Irinotecan plus raltitrexed as second‑line chemotherapy for metastatic colorectal cancer: A retrospective study
- Authors:
- Published online on: June 28, 2024 https://doi.org/10.3892/ol.2024.14542
- Article Number: 409
-
Copyright: © Lian et al. This is an open access article distributed under the terms of Creative Commons Attribution License.
Abstract
Introduction
Colorectal cancer (CRC) currently has the fourth highest incidence of all cancer types and is the second leading cause of cancer-related mortality in the US (1). Given that the 5-year overall survival (OS) rate of patients with CRC is ~52.37% (2), it is necessary to identify more effective treatments for these patients. At present, the standard first-line chemotherapy regimens are combinations of 5-fluorouracil and oxaliplatin (CAPEOX/FOLFOX) or 5-fluorouracil and irinotecan (FOLFIRI). If one first-line treatment fails, the other regimen is used for second-line treatment. However, the reported incidence of fluoropyrimidine-related cardiac toxicity varies from 1.5–18% (3). It is therefore necessary to develop effective alternatives to fluoropyrimidines.
The ARCTIC study reported that raltitrexed, alone or in combination with oxaliplatin or irinotecan, provided a safe option for patients who had previously developed cardiac toxicity from 5-fluorouracil or capecitabine (3). Raltitrexed has been recommended by the European Society for Medical Oncology, National Institute for Health and Clinical Excellence and Chinese Society of Clinical Oncology as an alternative to fluoropyrimidines and is widely used as such (4,5). A phase II study of irinotecan combined with raltitrexed (TOMIRI) as second-line therapy for CRC was conducted in 2003 (6). The same group reported a median OS (mOS) of 11.9 months and median progression-free survival (mPFS) of 4.6 months. The main adverse events (AEs) of this regimen were diarrhea, weakness, vomiting, infection and neutropenia. Other studies have also reported the efficacy of this combination for treating CRC (7,8). However, the efficacy of TOMIRI chemotherapy in the real world still needs to be further elucidated.
Thus, in the present study, clinical experience with TOMIRI chemotherapy in a real-world setting was summarized. Furthermore, a series of possible prognostic factors were assessed to identify those factors that predicted beneficial outcomes.
Materials and methods
Study cohort
The cohort of the present single-institution, retrospective and observational study included 205 patients with advanced CRC who had received TOMIRI chemotherapy at Harbin Medical University Cancer Hospital (Harbin, China) between January 2017 and December 2019 and had provided written informed consent to this treatment. The inclusion criteria were as follows: i) Histological diagnosis of advanced CRC; and ii) patients had received TOMIRI chemotherapy with or without targeted therapy. The exclusion criteria were as follows: i) History of other malignancies; and ii) patients without efficacy evaluation every 3–4 cycles. In addition, 50 patients who had received FOLFIRI as second-line chemotherapy were used as controls. The inclusion criteria were as follows: i) Histological diagnosis of advanced CRC in Harbin Medical University Cancer Hospital; and ii) patients had received FOLFIRI chemotherapy with or without targeted therapy between May 2014 to December 2020.
Efficacy was compared between patients who had received TOMIRI as a first-line and third- or later-line treatment, after which the clinical efficacy of TOMIRI in patients who had received it as second-line chemotherapy was focused on. A flow chart of the study is presented in Fig. S1.
Data collection
Basic patient characteristics, such as chemotherapy regimen, treatment cycles, clinical efficacy and AEs, were independently extracted from medical records by two physicians, as were findings from imaging examinations, including abdomen, chest and pelvis enhanced computed tomography, and liver and rectal nuclear magnetic resonance imaging. These had been performed every two or three cycles as necessary to evaluate the efficacy of chemotherapy. Laboratory findings were collected to analyze AEs after treatment with TOMIRI, including white blood cell count, red blood cell count, hemoglobin, platelet count, urinalysis, liver function tests, renal function tests and electrocardiograms. Carcinoembryonic antigen (CEA) and carbohydrate antigen 19-9 (CA19-9) concentrations were recorded prior to the first cycle of TOMIRI. Age and pathologic tumor-node-metastasis (pTNM) stage at the time of initial diagnosis were recorded. The last follow-up was in December 2021.
Data assessment
The primary endpoints in the present study were OS, PFS, objective response rate (ORR) and disease control rate (DCR). The secondary endpoint was AEs. Tumor responses were assessed in accordance with the Response Evaluation Criteria in Solid Tumors version 1.1 (RECIST 1.1) (9), namely complete remission (CR), partial remission (PR), stable disease (SD) and progressive disease (PD), which were evaluated by two independent physicians. ORR was defined as the sum of patients with complete and partial remissions. The DCR was calculated as the sum of patients with complete and partial remissions and SD. PFS was defined as the time from the start of TOMIRI chemotherapy to detection of any form of disease progression. OS was defined as the time from the start of TOMIRI chemotherapy to mortality from any cause. Treatment-related toxicity was graded according to the Common Terminology Criteria for Adverse Events version 5.0 (10).
Statistical analysis
Basic patient characteristics were analyzed by descriptive statistics. Survival curves were constructed using the Kaplan-Meier method and compared using the log-rank test. In the event of late-stage crossover of curves, the two-stage test was performed using the R package ‘TSHRC’ (Version 0.1.6; R Foundation for Statistical Computing) (11). Pairwise comparisons were performed using the log-rank test with the R package ‘survminer’ (Version 3.5.5; R Foundation for Statistical Computing) and R package ‘survival’ (Version 0.4.9; R Foundation for Statistical Computing). Age, sex, tumor stage, treatment cycles, the use of targeted therapy, surgery (yes or no), tumor location, treatment interval, local treatment (yes or no), RAS, BRAF, first-line PFS, CEA and CA 19-9 were included in the Cox proportional hazards model for univariate and multivariate analysis. Statistical analysis was performed using R version 4.3.1 (R Foundation for Statistical Computing), GraphPad Prism Software Prism Version 7 for Windows (GraphPad Software; Dotmatics) and SPSS 18.0 (SPSS, Inc.). P<0.05 was considered to indicate a statistically significant difference.
Results
Patient characteristics
After excluding the cases that did not meet the inclusion criteria, 205 patients who had received TOMIRI were included in the retrospective analysis. TOMIRI chemotherapy was used as a first-line treatment in 23 patients, as a second-line treatment in 164 patients and as a third- or later-line treatment in 18 patients (Fig. S1). The baseline characteristics of all 205 patients are summarized in Table SI.
Comparison of efficacy
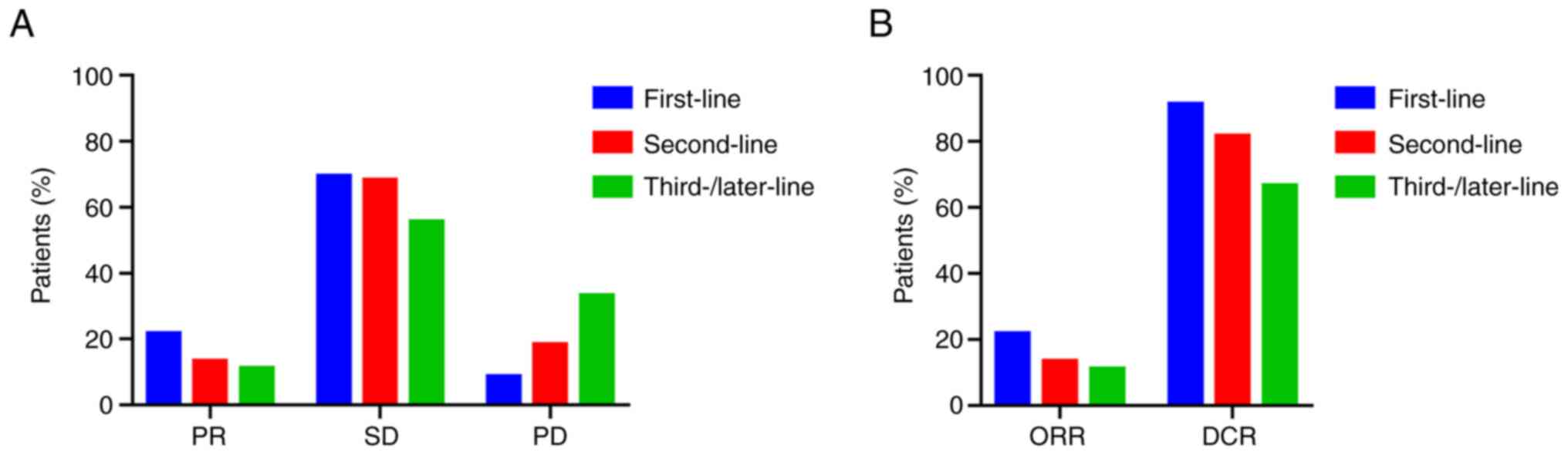
The efficacy of TOMIRI as a first, second and third- or later-line chemotherapy was compared (Fig. 1). The ratios of PR, SD and PD are provided in Fig. 1A. The ORRs of patients who received TOMIRI as a first, second and third- or later-line treatment was 21.7, 13.4 and 11.1%, respectively (Fig. 1B). DCRs were 91.3, 81.7 and 66.7%, respectively, as presented in Fig. 1B. The median PFS of TOMIRI as first-line chemotherapy was 9 months (95% CI, 5.3–12.7), whereas for TOMIRI as a second-line therapy, it was 7 months (95% CI, 6.2–7.8), and as third- or later-line, it was 6 months (95% CI, 4.8–7.2). The median OS was 37 months for TOMIRI as first-line chemotherapy (95% CI, 16.3–57.7), 21 months for TOMIRI as second-line therapy (95% CI, 16.6–25.4) and 17 months for TOMIRI as third- or later-line therapy (95% CI, 10.1–23.9). Earlier treatment with TOMIRI was associated with greater efficacy, suggesting that administering this regimen earlier may improve patient outcomes.
To further evaluate the efficacy of TOMIRI as a second-line treatment for CRC, differences in outcomes between the TOMIRI and FOLFIRI regimens were compared. As indicated in Fig. S2, the differences in PFS and OS were not statistically significant (Fig. S2A and B). However, TOMIRI with targeted therapy achieved longer mOS (25 months) than FOLFIRI with targeted therapy (20 months).
Details of the second-line treatment group
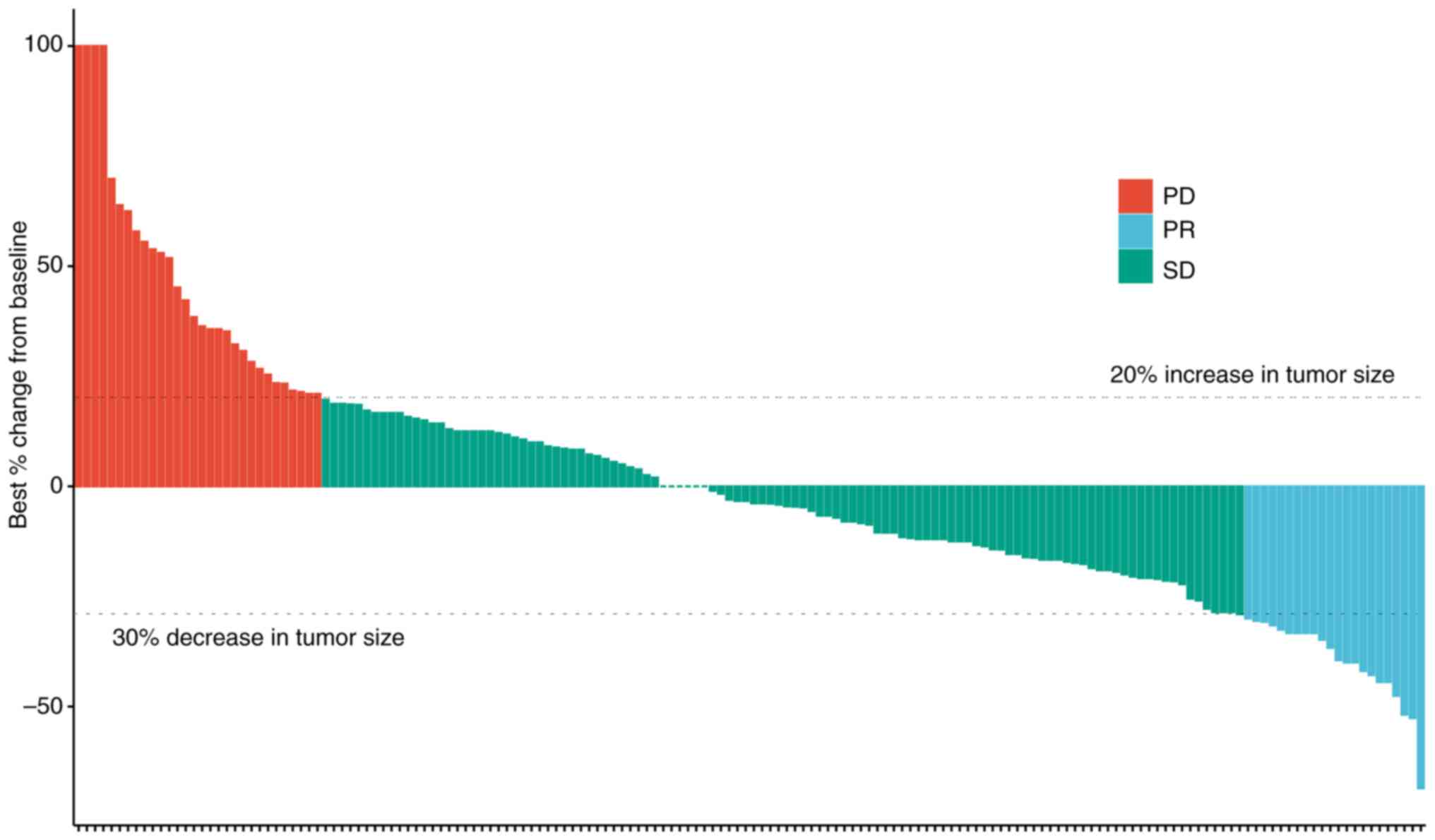
The efficacy of TOMIRI as a second-line chemotherapy treatment was further assessed (Fig. 2). The waterfall plot showed the efficacy of patients receiving TOMIRI, and most patients experienced tumor shrinkage after application. Details of previous treatments are indicated in Table SII. A total of 87 patients had undergone radical surgery, 36 palliative surgery and 15 local radical surgery without excision of metastases. CAPEOX and FOLFOX were administered as first-line chemotherapy in most patients (CAPEOX, 81.1%; FOLFOX, 11.6%). Table SIII presents the details and outcomes of second-line treatments. When TOMIRI was administered as a second-line therapy, PR, SD and PD was achieved in 13.4, 68.3 and 18.3% of patients, respectively. Patients received a median of four cycles of treatment (range, 2–27). Localized lesions had been resected or subjected to radiotherapy or radiofrequency ablation during second-line therapy in 11.0% of patients. The most common third- or later-line treatment was regorafenib (11.6%).
Prognostic factors
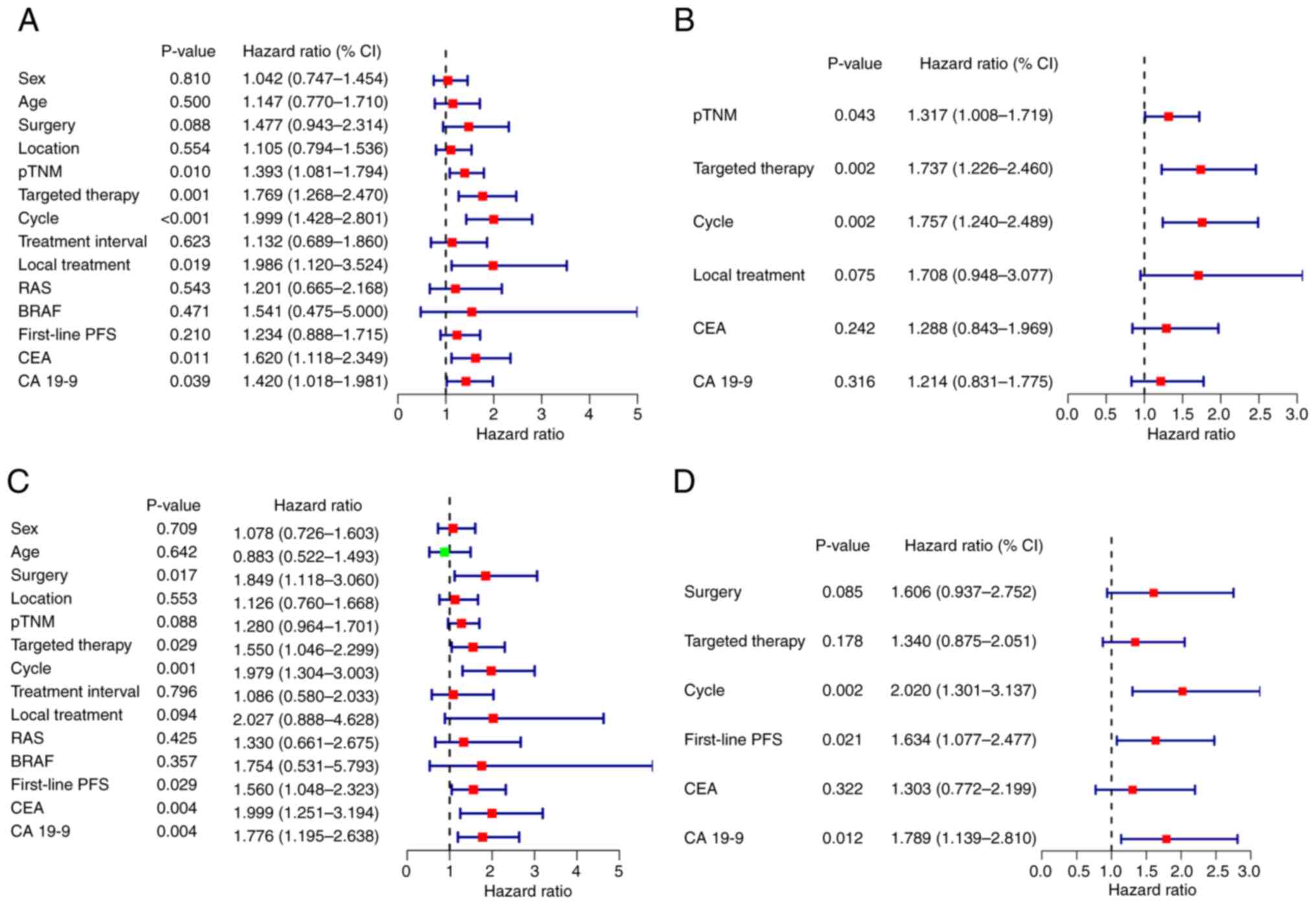
Univariate and multivariate analysis were performed to evaluate prognostic factors (Fig. 3). Multivariate analysis demonstrated that pTNM stage, targeted therapy and the number of treatment cycles were independent prognostic factors for PFS, and treatment cycle, first-line PFS and CA19-9 concentration were independent prognostic factors for OS.
Whether or not surgery had been performed on the primary tumor had no significant impact on PFS after second-line treatment (P=0.17; Fig. S3A). However, as shown in Fig. S3B, the mOS for no surgery, local surgery, palliative surgery and radical surgery was 14, 19, 22 and 25 months, respectively (P=0.091). Similarly, it was found that early diagnosis was associated with a significantly longer PFS. OS showed a similar trend; however, it was not statistically significant (PFS: P=0.006; OS: P=0.210; Fig. S3C and D). Fig. S4 shows the separate impacts of T, N and M stage on prognosis. It was demonstrated that T stage had no significant association with prognosis (PFS: P=0.94; OS: P=0.93), whereas N stage was significantly associated with OS (P=0.0012) and M stage with PFS (P=0.0089). This highlights the importance of N and M stage for prognosis of patients with advanced-stage disease.
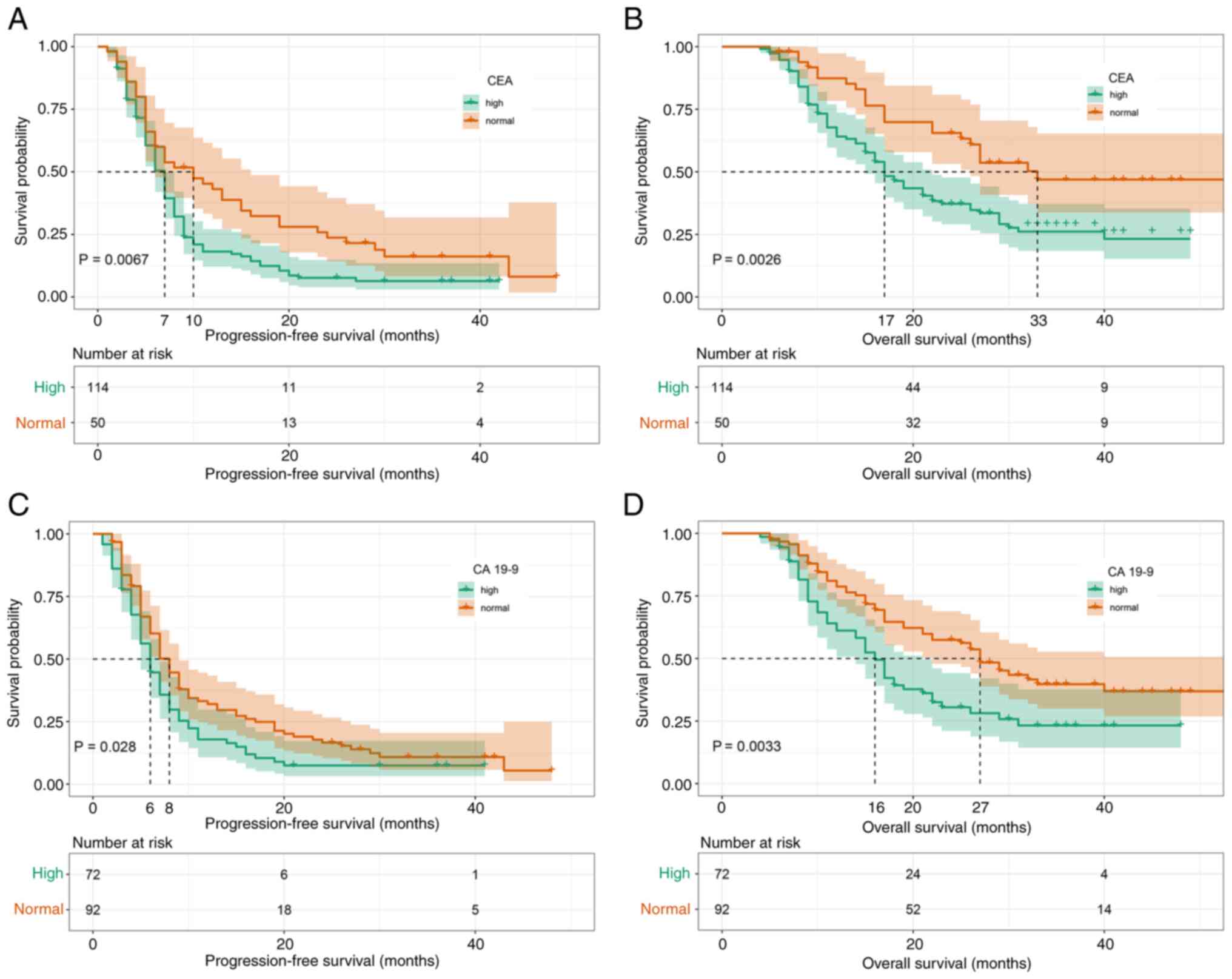
Subsequently, the prognostic effect of pre-second-line therapy CEA and CA19-9 concentrations were analyzed. It was found that high CEA concentrations were significantly associated with a shorter PFS (mPFS, 7 vs. 10 months; P=0.0067; Fig. 4A) and OS (mOS, 17 vs. 33 months; P=0.0026; Fig. 4B). The same was true for pre-second-line therapy CA19-9 concentrations [mPFS, 6 vs. 8 months; P=0.028 (Fig. 4C); and mOS, 16 vs. 27 months; P=0.0033 (Fig. 4D)].
The impact of selected factors on efficacy during second-line therapy was further assessed and significant associations were demonstrated between the number of treatment cycles and longer PFS (mPFS, 5 vs. 9 months; P<0.0001; Fig. 5A) and OS (mOS, 17 vs. 31 months; P=0.00085; Fig. 5B). As recommended by the Chinese Society of Clinical Oncology clinical guidelines (12), 87 patients received bevacizumab or cetuximab combined with chemotherapy, whereas the remaining 77 patients received TOMIRI alone. It was found that targeted therapy had significant positive effects on PFS (mPFS, 6 vs. 8 months; P=0.00033; Fig. 5C) and OS (mOS, 17 vs. 25 months; P=0.025; Fig. 5D). A total of 18 patients had undergone local treatment (resection of metastases, radiofrequency ablation or radiation therapy) during second-line chemotherapy. Local treatment had a significant association with favourable prognosis (Fig. 5E and F), particularly with regard to PFS (mPFS, 6 vs. 11 months; P=0.012).
There were no differences in survival time between patients who had twice-weekly vs. thrice-weekly treatment cycles (Fig. S5). Similarly, the efficacy of first-line treatment was not significantly associated with improved PFS or OS (Fig. S6). However, first-line PFS was significantly associated with improved OS (mOS, 17 vs. 27 months; P=0.024).
To evaluate the importance of prognostic factors in TOMIRI therapy, random forest regression was performed using the following factors: pTNM stage, CEA, CA19-9, treatment cycle, local treatment and targeted therapy. Each of these factors had been identified as prognostic factors in TOMIRI therapy by univariate analysis. The results are demonstrated in Fig. S7, which shows that the number of treatment cycles and the use of targeted therapy were the most important prognostic factors.
Safety
The occurrence of AEs for first-line, second-line and third- or later-line treatment with TOMIRI therapy is presented in Table SIV. Overall, treatment was well tolerated and most hematological toxicities were grade (G)1 or G2 (neutropenia, 12.2%; thrombocytopenia, 10.2%; anemia, 27.3%). Proteinuria (38.1%) and hematuria (21.0%) were also commonly encountered AEs. Hepatic dysfunction was the most commonly reported AE for both G1-2 (55.1%) and G3-4 (7.3%) categories. No cases of gastrointestinal perforation or severe heart failure were identified in the cohort.
Discussion
Irinotecan in combination with raltitrexed has been widely used in patients with CRC who have developed 5-fluoropyrimidine-associated cardiac toxicity (3,5,13). The retrospective analysis in the present study was conducted to evaluate the clinical benefit of TOMIRI chemotherapy. Data from 205 patients who had been treated with this regimen were analyzed. These patients were divided into three groups according to when TOMIRI had been administered: As a first-line treatment (n=23), second-line treatment (n=164) and third- or later-line treatment (n=18). The clinical benefits were evaluated using the primary endpoints of PFS, OS, ORR and DCR.
It was found that TOMIRI chemotherapy was most effective when administered as first-line treatment (ORR, 21.7%; DCR, 91.3%; mPFS, 10 months; and mOS, 37 months). The results for its administration as second-line treatment (ORR, 13.4%; DCR, 81.7%; mPFS, 7 months; and mOS, 21 months) indicated that this regimen may be more beneficial to administer earlier. This finding is supported by a previous meta-analysis demonstrating that, when administered as a first-line therapy, this combination had an ORR of 34.1%, mPFS of 6.7 months and mOS of 14.2 months (4). In another study, 75 patients were treated with raltitrexed alone, oxaliplatin + raltitrexed or TOMIRI with or without bevacizumab. The mPFS and mOS were 10.6 (95% CI, 8.2–13.1) and 27.4 months (95% CI, 24.1–38.1), respectively (14). Although the present study did not reach the ORR reported in the meta-analysis, the results for mPFS and mOS were improved as compared to a previous study. This discrepancy may be attribuTable to insufficient numbers of patients and different decades of treatment.
Most of the patients in the present study were in the second-line treatment group according to the relevant guidelines. The mOS and mPFS for FOLFIRI as a second-line therapy were 15.4 and 6.2 months, respectively, which is consistent with findings of previous studies (15). In another study, the mPFS was 6.4 months with bevacizumab/FOLFOX and 6.9 months with bevacizumab/FOLFIRI. As for mOS, it was 14.1 months with bevacizumab/FOLFOX and 15.7 months with bevacizumab/FOLFIRI (16). In a phase II trial, twice-weekly TOMIRI as a second-line therapy for metastatic CRC achieved a median PFS of 4.5 months (95% CI, 3.8–5.2) and a median OS of 12.0 months (95% CI, 8.5–15.5) (17). Thus, TOMIRI chemotherapy achieved greater benefits in the patients in the present study than in previous studies (15,16). In the present study, TOMIRI achieved noninferiority PFS and OS benefit as compared with FOFIRI. TOMIRI with targeted therapy achieved longer mOS than FOLFIRI with targeted therapy. As the sample of the present study was relatively small (more patients in the oncology centers of Harbin Medical University Cancer Hospital were either administered TOMIRI or had been enrolled in clinical trials), the findings need to be confirmed by further clinical studies.
Furthermore, factors that identified the patients that were most likely to benefit from TOMIRI administered as a second-line therapy after recurrence or progression after standard first-line chemotherapy were assessed. Kaplan-Meier curves on variables identified as significant by univariate analysis were analyzed, and then the results of the two statistical analyses were combined to reach the following conclusions: Surgical resection, even palliative surgery on the primary tumor, significantly and positively impacted long-term survival, which is consistent with previously published data (18,19). However, it had no effect on the outcomes of second-line treatment. Of note, tumor stage at first diagnosis was a greater predictor of efficacy of second-line treatment. However, N and M stage, but not T stage, were associated with a greater duration of survival. As reported in a previous study, the prognosis after second-line therapy varied considerably according to tumor biomarker status (20). Results from the present study demonstrated that patients with normal and high CEA concentrations before second-line treatment achieved an mPFS of 7 and 10 months, respectively, whereas they achieved an mOS of 17 and 31 months, respectively. Normal CA19-9 concentrations were also associated with a significant survival advantage compared with high CA19-9 concentrations (mPFS, 6 vs. 8 months; mOS, 17 vs. 33 months). Previous analyses have shown that patients who have better responses to first-line chemotherapy or longer PFS are more likely to benefit from second-line chemotherapy (21,22). In the present study, a comprehensive analysis of tumor responses demonstrated that patients who had longer PFS with first-line chemotherapy had longer OS, but not PFS, with second-line chemotherapy.
The efficacy of second-line chemotherapy is highly dependent on the combination used. Results of the present study showed that administration of targeted therapy, whether bevacizumab or cetuximab, was associated with prolongation of the survival time (OS, 25 vs. 17 months). However, as so few patients received targeted therapy, it was not possible to distinguish between the impacts of bevacizumab vs. cetuximab when used as a component of second-line treatment. Furthermore, the PRODIGE18 study reported a non-significant difference between bevacizumab and cetuximab with chemotherapy when administered to patients with wild-type RAS metastatic CRC (23). However, certain patients who underwent local treatment of metastases, including resection, radiofrequency ablation and radiation therapy, had improved outcomes. These findings need to be further validated by large prospective trials. Furthermore, the present study found no significant difference in efficacy and safety between twice-weekly and thrice-weekly cycles, which is consistent with previously reported findings (24,25).
The present study did not reveal any significant association between treatment time-line and clinical AEs. Overall, the incidence of G3/4 AEs was low. However, the patients did present with higher incidences of myelosuppression and liver dysfunction than previously reported (6). This indicates that it is important to protect liver function and prevent myelosuppression whilst using TOMIRI chemotherapy.
In conclusion, the findings of the present study suggest that irinotecan in combination with raltitrexed chemotherapy may be a superior choice than FOLFIRI for second-line chemotherapy in patients with CRC.
Supplementary Material
Supporting Data
Supporting Data
Acknowledgements
Not applicable.
Funding
The present work was supported by the National Natural Science Foundation of China (grant no. U20A20376), Beijing Award Foundation (grant no. YXJL-2020-0818-0478), Heilongjiang Province Postdoctoral Science Foundation (grant no. LBHZ21189), Harbin Medical University Innovative Science Research Funded Project (grant no. 2022-KYYWF-0289) and China Postdoctoral Science Foundation (grant no. 2022MD713747).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
JL, XP, BX and HL conceived and designed the analysis. JL, RW, XW, XP, BX, ST and JS collected the data. JL, RW and XW performed the analysis. JL and HL wrote the manuscript. All authors have read and approved the final manuscript. JL and HL confirm the authenticity of all the raw data.
Ethics approval and consent to participate
The research protocol was approved by Harbin Medical University Cancer Hospital (approval no. KY2017-19) and the study was conducted in accordance with the principles of the Declaration of Helsinki. Written informed consent for treatment with the TOMIRI or FOLFIRI regimen was obtained from the patients or their parents before the start of treatment.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
|
Siegel RL, Miller KD, Fuchs HE and Jemal A: Cancer statistics, 2021. CA Cancer J Clin. 71:7–33. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Li X, Zhou Y, Luo Z, Gu Y, Chen Y, Yang C, Wang J, Xiao S, Sun Q, Qian M and Zhao G: The impact of screening on the survival of colorectal cancer in Shanghai, China: A population based study. BMC Public Health. 19:10162019. View Article : Google Scholar : PubMed/NCBI | |
|
Ransom D, Wilson K, Fournier M, Simes RJ, Gebski V, Yip D, Tebbutt N, Karapetis CS, Ferry D, Gordon S and Price TJ: Final results of Australasian gastrointestinal trials group ARCTIC study: An audit of raltitrexed for patients with cardiac toxicity induced by fluoropyrimidines. Ann Oncol. 25:117–121. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
Barni S, Ghidini A, Coinu A, Borgonovo K and Petrelli F: A systematic review of raltitrexed-based first-line chemotherapy in advanced colorectal cancer. Anticancer Drugs. 25:1122–1128. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
Batra A, Rigo R, Hannouf MB and Cheung WY: Real-world safety and efficacy of raltitrexed in patients with metastatic colorectal cancer. Clin Colorectal Cancer. 20:e75–e81. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Aparicio J, Vicent JM, Maestu I, Garcerá S, Busquier I, Bosch C, Llorca C, Díaz R, Fernández-Martos C and Galán A: Multicenter phase II trial evaluating a three-weekly schedule of irinotecan plus raltitrexed in patients with 5-fluorouracil-refractory advanced colorectal cancer. Ann Oncol. 14:1121–1125. 2003. View Article : Google Scholar : PubMed/NCBI | |
|
Chiara S, Nobile MT, Tomasello L, Acquati M, Taveggia P, Murolo C, Percivale P and Rosso R: Phase II trial of irinotecan and raltitrexed in chemotherapy-naive advanced colorectal cancer. Anticancer Res. 25:1391–1396. 2005.PubMed/NCBI | |
|
Aparicio J, de las Peñas R, Vicent JM, Garcerá S, Llorca C, Maestu I, Yuste AL and Farrés J: Multicenter phase I study of irinotecan plus raltitrexed in patients with 5-fluorouracil-refractory advanced colorectal cancer. Oncology. 63:42–47. 2002. View Article : Google Scholar : PubMed/NCBI | |
|
Schwartz LH, Seymour L, Litière S, Ford R, Gwyther S, Mandrekar S, Shankar L, Bogaerts J, Chen A, Dancey J, et al: RECIST 1.1-standardisation and disease-specific adaptations: Perspectives from the RECIST working group. Eur J Cancer. 62:138–145. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Basch E, Dueck AC, Rogak LJ, Mitchell SA, Minasian LM, Denicoff AM, Wind JK, Shaw MC, Heon N, Shi Q, et al: Feasibility of implementing the patient-reported outcomes version of the common terminology criteria for adverse events in a multicenter trial: NCCTG N1048. J Clin Oncol. 36:JCO20187886202018. View Article : Google Scholar : PubMed/NCBI | |
|
Li H, Han D, Hou Y, Chen H and Chen Z: Statistical inference methods for two crossing survival curves: A comparison of methods. PLoS One. 10:e01167742015. View Article : Google Scholar : PubMed/NCBI | |
|
Dong C, Ding Y, Weng S, Li G, Huang Y, Hu H, Zhang Z, Zhang S and Yuan Y: Update in version 2021 of CSCO guidelines for colorectal cancer from version 2020. Chin J Cancer Res. 33:302–307. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Fohlen A, Bordji K, Assenat E, Gongora C, Bazille C, Boulonnais J, Naveau M, Breuil C, Pérès EA, Bernaudin M and Guiu B: Anticancer drugs for intra-arterial treatment of colorectal cancer liver metastases: In-vitro screening after short exposure time. Pharmaceuticals (Basel). 14:6392021. View Article : Google Scholar : PubMed/NCBI | |
|
Gallois C, Hafliger E, Auclin E, Perret A, Coutzac C, Turpin A, Pellat A, Randrian V, Basile D, Faroux R, et al: First-line chemotherapy with raltitrexed in metastatic colorectal cancer: An association des gastro-entérologues oncologues (AGEO) multicentre study. Dig Liver Dis. 54:684–691. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Clarke SJ, Yip S, Brown C, van Hazel GA, Ransom DT, Goldstein D, Jeffrey GM, Tebbutt NC, Buck M, Lowenthal RM, et al: Single-agent irinotecan or FOLFIRI as second-line chemotherapy for advanced colorectal cancer; results of a randomised phase II study (DaVINCI) and meta-analysis [corrected]. Eur J Cancer. 47:1826–1836. 2011. View Article : Google Scholar : PubMed/NCBI | |
|
Bendell JC, Tournigand C, Swieboda-Sadlej A, Barone C, Wainberg ZA, Kim JG, Pericay C, Pastorelli D, Tarazi J, Rosbrook B, et al: Axitinib or bevacizumab plus FOLFIRI or modified FOLFOX-6 after failure of first-line therapy for metastatic colorectal cancer: A randomized phase II study. Clin Colorectal Cancer. 12:239–247. 2013. View Article : Google Scholar : PubMed/NCBI | |
|
Cheng K, Zhou YW, Chen Y, Li ZP, Qiu M and Liu JY: Biweekly raltitrexed combined with irinotecan as second-line therapy for patients with metastatic colorectal cancer: A phase II trial. Cancer Control. 29:107327482210803322022. View Article : Google Scholar : PubMed/NCBI | |
|
Arhin ND, Shen C, Bailey CE, Matsuoka LK, Hawkins AT, Holowatyj AN, Ciombor KK, Hopkins MB, Geiger TM, Kam AE, et al: Surgical resection and survival outcomes in metastatic young adult colorectal cancer patients. Cancer Med. 10:4269–4281. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Wong EYT, Tan GHC, Ng DWJ, Koh TPT, Kumar M and Teo MCC: Surgical management of metastatic colorectal cancer: A single-centre experience on oncological outcomes of pulmonary resection vs cytoreductive surgery and HIPEC. J Gastrointest Cancer. 48:353–360. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Yoshino T, Obermannová R, Bodoky G, Garcia-Carbonero R, Ciuleanu T, Portnoy DC, Kim TW, Hsu Y, Ferry D, Nasroulah F and Tabernero J: Baseline carcinoembryonic antigen as a predictive factor of ramucirumab efficacy in RAISE, a second-line metastatic colorectal carcinoma phase III trial. Eur J Cancer. 78:61–69. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Palmieri LJ, Fihri A, Doat S, Dubreuil O, Manceau G, Karoui M, Wagner M, Lucidarme O and Bachet JB: Tumor-size responses to first-line is a predictor of overall survival in metastatic colorectal cancer. Eur Radiol. 29:3871–3880. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Lavacchi D, Roviello G, Giommoni E, Dreoni L, Derio S, Brugia M, Amedei A, Pillozzi S and Antonuzzo L: Aflibercept plus FOLFIRI as second-line treatment for metastatic colorectal cancer: A single-institution real-life experience. Cancers (Basel). 13:38632021. View Article : Google Scholar : PubMed/NCBI | |
|
Bennouna J, Hiret S, Bertaut A, Bouché O, Deplanque G, Borel C, François E, Conroy T, Ghiringhelli F, des Guetz G, et al: Continuation of bevacizumab vs cetuximab plus chemotherapy after first progression in KRAS wild-type metastatic colorectal cancer: The UNICANCER PRODIGE18 randomized clinical trial. JAMA Oncol. 5:83–90. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Matsuda C, Honda M, Tanaka C, Fukunaga M, Ishibashi K, Munemoto Y, Hata T, Bando H, Oshiro M, Kobayashi M, et al: Multicenter randomized phase II clinical trial of oxaliplatin reintroduction as a third- or later-line therapy for metastatic colorectal cancer-biweekly versus standard triweekly XELOX (the ORION study). Int J Clin Oncol. 21:566–572. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Hurwitz H, Mitchell E, Cartwright T, Kwok A, Hu S, McKenna E and Patt YZ: A randomized, phase II trial of standard triweekly compared with dose-dense biweekly capecitabine plus oxaliplatin plus bevacizumab as first-line treatment for metastatic colorectal cancer: XELOX-A-DVS (dense versus standard). Oncologist. 17:937–946. 2012. View Article : Google Scholar : PubMed/NCBI |