Exploration of the role of EMC3‑AS1 as a potential diagnostic and prognostic indicator in liver cancer
- Authors:
- Published online on: June 28, 2024 https://doi.org/10.3892/ol.2024.14545
- Article Number: 412
-
Copyright: © Liu et al. This is an open access article distributed under the terms of Creative Commons Attribution License.
Abstract
Introduction
According to 2020 Global Cancer Statistics, liver cancer accounts for 75–85% of primary liver tumors, with ~906,000 new cases diagnosed each year, and is the third most frequent cause of cancer-associated mortality worldwide (1). Due to the insidious onset of liver cancer and the absence of early clinical manifestations, the majority of patients with liver cancer are diagnosed at an advanced disease stage, which precludes surgical intervention (2). Despite advancements in treatment modalities such as liver transplantation, anatomical liver resection, interventional therapy, local ablation therapy, radiation therapy and targeted therapy, the 5-year overall survival (OS) rate remains at <15% (3).
Long non-coding RNAs (lncRNAs), a class of RNAs >200 nucleotides in length without protein translation capacity, play a pivotal role in tumorigenesis (4). Certain lncRNAs are known to be involved in various biological processes, including cell stemness, DNA damage, chemical resistance, immune escape, epithelial-mesenchymal transition (EMT) and metabolic disorders (5). The aberrant expression of lncRNA may contribute to the occurrence, progression, invasion and metastasis of diverse tumors, including liver cancer (6–9). For instance, Tang et al (10) identified that the expression of lncRNA CRNDE was upregulated in liver cancer, and promoted the proliferation and migration of liver cancer cells by sponging microRNA (miR)-337-3p, thereby upregulating the expression of sineoculis homeobox homolog 1. In another study, lncRNA CEBPA-DT was shown to interact with heterogeneous nuclear ribonucleoprotein C, thereby activating the interaction between discoidin domain receptor 2 and β-catenin, leading to liver cancer metastasis (11). Furthermore, lncRNA SNHG7 was indicated to induce the proliferation and migration of liver cancer cells by sponging miR-122-5p (12), and lncRNA ANRIL was demonstrated to regulate liver cancer cell proliferation, migration and invasion via the targeting of miR-384 and modulation of STAT3 (13).
The formation of N6-methyladenosine (m6A) is a frequently occurring epigenetic modification that is essential for mRNA splicing, export, translation and degradation, and has been shown to play an important role in the occurrence and progression of numerous malignancies (14). Previous studies have demonstrated that lncRNAs regulate m6A modifications in various types of cancer (15). Endoplasmic reticulum membrane protein complex subunit 3 antisense RNA 1 (EMC3-AS1) is an lncRNA located on chromosome 3q25. A correlation analysis performed in our preliminary screening analysis (unpublished data) revealed that the expression of EMC3-AS1 positively correlates with that of m6A-associated genes in liver cancer, including methyltransferase-like 3, RNA-binding motif protein 15B, YTH N6-methyladenosine RNA-binding protein F1, heterogeneous nuclear ribonucleoprotein A2/B1 and RNA-binding motif protein X-linked. However, the expression and function of lncRNA EMC3-AS1 have not yet been thoroughly explored in liver cancer.
In the current study, the differential expression of EMC3-AS1 between liver cancer tissues and adjacent normal (AN) liver tissues was identified by the analysis of data from The Cancer Genome Atlas (TCGA) and three Gene Expression Omnibus (GEO) datasets, and subsequently validated in a primary clinical cohort. The potential association of EMC3-AS1 expression with the prognosis and diagnosis of patients with liver cancer was then explored. The tumor microenvironment (TME) was also compared between liver cancer tissues with high (H) and low (L) expression of EMC3-AS1. Additionally, EMC3-AS1 was silenced using small interfering RNA (siRNA) in HepG2, Sk-Hep-1 and Huh-7 liver cancer cells, and cell proliferation, colony formation and migration were evaluated in vitro.
Materials and methods
Data acquisition and expression analysis
Gene expression data, comprising fragments per kilobase of transcripts per million mapped reads, and the respective clinical data of patients with liver cancer were obtained from TCGA (http://cancergenome.nih.gov/). Subsequently, the format of the gene expression data was converted into transcripts per million reads values for analysis. In addition, three datasets (GSE22058, GSE25097 and GSE64041) (16–18) containing data on 428 tumor specimens and 451 AN specimens were downloaded from the GEO database (https://www.ncbi.nlm.nih.gov/geo/) for the validation of EMC3-AS1 expression (Table SI). The expression of EMC3-AS1 was compared between tumor and AN tissues in the datasets from TCGA and the GEO, and 50 pairs of liver cancer tissues and AN tissues from the same patients in TCGA were compared as well.
Collection of patients and tissue specimens
A total of 42 liver cancer and 35 AN tissues (at least 2 cm away from the tumor edge) were acquired from patients with liver cancer (n=42; mean age, 60.1 years; age range, 35–80 years) who underwent liver resection in Sichuan Provincial People's Hospital (Chengdu, China) between January 2022 and September 2022. Information on each patient is listed in Table SII. Patients in this study were diagnosed with liver cancer by preoperative imaging examination or biopsy, and their preoperative liver function was classified as Child-Pugh grade A (19). None of the patients underwent preoperative therapy. Patients with cholangiocarcinoma or metastatic liver cancer were excluded. After resection, all tissue specimens were promptly frozen in liquid nitrogen and preserved at −80°C. The present study was conducted in compliance with the Declaration of Helsinki and was approved by the Ethics Committee of Sichuan Provincial People's Hospital (approval no. 2022-2). Before surgery, all patients provided written informed consent for the use of their tissue samples in the present study.
RNA extraction and reverse transcription-quantitative PCR (RT-qPCR)
Total RNA was isolated from liver cancer and normal liver tissues using TRIzol® (Invitrogen; Thermo Fisher Scientific, Inc.). A NanoDrop spectrophotometer (NanoDrop Technologies; Thermo Fisher Scientific, Inc.) was utilized to examine the yield and purity of the RNA samples. A 260/280 optical density ratio of 1.8–2.2 was considered to indicate acceptable RNA purity. Agarose gel electrophoresis was then conducted, and the integrity and diffusion of the bands were examined to assess the quality of the total RNA. Samples displaying RNA degradation during collection were excluded to ensure the accuracy and reliability of the experimental data. Subsequently, cDNA synthesis was conducted using a PrimeScript™ RT Reagent kit with DNA Eraser (Takara Bio, Inc.) according to the manufacturer's instructions. qPCR was then conducted using TB-Green®-Premix-Ex-Taq™-II (Takara Bio, Inc.). EMC3-AS1 and GAPDH gene products were amplified, with the latter serving as the reference gene. qPCR was performed under the following conditions: 95°C for 30 sec, then 40 cycles of 95°C for 5 sec, 55°C for 30 sec and 72°C for 1 min, followed by 95°C for 10 sec and 65°C for 5 sec. The primers used were as follows: GAPDH forward, 5′-ACATCGCTCAGACACCATG-3′ and reverse, 5′-ACCAGAGTTAAAAGCAGCCC-3′; and EMC3-AS1 forward, 5′-TGCCTCAGTATCTGAACACAAG-3′ and reverse, 5′-TTGAGCCAGGGACATTTCTG-3′. Relative expression level was obtained using the 2−Δ∆Cq method (20).
Prognostic and diagnostic value of EMC3-AS1
Patients with liver cancer from TCGA were categorized into H-EMC3-AS1 and L-EMC3-AS1 expression groups according to the median EMC3-AS1 expression value. Kaplan-Meier analysis and the log-rank test were used to compare OS and disease-free survival (DFS) between the H- and L-EMC3-AS1 expression groups using the survival (version 3.3) package in the R platform. The pROC (version 1.18) (21) package was used to perform receiver operating characteristic (ROC) curve analysis on the aforementioned datasets and the present cohort.
Functional enrichment analysis
Gene set variation analysis (GSVA) was performed to assess the variation of pathways between the H- and L-EMC3-AS1 expression groups using the GSVA (version 1.44.2) package in R (22), and the data were displayed using heatmaps. Hallmark and Kyoto Encyclopedia of Genes and Genomes (KEGG) gene sets were acquired from MSigDB (23). Gene set enrichment analysis (GSEA) was carried out to determine the differential functional phenotype between the H- and L-EMC-AS1 expression groups using the clusterProfiler (version 4.4.4) (24) package. A false discovery rate (FDR) of <0.05 was used as the cut-off threshold for significantly enriched gene sets. The limma (version 3.52.1) (25) package was employed to identify differentially expressed mRNAs (DEmRNAs) between the H- and L-EMC-AS1 expression groups. Genes with a |log2 fold-change (FC)|>1 and FDR <0.05 were selected as DEmRNAs. Gene Ontology (GO) and KEGG pathway analyses were performed using the clusterProfiler package. Adjusted P<0.05 was used as the cut-off threshold for GO terms and KEGG pathways.
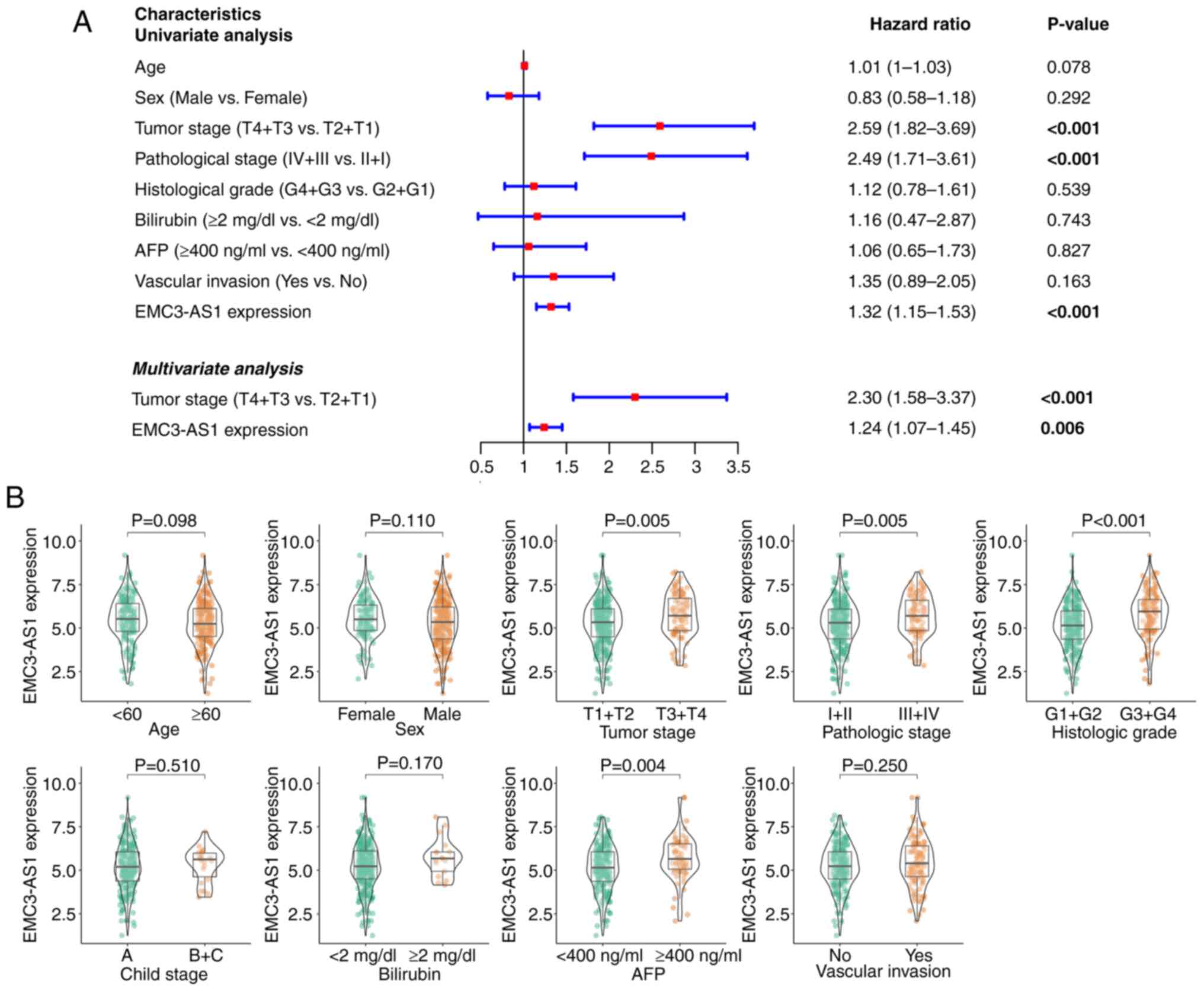
Analysis of EMC3-AS1 as a risk factor for OS and its association with clinicopathological features
Univariate and multivariate Cox regression analyses were conducted to screen for the risk factors for poor prognosis (shorter OS time) in patients with liver cancer. Subgroup analyses for EMC3-AS1 expression were conducted based on age, sex, tumor stage, pathological stage, tumor grade, Child-Pugh stage, bilirubin level, α-fetoprotein (AFP) level and vascular invasion status. The 7th American Joint Committee on Cancer staging system was used for staging (26).
Association of EMC3-AS1 expression with tumor-infiltrating immune cells (TIICs)
CIBERSORT (version 1.06) (27) is a deconvolution algorithm used to distinguish 22 human immune cell subtypes based on the gene expression in complex tissues. The CIBERSORT algorithm was used to determine the fraction of TIICs in the tumor environment, and the associations between the infiltration of 22 subtypes of immune cells and EMC3-AS1 expression in liver cancer were determined.
Immune checkpoint (IC) inhibitors (ICIs) have markedly changed cancer treatment in recent decades (28). Thus, the mRNA expression levels of 35 ICs, such as cytotoxic T-lymphocyte antigen 4 (CTLA4), programmed cell death protein 1 (PDCD1), CD86, CD274, CD276, hepatitis A virus cellular receptor 2 (HAVCR2), lymphocyte-activation gene 3 (LAG3), galectin 9 (LGALS9), neuropilin 1 (NRP1) and T cell immunoreceptor with Ig and ITIM domains (TIGIT), among others, were compared between the H- and L-EMC-AS1 expression groups in the present study.
The activity of cancer stem cells (CSCs) can be quantified using the mRNA stemness index (mRNAsi) and epigenetically regulated mRNAsi (EREG-mRNAsi) (29), which reflect the dedifferentiation capacity of CSCs. Therefore, the score of mRNAsi and EREG-mRNAsi obtained from previous research (29) were compared between the H- and L-EMC3-AS1 expression groups. Pearson's correlation analysis was applied to investigate the correlations between the expression of EMC3-AS1 and mRNAsi or EREG-mRNAsi. Furthermore, ESTIMATE score, immune score and stromal score generated by ESTIMATE algorithm (30) could reflect tumor immune infiltration level. These scores of patients with liver cancer in TCGA were obtained from the ESTIMATE database (https://bioinformatics.mdanderson.org/estimate/) and were compared between H- and L-EMC3-AS1 expression groups, respectively. Additionally, the activities of 13 immune-associated pathways were evaluated using the single-sample GSEA (ssGSEA) algorithm in GSVA (version 1.44.2) package (21), and comparisons between the H- and L-EMC3-AS1 expression groups were made.
Therapeutic correlation analyses
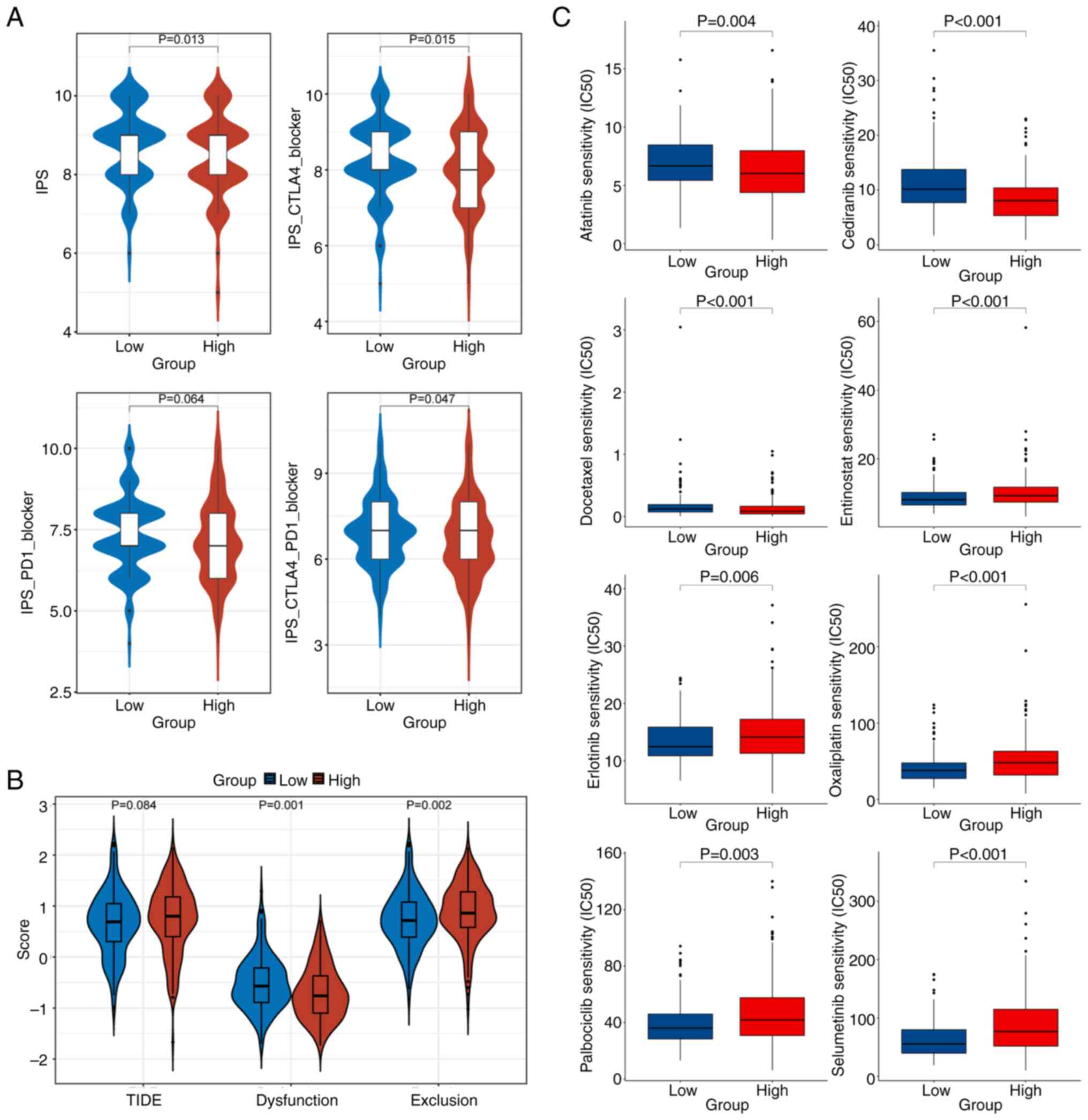
The immunophenoscore (IPS) is a scoring scheme ranging from 0 to 10 that reflects the response of patients with cancer to anti-CTLA4 and anti-programmed cell death protein 1 (PD-1) therapy (31). IPSs were obtained from The Cancer Immunome Atlas (TCIA; http://tcia.at/home), and were compared between the H- and L-EMC3-AS1 expression groups to assess the association between immunotherapy response and EMC3-AS1 expression in liver cancer.
The tumor immune dysfunction and exclusion (TIDE) scoring scheme (32) is a predictor of the response to IC blockade in patients with cancer. The TIDE score of patients with liver cancer was computed using the TIDE online tool (http://tide.dfci.harvard.edu), and the TIDE scores of the H- and L-EMC3-AS1 expression groups were compared. Furthermore, the oncoPredict (version 0.2) package (33) was used to compute the half-maximal inhibitory concentrations of various chemotherapeutic and molecular targeted drugs for the treatment of liver cancer, and the difference in drug sensitivity between the H- and L-EMC3-AS1 expression groups was then compared.
Cell culture, transfection and treatment
HepG2, Sk-Hep-1 and Huh-7 cells were obtained from The Cell Bank of Type Culture Collection of The Chinese Academy of Sciences and were authenticated using STR profiling. The HepG2 and Huh-7 cell lines were cultured in high-glucose DMEM (Gibco; Thermo Fisher Scientific, Inc.) containing 10% FBS (Biological Industries; Sartorius AG) and 1% streptomycin-penicillin (Biological Industries; Sartorius AG), while the Sk-Hep-1 cells were cultured in minimum essential medium (Gibco; Thermo Fisher Scientific, Inc.) containing 10% FBS and 1% streptomycin-penicillin. Cells were maintained in a 37°C incubator with 5% CO2.
The HepG2, Sk-Hep-1 and Huh-7 cells were transfected with siRNAs (Shanghai GenePharma Co., Ltd.) using Lipofectamine® 2000 (Invitrogen; Thermo Fisher Scientific, Inc.) at a final concentration of 200 nM. After 48 h of transfection at 37°C, total RNA was extracted. The efficacy of the siRNA transfection was assessed to determine the interference efficiency. The siRNAs demonstrating the highest interference efficacy were chosen for further analyses. The siRNA sequences were as follows: EMC3-AS1-negative control (NC) sense, 5′-UUCUCCGAACGUGUCACGUTT-3′ and antisense, 5′-ACGUGACACGUUCGGAGAATT-3′; EMC3-AS1-1739 sense, 5′-GUGCCACCAUGGAUAUUCATT-3′ and antisense, 5′-UGAAUAUCCAUGGUGGCACTT-3′; EMC3-AS1-2842 sense, 5′-CCACCAGAUAUACCACUAUTT-3′ and antisense, 5′-AUAGUGGUAUAUCUGGUGGTT-3′.
Colony formation assay
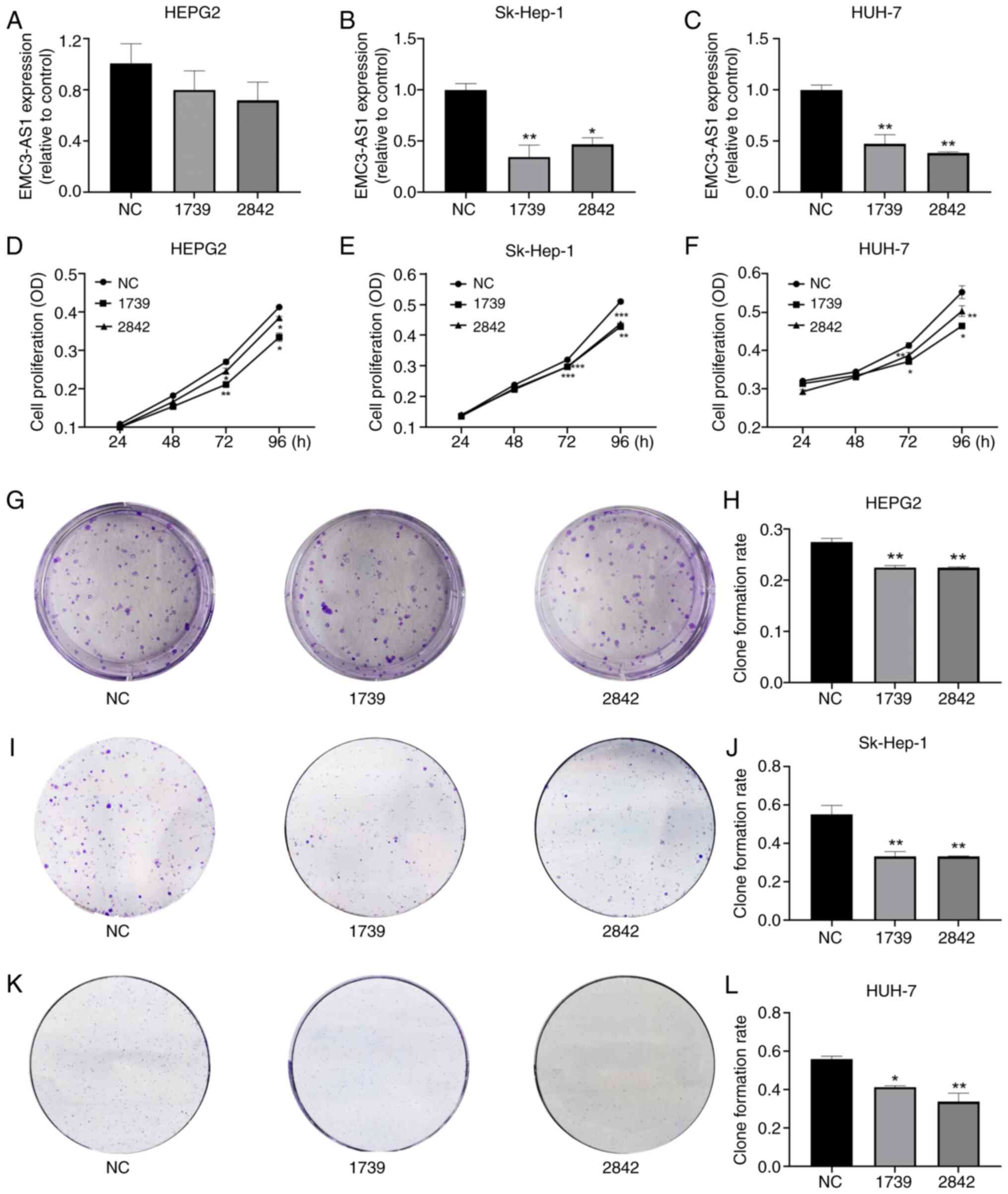
Following transfection, the HepG2, Sk-Hep-1 and Huh-7 cells (2,000 cells/well) were independently cultured in 6-well plates and incubated for 14 days. Subsequently, the cells were fixed with 4% paraformaldehyde for 20 min at room temperature and stained with crystal violet (0.5%; Sigma-Aldrich; Merck KGaA) for 15–20 min at room temperature. A cluster with >50 cells was defined as a colony. Colony numbers were calculated using ImageJ software (Version 1.52; National Institutes of Health), and representative images were captured.
Cell proliferation assay
To detect cell proliferation, MTT (Sigma-Aldrich; Merck KGaA) was added to transfected HepG2, Sk-Hep-1 and Huh-7 cells. The cells (4,000 cells/well) were seeded in a 96-well plate (100 µl/well) and cultured. After 24, 48, 72 or 96 h of transfection, 100 µl MTT was added and the cells were incubated for another 4 h. Next, the supernatant was removed, and DMSO was added (150 µl/well). The absorbance at 490 nm was recorded using a microplate reader after shaking on an orbital shaker for 10 min.
Wound healing assay
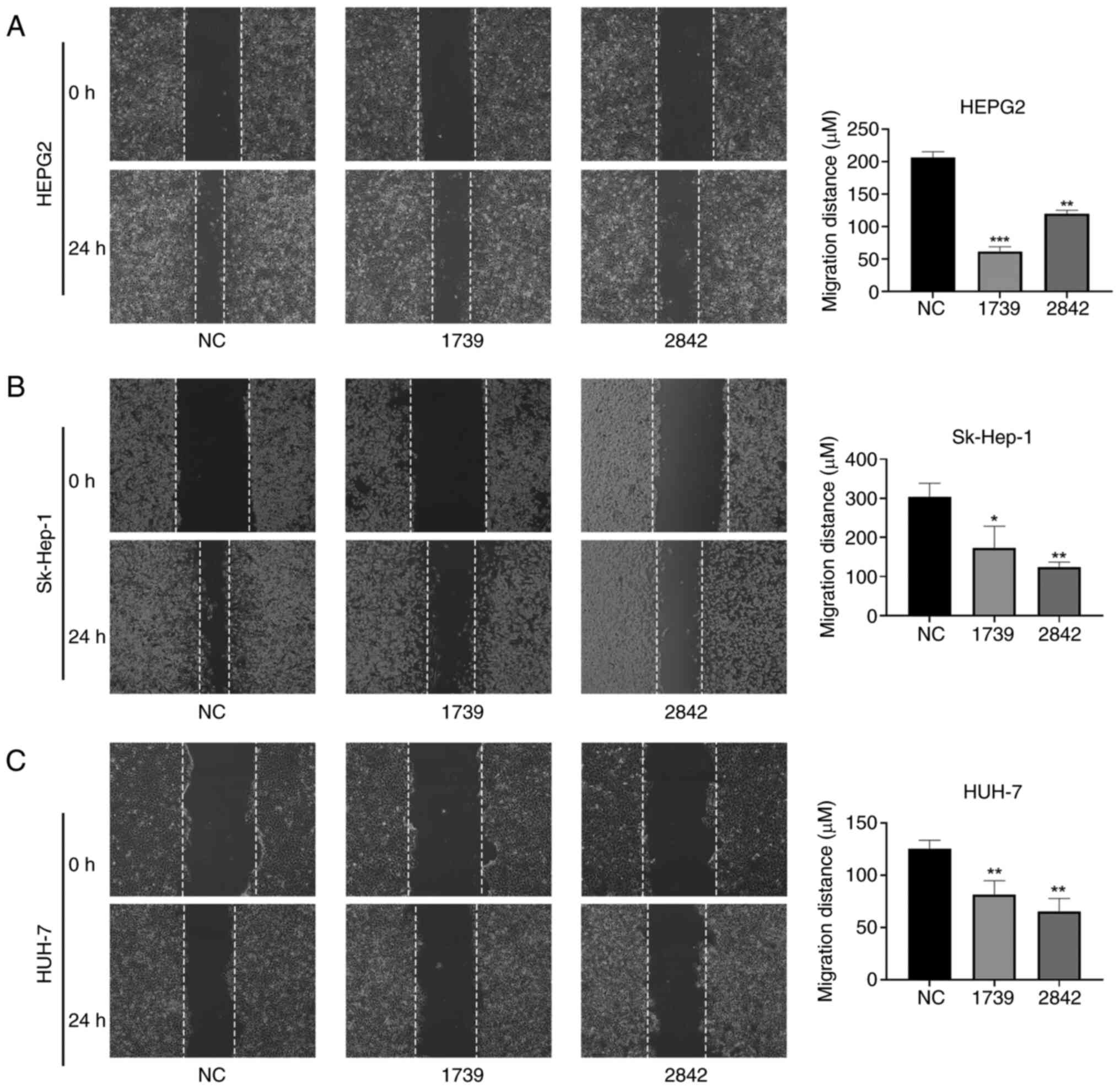
Transfected cells were plated in a 6-well culture plate after transfection. Upon reaching >90% cell confluence, the wound was gently scraped with a 100-µl pipette tip and a sterile ruler. After scraping, the cells were rinsed three times with PBS to eliminate the detached cells, and 2 ml DMEM containing 2% FBS was added to each well followed by incubation. Images were captured and the migration distance was measured by comparing the images at 0 h and after culturing the cells for 24 h. An inverted microscope (Olympus Corporation) was used to calculate the number of migrated cells.
Transwell assay
Cell suspensions containing 5×104 cells were added to the upper chamber of a 24-well culture plate with Transwell inserts and incubated with serum-free DMEM (200 µl) at 24 h post-transfection. In the lower chamber of the 24-well plates, 500 µl complete DMEM was added. Following 24 h of incubation at 37°C, the chambers were removed and rinsed once with sterile PBS. Cells on the upper side were wiped away with a cotton swab. The remaining cells were fixed for 15 min and stained with crystal violet (0.5%) for 20 min at room temperature. Finally, a light microscope was employed to collect images and count the number of migrated cells.
Statistical analysis
Statistical analysis and plotting were performed in R software (version 4.0) and GraphPad Prism (version 8.0; Dotmatics). Univariate and multivariate Cox regression analyses were used to identify prognostic factors associated with OS. Survival curves were generated and compared between different EMC3-AS1 expression groups using Kaplan-Meier analysis and the log-rank test. For continuous variables with non-normal distributions, Wilcoxon rank-sum test was applied for comparing differences between two independent groups, while Wilcoxon signed-rank test was used for paired groups. All experiments were performed in triplicate. Data from the experiments are expressed as the mean ± standard deviation. Differences among multiple groups were compared by one-way analysis of variance followed by Dunnett's post hoc test. P<0.05 was considered to indicate a statistically significant result.
Results
EMC3-AS1 is upregulated in liver cancer tissues
Analysis of data from TCGA revealed a significant upregulation of EMC3-AS1 in 374 liver cancer tissues compared with that in 50 AN tissues, as well as in 50 paired liver cancer samples compared with AN tissues (P<0.001; Fig. 1A and B). EMC3-AS1 upregulation was also evident in the tumor tissues in the three GEO datasets containing 428 liver cancer and 451 AN tissues (P<0.001; Fig. 1C-E). In addition, based on the results of RT-qPCR analysis of the current study cohort, EMC3-AS1 expression was upregulated in the 42 liver cancer tissues compared with that in the 35 AN liver tissues (P<0.001; Fig. 1F).
EMC3-AS1 is both an unfavorable prognostic biomarker and an effective diagnostic biomarker
Kaplan-Meier analysis revealed that the patients in the H-EMC3-AS1 group exhibited shorter OS (P=0.005) and DFS times (P<0.001) compared with those in the L-EMC3-AS1 group (Fig. 2A and B). In the ROC analysis based on EMC3-AS1 expression in HCC and adjacent tissues, the area under the curve (AUC) in TCGA cohort was 0.866 (Fig. 2C). The good performance of EMC3-AS1 was validated in the GEO datasets GSE22058, GSE25097 and GSE64041, with AUCs of 0.892, 0.824 and 0.703, respectively (Fig. 2D-F). In addition, ROC analysis was conducted to examine the ability of EMC3-AS1 expression to distinguish liver cancer in the current study cohort. Notably, it was found that EMC3-AS1 exhibited good diagnostic ability, with an AUC of 0.785 (Fig. 2G).
EMC3-AS1 is a significant prognostic factor
The univariate Cox regression analysis (Fig. 3A) indicated that poor prognosis was associated with the expression of EMC3-AS1 [hazard ratio (HR), 1.32; 95% confidence interval (CI), 1.15–1.53; P<0.001], tumor stage (HR, 2.59; 95% CI, 1.82–3.69; P<0.001) and pathological stage (HR, 2.49; 95% CI, 1.71–3.61; P<0.001). In the multivariate Cox regression analysis (Fig. 3A), shorter OS time was found to be independently associated with the expression of EMC3-AS1 (HR, 1.24; 95% CI, 1.07–1.45; P=0.006) and advanced tumor stage (HR, 2.30; 95% CI, 1.58–3.37; P<0.001). In the subgroup analysis, patients with liver cancer who had an advanced tumor stage (P=0.005), advanced pathological stage (P=0.005), advanced histological grade (P<0.001) and higher AFP level (P=0.004) were found to have significantly elevated EMC3-AS1 expression levels (Fig. 3B). However, no notable differences in EMC3-AS1 expression were observed between the subgroups based on age, sex, Child-Pugh stage, bilirubin level or vascular invasion status.
Functional enrichment analysis
According to the result of GSVA, the Hallmark gene sets ‘G2M checkpoint’, ‘E2F targets’ and ‘mitotic spindle’ were markedly activated in the H-EMC3-AS1 expression group (Fig. 4A). In the KEGG gene sets, pathways associated with cell division and proliferation, including ‘DNA replication’ and ‘cell cycle’, were markedly enriched in the H-EMC3-AS1 expression group (Fig. 4B). A total of 222 DEmRNA (180 upregulated and 42 downregulated genes) were identified between the H- and L-EMC3-AS1 expression groups. For these DEGs, GO enrichment analysis revealed that ‘nuclear division’, ‘chromosomal region’ and ‘tubulin binding’ were the most enriched terms in the biological process, cellular component and molecular function categories (Fig. 4C; Table SIII). ‘Progesterone-mediated oocyte maturation’, ‘cell cycle’ and ‘oocyte meiosis’ were found to be the most highly enriched pathways in the KEGG pathway enrichment analysis (Fig. 4E; Table SIV).
In the GSEA, ‘E2F targets’, ‘G2M checkpoint’ and ‘mitotic spindle’ were the top three enriched pathways in the Hallmark gene sets, while ‘intrinsic pathway of fibrin clot formation’, ‘cytochrome p450 arranged by substrate type’ and ‘formation of fibrin clot clotting cascade’ were the most enriched Reactome gene sets (Fig. 4D and F).
Association of EMC3-AS1 expression with immune status
The expression of EMC3-AS1 was found to be positively correlated with the infiltration of activated memory CD4 T cells (R=0.17, P=0.001), follicular helper T cells (R=0.15, P=0.004), regulatory T cells (R=0.15, P=0.003), M0 macrophages (R=0.18, P<0.001) and neutrophils (R=0.15, P=0.004), and negatively correlated with the infiltration of resting memory CD4 T cells (R=−0.12, P=0.026) and monocytes (R=−0.14, P=0.007) (Fig. 5A). The mRNA levels of the ICs CTLA4, PDCD1, CD86, CD274, CD276, HAVCR2, LAG3, LGALS9, NRP1 and TIGIT were significantly higher in the H-EMC3-AS1 expression group compared with those in the L-EMC3-AS1 expression group (P<0.05; Fig. 5B). In addition, the mRNAsi of the H-EMC3-AS1 group was higher than that of the L-EMC3-AS1 group (P=0.004; Fig. 5C) and was positively correlated with EMC3-AS1 expression (R=0.17, P=0.001). By contrast, the EREG-mRNAsi of the H-EMC3-AS1 group was lower than that of the L-EMC3-AS1 group (P=0.001), and was negatively correlated with EMC3-AS1 expression (R=−0.21, P<0.001; Fig. 5D). Additionally, the Stromal score (P=0.003) and Estimate score (P=0.023) of patients in the H-EMC3-AS1 group were significantly lower than those of patients in the L-EMC3-AS1 group (Fig. 5E). According to the result of the ssGSEA for 13 immune-related pathways, the activity of MHC class I molecules was greater in the H-EMC3-AS1 group compared with that in the L-EMC3-AS1 group (P<0.001), whereas type I/II IFN responses and cytolytic activity were restrained in the H-EMC3-AS1 expression group (P<0.001, Fig. 5F).
Comprehensive therapeutic analysis
IPS analysis indicated that the IPS level of patients in the L-EMC3-AS1 group was elevated compared with that of the H-EMC3-AS1 group, suggesting that patients in the L-EMC3-AS1 expression group were more suitable for immunotherapy (P=0.013). Furthermore, patients in L-EMC3-AS1 expression group also demonstrated significantly higher IPS-CTLA4 blocker scores (P=0.015) and IPS-CTLA4-PD1 blocker scores (P=0.047), which suggested that these patients might experience an effective response when treated with anti-CTLA4 or anti-PD1 plus anti-CTLA4 therapy (Fig. 6A). In the TIDE analysis, lower dysfunction scores and higher exclusion scores of T cells in the tumor microenvironment were found in patients with high EMC3-AS1 expression compared with those in patients with low EMC3-AS1 expression (P=0.001 and P=0.002, respectively; Fig. 6B). Furthermore, the oncoPredict analysis indicated that the patients in the L-EMC3-AS1 group would be more sensitive to treatment with entinostat, erlotinib, oxaliplatin, palbociclib and selumetinib compared with those in the H-EMC3-AS1 group (all P<0.05; Fig. 6C).
Silencing EMC3-AS1 inhibits liver cancer cell proliferation
HepG2, Sk-Hep-1 and Huh-7 cells were transfected with non-targeting siRNA (siRNA-NC) or EMC3-AS1-specific siRNAs (siRNA-1739/2842) to silence the expression of EMC3-AS1. According to the results of RT-qPCR, EMC3-AS1 expression was successfully knocked down via transfection with siRNA-1739 and siRNA-2842 in the HepG2, Sk-Hep-1 and Huh-7 cells (Fig. 7A-C), indicating that the siRNA-silenced cells were suitable for use in subsequent assays. Transfection with siRNA-1739 and siRNA-2842 was shown to reduce the proliferation and colony forming ability of HepG2, Sk-Hep-1 and Huh-7 cells compared with that of the siRNA-NC group (Fig. 7D-L).
Silencing EMC3-AS1 suppresses liver cancer cell migration
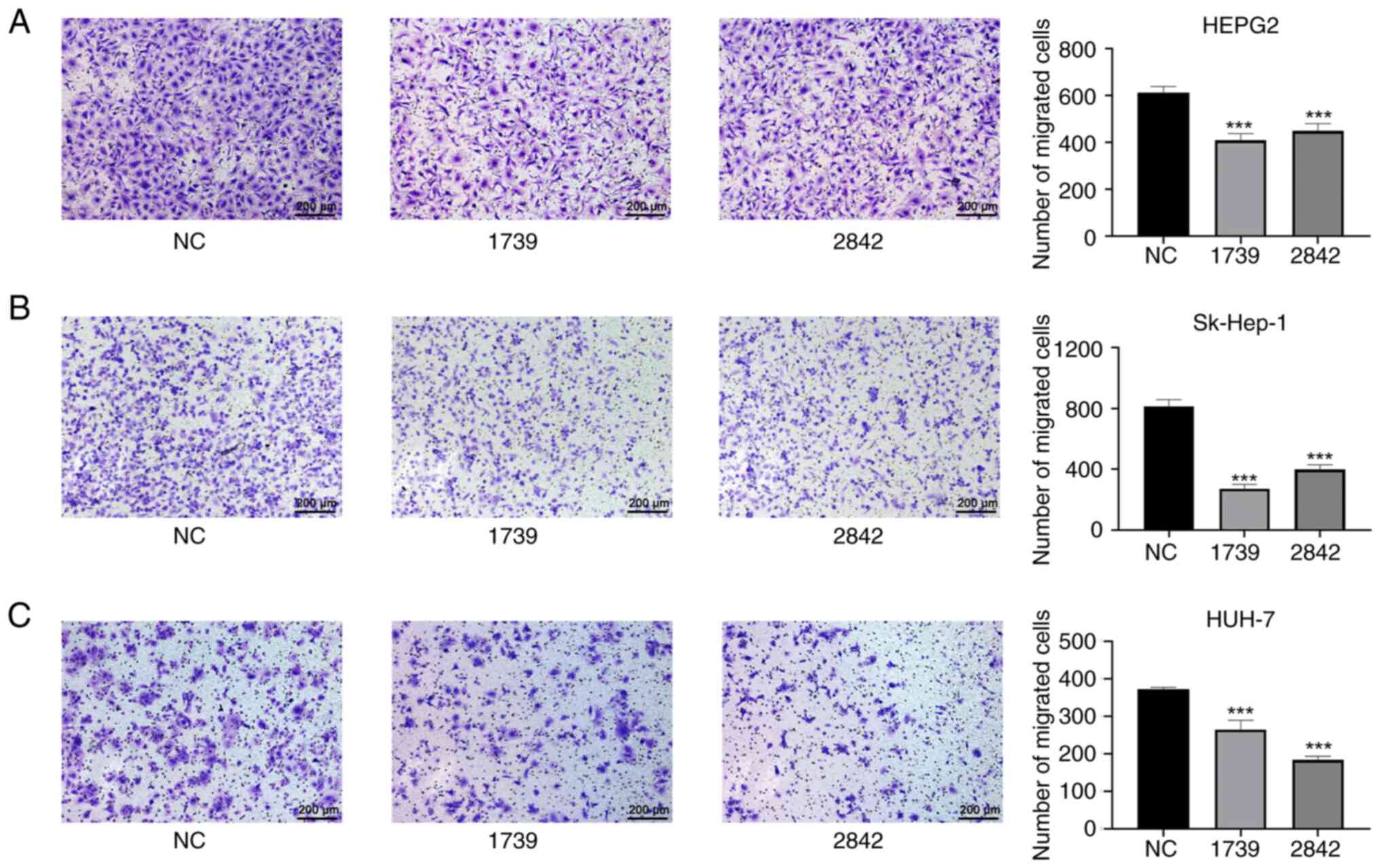
As shown in Fig. 8, the migration ability of the HepG2, Sk-Hep-1 and Huh-7 liver cancer cells in the wound healing assay was significantly reduced by the knockdown of EMC3-AS1 expression (Fig. 8). The migration of HepG2, Sk-Hep-1 and Huh-7 cells in the Transwell assay was also significantly inhibited after the knockdown of EMC3-AS1 (Fig. 9). These results suggest that a deficiency in EMC3-AS1 affects the vital functions of liver cancer cells, and that EMC3-AS1 may serve as a novel target for liver cancer treatment.
Discussion
The current study demonstrated that the expression of EMC3-AS1 was upregulated in liver cancer tissues compared with that in AN tissues in various datasets, including TCGA and GEO datasets, as well as in a primary clinical cohort. The expression of EMC3-AS1 was also higher in patients with more advanced liver cancer. Higher expression of EMC3-AS1 was associated with shorter OS and DFS times in patients with liver cancer. Furthermore, EMC3-AS1 demonstrated the ability to effectively discriminate between liver cancer and normal tissues, exhibiting a good performance not only in TCGA and GEO datasets, but also in the current clinical cohort. These findings suggest that EMC3-AS1 holds potential as a diagnostic and prognostic biomarker. Multivariate Cox regression analysis indicated that higher expression of EMC3-AS1 was independently associated with a poor prognosis in patients with liver cancer. Furthermore, GSVA, GSEA, GO and KEGG enrichment analyses showed significant enrichment and activation of several pathways associated with the tumorigenesis and progression of liver cancer, including ‘chromosome segregation’, ‘organelle fission’, ‘nuclear division’ and ‘cell cycle’.
The TME is primarily composed of immune cells, extracellular matrix, cytokines and cancer cells (34). In addition to playing a crucial role in tumor recognition and clearance, the TME also contributes to immune escape and cancer progression (35). The TME of liver cancer is strongly suppressive, with aberrant accumulation of immunosuppressive cells (36). Immunotherapy targeting immunosuppressive cells and pathways is a promising approach for cancer treatment (37). Tumor-associated neutrophils have been identified as key drivers of progression and immunosuppression in liver cancer (38,39). Wang et al (40) suggested that the resistance of hepatocellular carcinoma cells to sorafenib could be ascribed to the activation of CXCR2 signaling facilitated by tumor-associated macrophages. Regulatory T cells have also been shown to be associated with liver cancer invasion and to be crucial in hindering the development of effective antitumor responses (41). In the present study, EMC3-AS1 expression was shown to be positively correlated with the infiltration of follicular helper T cells, activated memory CD4 T cells, M0 macrophages, regulatory T cells and neutrophils in the liver. Conversely, a significant negative correlation was observed between EMC3-AS1 expression and the infiltration of monocytes and resting memory CD4 T cells.
ICIs have demonstrated marked therapeutic efficacy for a variety of cancer types, including liver cancer (42–44). Assessing the expression of ICs and their ligands in the TME is often the initial step when making decisions regarding immunotherapy in patients with cancer (45). In liver cancer, the upregulation of PD ligand 1 (PD-L1) expression is a significant predictor of poor survival (46–48). Enhancing the response of a patient to anti-PD-1 or anti-PD-L1 treatment is a pivotal therapeutic strategy for patients with liver cancer (48). Notably, in the current study, the mRNA expression of several key ICIs, including CTLA4, CD86, CD274, LGALS9, PDCD1 and TIGIT, was significantly higher in the H-EMC3-AS1 expression group than that in the L-EMC3-AS1 expression group. This finding suggests that patients exhibiting elevated levels of EMC3-AS1 also demonstrate increased expression of various ICs in the TME, which would be clinically helpful in improving patient stratification for IC blockade immunotherapy.
Clinical impact assessments of ICIs have been conducted in various cancer types, and drugs such as nivolumab, pembrolizumab and atezolizumab have been recommended as first-line treatments for patients with liver cancer (49). However, positive responses to immunotherapy are observed in only a small proportion of patients with liver cancer, highlighting the importance of identifying responsive patients and understanding resistance mechanisms (50). In the present study, patients with liver cancer with low EMC3-AS1 expression had higher IPSs when strategies to block CTLA4 or co-block PD1 and CTLA4 were employed. Additionally, patients with low EMC3-AS1 expression exhibited higher dysfunction scores and lower exclusion scores for T cells, indicating that they were more likely to benefit from immunotherapy.
Further experiments were performed in the present study to investigate the influence of EMC3-AS1 on the biological behavior of liver cancer cells in vitro. Transfection with siRNA targeting EMC3-AS1 markedly reduced its expression in HepG2, Sk-Hep-1 and Huh-7 cells, leading to decreased cell proliferation and colony-forming ability. In addition, the results of wound healing and Transwell assays revealed that the migration capabilities of HepG2, Sk-Hep-1 and Huh-7 cells were notably suppressed following the downregulation of EMC3-AS1 expression. These findings imply that EMC3-AS1 may act as a potential target for the treatment of liver cancer.
However, the current study has several limitations that should be acknowledged. Firstly, multicenter studies with larger sample sizes are required to confirm the expression of EMC3-AS1 in liver cancer. Secondly, additional experiments are required to explore the potential in vitro and in vivo mechanisms of EMC3-AS1 to support the present findings. Thirdly, the CIBERSORT algorithm relies on the fidelity of the reference profiles, which could deviate in cells involved in heterotypic interactions, phenotypic plasticity or disease-induced dysregulation. It is necessary to verify results in independent cohorts or experimental models in future research. Finally, prospective studies are warranted to validate the prognostic and diagnostic value of EMC3-AS1 in patients with liver cancer.
In summary, lncRNA EMC3-AS1 is upregulated in liver cancer and is associated with a poor prognosis, making it a potential diagnostic and prognostic biomarker for patients with liver cancer. Silencing EMC3-AS1 is potentially a promising therapeutic approach for liver cancer treatment.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by the Scientific Research Project of Chengdu Health Commission (grant no. 2020118), the Scientific Research Project of Sichuan Medical Association of China (grant no. S20073) and the Joint Fund of Chengdu Medical University and Pidu District People's Hospital (grant no. 2021LHZD-02).
Availability of data and materials
The data generated in the present study may be requested from the corresponding author.
Authors' contributions
BL designed the study and analyzed the data. XY performed the experiments. JZ and KD were involved in the acquisition and management of data. TF analysed the data and interpreted the results. BL, XY and TF revised the manuscript. CD designed the study and wrote the manuscript. BL and XY confirm the authenticity of all the raw data. All authors read and approved the final manuscript.
Ethics approval and consent to participate
This study was performed in accordance with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of Sichuan Provincial People's Hospital (Chengdu, China; approval no. 2022-2). Written informed consent for the use of their tissues was acquired from patients before surgery.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
|
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Nagtegaal ID, Odze RD, Klimstra D, Paradis V, Rugge M, Schirmacher P, Washington KM, Carneiro F and Cree IA; WHO Classification of Tumours Editorial Board, : The 2019 WHO classification of tumours of the digestive system. Histopathology. 76:182–188. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Zhang W, Hu B, Han J, Wang Z, Ma G, Ye H, Yuan J, Cao J, Zhang Z, Shi J, et al: Surgery after conversion therapy with PD-1 Inhibitors plus tyrosine kinase inhibitors are effective and safe for advanced hepatocellular carcinoma: A pilot study of ten patients. Front Oncol. 11:7479502021. View Article : Google Scholar : PubMed/NCBI | |
|
Caley DP, Pink RC, Trujillano D and Carter DRF: Long noncoding RNAs, chromatin, and development. ScientificWorldJournal. 10:90–102. 2010. View Article : Google Scholar : PubMed/NCBI | |
|
Jiang MC, Ni JJ, Cui WY, Wang BY and Zhuo W: Emerging roles of lncRNA in cancer and therapeutic opportunities. Am J Cancer Res. 9:1354–1366. 2019.PubMed/NCBI | |
|
Huang Z, Zhou JK, Peng Y, He W and Huang C: The role of long noncoding RNAs in hepatocellular carcinoma. Mol Cancer. 19:772020. View Article : Google Scholar : PubMed/NCBI | |
|
Liu Y, Liu X, Lin C, Jia X, Zhu H, Song J and Zhang Y: Noncoding RNAs regulate alternative splicing in cancer. J Exp Clin Cancer Res. 40:112021. View Article : Google Scholar : PubMed/NCBI | |
|
Malakoti F, Targhazeh N, Karimzadeh H, Mohammadi E, Asadi M, Asemi Z and Alemi F: Multiple function of lncRNA MALAT1 in cancer occurrence and progression. Chem Biol Drug Des. 101:1113–1137. 2023. View Article : Google Scholar : PubMed/NCBI | |
|
Si C, Yang L and Cai X: LncRNA LINC00649 aggravates the progression of cervical cancer through sponging miR-216a-3p. J Obstet Gynaecol Res. 48:2853–2862. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Tang D, Zhao L, Peng C, Ran K, Mu R and Ao Y: LncRNA CRNDE promotes hepatocellular carcinoma progression by upregulating SIX1 through modulating miR-337-3p. J Cell Biochem. 120:16128–16142. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Cai Y, Lyu T, Li H, Liu C, Xie K, Xu L, Li W, Liu H, Zhu J, Lyu Y, et al: LncRNA CEBPA-DT promotes liver cancer metastasis through DDR2/β-catenin activation via interacting with hnRNPC. J Exp Clin Cancer Res. 41:3352022. View Article : Google Scholar : PubMed/NCBI | |
|
Zhao Z, Gao J and Huang S: LncRNA SNHG7 promotes the HCC progression through miR-122-5p/FOXK2 axis. Dig Dis Sci. 67:925–935. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Ji Y, Sun H, Liang H, Wang Y, Lu M, Guo Z, Lv Z and Ren W: Evaluation of LncRNA ANRIL potential in hepatic cancer progression. J Environ Pathol Toxicol Oncol. 38:119–131. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Dai D, Wang H, Zhu L, Jin H and Wang X: N6-methyladenosine links RNA metabolism to cancer progression. Cell Death Dis. 9:1242018. View Article : Google Scholar : PubMed/NCBI | |
|
Ma S, Chen C, Ji X, Liu J, Zhou Q, Wang G, Yuan W, Kan Q and Sun Z: The interplay between m6A RNA methylation and noncoding RNA in cancer. J Hematol Oncol. 12:1212019. View Article : Google Scholar : PubMed/NCBI | |
|
Burchard J, Zhang C, Liu AM, Poon RT, Lee NP, Wong KF, Sham PC, Lam BY, Ferguson MD, Tokiwa G, et al: microRNA-122 as a regulator of mitochondrial metabolic gene network in hepatocellular carcinoma. Mol Syst Biol. 6:4022010. View Article : Google Scholar : PubMed/NCBI | |
|
Sung WK, Zheng H, Li S, Chen R, Liu X, Li Y, Lee NP, Lee WH, Ariyaratne PN, Tennakoon C, et al: Genome-wide survey of recurrent HBV integration in hepatocellular carcinoma. Nat Genet. 44:765–769. 2012. View Article : Google Scholar : PubMed/NCBI | |
|
Makowska Z, Boldanova T, Adametz D, Quagliata L, Vogt JE, Dill MT, Matter MS, Roth V, Terracciano L and Heim MH: Gene expression analysis of biopsy samples reveals critical limitations of transcriptome-based molecular classifications of hepatocellular carcinoma. J Pathol Clin Res. 2:80–92. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Pugh RN, Murray-Lyon IM, Dawson JL, Pietroni MC and Williams R: Transection of the oesophagus for bleeding oesophageal varices. Br J Surg. 60:646–649. 1973. View Article : Google Scholar : PubMed/NCBI | |
|
Livak KJ and Schmittgen TD: Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001. View Article : Google Scholar : PubMed/NCBI | |
|
Robin X, Turck N, Hainard A, Tiberti N, Lisacek F, Sanchez JC and Müller M: pROC: An open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinformatics. 12:772011. View Article : Google Scholar : PubMed/NCBI | |
|
Hänzelmann S, Castelo R and Guinney J: GSVA: Gene set variation analysis for microarray and RNA-seq data. BMC Bioinformatics. 14:72013. View Article : Google Scholar : PubMed/NCBI | |
|
Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, Paulovich A, Pomeroy SL, Golub TR, Lander ES and Mesirov JP: Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci USA. 102:15545–15550. 2005. View Article : Google Scholar : PubMed/NCBI | |
|
Yu G, Wang LG, Han Y and He QY: clusterProfiler: An R package for comparing biological themes among gene clusters. OMICS. 16:284–287. 2012. View Article : Google Scholar : PubMed/NCBI | |
|
Ritchie ME, Phipson B, Wu D, Hu Y, Law CW, Shi W and Smyth GK: limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 43:e472015. View Article : Google Scholar : PubMed/NCBI | |
|
Edge SB and Compton CC: The American joint committee on cancer: The 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 17:1471–1474. 2010. View Article : Google Scholar : PubMed/NCBI | |
|
Newman AM, Steen CB, Liu CL, Gentles AJ, Chaudhuri AA, Scherer F, Khodadoust MS, Esfahani MS, Luca BA, Steiner D, et al: Determining cell type abundance and expression from bulk tissues with digital cytometry. Nat Biotechnol. 37:773–782. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Salmaninejad A, Valilou SF, Shabgah AG, Aslani S, Alimardani M, Pasdar A and Sahebkar A: PD-1/PD-L1 pathway: Basic biology and role in cancer immunotherapy. J Cell Physiol. 234:16824–16837. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Malta TM, Sokolov A, Gentles AJ, Burzykowski T, Poisson L, Weinstein JN, Kamińska B, Huelsken J, Omberg L, Gevaert O, et al: Machine learning identifies stemness features associated with oncogenic dedifferentiation. Cell. 173:338–354.e15. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Yoshihara K, Shahmoradgoli M, Martínez E, Vegesna R, Kim H, Torres-Garcia W, Treviño V, Shen H, Laird PW, Levine DA, et al: Inferring tumour purity and stromal and immune cell admixture from expression data. Nat Commun. 4:26122013. View Article : Google Scholar : PubMed/NCBI | |
|
Charoentong P, Finotello F, Angelova M, Mayer C, Efremova M, Rieder D, Hackl H and Trajanoski Z: Pan-cancer immunogenomic analyses reveal genotype-immunophenotype relationships and predictors of response to checkpoint blockade. Cell Rep. 18:248–262. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Jiang P, Gu S, Pan D, Fu J, Sahu A, Hu X, Li Z, Traugh N, Bu X, Li B, et al: Signatures of T cell dysfunction and exclusion predict cancer immunotherapy response. Nat Med. 24:1550–1558. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Maeser D, Gruener RF and Huang RS: oncoPredict: An R package for predicting in vivo or cancer patient drug response and biomarkers from cell line screening data. Brief Bioinform. 22:bbab2602021. View Article : Google Scholar : PubMed/NCBI | |
|
Chew V, Lai L, Pan L, Lim CJ, Li J, Ong R, Chua C, Leong JY, Lim KH, Toh HC, et al: Delineation of an immunosuppressive gradient in hepatocellular carcinoma using high-dimensional proteomic and transcriptomic analyses. Proc Natl Acad Sci USA. 114:E5900–E5909. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Dunn GP, Bruce AT, Ikeda H, Old LJ and Schreiber RD: Cancer immunoediting: From immunosurveillance to tumor escape. Nat Immunol. 3:991–998. 2002. View Article : Google Scholar : PubMed/NCBI | |
|
Shlomai A, de Jong YP and Rice CM: Virus associated malignancies: The role of viral hepatitis in hepatocellular carcinoma. Semin Cancer Biol. 26:78–88. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
Pardoll DM: The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 12:252–264. 2012. View Article : Google Scholar : PubMed/NCBI | |
|
Margetts J, Ogle LF, Chan SL, Chan AWH, Chan KCA, Jamieson D, Willoughby CE, Mann DA, Wilson CL, Manas DM, et al: Neutrophils: Driving progression and poor prognosis in hepatocellular carcinoma? Br J Cancer. 118:248–257. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Arvanitakis K, Mitroulis I and Germanidis G: Tumor-associated neutrophils in hepatocellular carcinoma pathogenesis, prognosis, and therapy. Cancers (Basel). 13:28992021. View Article : Google Scholar : PubMed/NCBI | |
|
Wang HC, Haung LY, Wang CJ, Chao YJ, Hou YC, Yen CJ and Shan YS: Tumor-associated macrophages promote resistance of hepatocellular carcinoma cells against sorafenib by activating CXCR2 signaling. J Biomed Sci. 29:992022. View Article : Google Scholar : PubMed/NCBI | |
|
You M, Gao Y, Fu J, Xie R, Zhu Z, Hong Z, Meng L, Du S, Liu J, Wang FS, et al: Epigenetic regulation of HBV-specific tumor-infiltrating T cells in HBV-related HCC. Hepatology. 78:943–958. 2023. View Article : Google Scholar : PubMed/NCBI | |
|
Zhu AX, Finn RS, Edeline J, Cattan S, Ogasawara S, Palmer D, Verslype C, Zagonel V, Fartoux L, Vogel A, et al: Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): A non-randomised, open-label phase 2 trial. Lancet Oncol. 19:940–952. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Romano E, Kusio-Kobialka M, Foukas PG, Baumgaertner P, Meyer C, Ballabeni P, Michielin O, Weide B, Romero P and Speiser DE: Ipilimumab-dependent cell-mediated cytotoxicity of regulatory T cells ex vivo by nonclassical monocytes in melanoma patients. Proc Natl Acad Sci USA. 112:6140–6145. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Powles T, Eder JP, Fine GD, Braiteh FS, Loriot Y, Cruz C, Bellmunt J, Burris HA, Petrylak DP, Teng SL, et al: MPDL3280A (anti-PD-L1) treatment leads to clinical activity in metastatic bladder cancer. Nature. 515:558–562. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
Rotte A, Jin JY and Lemaire V: Mechanistic overview of immune checkpoints to support the rational design of their combinations in cancer immunotherapy. Ann Oncol. 29:71–83. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Shi J, Liu J, Tu X, Li B, Tong Z, Wang T, Zheng Y, Shi H, Zeng X, Chen W, et al: Single-cell immune signature for detecting early-stage HCC and early assessing anti-PD-1 immunotherapy efficacy. J Immunother Cancer. 10:e0031332022. View Article : Google Scholar : PubMed/NCBI | |
|
Pfister D, Núñez NG, Pinyol R, Govaere O, Pinter M, Szydlowska M, Gupta R, Qiu M, Deczkowska A, Weiner A, et al: NASH limits anti-tumour surveillance in immunotherapy-treated HCC. Nature. 592:450–456. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Cheng AL, Hsu C, Chan SL, Choo SP and Kudo M: Challenges of combination therapy with immune checkpoint inhibitors for hepatocellular carcinoma. J Hepatol. 72:307–319. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Benson AB, D'Angelica MI, Abbott DE, Anaya DA, Anders R, Are C, Bachini M, Borad M, Brown D, Burgoyne A, et al: Hepatobiliary cancers, version 2.2021, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 19:541–565. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Federico P, Petrillo A, Giordano P, Bosso D, Fabbrocini A, Ottaviano M, Rosanova M, Silvestri A, Tufo A, Cozzolino A and Daniele B: Immune checkpoint inhibitors in hepatocellular carcinoma: Current status and novel perspectives. Cancers (Basel). 12:30252020. View Article : Google Scholar : PubMed/NCBI |