Quality of life assessment and cost‑utility analysis of initial chemotherapy for patients with non‑Hodgkin's lymphoma: A prospective analysis
- Authors:
- Published online on: July 11, 2024 https://doi.org/10.3892/ol.2024.14564
- Article Number: 430
-
Copyright: © Tanaka et al. This is an open access article distributed under the terms of Creative Commons Attribution License.
Abstract
Introduction
The management of patients with malignant lymphoma entails developing a treatment strategy based on clinical factors, including age and prognostic factors (1,2), as well as incorporating treatment evidence from patient backgrounds and guidelines. Clinical management approaches range from immediate treatment with chemotherapy or radiation therapy, even without symptoms, to a watch-and-wait (W&W) approach, where treatment is initiated when symptoms manifest (3,4). Before the advent of chemotherapy, patients with diffuse large B-cell lymphoma often succumbed to the disease within weeks to months of diagnosis (5). In the 1970s, the introduction of cyclophosphamide, doxorubicin, vincristine, and prednisolone (CHOP) chemotherapy marked a major advancement, achieving a complete response in approximately 50% of patients and disease-free survival in 30–40% of patients (6).
With the considerable prolongation of survival periods through chemotherapy, the emphasis on quality of life (QOL) increased (7–9). Accordingly, with the development of new drugs, measuring QOL as a secondary endpoint (10) and comparing the QOL between patients receiving chemotherapy and those managed with the W&W approach (11) have become integral research components. Such studies show that long-term survival may be achieved at the cost of a gradual decrease in QOL (12). However, to the best of our knowledge, no study has examined the effect of initial chemotherapy on QOL or the early cost-effectiveness of chemotherapy for malignant lymphoma, despite the high response rates and early treatment effects (13). The transition from everyday life to an altered state because of treatment represents a major aspect of initial chemotherapy for patients with malignant lymphoma. This transition can influence QOL owing to restrictions in daily activities, anxiety related to treatment, and discomfort caused by adverse effects (14).
Economic issues also affect health-related QOL in non-Hodgkin lymphoma survivors (15). Thus, healthcare providers must monitor and respond to changes in the QOL of patients, particularly because rising healthcare costs impose heavier societal burdens (16). Although CHOP has been a universal first-line treatment since its effectiveness was established in the 1970s, there are no reports on its cost-effectiveness. In clinical practice, it is crucial to prioritize the efficacy of chemotherapy; however, the landscape of treatment has drastically changed since the efficacy of CHOP was first established. In recent years, the inclusion of cost-effectiveness calculations in the review process has become standard practice when applying for approval of a new drug in other countries. In addition, considering the challenging financial situation of Japan's public healthcare services, it is imperative to allocate the limited available budgets appropriately based on cost-effectiveness of treatments. Thus, clarifying the cost-effectiveness of established treatments is crucial. Additionally, long-term declines in QOL (17–19) have not captured changes in the QOL of patients undergoing traumatic life changes. Understanding the changes that occur during periods of considerable change in the lives of patients can help healthcare providers improve the quality of care. Access to real-world analysis results that reflect the cost-effectiveness associated with substantial life changes remains crucial. In this milieu, we conducted a prospective patient-reported QOL survey to clarify the effect of initial chemotherapy on QOL and its cost-effectiveness in patients with malignant lymphoma.
Patients and methods
Participants
Patients with malignant lymphoma who received initial chemotherapy at Gifu Municipal Hospital from January 2021 to December 2022 were included. Inclusion criteria comprised patients diagnosed with all types of lymphoma and those scheduled to begin chemotherapy via intravenous infusion. Exclusion criteria comprised patients diagnosed with lymphoma but treated with oral anticancer agents and patients who did not receive chemotherapy, such as those managed using the W&W approach. Consent to participate in the study was obtained after the treatment plan was determined and before the first dose of chemotherapy was administered. We also administered pre- and post-treatment questionnaires. The pretreatment questionnaire was administered before the start of chemotherapy on day 1, and the post-treatment questionnaire was administered on the last day of each regimen's defined treatment period (e.g., for a 3-week regimen, pre- and post-treatment questionnaires were administered on days 1 and 21, respectively).
Patient characteristics
Data on patient characteristics, including sex, age, performance status, cancer stage, B symptom, classification, treatment regimen, administration date, days from administration to discharge, drugs used, prescribed drug dose, and administered drug dose, were retrieved from electronic medical records. Pain management during treatment involved the use of analgesics when the pain was intense and based on patient's request. For psychological symptoms, such as depression, patients are assessed by the medical staff. If severe depression is diagnosed, counselors are made available under the guidance of the attending physician; however, counselors were not available for this study. These procedures for psychological symptoms and pain are standard methods in daily medical practice and were adhere to by the participants in our study. No special, unique interventions were applied to the participants.
Regimen
Chemotherapy involved administering drug therapy based on a predetermined treatment plan (regimen) that detailed the dosage, administration method, administration date, and treatment intervals. Certain regimens were considered clinically equivalent, with similar effectiveness, and were classified accordingly. CHOP; CHOP + rituximab (R); and pirarubicin, cyclophosphamide, vincristine, prednisolone were classified as CHOP ± R therapy. The relative dose intensity (RDI) was calculated by dividing the administered drug dose by the prescribed drug dose during the specified period. In addition, the molecular-targeted drugs rituximab, obinutuzumab, and polatuzumab vedotin were administered to all patients prior to the administration of antihistamines, antipyretic analgesics, and corticosteroids, as specified in the package insert to reduce infusion reactions. As an antiemetic measure for cytotoxic anticancer drugs, 5-hydroxytryptamine and corticosteroids were administered to all patients according to the risk classification of The Japan Society of Clinical Oncology Clinical Practice Guidelines for Antiemesis (20). These antiemetics were administered in advance to all patients, and anticancer agents were administered after them. If nausea and vomiting occurred despite these premedications, prochlorperazine was added at the discretion of the attending physician.
Quality of life
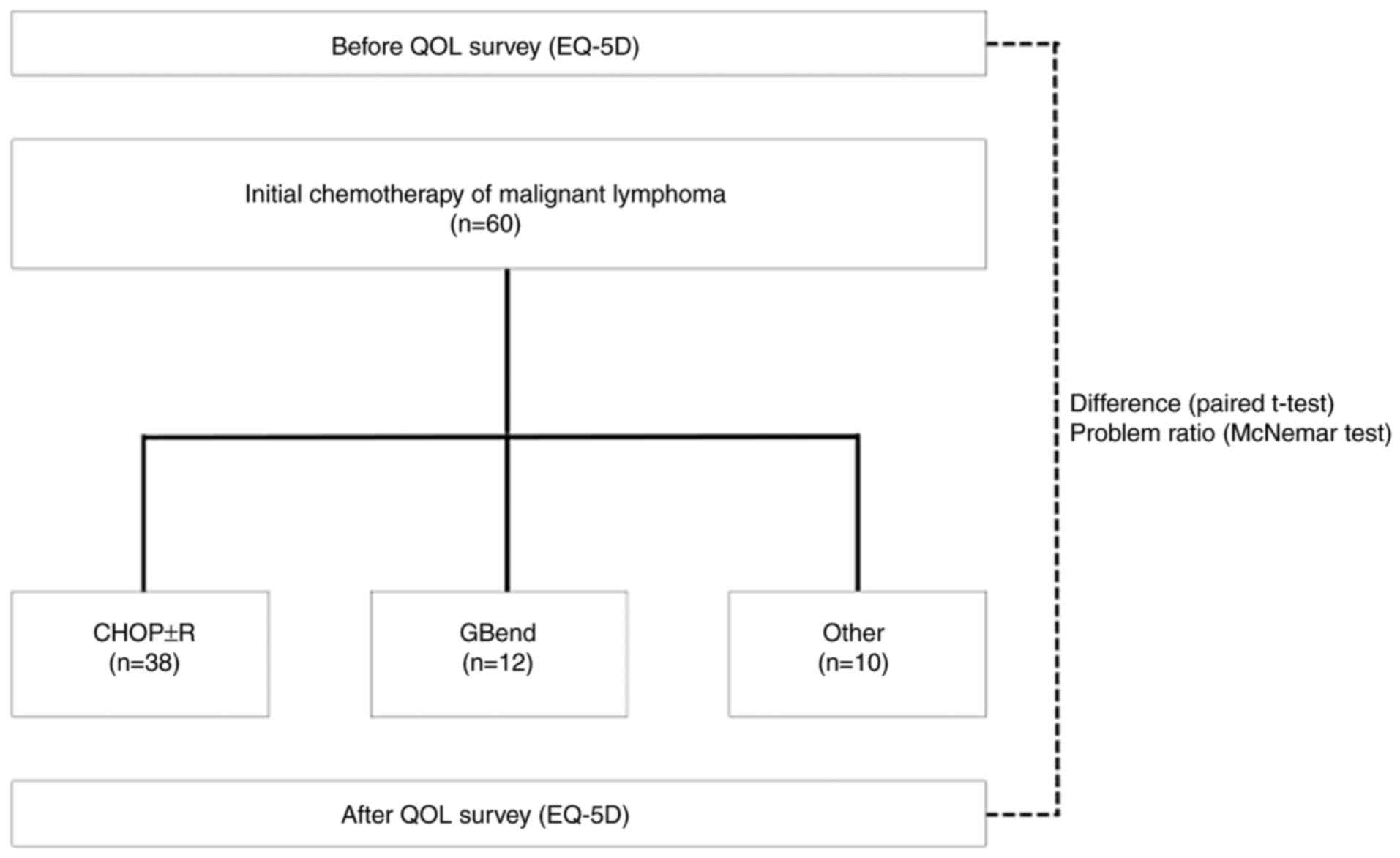
We conducted a self-reported patient questionnaire survey using EuroQol-5 dimensions (EQ-5D) (21) before and after initial chemotherapy. The utility values and percentage of issues in each of the five dimensions were compared before and after chemotherapy. Due to the varying adverse effects across different regimens that affect the QOL, the analysis was stratified by regimen. As chemotherapy involves the selection of a regimen tailored to the conditions of individual patients, we analyzed CHOP ± R therapy and all regimens (all patients) as the target for analysis; regimens administered to <20% of the total patients were excluded from the analysis owing to a lack of accuracy in utility value changes and average cost values. This exclusion minimizes potential errors in comparing regimens, which may compromise the precision of the analysis. Fig. 1 shows a schematic for the experimental design.
Cost-effectiveness
We utilized the official drug prices, and the analysis was conducted from the perspective of the cost payer (the standpoint of public healthcare) for cost calculation of the administered drug dose. In Japan, the Ministry of Health, Labour and Welfare (MHLW) recommends the use of generic drugs, including cytotoxic anticancer drugs. Generic drugs are products that contain the identical amount of the active ingredient found in the patented products (brand-name drug), offering the same efficacy, dosage, and method of administration. They were approved by the MHLW as therapeutically ‘equivalent to’ and ‘substitutable for’ the patented drug. The Japanese MHLW recommends the use of generic drugs for all applications except when only brand-name drugs are available on the market. This is widely practiced in the Japanese medical field as a measure to control healthcare costs, as national healthcare costs in Japan continue to increase. The MHLW also recommends the use of biosimilars for molecular-targeted drugs. At our institution, we use generic drugs and biosimilars when they are available in the Japanese market, but when they are not available, we use brand-name drugs in our daily practice. In Japan, generic drugs and biosimilars are manufactured under Good Manufacturing Practices as stipulated by the MHLW, which assures their quality and that the efficacy and safety of both drugs are equivalent. As a result, several medical institutions in Japan use generic drugs, leading to the widespread use of such drugs in Japan. In addition, due to the uniform pricing of generic drugs among several pharmaceutical manufacturers for the same ingredients and specifications, the price of generic drugs is almost consistent. Because of these factors, the price of the least expensive version of the drug is selected for the commonly used version of the drug, and the estimates in this study also reflect these conditions. The cost-utility analysis was employed for cost-effectiveness analysis. Incremental cost-effectiveness ratio (ICER) was calculated to determine the cost required per quality-adjusted life year (QALY). The ICER threshold was set at 7.5 million yen, a value recognized by the Japanese MHLW as indicating favorable cost-effectiveness for anticancer drugs (22). In cases where the cost was ~7.5 million yen, sensitivity analysis was conducted to account for potential errors. However, if the cost significantly exceeded 7.5 million yen (such as by more than three times), sensitivity analysis was not performed as the regimen was considered not to be cost-effective. Owing to variations in policies regarding hospitalization, supportive therapy, and monitoring among facilities, the cost calculation solely focused on the administered anticancer drugs to eliminate differences in the surrounding environment. As no control group was established, we considered the scenario of chemotherapy non-administration, evaluating cost-effectiveness under the assumption of no change in QOL during the same period as chemotherapy administration.
Sensitivity analysis
Classifying patients receiving chemotherapy as a control group without treatment was considered unethical. Therefore, the control group was established under the assumption of no treatment and no change in utility values. Sensitivity analysis was conducted assuming that QOL changes would occur in the control group under the hypothetical conditions. Moreover, the drugs used and doses administered were considered to rarely change as they are specified in the regimen. Additionally, drug costs remain relatively stable as they were based on the publicly set prices by the MHLW. As there have been reports on changes in QOL and as these reports mostly specified standard deviations, QOL values were used as parameters in the sensitivity analysis. The previously described QOL change values (23) were adopted for this analysis. The QOL change values were converted to 0.0025 and double of that value (0.005), for 3 weeks, the same duration as for most regimens. These values were varied on the ascending and descending sides, respectively. We compared the results with the scenario of no reported change in QOL to analyze the effect of these variations.
Statistical analysis
The McNemar test was employed to compare the proportion of issues in the EQ-5D before and after chemotherapy. A paired t-test was used to compare the utility values of the EQ-5D before and after chemotherapy. Results with a significance level <5% were considered statistically significant. Statistical analyses were performed using SPSS Statistics 29.0.
Ethics
This study was approved by the research ethics committees of Gifu Municipal Hospital (approval number 663) and Gifu Pharmaceutical University (approval number 2–23). Participation was restricted to patients who provided written informed consent for their enrollment after receiving a written description of the study.
Results
Patient characteristics
This study involved 60 patients (33 male and 27 female individuals) aged 69.7±10.9 years [mean ± standard deviation (SD)]. Seventeen patients had performance status 0, 23 had stage IV tumor, 51 showed B symptom absence, 28 had diffuse large B-cell lymphoma, 51 had B-cell type, and 33 had aggressive lymphoma (Table I).
Regimen
The RDI for all the regimens was 90.8%. The most common regimen was CHOP ± R therapy (cyclophosphamide 750 mg/m2 (day 2), doxorubicin 50 mg/m2 (day 2), vincristine 1.4 mg/m2 (day 2), prednisolone, and rituximab 375 mg/m2 (day 1)), administered to 38 patients, with an RDI of 88.4±11%. The second most common regimen was obinutuzumab and bendamustine therapy [GBend; obinutuzumab 1,000 mg/body (days 1, 8, and 15) and bendamustine 90 mg/m2 (days 1 and 2)], administered to 12 patients, with an RDI of 99.0±0.9%. Drugs with different adverse effect profiles from the cytotoxic anticancer drug (inhibits cell division and proliferation) included the molecularly targeted agents (specific action on unique target molecules involved in cancer cell growth) rituximab (anti-CD20 monoclonal antibody), obinutuzumab (humanized anti-CD20 monoclonal antibody), brentuximab vedotin (anti-CD30 monoclonal antibody), and polatuzumab vedotin (anti-CD79b monoclonal antibody), which were used in combination with the cytotoxic anticancer drug (Table II). The mean cost of the administered drugs was 267,577 yen for all regimens and 90,568 yen for CHOP ± R therapy (Table III).
Quality of life
The utility values before chemotherapy were 0.853±0.149 (mean ± SD) for all regimens and 0.841±0.135 for CHOP ± R therapy. After chemotherapy, the utility values were 0.868±0.123 for all regimens and 0.876±0.117 for CHOP ± R therapy. The differences in utility values before and after chemotherapy were 0.016±0.142 (P=0.397) for all regimens and 0.035±0.103 (P=0.043) for CHOP ± R therapy (Table III). For each dimension of the EQ-5D, the proportion of patients answering ‘having issues’ before and after chemotherapy is presented below (the McNemar test was used). For all regimens, the proportions of ‘Mobility’ were 21.7 and 30% (P=0.302), ‘Self-care’ were 6.7 and 6.7% (P=1.000), ‘Usual activities’ were 20 and 35% (P=0.049), ‘Pain/Discomfort’ were 43.3 and 31.7% (P=0.167), and ‘Anxiety/Depression’ were 46.7 and 28.3% (P=0.003). For CHOP ± R therapy, the proportions for ‘Mobility’ were 26.3 and 31.6% (P=0.754), ‘Self-care’ were 5.3 and 7.9% (P=1.000), ‘Usual activities’ were 26.3 and 28.9% (P=1.000), ‘Pain/Discomfort’ were 39.5 and 28.9% (P=0.344), and ‘Anxiety/Depression’ were 52.6 and 31.6% (P=0.008) (Table IV).
Cost-effectiveness
Under the conditions of assumption, the ICER was 34,323,051 yen/QALY for all regimens and 5,152,457 yen/QALY for CHOP ± R therapy (Table V).
Sensitivity analysis
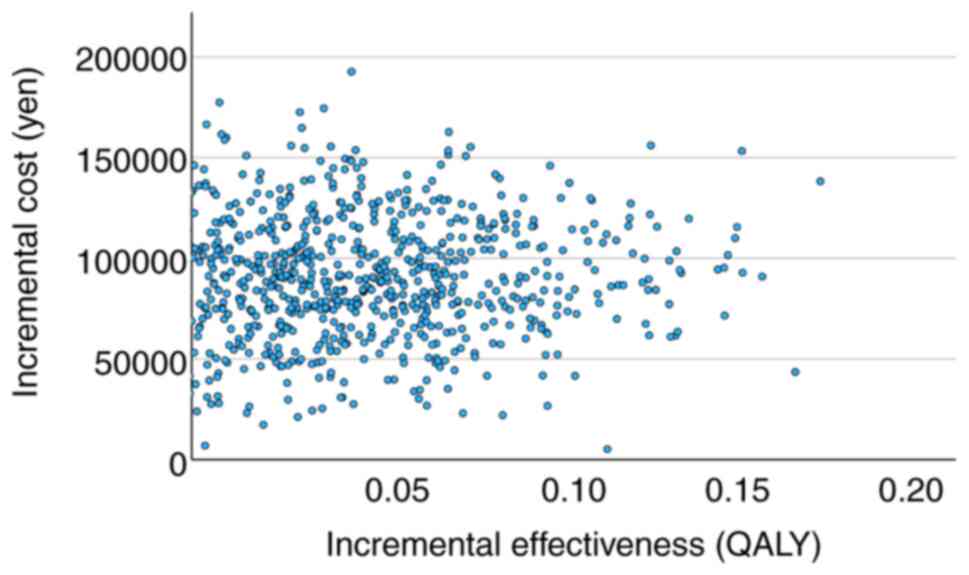
Varying the utility values caused the ICER to fluctuate from 25,988,842 to 50,525,908 yen/QALY for all regimens and 4,510,890 to 6,006,778 yen/QALY for CHOP ± R therapy (Table IV). Fig. 2 shows the relationship between incremental utility and incremental cost and Fig. 3 shows the relationship between willingness to pay and cost-effectiveness based on data from the study on CHOP ± R therapy.
Discussion
We conducted a prospective patient-reported QOL survey to clarify the effect of initial chemotherapy on QOL and its cost-effectiveness. The male-to-female ratio and mean age of patients for all regimens did not significantly differ from the epidemiological survey results in the JSH Practical Guidelines for Hematological Malignancies 2018 (24), and the tumor subtypes and regimens used for treatment generally tended to be consistent with the treatment strategies outlined in the guidelines. After initial chemotherapy for patients with malignant lymphoma, the utility values tended to increase compared with those before administration. In particular, CHOP ± R therapy showed a significant increase in utility values. For regimens other than CHOP ± R therapy, the baseline utility values were high, permitting limited room for further increase because of chemotherapy. These regimens include GBend therapy, which is not expected to significantly increase the utility values of chemotherapy. Additionally, there are more potent regimens, including those administered to prospective transplant recipients (25–27) and those used for treating extranodal natural killer/T-cell lymphoma, nasal type (ENKL), and Burkitt lymphoma. These potent regimens (particularly those used for ENKL and Burkitt lymphoma) often involve administering anticancer drugs over multiple days, dispersed (split) within a single cycle. In contrast, the CHOP ± R regimen typically specifies early administration within a single cycle. Anticancer drugs administered over multiple days and dispersed (split) are associated with a higher incidence of certain adverse effects, such as gastrointestinal symptoms and fatigue (28). As chemotherapy was administered at a high RDI, a higher proportion of patients responded that they had problems with their ‘Usual activities’ in the questionnaire survey after chemotherapy, which is thought to have contributed to the decrease in utility values.
In the five dimensions of the EQ-5D 5 questionnaire used for the QOL survey, the ‘Mobility’ and ‘Usual activities’ domain showed a worsening trend, whereas the ‘Personal care’ domains showed negligible changes. A trend toward decreased prevalence of ‘Pain/Discomfort’ was observed after chemotherapy compared with that before treatment, whereas the proportion of patients reporting ‘Anxiety/Depression’ significantly decreased. The effectiveness of chemotherapy for malignant lymphoma, which shows high response rates and rapid treatment effects (13), is thought to alleviate patient-perceived symptoms, such as pain from swelling or tumors at the lesion site, fever from B-symptoms due to disease progression, weight loss, and night sweats. Patients may experience relief from disease-related symptoms and decreased anxiety (29,30), potentially arising from the awareness of symptoms and a clear understanding of the disease. Moreover, improvement in ‘Anxiety/Depression’ may increase the motivation and expectations of a patient to resume daily life like that before the onset of the disease. However, all patients with malignant lymphoma who initiated intravenously administered anticancer therapy at our facility were treated as inpatients. This approach was necessary for comprehensive disease management and effectively addressing adverse effects of chemotherapy, such as febrile neutropenia. Chemotherapy administered within an inpatient setting is preferred in Japan (31), leading to restrictions in ‘Usual activities’, such as work, study, household chores, family, and leisure activities that patients engage in when at home. Because the mean hospitalization period exceeding 16 days, a gap between expectations and reality, significantly increased the number of patients reporting problems with their ‘Usual activities’. It is suggested that if healthcare providers offer assistance and support in the area of ‘Usual activities’ to patients undergoing initial chemotherapy, preventing a decline in QOL and maintaining it while administering chemotherapy treatment is possible.
Similar to the above findings, the proportion of patients reporting ‘Pain/Discomfort’ and ‘Anxiety/Depression’ decreased among patients receiving CHOP ± R therapy. Although the proportion of patients perceiving issues with ‘Usual activities’ significantly increased for all regimens, it only slightly increased for CHOP ± R therapy. This difference may be attributed to the inclusion of potent regimens with multiple days of dispersed (split) administration of anticancer drugs for all regimens, contrasting with CHOP ± R therapy, which involves early administration on a single day.
The ICER for the initial chemotherapy in patients with malignant lymphoma was 34.3 million yen/QALY for all regimens and 5.15 million yen/QALY for CHOP ± R therapy. CHOP ± R remains widely used in the treatment of malignant lymphoma because it is the standard treatment and has been approved for use for approximately 60 years (6). The drugs comprising CHOP ± R therapy were introduced in Japan several decades ago, with cyclophosphamide in 1962, doxorubicin in 1975, vincristine in 1968, prednisolone in 1956, and the most expensive, rituximab, in 2001. In Japan, the MHLW establishes official drug prices that decrease (discount) with time following a drug's launch. However, the prices of drugs have remained consistent for many years, reaching the lowest price to date except for rituximab. Furthermore, the use of biosimilars for rituximab, as recommended by the MHLW, has helped maintain the cost of CHOP ± R therapy low. The drug costs for polatuzumab vedotin with rituximab, cyclophosphamide, doxorubicin, and prednisolone (Pola-R-CHP) therapy for one person and brentuximab vedotin with cyclophosphamide, doxorubicin, and prednisolone (A-CHP) therapy for two people exceed one million yen per person. When these two regimens (three patients) were excluded, the mean drug cost for all regimens was approximately 190,000 yen, with an ICER of approximately 25 million yen/QALY. When the 12 patients with the next highest-cost GBend therapy were excluded, the mean drug cost for all regimens was approximately 160,000 yen and the ICER was 18 million yen/QALY. When the 15 patients with the three highest-cost regimens were excluded, the average drug cost for all regimens was approximately 100,000 yen and the ICER was 10 million yen/QALY. Thus, the drug cost must be reduced to approximately 100,000 yen to maintain the ICER within the threshold based on the increase in utility values. The high cost of recently introduced drugs (such as molecular-targeted drugs) for treating malignant lymphoma is considered a factor contributing to the increased ICER threshold. However, the threshold for the ICER varies from country to country, and its acceptability depends on the healthcare system and its policies. The cost-effectiveness calculations in this study were based on drug costs alone, without including the costs associated with hospitalization. Therefore, it is possible to divert the cost-effectiveness results even for outpatient chemotherapy because the results of this study apply to patients receiving R-CHOP therapy, which is administered worldwide, if the conditions with equivalent drug costs and equivalent patient backgrounds are met. In some countries, such as the United Kingdom, it is common to incorporate an economic evaluation into insurance coverage and drug pricing decisions, with ICER quantifying the costs required to improve one QALY unit, which is used to determine whether a drug can be covered by the insurance and its price. In Japan, the insurance coverage and drug prices are not determined on the basis of the ICER. Because an economic evaluation is not mandatory in Japan for insurance coverage and determination of drug price, there is a lack of evidence using QALYs, and cost-effectiveness has not been examined for the treatment of patients with malignant lymphoma. QALYs can be analyzed in terms of health-related QOL and can be compared across treatments, including those of different diseases. It can be used to make policy decisions on the selection of cost-effective treatment, such as selecting a lower cost treatment when there are multiple treatments with the same effectiveness, or selecting a treatment with higher effectiveness when multiple treatments are available at the same cost. In addition, incremental cost-effectiveness costs by QALYs can be compared to thresholds set by each country and can be used to inform healthcare policy decisions such as insurance reimbursement. Health economic evaluation based on QALY provides information that will lead to the selection of more effective and cost-effective treatments, thereby improving patients' QOL and optimizing medical finances. We believe that the results obtained from the patient-reported outcomes in this study may provide useful information for comparison and consideration of existing and future new treatment options.
In the sensitivity analysis, where the utility values varied by ±0.0025 and ±0.005 in both upward and downward directions, the ICERs for all regimens considerably exceeded the threshold. This finding is consistent with the survey results. However, the ICER remained within the Japanese threshold of 7.5 million yen per the survey results for CHOP ± R therapy, demonstrating its robustness.
Following initial chemotherapy, improved QOL was observed; however, this improvement is not expected to continue throughout repeated chemotherapy. In other words, initial chemotherapy may have contributed to an improvement in discomfort symptoms caused by the illness (32), with the recovery from chemotherapy-related adverse effects likely influencing the observed trend in QOL improvement.
This study has certain limitations. First, it was a single-center study; therefore, institutional policies may have influenced the treatment choices and treatment environment. Furthermore, the study duration had to be extended to increase the number of cases owing to the low incidence of malignant lymphoma compared with that of solid tumors. This constraint arises from the evolving treatment approaches over time, necessitating a constrained study period. Therefore, the small number of patients and short follow-up period may have influenced our findings.
This study sheds light on the dramatic changes in QOL experienced by patients receiving initial chemotherapy, which was not evident in previously reported long-term QOL studies. Hence, the findings provide valuable insights into potentially beneficial changes that can be implemented by healthcare providers throughout the treatment course of a patient. Furthermore, CHOP ± R was found to have superior cost-effectiveness, considering the new findings on QOL changes from real-world data. Collectively, this study provides valuable insights to guide patient treatment options and influence national healthcare policies.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be requested from the corresponding author.
Authors' contributions
KT, MY, TT, SI, JK, YN, HT and SK designed the study. KT and MY collected EuroQol 5 dimensions questionnaires. KT, TT and YT collected clinical data. KT and YT confirm the authenticity of all the raw data. KT, MY, TT, YI, YT and TY performed statistical analysis. KT, MY, YI and TY generated figures and tables. KT, MY, YI and TY validated and visualized the study. KT, MY and SK wrote the first draft of the manuscript. TT, YT, SI, YI, JK, YN, TY and HT critically revised the manuscript and provided valuable feedback. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The present study was approved by the Gifu Municipal Hospital Clinical Research Review Board (approval number 663; Gifu, Japan) and Gifu Pharmaceutical University Ethics Committee (approval number 2–23; Gifu, Japan). Participation was restricted to patients who, after receiving a written description of the study, provided written informed consent for their enrollment.
Patient consent for publication
Written informed consent for publication of the paper was obtained from patients at the time of study participation.
Competing interests
The authors declare that they have no competing interests.
Glossary
Abbreviations
Abbreviations:
|
QOL |
quality of life |
|
EQ-5D |
EuroQol 5 dimensions |
|
CHOP |
cyclophosphamide, doxorubicin, vincristine, prednisolone |
|
RDI |
relative dose intensity |
|
MHLW |
Ministry of Health, Labour and Welfare |
|
ICER |
incremental cost-effectiveness ratio |
|
QALY |
quality-adjusted life year |
|
GBend |
obinutuzumab, bendamustine |
|
ENKL |
extranodal natural killer/T-cell lymphoma, nasal type |
References
|
Zhou Z, Sehn LH, Rademaker AW, Gordon LI, Lacasce AS, Crosby-Thompson A, Vanderplas A, Zelenetz AD, Abel GA, Rodriguez MA, et al: An enhanced international prognostic index (NCCN-IPI) for patients with diffuse large B-cell lymphoma treated in the rituximab era. Blood. 123:837–842. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
Solal-Celigny P: Follicular lymphoma international prognostic index. Curr Treat Options Oncol. 7:270–275. 2006. View Article : Google Scholar : PubMed/NCBI | |
|
Ardeshna KM, Smith P, Norton A, Hancock BW, Hoskin PJ, MacLennan KA, Marcus RE, Jelliffe A, Vaughan G, Hudson, et al: Long-term effect of a watch and wait policy versus immediate systemic treatment for asymptomatic advanced-stage non-Hodgkin lymphoma: A randomised controlled trial. Lancet. 362:516–522. 2003. View Article : Google Scholar : PubMed/NCBI | |
|
Martin P, Chadburn A, Christos P, Weil K, Furman RR, Ruan J, Elstrom R, Niesvizky R, Ely S, Diliberto M, et al: Outcome of deferred initial therapy in mantle-cell lymphoma. J Clin Oncol. 27:1209–1213. 2009. View Article : Google Scholar : PubMed/NCBI | |
|
DeVita VT Jr, Canellos GP, Chabner B, Schein P, Hubbard SP and Young RC: Advanced diffuse histiocytic lymphoma, a potentially curable disease. Lancet. 1:248–250. 1975. View Article : Google Scholar : PubMed/NCBI | |
|
Coltman CA, Dahlberg S and Jones SE: CHOP is curative in thirty percent of patients with large cell lymphoma: A twelve-year Southwest oncology group follow-up. Advances in cancer chemotherapy: Update on treatment for diffuse large cell lymphoma. Skarin AT: Wiley; New York, NY: pp. 71–77. 1986 | |
|
Johnsen AT, Tholstrup D, Petersen MA, Pedersen L and Groenvold M: Health related quality of life in a nationally representative sample of haematological patients. Eur J Haematol. 83:139–148. 2009. View Article : Google Scholar : PubMed/NCBI | |
|
Montgomery C, Pocock M, Titley K and Lloyd K: Individual quality of life in patients with leukaemia and lymphoma. Psychooncology. 11:239–243. 2002. View Article : Google Scholar : PubMed/NCBI | |
|
Parker PA, Baile WF, de Moor CD and Cohen L: Psychosocial and demographic predictors of quality of life in a large sample of cancer patients. Psychooncology. 12:183–193. 2003. View Article : Google Scholar : PubMed/NCBI | |
|
Friedberg JW, Thompson CA, Trněný M, Morschhauser F, Salles G, Reagan PM, Hertzberg M, Smolewski P, Zhang H, Thieblemont C, et al: Health-related quality of life (HRQoL) in patients with diffuse large B-cell lymphoma (DLBCL) treated with polatuzumab vedotin, rituximab, cyclophosphamide, doxorubicin and prednisone (Pola-R-CHP) versus rituximab, cyclophosphamide, doxorubicin, vincristine and prednisone (R-CHOP) in the Phase III POLARIX Study. Blood. 140 (Suppl 1):S6623–S6626. 2022. View Article : Google Scholar | |
|
Ardeshna KM, Qian W, Smith P, Braganca N, Lowry L, Patrick P, Warden J, Stevens L, Pocock CFE, Miall F, et al: Rituximab versus a watch-and-wait approach in patients with advanced-stage, asymptomatic, non-bulky follicular lymphoma: An open-label randomised phase 3 trial. Lancet Oncol. 15:424–435. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
Brandt J, Dietrich S, Meissner J, Neben K, Ho AD and Witzens-Harig M: Quality of life of long-term survivors with Hodgkin lymphoma after high-dose chemotherapy, autologous stem cell transplantation, and conventional chemotherapy. Leuk Lymphoma. 51:2012–2020. 2010. View Article : Google Scholar : PubMed/NCBI | |
|
Coiffier B, Lepage E, Briere J, Herbrecht R, Tilly H, Bouabdallah R, Morel P, Van Den Neste E, Salles G, Gaulard P, et al: CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med. 346:235–242. 2002. View Article : Google Scholar : PubMed/NCBI | |
|
El Haidari RE, Anota A, Dabakuyo-Yonli TS, Guillemin F, Conroy T, Velten M, Jolly D, Causeret S, Cuisenier J, Graesslin O, et al: Utility values and its time to deterioration in breast cancer patients after diagnosis and during treatments. Qual Life Res. 31:3077–3085. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Wasse SK, Mounier M, Assogba E, Rossi C, Adnet J, Gauthier S, Girard S, Atsou KM, Dabakuyo-Yonli TS and Maynadie M: Factors affecting health-related quality of life among survivors of non-Hodgkin lymphoma: A population-based study. Cancers (Basel). 15:38852023. View Article : Google Scholar : PubMed/NCBI | |
|
Sasaki T, Izawa M and Okada Y: Current trends in health insurance systems: OECD countries vs Japan. Neurol Med Chir (Tokyo). 55:267–275. 2015. View Article : Google Scholar | |
|
Paunescu AC, Copie CB, Malak S, Gouill SL, Ribrag V, Bouabdallah K, Sibon D, Rumpold G, Preau M, Mounier N, et al: Quality of life of survivors 1 year after the diagnosis of diffuse large B-cell lymphoma: A LYSA study. Ann Hematol. 101:317–332. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Oerlemans S, Issa DE, van den Broek EC, Nijziel MR, Coebergh JWW, Huijgens PC, Mols F and van de Poll-Franse LV: Health-related quality of life and persistent symptoms in relation to (R-)CHOP14, (R-)CHOP21, and other therapies among patients with diffuse large B-cell lymphoma: Results of the population-based PHAROS-registry. Ann Hematol. 93:1705–1715. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
Mounier N, Anthony S, Busson R, Thieblemont C, Ribrag V, Tilly H, Haioun C, Casasnovas RO, Morschhauser F, Feugier P, et al: Long-term fatigue in survivors of non-Hodgkin lymphoma: The lymphoma study association SIMONAL cross-sectional study. Cancer. 125:2291–2299. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Aogi K, Takeuchi H, Saeki T, Aiba K, Tamura K, Iino K, Imamura CK, Okita K, Kagami Y, Tanaka R, et al: Optimizing antiemetic treatment for chemotherapy-induced nausea and vomiting in Japan: Update summary of the 2015 Japan society of clinical oncology clinical practice guidelines for antiemesis. Int J Clin Oncol. 26:1–17. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Herdman M, Gudex C, Lloyd A, Janssen M, Kind P, Parkin D, Bonsel G and Badia X: Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res. 20:1727–1736. 2011. View Article : Google Scholar : PubMed/NCBI | |
|
Hasegawa M, Komoto S, Shiroiwa T and Fukuda T: Formal implementation of cost-effectiveness evaluations in Japan: A unique health technology assessment system. Value Health. 23:43–51. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Doorduijn J, Buijt I, Holt B, Steijaert M, Uyl-de Groot C and Sonneveld P: Self-reported quality of life in elderly patients with aggressive non-Hodgkin's lymphoma treated with CHOP chemotherapy. Eur J Haematol. 75:116–123. 2005. View Article : Google Scholar : PubMed/NCBI | |
|
Omachi K: JSH practical guidelines for hematological malignancies, 2018: II. Lymphoma-overview. Int J Hematol. 110:3–10. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Wilson WH, Grossbard ML, Pittaluga S, Cole D, Pearson D, Drbohlav N, Steinberg SM, Little RF, Janik J, Gutierrez M, et al: Dose-adjusted EPOCH chemotherapy for untreated large B-cell lymphomas: A pharmacodynamic approach with high efficacy. Blood. 99:2685–2693. 2002. View Article : Google Scholar : PubMed/NCBI | |
|
Kantarjian HM, O'Brien S, Smith TL, Cortes J, Giles FJ, Beran M, Pierce S, Huh Y, Andreeff M, Koller C, et al: Results of treatment with hyper-CVAD, a dose-intensive regimen, in adult acute lymphocytic leukemia. J Clin Oncol. 18:547–561. 2000. View Article : Google Scholar : PubMed/NCBI | |
|
Romaguera JE, Fayad L, Rodriguez MA, Broglio KR, Hagemeister FB, Pro B, McLaughlin P, Younes A, Samaniego F, Goy A, et al: High rate of durable remissions after treatment of newly diagnosed aggressive mantle-cell lymphoma with rituximab plus hyper-CVAD alternating with rituximab plus high-dose methotrexate and cytarabine. J Clin Oncol. 23:7013–7023. 2005. View Article : Google Scholar : PubMed/NCBI | |
|
Markman M: Toxicities of the platinum antineoplastic agents. Expert Opin Drug Saf. 2:597–607. 2003. View Article : Google Scholar : PubMed/NCBI | |
|
Devlen J, Maguire P, Phillips P, Crowther D and Chambers H: Psychological problems associated with diagnosis and treatment of lymphomas. I: Retrospective study. Br Med J (Clin Res Ed). 295:953–954. 1987. View Article : Google Scholar : PubMed/NCBI | |
|
Oerlemans S, Mols F, Nijziel MR, Zijlstra WP, Coebergh JWW and van de Poll-Franse LV: The course of anxiety and depression for patients with Hodgkin's lymphoma or diffuse large B cell lymphoma: A longitudinal study of the PROFILES registry. J Cancer Surviv. 8:555–564. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
Ohno S, Shoji A, Hatake K, Oya N and Igarashi A: Cost-effectiveness analysis of treatment regimens with obinutuzumab plus chemotherapy in Japan for untreated follicular lymphoma patients. J Med Econ. 23:1130–1141. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Tang AL and Thomas SJ: Relationships between depressive symptoms, other psychological symptoms, and quality of life. Psychiatry Res. 289:1130492020. View Article : Google Scholar : PubMed/NCBI |