Cross‑talk between lymphangiogenesis and malignant melanoma cells: New opinions on tumour drainage and immunization (Review)
- Authors:
- Published online on: January 5, 2024 https://doi.org/10.3892/ol.2024.14215
- Article Number: 81
-
Copyright: © Ju et al. This is an open access article distributed under the terms of Creative Commons Attribution License.
Abstract
Introduction
Malignant melanoma (MM) is caused by the transformation of pigment-producing melanocytes into cancer cells. It commonly develops in the basal layer of the skin and mucous membranes, but it can also occur in the uvea, meninges, gastrointestinal tract, and other tissues (1). The incidence of MM has continued to rise worldwide, and the 5-year survival rate is only 25% (2–4). Major treatment options include surgical resection, immunotherapy, and targeted therapy (4,5). Since MM is prone to lymphatic metastasis at the early stages, the 12-month progression-free survival rate after targeted therapy is ~35%, and the median overall survival is 23 months (6). Therefore, the remission time and the overall survival rate do not demonstrate significant improvement (5–8). Lymphatic vessel density (LVD) in patients with MM was positively associated with a poor prognosis (9,10). This means that when the number of lymph vessels (LVs) adjacent to the tumour is higher, the probability of tumour metastasis is higher. At the same time, LVD is also associated with tumour-infiltrating lymphocytes (TILs), which have predictive value for therapy. When the number of paracancerous LVs increases, the number of TILs in the tissue also increases. The increased TIL number could have a better response to immunotherapy in MM research, which is beneficial to the prognosis of patients (6,7). Therefore, lymphatic endothelial cells (LECs) not only serve as a suitable conduit for metastasis but also play a multifaceted role in the metastasis of MM and the host immune response. In the present review, the interactions between melanoma cells (MMCs) and LECs during MM metastasis were discussed.
Features of tumour lymphatic drainage
The lymphatic system is a unidirectional circulatory network involved in maintaining tissue fluid balance, absorbing lipids and conducting immune surveillance (11). Capillary lymphatic vessels (CLVs) of blind-ended origin are permeable, with only a loosely single endothelial layer (12,13). Thin fibrous structures attached to the surfaces of LECs on CLVs can sense interstitial pressure, further increasing the permeability of CLVs in inflammatory or tumour situations (11). All of the aforementioned features make nascent CLVs the best channel for tumour cells to invade. In addition, a high endothelial venules (HEVs) of the lymph node (LN) also serves as a convenient exit for metastatic MMCs (13). In a mouse model, B16 melanoma was found to enter the blood circulation through LNs and finally metastasize to the lung (14).
It is well documented that LECs have multiple functions, including the secretion of chemokines that actively recruit cells. Single-cell sequencing of LNs confirmed the presence of six functional types of LECs mapped to specific locations, lining the floor and ceiling of the subcapsular sinuses (SCSs), medullary sinuses (MSs), and valve, expressing different chemokines (15–17). LECs of the SCSs and MSs express high levels of neutrophil chemoattractants to maintain chemokine signaling gradients (15,16). Chemokines and their ligands play crucial roles in leukocyte trafficking and are involved in the metastasis of cancer to specific organs. In MM, LECs serve as the primary source for the chemokine CCL21. This chemokine attracts CCR7+ MMCs towards CCL21-expressing LECs but not blood endothelial cells (18,19). As a result, it further promotes LN metastasis. Secreting CXCL12 by tumour-associated LECs at metastatic sites has been reported to attract MMCs expressing CXCR4 and promote the growth of metastases. This process can also convert tumour immunogenicity into immune tolerance, thereby promoting tumour progression (20,21). Production of the chemokine CCL1 by the lymphatic sinus within the LN mediates the entry of MMCs expressing CCR8 into the LNs. Blocking CCR8 has been shown to reduce LN metastasis (22). At the same time, high expression of the chemokines CXCL5, CXCR3 and CXCR4 has been found in a variety of melanoma experiments (23–25). Further blockade of these chemokines or their receptors with antagonists or neutralizing antibodies reduced the metastasis of MMCs (23–25).
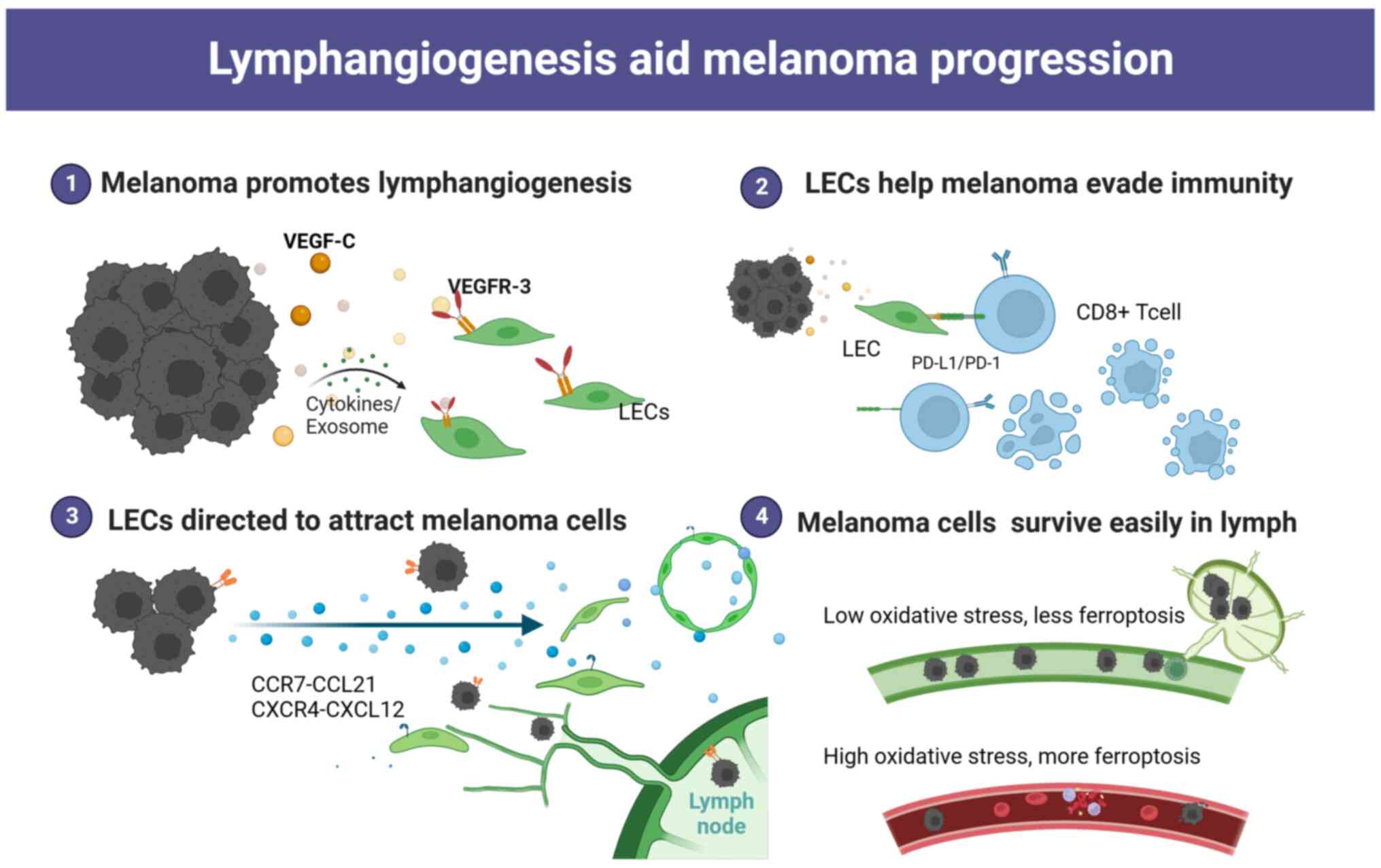
A previous study showed that lymph from patients with MM is a rich source of tumour-derived factors including melanoma biomarkers such as LDH, S100B and S100A8, metastasis-associated proteins such as CSF-1, galectin-3, MMP-2 and MMP-9, tumor-derived factors such as IL-6, IL-8, IL-1β, IL-4, IL-10, TNF-α and extracellular vesicles. This can offer a valuable proteomic signatures in comparison to plasma contents (26). Low levels of free iron along with high levels of glutathione and oleic acid in lymph protect MMCs from oxidative stress and ferroptosis, thereby preventing subsequent metastasis (27,28). Compared with the highly oxidized state in the blood, the lymphatic circulation provides a more suitable environment for the survival and colonization of MMCs (27). When MMCs were injected into mice, the efficiency of tumour cell metastasis following intranodal injection was notably higher compared with that following intravenous injection (27). The efficiency of intravenous metastasis also increased after MMCs had been disposed of in lymph. The lymphatic system, to some extent, provides a favorable environment for tumour metastasis.
MM promotes lymphangiogenesis and LN education
To facilitate invasion and metastasis, MMCs, tumour-associated macrophages (TAMs) and stromal cells in the tumour microenvironment can release multiple cytokines that promote the proliferation of LECs and induce lymphangiogenesis (29–31). There is a large number of micro-LVs in MM paracancerous tissues, and quantitative studies have shown that the mean LVD in MM nests and paracancerous areas is 6.3 and 12.5 per mm2, respectively (32,33). The LVD in the paracancerous region is notably higher than that in central areas, and the LVD in metastatic MM is higher than that in primary MM (34,35). Although the link between lymphangiogenesis and metastasis has received strong support, the precise molecular mechanisms driving tumour lymphangiogenesis remain poorly understood.
VEGF-C/D-VEGFR3 is the most prominent and well-investigated signaling pathway that plays an important role in lymphangiogenesis. VEGF-C and VEGF-D are growth factors that stimulate LEC proliferation and lymphatic remodeling. These factors have been found to be upregulated in MMCs (36,37). Inhibition of lymphangiogenesis by blocking VEGFR-3 or VEGF-C/D could reduce LN colonization and distant metastasis (38,39).
In addition to VEGF family members, CXCL5 is upregulated in T4-stage MMCs, leading to a notable increase in lymphangiogenesis (25). Other factors that can also directly or indirectly promote lymphangiogenesis include angiopoietins, SRY-box transcription factor 18, fibroblast growth factor and epidermal growth factor (10,40,41).
MMCs are highly plastic and can dynamically switch phenotypes (42,43). MM promotes natural killer (NK) cell evasion and T-cell suppression by expressing major histocompatibility complex (MHC)-I and programmed death-ligand 1 (PD-L1) at high levels to facilitate LN metastasis. After LN metastasis, T-cell responses to tumours are suppressed, regulatory T cells (Tregs) are induced, and distant organ metastasis is promoted (44). Extracellular vesicles, inflammatory factors and cytokines secreted by MMCs reach the premetastatic LNs. They not only act on LECs but also cause the microenvironment to be reprogrammed into a favorable niche that facilitates later colonization and supports the development of metastasis (31,45–47). Fibroblast reticular cells (FRCs) in LNs can control the LN elasticity and microarchitecture (48). Recent studies have shown that IL-1 secreted by dedifferentiated MMCs inhibits the JAK1-STAT3 and YAP pathways, which drive actomyosin contraction in FRCs (48,49). This inhibition leads to LN swelling and promotes tumour spreading in the premetastatic LN niche.
Role of LECs in immunomodulation
Under physiological conditions, LVs control immune cell trafficking and initiate the immune response to inhibit tumour progression. However, tumour-associated LECs display a remarkable degree of phenotypic plasticity that regulates immunotolerance (Fig. 1). LECs isolated from highly metastatic tumours showed a unique expression profile and transcriptional program compared with those from non-tumour tissues (50). Characterization of the tumour-associated LEC secretome by RNA sequencing and cytokine array revealed that IL6 is one of the most markedly regulated molecules in promoting primary tumour growth, and its production is negligible in unexposed LECs (51).
In several MM models, LECs maintain peripheral tolerance by directly upregulating PD-L1, which interacts with PD-1 on T cells to inhibit autoreactive T cells (52). The observed upregulation of PD-L1 on LECs may be stimulated by IFN-γ released in the tumour microenvironment (52,53). LECs are capable of scavenging and cross-presenting tumour-associated antigens on MHC-I, which in turn causes dysfunctional activation of CD8+ T cells (54). It was shown that LECs in LN could cross-present the exogenous tumour antigen OVA to CD8+ T cells, and naive LECs scavenge and cross-present OVA in vitro, leading to loss of function in a B16F10 melanoma model (55). In other aspects, LECs play an immunosuppressive role by dampening dendritic cell (DC) maturation, thereby reducing the ability of DCs to activate effector T cells (56,57). LECs upregulate the expression of MHC-II in human melanoma specimens and depend on IFN-γ to promote Treg proliferation and exert immunosuppressive effects in the tumour microenvironment (54,58–60). All results suggest that LECs could play a critical role in developing an immunosuppressive environment.
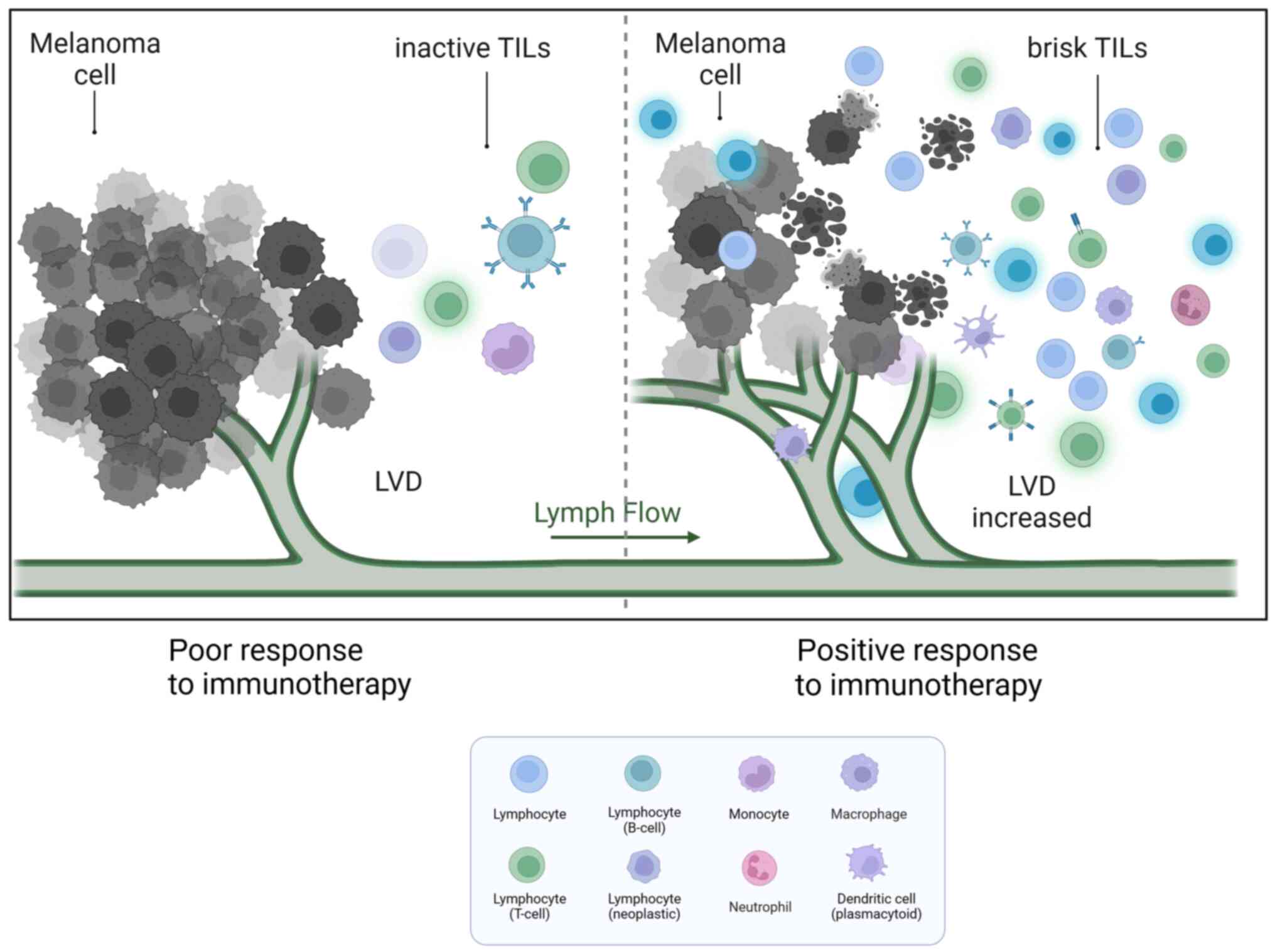
High immunogenicity and immune escape of MMCs
MM is an immunogenic malignancy, and there have been attempts to exploit this specificity to develop novel therapeutic strategies. A study found a notable number of immune cells, including different subsets of T cells, DCs, lymphocytes, macrophages, neutrophils and other cells, infiltrating around the MM tissue. This infiltration may be attributed to tumour-host interactions (61). There is a growing interest in the antitumor immune response exerted by tumour-infiltrating immune cells (TIICs) (62–64). TIIC proportion notably varies among different individuals. As a result, a certain percentage of patients with melanoma do not respond to checkpoint immunotherapy (Fig. 2). A study cohort of 2,624 patients with cutaneous melanoma found that TILs were an important histopathological characteristic reflecting host immune response. A total of 16.5% of patients had no TILs, 73.0% had inactive TILs and 10.4% had active TILs. The 5-year survival rate was 71.0% among patients without TILs and 85.2% among patients with brisk TILs. Brisk TILs were notably associated with improved overall survival (OS) (65). The presence of various subpopulations of TIICs has also been reported to predict patient response to immune checkpoint therapy.
MMCs manipulate their heterogeneity and plasticity in some recurrent cases, leading to the loss of expression of multiple tumour antigens or complete loss of HLA class I expression which allows them to evade functional antigen-specific immune recognition (66–70). The effectiveness of T-cell cytotoxicity requires proper antigen presentation by DCs. Insufficiently presented antigens cannot activate T cells and induce immunological ignorance. Altered expression of MHC-I is frequently observed on MMCs, which allows them to evade recognition by NK cells and reduces their cytolytic activity (70). Mediators including IL-8, IL-10, TGF-1 and VEGF released by MMCs or TAMs limit the maturation of normal DCs and, as a result, hinder their ability to present and activate CD8+ T cells (70,71).
Activation of negative immune checkpoint molecules on MMCs shields them from immune attacks and enables further proliferation. PD-L1 is expressed at high levels on MMCs (72), and the combination of PD-1 and PD-L1 can initiate CD8+ T-cell apoptosis and stimulate the differentiation of CD4+ T cells into Tregs, allowing tumour cells to evade the immune system (72). CD8+ T cells/Tregs in the tumour microenvironment could be used to predict the survival of patients with MM (73). Increased expression and higher affinity of cytotoxic T-lymphocyte associated protein 4 (CTLA-4) molecules on activated effector T cells in MM can prevent CD28 receptor binding to B7.1 and B7.2 on antigen presenting cells, leading to T lymphocyte deactivation (74). M2 macrophages have low antigen-presenting activity, inhibit CD8+ T-cell and NK cell activity, and promote tumour cell migration (44,75). In addition, Tregs are deregulated in MM and suppress the immune system by overproducing TGF-β, IL-10 and IDO. This excessive production hampers the function of CD4+ and CD8+ T cells, as well as NK cells (44). MMC-derived exosomes also carry PD-L1, which can bind to T cells to suppress antitumor immunity (31,46,76).
Immune cell infiltration enhanced immunotherapy
Although increased LVs can promote MM spread, increased tumour-associated LECs can assist MMCs in escaping host immunity. The paradox is that tumour lymphangiogenesis promotes T-cell infiltration and potentiates immunotherapy in melanoma (77,78). There were strong positive associations between the expression levels of the lymphatic genes PDPN, LYVE1 and VEGFC, and the immune cell-specific genes CD45, CD11B, F480, KLRB1, CD3D, CD8A, CD4 and FOXP3 in human metastatic melanoma (79). Immune cell infiltration was notably reduced after MM implantation in a mouse model lacking dermal CLVs. In addition, inflammatory cytokines were markedly lower, and MM was found to be more susceptible to CD8+ T-cell attack. These findings suggest that lymphangiogenesis in MM is associated with immunosuppression (80). In human metastatic melanoma, the expression of VEGF-C showed a strong association with CCL21 and T-cell inflammation. Additionally, serum concentrations of VEGF-C were found to be associated with both T-cell activation and expansion (77,80).
Tertiary lymphatic structures (TLS) in MM
The formation of ectopic lymphatic aggregates found in tumour or inflammatory tissues is defined as tertiary lymphoid structures (TLS). They are anatomically similar to LNs and contain T-cell areas, germinal centers with proliferating B cells, and high endothelial venules (HEVs), among others (81). TLS are typically found in the paracancerous or stromal regions rather than the core of the tumour. They also express chemokines, adhesion molecules, and integrins such as intercellular adhesion molecule (ICAM) 2, ICAM3, vascular cell adhesion molecule 1 and integrins (αL, α4, and αD), as well as CCL21, CXCL13, CCL17, CCL22 and IL-16 to facilitate lymphocyte recruitment (81). In human MM, it has been found that the presence of functional TLS activates local anti-melanoma immune responses and generally indicates a positive prognosis. The histological evaluation highlighted that metastatic melanoma contains B-cell lymphoid follicles, indicating the presence of complete TLS. By contrast, primary melanoma does not contain B-cell lymphoid follicles in TLS (82,83). Other studies have shown that TLS does occur in primary melanomas, although at a lower frequency compared with what has been reported in metastases (83–85). Using clinical samples of metastatic melanomas, it was found that B-cell markers in TLS were the most differentially expressed genes in distinguishing patients with MM with and without immunotherapeutic response, as determined by bulk RNA sequencing. Additionally, TLS also influence various T-cell phenotypes and play a crucial role in enhancing the survival of patients with MM (85,86). Therefore, the induction of B-cell-rich TLS formation to enhance the tumour response to immunotherapy can be explored as a novel strategy for treating MM.
Conclusion
During MM progression, MMCs reduce the expression of tumour-associated antigens to avoid their presentation and recognition. However, they also express multiple inhibitory antigens to induce immune tolerance when interacting with immune cells. MM could enhance access to tumour drainage by stimulating lymphangiogenesis through various mechanisms, which leads to increased proliferation of LECs and elevated paracancerous LVD. In the tumour microenvironment, the increased number of tumour-associated LECs recruits MMCs and immune cells, assisting MMCs in evading immune surveillance. TILs were found to have a positive association with LVD and played a role in enhancing the effectiveness of immunotherapy in MM.
In summary, increased LVD in MM promotes tumour drainage and increases TILs. While increased drainage can lead to a poor prognosis, TILs enhance the patients' response to immunotherapy and improve OS. The interaction between lymphangiogenesis and MM is complex and dynamic, and the precise mechanisms remain an open question. It may also be extended to other malignant tumours that are prone to lymphatic metastasis, such as breast cancer and squamous cell carcinoma.
Acknowledgements
Not applicable.
Funding
The present review was supported by the National Natural Science Foundation of China (grant no. 81641174), the Jiangsu Province Postgraduate Practice Innovation Program (grant no. SJCX22_1879), the National Natural Science Foundation of China (grant no. 82173380), the Doctoral Program for Entrepreneurship and Innovation of Jiangsu Province (grant no. JSSCBS20211596) and the Social Development Project of Zhenjiang Key Research and Development Program (grant no. SH2021073).
Availability of data and materials
Not applicable.
Authors' contributions
WJ, XHY, ZXY conceived and designed the study. WJ, HHC, WZ, DML, WZ collected information. WJ and HHC drew images and wrote the manuscript. All authors have read and approved the final version of the manuscript. Data authentication is not applicable.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
|
Hussein Al-Janabi M, Mohammad JG, Mohsen AY, Saad A and Issa R: Metastatic melanoma to the gallbladder presented as a polyp with acute cholecystitis: A case report from Syria. Ann Med Surg (Lond). 76:1035142022.PubMed/NCBI | |
|
Eddy K and Chen S: Overcoming immune evasion in melanoma. Int J Mol Sci. 21:89842020. View Article : Google Scholar : PubMed/NCBI | |
|
Schadendorf D, van Akkooi ACJ, Berking C, Griewank KG, Gutzmer R, Hauschild A, Stang A, Roesch A and Ugurel S: Melanoma. Lancet. 392:971–984. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Falk Delgado A, Zommorodi S and Falk Delgado A: Sentinel lymph node biopsy and complete lymph node dissection for melanoma. Curr Oncol Rep. 21:542019. View Article : Google Scholar : PubMed/NCBI | |
|
Hartman RI and Lin JY: Cutaneous melanoma-a review in detection, staging, and management. Hematol Oncol Clin North Am. 33:25–38. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Ribas A, Hamid O, Daud A, Hodi FS, Wolchok JD, Kefford R, Joshua AM, Patnaik A, Hwu WJ, Weber JS, et al: Association of pembrolizumab with tumor response and survival among patients with advanced melanoma. JAMA. 315:1600–1609. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Knackstedt T, Knackstedt RW, Couto R and Gastman B: Malignant melanoma: Diagnostic and management update. Plast Reconstr Surg. 142:202e–216e. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Faries MB, Thompson JF, Cochran AJ, Andtbacka RH, Mozzillo N, Zager JS, Jahkola T, Bowles TL, Testori A, Beitsch PD, et al: Completion dissection or observation for sentinel-node metastasis in melanoma. N Engl J Med. 376:2211–2222. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Pasquali S, van der Ploeg APT, Mocellin S, Stretch JR, Thompson JF and Scolyer RA: Lymphatic biomarkers in primary melanomas as predictors of regional lymph node metastasis and patient outcomes. Pigment Cell Melanoma Res. 26:326–337. 2013. View Article : Google Scholar : PubMed/NCBI | |
|
Ma Q, Dieterich LC, Ikenberg K, Bachmann SB, Mangana J, Proulx ST, Amann VC, Levesque MP, Dummer R, Baluk P, et al: Unexpected contribution of lymphatic vessels to promotion of distant metastatic tumor spread. Sci Adv. 4:eaat47582018. View Article : Google Scholar : PubMed/NCBI | |
|
Oliver G, Kipnis J, Randolph GJ and Harvey NL: The lymphatic vasculature in the 21st century: Novel functional roles in homeostasis and disease. Cell. 182:270–296. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Petrova TV and Koh GY: Organ-specific lymphatic vasculature: From development to pathophysiology. J Exp Med. 215:35–49. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Johnson LA: In sickness and in health: The immunological roles of the lymphatic system. Int J Mol Sci. 22:44582021. View Article : Google Scholar : PubMed/NCBI | |
|
Pereira ER, Kedrin D, Seano G, Gautier O, Meijer EFJ, Jones D, Chin SM, Kitahara S, Bouta EM, Chang J, et al: Lymph node metastases can invade local blood vessels, exit the node, and colonize distant organs in mice. Science. 359:1403–1407. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Takeda A, Hollmén M, Dermadi D, Pan J, Brulois KF, Kaukonen R, Lönnberg T, Boström P, Koskivuo I, Irjala H, et al: Single-cell survey of human lymphatics unveils marked endothelial cell heterogeneity and mechanisms of homing for neutrophils. Immunity. 51:561–572.e5. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Rodda LB, Lu E, Bennett ML, Sokol CL, Wang X, Luther SA, Barres BA, Luster AD, Ye CJ and Cyster JG: Single-cell RNA sequencing of lymph node stromal cells reveals niche-associated heterogeneity. Immunity. 48:1014–1028.e6. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Fujimoto N, He Y, D'Addio M, Tacconi C, Detmar M and Dieterich LC: Single-cell mapping reveals new markers and functions of lymphatic endothelial cells in lymph nodes. PLoS Biol. 18:e30007042020. View Article : Google Scholar : PubMed/NCBI | |
|
Zhang L, Zhu L, Yao X, Lou X, Wan J, Duan X, Pan L, Li A, Gu Z, Wang M, et al: Paclitaxel treatment enhances lymphatic metastasis of B16F10 melanoma cells via CCL21/CCR7 axis. Int J Biol Sci. 18:1476–1490. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Cristiani CM, Turdo A, Ventura V, Apuzzo T, Capone M, Madonna G, Mallardo D, Garofalo C, Giovannone ED, Grimaldi AM, et al: Accumulation of circulating CCR7+ natural killer cells marks melanoma evolution and reveals a CCL19-dependent metastatic pathway. Cancer Immunol Res. 7:841–852. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Mendt M and Cardier JE: Activation of the CXCR4 chemokine receptor enhances biological functions associated with B16 melanoma liver metastasis. Melanoma Res. 27:300–308. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
McConnell AT, Ellis R, Pathy B, Plummer R, Lovat PE and O'Boyle G: The prognostic significance and impact of the CXCR4-CXCR7-CXCL12 axis in primary cutaneous melanoma. Br J Dermatol. 175:1210–1220. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Korbecki J, Grochans S, Gutowska I, Barczak K and Baranowska-Bosiacka I: CC chemokines in a tumor: A review of pro-cancer and anti-cancer properties of receptors CCR5, CCR6, CCR7, CCR8, CCR9, and CCR10 ligands. Int J Mol Sci. 21:76192020. View Article : Google Scholar : PubMed/NCBI | |
|
Alimohammadi M, Rahimi A, Faramarzi F, Alizadeh-Navaei R and Rafiei A: Overexpression of chemokine receptor CXCR4 predicts lymph node metastatic risk in patients with melanoma: A systematic review and meta-analysis. Cytokine. 148:1556912021. View Article : Google Scholar : PubMed/NCBI | |
|
Doron H, Amer M, Ershaid N, Blazquez R, Shani O, Lahav TG, Cohen N, Adler O, Hakim Z, Pozzi S, et al: Inflammatory activation of astrocytes facilitates melanoma brain tropism via the CXCL10-CXCR3 signaling axis. Cell Rep. 28:1785–1798.e6. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Soler-Cardona A, Forsthuber A, Lipp K, Ebersberger S, Heinz M, Schossleitner K, Buchberger E, Gröger M, Petzelbauer P, Hoeller C, et al: CXCL5 facilitates melanoma cell-neutrophil interaction and lymph node metastasis. J Invest Dermatol. 138:1627–1635. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Broggi MAS, Maillat L, Clement CC, Bordry N, Corthésy P, Auger A, Matter M, Hamelin R, Potin L, Demurtas D, et al: Tumor-associated factors are enriched in lymphatic exudate compared to plasma in metastatic melanoma patients. J Exp Med. 216:1091–1107. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Ubellacker JM, Tasdogan A, Ramesh V, Shen B, Mitchell EC, Martin-Sandoval MS, Gu Z, McCormick ML, Durham AB, Spitz DR, et al: Lymph protects metastasizing melanoma cells from ferroptosis. Nature. 585:113–118. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Piskounova E, Agathocleous M, Murphy MM, Hu Z, Huddlestun SE, Zhao Z, Leitch AM, Johnson TM, DeBerardinis RJ and Morrison SJ: Oxidative stress inhibits distant metastasis by human melanoma cells. Nature. 527:186–191. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Habenicht LM, Kirschbaum SB, Furuya M, Harrell MI and Ruddell A: Tumor regulation of lymph node lymphatic sinus growth and lymph flow in mice and in humans. Yale J Biol Med. 90:403–415. 2017.PubMed/NCBI | |
|
Peppicelli S, Bianchini F and Calorini L: Inflammatory cytokines induce vascular endothelial growth factor-C expression in melanoma-associated macrophages and stimulate melanoma lymph node metastasis. Oncol Lett. 8:1133–1138. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
Leary N, Walser S, He Y, Cousin N, Pereira P, Gallo A, Collado-Diaz V, Halin C, Garcia-Silva S, Peinado H and Dieterich LC: Melanoma-derived extracellular vesicles mediate lymphatic remodelling and impair tumour immunity in draining lymph nodes. J Extracell Vesicles. 11:e121972022. View Article : Google Scholar : PubMed/NCBI | |
|
Dadras SS, Paul T, Bertoncini J, Brown LF, Muzikansky A, Jackson DG, Ellwanger U, Garbe C, Mihm MC and Detmar M: Tumor lymphangiogenesis: A novel prognostic indicator for cutaneous melanoma metastasis and survival. Am J Pathol. 162:1951–1960. 2003. View Article : Google Scholar : PubMed/NCBI | |
|
Pastushenko I, Van den Eynden GG, Vicente-Arregui S, Prieto-Torres L, Alvarez-Alegret R, Querol I, Dirix LY, Carapeto FJ, Vermeulen PB and Van Laere SJ: Increased angiogenesis and lymphangiogenesis in metastatic sentinel lymph nodes is associated with nonsentinel lymph node involvement and distant metastasis in patients with melanoma. Am J Dermatopathol. 38:338–346. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Ayubi E and Safiri S: Lymphatic vessel density and VEGF-C expression as independent predictors of melanoma metastases: Methodological issues. J Plast Reconstr Aesthet Surg. 71:604–605. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Pastushenko I, Vermeulen PB, Carapeto FJ, Van den Eynden G, Rutten A, Ara M, Dirix LY and Van Laere S: Blood microvessel density, lymphatic microvessel density and lymphatic invasion in predicting melanoma metastases: Systematic review and meta-analysis. Br J Dermatol. 170:66–77. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
Špirić Z, Eri Ž and Erić M: Lymphatic vessel density and VEGF-C expression as independent predictors of melanoma metastases. J Plast Reconstr Aesthet Surg. 70:1653–1659. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Skobe M, Hamberg LM, Hawighorst T, Schirner M, Wolf GL, Alitalo K and Detmar M: Concurrent induction of lymphangiogenesis, angiogenesis, and macrophage recruitment by vascular endothelial growth factor-C in melanoma. Am J Pathol. 159:893–903. 2001. View Article : Google Scholar : PubMed/NCBI | |
|
Wang M, Xu Y, Wen GZ, Wang Q and Yuan SM: Rapamycin suppresses angiogenesis and lymphangiogenesis in melanoma by downregulating VEGF-A/VEGFR-2 and VEGF-C/VEGFR-3 expression. Onco Targets Ther. 12:4643–4654. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Lee JY, Hong SH, Shin M, Heo HR and Jang IH: Blockade of FLT4 suppresses metastasis of melanoma cells by impaired lymphatic vessels. Biochem Biophys Res Commun. 478:733–738. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Korhonen EA, Murtomäki A, Jha SK, Anisimov A, Pink A, Zhang Y, Stritt S, Liaqat I, Stanczuk L, Alderfer L, et al: Lymphangiogenesis requires Ang2/Tie/PI3K signaling for VEGFR3 cell-surface expression. J Clin Invest. 132:e1554782022. View Article : Google Scholar : PubMed/NCBI | |
|
Rezzola S, Sigmund EC, Halin C and Ronca R: The lymphatic vasculature: An active and dynamic player in cancer progression. Med Res Rev. 42:576–614. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Wouters J, Kalender-Atak Z, Minnoye L, Spanier KI, De Waegeneer M, Bravo González-Blas C, Mauduit D, Davie K, Hulselmans G, Najem A, et al: Robust gene expression programs underlie recurrent cell states and phenotype switching in melanoma. Nat Cell Biol. 22:986–998. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Arozarena I and Wellbrock C: Phenotype plasticity as enabler of melanoma progression and therapy resistance. Nat Rev Cancer. 19:377–391. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Reticker-Flynn NE, Zhang W, Belk JA, Basto PA, Escalante NK, Pilarowski GOW, Bejnood A, Martins MM, Kenkel JA, Linde IL, et al: Lymph node colonization induces tumor-immune tolerance to promote distant metastasis. Cell. 185:1924–1942.e23. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
García-Silva S, Benito-Martín A, Nogués L, Hernández-Barranco A, Mazariegos MS, Santos V, Hergueta-Redondo M, Ximénez-Embún P, Kataru RP, Lopez AA, et al: Melanoma-derived small extracellular vesicles induce lymphangiogenesis and metastasis through an NGFR-dependent mechanism. Nat Cancer. 2:1387–1405. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Gowda R, Robertson BM, Iyer S, Barry J, Dinavahi SS and Robertson GP: The role of exosomes in metastasis and progression of melanoma. Cancer Treat Rev. 85:1019752020. View Article : Google Scholar : PubMed/NCBI | |
|
Wakisaka N, Hasegawa Y, Yoshimoto S, Miura K, Shiotani A, Yokoyama J, Sugasawa M, Moriyama-Kita M, Endo K and Yoshizaki T: Primary tumor-secreted lymphangiogenic factors induce pre-metastatic lymphvascular niche formation at sentinel lymph nodes in oral squamous cell carcinoma. PLoS One. 10:e01440562015. View Article : Google Scholar : PubMed/NCBI | |
|
Li L, Wu J, Abdi R, Jewell CM and Bromberg JS: Lymph node fibroblastic reticular cells steer immune responses. Trends Immunol. 42:723–734. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Rovera C, Berestjuk I, Lecacheur M, Tavernier C, Diazzi S, Pisano S, Irondelle M, Mallavialle A, Albrengues J, Gaggioli C, et al: Secretion of IL1 by dedifferentiated melanoma cells inhibits JAK1-STAT3-driven actomyosin contractility of lymph node fibroblastic reticular cells. Cancer Res. 82:1774–1788. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Clasper S, Royston D, Baban D, Cao Y, Ewers S, Butz S, Vestweber D and Jackson DG: A novel gene expression profile in lymphatics associated with tumor growth and nodal metastasis. Cancer Res. 68:7293–7303. 2008. View Article : Google Scholar : PubMed/NCBI | |
|
Van de Velde M, Ebroin M, Durré T, Joiret M, Gillot L, Blacher S, Geris L, Kridelka F and Noel A: Tumor exposed-lymphatic endothelial cells promote primary tumor growth via IL6. Cancer Lett. 497:154–164. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Dieterich LC, Ikenberg K, Cetintas T, Kapaklikaya K, Hutmacher C and Detmar M: Tumor-associated lymphatic vessels upregulate PDL1 to inhibit T-cell activation. Front Immunol. 8:662017. View Article : Google Scholar : PubMed/NCBI | |
|
Lane RS, Femel J, Breazeale AP, Loo CP, Thibault G, Kaempf A, Mori M, Tsujikawa T, Chang YH and Lund AW: IFNγ-activated dermal lymphatic vessels inhibit cytotoxic T cells in melanoma and inflamed skin. J Exp Med. 215:3057–3074. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Nörder M, Gutierrez MG, Zicari S, Cervi E, Caruso A and Guzmán CA: Lymph node-derived lymphatic endothelial cells express functional costimulatory molecules and impair dendritic cell-induced allogenic T-cell proliferation. FASEB J. 26:2835–2846. 2012. View Article : Google Scholar : PubMed/NCBI | |
|
Lund AW, Duraes FV, Hirosue S, Raghavan VR, Nembrini C, Thomas SN, Issa A, Hugues S and Swartz MA: VEGF-C promotes immune tolerance in B16 melanomas and cross-presentation of tumor antigen by lymph node lymphatics. Cell Rep. 1:191–199. 2012. View Article : Google Scholar : PubMed/NCBI | |
|
de Winde CM, Munday C and Acton SE: Molecular mechanisms of dendritic cell migration in immunity and cancer. Med Microbiol Immunol. 209:515–529. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Swartz MA and Lund AW: Lymphatic and interstitial flow in the tumour microenvironment: Linking mechanobiology with immunity. Nat Rev Cancer. 12:210–219. 2012. View Article : Google Scholar : PubMed/NCBI | |
|
Dubrot J, Duraes FV, Harlé G, Schlaeppi A, Brighouse D, Madelon N, Göpfert C, Stokar-Regenscheit N, Acha-Orbea H, Reith W, et al: Absence of MHC-II expression by lymph node stromal cells results in autoimmunity. Life Sci Alliance. 1:e2018001642018. View Article : Google Scholar : PubMed/NCBI | |
|
Li CY, Park HJ, Shin J, Baik JE, Mehrara BJ and Kataru RP: Tumor-associated lymphatics upregulate MHC-II to suppress tumor-infiltrating lymphocytes. Int J Mol Sci. 23:134702022. View Article : Google Scholar : PubMed/NCBI | |
|
Lukacs-Kornek V, Malhotra D, Fletcher AL, Acton SE, Elpek KG, Tayalia P, Collier AR and Turley SJ: Regulated release of nitric oxide by nonhematopoietic stroma controls expansion of the activated T cell pool in lymph nodes. Nat Immunol. 12:1096–1104. 2011. View Article : Google Scholar : PubMed/NCBI | |
|
Antohe M, Nedelcu RI, Nichita L, Popp CG, Cioplea M, Brinzea A, Hodorogea A, Calinescu A, Balaban M, Ion DA, et al: Tumor infiltrating lymphocytes: The regulator of melanoma evolution. Oncol Lett. 17:4155–4161. 2019.PubMed/NCBI | |
|
Mihm MC Jr and Mulé JJ: Reflections on the histopathology of tumor-infiltrating lymphocytes in melanoma and the host immune response. Cancer Immunol Res. 3:827–835. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Durante MA, Rodriguez DA, Kurtenbach S, Kuznetsov JN, Sanchez MI, Decatur CL, Snyder H, Feun LG, Livingstone AS and Harbour JW: Single-cell analysis reveals new evolutionary complexity in uveal melanoma. Nat Commun. 11:4962020. View Article : Google Scholar : PubMed/NCBI | |
|
Li H, van der Leun AM, Yofe I, Lubling Y, Gelbard-Solodkin D, van Akkooi ACJ, van den Braber M, Rozeman EA, Haanen JBAG, Blank CU, et al: Dysfunctional CD8 T cells form a proliferative, dynamically regulated compartment within human melanoma. Cell. 176:775–789.e18. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Yang J, Lian JW, Chin YH, Wang L, Lian A, Murphy GF and Zhou L: Assessing the prognostic significance of tumor-infiltrating lymphocytes in patients with melanoma using pathologic features identified by natural language processing. JAMA Netw Open. 4:e21263372021. View Article : Google Scholar : PubMed/NCBI | |
|
Khong HT, Wang QJ and Rosenberg SA: Identification of multiple antigens recognized by tumor-infiltrating lymphocytes from a single patient: Tumor escape by antigen loss and loss of MHC expression. J Immunother. 27:184–190. 2004. View Article : Google Scholar : PubMed/NCBI | |
|
Maeurer MJ, Gollin SM, Martin D, Swaney W, Bryant J, Castelli C, Robbins P, Parmiani G, Storkus WJ and Lotze MT: Tumor escape from immune recognition: Lethal recurrent melanoma in a patient associated with downregulation of the peptide transporter protein TAP-1 and loss of expression of the immunodominant MART-1/Melan-A antigen. J Clin Invest. 98:1633–1641. 1996. View Article : Google Scholar : PubMed/NCBI | |
|
Al-Batran SE, Rafiyan MR, Atmaca A, Neumann A, Karbach J, Bender A, Weidmann E, Altmannsberger HM, Knuth A and Jäger E: Intratumoral T-cell infiltrates and MHC class I expression in patients with stage IV melanoma. Cancer Res. 65:3937–3941. 2005. View Article : Google Scholar : PubMed/NCBI | |
|
Rodig SJ, Gusenleitner D, Jackson DG, Gjini E, Giobbie-Hurder A, Jin C, Chang H, Lovitch SB, Horak C, Weber JS, et al: MHC proteins confer differential sensitivity to CTLA-4 and PD-1 blockade in untreated metastatic melanoma. Sci Transl Med. 10:eaar33422018. View Article : Google Scholar : PubMed/NCBI | |
|
Passarelli A, Mannavola F, Stucci LS, Tucci M and Silvestris F: Immune system and melanoma biology: A balance between immunosurveillance and immune escape. Oncotarget. 8:106132–106142. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Failli A, Legitimo A, Orsini G, Romanini A and Consolini R: Numerical defect of circulating dendritic cell subsets and defective dendritic cell generation from monocytes of patients with advanced melanoma. Cancer Lett. 337:184–192. 2023. View Article : Google Scholar : PubMed/NCBI | |
|
Garcia-Diaz A, Shin DS, Moreno BH, Saco J, Escuin-Ordinas H, Rodriguez GA, Zaretsky JM, Sun L, Hugo W, Wang X, et al: Interferon receptor signaling pathways regulating PD-L1 and PD-L2 expression. Cell Rep. 19:1189–1201. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Jacobs JFM, Nierkens S, Figdor CG, de Vries IJM and Adema GJ: Regulatory T cells in melanoma: The final hurdle towards effective immunotherapy? Lancet Oncol. 13:e32–e42. 2012. View Article : Google Scholar : PubMed/NCBI | |
|
Petrova V, Arkhypov I, Weber R, Groth C, Altevogt P, Utikal J and Umansky V: Modern aspects of immunotherapy with checkpoint inhibitors in melanoma. Int J Mol Sci. 21:23672020. View Article : Google Scholar : PubMed/NCBI | |
|
Falleni M, Savi F, Tosi D, Agape E, Cerri A, Moneghini L and Bulfamante GP: M1 and M2 macrophages' clinicopathological significance in cutaneous melanoma. Melanoma Res. 27:200–210. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Chen G, Huang AC, Zhang W, Zhang G, Wu M, Xu W, Yu Z, Yang J, Wang B, Sun H, et al: Exosomal PD-L1 contributes to immunosuppression and is associated with anti-PD-1 response. Nature. 560:382–386. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Fankhauser M, Broggi MAS, Potin L, Bordry N, Jeanbart L, Lund AW, Da Costa E, Hauert S, Rincon-Restrepo M, Tremblay C, et al: Tumor lymphangiogenesis promotes T cell infiltration and potentiates immunotherapy in melanoma. Sci Transl Med. 9:eaal47122017. View Article : Google Scholar : PubMed/NCBI | |
|
Moussion C and Turley SJ: Tumour lymph vessels boost immunotherapy. Nature. 552:340–342. 2017. View Article : Google Scholar | |
|
Lund AW, Wagner M, Fankhauser M, Steinskog ES, Broggi MA, Spranger S, Gajewski TF, Alitalo K, Eikesdal HP, Wiig H and Swartz MA: Lymphatic vessels regulate immune microenvironments in human and murine melanoma. J Clin Invest. 126:3389–3402. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Bordry N, Broggi MAS, de Jonge K, Schaeuble K, Gannon PO, Foukas PG, Danenberg E, Romano E, Baumgaertner P, Fankhauser M, et al: Lymphatic vessel density is associated with CD8+ T cell infiltration and immunosuppressive factors in human melanoma. Oncoimmunology. 7:e14628782018. View Article : Google Scholar : PubMed/NCBI | |
|
Sautès-Fridman C, Petitprez F, Calderaro J and Fridman WH: Tertiary lymphoid structures in the era of cancer immunotherapy. Nat Rev Cancer. 19:307–325. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Helmink BA, Reddy SM, Gao J, Zhang S, Basar R, Thakur R, Yizhak K, Sade-Feldman M, Blando J, Han G, et al: B cells and tertiary lymphoid structures promote immunotherapy response. Nature. 577:549–555. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Cipponi A, Mercier M, Seremet T, Baurain JF, Théate I, van den Oord J, Stas M, Boon T, Coulie PG and van Baren N: Neogenesis of lymphoid structures and antibody responses occur in human melanoma metastases. Cancer Res. 72:3997–4007. 2012. View Article : Google Scholar : PubMed/NCBI | |
|
Ladányi A, Sebestyén T, Mohos A, Liszkay G, Somlai B, Tóth E and Tímár J: Ectopic lymphoid structures in primary cutaneous melanoma. Pathol Oncol Res. 20:981–985. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
Cabrita R, Lauss M, Sanna A, Donia M, Skaarup Larsen M, Mitra S, Johansson I, Phung B, Harbst K, Vallon-Christersson J, et al: Tertiary lymphoid structures improve immunotherapy and survival in melanoma. Nature. 577:561–565. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Maibach F, Sadozai H, Seyed Jafari SM, Hunger RE and Schenk M: Tumor-infiltrating lymphocytes and their prognostic value in cutaneous melanoma. Front Immunol. 11:21052020. View Article : Google Scholar : PubMed/NCBI |