Infection‑associated bile acid disturbance contributes to macrophage activation in patients with cirrhosis
- Authors:
- Published online on: June 28, 2024 https://doi.org/10.3892/mmr.2024.13274
- Article Number: 150
-
Copyright: © Su et al. This is an open access article distributed under the terms of Creative Commons Attribution License.
Abstract
Introduction
Cirrhosis causes ~1.2 million deaths each year, accounting for 3.5% of all deaths worldwide (1,2). Cirrhosis is the end-stage of various chronic liver diseases, such as non-alcoholic fatty liver disease, alcoholic liver disease, viral hepatitis, autoimmune diseases and biliary disease (2). Regardless of the cause of cirrhosis, infection is its most common complication, occurring in up to 35% of patients with cirrhosis (1). Spontaneous bacterial peritonitis (SBP) is one of the most common types of infections in patients with cirrhosis, followed by lung infection, urinary tract infections, bacteraemia, and skin and soft tissue infections (3,4). Infections lead to decompensation, such as variceal haemorrhage and hepatorenal syndrome, which can increase the risk of acute chronic liver failure (3–5). Studies have shown that the mortality rate of patients with cirrhosis and infection is four times that of uninfected patients, accounting for 50% of fatalities in patients with cirrhosis (1,4).
Patients with cirrhosis have an increased susceptibility to infection due to intestinal microbiome disturbance and immunodeficiency syndrome (6,7). Therefore, the translocation of intestinal bacteria is the main pathogenesis of SBP and bacteraemia, during which the immune system may be activated by lipopolysaccharides (LPS), which are also termed endotoxins, that are released from the leaky gut (3). Immune dysfunction is a common symptom in patients with cirrhosis and is known as cirrhosis-associated immune dysfunction (CAID) (7). CAID causes innate and acquired immune disorders by affecting the liver and systemic immune functions, including the function of various immune cells such as neutrophils, macrophages, B lymphocytes and T lymphocytes (6,7). Macrophages, derived from monocytes, are the first line of defence against pathogens, and are divided into two subgroups based on differences in their functional and transcriptional characteristics. Namely, M1 macrophages induce a proinflammatory response, and M2 macrophages induce an anti-inflammatory response (8). In the early stages of infection, LPS drives macrophages towards the M1 phenotype (8). M1 macrophages engulf bacteria and present antigens to T cells, which secrete a variety of inflammatory factors, including interleukin (IL-)1β, IL-6, tumour necrosis factor α (TNFα), and C-X-C motif chemokine ligand 9, thereby activating an immune response (9). M1 activation is dependent on the transcriptional activity of nuclear factor κB (NF-κB) (9). In contrast to M1 macrophages, M2 macrophages suppress inflammatory responses, clear debris and apoptotic cells, and facilitate tissue repair and wound healing (8). In patients with cirrhosis, impairment of the ability of macrophages to recognise and eliminate harmful microbiota is a key factor that determines the susceptibility of patients to infection (1,3,10).
Bile acids are synthesised in the liver via cytochrome P450 (CYP450)-mediated cholesterol oxidation (11). Primary bile acids, namely cholic acid (CA) and chenodeoxycholic acid (CDCA), and secondary bile acids, namely deoxycholic acid (DCA) and lithocholic acid (LCA), are the major constituents of bile acids in humans (11). Owing to abnormalities in bile acid synthesis, metabolism and excretion, the bile acid profile of patients with cirrhosis is significantly different compared with the bile acid profile of the healthy population (12,13). Among the various types of bile acid, CDCA inhibits LPS-induced IL-6 expression in RAW264.7 cells, a widely used murine macrophage cell line (14), while DCA increases NLR family pyrin domain containing 3 (NLRP3) and IL-1β expression in primary mouse macrophages and RAW264.7 cells (15). These findings suggest that an abnormal bile acid profile may contribute to impaired macrophage function in patients with cirrhosis. Although an abnormal bile acid profile in patients with cirrhosis has been reported previously, the effect of infection on the bile acid profile in these patients and how bile acid disturbance by infection modulates macrophage function remains unclear. In the present study, the changes in the bile acid profile of cirrhotic patients with infection and the association between changes in bile acids and infection were explored. In addition, the effects of bile acids on macrophage function in vitro were investigated.
Materials and methods
Study population and design
A total of 57 patients with cirrhosis (without infection, n=18; with infection, n=39) attending the Departments of Gastroenterology and Infection of The First Affiliated Hospital of Anhui Medical University (Hefei, China) from June 2020 to October 2020 were selected for the present study. A further 20 healthy individuals who underwent physical examination at The First Affiliated Hospital of Anhui Medical University during the same period were included in the control group. The study was approved by The Ethics Committee of the First Affiliated Hospital of Anhui Medical University (Hefei, China; approval no. PJ2020-13-07) and was conducted in accordance with the principles of the Declaration of Helsinki (2008). All patients provided written informed consent before enrolment in the study. Biochemical index data were extracted from the medical records of patients. To analyse the serum bile acid profile of patients, 200 µl discarded serum samples from liver function tests were collected.
Patients with cirrhosis were identified by liver biopsy, transient elastography, or a combination of clinical signs and various findings provided by laboratory tests, endoscopy and radiological imaging (16).
The inclusion criteria for cirrhosis with infection included one of the following signs, symptoms or abnormal laboratory test results (17–19): i) Acute peritonitis; ii) manifestations of systemic inflammatory response syndrome; iii) deterioration of liver function without an obvious cause; iv) hepatic encephalopathy; v) shock; vi) intractable ascites or unresponsiveness to diuretics or renal failure; vii) acute gastrointestinal bleeding; and viii) abnormal laboratory test results, namely an ascites polymorphonuclear neutrophil count ≥0.25×109/l, a positive ascites bacterial culture or procalcitonin levels >0.5 ng/ml, excluding other sites of infection.
The exclusion criteria were as follows: i) Patients with hepatocellular carcinoma; ii) patients with recent severe trauma, surgery or other serious infections caused by liver disease; iii) patients aged <20 or >60 years; iv) incomplete case histories; v) recent severe trauma, surgery or other severe infections due to liver disease; vi) autoimmune diseases of the liver (such as primary biliary cirrhosis, autoimmune hepatitis and primary sclerosing cholangitis); and vii) severe extrahepatic disease (such as decompensated cardiac insufficiency, severe obstructive pulmonary disease and psychiatric disease).
Reagents and chemicals
LPS (cat. no. L2630), DCA (cat. no. D2510), hyodeoxycholic acid (HDCA; cat. no. H3878), phorbol 12-myristate 13-acetate (PMA; cat. no. P1585) and FITC-dextran (cat. no. FD40S) were obtained from Sigma-Aldrich. High-glucose Dulbecco's Modified Eagle's Medium (DMEM; cat. no. 10313039), Roswell Park Memorial Institute (RPMI) 1640 medium (cat. no. 11875085), foetal bovine serum (cat. no. 10270106) and penicillin-streptomycin (cat. no. 15140122) were purchased from Gibco (Thermo Fisher Scientific, Inc.). Bovine serum albumin (BSA; cat. no. 4240) was purchased from BioFroxx (neoFroxx GmbH). The following antibodies were used in the present study: anti-iNOS antibody (1:1,000; cat. no. AF0199; Affinity Biosciences), anti-Arg-1 antibody (1:1,000; cat. no. 16001-1-AP; Proteintech Group, Inc.), anti-p65 antibody (1:1,000; cat. no. 380172; Chengdu Zen-Bioscience Co., Ltd.), anti-IκBα (1:750; cat. no. WL01936; Wanleibio Co., Ltd.), anti-phospho-IκBα (1:750; cat. no. WL02495; Wanleibio Co., Ltd.) and anti-histone H3 (1:1,000; cat. no. WL0984a; Wanleibio Co., Ltd.). TRIzol® reagent (cat. no. 15596026) was procured from Invitrogen (Thermo Fisher Scientific, Inc.). SYBR Green premix (cat. no. AG11701) and Evo M-MLV RT premix (cat. no. AG11706) were purchased from Hunan Accurate Bio-Medical Co., Ltd.). Finally, APC anti-mouse CD86 (1:150; cat. no. 105012), PE anti-mouse CD163 (1:50; cat. no. 155308), APC anti-human CD86 (1:150; cat. no. 374207) and PE anti-human CD163 (1:50; cat. no. 333605) were purchased from BioLegend, Inc. The antibody dilution ratios specified in this section were used for Western blot analysis.
Detection of the bile acid profile
The bile acid profile was determined as described previously (13). Briefly, 100 µl of the serum was diluted with 500 µl 0.01% formic acid, with dehydrocholic acid used as an internal standard. The diluted samples were added to Oasis-HLB cartridges and washed with 1 ml water, and then eluted in 1.5 ml methanol elution. The eluate was evaporated and redissolved in methanol (100 µl). The analysis was performed using a high-performance liquid chromatography (HPLC) system (Shimadzu Corporation) coupled with a triple time-of-flight (TOF) 5600 mass spectrometer (HPLC-QTOF-MS; Shanghai AB SCIEX Analytical Instrument Trading Co., Ltd.). All chromatographic separations were performed with a ZOEBAX Eclipse Plus C18 column (2.1×150 mm; 3.5 µm; Agilent Technologies, Inc.). The column was maintained at 40°C and the injection volume of all samples was 5 µl. The mobile phase consisted of 2.6 mmol/l ammonium acetate in water adjusted to pH 6.8 using ammonium hydroxide (mobile phase A) and acetonitrile (mobile phase B). The flow rate was 0.2 ml/min with the following mobile phase gradient: 0–5 min (20% B), 5–10 min (20–25% B), 10–20 min (25% B), 20–25 min (25–55% B), 25–29 min (55% B), 29–31 min (55–20% B) and 31–34 min (20% B). The mass spectrometer was operated with an atmospheric pressure chemical ionization source in the negative ion mode. The detection range was m/z 300–800. The following optimized condition was used: spray voltage, 5,500 V; nebulizer gas (gas 1), 50 psi; heater gas (gas 2), 50 psi; curtain gas, 30 psi; capillary temperature, 400°C; declustering potential, 70 V; and collision energy, 40eV. Nitrogen was used as nebulizer and auxiliary gas. The data were collected and analyzed with PeakView software 1.2 (Shanghai AB SCIEX Analytical Instrument Trading Co.) and MultiQuant software 2.1.1 (Shanghai AB SCIEX Analytical Instrument Trading Co.).
Cell culture
The RAW264.7 macrophage cell line, purchased from Wuhan Pronoxel, was maintained in DMEM supplemented with 10% foetal bovine serum and 1% penicillin-streptomycin in an incubator at 37°C and 5% CO2. The human acute monocytic leukaemia cell line THP-1 was provided by Cell Bank/Stem Cell Bank, Chinese Academy of Sciences, and cultured in RPMI-1640 medium with 10% foetal bovine serum, 100 U/ml streptomycin and 100 U/ml penicillin in an incubator at 37°C and 5% CO2. Differentiation of THP-1 monocytes into macrophages was induced by exposure to 100 ng/ml PMA for 48 h at 37°C. The medium containing PMA was aspirated and the differentiated THP-1 macrophages were washed with phosphate-buffered saline (PBS). RAW264.7 and differentiated THP-1 cells were stimulated with DCA, HDCA and LPS alone or in combination for 4 h at 37°C. The stimulatory LPS concentration was 100 ng/ml. Based on the cell viability assay results, the DCA concentration was 200 µM for RAW264.7 cells and 100 µM for THP-1 cells, and the HDCA concentration was 100 µM for both cell lines. The CCK-8 kit (cat. no. K101815133EF5E) was purchased from APeXBIO Technology LLC, and cells were incubated with the CCK-8 reagent for 2 h at 37°C.
Western blotting
Western blotting was performed as described previously (15). RAW264.7 and THP-1 cells were collected, lysed with RIPA Lysis Buffer (cat. no. P0013C; Beyotime Institute of Biotechnology) containing PMSF (cat. no. ST507; Beyotime Institute of Biotechnology) and phosphatase inhibitors (cat. no. P1081; Beyotime Institute of Biotechnology), and centrifuged at 13,000 × g for 20 min at 4°C. The protein concentration in the supernatant was measured using a BCA protein assay kit (cat. no. P0010S; Beyotime Institute of Biotechnology). Equal amounts of protein (30 µg/lane) were separated by sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE, 8 or 12% gel concentration for different molecular weight proteins) and transferred to polyvinylidene difluoride membranes. After incubation in 5% non-fat milk in Tris-buffered saline with 0.05% Tween-20 (TBST) for 1 h at room temperature (to block non-specific binding), the membranes were incubated overnight at 4°C with the appropriate primary antibody. The following day, the membranes were washed three times with TBST and incubated with the respective secondary antibody (1:10,000; HRP-labelled Goat Anti-Rabbit IgG, cat. no. A0208; or HRP-labelled Goat Anti-Mouse IgG, cat. no. A0216; Beyotime Institute of Biotechnology) for 1 h at room temperature. Protein bands were captured using a Bioshine ChemiQ 4600 Mini Chemiluminescence Imaging System (Bioshine Co., Ltd.). The optical density of each band was quantitated using ImageJ software 1.45S (National Institutes of Health) and normalised to the intensity of β-actin.
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted from cultured RAW264.7 or THP-1 cells with TRIzol® reagent (cat. no. 10296010; Thermo Fisher Scientific, Inc.) and reverse transcribed into cDNA using Evo M-MLV Reagent Premix (cat. no. AG11706; Hunan Accurate Bio-Medical Co., Ltd.), under the following conditions: 37°C for 15 min, followed by a brief incubation at 85°C for 5 sec, and then incubated at 4°C. The mRNA expression levels of Tnfα, Il6, Il1b and Il10 were determined using RT-qPCR. The primer sequences (Sangon Biotech Co., Ltd.) are shown in Table SI. Actb and Gapdh were used as internal references for the mouse and human cell lines, respectively, and a total of three biological replicates were used for each sample. RT-qPCR was conducted using the SYBR Green Premix (cat. no. AG11701; Accurate Bio-Medical Co., Ltd.) on a Fluorescence Quantitative PCR Instrument (cat. no. LC480; Roche Diagnostics). The PCR thermal cycling parameters were as follows: 95°C for 30 sec; 40 cycles of 95°C for 5 sec and 60°C for 30 sec. Gene expression was normalised to that of the internal controls and quantified relative to its expression in cells using the 2−ΔΔCq method (20).
Flow cytometry
Each well of a 24-well plate was inoculated with RAW264.7 or THP-1, and cultured for 24 h at 37°C. After which, 1×106 macrophages were treated with 1.3 µg/ml APC anti-mouse CD86 and 4 µg/ml PE anti-mouse CD163 antibodies. After incubation in the dark at 4°C for 30–45 min, 200 µl PBS was added and the samples was analysed with flow cytometry. FITC-dextran was added to the cells and incubated at 37°C for 1.5 h; a blank was also prepared. Cells incubated at 4°C were used as controls to normalise the phagocytic capacity of macrophages. After washing the cells three times with PBS containing 0.25% BSA, 200 µl PBS was added, and the assay was performed on a flow cytometer (CytoFLEX; Beckman Coulter, Inc.). Analysis was performed using CytExpert software 2.4 (Beckman Coulter, Inc.). The gating strategy for flow cytometry is described in Fig. S1 and Fig. S2.
Immunofluorescence
RAW264.7 or THP-1 cell were stimulated with DCA, HDCA or LPS for 4 h at 37°C. The stimulated concentrations of DCA, HDCA, and LPS were consistent with those described in the Cell culture section. After washing three times with PBS, the cells were fixed in 4% paraformaldehyde for 30 min at room temperature. After which, the cells were washed once with PBS and permeabilised with a 0.5% Triton X-100 solution for 15 min at room temperature. The cells were incubated with a solution containing 0.25% BSA for 1 h at room temperature. After which, the cells were incubated with the primary antibody, anti-p65 (1:50), overnight at 4°C. The following day, the cells were washed and incubated with the diluted fluorescent secondary antibody (1:500; Cy3 labelled goat anti-rabbit IgG; cat. no. A0516; Beyotime Institute of Biotechnology) for 1 h at room temperature in the dark. After washing three times with PBS, the cells were incubated with 4′,6-diamidino-2-phenylindole for 10 min in the dark to stain the nuclei at room temperature. Images were obtained using a CX23 fluorescence microscope (Olympus Corporation), and analysis was ImageJ software 1.45S.
Wound healing assay
A sterile pipette tip was used to scratch a straight line across the macrophage monolayer. After washing away excess cells with PBS, the medium containing 10% foetal bovine serum was replaced with serum-free medium, and images of the scratch wound were taken with a CX23 fluorescence microscope (Olympus Corporation) at 0 and 24 h. Quantification was performed by evaluating the area of the scratch blank area after 24 h using ImageJ 1.45S.
Transwell assay
Transwell plates with 8-µm membrane pores were used for the cell migration experiments. For each Transwell plate, RAW264.7 or THP-1 cells were seeded in the upper chambers, with 200 µl cell suspension (1×105 cells) in serum-free medium plated. The lower chambers were filled with 500 µl complete medium with 10% foetal bovine serum, supplemented with DCA, HDCA or LPS. Incubation was at 37°C for 24 h. Fixation was achieved by adding 500 µl 4% paraformaldehyde to the lower chamber for 30 min at room temperature, followed by staining with 500 µl crystal violet for 15 min at room temperature. Images were captured with a CX23 fluorescence microscope (Olympus Corporation).
Statistical analysis
The data are presented as the mean ± standard error of the mean. Differences between the groups were analysed using one-way analysis of variance followed by Tukey's test for normally distributed data and the Kruskal-Wallis test for non-normally distributed data. The chi-square test was used to compare the differences between groups for the categorical data. Each experiment was repeated three times. P<0.05 was considered to indicate a statistically significant difference. Data analysis was conducted using SPSS 25.0 (IBM Corp.). Variable importance projection (VIP) analysis was performed using SIMCA 14.1 software (Umetrics). Receiver operating characteristic (ROC) curves were analysed using MedCalc 19.6 software (MedCalc Software Ltd.). Principal component analysis (PCA) and heatmap analyses were conducted using the MetaboAnalyst 5.0 online platform (www.metaboanalyst.ca).
Results
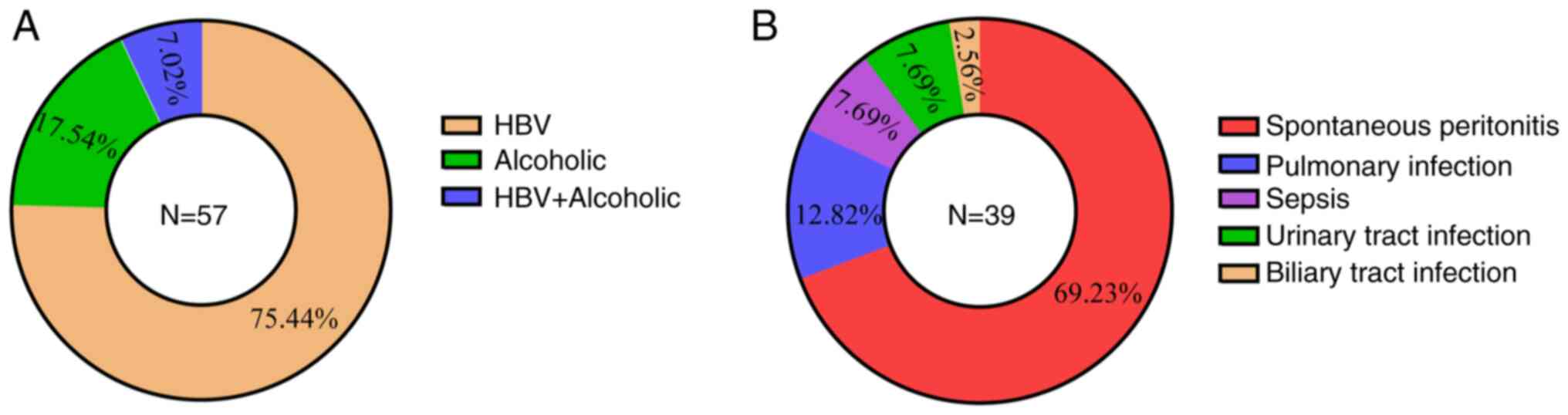
Demographic characteristics of the patients
A total of 20 healthy individuals, 18 patients with cirrhosis but without infection and 39 patients with cirrhosis and infection were included in the present study. The causes of cirrhosis and secondary infections are presented in Fig. 1. The demographic and routine biochemical parameters of the recruited subjects were recorded and age matched among the three groups (Table I). There were more men in the sample, consistent with the fact that the incidence of cirrhosis is higher in men (21). In addition, infected patients with cirrhosis exhibited a higher proportion of Child-Pugh C classification compared with uninfected patients (Table I), indicating a worse prognosis compared with those with Child-Pugh B (22). Compared with healthy subjects, patients with cirrhosis had significant abnormalities in liver functions and total bile acid (TBA) levels, which was in line with previous studies (12,13). There were lower white blood cell (WBC) and platelet counts in patients with cirrhosis but without infection compared with healthy subjects due to hypersplenism in patients with cirrhosis, resulting in increased destruction of WBCs and platelets, a common clinical manifestation in cirrhosis (23). Compared with the patients with cirrhosis but without infection, patients with cirrhosis and infection appeared to have higher serum aspartate aminotransferase (AST), γ-glutamyltrasferase (ALP) and bilirubin levels, and lower haemoglobin levels (Table I). However, there was no statistically significant difference in AST and ALP levels between patients with cirrhosis and those with cirrhosis and infection. This suggests a deteriorating physical state and impaired liver function in patients with cirrhosis and infection. Indicators such as WBCs, and the percentage of neutrophils and inflammatory factors were used to characterise the infection status (Table I and Fig. S3). Notably, serum levels of TBA appeared to be higher in patients with cirrhosis and infection than in those without infection, although the difference was not statistically significant. The TBA levels increased progressively in all three groups of patients (Table I), which suggests that the progression of patients with cirrhosis and infection is accompanied by abnormalities in bile acid metabolism.
Abnormal bile acid profile in patients with cirrhosis and infection
Next, how the detailed bile acid profile was changed by infection in patients with cirrhosis was investigated, and the differences in the bile acid profile among the three groups of patients was examined. PCA showed a significant separation of the three groups of patients, and the comparison between each of the two groups using orthogonal partial least squares discriminant analysis (OPLS-DA) further supported this conclusion (Fig. 2A and Table SII). Although the Q2 for OPLS-DA between cirrhotic patients and cirrhotic patients with infection was only 0.207 (P<0.05), however this indicated a difference in the bile acid profile between the two groups, which was further shown in the heatmap analyses (Fig. 2B). As shown in Fig. 2B and Table II, there were significant changes in the bile acid profile between healthy subjects and patients with cirrhosis. Specifically, there were alterations in DCA, glycoursodeoxycholic acid (GUDCA), glycocholic acid (GCA) and taurochenodeoxycholic acid (TCDCA), which was consistent with previous studies (12,13). Furthermore, the bile acid profile was different between patients with cirrhosis and patients with cirrhosis and infection. Compared with patients with cirrhosis without infection, some bile acid species tended to change although without a statistical significance; in particular, HDCA and DCA were consistently decreased across the healthy population, patients with cirrhosis and patients with cirrhosis and infection, although DCA levels were not statistically different between patients with cirrhosis and patients with cirrhosis and infection. Glycochenodeoxycholic acid, taurocholic acid (TCA), and TCDCA were consistently elevated among the three groups, although there was no statistical difference in these levels between the patients with cirrhosis and patients with cirrhosis and infection.
Reduction in HDCA and DCA associated with infection
The altered bile acids were screened based on the VIP scores, with VIP >1 and P<0.05 as the screening conditions (24). The bile acid species that were different between healthy subjects and patients with cirrhosis were glycingoose DCA (GCDCA), TCDCA, TCA, GCA, taurine ursodeoxycholic acid (TUDCA) and GUDCA. In addition, the ROC curves suggested that HDCA, DCA, GCDCA, TCDCA and taurine DCA (TDCA) could be well distinguished between healthy subjects and patients with cirrhosis. The area under the ROC curve (AUC) for each aforementioned bile acid was 0.731, 0.819, 0.942, 0.967 and 0.751, respectively (Fig. 3A). HDCA, TDCA, DCA, TCDCA and GCDCA differed between patients with cirrhosis and infection, and healthy subjects. The AUC was 0.946, 0.974, 0.908, 0.983 and 0.969, respectively (Fig. 3B). The VIP scores also showed that HDCA, TCDCA, TDCA, DCA, GCDCA and TCA were different between patients with cirrhosis, with or without infection. After which, the association between disturbed bile acids and infection was assessed based on ROC curves. The AUC for HDCA, DCA, GCDCA, TCDCA and TDCA was 0.769, 0.583, 0.618, 0.630 and 0.641, respectively (Fig. 3C). Considering the effect of unconjugated bile acids on immune function (12,15), it was hypothesized that the decrease in HDCA and DCA may be closely related to infection in patients with cirrhosis.
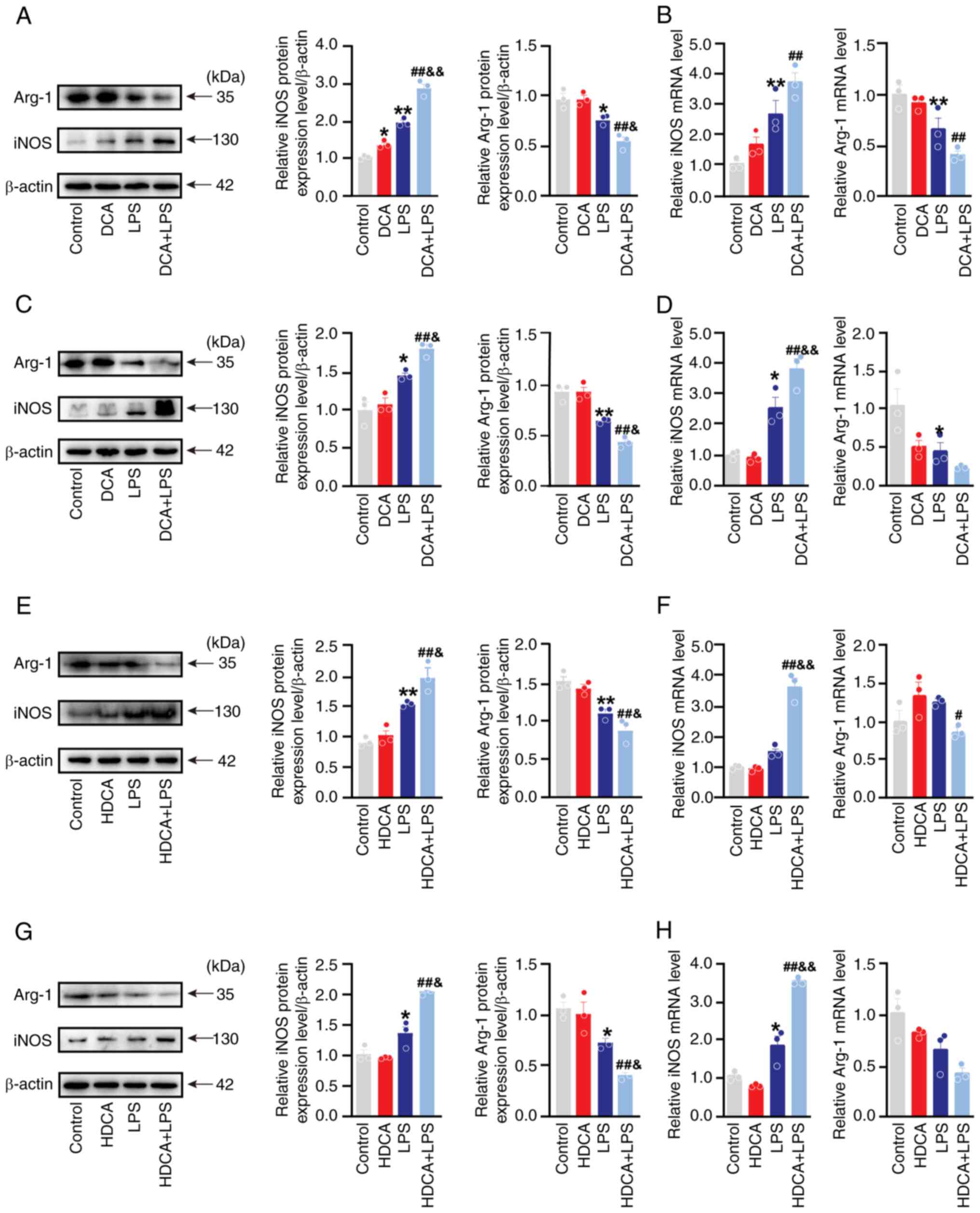
DCA and HDCA increase LPS-induced M1 polarisation
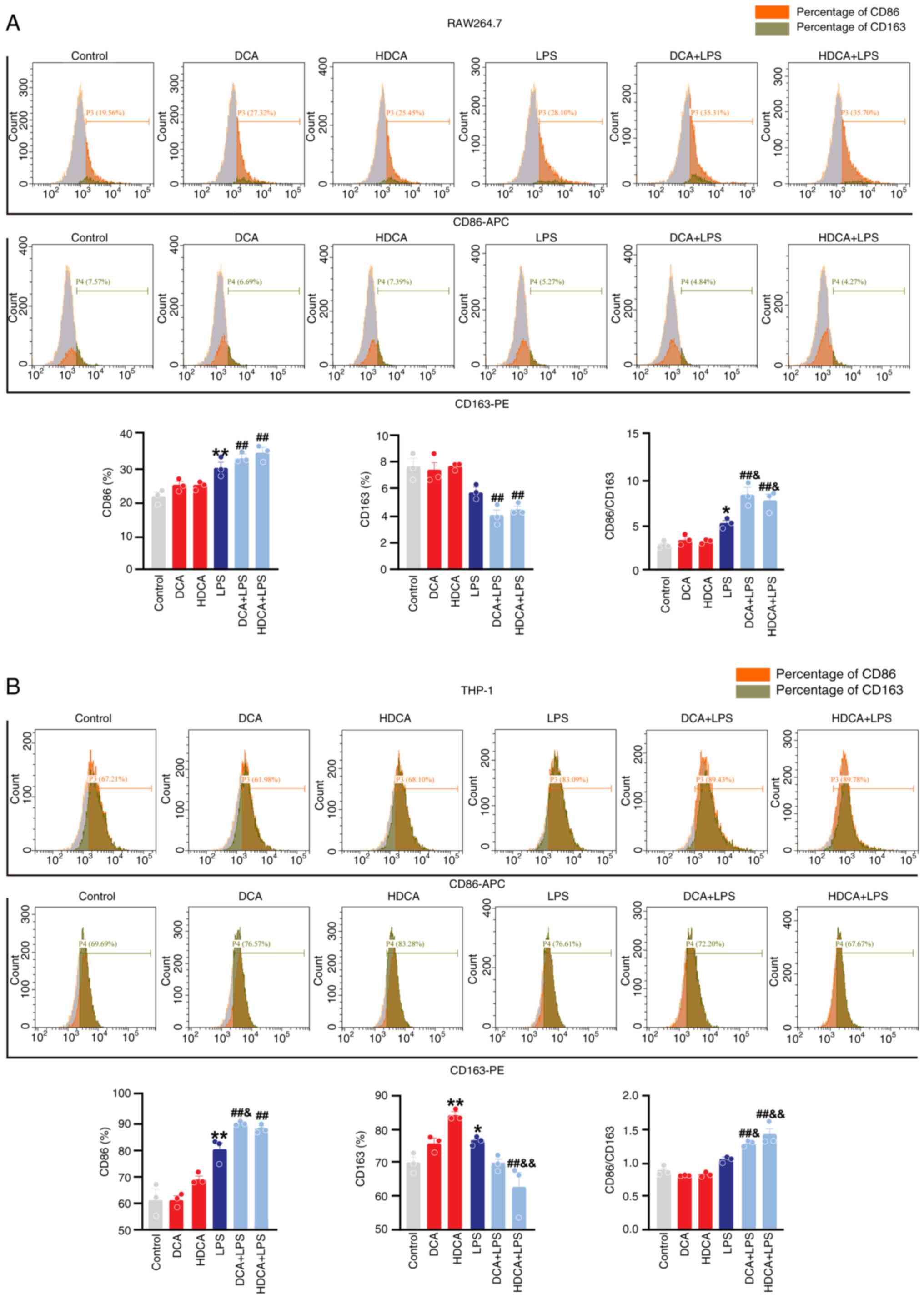
Macrophages play an important role in the clearance of infections. CDCA can influence macrophage polarisation and function, but the role of DCA and HDCA in modulating macrophage polarisation remains largely unknown (15). Thus, the present study assessed the role of DCA and HDCA in macrophage polarisation by evaluating the expression of the M1 macrophage markers iNOS and CD86 and the M2 macrophage markers Arg-1 and CD163 (25,26). The effects of DCA (25–400 µM) and HDCA (25–400 µM) on cell viability were assessed using the cell counting kit 8 (CCK-8) and their stimulating concentrations were selected based on the results of CCK-8 (Fig. S4). Following single DCA stimulation, there was a slight increase observed in iNOS protein expression in RAW264.7 cells, with no significant effect on iNOS mRNA levels. Notably, DCA alone did not induce changes in either iNOS protein or mRNA expression in THP-1 cells. Similarly, independent stimulation with DCA did not impact the expression of Arg-1 protein or mRNA in either RAW264.7 or THP-1 cells in vitro (Fig. 4A-D). Likewise, HDCA stimulation alone did not alter the expression of iNOS or Arg-1 protein and mRNA in RAW264.7 and THP-1 cells in vitro (Fig. 4E-H). However, after co-stimulation with LPS, both DCA and HDCA significantly enhanced the LPS-induced induction of iNOS protein and mRNA expression compared with LPS treatment alone, although the increase in iNOS mRNA expression by DCA in RAW264.7 cells was not statistically significant (Fig. 4A-H). Additionally, DCA and HDCA acted synergistically with LPS to further increase the trend of downregulation of Arg-1 protein expression, although the decrease in Arg-1 mRNA was not statistically significant. (Fig. 4A-H). Nonetheless, they acted synergistically with LPS to increase the value of iNOS/Arg-1 (Fig. S5). Similar to the western blotting and RT-qPCR data, stimulation of RAW264.7 with DCA or HDCA alone did not affect CD86 and CD163 expression, and HDCA mildly increased CD163 expression in THP-1 cells (Fig. 5). DCA and HDCA enhanced the LPS-induced increase in CD86 expression and facilitated the LPS-induced downregulation of CD163 in RAW264.7 cells, though these changes were not statistically significant. However, both DCA and HDCA significantly elevated the LPS-induced CD86/CD163 ratio (Fig. 5A). A similar effect was observed in THP-1 cells (Fig. 5B). Thus, DCA or HDCA enhanced the effect of LPS on the induction of the CD86/163 ratio in RAW264.7 and THP-1 cells. The results of the present study suggested that DCA and HDCA could act synergistically with LPS to enhance macrophage M1 polarisation; however, neither of them were effective when used alone.
DCA and HDCA increase LPS-induced secretion of inflammatory factors
Cytokines are important components of the macrophage response and established indicators of the macrophage phenotype (8). RT-qPCR was used to determine the expression of cytokine mRNA levels in RAW264.7 and THP-1 cells treated with DCA/HDCA in the presence or absence of LPS. DCA stimulation alone mildly upregulated Il6 mRNA expression in RAW264.7 cells, but the difference was not statistically significant. This effect was not observed in THP-1 cells (Fig. 6A and B). In addition, HDCA stimulation alone did not affect the mRNA expression of the proinflammatory factors in RAW264.7 and THP-1 cells (Fig. 6C and D). However, co-stimulation with LPS and DCA significantly increased LPS-induced Il6 mRNA expression in RAW264.7 cells, elevated Tnfα mRNA expression and decreased Il10 mRNA expression in THP-1 cells (Fig. 6A and B). In contrast, co-stimulation of HDCA with LPS increased Il1b and Il6 mRNA expression in RAW264.7 cells and enhanced Il6 mRNA expression in THP-1 cells (Fig. 6C and D).
DCA and HDCA enhance phagocytosis and migration of macrophages
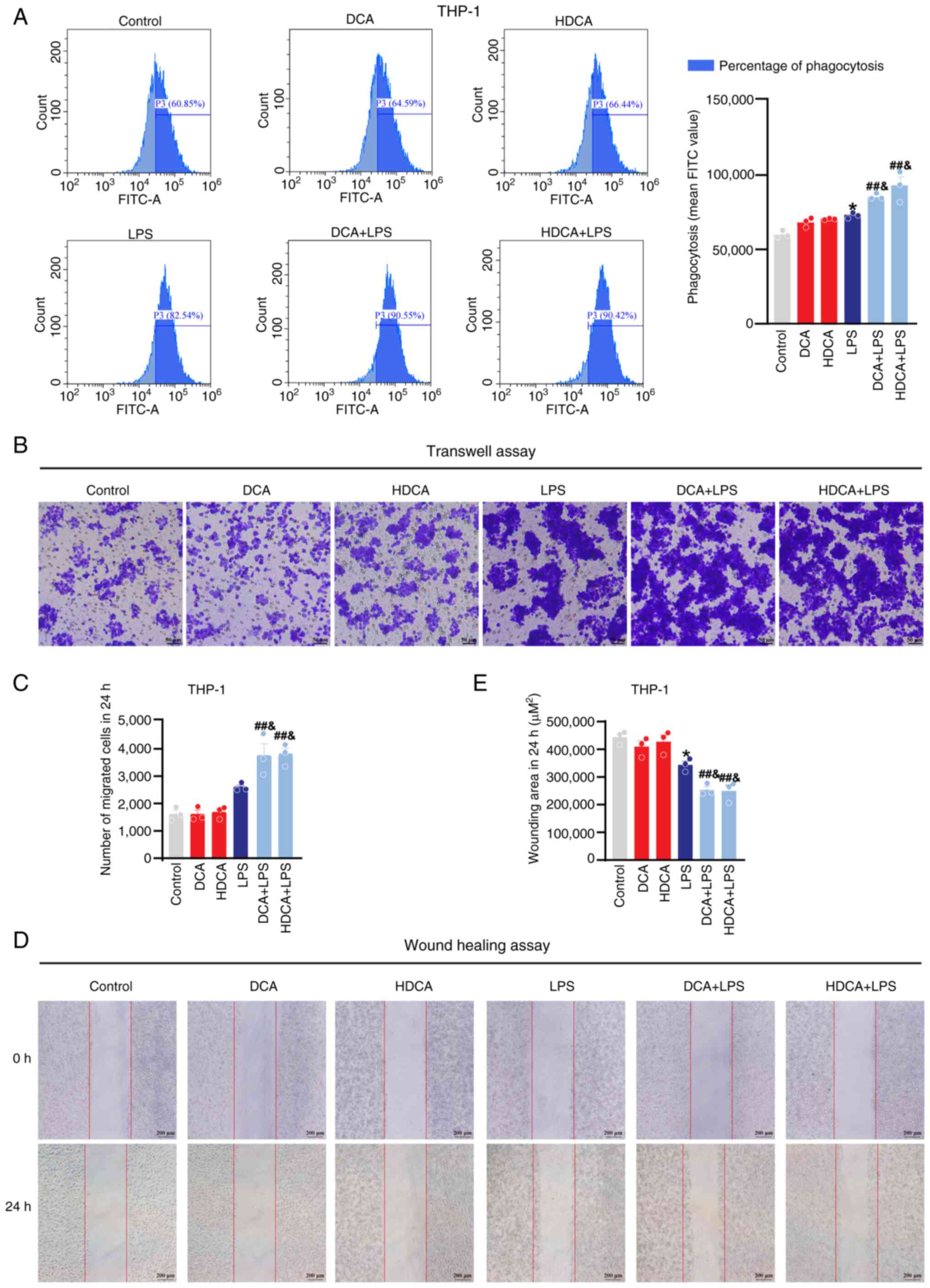
Flow cytometry and migration assays were used to evaluate macrophage phagocytosis and migration, and to further assess the effect of bile acids on macrophage function. The flow cytometry results showed that macrophage phagocytosis was enhanced after LPS stimulation, and DCA and HDCA further enhanced the LPS-induced phagocytosis compared with LPS stimulation alone; DCA or HDCA treatment alone had no effect in RAW264.7 cells (Fig. 7A). Meanwhile, the wound healing and Transwell assays showed a significant increase in the migration capacity of RAW264.7 cells after LPS stimulation, which was further enhanced by DCA or HDCA, although neither HDCA nor DCA treatment alone showed a significant effect on the migration behaviour of macrophages (Fig. 7B-E). In THP-1 cells, the effects of DCA and HDCA on phagocytosis and migration were consistent with those in RAW264.7 cells (Fig. 8). These results suggest that DCA or HDCA alone did not affect the phagocytosis and migration of macrophages but enhanced the LPS-induced induction of phagocytosis and migration when used in combination with LPS.
DCA and HDCA enhance LPS-induced NF-κB activation
NF-κB activation is essential for functions such as M1 polarisation and inflammatory factor secretion by macrophages (8). To further validate the effect of bile acids on the NF-κB signalling pathway in macrophages, the expression levels of proteins related to the NF-κB signalling pathway were analysed. First, the expression of IκBα and p-IκBα was assessed by western blotting; IκBα expression decreased and p-IκBα expression increased in RAW264.7 and THP-1 cells after LPS stimulation. DCA and HDCA stimulation alone had no significant effect on the expression of IκBα and p-IκBα, but after co-stimulation with LPS, the expression of IκBα in RAW264.7 and THP-1 cells was further decreased, while the expression of p-IκBα was further elevated compared with LPS stimulation alone (Fig. 9A-D). This suggested that DCA and HDCA act synergistically with LPS and reduce the binding of IκBα to p65 in the cytoplasm and promote the translocation of p65 into the nucleus. To test this hypothesis, the expression of p65 in the nucleus was analysed using histone H3 as an internal reference. DCA and HDCA acted synergistically with LPS to increase the expression of p65 in the nucleus (Fig. 9A-D). In addition, immunofluorescence experiments confirmed the phenomenon (Fig. 9E-H).
Discussion
Patients with cirrhosis are at a higher risk of infection, with the incidence of bacterial infections reaching as high as 32–34% (27). While nearly two thirds of patients with cirrhosis face the threat of sepsis, the complications associated with infection lead to a 3-fold increase in mortality in patients with cirrhosis and 50% of inpatients die (27). The compromised intestinal barrier and the presence of microbiota-derived LPS in patients with cirrhosis dysregulate macrophage function and disrupt immune homeostasis, thereby heightening the susceptibility to infections (3,6,7). Macrophages play crucial roles in phagocytosis, antigen presentation and the secretion of inflammatory factors. Reduced macrophage efficacy in pathogen recognition and clearance is strongly linked to the increased incidence of infections and mortality in patients with cirrhosis (1,7,27). However, the factors by which infection alter macrophage function remain largely unknown.
In the present study, it was demonstrated that DCA and HDCA were further dysregulated by infection in patients with cirrhosis, while these two bile acids could act synergistically with LPS to enhance macrophage polarisation, migration and activation, accompanied by increased induction of proinflammatory cytokines. A key finding of the present study is that infection could further disturb bile acid homeostasis in patients with cirrhosis. Bile acid synthesis, metabolism and transport are impaired as cirrhosis progresses, making the bile acid profile a potential biomarker of the disease (28,29). Reportedly, levels of CDCA and CA are increased, while levels of DCA are decreased in patients with cirrhosis compared with healthy controls (13). In line with previous studies, the results of the present study replicated the differences in the bile acid profile observed between healthy individuals and patients with cirrhosis (13,30). Additionally, the present study revealed novel distinctions in the bile acid profile between patients with cirrhosis, with or without infection. There were differences in HDCA, DCA, GCDCA and TDCA, among which the AUC for HDCA, DCA and TDCA was 0.769, 0.583 and 0.641, respectively, indicating a strong association between bile acid levels and infection.
Another important finding of the present study is the key role of HDCA and DCA, two of the bile acids altered by infection, in modulating macrophage polarisation, migration and function. Bile acids modulate various signal transduction pathways, such as apoptosis, autophagy and stress management (31–35), whereas several toxic bile acids act as potential damage-associated molecular patterns (DAMPs) that regulate the immune system, particularly macrophages, especially under infection or proinflammatory conditions (36). Taurine conjugates of DCA and CDCA were significantly elevated in the serum, and these conjugates increase IL-1β expression in both RAW264.7 and THP-1 cells in vitro (15). TCA promotes IL-4-induced M2-like polarisation of bone marrow-derived macrophages in vitro, and the mechanism may be related to gene expression of the farnesoid X receptor (FXR), an important bile acid receptor (37). TUDCA and taurolithocholic acid inhibit LPS-induced iNOS expression in RAW264.7 cells potentially by activating Takeda G protein-coupled receptor 5 (TGR5) (38). These studies suggest that bile acid metabolites act as proinflammatory DAMPs or immunoregulators to regulate macrophage function. NF-κB stands out as a pivotal transcription factor governing immunity and inflammation regulation. Previous studies have shown that the role of bile acids in the regulation of immune cells is closely related to NF-κB (39–41). In the present study, a novel role of HDCA and DCA in sensitising LPS-induced macrophage activation was introduced, accompanied by enhanced NF-κB activation, while HDCA and DCA treatment alone showed no significant effect. These findings suggested that the progressive reduction of HDCA and DCA in patients with cirrhosis at least partially contributes to the impaired responsiveness of macrophages to LPS, thus increasing the susceptibility of patients with cirrhosis to infection. By contrast, another study demonstrated that DCA treatment alone for 24 h promoted M1 polarisation in RAW264.7 macrophages at least partially through Toll-like receptor 2 transactivated by the M2 muscarinic acetylcholine receptor/Src pathway, but not in association with activation of FXR and TGR5 (39). On the other hand, HDCA inhibited LPS-induced inflammatory responses in BV2 cells via TGR5 (42). Similar to the results of the present study, Li et al (43) found that HDCA plasma levels were significantly lower in patients with sepsis and negatively correlated with disease severity. However, HDCA acted as an endogenous TLR4 antagonist and inhibited excessive macrophage activation. In a mouse model of liver cancer, taurine-CA (T-CA), tauro-β-muricholic acid and tauro-ω-muricholic acid levels increased, and T-CA induced macrophage M2 polarisation (37). CDCA and TCDCA promote IL-1β and IL-18 secretion through activation of the macrophage NLRP3 inflammasome, which is associated with FXR binding to NLRP3 and reduced NLRP3 activity (15), whereas low concentrations of CDCA induced FXR binding to the IL-6 promoter, which in turn reduced IL-6 expression in macrophages (14). Thus, the effects of different bile acids on macrophage phenotypes depends on the experimental context.
While controversial, recent studies increasingly suggest that bile acids influence macrophage function via signalling regulated by their receptors, namely FXR or TGR5 (44). Bile acids exhibit differing affinity for FXR or TGR5. The order of potency for natural FXR agonists is CDCA > DCA > CA > LCA, with UDCA acting as an antagonist (45). Regarding the TGR5 agonistic potency, the order is LCA > DCA > CDCA > CA (45). Additionally, other signalling molecules such as reactive oxygen species and mitofusin 2 may play a role (46,47). This may explain the varied effects of different bile acids on macrophages. Furthermore, differences in the origin of macrophages and the methods used to induce polarisation contribute to the diverse experimental outcomes (48,49). Thus, in the presence of multiple bile acid changes in vivo, the effect of bile acids on macrophage function could become complex. Further investigation is necessary to fully assess the roles of bile acids on the immune status.
Bile acid production is intricately regulated by a variety of metabolic enzymes, transporters and intestinal flora. As secondary bile acids, DCA and HDCA are derived from CA and CDCA, respectively, through bacterial metabolism in the intestine (45). The CA and CDCA levels gradually increase in patients with cirrhosis, with or without infection, indicating a decrease in DCA and HDCA production likely due to disturbances in the intestinal flora (45). Intestinal flora disorders are well recognised in patients with cirrhosis, with recent studies implicating intestinal flora and their metabolites as causative factors of infections in this population (50). Restoring intestinal flora homeostasis through supplementation with beneficial bacterial strains holds promise as a therapeutic approach for patients with cirrhosis and infectious complications (51,52). The findings of the present study also suggest the potential of supplementing bile-acid-metabolising flora, although additional studies are needed to elucidate the specific flora involved in DCA or HDCA metabolism in infected states. This will necessitate conducting 16S rRNA sequencing and macrogenomic sequencing of the gut flora of clinical patients to comprehensively assess the composition and function of the gut flora in patients with cirrhosis under infectious conditions, along with their associations with DCA or HDCA metabolism. Additionally, further exploration of the potential of transplanting flora associated with DCA or HDCA metabolism in preventing and treating infections in patients with cirrhosis is warranted.
The limitations of the present study include the lack of statistical significance regarding the levels of certain bile acid species, attributed to substantial individual variations in bile acids and the small sample size of patients with cirrhosis. Due to ethical requirements, the serum from patients mentioned in the manuscript was discarded specimens after clinical testing in 2020 and analysis of bile acid profiles was completed in 2021. Introducing new patient samples would necessitate a fresh ethical review. Furthermore, detection errors in the bile acid profile assay from different test lots prevent the presentation of data from additional clinical patients. Moreover, the diverse factors related to infection and antibiotic utilization in various cohorts of patients with cirrhosis may introduce confounding variables, potentially influencing bias in bile acid profiles. Therefore, further subgroup studies with larger sample sizes are indispensable to validate the credibility of the observations of the present study. In addition, direct assessment of macrophage function in patients with cirrhosis, with or without infection is crucial to elucidate the role of bile acids in regulating macrophage function under clinically complex conditions. Regrettably, the inability to collect anticoagulated whole blood from the patients in the present study impeded further exploration in this area. This underscores the necessity for more comprehensive clinical studies to delve deeper into this aspect. Building on this foundation, a dynamic evaluation of changes in bile acids and macrophage function would be more conducive to elucidating the underlying mechanisms and identifying potential biomarkers. Moreover, the downstream signalling pathways by which DCA and HDCA regulate macrophage function require elucidation. Recent literature has highlighted the crucial role of bile acids in governing mitochondrial homeostasis (53,54). Given the pivotal significance of energy metabolism in upholding macrophage viability (55), a comprehensive investigation into how DCA or HDCA influences macrophage function through the modulation of mitochondrial energy metabolism presents a promising avenue for elucidating their mechanisms of action. The available evidence suggests that bile acid-mediated regulation of mitochondrial homeostasis is intricately linked to the downstream signals of TGR5, prominently involving protein kinase C and the mitochondrial calcium signaling pathway IP3R1-GRP75-VDAC1 (56,57). This emphasizes the existence of a sophisticated signaling regulatory pathway between bile acids and mitochondria. Hence, additional experiments are necessary to thoroughly investigate the relationship among different types of bile acids, mitochondrial metabolism and macrophage function.
In summary, in the present study it was found that the bile acid profiles of patients with cirrhosis and infection were altered compared with patients with cirrhosis but without infection, with significantly lower levels of HDCA, DCA, TDCA and GUDCA, and higher levels of GCDCA, TCDCA and TCA. Among these bile acids, HDCA and DCA were closely related to the occurrence of infection. Notably, HDCA and DCA increased macrophage sensitivity to LPS stimulation. An infection-induced decrease in HDCA and DCA may impair the sensitivity of macrophages to LPS stimulation, which partially explains the infection-induced vulnerability of patients with cirrhosis to immune disorders and death. However, changes in other bile acids or factors may also contribute, which cannot be neglected. These findings suggest that the bile acid profile, particularly the HDCA and DCA levels, may be potential biomarkers for patients with cirrhosis and infection, although their roles and mechanisms of action on the immune system still need to be explored further. Assessing the DCA and HDCA levels in patients with cirrhosis may provide insights into the risk of infection and prognosis. Additionally, restoring metabolic homeostasis of bile acids through supplementation of beneficial flora holds promise as a potential therapeutic strategy to address the infectious complications associated with cirrhosis.
Supplementary Material
Supporting Data
Supporting Data
Acknowledgements
The authors would like to thank Dr Yuan Che, Dr Shuang Cui and Dr Chujie Ding (China Pharmaceutical University, Jiangsu, China) for providing technical assistance.
Funding
This study was financially supported by grants from the National Nature Science Foundation of China (grant no. 82204703), National Nature Science Foundation of Anhui Province (grant no. 2008085MH287), Anhui Province Traditional Chinese Medicine Inheritance and Innovation Scientific Research Project (grant no. 2020cczd03), Natural Science Foundation of Anhui Medical University (grant no. 2022×kj158) and The Beijing Kangmeng Charity Foundation Medical Research Development Fund Project (grant no. HS202007).
Availability of data and materials
The data generated in the present study may be requested from the corresponding author.
Authors' contributions
CG and YS designed the study; QZ collected the serum from the patients; CG tested the levels of bile acid; YS, QZ, QW and MJ performed the western blotting, RT-qPCR and flow cytometry experiments; XZ, YD and JW performed the immunofluorescence, wound healing and Transwell experiments; and XW completed the statistical analysis of the data and the production of the graphs. YS and CG confirm the authenticity of all the raw data. All authors read and approved the final version of the manuscript.
Ethics approval and consent to participate
The study was approved by The Ethics Committee of The First Affiliated Hospital of Anhui Medical University (approval no. PJ2020-13-07; Hefei, China) and was conducted in accordance with the principles of the Declaration of Helsinki (2008). All patients provided written informed consent before enrollment in the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
|
Wilde B and Katsounas A: Immune dysfunction and albumin-related immunity in liver cirrhosis. Mediators Inflamm. 2019:75376492019. View Article : Google Scholar : PubMed/NCBI | |
|
Ginès P, Krag A, Abraldes JG, Solà E, Fabrellas N and Kamath PS: Liver cirrhosis. Lancet. 398:1359–1376. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Irvine KM, Ratnasekera I, Powell EE and Hume DA: Causes and consequences of innate immune dysfunction in cirrhosis. Front Immunol. 10:2932019. View Article : Google Scholar : PubMed/NCBI | |
|
Haidar G and Singh N: The evolving challenge of infections in cirrhosis. N Engl J Med. 385:1150–1151. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Fernández J, Piano S, Bartoletti M and Wey EQ: Management of bacterial and fungal infections in cirrhosis: The MDRO challenge. J Hepatol. 75 (Suppl 1):S101–S117. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Albillos A, Lario M and Álvarez-Mon M: Cirrhosis-associated immune dysfunction: Distinctive features and clinical relevance. J Hepatol. 61:1385–1396. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
Noor MT and Manoria P: Immune dysfunction in cirrhosis. J Clin Transl Hepatol. 5:50–58. 2017.PubMed/NCBI | |
|
Yunna C, Mengru H, Lei W and Weidong C: Macrophage M1/M2 polarization. Eur J Pharmacol. 877:1730902020. View Article : Google Scholar : PubMed/NCBI | |
|
Geiß C, Salas E, Guevara-Coto J, Régnier-Vigouroux A and Mora-Rodriguez RA: Multistability in macrophage activation pathways and metabolic implications. Cells. 11:4042022. View Article : Google Scholar | |
|
Van der Merwe S, Chokshi S, Bernsmeier C and Albillos A: The multifactorial mechanisms of bacterial infection in decompensated cirrhosis. J Hepatol. 75 (Suppl 1):S82–S100. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Jia W, Xie G and Jia W: Bile acid-microbiota crosstalk in gastrointestinal inflammation and carcinogenesis. Nat Rev Gastroenterol Hepatol. 15:111–128. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Balazs I, Horvath A, Leber B, Feldbacher N, Sattler W, Rainer F, Fauler G, Vermeren S and Stadlbauer V: Serum bile acids in liver cirrhosis promote neutrophil dysfunction. Clin Transl Med. 12:e7352022. View Article : Google Scholar : PubMed/NCBI | |
|
Zhou J, Huang N, Guo Y, Cui S, Ge C, He Q, Pan X, Wang G, Wang H and Hao H: Combined obeticholic acid and apoptosis inhibitor treatment alleviates liver fibrosis. Acta Pharm Sin B. 9:526–536. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Cao S, Meng X, Li Y, Sun L, Jiang L, Xuan H and Chen X: Bile acids elevated in chronic periaortitis could activate farnesoid-X-receptor to suppress IL-6 production by macrophages. Front Immunol. 12:6328642021. View Article : Google Scholar : PubMed/NCBI | |
|
Hao H, Cao L, Jiang C, Che Y, Zhang S, Takahashi S, Wang G and Gonzalez FJ: Farnesoid X receptor regulation of the NLRP3 inflammasome underlies cholestasis-associated sepsis. Cell Metab. 25:856–867.e5. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Yoshiji H, Nagoshi S, Akahane T, Asaoka Y, Ueno Y, Ogawa K, Kawaguchi T, Kurosaki M, Sakaida I, Shimizu M, et al: Evidence-based clinical practice guidelines for liver cirrhosis 2020. J Gastroenterol. 56:593–619. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
European Association for the Study of the Liver, . EASL clinical practice guidelines on the management of ascites, spontaneous bacterial peritonitis, and hepatorenal syndrome in cirrhosis. J Hepatol. 53:397–417. 2010. View Article : Google Scholar : PubMed/NCBI | |
|
Aithal GP, Palaniyappan N, China L, Härmälä S, Macken L, Ryan JM, Wilkes EA, Moore K, Leithead JA, Hayes PC, et al: Guidelines on the management of ascites in cirrhosis. Gut. 70:9–29. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Chinese Society of Hepatology Chinese Medical Association, ; Xu X, Duan Z, Ding H, Li W, Jia J, Wei L, Linghu E and Zhuang H: Chinese guidelines on the management of ascites and its related complications in cirrhosis. Hepatol Int. 13:1–21. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Fuenzalida B, Yañez MJ, Mueller M, Mistry HD, Leiva A and Albrecht C: Evidence for hypoxia-induced dysregulated cholesterol homeostasis in preeclampsia: Insights into the mechanisms from human placental cells and tissues. FASEB J. 38:e234312024. View Article : Google Scholar : PubMed/NCBI | |
|
Buzzetti E, Parikh PM, Gerussi A and Tsochatzis E: Gender differences in liver disease and the drug-dose gender gap. Pharmacol Res. 120:97–108. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Durand F and Valla D: Assessment of the prognosis of cirrhosis: Child-Pugh versus MELD. J Hepatol. 42 (Suppl):S100–S107. 2005. View Article : Google Scholar : PubMed/NCBI | |
|
Qamar AA, Grace ND, Groszmann RJ, Garcia-Tsao G, Bosch J, Burroughs AK, Ripoll C, Maurer R, Planas R, Escorsell A, et al: Incidence, prevalence, and clinical significance of abnormal hematologic indices in compensated cirrhosis. Clin Gastroenterol Hepatol. 7:689–695. 2009. View Article : Google Scholar : PubMed/NCBI | |
|
Deng W, Rao J, Chen X, Li D, Zhang Z, Liu D, Liu J, Wang Y and Huang O: Metabolomics study of serum and urine samples reveals metabolic pathways and biomarkers associated with pelvic organ prolapse. J Chromatogr B Analyt Technol Biomed Life Sci. 1136:1218822020. View Article : Google Scholar : PubMed/NCBI | |
|
Xia L, Zhang C, Lv N, Liang Z, Ma T, Cheng H, Xia Y and Shi L: AdMSC-derived exosomes alleviate acute lung injury via transferring mitochondrial component to improve homeostasis of alveolar macrophages. Theranostics. 12:2928–2947. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Weber M, Lutz R, Olmos M, Glajzer J, Baran C, Nobis CP, Möst T, Eckstein M, Kesting M and Ries J: Beyond PD-L1-identification of further potential therapeutic targets in oral cancer. Cancers (Basel). 14:18122022. View Article : Google Scholar : PubMed/NCBI | |
|
Bonnel AR, Bunchorntavakul C and Reddy KR: Immune dysfunction and infections in patients with cirrhosis. Clin Gastroenterol Hepatol. 9:727–738. 2011. View Article : Google Scholar : PubMed/NCBI | |
|
Garrido A, Kim E, Teijeiro A, Sánchez Sánchez P, Gallo R, Nair A, Matamala Montoya M, Perna C, Vicent GP, Muñoz J, et al: Histone acetylation of bile acid transporter genes plays a critical role in cirrhosis. J Hepatol. 76:850–861. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Rauchbach E, Zeigerman H, Abu-Halaka D and Tirosh O: Cholesterol induces oxidative stress, mitochondrial damage and death in hepatic stellate cells to mitigate liver fibrosis in mice model of NASH. Antioxidants (Basel). 11:5362022. View Article : Google Scholar : PubMed/NCBI | |
|
Aliwa B, Horvath A, Traub J, Feldbacher N, Habisch H, Fauler G, Madl T and Stadlbauer V: Altered gut microbiome, bile acid composition and metabolome in sarcopenia in liver cirrhosis. J Cachexia Sarcopenia Muscle. 14:2676–2691. 2023. View Article : Google Scholar : PubMed/NCBI | |
|
Bertolini A, Fiorotto R and Strazzabosco M: Bile acids and their receptors: Modulators and therapeutic targets in liver inflammation. Semin Immunopathol. 44:547–564. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Režen T, Rozman D, Kovács T, Kovács P, Sipos A, Bai P and Mikó E: The role of bile acids in carcinogenesis. Cell Mol Life Sci. 79:2432022. View Article : Google Scholar : PubMed/NCBI | |
|
Wang H, Ge C, Zhou J, Guo Y, Cui S, Huang N, Yan T, Cao L, Che Y, Zheng Q, et al: Noncanonical farnesoid X receptor signaling inhibits apoptosis and impedes liver fibrosis. EBioMedicine. 37:322–333. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Thomas JP, Modos D, Rushbrook SM, Powell N and Korcsmaros T: The emerging role of bile acids in the pathogenesis of inflammatory bowel disease. Front Immunol. 13:8295252022. View Article : Google Scholar : PubMed/NCBI | |
|
Thibaut MM and Bindels LB: Crosstalk between bile acid-activated receptors and microbiome in entero-hepatic inflammation. Trends Mol Med. 28:223–236. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Yan T, Yan N, Wang H, Yagai T, Luo Y, Takahashi S, Zhao M, Krausz KW, Wang G, Hao H and Gonzalez FJ: FXR-deoxycholic Acid-TNF-α axis modulates acetaminophen-induced hepatotoxicity. Toxicol Sci. 181:273–284. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Sun R, Zhang Z, Bao R, Guo X, Gu Y, Yang W, Wei J, Chen X, Tong L, Meng J, et al: Loss of SIRT5 promotes bile acid-induced immunosuppressive microenvironment and hepatocarcinogenesis. J Hepatol. 77:453–466. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Romero-Ramirez L, Garcia-Rama C, Wu S and Mey J: Bile acids attenuate PKM2 pathway activation in proinflammatory microglia. Sci Rep. 12:14592022. View Article : Google Scholar : PubMed/NCBI | |
|
Wang L, Gong Z, Zhang X, Zhu F, Liu Y, Jin C, Du X, Xu C, Chen Y, Cai W, et al: Gut microbial bile acid metabolite skews macrophage polarization and contributes to high-fat diet-induced colonic inflammation. Gut Microbes. 12:1–20. 2020. View Article : Google Scholar | |
|
Pi Y, Wu Y, Zhang X, Lu D, Han D, Zhao J, Zheng X, Zhang S, Ye H, Lian S, et al: Gut microbiota-derived ursodeoxycholic acid alleviates low birth weight-induced colonic inflammation by enhancing M2 macrophage polarization. Microbiome. 11:192023. View Article : Google Scholar : PubMed/NCBI | |
|
Mobraten K, Haugbro T, Karlstrom E, Kleiveland CR and Lea T: Activation of the bile acid receptor TGR5 enhances LPS-induced inflammatory responses in a human monocytic cell line. J Recept Signal Transduct Res. 35:402–409. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Zhu H, Bai Y, Wang G, Su Y, Tao Y, Wang L, Yang L, Wu H, Huang F, Shi H and Wu X: Hyodeoxycholic acid inhibits lipopolysaccharide-induced microglia inflammatory responses through regulating TGR5/AKT/NF-κB signaling pathway. J Psychopharmacol. 36:849–859. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Li J, Chen Y, Li R, Zhang X, Chen T, Mei F, Liu R, Chen M, Ge Y, Hu H, et al: Gut microbial metabolite hyodeoxycholic acid targets the TLR4/MD2 complex to attenuate inflammation and protect against sepsis. Mol Ther. 31:1017–1032. 2023. View Article : Google Scholar : PubMed/NCBI | |
|
Fiorucci S, Biagioli M, Zampella A and Distrutti E: Bile acids activated receptors regulate innate immunity. Front Immunol. 9:18532018. View Article : Google Scholar : PubMed/NCBI | |
|
Cai J, Rimal B, Jiang C, Chiang JYL and Patterson AD: Bile acid metabolism and signaling, the microbiota, and metabolic disease. Pharmacol Ther. 237:1082382022. View Article : Google Scholar : PubMed/NCBI | |
|
Che Y, Xu W, Ding C, He T, Xu X, Shuai Y, Huang H, Wu J, Wang Y, Wang C, et al: Bile acids target mitofusin 2 to differentially regulate innate immunity in physiological versus cholestatic conditions. Cell Rep. 42:1120112023. View Article : Google Scholar : PubMed/NCBI | |
|
Liu J, Wei Y, Jia W, Can C, Wang R, Yang X, Gu C, Liu F, Ji C and Ma D: Chenodeoxycholic acid suppresses AML progression through promoting lipid peroxidation via ROS/p38 MAPK/DGAT1 pathway and inhibiting M2 macrophage polarization. Redox Biol. 56:1024522022. View Article : Google Scholar : PubMed/NCBI | |
|
Vogel DY, Glim JE, Stavenuiter AW, Breur M, Heijnen P, Amor S, Dijkstra CD and Beelen RH: Human macrophage polarization in vitro: Maturation and activation methods compared. Immunobiology. 219:695–703. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
Li P, Hao Z, Wu J, Ma C, Xu Y, Li J, Lan R, Zhu B, Ren P, Fan D and Sun S: Comparative proteomic analysis of polarized human THP-1 and mouse RAW264.7 macrophages. Front Immunol. 12:7000092021. View Article : Google Scholar : PubMed/NCBI | |
|
Bajaj JS and Khoruts A: Microbiota changes and intestinal microbiota transplantation in liver diseases and cirrhosis. J Hepatol. 72:1003–1027. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Liu J, Yang D, Wang X, Asare PT, Zhang Q, Na L and Shao L: Gut microbiota targeted approach in the management of chronic liver diseases. Front Cell Infect Microbiol. 12:7743352022. View Article : Google Scholar : PubMed/NCBI | |
|
Philips CA, Ahamed R, Abduljaleel JKP, Rajesh S and Augustine P: Identification and analysis of gut microbiota and functional metabolism in decompensated cirrhosis with infection. J Clin Transl Hepatol. 11:15–25. 2023.PubMed/NCBI | |
|
Abrigo J, Olguin H, Tacchi F, Orozco-Aguilar J, Valero-Breton M, Soto J, Castro-Sepúlveda M, Elorza AA, Simon F and Cabello-Verrugio C: Cholic and deoxycholic acids induce mitochondrial dysfunction, impaired biogenesis and autophagic flux in skeletal muscle cells. Biol Res. 56:302023. View Article : Google Scholar : PubMed/NCBI | |
|
Zhao Q, Dai MY, Huang RY, Duan JY, Zhang T, Bao WM, Zhang JY, Gui SQ, Xia SM, Dai CT, et al: Parabacteroides distasonis ameliorates hepatic fibrosis potentially via modulating intestinal bile acid metabolism and hepatocyte pyroptosis in male mice. Nat Commun. 14:18292023. View Article : Google Scholar : PubMed/NCBI | |
|
Chen Y, Yang M, Huang W, Chen W, Zhao Y, Schulte ML, Volberding P, Gerbec Z, Zimmermann MT, Zeighami A, et al: Mitochondrial metabolic reprogramming by CD36 signaling drives macrophage inflammatory responses. Circ Res. 125:1087–1102. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Li Y, Zhu L, Cai MX, Wang ZL, Zhuang M, Tan CY, Xie TH, Yao Y and Wei TT: TGR5 supresses cGAS/STING pathway by inhibiting GRP75-mediated endoplasmic reticulum-mitochondrial coupling in diabetic retinopathy. Cell Death Dis. 14:5832023. View Article : Google Scholar : PubMed/NCBI | |
|
Zhang MY, Zhu L, Zheng X, Xie TH, Wang W, Zou J, Li Y, Li HY, Cai J, Gu S, et al: TGR5 activation ameliorates mitochondrial homeostasis via regulating the PKCδ/Drp1-HK2 signaling in diabetic retinopathy. Front Cell Dev Biol. 9:7594212022. View Article : Google Scholar : PubMed/NCBI |