves (ROC) were used to evaluate biomarker performance. A value of P≤0.05 was considered to indicate a statistically significant difference.
Results
Descriptive analysis
The descriptive analysis in the present study did not reveal any significant differences in the mean age of the patients in the different the groups (P>0.05), whereas statistically significant differences were observed in the duration of SLT use between groups 2, 3 and 4. The maximum duration of SLT use was 14.15±5.81 years, observed in SLT users who developed oral cancer (group 4), whereas the minimum duration of SLT use was 6.15±2.35 years, observed in SLT users with precancerous oral lesions. All groups had non-significant distributions of males and females, although the habit of SLT use was more prevalent in males than in females. Gutka chewing was the most common form of SLT, followed by betel quid chewing. The frequency of SLT chewing/per day was the highest in group 4, followed by groups 3 and 2. The majority of the participants in group 2 chewed SLT for <5 min (70%), whereas the majority of the participants in group 4 chewed SLT for >5 min (63%), as depicted in Table I. Post hoc analysis revealed that there were statistically notable disparities in the duration of SLT use between groups 2 and 3 in comparison to group 4. Nevertheless, there were no significant differences between groups 2 and 3. Statistically significant disparities were observed among all experimental groups in terms of the frequency of SLT use (Table II).
The most common precancerous lesion in group 3 was leukoplakia (60%), followed by oral submucosal fibrosis (23%) and erythroplakia (17%). The majority of the participants in group 4 had well-differentiated oral squamous cell carcinoma (52%) (Table III). The majority of oral lesions were present on the buccal mucosa, labial mucosa and gingivobuccal corridor.
Hematological analysis
Statistically significant differences were observed in all hematological parameters between the groups. Group 4 exhibited a significant decrease in mean hemoglobin levels, total platelet count and total leukocyte count, followed by groups 3 and 2 compared to the control group. The total red blood cell (RBC) count, erythrocyte sedimentation rate, mean corpuscular hemoglobin and high-sensitivity C-reactive protein (hs-CRP) levels were significantly higher in group 4, followed by groups 3 and 2, compared with the control group (Table IV). Post hoc analysis revealed significant differences between groups 1 and 4 in terms of Hb levels. Moreover, there were significant differences between all groups, except for groups 3 and 4, in terms of the total RBC count. Additionally, significant differences were found between groups 1 and 3, groups 1 and 4, and groups 2 and 4 in terms of total platelet count. Furthermore, there were significant differences between all groups, except groups 2 and 3, as well as groups 2 and 4, in terms of the total leukocyte count. Moreover, significant differences were found between all groups, except for groups 2 and 3, as well as groups 3 and 4, in terms of MCH. Lastly, there were significant differences between all groups, except for Groups 1 and 2, in terms of hs-CRP levels (Table IV).
Table IVComparison of hematological investigations of the study groups using one-way ANOVA followed by post hoc analysis. |
Inferential analysis
Analysis between the groups using ANOVA revealed statistically significant differences in the mean values of GSH, serum protein and albumin levels (P<0.001). The maximum levels of reduced GSH (9.61±0.86 mg/Hb) were observed in healthy individuals with no history of SLT use (group 1), and the minimum levels (2.63±0.43) were found in group 4, as shown in Table V. Post hoc analysis using Tukey's test revealed a statistically significant difference in serum GSH levels between all groups (P<0.001; Table VI). No significant differences were observed in the serum protein levels between groups 1 and 2, groups 1 and 4, and groups 2 and 4, whereas no significant differences were observed in the serum albumin levels between groups 2 and 3, as shown in Table VI.
Results of ROC analysis
ROC analysis was conducted to determine the sensitivity and specificity of GSH in the blood and albumin and total protein levels in the serum at different thresholds. The optimum threshold values of GSH was found to be ≤7.96 for group 2 (Fig. 2A), ≤5.96 for group 3 (Fig. 2D) and ≤3.43 for group 4 (Fig. 2G), and having a sensitivity and specificity of 100%. Albumin had a threshold value of ≤7.9 for group 2 with 70% sensitivity and 80% specificity (Fig. 2B), ≤5.9 for group 3 with 70% sensitivity and 85% specificity (Fig. 2E), and ≤3.5 for group 4 (Fig. 2H), with 100% sensitivity and 100% specificity. The threshold values of total serum protein levels were >7.9 for group 2 (Fig. 2C) at 50% sensitivity and 55% specificity, ≤5.96 for group 3 (Fig. 2F) at 70% sensitivity and specificity of 95%, and ≤5.81, at 15% sensitivity and 100% specificity for group 4 (Fig. 2I). This indicates that GSH and albumin levels are reliable diagnostic biomarkers, whereas the total protein content is a weak biomarker.
Regression analysis
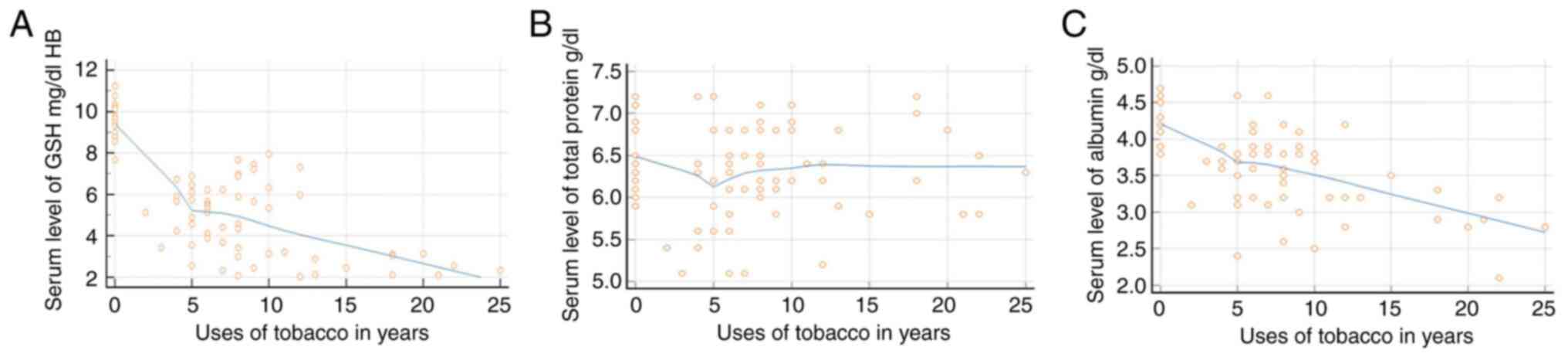
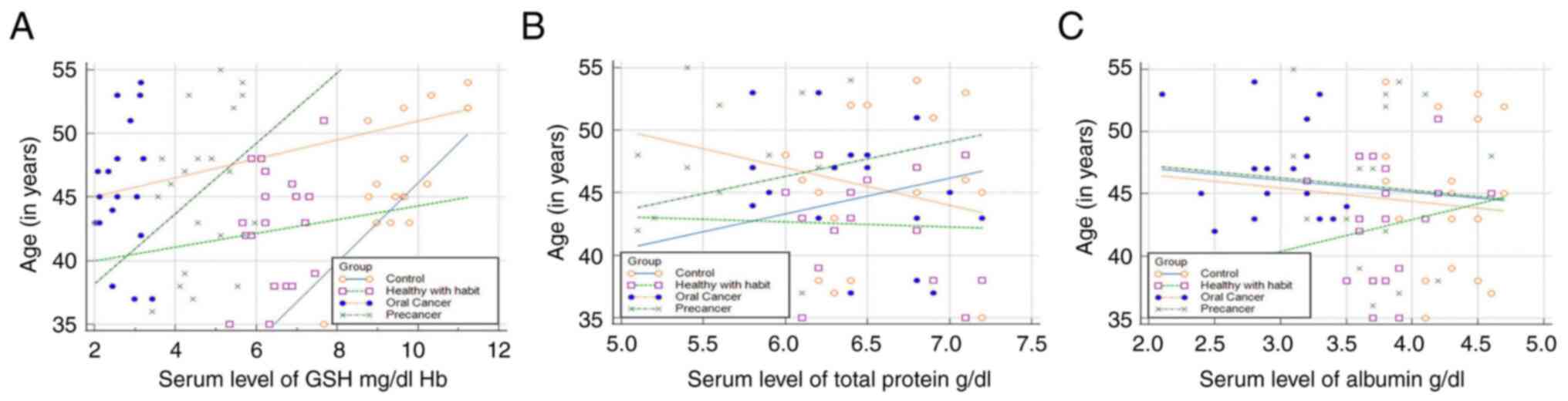
Regression analysis revealed that the GSH levels in the blood and serum albumin levels decreased as the duration of SLT use increased; however, the total protein level only exhibited slight fluctuations in the initial phases of tobacco use (Fig. 3). The GSH levels exhibited an increasing trend with age, indicating that the GSH levels increased in all groups as the age of the patients increased. The total protein level decreased in group 4, whereas groups 3 exhibited an increasing trend with age. The serum albumin levels exhibited an increasing trend with age only in group 2 (Fig. 4).
Discussion
In healthy humans, a state of equilibrium is maintained between oxidants and antioxidants. Nevertheless, in an atypical state, an over-abundance of oxidizing agents is generated, leading to the inhibition of antioxidant defenses. Consequently, this results in a deviation in the ratio favoring pro-oxidants (8). SLT alters the activity of antioxidants in saliva. Oxidative stress can function as a biomarker for the diagnosis and prediction of damage and the abnormal growth of oral tissues. Additionally, it can serve as an early indicator to prevent abnormal changes in the oral cavity (9).
The present study found the increased use of SLT in males aged 45 years, which was in agreement with the findings of previous studies (1,2,4,7). There is substantial evidence to indicate that the enactment of smoke-free laws, the augmentation of smoking taxes and the prevalence of a favorable societal attitude towards SLT during working hours have been positively associated with the rate of SLT consumption among males (4). Substances such as lime and catechu, which are employed in the formulation of SLT commodities, participate in the generation of ROS within the cellular environment (14).
In the present study, GSH levels were higher in non-tobacco users than in tobacco users. Tobacco snuffs can generate free radicals, causing protein nitration, lipid peroxidation and DNA adduct formation. Exposure to SLT leads to the generation of ROS, which are considerably higher in SLT users than in non-tobacco users (12,13). GSH, a vital antioxidant that dissolves in water, is produced through the synthesis of the amino acids, glycine, glutamate and cysteine. GSH exhibits an elevated redox potential and effectively operates as a potent antioxidant (10). It facilitates the detoxification and breakdown of ROS, thereby inhibiting cellular oxidative damage and cancer development.
Herein, GSH levels were found to be decreased in SLT users with precancerous and cancerous lesions. The lowest GSH levels were observed in SLT users with oral cancer. The findings of the present study are consistent with those of previous studies (5,17,21,22). Glutathione consists of a reduced form known as GSH and a form that has been oxidized, referred to as GSH disulfide (GSSG). The ability of GSH to be regenerated is directly related to the redox state of the GSSG-GSH couple (GSSG/2GSH). As a result, GSH provides primary support for intracellular ‘redox homeostasis’ or ‘redox buffering’ capacity (22). When subjected to excessive xenobiotics, including carcinogens, there is an increased utilization of GSH for conjugation, which in turn leads to detoxification. This process results in a decrease in the GSH/GSSG ratio, rendering GSH less available, and consequently reducing the body's defense against free radicals. Notably, the depletion of GSH is adequate to sensitize cancer cells to both oxidative and nitrative stress. This sensitization leads to DNA damage. Consequently, DNA degeneration can occur, which can activate carcinogens and ultimately initiate and progress into cancer. Depletion of GSH may sensitize tumors to chemotherapy and radiotherapy (10,16). ROC analysis revealed GSH to be a reliable biomarker for predicting oral cancer. In the present study, the mean GSH levels decreased with age and increased with the duration of SLT use, which is in agreement with the findings of previous studies (23,24).
Serum proteins have been widely acknowledged for their antioxidant characteristics owing to the presence of unbound thiol groups. Of all the proteins, albumin stands out as the most efficacious and abundant extracellular antioxidant. In the present study, it was found to be a reliable biomarker. The findings of the present study indicated a significant decrease in serum albumin levels in SLT users compared to healthy controls, and it decreased further in patients with precancerous and cancerous lesions. This finding is in accordance with that of previous studies (9,15,25). The effect of free radicals formed by SLT has been demonstrated to induce modifications to DNA bases, causing fractures in the DNA strand, impairing the integrity of tumor suppressor genes and promoting the expression of proto-oncogenes. Additionally, these free radicals can disrupt antioxidant defense mechanisms. Albumin is considered to possess the ability to function as an antioxidant, which may be attributed to the abundant presence of sulfhydryl and free thiol groups within the molecular structure of albumin. Oral cancers are associated with inflammatory mediators, such as IL-6 and TNF, which potentially exert their effects through dual mechanisms, namely, by enhancing the extravasation of albumin across the capillary endothelium at the tumor site and dampening the hepatic production of albumin. A decrease in serum albumin levels can lead to a reduction in the sequestration of unbound reactive molecules, thereby enhancing the probability of deleterious cellular damage that can induce cancer development (26).
In the present study, serum protein levels also decreased with the use of SLT and during the precancerous phase. However, there was no significant difference in serum protein levels between the control group and SLT users with oral cancer. This finding is in agreement with the findings of previous studies (13,27). The decrease in the serum protein concentration can potentially be elucidated in relation to the inflammatory response associated with oral malignancies. The total serum protein level was found to be a weak biomarker in the present study.
The findings of the present study revealed that reduced glutathione and serum albumin are reliable biomarkers for the early diagnosis and the assessment of the prognosis of oral premalignant and malignant lesions, and for planning specific treatment strategies for their prevention and management. These findings are of particular importance for subjects who have the habit of using SLT. They should be encouraged to quit these habits, and awareness campaigns should be organized where these biomarkers can be used efficiently for mass screening.
The altered hematological parameters in SLT users suggest the selective toxicity of SLT and its components. The increase in the RBC count in SLT users observed herein indicated erythropoiesis, which may be due to insufficient pulmonary function with the long-term use of SLT. Elevated hs-CRP levels also suggest the presence of chronic inflammation and an increased risk of cardiovascular disease in SLT users (28).
A limitation of the present study was the evaluation of GSH, albumin and serum proteins in the blood samples of patients, but not in salivary samples. The primary challenge hindering the development of a salivary diagnostic protocol lies in the fact that although numerous biomarkers identified in the blood serum can also be detected in saliva, their concentrations are so minimal that they offer limited value to the diagnostic process. Moreover, saliva collection is highly sensitive and affects the salivary biomarker levels. The present study also did not consider variations in biomarker levels at the different stages of oral squamous cell carcinoma. The present study evaluated GSH levels in the blood samples of patients; however, the estimation of GSH levels in blood and tissue samples of patients could have provided more information on oxidative stress. As GSH levels have been associated with oxidative stress (29), future studies should be conducted to assess ROS, and enzymes such as glutathione-peroxidase, and catalase at the cellular level. The present study measured the total serum protein levels, which were found to be weak biomarkers. Therefore, future prospective studies need to be conducted to evaluate specific proteins in salivary or serum samples from patients at different stages of oral cancer.
In conclusion, the findings of the present study draw a conclusion regarding the impact of oral premalignant and malignant tumors, on the concentration of GSH and proteins in the sera in comparison with individuals without any health issues or tobacco habits, indicating the possibility of utilizing these changes in GSH and protein profiles to diagnose and predict the course of oral cancer.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
AN conceived and designed the study. BA and AV recruited the patients and assessed the variables. PV performed the data analysis. ABW and AAP assessed the laboratory data and drafted the manuscript. All authors have reviewed the manuscript, and all authors have read and approved the final manuscript. AV and PV confirm the authenticity of all the raw data.
Ethical approval and consent to participate
Ethical approval for the study was obtained from the Institutional Ethical Committee at Jawahar Medical Foundation's Annasaheb Chudaman Patil Memorial Dental College Dhule (EC/NEW/INST/2022/2959/Y22/212) and the study was conducted in accordance with the ethical principles outlined in the Declaration of Helsinki. Written informed consent was obtained from all participants after the study protocol was explained to them.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
|
Acharya S, Singh S and Bhatia SK: Association between smokeless tobacco and risk of malignant and premalignant conditions of oral cavity: A systematic review of Indian literature. J Oral MaxillofacPathol. 25(371)2021.PubMed/NCBI View Article : Google Scholar | |
|
Diaz MC, Kierstead EC, Edwards D, Kim Y, Rose SW, Emery S, Khatib B, Liu M and Kostygina G: Online tobacco advertising and current chew, dip, snuff and snus use among youth and young adults, 2018-2019. Int J Environ Res Public Health. 19(4786)2022.PubMed/NCBI View Article : Google Scholar | |
|
Niaz K, Maqbool F, Khan F, Bahadar H, Hassan FI and Abdollahi M: Smokeless tobacco (paan and gutkha) consumption, prevalence, and contribution to oral cancer. Epidemiol Health. 9(e2017009)2017.PubMed/NCBI View Article : Google Scholar | |
|
Gupta PC and Ray CS: Smokeless tobacco and health in India and south Asia. Respirology. 8:419–431. 2003.PubMed/NCBI View Article : Google Scholar | |
|
Garg S, Pandeshwar P and Padmashree S: Estimation of serum superoxide dismutase and glutathione peroxidase levels in tobacco chewers and smokers: A comparative study. J Oral Med Oral Surg Oral Pathol Oral Radiol. 4:147–154. 2018. | |
|
IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Smokeless tobacco and some tobacco-specific N-nitrosamines. IARC Monogr Eval Carcinog Risks Hum. 89:1–592. 2007.PubMed/NCBI | |
|
Tilashalski K, Rodu B and Cole P: A pilot study of smokeless tobacco in smoking cessation. Am J Med. 104:456–458. 1998.PubMed/NCBI View Article : Google Scholar | |
|
Young I and Woodside J: Antioxidants in health and disease. J Clin Pathol. 54:176–186. 2001.PubMed/NCBI View Article : Google Scholar | |
|
Bagchi M, Bagchi D, Hassoun EA and Stohs SJ: Subchronic effects of smokeless tobacco extract (STE) on hepatic lipid peroxidation, DNA damage and excretion of urinary metabolites in rats. Toxicology. 127:29–38. 1998.PubMed/NCBI View Article : Google Scholar | |
|
Zitka O, Skalickova S, Gumulec J, Masarik M, Adam V, Hubalek J, Trnkova L, Kruseova J, Eckschlager T and Kizek R: Redox status expressed as GSH:GSSG ratio as a marker for oxidative stress in paediatric tumour patients. Oncol Lett. 4:1247–1253. 2012.PubMed/NCBI View Article : Google Scholar | |
|
Pizzorno J: Glutathione! Integr Med (Encinitas). 13:8–12. 2014.PubMed/NCBI | |
|
Lobo V, Patil A, Phatak A and Chandra N: Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn Rev. 4:118–126. 2010.PubMed/NCBI View Article : Google Scholar | |
|
Gupta N, Verma K, Nalla S, Kulshreshtha A, Lall R and Prasad S: Free radicals as a double-edged sword: The cancer preventive and therapeutic roles of curcumin. Molecules. 25(5390)2020.PubMed/NCBI View Article : Google Scholar | |
|
Benowitz NL: Smokeless tobacco as a nicotine delivery device: Harm or harm reduction? Clin Pharmacol Ther. 90:491–493. 2011.PubMed/NCBI View Article : Google Scholar | |
|
Kosova F, Cetin B, Akinci M, Aslan S, Ari Z, Sepici A, Altan N and Cetin A: Advanced oxidation protein products, ferrous oxidation in xylenol orange, and malondialdehyde levels in thyroid cancer. Ann Surg Oncol. 14:2616–2620. 2007.PubMed/NCBI View Article : Google Scholar | |
|
Patidar KA, Parwani RN and Wanjari SP: Correlation of salivary and serum IgG, IgA levels with total protein in oral submucous fibrosis. J Oral Sci. 53:97–102. 2011.PubMed/NCBI View Article : Google Scholar | |
|
Rasool M, Khan SR, Malik A, Khan KM, Zahid S, Manan A, Qazi MH and Naseer MI: Comparative studies of salivary and blood sialic acid, lipid peroxidation and antioxidative status in oral squamous cell carcinoma (OSCC). Pak J Med Sci. 30:466–471. 2014.PubMed/NCBI View Article : Google Scholar | |
|
Beutler E, Duron O and Kelly BM: Improved method for the determination of blood glutathione. J Lab Clin Med. 61:882–888. 1963.PubMed/NCBI | |
|
Wokes F and Still BM: The estimation of protein by the biuret and Greenberg methods. Biochem J. 36:797–806. 1942.PubMed/NCBI View Article : Google Scholar | |
|
Rodkey FL: Direct spectrophotometric determination of albumin in human serum. Clin Chem. 11:478–487. 1965.PubMed/NCBI | |
|
Sharma M, Rajappa M, Kumar G and Sharma A: Oxidant-antioxidant status in Indian patients with carcinoma of posterior one-third of tongue. Cancer Biomark. 5:253–260. 2009.PubMed/NCBI View Article : Google Scholar | |
|
Mohideen K, Sudhakar U, Jeddy N, Sankari SL, Radhika T and Vani N: Assessment of the anti-oxidant reduced glutathione in oral squamous cell carcinoma-Systematic review and meta-analysis. J Oral Maxillofac Pathol. 26(592)2022.PubMed/NCBI View Article : Google Scholar | |
|
Zhu Y, Carvey PM and Ling Z: Age-related changes in glutathione and glutathione-related enzymes in rat brain. Brain Res. 1090:35–44. 2006.PubMed/NCBI View Article : Google Scholar | |
|
Begum SF, Nagajothi G, Latha KS, Sandeep G, Sreekanth B, Kumar CS, Rajendra W and Maddu N: Possible role of nicotine and cotinine on nitroxidative stress and antioxidant content in saliva of smokeless tobacco consumers. Pract Lab Med. 12(e00105)2018.PubMed/NCBI View Article : Google Scholar | |
|
Metgud R and Patel S: Serum and salivary levels of albumin as diagnostic tools for oral pre-malignancy and oral malignancy. Biotech Histochem. 89:8–13. 2014.PubMed/NCBI View Article : Google Scholar | |
|
Mohanty V, Subbannayya Y, Patil S, Abdulla R, Ganesh MS, Pal A, Ray JG, Sidransky D, Gowda H, Prasad TSK and Chatterjee A: Molecular alterations in oral cancer between tobacco chewers and smokers using serum proteomics. Cancer Biomark. 31:361–373. 2021.PubMed/NCBI View Article : Google Scholar | |
|
More CB, Shah PH and Venkatesh R: Estimation of serum protein in oral potentially malignant disorders and oral malignancy-A cross-sectional study. J Clin Diagn Res. 11:ZC17–ZC19. 2017.PubMed/NCBI View Article : Google Scholar | |
|
Shukla AK, Khaitan T, Gupta P and Naik SR: Smokeless tobacco and its adverse effects on hematological parameters: A cross-sectional study. Adv Prev Med. 2019(3182946)2019.PubMed/NCBI View Article : Google Scholar | |
|
Cheng SB, Liu HT, Chen SY, Lin PT, Lai CY and Huang YC: Changes of oxidative stress, glutathione, and its dependent antioxidant enzyme activities in patients with hepatocellular carcinoma before and after tumor resection. PLoS One. 12(e0170016)2017.PubMed/NCBI View Article : Google Scholar |