Protease activity at invadopodial focal digestive areas is dependent on NHE1-driven acidic pHe
- Authors:
- Published online on: December 13, 2013 https://doi.org/10.3892/or.2013.2923
- Pages: 940-946
Abstract
Introduction
The tumor microenvironment plays a fundamental role in promoting tumor progression and metastasis (1). One of the primary forces of the metabolic tumor microenvironment guiding an invasive and metastatic phenotype is the low extracellular pH (pHe) produced by tumor cells (2,3). Studies using in vitro cell-culture and in situ tumor spectroscopy have revealed that, while having an alkaline intracellular pH (pHi), tumors can reach an acidic interstitial pHe 0.5–1 units lower than normal tissue (tumor pHe of 6.5–7 vs. a normal tissue pHe of 7.4) (4). This acidic pHe was previously demonstrated to stimulate in vitro invasion (5) and in vivo metastasis (6), but the mechanism(s) underlying acidic pHe-induced effects are only recently being defined. According to the ‘acid-mediate invasion hypothesis’ (7), acidic conditions promote extracellular release and activity of key proteases such as cathepsin B and matrix metalloproteinase-2 and -9 (MMP-2, -9) (8), which break down both extracellular matrix (ECM) and basement membrane, thereby promoting migration, invasion and metastasis (9). Therefore, we initiated studies to elucidate the nature of the molecular pathways and mechanisms that regulate these events.
Aggressive tumor cells from multiple malignancies localize and concentrate a series of different proteases including the secreted MMP-2 and -9 and the transmembrane proteases (such as MT1-MMP) at actin-rich invasive protrusions called invadopodia that precisely regulate the directed proteolysis of the ECM and facilitate invasion (10). A wide variety of actin-interacting proteins, scaffolding proteins, signaling proteins and ion transporters are involved in invadopodia formation and functioning (11). In particular, the plasma membrane Na+/H+ exchanger 1 (NHE1) is localized at cancer cell invadopodia, where it plays an integrated role in both invadopodia formation and proteolytic activity (12). Taken together, these data suggest that there exists at invadopodia a concordance between NHE1 localization, extracellular acidification, protease activity on the cell surface at invadopodia and the cytoskeleton reorganization necessary for invadopodial maturation in human malignant breast carcinoma cells. There is currently no report on the effect of NHE1-induced acid pHe on protease activity at the most important sites of ECM digestion by cancer cells, the invadopodia. This is due to the fact that, although biochemical studies can determine net protease activity in the tumor extracellular medium, they do not provide any information if the localization and activation of proteases may occur at invadopodia. Here, by using in situ invadopodial zymography, we measured the individual activity of the different proteases and related this invadopodial-localized protease expression and activity to NHE1 and pHe at invadopodia of the human metastatic breast cancer cell line, MDA-MB-231.
Materials and methods
Reagents
Porcine skin gelatin, type A, was from Sigma. Matrigel growth factor reduced w/o phenol red from BD Biosciences. DQ™Green BSA, DQ™Red BSA and DQ™pig skin gelatin fluorescein conjugated from Molecular Probes. Primary antibodies: monoclonal [4E9] anti-NHE1 (Abcam), monoclonal anti-Cortactin (p80/85) clone 4F1 (Millipore), polyclonal anti-MMP-2 and anti-MMP-9 (Cell Signaling Technology, Inc.), polyclonal anti-cathepsin B (Fitzgerald), monoclonal anti-β actin (Sigma). Rhodamine B, isothiocyanate, mixed isomers (TRITC) was from Sigma. Immunofluorescence: polyclonal anti-NHE1 (Alpha Diagnostic). Secondary antibodies: anti-mouse (Sigma) and anti-rabbit (Cell Signaling Technology, Inc.) HRP linked antibodies, goat anti-mouse and anti-rabbit Alexa Fluor 488- and Alexa Fluor 568-linked (Molecular Probes, Inc., Eugene, OR, USA).
Cell culture and preparation of different pHe in growth medium and protease inhibitors
MDA-MB-231 cells were grown in Dulbecco’s modified Eagle’s medium (DMEM) high glucose (4,500 mg/l) supplemented with NaHCO3 (3,700 mg/l), 10% (v/v) heat-inactivated fetal bovine serum, L-glutamine (2 mM), sodium-pyruvate (1 mg/ml) and penicillin (100 units)/streptomycin (100 mg/ml) in a 5% CO2/95% air humidified incubator at 37°C.
Protease expression levels and in situ protease zymography (i.e. invadopodia-dependent ECM digestion) were measured at neutral (pHe 7.4), acidic (pHe 6.7) or basic (pHe 7.8) growth mediums, in the absence or presence of the following protease inhibitors: cathespin B inhibitor CA-074 (5 μM), Calbiochem (cat. no. 205030), MMP-9 inhibitor 1 (7.5 nM) Calbiochem (cat. no. 444278) or MMP-2 inhibitor 1 (15 μM) Calbiochem (cat. no. 444244), Batimastat (Sigma) and Cariporide (Sanofi-Aventis).
Degradation assay and in situ protease zymography
The activity of each protease was assayed at invadopodia by measuring the quantitative levels of focal and pericellular ECM digestion via in situ zymography in cells plated on Matrigel containing quenched DQ-BSA, as previously described (12), in the absence and presence of the specific inhibitor of each protease.
Invadopodia cell fractionation
Cytosol, membrane and invadopodia fractions were obtained from cells grown on gelatin, as previously described (12).
Preparation of conditioned media, cell lysates and western blotting
These procedures were performed as previously described (13).
Image acquisition and analysis
Images were acquired and analyzed as previously described (14) with a ×60 oil objective using a Nikon Eclipse TE 2000S epifluorescence microscope or a laser scanning confocal microscope (LSCM) (C1/TE2000-U; Nikon Instruments S.p.A., Sesto Fiorentino-FI, Italy). Confocal images were analyzed using ImageJ (http://rsb.info.nih.gov/ij/).
Duolink proximity ligation assay (PLA)
Cells were plated for the invadopodial matrix degradation assay, fixed, permeabilized and stained with primary antibodies (NHE1, MMP-2, MMP-9 and cathepsin B) at the recommended immunofluorescence dilution (or 1:400 if no dilution was given). Proximity ligation was performed according to the manufacturer’s protocol using the Duolink Detection kit with PLA PLUS and MINUS probes for mouse and rabbit (Olink Biosciences; ref. 43). Samples were analyzed with a confocal microscope (LSM 510 Meta; Carl Zeiss Inc.) under a ×63 oil objective.
Statistical analysis
Student’s t-test was applied to analyze the statistical significance between treatments and p<0.05 was considered to indicate a statistically significant difference, assuming equal variances on all experimental data sets. All comparisons were performed with InStat (GraphPad Software, San Diego, CA, USA).
Results
Acidic pHe increases MMP-2, -9 and cathepsin B secretion
A correlation between tumor progression and metastasis and elevated levels of the zinc-dependent matrix metalloproteases MMP-2 and -9 (15) and/or the acidic cysteine proteases (16) has been reported in several experimental and clinical studies. Furthermore, the acidic microenvironment has been shown to be essential for promoting expression and activity of these proteases in both cancer and stromal cells during tumor invasion, angiogenesis and metastasis (6,17). Therefore, we focused on MMP-2, -9 and cathepsin B to first assess how shifting the pHe of culture medium of breast cancer cells towards acidic or alkaline values changes their cellular levels and extracellular secretion. We incubated metastatic breast cancer cells, MDA-MB-231, with media at various pH (pH 6.7, pH 7.4 and pH 7.8) for 8 h and measured the expression levels of MMP-2, -9 and cathepsin B by western blot analysis in both the total cell homogenates and in the collected tumor conditioned medium. As seen in Fig. 1, cells incubated with acidic medium (pH 6.7) in comparison to neutral medium (pH 7.4), exhibited a decrease of the MMP-2 (72 kDa) and MMP-9 (92 kDa) inactive pro-forms in the total cell homogenates and a marked increase of their mature forms (64 and 84 kDa respectively) released into the tumor conditioned medium. Similarly, extracellular acidosis (pH 6.7) reduced cathepsin B expression in the cellular homogenate while highly inducing its secretion into the tumor extracellular medium. On the contrary, when cells were incubated with alkaline medium (pH 7.8), we found that MMP-9 and cathepsin B did not change their expression in homogenates or secretion into conditioned media when compared to cells cultured at neutral pH, while MMP-2 continued to show decreased levels in the cellular homogenates and an increased secretion into the tumor conditioned medium. The data presented here confirm that extracellular acidic conditions increase the secretion of the activated forms of MMP-2, MMP-9 and cathepsin B. However, it remains unknown whether this increased secretion occurs globally for the cell or preferentially at invadopodia, known to be centers of enrichment for a variety of proteases.
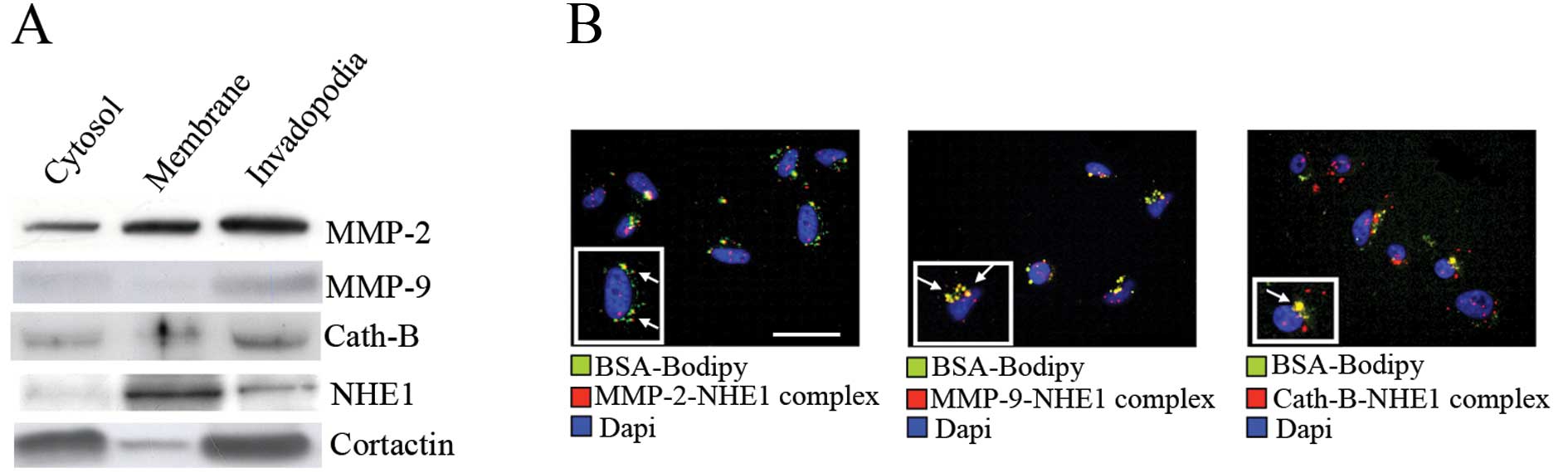
Cathepsin B, MMP-2 and -9 directly interact with NHE1 at matrix-degrading invadopodia
As we recently demonstrated that invadopodia are focal hotspots of very acidic pHe driven by high levels of NHE1 activity (12) and that this NHE1 activity is necessary for the digestive activity of the invadopodia, we explored whether the lowered peri-invadopodia pHe driving focal ECM degradation is through the regulation of the expression and/or activity of proteases at invadopodia. We first determined the presence of the three major proteases, cathepsin B, MMP-2 and -9, in invadopodia by fractionating cells plated on cross-linked porcine gelatin into cytosol, cell membrane or invadopodia fractions (12). The invadopodia compartment was identified on the basis of a strong cortactin overexpression when compared to the membrane fraction (12,18,19). As can be seen in Fig. 2A, all three proteases were enriched in the invadopodia although with different relative distributions. Furthermore, as previously reported (12), NHE1 was also expressed in the invadopodia compartment. To further analyze the potential association among NHE1, MMP-2, -9 and cathepsin B at proteolytically active invadopodia, a PLA, which can detect endogenous protein-protein interactions that occur within 40 nm (20), was combined with a Matrigel degradation assay, in which cells are plated on a mixture of Matrigel containing DQ Green BSA-Bodipy and a green fluorescent emission staining is indicative of invadopodia driven-ECM digestion (12). The advantages of PLA are that this technique provides a fluorescent signal (red) only when two target proteins are colocalized, allowing improved sensitivity for establishing endogenous protein-protein interactions and giving in situ information whether these colocalizations occur in specific intracellular compartments. As shown by the red fluorescent staining reported in Fig. 2B, NHE1 associated with all three proteases at the level of matrix-degrading invadopodia (shown in green, while the merged area in yellow are indicated with arrows). These results demonstrate that some sub-populations of MMP-2, -9 and cathepsin B may reside at the level of functionally active invadopodia where they interact with NHE1.
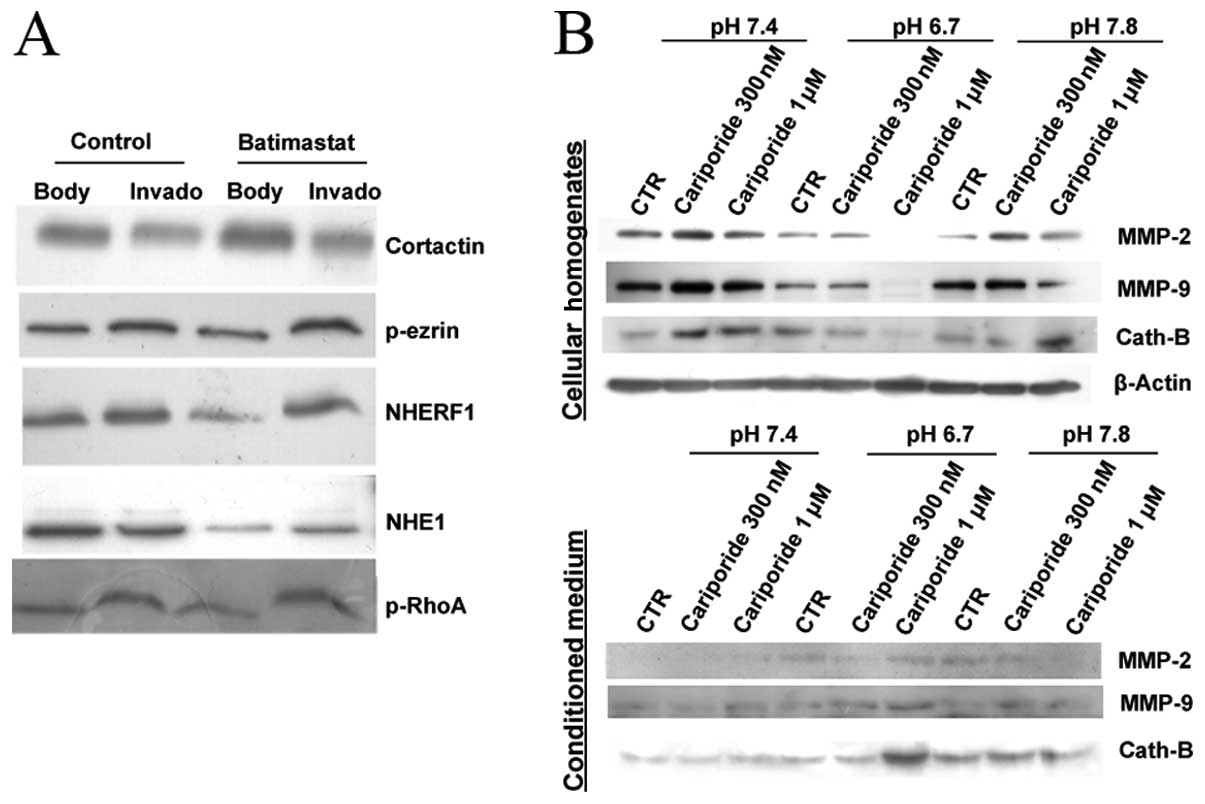
Protease activity suppression increases relative invadopodial NHE1 expression, while NHE1 inhibition increases acid-induced protease secretion
As both NHE1 and MMPs are involved in the invadopodia mediated-ECM degradation, and having demonstrated in Fig. 2A that breast cancer cells have an enrichment of NHE1 and protease expression at invadopodia when compared to cellular bodies, we investigated if proteases and NHE1 exert a reciprocal transmodulation of their expression and/or activities. We first evaluated the role of MMP activity on NHE1 compartmentalization in invadopodia by using a general MMP inhibitor, batimastat. As can be observed in the invadopodia fractionation experiments reported in Fig. 3A, treatment with 5 μM batimastat for 6 h significantly redistributed NHE1 from cellular bodies to invadopodia while having no effect on the distribution of a series of other invadopodia located proteins, suggesting a feed-back role of these enzymes on invadopodia proteolytic activity in breast cancer cells by controlling NHE1 expression and localization. These data are consistent with evidence that: (i) inhibiting NHE1 activity reduced MMP-2 and -9 activation (21) and cathepsin B activation (5) in the extracellular medium, and (ii) that the overexpression of a non-transporting NHE1 mutant reduced MMP-9 expression and activity (22).
To determine whether inhibition of NHE1 by different doses of cariporide affects protease release and if there is a pH-dependence of this process, we examined the effect of cariporide on MMP-2, -9 and cathepsin B expression at the same pHe values utilized in the previous experiments shown in Fig. 1. As shown in Fig. 3B, when NHE1 activity was inhibited by 300 nM cariporide in cells grown at physiological pH (pH 7.4), we observed an intracellular accumulation of the three proteases and a corresponding reduction of their secretion in the tumor conditioned medium. As previously, we found that acidic pHe increased the release of MMP-2, -9 and cathepsin B and inhibition of NHE1 by cariporide (especially at 1 μM) strongly stimulated the release of the proteases almost completely emptying the cells of all three proteases resulting in peaks of their levels in the conditioned medium. The use of cariporide concentrations near its IC50 value (~280 nM) and at a near maximal concentration (1 μM) for inhibiting NHE1 activity (12), demonstrated that the release of the proteases have somewhat different interaction kinetics with NHE1 activity.
Acidic pHe-dependent protease activity is localized to the invadopodia
We finally determined the activity and role of the three proteases at the level of invadopodia. Similar to studies of experimental extracellular acidification in determining osteoclastic proteolytic programs (23,24), we examined whether exposure to an experimental acidosis may increase focal, invadopodia-dependent ECM degradation in MDA-MB-231 cells plated on Matrigel containing DQ Green BSA-Bodipy and if this increase was due, at least in part, to a direct increase in protease activity. To determine the pH-dependence of the activity of the specific proteases MMP-2, -9 and cathepsin B at the invadopodia, we performed focal digestion experiments via in situ zymography in Matrigel by incubating the cells in acidic (pHe 6.7), neutral (pHe 7.4) or basic (pHe 7.8) growth mediums, in the absence or presence of the following small-molecule inhibitors to pharmacologically knock-out protease function: cathepsin B inhibitor CA-074 (5 μM), MMP-9 inhibitor 1 (7.5 nM) or MMP-2 inhibitor 1 (15 μM). Proteolysis of individual cells was followed as previously described (12) with invadopodia-dependent proteolysis being defined as the pixel density of focal zones of digestion for each cell. Fig. 4A shows confocal immunofluorescence images, using this in situ zymographic assay, of the actin cytoskeleton (red) and digestion (green) from typical cells of the different pHe treatments, while Fig. 4B shows the histogram of the cumulative data of total invadopodia (focal) ECM proteolysis from four independent experiments. Exposure to extracellular acidification produced a significant increase in both invadopodia expression/number (percent of proteolytically active cells, data not shown) and total invadopodial activity in control cells (red bars in Fig. 4B) when compared to cells treated at neutral or basic pHe. These results confirm data from previous studies showing the pH dependence of ECM digestion at invadopodia (12). Notably, the ability of all three protease inhibitors to block the invadopodia-localized proteolysis was much greater in the acidic medium and again, declined with increasing pHe demonstrating that, indeed, MMP-2 (green bars), MMP-9 (blue bars) and cathepsin B (yellow bars) are more active at acidic pHe.
Collectively, these data show that, as in osteoclasts, an experimental acidification increases tumor cell proteolytic activity by turning on intrinsic invadopodial programs and that an important future direction will be to identify the molecular components of these specific invasive programs.
Discussion
Matrix metalloproteases (i.e. MMP-2, -9 and MT1-MMP) and cysteine proteases such as cathepsin B are key invasion markers due to their critical role in digesting both extracellular matrix (ECM) and basement membrane proteins (25). However, except for the membrane anchored MT1-MMP, there is some controversy concerning the respective importance of each protease in ECM digestion, their interaction and the role of extracellular pH (pHe) in driving or regulating protease activity and action at the most important sites of ECM digestion by cancer cells, the invadopodia.
While a large series of observations demonstrate that NHE1-dependent acidification of the extracellular space functions together with extracellular proteases to digest the ECM in a controlled manner, the experiments were performed with whole conditioned medium; therefore, the structural and functional mechanisms/determinants linking the extracellular acidification to the ECM proteolysis remain unknown. In this context, data from various groups has shown that NHE1-dependent proton extrusion at the invadopodia site is a crucial event for localized ECM digestion (12,26,27). However, while it has been demonstrated for MT1-MMP that its accumulation at mature-ECM digesting invadopodia is a pH dependent-event (21), the presence, pH dynamics and interaction with NHE1 of the other proteases at invadopodia are unknown. The goal of the present study was to elucidate other proteases present at or surrounding the invadopodia and the dynamics of both their regulation by pHe and their relationship with NHE1.
To highlight these three key processes, we applied the following experimental strategies; first, we measured the cellular expression and secretion of cathepsin B, MMP-2 and -9 in cells incubated in neutral (pHe 7.4), in acidic (pHe 6.7) or in basic (pHe 7.8) growth medium and these experiments confirmed that all three proteases are secreted at higher levels at lower pHe (Fig. 1). Furthermore, we reported for the first time in a single study by invadopodia fractionation experiments that all these proteases compartmentalize with NHE1 at invadopodia (Fig. 2A). In this respect, an important novel observation of the present study was that, in ECM-digesting invadopodia, NHE1 was very closely associated with the three proteases, such that we observed a positive interaction signal in an in situ assay (proximity ligation assay) that measures interactions between two proteins in close proximity (under 40 nm) with high spatial resolution (28) (Fig. 2B). This suggests that a tight physical and functional association between the major enzyme responsible for extra-invadopodial acidification, NHE1, and the acidic-driven proteases in that microspace exists in invadopodia. Also, as NHE1 is associated with MMP-2/-9 and cathepsin B at invadopodia, we investigated whether NHE1 activity is important for the secretion of the three proteases into the peri-invadopodial space and if protease activity may possibly reciprocally regulate NHE1 expression at the invadopodia. We observed a dose-dependent stimulation of the release of cathepsin B, MMP-2 and -9 by NHE1 inhibitor, cariporide (Fig. 3B) at acidic pHe, indicating a strict control of NHE1 over the protease secretion pathway. Lastly, as a direct measure of a pH-dependent regulation of protease activity at the invadopodia site of ECM digestion is still lacking, we performed a series of in situ zymogram experiments in the absence and presence of specific inhibitor of each protease, confirming that the major site of protease digestion of the ECM occurs at invadopodia (Fig. 4A and B). These data indicate that more than one protease is functioning to digest the ECM at invadopodia and that the proteases have a functional pHe optimum that is acidic not only in vitro but also in situ. Indeed, our use of small-molecule inhibitors to pharmacologically knock-out protease function revealed that each of the proteases plays important roles in invadopodia proteolysis of the ECM and, therefore, presumably in proteolysis-dependent functions such as tumor growth, tumor vascularity and invasion.
In conclusion, our data demonstrate for the first time that proton extrusion at the invadopodial site is a crucial event for proteolytic ECM digestion in that at the invadopodial sites of ECM proteolysis, more than one protease is functioning to digest the ECM and these proteases have a functional pHe optimum that is acidic. These data provide a structural basis for the well known role of the NHE1 in tumor cell invasion and the regulation of protease activity localized at invadopodia.
Acknowledgements
This study was supported by ‘Associazione Italiana per la Ricerca sul Cancro’ (AIRC) grant no. 11348 and PRIN Grant 2009 no. 1341 to S.J.R. The SJR laboratory is part of the Italian network ‘Istituto Nazionale Biostrutture e Biosistemi’ (INBB), and the ‘Centro di Eccellenza di Genomica in Campo Biomedico ed Agrario’ of the University of Bari and the project ‘BioBoP’ of the Region Puglia.
References
|
Spano D and Zollo M: Tumor microenvironment: a main actor in the metastasis process. Clin Exp Metastasis. 29:381–395. 2012. View Article : Google Scholar : PubMed/NCBI | |
|
Cardone RA, Casavola V and Reshkin SJ: The role of disturbed pH dynamics and the Na+/H+ exchanger in metastasis. Nat Rev Cancer. 5:786–795. 2005. View Article : Google Scholar : PubMed/NCBI | |
|
Bailey KM, Wojtkowiak JW, Hashim AI and Gillies RJ: Targeting the metabolic microenvironment of tumors. Adv Pharmacol. 65:63–107. 2012. View Article : Google Scholar | |
|
Martin NK, Robey IF, Gaffney EA, Gillies RJ, Gatenby RA and Maini PK: Predicting the safety and efficacy of buffer therapy to raise tumour pHe: an integrative modelling study. Br J Cancer. 106:1280–1287. 2012. View Article : Google Scholar : PubMed/NCBI | |
|
Bourguignon LY, Singleton PA, Diedrich F, Stern R and Gilad E: CD44 interaction with Na+-H+ exchanger (NHE1) creates acidic microenvironments leading to hyaluronidase-2 and cathepsin B activation and breast tumor cell invasion. J Biol Chem. 279:26991–27007. 2004.PubMed/NCBI | |
|
Rofstad EK, Mathiesen B, Kindem K and Galappathi K: Acidic extracellular pH promotes experimental metastasis of human melanoma cells in athymic nude mice. Cancer Res. 66:6699–6707. 2006. View Article : Google Scholar : PubMed/NCBI | |
|
Estrella V, Chen T, Lloyd M, Wojtkowiak J, Cornnell HH, Ibrahim-Hashim A, et al: Acidity generated by the tumor microenvironment drives local invasion. Cancer Res. 73:1524–1535. 2013. View Article : Google Scholar : PubMed/NCBI | |
|
Reshkin SJ, Cardone RA and Harguindey S: Na+-H+ exchanger, pH regulation and cancer. Recent Pat Anticancer Drug Discov. 8:85–99. 2013. | |
|
Mason SD and Joyce JA: Proteolytic networks in cancer. Trends Cell Biol. 21:228–237. 2011. View Article : Google Scholar : PubMed/NCBI | |
|
Eckert MA and Yang J: Targeting invadopodia to block breast cancer metastasis. Oncotarget. 2:562–568. 2011.PubMed/NCBI | |
|
Yamaguchi H: Pathological roles of invadopodia in cancer invasion and metastasis. Eur J Cell Biol. 91:902–907. 2012. View Article : Google Scholar : PubMed/NCBI | |
|
Busco G, Cardone RA, Greco MR, Bellizzi A, Colella M, Antelmi E, et al: NHE1 promotes invadopodial ECM proteolysis through acidification of the peri-invadopodial space. FASEB J. 24:3903–3915. 2010. View Article : Google Scholar : PubMed/NCBI | |
|
Cardone RA, Greco MR, Capulli M, Weinman EJ, Busco G, Bellizzi A, et al: NHERF1 acts as a molecular switch to program metastatic behavior and organotropism via its PDZ domains. Mol Biol Cell. 23:2028–2040. 2012. View Article : Google Scholar : PubMed/NCBI | |
|
Cardone RA, Bagorda A, Bellizzi A, Busco G, Guerra L, Paradiso A, et al: Protein kinase A gating of a pseudopodial-located RhoA/ROCK/p38/NHE1 signal module regulates invasion in breast cancer cell lines. Mol Biol Cell. 16:3117–3127. 2005. View Article : Google Scholar : PubMed/NCBI | |
|
Roomi MW, Monterrey JC, Kalinovsky T, Rath M and Niedzwiecki A: Distinct patterns of matrix metalloproteinase-2 and -9 expression in normal human cell lines. Oncol Rep. 21:821–826. 2009.PubMed/NCBI | |
|
Matarrese P, Ascione B, Ciarlo L, Vona R, Leonetti C, Scarsella M, et al: Cathepsin B inhibition interferes with metastatic potential of human melanoma: an in vitro and in vivo study. Mol Cancer. 9:2072010. View Article : Google Scholar : PubMed/NCBI | |
|
Brisson L, Gillet L, Calaghan S, Besson P, Le Guennec JY, Roger S and Gore J: NaV1.5 enhances breast cancer cell invasiveness by increasing NHE1-dependent H+ efflux in caveolae. Oncogene. 30:2070–2076. 2011. | |
|
Bowden ET, Barth M, Thomas D, Glazer RI and Mueller SC: An invasion-related complex of cortactin, paxillin and PKCμ associates with invadopodia at sites of extracellular matrix degradation. Oncogene. 18:4440–4449. 1999.PubMed/NCBI | |
|
Caldieri G, Ayala I, Attanasio F and Buccione R: Cell and molecular biology of invadopodia. Int Rev Cell Mol Biol. 275:1–34. 2009. View Article : Google Scholar | |
|
Söderberg O, Gullberg M, Jarvius M, Ridderstråle K, Leuchowius KJ, Jarvius J, et al: Direct observation of individual endogenous protein complexes in situ by proximity ligation. Nat Methods. 3:995–1000. 2006.PubMed/NCBI | |
|
Lin Y, Chang G, Wang J, Jin W, Wang L, Li H, et al: NHE1 mediates MDA-MB-231 cells invasion through the regulation of MT1-MMP. Exp Cell Res. 317:2031–2040. 2011. View Article : Google Scholar : PubMed/NCBI | |
|
Putney LK and Barber DL: Expression profile of genes regulated by activity of the Na-H exchanger NHE1. BMC Genomics. 5:462004. View Article : Google Scholar : PubMed/NCBI | |
|
Komarova SV, Pereverzev A, Shum JW, Sims SM and Dixon SJ: Convergent signaling by acidosis and receptor activator of NF-κB ligand (RANKL) on the calcium/calcineurin/NFAT pathway in osteoclasts. Proc Natl Acad Sci USA. 102:2643–2648. 2005.PubMed/NCBI | |
|
Pereverzev A, Komarova SV, Korcok J, Armstrong S, Tremblay GB, Dixon SJ and Sims SM: Extracellular acidification enhances osteoclast survival through an NFAT-independent, protein kinase C-dependent pathway. Bone. 42:150–161. 2008. View Article : Google Scholar | |
|
Monet M, Lehen’kyi V, Gackiere F, Firlej V, Vandenberghe M, Roudbaraki M, et al: Role of cationic channel TRPV2 in promoting prostate cancer migration and progression to androgen resistance. Cancer Res. 70:1225–1235. 2010. View Article : Google Scholar : PubMed/NCBI | |
|
Lucien F, Brochu-Gaudreau K, Arsenault D, Harper K and Dubois CM: Hypoxia-induced invadopodia formation involves activation of NHE-1 by the p90 ribosomal S6 kinase (p90RSK). PLoS One. 6:e288512011. View Article : Google Scholar : PubMed/NCBI | |
|
Magalhaes MA, Larson DR, Mader CC, Bravo-Cordero JJ, Gil-Henn H, Oser M, et al: Cortactin phosphorylation regulates cell invasion through a pH-dependent pathway. J Cell Biol. 195:903–920. 2011. View Article : Google Scholar : PubMed/NCBI | |
|
Fredriksson S, Gullberg M, Jarvius J, Olsson C, Pietras K, Gústafsdóttir SM, et al: Protein detection using proximity-dependent DNA ligation assays. Nat Biotechnol. 20:473–477. 2002. View Article : Google Scholar : PubMed/NCBI |