α‑Phellandrene enhances the apoptosis of HT‑29 cells induced by 5‑fluorouracil by modulating the mitochondria‑dependent pathway
- Authors:
- Published online on: March 5, 2024 https://doi.org/10.3892/or.2024.8720
- Article Number: 61
-
Copyright: © Susanto et al. This is an open access article distributed under the terms of Creative Commons Attribution License.
Abstract
Introduction
α-Phellandrene (α-PA) is a monoterpene compound, which is found in essential oils or extracts from natural plants, particularly in spices and herbs such as pine (1), ginger (2), dill (3) and peppermint (4). Previous studies have shown that α-PA has antidepressant (5), anti-inflammatory (6), immunoregulatory (7) and anticancer (8) effects. Our previous research demonstrated that α-PA can induce cell death by decreasing ATP levels (9) and trigger autophagy by affecting AKT/LC3II/mTOR signaling in human liver cancer cells (8). In addition, α-PA induces apoptosis in leukemia cells by regulating p53 signaling activation and the mitochondria-dependent pathway (10,11). Therefore, α-PA may enhance the efficacy of anticancer medicine via the induction of apoptotic pathways.
In 2020, colorectal cancer (CRC) was reported to have a global incidence rate of 10% among cancers of the gastrointestinal tract and 9.4% of cancer deaths (12). A number of therapies, such as surgery, radiation, immunotherapy, targeted therapy and chemotherapy, have been used to treat patients with CRC (13). 5-Fluorouracil (5-FU) is one of the most widely used chemotherapeutic medicines for treating CRC (14); it is a derivative of uracil, where a fluorine atom is substituted for a hydrogen atom at the C-5 position. The major mechanism of 5-FU in chemotherapy is interference with DNA and RNA synthesis in cancer cells through thymidylate synthase (TS), which is needed to replicate and repair DNA (14). 5-FU irreversibly inhibits TS, leading to a deoxyribonucleoside triphosphate imbalance, resulting in DNA damage and cell death (14). The aforementioned nuclear stress is the underlying mechanism by which 5-FU causes p53 activation and triggers mitochondria-dependent apoptosis (15). The two main apoptotic pathways are extrinsic and intrinsic pathways (16). The extrinsic apoptosis pathway begins outside of the cell, with some ligands, such as TNF-α, binding death receptor (DRs) on cell membranes, such as Fas and DR2, which triggers FADD binding to caspase-8 to induce apoptosis. The intrinsic apoptotic pathway, which begins when some injury, such as stress or extra calcium ions, occurs within the cell, resulting in the activation of the Bcl-2 family, leaking mitochondrial membrane and a lower mitochondrial membrane potential, and activation of caspase family expression, thus leading to the induction of apoptosis (16). Notably, the Bcl-2 family members, such as Bcl-2, Bax and Bid (17), and mitochondria membrane molecules, such as voltage-dependent anion channel (VDAC)/hexokinase-2 (HK-2) modulators (18), are activated by intrinsic stress and then trigger the mitochondria-dependent intrinsic apoptosis pathway. Moreover, caspase-8 can be activated by TLR4 on the cell membrane, and can directly activate caspase-3 and trigger the extrinsic apoptotic pathway. Both extrinsic and intrinsic apoptotic pathways can initiate different stages of the caspase family cascade (19), such as caspse-8 via the extrinsic apoptotic pathway, and caspase-9 and −3 via the intrinsic apoptotic pathway, which serve critical roles in activating apoptosis.
Notably, 5-FU may cause drug resistance and numerous side effects (20). DNA damage by 5-FU can also occur in normal cells, leading to cytotoxicity and apoptosis (21). There are three significant side effects of 5-FU: Cytotoxicity, especially neurotoxicity and cardiotoxicity (22); oral mucositis (23); and bone marrow disorder (24). In the clinic, oncologists have used nonsteroidal anti-inflammatory drugs (NSAIDs) to relieve side effects, such as pain, inflammation and fever, and as anticancer drugs (25). However, the prolonged use of NSAIDs can also cause side effects due to their chemical composition (26). Using active compounds from food or phytochemicals to enhance anticancer medicine efficacy is a promising approach to reducing the side effects of 5-FU.
The present study aimed to examine how the combination of α-PA and 5-FU affects the induction of apoptosis in colon cancer. In addition, whether α-PA can enhance 5-FU-induced apoptosis by regulating the mitochondria-dependent pathway remains to be elucidated.
Materials and methods
Chemicals and reagents
α-PA and dimethyl sulfoxide (DMSO) were purchased from MilliporeSigma. 5-FU was obtained from Enzo Life Sciences, Inc. RPMI 1640 medium and fetal bovine serum were purchased from Gibco (Thermo Fisher Scientific, Inc.). Penicillin (10,000 U/ml) and streptomycin (10 mg/ml) were obtained from Cytiva.
Cell line and treatments
HT-29 cells are a human colon adenocarcinoma cell line sensitive to 5-FU, which have an epithelial morphology (27). In the present study, HT-29 cells were obtained from the Bioresource Collection and Research Center. To authenticate the cell line to ensure that it matches the original HT-29 cell line, multiplex PCR was performed using AmpFLSTR Identifiler PLUS PCR Amplification Kit (Applied Biosystems; Thermo Fisher Scientific, Inc.) containing 16 STR loci. The PCR products labeled with different fluorescence were analyzed with GeneMapper ID v3.1 on the capillary Genetic DNA analyzer 3730 (Applied Biosystems; Thermo Fisher Scientific, Inc.). The genotyping results of the sample were searched in human STR profile databases (https://www.dsmz.de/services/services-human-and-animal-cell-lines/online-str-analysis.html; https://web.expasy.org/cellosaurus-str-search/; http://www.culturecollections.org.uk/services/authenticell/search-by-profile/#). Searching the sample genotypes in the ATCC/DSMZ/JCRB/RIKEN/ECACC/ExPASy STR database, the test sample result matched the HT-29 cell line. The cells were cultured in RPMI 1640 medium supplemented with 5% fetal bovine serum and 1% antibiotic solution (10,000 U/ml penicillin and 10 mg/ml streptomycin) at 37°C in an humidified incubator (Thermo Fisher Scientific, Inc.) containing 5% CO2.
HT-29 cells were seeded at 1.0×105 cells/30 mm plate and were incubated for 24 h. The cells were then treated with 50, 100 or 250 µM α-PA combined with 5 µM 5-FU, followed by incubation for a further 72 h. These treatment conditions were identified through a series of pre-tests of cell viability, in which HT-29 cells were treated with 5, 10, 50, 100, 250 or 500 µM α-PA combined with 5 µM 5-FU for 24, 48 or 72 h. Finally, the model of 50, 100 or 250 µM α-PA combined with 5-FU for 72 h was considered the optimum study condition. In response to this study condition, the cell viability was not too low to detect normal physiological molecule levels, but also the cells did not trend towards excessive cell death. α-PA was dissolved in DMSO; therefore, DMSO was used to treat the control group. As additional control groups, HT-29 cells were subjected to a 72-h treatment with 5 µM 5-FU and 250 µM α-PA alone.
Cell viability assay
The MTT assay (28) and morphological examinations were used to evaluate cell viability. HT-29 cells were treated with different doses of α-PA combined with 5-FU for 72 h. After removing the culture medium, the cells were washed with sterilized cold PBS (3.2 mM sodium phosphate dibasic heptahydrate, 0.5 mM potassium dihydrogen phosphate, 1.3 mM potassium chloride, 135 mM sodium chloride, pH 7.4) and were then incubated with 5 mg/ml MTT reagent for 3 h at 37°C, and isopropanol was used to dissolve purple formazan. The resulting isopropanol fraction was analyzed at 570 nm using a Microplate Biokinetics Reader (BioTek Instruments, Inc.). Cell morphology was assessed using a phase-contrast inverted fluorescence microscope (Olympus IX 51; Olympus Corporation).
Evaluation of the combined effects
Chou and Talalay's method was used to quantitatively analyze dose-effect relationships (29). The combination index (CI), in which CI=(D1/DX1) + (D2/DX2), was calculated and assessed to analyze the interaction between 5-FU and α-PA. In the equation, D1 and D2 are the respective combination doses of 5-FU and α-PA that yield an effect of 50% growth inhibition, with DX1 and DX2 being the corresponding single doses of 5-FU and α-PA that result in the same effect. CI<1, CI=1 and CI>1 indicate synergistic, additive and antagonistic effects.
Analysis of apoptosis
The culture medium was removed after exposing HT-29 cells to different doses of α-PA combined with 5-FU for 72 h. The cells were rinsed twice with sterilized cold PBS. For immunocytochemical staining, cells were fixed with 5% paraformaldehyde at 4°C for 20 min. Subsequently, the cells were exposed to 0.5% Triton X-100 in PBS for 15 min and to 1% bovine serum albumin (BSA; Gibco; Thermo Fisher Scientific, Inc.) for 30 min at room temperature. Subsequently, to assess apoptosis, the cells were stained using an Annexin V Apoptosis Detection Kit I (cat. no. E-CK-A211; Elabscience Biotechnology, Inc.) according to the manufacturer's instructions. HT-29 cells were stained with Annexin V-FITC (2.5 µl/1 ml 1X binding buffer) and PI (2.5 µl/1 ml 1X binding buffer) for 15 min in the dark at room temperature. Automated image capture was performed using a Cytation™ 1 Cell Imaging Multi-Mode Reader (BioTek Instruments, Inc.) and Gen5 software (BioTek Instruments, Inc.). Annexin V was employed to detect early apoptotic cells, whereas PI was used to detect late apoptotic cells (30). Quantitative values for fluorescence intensity were obtained using the Cytation 1 Cell Imaging Multi-Mode Reader and were normalized to cell count. The percentage for the 5-FU group was set at 100%, and levels relative to the 5-FU group were calculated for the other groups.
Analysis of intracellular p53, Bax and Bcl-2 protein expression levels
The culture medium was removed after exposing HT-29 cells to different doses of α-PA combined with 5-FU for 72 h. The cells were then rinsed twice with sterilized cold PBS and 200 µl lysis buffer (10 mM Tris-HCl, 5 mM EDTA, 0.2 mM phenylmethylsulfonyl fluoride, 20 µg/ml aprotinin; pH 7.4) was used for protein extraction. The total protein concentration was determined using the method outlined by Lowry et al (31). Subsequently, ~20 µg cellular protein was separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis using 10% gels (32) and proteins were then transferred to polyvinylidene difluoride membranes (33). For blocking, 5% (w/v) non-fat dry milk suspended in PBS was used for 2 h at room temperature. Then, the membranes were exposed to the following primary antibodies diluted in PBS: Anti-p53 (cat. no. sc-126; 1:1,000), anti-Bax (cat. no. sc-7480; 1:1,000), anti-Bcl-2 (cat. no. sc-7382; 1:1,000), GADPH (cat. no. sc-32233; 1:1,000) and β-tubulin (cat. no. sc-166729; 1:1,000) (all from Santa Cruz Biotechnology, Inc.) for 12 h at 4°C. After three washes with PBS/0.1% (v/v) Tween-20 (5 min/wash), the membranes were incubated with a goat-anti mouse HRP-conjugated IgG secondary antibody (cat. no. E-AB-1001; 1:5,000 diluted in PBS; Elabscience Biotechnology, Inc.) for 2 h at room temperature. After three washes with PBS/0.1% (v/v) Tween-20 (5 min/wash), an enhanced chemiluminescence detection kit (cat. no. RPN3243; Amersham; Cytiva) was used to observe the bands and they were visualized and band densities were semi-quantified using a ChemiDoc XRSþ System and ImageLab 5.2.1 software (both from Bio-Rad Laboratories, Inc.).
MMP (ΔΨm) analysis
The culture medium was removed after exposing HT-29 cells to different doses of α-PA combined with 5-FU for 72 h. The cells were rinsed twice with sterilized cold PBS. Subsequently, the samples were incubated in medium containing 1 µM DiOC6 (Sigma-Aldrich; Merck KGaA) (34) and 1 µl/ml Hoechst 33342 (MilliporeSigma) for 20 min at room temperature in the dark, followed by three washes with PBS. The Cytation 1 Cell Imaging Multi-Mode Reader and Gen5 software were used for automated image capture. The fluorescence intensity of DiOC6 was determined by dividing its fluorescence intensity by that of Hoechst 33342. This method facilitated the standardization of the fluorescence intensity of the target molecule by considering the relative cell counts in each group.
Intracellular VDAC-1 and HK-2 protein distribution and quantification
The culture medium was removed after exposing HT-29 cells to different doses of α-PA combined with 5-FU for 72 h. The cells were rinsed twice with sterilized cold PBS. For staining, cells were fixed with 5% paraformaldehyde at 4°C for 20 min. Subsequently, the cells were treated with 0.5% Triton X-100 in PBS for 15 min and 1% BSA for 30 min at room temperature. To conduct immunostaining, the cells were exposed to the following primary antibodies diluted in PBS: Anti-VDAC-1 (cat. no. AF5478; 1:1,000) and anti-HK-2 (cat. no. BF0283; 1:1,000) (Affinity Biosciences, Ltd.) overnight at 4°C. The cells were rinsed with PBS and were subsequently exposed to an Alexa Fluor® 488 conjugated goat anti-rabbit IgG (H+L) highly cross-adsorbed secondary antibody (green; 1:1,000; cat. no. A-11034; Thermo Fisher Scientific, Inc.) or Alexa Fluor® 488 conjugated goat anti-mouse IgG (H+L) highly cross-adsorbed secondary antibody (green; 1:1,000; cat. no. A-32723; Thermo Fisher Scientific, Inc.) for 2 h at room temperature in the dark. Subsequently, the cells were stained with 1 µg/ml Hoechst 33342 (blue) at room temperature and were then washed three times with PBS to remove excess Hoechst. The Cytation 1 Cell Imaging Multi-Mode Reader and Gen5 software were used for automated image capture. The fluorescence intensity of a particular target molecule (VDAC-1 and HK-2) was determined by dividing its fluorescence intensity by that of Hoechst 33342. This method facilitated the standardization of the fluorescence intensity of the target molecule by considering the relative cell counts in each group.
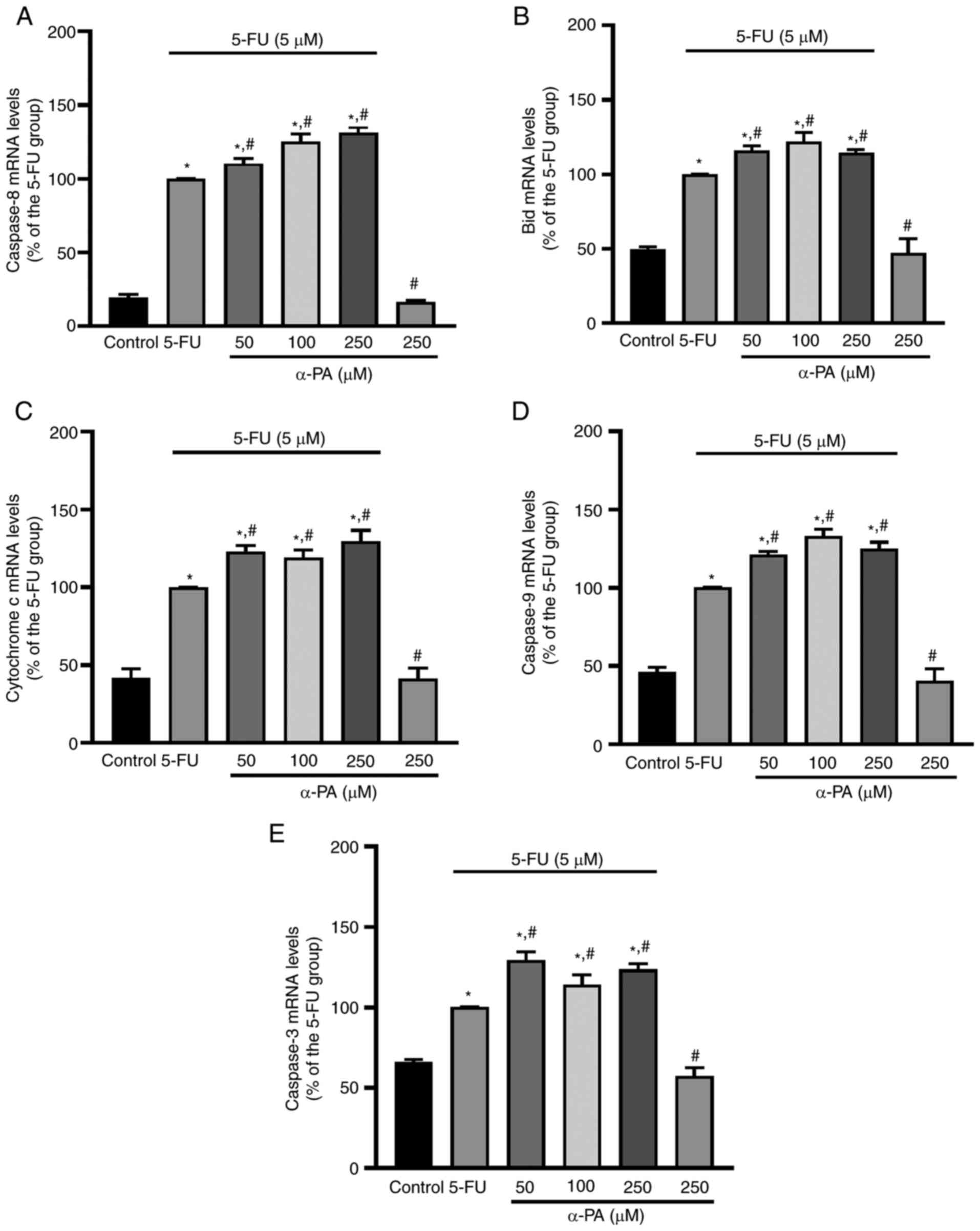
Cytochrome c, caspase-9, −3, −8 and Bid mRNA expression analysis
The culture medium was removed after exposing HT-29 cells to different doses of α-PA combined with 5-FU for 72 h. The cells were rinsed twice with sterilized cold PBS. The total RNA was extracted using a modified version of the method outlined by Chomczynski and Sacchi (35). Briefly, 1 ml TRIzol® reagent (Invitrogen; Thermo Fisher Scientific, Inc.) was added to the cells and mixed. Subsequently, 200 µl chloroform was added and mixed. The mixture was cooled on ice for 5 min and was then centrifuged at 16,200 × g for 15 min at 4°C. The aqueous phase (500 µl) was transferred to a fresh tube and mixed with 500 µl isopropanol, which was cooled on ice for 5 min. The solution was then centrifuged at 16,200 × g for 15 min at 4°C. The RNA pellet was washed with 75% (v/v) ethanol and the supernatant was removed. The pellet was air-dried at room temperature, resuspended in 30–50 µl DEPC-treated water and stored at −80°C until use. Total RNA (5 µg) was used for reverse transcription to synthesize cDNA, which was performed using Superscript III reverse transcriptase (Thermo Fisher Scientific, Inc.) and oligo d(T)21 as a primer; the reaction conditions were performed as recommended by the manufacturer. The expression levels of cytochrome c, caspase-9, −3, −8, Bid and GAPDH were measured using 2X Rotor-Gene SYBR Green PCR Master Mix (cat. no. 204076; Qiagen GmbH). The primer sequences are listed in Table I. A 10-µl reaction mixture was prepared by combining cDNA aliquots, 3 µl each forward and reverse primers, and 5 µl 2X Rotor-Gene SYBR Green PCR Master Mix. The thermocycling conditions for quantitative PCR (qPCR) were as follows: 95°C for 2 min and 95°C for 10 min, followed by 40 cycles at 95°C for 15 sec and 60°C for 1 min, according to the manufacturer's protocol. Gene expression is presented as the fold change relative to the expression levels of GAPDH, and was calculated as 2−ΔΔCq. The results were obtained from three independent experiments (n=3). qPCR was performed using an Applied Biosystems 7300 Real-Time PCR System (Applied Biosystems; Thermo Fisher Scientific, Inc.).
The qPCR data were used to determine the expression levels of the caspase-3, −8, −9, cytochrome c and Bid genes using the 2−ΔΔCq method (36). The ΔCq value was determined by subtracting the Cq value obtained using the primers for a specific gene from the Cq value obtained using the primers for GAPDH. The ΔΔCq value was then calculated as the difference between the ΔCq value of each treatment and the ΔCq value of the control group. The 2−ΔΔCq method was used to calculate fold changes between the treated groups and the control group.
Statistical analysis
The statistical analysis was performed using SPSS software for Windows, version 13.0 (SPSS Inc.). Data are presented as the mean ± SD (n=3-5). A one-way analysis of variance and a Tukey's multiple-range test were used to evaluate the significance of differences between two mean values. P<0.05 was considered to indicate a statistically significant difference.
Results
A combination of α-PA and 5-FU decreases cell viability and triggers apoptosis in HT-29 cells
As shown in Fig. 1A, compared with that in the control group, cell viability was significantly decreased by ~41% in the 5-FU group and by 54–62% in the 5-FU combined with α-PA groups (P<0.05). In addition, cell viability was significantly lower in the 50, 100 and 250 µM α-PA combined with 5-FU groups (46.0±4.0, 43.7±1.3 and 38.4±4.4%, respectively) than in the 5-FU alone group (58.9±4.6%) (P<0.05). The morphological examination showed that, after treatment with 5-FU in combination with α-PA or alone, there were fewer HT-29 cells and morphological changes, such as shrinkage or contraction, were observed (Fig. 1B). As shown in Fig. 1A and B, the viability and morphology of cells in the 250 µM α-PA alone group were similar to those in the control group.
In the quantitative analysis of dose-effect relationships, the CI value was 0.869 for the interaction between 5-FU and α-PA. This means that the treatment combination of 5-FU and α-PA synergistically antitumor effects.
In the present study, early-stage (Annexin V, green staining) and late-stage (PI, red staining) apoptotic cells were analyzed by immunocytochemical staining. As shown in Fig. 1C, Annexin V and PI fluorescence were more intense in the 5-FU alone group and the groups treated with 50, 100 or 250 µM α-PA for 72 h than in the control and α-PA alone groups. The data showed that compared with in the control group, in the 5-FU alone group, both early-stage and late-stage apoptosis were significantly increased by 43 and 58%, respectively (P<0.05; Fig. 1D and E). As shown in Fig. 1D, early-stage apoptosis was significantly higher following treatment with 100 and 250 µM α-PA combined with 5-FU for 72 h (119±6.8 and 217±14.8%, respectively) than after treatment with 5-FU alone (100%) and the effect was dose-dependent (P<0.05). Late-stage apoptosis was also significantly higher after treatment with 50, 100 and 250 µM α-PA combined with 5-FU (162±3.1, 168±3.5 and 253±14.7%, respectively) than after treatment with 5-FU alone (100%), and the effect was dose-dependent (Fig. 1E).
These results indicated that 50–250 µM α-PA combined with 5-FU reduced cell viability more effectively than 5-FU alone. The reduction in HT-29 cell viability following treatment with α-PA combined with 5-FU may be caused by apoptosis.
α-PA combined with 5-FU regulates mitochondrial integrity in HT-29 cells
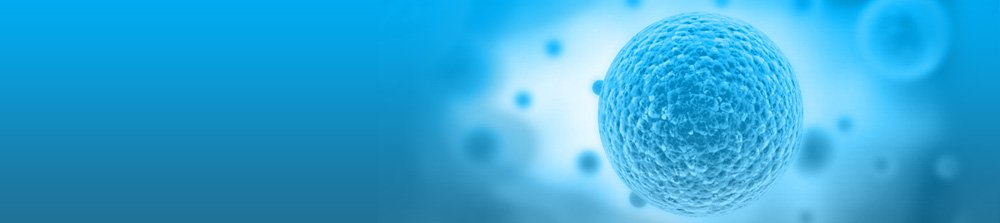
p53 protein expression levels were significantly higher in HT-29 cells after treatment with 5-FU alone (100±0%), or with 50, 100 or 250 µM α-PA combined with 5-FU for 72 h (104±4.7, 103±2.7 and 103±10.2%, respectively) compared with those in the control group (84.9±2.5%) or after treatment with 250 µM α-PA alone (80.3±4.5%) (P<0.05; Fig. 2A). There are two bands present in the western blotting results of Bax; therefore, the present study identified and semi-quantified both bands as whole Bax protein expression. The protein expression levels of Bax were significantly higher in HT-29 cells after treatment with 5-FU alone, or with 50, 100 or 250 µM α-PA combined with 5-FU treatment compared with those the control group or after treatment with 250 µM α-PA alone (P<0.05; Fig. 2B). Bax protein expression levels were also significantly higher in HT-29 cells following treatment with 50, 100 or 250 µM α-PA combined with 5-FU (109±4.9, 110±4.4 and 110±5.5%, respectively) compared with after treatment with 5-FU alone (100%) (P<0.05; Fig. 2B). As shown in Fig. 2C, Bcl-2 protein expression levels were significantly lower after treatment with 5-FU alone, or with 50, 100 or 250 µM α-PA combined with 5-FU compared with those in the control group (P<0.05). Compared with 5-FU alone, treatment with 50–250 µM α-PA combined with 5-FU significantly decreased Bcl-2 protein expression levels by 20–21% (P<0.05; Fig. 2C).
The fluorescence intensity of DiOC6 in HT-29 cells was lower following treatment with 5-FU alone or with 50–250 µM α-PA combined with 5-FU compared with that in the control group or following treatment with 250 µM α-PA alone (Fig. 2D and E). The quantitative results demonstrated that the MMP levels were significantly lower in the 5-FU alone group (100%) and in the 100 and 250 µM α-PA combined with 5-FU groups (85.0±3.2 and 76.8±5.8%) than those in the control group (113±2.9%) and 250 µM α-PA alone group (108±4.4%) (P<0.05; Fig. 2D).
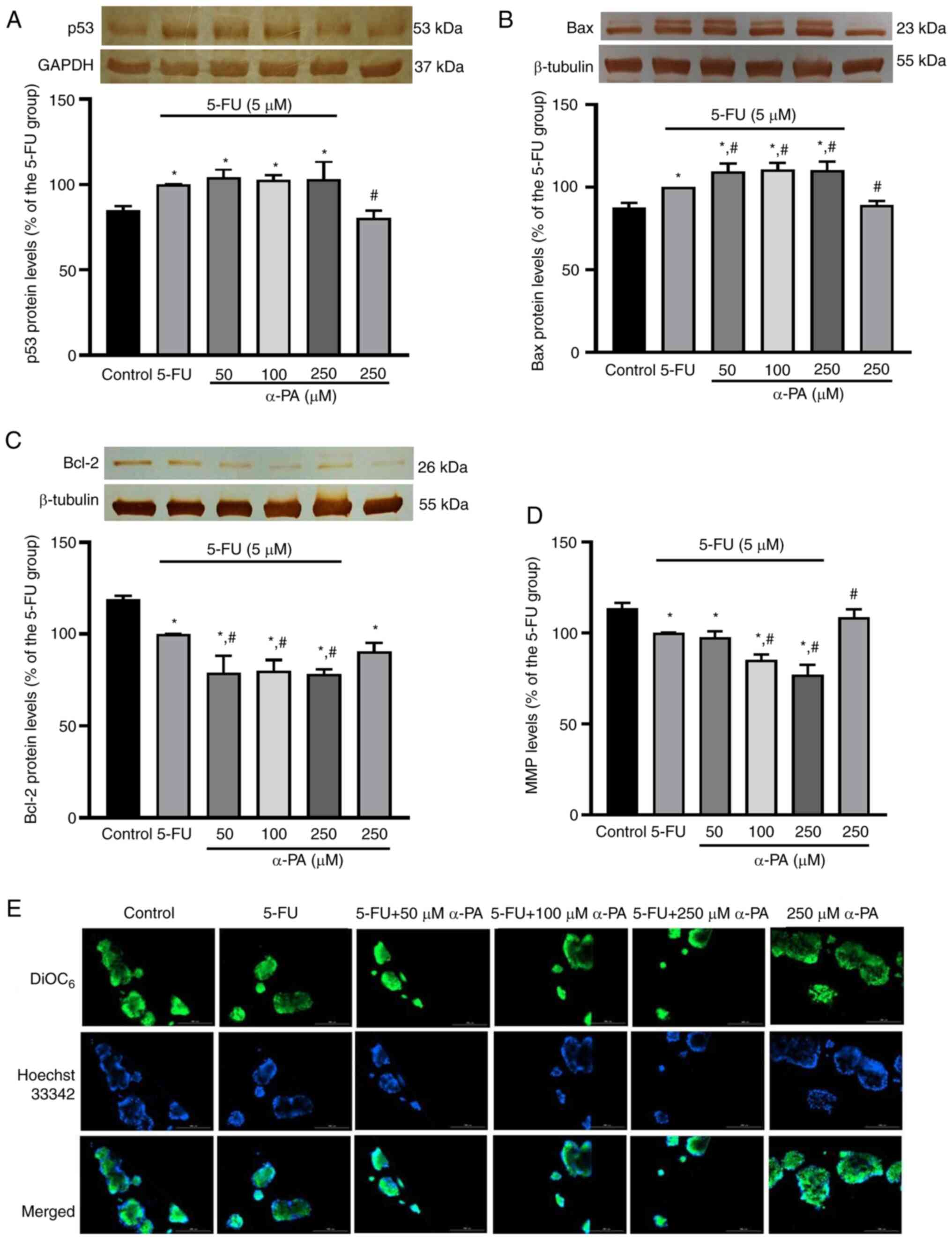
The fluorescence intensity of HK-2 (green staining) in HT-29 cells was lower after treatment with 5-FU alone or with α-PA combined with 5-FU than that in the control group or following treatment with 250 µM α-PA alone (Fig. 3A and B). However, the fluorescence intensity of VDAC-1 (red staining) did not seem to change in any treatment group (Fig. 3A). The quantitative results revealed that compared with 5-FU alone, 5-FU combined with 50, 100 or 250 µM α-PA significantly decreased the protein expression levels of HK-2 by 12–17% (P<0.05; Fig. 3B). The quantitative results of VDAC-1 staining showed that the protein expression levels of VDAC-1 were unaffected by treatment with the control, 5-FU alone, α-PA combined with 5-FU or α-PA alone.
These results indicated that 100 or 250 µM α-PA combined with 5-FU lowered the MMP levels more effectively than 5-FU alone. The reduction in MMP in HT-29 cells may be caused by a decrease in HK-2 levels, leading to a less stable VDAC-HK-2 complex.
α-PA combined with 5-FU enhances apoptosis-inducing molecule expression in HT-29 cells
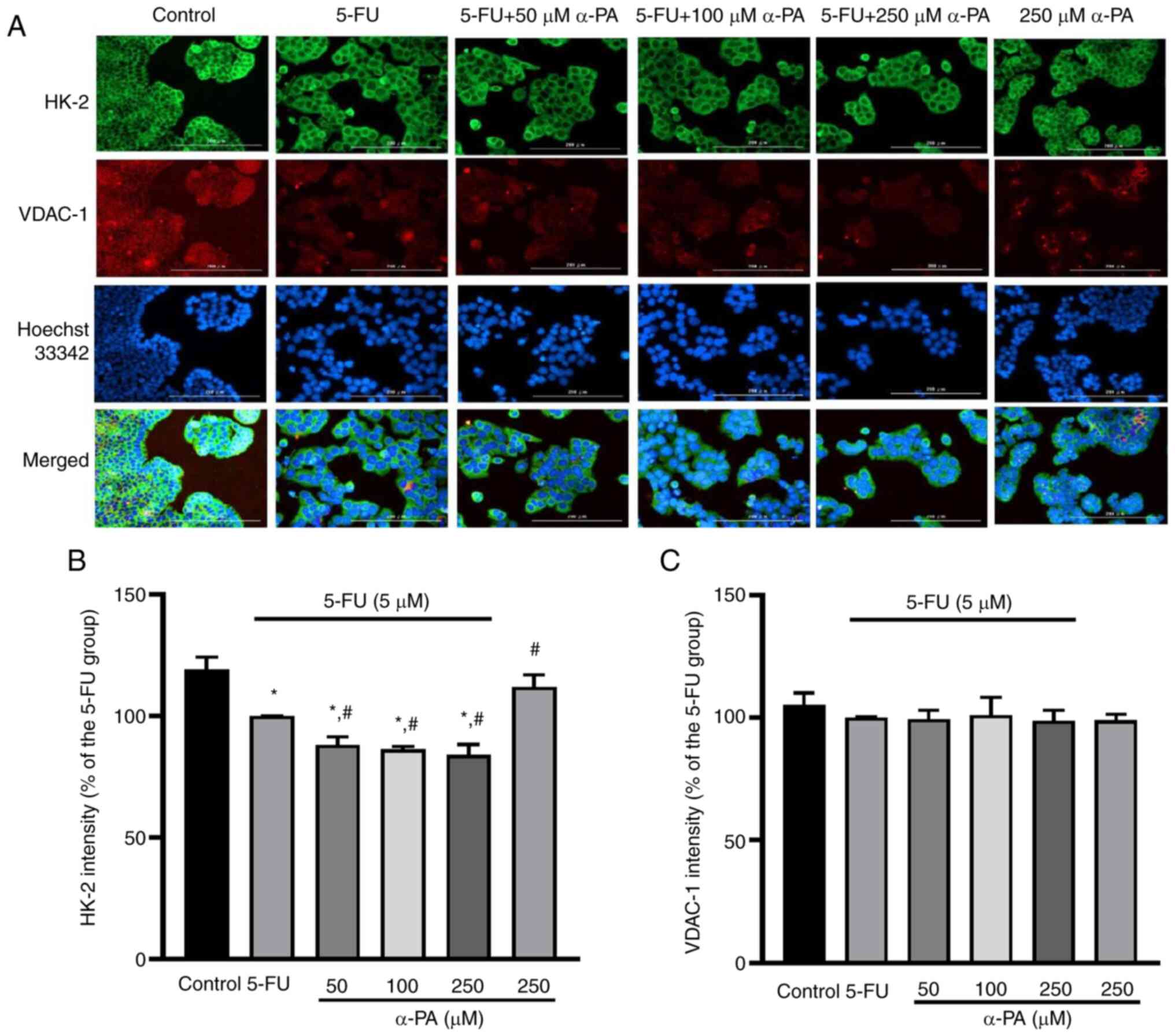
As shown in Fig. 4A, compared with those in the control group, after treatment with 5-FU alone, the mRNA expression levels of caspase-8 were significantly increased by ~81% (P<0.05). In addition, compared with those in the 5-FU alone group (100±0%), the caspase-8 mRNA expression levels were significantly higher in cells treated with 5-FU combined with 50, 100 or 250 µM α-PA (110±3.3, 125±5.0 and 131±3.7%, respectively) (P<0.05). In the Bid mRNA expression analysis, compared with those in the 5-FU alone group, 5-FU combined with 50, 100 or 250 µM α-PA significantly increased the mRNA expression levels of Bid by 14–21% (P<0.05; Fig. 4B). Furthermore, compared with those in the control group, 5-FU alone significantly increased the mRNA expression levels of cytochrome c, caspase-9 and caspase-3 in HT-29 cells by ~58, 54 and 44%, respectively (P<0.05; Fig. 4C-E). Moreover, compared with in cells treated with 5-FU alone (100%), 50, 100 or 250 µM α-PA combined with 5-FU significantly increased the mRNA expression levels of cytochrome c (122±4.1, 119±4.1 and 129±7.2%, respectively), caspase-9 (121±1.7, 133±4.7 and 125±4.1%, respectively), and caspase-3 (129±5.3, 114±6.1 and 124±3.5%, respectively) in HT-29 cells (P<0.05).
These results suggested that 50–250 µM α-PA combined with 5-FU increased apoptosis-inducing molecule expression in HT-29 cells.
Discussion
The findings of the present study indicated that the combination of α-PA and 5-FU may exhibit a synergistic effect in promoting apoptosis, thereby decreasing the viability of human colon cancer HT-29 cells. The underlying mechanism may involve triggering the mitochondria-dependent apoptotic pathway by regulating the expression of Bcl-2 family members, including Bid, Bax, Bcl-2 and Bid; reducing the levels of mitochondrial membrane regulatory factors, including HK-2 protein expression and MMP; and increasing the expression of caspase family members, including caspase-8, −9, and −3. These results showed that combining α-PA and 5-FU may reduce the required dosage of 5-FU for patients treated via chemotherapy by reducing cancer cell viability. This may reduce the toxicity and side effects of 5-FU in normal cells. The present findings are promising for patients undergoing cancer therapy, as it may minimize toxicity from 5-FU. However, further studies are needed to determine the optimum lower dosage of 5-FU under cotreatment with α-PA and its mechanism.
The present study investigated the potential of α-PA to reduce the toxicity and side effects of 5-FU treatment. The IC50 values for 5-FU alone and 5-FU combined with α-PA were 16.67 and 133 µM, respectively, after treating HT-29 cells for 72 h (data not shown). The lower IC50 value of 5-FU indicates that it has higher toxicity against HT-29 cells. On the other hand, the IC50 value of 5-FU combined with α-PA is higher, indicating that using a high dosage of α-PA along with a lower dosage of 5-FU may help reduce the toxicity of 5-FU and inhibit the proliferation of human HT-29 colon cancer cells. T-Johari et al (37) determined the degree of toxicity of 5-FU after 72 h treatment according to the respective concentration that reduced cell populations to 50% (CD50). 5-FU reduced the number of viable HT-29 cells at a CD50 of 15.5 µg/ml (118.5 µM), whereas the CD50 of 5-FU in normal colon CCD-18Co cells was 203.1 µg/ml (1.55 mM) (37). Yusefi et al (38) also demonstrated that, after 72 h treatment, the IC50 of 5-FU in the human colorectal cancer HCT116 cells is 0.87 µg/ml (6.74 µM), and in the normal human colon CCD112 cells it is 3.83 µg/ml (20.9 µM). These studies and the present results demonstrated that 5-FU has a higher toxicity in cancer cells than in normal colon cells. In addition, 5-FU combined with α-PA treatment can reduce the dosage of 5-FU treatment. However, to the best of our knowledge, there have been no human studies assessing α-PA plasma levels. In the future, the toxicity of 5-FU in different types of normal and cancerous colon cells needs to be investigated, and how the percentage of 5-FU dosage can be reduced when 5-FU is combined with α-PA treatment should be assessed. Furthermore, the pharmacokinetics of α-PA in humans requires further study.
In addition to NSAIDs, using dietary materials to reduce the side effects of chemotherapeutic medicines is essential for improving patient prognosis. Some phytochemicals from plants, such as curcumin (39), apple oligogalactan (40), rutin (41), gypenosides (42) and allicin (43), can enhance the anticancer effects of 5-FU by regulating the NF-κB signaling pathway, inducing anti-inflammatory effects and antioxidative stress, affecting the cell cycle and inducing caspase family activation. Among natural phytochemicals, α-PA is a monoterpenoid compound from fruits, vegetables and medicinal plants (3,44). Previous studies have shown that some terpenoids inhibit cell proliferation and tumor growth in various types of human cancer. Yang and Dou (45) and Pratheeshkumar and Kuttan (46) showed that terpenoids can trigger p53-induced, caspase-3-mediated proapoptotic signaling, and suppress Bcl-2-mediated apoptosis. Our previous study revealed that α-PA can increase phosphorylated (p)-p53, p-H2A.X, 14-3-3-σ and MDC1 protein expression, but can inhibit p53, MGMT, DNA-PK and BRCA-1 protein expression in human leukemia WEHI-3 cells (10). α-PA also induces apoptosis by increasing the intracellular p53, reactive oxygen species and Ca2+ levels, decreasing MMP (ΔΨm) levels, and promoting the release of cytochrome c, apoptosis-inducing factor (AIF) and Endo G from the mitochondria in WEHI-3 cells (11). In addition, our previous studies reported that in human liver cancer J5 cells, α-PA can induce necrosis and autophagy by reducing ATP production (9) and inducing the AKT/mTOR/LC3 pathway (8), respectively. In the present study, 250 µM α-PA induced early-stage apoptosis (stained with Annexin V) and late-stage apoptosis (stained with PI). Moreover, α-PA combined with 5-FU caused early- and late-stage apoptosis more effectively by regulating Bcl-2, caspase-family gene expression and mitochondria-related molecules.
The majority of mitochondria-dependent apoptotic pathways usually include one or more of the following pathways: Bcl-2 family regulation by p53 after DNA damage (47,48), mitochondrial membrane dysfunction (49), and mitochondria-mediated caspase family activation (50).
The p53 gene is a tumor suppressor gene, and its activity stops the formation of tumors. When DNA synthesis is disrupted or damaged by multiple stimuli, such as oxidative stress, radiation and metabolic disorders, p53 is essential in regulating Bcl-2, Bax and caspase 8 for cell cycle arrest for DNA repair or in inducing apoptosis and autophagy, leading to cell death (47,48). In cancer treatment, administering 5-FU can cause DNA synthesis disorders and damage, which activates the expression of p53 and p21 genes for DNA repair or apoptosis induction. It is one of the ways in which 5-FU is effective in cancer therapy (51). In addition, our previous studies have shown that α-PA can regulate p53 expression in various experimental models. It can inhibit p53 protein levels, but increase p-IκB protein levels, thus enhancing Bax levels and apoptosis in human leukemia WEHI3 cells (10). However, in human liver J5 cells, α-PA can increase nuclear p53 protein levels and decrease cytoplasmic p53 expression to induce autophagy (8). In the present study, α-PA alone did not affect p53 levels. However, α-PA combined with 5-FU could increase p53 and Bax expression levels, enhancing apoptosis in HT-29 cells. Furthermore, orientin, a water-soluble glycosyl flavonoid, has been shown to activate p53 and induce Bax protein expression, reducing Bcl-2 protein expression and inducing apoptosis in HT-29 cells (52). Sionov and Haupt (53) reported on various factors that trigger the cellular response to regulated p53. These include the cell type, the extracellular stimuli, the intensity of the stress signals, the post-transcription/translation modification of p53 and the interaction of p53 with specific proteins (53). In the present study, the α-PA alone group at 250 µM did not exhibit an increase in p53 levels, and when α-PA and 5-FU were combined, the expression levels of p53 were not significantly higher than in the cells treated with 5-FU alone. Therefore, the increased levels of Bax, decreased levels of Bcl-2 and reduced mitochondrial function, does not seem to be due to p53 activation but may result from other factors, such as cell type, extracellular stimuli or the intensity of stress signals.
When focusing on apoptosis induced by mitochondrial membrane dysfunction, caspase-8-regulated Bid and Bcl-2 are key pathways. The extrinsic apoptotic pathway is activated by p53 or activated by death receptors to regulate apoptotic proteins, such as caspase-8, which then stimulate apoptosis by directly cleaving and activating caspase-3 or cleaving Bid, a proapoptotic Bcl-2 family protein (47,48). Among these target genes, the Bcl-2 family serves an important role in inducing apoptosis by regulating mitochondrial function. Specifically, Bax and Bak undergo oligomerization at the mitochondrial outer membrane of the mitochondria, forming pores that cause mitochondrial outer membrane permeabilization (MOMP) and the subsequent release of cytochrome c. These events trigger the caspase family activation, ultimately leading to apoptosis (54). Paeoniflorin-6′-O-benzene sulfonate, a potential therapeutic medicine for hepatocellular carcinoma, combined with 5-FU can activate the intrinsic mitochondrial apoptotic pathway induced by p53, inhibit anti-apoptotic Bcl-2 expression, and induce proapoptotic Bax, cytochrome c and caspase family expression (55).
When 5-FU is administered, caspase-8 is activated by p53, Bid is activated and MOMP is regulated (56,57). A reduction in MOMP is one of the leading causes of cytochrome c release from the mitochondria and triggers caspase family expression (57). Allavena et al (58) showed that malnutrition and limited nutrients cause caspase-8 activation and reduce MOMP levels, leading to apoptosis but not autophagy. After caspase-8 and Bid activation leads to Bax and Bak activation, the modification of fatty acids in the mitochondrial membrane reduces MOMP; therefore, under limited nutrients or stress, it is worth assessing which mechanism regulates cellular apoptosis or autophagy (59). In the present study, α-PA combined with 5-FU treatment significantly increased caspase-8 and Bid expression, and reduced MMP. A novel finding of the present study is that phytochemicals and 5-FU may regulate caspase-8 signaling to induce apoptosis in human colon cancer HT29 cells.
In the present study, α-PA combined with 5-FU treatment not only enhanced caspase-8 to initiate the extrinsic pathway of apoptosis, but also regulated VDAC-HK-2 complex expression on the mitochondria surface to regulate mitochondrial function. Positioned at the outer membrane of mitochondria, VDAC plays an important role in regulating the permeability of ATP/ADP, regulation of calcium homeostasis, and calcium flux within ER-mitochondria contact sites. VDAC has also been recognized to be involved in the release of the Bcl2-family and HK. VDAC binding cytosolic HK-2 forms a VDAC-HK complex, which is essential for maintaining mitochondria integrity and normal function (60). Klepinin et al (61) also showed that the VDAC binding sites for tubulin and HK within the membrane protein super complex modify mitochondrial membrane integrity and increase permeability. On the mitochondrial membrane, VDAC-1-bound HK facilitates and promotes apoptosis capacity. Tewari et al (62) showed that silencing VDAC-1 protects HeLa cells from aspirin-induced cell death; upon aspirin treatment, VDAC-1 knockdown cells exhibited a smaller mitochondrial membrane potential change than wild-type cells. Aspirin has the capability to alter VDAC-1, and the interaction between VDAC and HK may be linked to cellular apoptosis and potential anticancer properties. Furthermore, VDAC has been suggested to be a target for the pro- and anti-apoptotic Bcl-2 family of proteins, and its significance lies in its role in the discharge of apoptotic proteins situated in the intermembrane space (60). Regulation of the expression and functions of VDAC and HK may be a potential target for chemoprevention and for developing cancer therapies. In the present study, compared with 5-FU alone, α-PA combined with 5-FU significantly increased caspase-8 and Bid mRNA expression levels, and significantly decreased MMP and HK-2 expression levels in HT-29 cells. These results indicated that α-PA combined with 5-FU may lead to a change in the mitochondrial outer membrane permeability and Bcl-2 family release.
Mitochondria-mediated caspase family activation is another important component in the mitochondria-dependent apoptotic pathway. Once mitochondrial membrane dysfunction occurs, cytochrome c is released from mitochondria, and caspase-9 and caspase-3 are activated, leading to apoptosis (63). A previous study showed that gambogenic acid combined with 5-FU can upregulate caspase-3, caspase-9, Bax, RIP1, AIF, VDAC, cytochrome c and cyclophilin D, and downregulate Bcl-2 (64). Cheng et al (65) also showed that casticin, a natural polymethoxyflavone from Vitex rotundifolia, combined with 5-FU reduces Bcl-2, but increases caspase-8 and caspase-3 in mouse leukemia WEHI-3 cells. Compared with 5-FU, α-PA combined with 5-FU significantly increased cytochrome c, caspase-9 and caspase-3 expression levels in the present study, showing that α-PA combined with 5-FU can induce apoptosis by mitochondria-mediated caspase family activation.
The findings of the present study have important implications for patients undergoing cancer therapy who are seeking to minimize the toxicity of the chemotherapeutic drug 5-FU. 5-FU not only induces apoptosis but also regulates cancer cell DNA synthesis (14). In the future, to determine the optimum lower dosage of 5-FU combined with α-PA, we aim to assess cell viability and DNA synthesis efficiency rate in cell models; tumor incidence rate and tumor size in animal models; and to potentially perform clinical trials to study its mechanism. Additionally, we need to assess α-PA and/or 5-FU plasma levels and pharmacokinetics in animal models or clinical trials. As well as the study of the synergistic effect of α-PA combined with 5-FU treatment on apoptosis, future studies may assess whether α-PA reduces the side effects of 5-FU, such as mucositis.
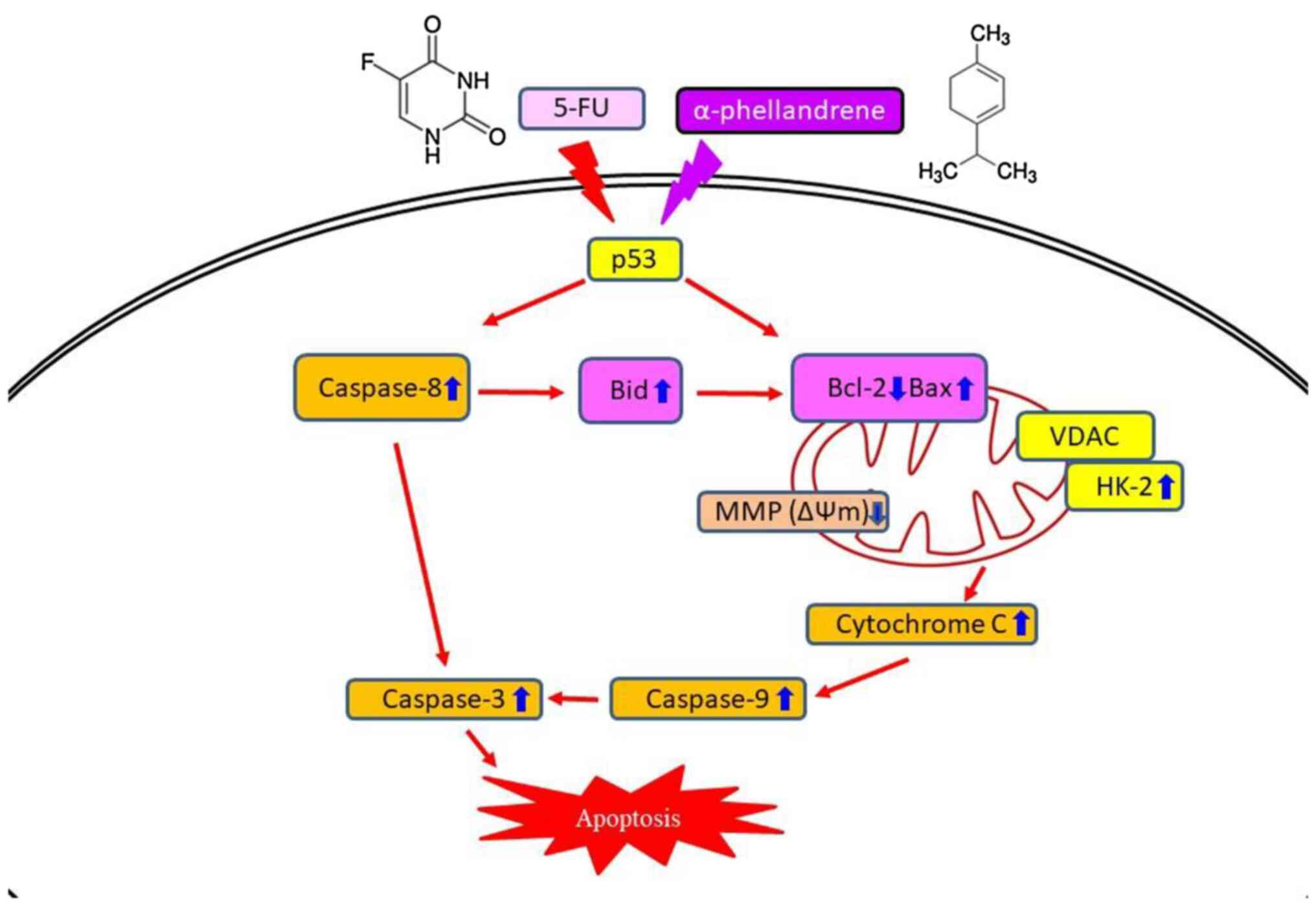
In conclusion, the findings of the present study indicated that the combination of α-PA and 5-FU exhibited a synergistic impact on reducing the viability of human colon cancer HT-29 cells via the induction of apoptosis. As shown in Fig. 5, the mechanism involves the mitochondria-dependent apoptotic pathway, including regulating the expression of members of the Bcl-2 family, such as Bax, Bcl-2 and Bid, regulating MMP and HK-2 expression levels, and increasing the expression levels of caspase cascade proteins, including caspase-9 and caspase-3.
Acknowledgements
Not applicable.
Funding
This study was financially supported by the Ministry of Science and Technology of Taiwan (grant no. NSTC 112-2320-B-126-001-).
Availability of data and materials
The data generated in the present study may be requested from the corresponding author.
Authors' contributions
CCW was responsible for ensuring the integrity of the entire study, and contributed to the editing and reviewing of the manuscript. LH contributed to the conception and design of the study concepts, as well as to the development of intellectual content and literature research. ACS and CCW contributed to the experiments. ACS and CCW contributed to data acquisition and data analysis. ACS and CCW confirm the authenticity of all the raw data. All authors have read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
|
Asaro C, Sullivan BT, Dalusky MJ and Berisford CW: Volatiles associated with preferred and nonpreferred hosts of the Nantucket pine tip moth, Rhyacionia frustrana. J Chem Ecol. 30:977–990. 2004. View Article : Google Scholar : PubMed/NCBI | |
|
Riyazi A, Hensel A, Bauer K, Geissler N, Schaaf S and Verspohl EJ: The effect of the volatile oil from ginger rhizomes (Zingiber officinale), its fractions and isolated compounds on the 5-HT3 receptor complex and the serotoninergic system of the rat ileum. Planta Med. 73:355–362. 2007. View Article : Google Scholar : PubMed/NCBI | |
|
Dimov M, Petkova Z, Stoyanova M, Dobreva K and Stoyanova A: Comparative chemical composition of dill fruits of different origins. Bulg J Agric Sci. 26:696–700. 2020. | |
|
Prasannakumar NR, Jyothi N, Saroja S and Lokesha AN: Insecticidal properties of Ocimum basilicum and Mentha piperita essential oils against South American Tomato moth, Phthorimaea absoluta (Meyrick) (Lepidoptera: Gelichiidae). Pestic Biochem Physiol. 190:1053292023. View Article : Google Scholar : PubMed/NCBI | |
|
Zhang Y, Long Y, Yu S, Li D, Yang M, Guan Y, Zhang D, Wan J, Liu S, Shi A, et al: Natural volatile oils derived from herbal medicines: A promising therapy way for treating depressive disorder. Pharmacol Res. 164:1053762021. View Article : Google Scholar : PubMed/NCBI | |
|
Siqueira HDS, Neto BS, Sousa DP, Gomes BS, da Silva FV, Cunha FVM, Wanderley CWS, Pinheiro G, Cândido AGF, Wong DVT, et al: α-Phellandrene, a cyclic monoterpene, attenuates inflammatory response through neutrophil migration inhibition and mast cell degranulation. Life Sci. 160:27–33. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Lin JJ, Lin JH, Hsu SC, Weng SW, Huang YP, Tang NY, Lin JG and Chung JG: Alpha-phellandrene promotes immune responses in normal mice through enhancing macrophage phagocytosis and natural killer cell activities. In Vivo. 27:809–814. 2013.PubMed/NCBI | |
|
Hsieh LC, Hsieh SL, Chen CT, Chung JG, Wang JJ and Wu CC: Induction of α-phellandrene on autophagy in human liver tumor cells. Am J Chin Med. 43:121–136. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Hsieh SL, Li YC, Chang WC, Chung JG, Hsieh LC and Wu CC: Induction of necrosis in human liver tumor cells by α-phellandrene. Nutr Cancer. 66:970–979. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
Lin JJ, Wu CC, Hsu SC, Weng SW, Ma YS, Huang YP, Lin JG and Chung JG: Alpha-phellandrene-induced DNA damage and affect DNA repair protein expression in WEHI-3 murine leukemia cells in vitro. Environ Toxicol. 30:1322–1330. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Lin JJ, Hsu SC, Lu KW, Ma YS, Wu CC, Lu HF, Chen JC, Lin JG, Wu PP and Chung JG: Alpha-phellandrene-induced apoptosis in mice leukemia WEHI-3 cells in vitro. Environ Toxicol. 31:1640–1651. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Xi Y and Xu P: Global colorectal cancer burden in 2020 and projections to 2040. Transl Oncol. 14:1011742021. View Article : Google Scholar : PubMed/NCBI | |
|
Florescu-Ţenea RM, Kamal AM, Mitruţ P, Mitruţ R, Ilie DS, Nicolaescu AC and Mogoantă L: Colorectal cancer: An update on treatment options and future perspectives. Curr Health Sci J. 45:134–141. 2019.PubMed/NCBI | |
|
Longley DB, Harkin DP and Johnston PG: 5-Fluorouracil: Mechanisms of action and clinical strategies. Nat Rev Cance. 3:330–338. 2003. View Article : Google Scholar | |
|
Alzahrani SM, Al Doghaither HA, Al-Ghafari AB and Pushparaj PN: 5-Fluorouracil and capecitabine therapies for the treatment of colorectal cancer (review). Oncol Rep. 50:1752023. View Article : Google Scholar : PubMed/NCBI | |
|
Debatin KM: Apoptosis pathways in cancer and cancer therapy. Cancer Immunol Immunother. 53:153–159. 2004. View Article : Google Scholar : PubMed/NCBI | |
|
Tsujimoto Y: Role of Bcl-2 family proteins in apoptosis: Apoptosomes or mitochondria? Genes Cells. 3:697–707. 1998. View Article : Google Scholar : PubMed/NCBI | |
|
Shoshan-Barmatz V, De S and Meir A: The mitochondrial voltage-dependent anion channel 1, Ca2+ transport, apoptosis, and their regulation. Front Oncol. 7:602017. View Article : Google Scholar : PubMed/NCBI | |
|
Li J and Yuan J: Caspases in apoptosis and beyond. Oncogene. 20:6194–6206. 2008. View Article : Google Scholar : PubMed/NCBI | |
|
Blondy S, David V, Verdier M, Mathonnet M, Perraud A and Christou N: 5-Fluorouracil resistance mechanisms in colorectal cancer: From classical pathways to promising processes. Cancer Sci. 111:3142–3154. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Mhaidat NM, Bouklihacene M and Thorne RF: 5-Fluorouracil-induced apoptosis in colorectal cancer cells is caspase-9-dependent and mediated by activation of protein kinase C-δ. Oncol Lett. 8:699–704. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
Cordier PY, Nau A, Ciccolini J, Oliver M, Mercier C, Lacarelle B and Peytel E: 5-FU-induced neurotoxicity in cancer patients with profound DPD deficiency syndrome: A report of two cases. Cancer Chemother Pharmacol. 68:823–826. 2011. View Article : Google Scholar : PubMed/NCBI | |
|
Baydar M, Dikilitas M, Sevinc A and Aydogdu I: Prevention of oral mucositis due to 5-fluorouracil treatment with oral cryotherapy. J Natl Med Assoc. 97:1161–1164. 2005.PubMed/NCBI | |
|
VanderVeen BN, Sougiannis AT, Velazquez KT, Carson JA, Fan D and Murphy EA: The acute effects of 5 fluorouracil on skeletal muscle resident and infiltrating immune cells in mice. Front Physiol. 11:5934682020. View Article : Google Scholar : PubMed/NCBI | |
|
Nicolatou-Galitis O, Sarri T, Bowen J, Di Palma M, Kouloulias VE, Niscola P, Riesenbeck D, Stokman M, Tissing W, Yeoh E, et al: Systematic review of anti-inflammatory agents for the management of oral mucositis in cancer patients. Support Care Cancer. 21:3179–3189. 2013. View Article : Google Scholar : PubMed/NCBI | |
|
Wong RSY: Role of nonsteroidal anti-inflammatory drugs (NSAIDs) in cancer prevention and cancer promotion. Adv Pharmacol Sci. 2019:34189752019.PubMed/NCBI | |
|
Nautiyal J, Kanwar SS, Yu Y and Majumdar AP: Combination of dasatinib and curcumin eliminates chemo-resistant colon cancer cells. J Mol Signal. 6:72011. View Article : Google Scholar : PubMed/NCBI | |
|
Denizot F and Lang R: Rapid colorimetric assay for cell growth and survival. Modifications to the tetrazolium dye procedure giving improved sensitivity and reliability. J Immunol Methods. 89:271–277. 1986. View Article : Google Scholar : PubMed/NCBI | |
|
Chou TC and Talalay P: Quantitative analysis of dose-effect relationships: The combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 22:27–55. 1984. View Article : Google Scholar : PubMed/NCBI | |
|
Rieger AM, Nelson KL, Konowalchuk JD and Barreda DR: Modified annexin V/propidium iodide apoptosis assay for accurate assessment of cell death. J Vis Exp. 50:e25972011. | |
|
Lowry OH, Rosebrough NJ, Farr AL and Randall RJ: Protein measurement with Folin phenol reagent. J Biol Chem. 193:265–275. 1951. View Article : Google Scholar : PubMed/NCBI | |
|
Laemmli UK: Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 227:680–685. 1970. View Article : Google Scholar : PubMed/NCBI | |
|
Towbin H, Staehelin T and Gordon J: Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: Procedure and some applications. Proc Natl Acad Sci USA. 76:4350–4354. 1979. View Article : Google Scholar : PubMed/NCBI | |
|
Sakamuru S, Attene-Ramos MS and Xia M: Mitochondrial membrane potential assay. Methods Mol Biol. 1473:17–22. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Chomczynski P and Sacchi N: Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 162:156–159. 1987. View Article : Google Scholar : PubMed/NCBI | |
|
Livak KJ and Schmittgen TD: Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001. View Article : Google Scholar : PubMed/NCBI | |
|
T-Johari SAT, Hashim F, Ismail WI and Ali AM: Combinatorial cytotoxic effects of gelam honey and 5-fluorouracil against human adenocarcinoma colon cancer HT-29 cells in vitro. Int J Cell Biol. 2019:30596872019. View Article : Google Scholar : PubMed/NCBI | |
|
Yusefi M, Shameli K, Jahangirian H, Teow SY, Umakoshi H, Saleh B, Rafiee-Moghaddam R and Webster TJ: The potential anticancer activity of 5-fluorouracil loaded in cellulose fibers isolated from rice straw. Int J Nanomedicine. 15:5417–5432. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Yang H, Huang S, Wei Y, Cao S, Pi C, Feng T, Liang J, Zhao L and Ren G: Curcumin enhances the anticancer effect of 5-fluorouracil against gastric cancer through down-regulation of COX-2 and NF-κB signaling pathways. J Cancer. 8:3697–3706. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Li Y, Fan L, Niu Y, Mian W, Zhang F, Xie M, Sun Y and Mei Q: An apple oligogalactan enhances the growth inhibitory effect of 5-fluorouracil on colorectal cancer. Eur J Pharmacol. 804:13–20. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
de Sousa Fideles L, de Miranda JAL, Martins CDS, Barbosa MLL, Pimenta HB, Pimentel PVS, Teixeira CS, Scafuri MAS, Façanha SO, Barreto JEF, et al: Role of rutin in 5-fluorouracil-induced intestinal mucositis: Prevention of histological damage and reduction of inflammation and oxidative stress. Molecules. 25:27862020. View Article : Google Scholar | |
|
Kong L, Wang X, Zhang K, Yuan W, Yang Q, Fan J, Wang P and Liu Q: Gypenosides synergistically enhances the anti-tumor effect of 5-fluorouracil on colorectal cancer in vitro and in vivo: A role for oxidative stress-mediated DNA damage and p53 activation. PLoS One. 10:e01378882015. View Article : Google Scholar : PubMed/NCBI | |
|
Țigu AB, Toma VA, Moț AC, Jurj A, Moldovan CS, Fischer-Fodor E, Berindan-Neagoe I and Pârvu M: The synergistic antitumor effect of 5-fluorouracil combined with allicin against lung and colorectal carcinoma cells. Molecules. 25:19472020. View Article : Google Scholar : PubMed/NCBI | |
|
Jirovetz L, Buchbauer G, Stoyanova AS, Georgiev EV and Damianova ST: Composition, quality control, and antimicrobial activity of the essential oil of long-time stored dill (Anethum graveolens L.) seeds from Bulgaria. J Agric Food Chem. 51:3854–3857. 2003. View Article : Google Scholar : PubMed/NCBI | |
|
Yang H and Dou QP: Targeting apoptosis pathway with natural terpenoids: Implications for treatment of breast and prostate cancer. Curr Drug Targets. 11:733–744. 2010. View Article : Google Scholar : PubMed/NCBI | |
|
Pratheeshkumar P and Kuttan G: Vernolide-A, a sesquiterpene lactone from Vernonia cinerea, induces apoptosis in B16F-10 melanoma cells by modulating p53 and caspase-3 gene expressions and regulating NF-κB-mediated bcl-2 activation. Drug Chem Toxicol. 34:261–270. 2011. View Article : Google Scholar : PubMed/NCBI | |
|
Matt S and Hofmann TG: The DNA damage-induced cell death response: A roadmap to kill cancer cells. Cell Mol Life Sci. 73:2829–2850. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Czabotar PE and Garcia-Saez AJ: Mechanisms of BCL-2 family proteins in mitochondrial apoptosis. Nat Rev Mol Cell Biol. 24:732–748. 2023. View Article : Google Scholar : PubMed/NCBI | |
|
Bonora M, Patergnani S, Ramaccini D, Morciano G, Pedriali G, Kahsay AE, Bouhamida E, Giorgi C, Wieckowski MR and Pinton P: Physiopathology of the permeability transition pore: Molecular mechanisms in human pathology. Biomolecules. 10:9982020. View Article : Google Scholar : PubMed/NCBI | |
|
Boice A and Bouchier-Hayes L: Targeting apoptotic caspases in cancer. Biochim Biophys Acta Mol Cell Res. 1867:1186882020. View Article : Google Scholar : PubMed/NCBI | |
|
Osaki M, Tatebe S, Goto A, Hayashi H, Oshimura M and Ito H: 5-Fluorouracil (5-FU) induced apoptosis in gastric cancer cell lines: Role of the p53 gene. Apoptosis. 2:221–226. 1997. View Article : Google Scholar : PubMed/NCBI | |
|
Thangaraj K, Balasubramanian B, Park S, Natesan K, Liu W and Manju V: Orientin induces G0/G1 cell cycle arrest and mitochondria mediated intrinsic apoptosis in human colorectal carcinoma HT29 cells. Biomolecules. 9:4182019. View Article : Google Scholar : PubMed/NCBI | |
|
Sionov RV and Haupt Y: The cellular response to p53: The decision between life and death. Oncogene. 18:6145–6157. 1999. View Article : Google Scholar : PubMed/NCBI | |
|
Renault TT, Teijido O, Antonsson B, Dejean LM and Manon S: Regulation of Bax mitochondrial localization by Bcl-2 and Bcl-x(L): Keep your friends close but your enemies closer. Int J Biochem Cell Biol. 45:64–67. 2013. View Article : Google Scholar : PubMed/NCBI | |
|
Chang W, Jiang XP, Jin S, Li PP, Song SS, Yuan PF, Wei W and Lu JT: Synergistic effects of CP-25 and 5-fluorouracil on the hepatocellular carcinoma in vivo and in vitro through regulating activating mitochondrial pathway. J Cancer. 13:1005–1018. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Schug ZT, Gonzalvez F, Houtkooper RH, Vaz FM and Gottlieb E: BID is cleaved by caspase-8 within a native complex on the mitochondrial membrane. Cell Death Differ. 18:538–548. 2011. View Article : Google Scholar : PubMed/NCBI | |
|
Vaseva AV and Moll UM: The mitochondrial p53 pathway. Biochim Biophys Acta. 1787:414–420. 2009. View Article : Google Scholar : PubMed/NCBI | |
|
Allavena G, Cuomo F, Baumgartner G, Bele T, Sellgren AY, Oo KS, Johnson K, Gogvadze V, Zhivotovsky B and Kaminskyy VO: Suppressed translation as a mechanism of initiation of CASP8 (caspase 8)-dependent apoptosis in autophagy-deficient NSCLC cells under nutrient limitation. Autophagy. 14:252–268. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Nikoletopoulou V, Markaki M, Palikaras K and Tavernarakis N: Crosstalk between apoptosis, necrosis and autophagy. Biochim Biophys Acta. 1833:3448–3459. 2013. View Article : Google Scholar : PubMed/NCBI | |
|
Shoshan-Barmatz V, Keinan N and Zaid H: Uncovering the role of VDAC in the regulation of cell life and death. J Bioenerg Biomembr. 40:183–191. 2008. View Article : Google Scholar : PubMed/NCBI | |
|
Klepinin A, Ounpuu L, Mado K, Truu L, Chekulayev V, Puurand M, Shevchuk I, Tepp K, Planken A and Kaambre T: The complexity of mitochondrial outer membrane permeability and VDAC regulation by associated proteins. J Bioenerg Biomembr. 50:339–354. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Tewari D, Majumdar D, Vallabhaneni S and Bera AK: Aspirin induces cell death by directly modulating mitochondrial voltage-dependent anion channel (VDAC). Sci Rep. 7:451842017. View Article : Google Scholar : PubMed/NCBI | |
|
Sun G: Death and survival from executioner caspase activation. Semin Cell Dev Biol. 156:66–73. 2024. View Article : Google Scholar : PubMed/NCBI | |
|
Su J, Cheng H, Zhang D, Wang M, Xie C, Hu Y, Chang HCW and Li Q: Synergistic effects of 5-fluorouracil and gambogenic acid on A549 cells: Activation of cell death caused by apoptotic and necroptotic mechanisms via the ROS-mitochondria pathway. Biol Pharm Bull. 37:1259–1268. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
Cheng ZY, Chueh FS, Peng SF, Lin CH, Kuo CL, Huang WW, Chen PY, Way TD and Chung JG: Combinational treatment of 5-fluorouracil and casticin induces apoptosis in mouse leukemia WEHI-3 cells in vitro. Environ Toxicol. 35:911–921. 2020. View Article : Google Scholar : PubMed/NCBI |