The dynamic duo: A narrative review on the synergy between stereotactic body radiotherapy and immunotherapy in lung cancer treatment (Review)
- Authors:
- Published online on: June 11, 2024 https://doi.org/10.3892/or.2024.8755
- Article Number: 96
-
Copyright: © Cheng et al. This is an open access article distributed under the terms of Creative Commons Attribution License.
Abstract
Introduction
Despite great strides in cancer treatment over the past decade, lung cancer remains the leading cause of cancer-associated death worldwide (1). There are two main types of lung cancer: Non-small-cell lung cancer (NSCLC) and SCLC, each with a distinct prognosis. If discovered early, both lung cancer types can be treated with aggressive local therapies; however, once progressed to advanced stages, the prognoses are usually poor. Over the past decade, advances in targeted therapies, immunotherapies and radiotherapy (RT) have improved patient survival, bringing hope to those with advanced lung cancer. Among these novel therapeutic strategies is the multimodality treatment of stereotactic body RT (SBRT) plus immunotherapy.
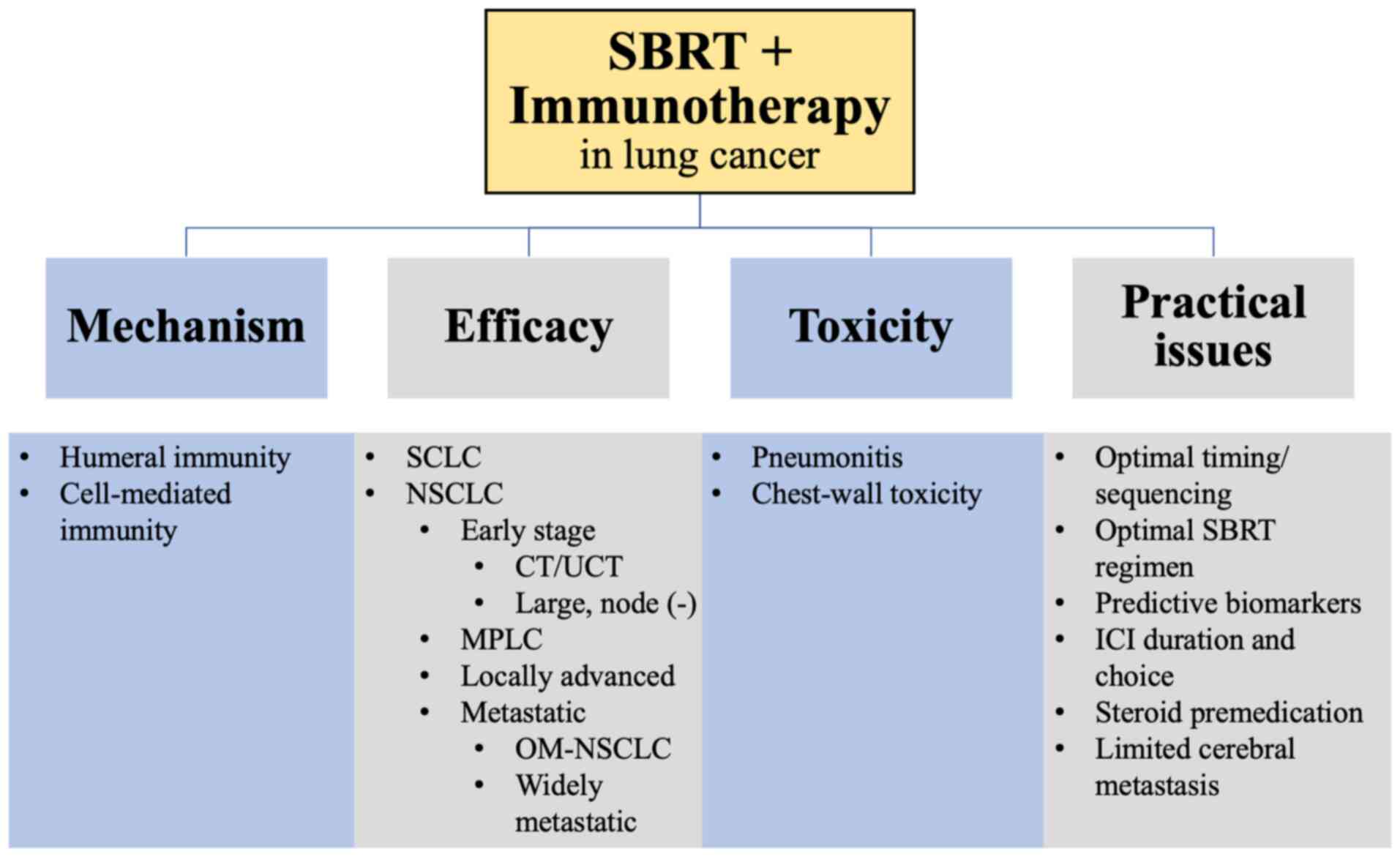
RT has long been a traditional means of treatment for cancer, depositing intense physical energy of ionizing radiation to rapidly growing cancer cells, thereby creating free radicals that induce DNA damage and subsequent cancer cell death (2). SBRT, also known as stereotactic ablative RT (SABR), is an extension of stereotactic radiosurgery (SRS) and stereotactic RT. The treatment is based on the use of an external 3-dimensional reference system (stereotactic system) for accurate localization of the target, as well as for directing the RT. It has been shown that in addition to DNA damage, SBRT is also able to prime the immune system for antitumor activity, which augments patient response to subsequently administered immunotherapy (3). This discovery has important implications, as numerous patients with lung cancer do not respond to immunotherapy alone. As one of the hottest areas in lung cancer research, a tremendous amount of preclinical and clinical studies has been published (4–14), although much more remains to be clarified and explored. The current study provided a review of the literature and summarized recent advances in SBRT + immunotherapy synergy in lung cancer treatment (Fig. 1).
The current review provides the following advance in knowledge. At the time of the writing of this manuscript, most clinical research on SBRT and immunotherapy synergy focused on immune checkpoint inhibitor (ICI) immunotherapy. As such, there have been numerous excellent reviews on SBRT with ICI immunotherapy (15–22). However, discussions around SBRT and non-ICI immunotherapy synergy have been lacking. Since entering the era of precision medicine, numerous excellent works in the preclinical phase have been published, which revealed various promising new molecular targets for future immunotherapies (23–26). Soon, some of these research efforts will start to enter clinical studies. Non-ICI immunotherapy may one day become just as important as ICI immunotherapy. As such, an understanding of their current roles and development in the grand scheme of lung cancer immuno-RT research is vital for understanding future research. In this light, the present review fills the gap in knowledge regarding the status of SBRT and non-ICI immunotherapy clinical research in lung cancer management. By encompassing both ICI and non-ICI immunotherapies, the present review provides a broader and more comprehensive discussion on the latest development in lung cancer immuno-RT with the aim of providing a further understanding of this latest treatment strategy and inspiring more preclinical and translational research in the area.
Mechanism
The tumor microenvironment (TME)
Simply put, the TME is the immediate environment surrounding a tumor, which includes the blood vessels, signalling molecules and extracellular matrix. The TME is a dynamic environment, such that as the tumor grows and metastasizes, it too evolves to enable tumor progression (2). The immune cells within the TME make up the tumor immune microenvironment (TIME), which is further divided into immunoreactive and immunosuppressive components. The former is made up of effector cells of the innate and adaptive responses, while the latter is comprised of adaptive immune cells such as regulatory T cells (Tregs), myeloid-derived suppressor cells and anti-inflammatory macrophages (2). Throughout the course of RT, the composition of the TIME changes as the more radioresistant immune cells survive, while the more radiosensitive cells perish (2); however, the effect of radiation on the TIME goes beyond just changing its composition. Evidence has shown that an additional immune effect is also taking place: The ‘abscopal effect’, which refers to a phenomenon whereby irradiation of a localized tumor results in the regression of distant tumors, even though the latter were not irradiated in the first place (16), and, although rare, this phenomenon is especially associated with SBRT compared to conventionally fractionated RT.
The immune effect of RT
Typically, tumor cells keep their TME immunologically ‘cold’ by downregulating their immunogenic surface markers such as major histocompatibility complex-1 and Fas, which allows tumor cells to escape immune surveillance (15). However, RT is able to reverse this immunosuppressive effect by acting as an in-situ vaccine, as, when it induces irreversible DNA damage and tumor cell death, it also causes the release of tumor antigens into the TME that are then engulfed by antigen-presenting cells, which upregulate the immunogenic surface markers and cross-present the tumor antigen to T cells. The process activates cell-mediated immunity and recruits even more lymphocytes from the bloodstream (2,15,16). These activated lymphocytes then display a homing tendency by roaming through the body looking for distant tumors with the same tumor antigens for elimination, and with distant tumors shrinking, the elusive abscopal effect is achieved (16). Several published preclinical and clinical studies have supported the immunogenic effect of SBRT and are summarized in Table I (4–14).
In addition to cell-mediated immunity, RT was also found to promote humoral immunity (12–14), which, although having traditionally been thought of as only playing a synergistic role in antitumor immunity, Xiao and Zhuang (2) pointed out that in weakly immunogenic cancers, non-specific immune responses such as humoral immunity become more important. As tumor cells die from radiation-induced apoptosis, they release tumor-associated antigen fragments that stimulate and activate B cells to differentiate into plasma B cells, which then produce antibodies to bind to distant tumor cells (2). The resulting antigen-antibody complexes are then phagocytosed by macrophages (2). Unfortunately, by conventional fractionated RT, the abscopal effect rarely occurs, with only case series and anecdotal evidence found in the literature (16). Hypo-fractionated high-dose radiation such as SBRT and immunotherapy synergy are thought to be ways to increase the occurrence of abscopal effects (2,16).
Immunotherapy for lung cancer
A category of immunotherapy that is of interest to researchers is that of therapeutic tumor vaccines. However, although generally tolerable, tumor vaccines have not always been shown to be effective in clinical trials thus far. For example, both Belagenpumetucel-L and SRL172, a killed mycobacterium vaccae, have been studied in phase III NSCLC trials (27,28), but without either showing any difference in overall survival (OS) and progression-free survival (PFS) compared to placebo. However, in a meta-analysis of various classes of immunotherapy in advanced NSCLC, therapeutic vaccines were still found to have an OS benefit compared to other immunomodulators such as cytokines and biological response modifiers (29). Today, new preclinical studies continue to identify potential molecular targets for new vaccines; for instance, molecular targets such as T-cell immunoglobulin and mucin domain-containing protein 3, tumor necrosis factor receptor superfamily, member 4 and indoleamine 2,3-dioxygenase 1 gene expression have been shown to promote anti-tumor activities in animal models of NSCLC and other poorly immunogenic tumors (23–26). Although further preclinical and clinical studies are still needed to confirm their efficacy, they are a promising avenue for research in cancer immunotherapy.
The major immunotherapy used in lung cancer treatment is ICIs, which include programmed death-ligand 1 (PD-L1)/programmed death-1 (PD-1) and cytotoxic T-lymphocyte antigen-4 (CTLA-4) antagonists. CTLA-4 is presented on T cells and binds to CD80 and CD86 on antigen-presenting cells, while on the other hand, PD-1 is a transmembrane receptor of members of both innate and adaptive immune cells, binding to PD-L1 and PD-L2, which are expressed widely on non-immune cells, including tumor cells (16). In lung cancer research, PD-1/PD-L1 inhibitors have been the most systematically researched, both as monotherapy and as an adjunct to RT, having also consistently demonstrated efficacy and safety as a primary lung cancer treatment (30). However, a detailed review of current evidence for ICI monotherapy in lung cancer treatment is beyond the scope of this review.
The advent of ICI immunotherapy has revolutionized lung cancer treatment, now being a part of the standard-of-care treatment for both NSCLC and SCLC, but still possesses certain limitations. For instance, numerous patients do not have high PD-L1 expression levels, which makes them less responsive to ICI (15,31), and furthermore, as discussed, TMEs are generally immunosuppressive, which severely dampens immune reactions to ICI. As such, much research effort has been directed at improving ICI's therapeutic efficacy, including a synergy with SBRT.
Synergistic effect of RT and immunotherapy
Early clinical evidence has already hinted at a synergy between RT and immunotherapy. The phase III START trial, which enrolled patients with stable stage III NSCLC on chemo-RT, aims to investigate whether tecemotide plus best supportive care could prolong survival compared to best supportive care alone. Tecemotide is a vaccine against mucin-1 protein antigen, which is designed to stimulate T-cell response. The study ultimately found no OS difference between the two arms; however, in a subgroup receiving concurrent chemo-RT (CCRT), improved OS was noted (32). On the other hand, perhaps the most well-known early evidence for RT + immunotherapy synergy is the secondary analysis of the KEYNOTE-001 trial, a large multicentre phase I trial for pembrolizumab (PD-1 inhibitor) in advanced NSCLC, wherein it was found that themedian PFS was significantly longer in patients receiving any type of RT prior to pembrolizumab (4.4 vs. 2.1 months, P=0.019) (33–35); in addition, the OS was also more favorable for the group with prior RT (10.6 vs. 5.3 months, P=0.026) (33–35). This evidence for RT + immunotherapy synergy paved the way for combining SBRT and immunotherapy.
SBRT differs from conventional RT protocols in that a larger dose of radiation with fewer fractions is delivered to the tumor site. Confidence in SBRT is accomplished by the integration of modern imaging, simulation, treatment planning and delivery technologies into all phases of the treatment process; from treatment simulation, planning and continuation throughout beam delivery. Fig. 2 illustrates a typical SBRT treatment plan, which is characterized by large RT doses (6–30 Gy) given in 1–5 fractions to well-circumscribed tumors (diameter, 5 cm or less). Due to the stringent need for patient immobilization and respiratory motion management, image guidance was utilized. Compared to conventional RT, SBRT seems to have a more potent in-situ vaccination, or immune activation, effect (2,15,16). While the radiobiological effect behind this observation remains to be fully elucidated, several studies have revealed a dose-dependent pattern behind radiation-induced antitumor immunity (15). It appears that a higher radiation dose induces more powerful anti-tumor immunity (15), although, as the dosage increases, so do the side effects.
Currently, a hypo-fractionated RT protocol with 24 Gy in three fractions is a commonly used immunomodulatory protocol in clinical trials (2), notwithstanding when the regimen is increased to 20 Gy per fraction, the risk of fibrosis and other adverse events (AEs) begins to outweigh the added benefit (2). Furthermore, SBRT of 12 Gy and above in a single dose has been shown to induce more T-regulatory cells and PD-L1 expression, as well as other immunosuppressive components of the TIME, thereby dampening the antitumor response (15); accordingly, immunotherapy, with the ability to ‘release the inhibitory breaks’ on the immune systems, can be added to augment the antitumor immunity while limiting radiation-associated toxicity and unwanted immunosuppression (2,15), while in return, the immune-priming effect of SBRT can enhance the efficacy of immunotherapy.
Toxicity issues
Both SBRT and immunotherapy are generally well-tolerated. As a local therapy, SBRT toxicities tend to be localized to the site of irradiation and the immediate surrounding area, while in thoracic SBRT, the commonly reported AEs are pneumonitis, chest wall pain, rib fractures, esophagitis and brachial plexopathy (36). In view of that SBRT is usually reserved for fragile patients who are not surgical candidates or unable to withstand systemic cytotoxic therapies, attempts to predict SBRT toxicity have had mixed results due to the inconsistent definition of toxicity used in various studies (36). Chest wall toxicities, for example, were reported to have an incidence rate of 8–46% (36); however, certain significant predictors have been identified, including tumor size as a predictor for ≥ grade 2 chest wall pain and elevated body mass index as strong predictors for chest wall pain post-SBRT (37,38). Among these factors, regarding the role of SBRT for lung tumors in central and ultra-central locations, node-negative tumors remain controversial, as increased rates of treatment-related toxicity may negate the benefit of treatment (36,39); accordingly, the American Society of Radiation Oncology guideline recommends 4–5 fractions for central tumors while discouraging the use of SBRT for ultra-central tumors until higher-quality evidence is provided (40).
Out of all immunotherapy categories, ICI's toxicity has been the most systematically researched. Its documented AEs are fatigue, as well as gastrointestinal-related and respiratory-related events, with the rate of grade 3 or higher ICI toxicity reported from 7–17% (36). Among the reported fatal side effects is pneumonitis, which a retrospective review found to be 1.3% in 915 patients treated with single or dual ICIs (41). Pneumonitis is of interest to researchers, not only because it is potentially fatal, but because it is also a reported toxicity of SBRT, and combining SBRT and ICI may indicate an increased risk of pneumonitis. A multicenter chart review conducted by Tian et al (42) found that the risk of grade 3 or higher pneumonitis was higher in the SBRT + ICI cohort compared to SBRT alone (10.7 vs. 0%, P=0.007), while the risk of any pneumonitis appeared the same between the two cohorts.
Any-grade pneumonitis also appears higher among those receiving dual ICI therapy. The highest-grade subacute AE was more significant in the synergy group compared to SBRT alone (5.4 vs. 0%, P<0.001), and no statistically significant difference in highest-grade acute AEs was noted. The authors concluded that SBRT + ICI was safe, but clinicians should exercise caution when considering pneumonitis, particularly in patients receiving SBRT + dual ICIs (42). Korpics et al (43) also reviewed three consecutive phase I trials between 2016 to 2020 that combined SBRT and ICI to treat widely metastatic solid tumors where the overall rate of ≥ grade 3 pneumonitis was 8.1%, and the development of pneumonitis was significantly associated with the established dosimetry parameters. The authors concluded that if kept under the protocol-specified dosage limit, thoracic SBRT + ICI would neither impose a significantly higher risk of pneumonitis nor other severe AEs (43). Other phase I trials have also corroborated the tolerability of SBRT + ICI combination in lung cancer treatment of various stages. Various relevant trials will be mentioned in the subsequent chapters.
Current advances in SBRT and immunotherapies synergy in lung cancer
SCLC
Approximately 13% of newly diagnosed lung cancers are SCLC (44). There are two methods of staging for SCLC: The tumor-node-metastasis (TNM) classification (45) and the Veterans Administration Lung Study Group (VALSG) system (46). The American Joint Committee on Cancer (AJCC) TNM stage I to III diseases roughly correlate with the VALSG limited stage (LS) disease, whereas AJCC stage IV diseases were historically defined as extensive stage (ES) by the VALSG (44). Today, both classification systems are still in use. Only about one-third of patients are diagnosed with LS-SCLC at presentation and are therefore potentially curable with a combination of surgery, chemotherapy and RT, while the other two-thirds present with ES-SCLC, whose only treatment option is chemotherapy (44). However, despite these standard treatments, survival remains poor. According to an analysis from 2020, the median survival for LS-SCLC following chemotherapy was 18.1 months, and even with the addition of early thoracic RT, the survival rate was just 18.4 months, while for ES-SCLC, the post-chemotherapy median survival was 9.6 months and neither thoracic RT nor prophylactic cranial irradiation was able to improve survival (47). More effective treatment options are therefore urgently needed.
In recent years, chemoimmunotherapy has become the standard first-line treatment for ES-SCLC after several landmark trials, including KEYNOTE 604 and Impower133, revealed its ability to improve PFS and OS in this population (30). SBRT has also become more common in treating early-stage node-negative SCLC, despite there not being any completed randomized controlled trials (RCTs) to support its use in this population (44). This is largely due to SBRT's favorable tolerability and a significant proportion of SCLC patients not being operative candidates (44). Of the limited data available, a retrospective study found a local PFS of 96.1% at three years for patients with LS-SCLC treated with SBRT and only 59% of the studied patients had received chemotherapy (48).
Building on the above findings, it was postulated that the synergy between SBRT plus ICI could be harnessed for treating SCLC; however, the addition of SBRT to chemoimmunotherapy is still in its infancy and studies are lacking. Pakkala et al (49) conducted a signal-finding phase 2 randomized trial where 18 patients with relapsed SCLC received durvalumab (PD-L1 inhibitor) and tremelimumab (CTLA-4 inhibitor) with or without prior immune-sensitizing SBRT, although the study ultimately failed to demonstrate improved PFS and objective response rate (ORR) after the addition of SBRT, but a trend in efficacy was revealed. Furthermore, changes in peripheral blood lymphocyte composition were consistent with an immunologic response to RT, suggesting SBRT and ICI synergy could be a step in the right direction. The trial also demonstrated the tolerability of combining SBRT with ICI at the dosage employed (49).
Building on the work of Pakkala et al (49), four phase I and II clinical trials focusing on SBRT and chemoimmunotherapy in ES-SCLC are being conducted. The DARES trial (NCT 05068232) by the University of Chicago is a single-arm, phase I study that investigates chemotherapy + SBRT + durvalumab in the setting of treatment-naïve ES-SCLC; another phase I study (NCT05403723) is identifying the AEs of chemotherapy with durvalumab and SBRT; a phase II study (NCT04923776) is investigating whether chemoimmunotherapy + liver-directed SBRT can improve PFS in patients with ES-SCLC with liver involvement; while the Cornell University is conducting a phase II study (NCT04951115) of standard chemoimmunotherapy with sub-ablative doses of SBRT in stage IV SCLC. These studies will generate important data for this novel SCLC treatment.
Metastatic NSCLC
Contrary to SCLC, most newly diagnosed lung cancers are classified as NSCLC (80–90%) (33). Early research on SBRT + immunotherapy has focused on metastatic NSCLC. One of the first landmark studies that demonstrated the feasibility of combining SBRT with ICI was conducted by Luke et al (50) to establish the recommended SBRT dosage by organ site when used with pembrolizumab. Several metastatic solid tumors were studied, including NSCLC. As its secondary outcome, the study found an ORR of 13.2%, a median OS of 9.6 months and a PFS of 3.1 months. The combination was well-tolerated with acceptable toxicity. The clinical benefit was later supported by another landmark study, PEMBRO-RT, in which patients with metastatic NSCLC were randomized to receive pembrolizumab alone or pembrolizumab + SBRT (51), and although the result did not meet the study's specified end-point criteria, the multi-center phase II study still showed a doubling of the ORR (20% in the control arm vs. 50% in the experimental arm, P<0.10); in particular, it was found that PD-L1-negative patients who historically respond poorly to ICI treatment received the greatest benefit from the combination therapy (51).
This encouraging result has inspired a current randomized phase II/III clinical trial, Alliance A082002 (NCT04929041), which further investigates whether the addition of SBRT to immunochemotherapy improves PFS and OS for patients with PD-L1-negative, advanced NSCLC (31). There is also a retrospective analysis that directly compares response rates after anti-CTLA-4 and anti-PD-1 combined with SBRT in metastatic NSCLC, where anti-PD-1 appeared to have a higher global response rate and better PFS (52). The authors assumed that the observation was driven by a reduced development of new out-of-field lesions, suggesting anti-PD-1 may have better control over distant tumors compared to CTLA-4 inhibitors (52). Although only a small number of trials had directly compared anti-PD-1 and anti-CTLA-4, the result corresponds to that of a phase III trial on melanoma, which found better response and survival among patients treated with anti-PD-1 than those on anti-CTLA-4 monotherapy (53). Further studies on SBRT + ICI synergy in metastatic NSCLC, including a randomized phase III study, are underway (Table II).
Other immunotherapies have also been studied in the context of metastatic NSCLC. Huang et al (54) reported a case from China where a patient with stage IV anaplastic lymphoma kinase mutated NSCLC and progressed on multiple lines of TKI treatment achieved complete response and a PFS of >26 months after SBRT + a 23-valent pneumococcal polysaccharide vaccine. Another case report presented a patient whose metastatic myoepithelial parotid carcinoma underwent dramatic regression after receiving two doses of mRNA coronavirus disease 2019 (COVID-19) vaccine (55). Subsequent imaging mass cytometry comparing pre- and post-vaccination biopsy samples revealed robust anticancer immune responses, and although the authors speculated that the anticancer response was due to the COVID-19 vaccination, it is interesting to note that this patient had also completed a postoperative RT of 60 Gy half a year prior. Both case reports, although limited, suggest vaccines can stimulate adaptive immune reaction after RT and may possibly induce abscopal responses, but regardless of this optimism, the only clinical trial on RT and mRNA vaccine in lung cancer has yielded only one case of partial response out of the 26 patients studied, with the majority of participants achieving stable disease (56).
Oligometastatic NSCLC (OM-NSCLC)
Beside widely metastatic NSCLC, stage IV NSCLC also includes OM-NSCLC disease. OM-NSCLC is defined in several trials as the patient having between 1–5 metastatic lesions (57,58). OM-NSCLC is an intermediate entity between locally confined cancer and widespread metastatic disease and is increasingly being recognized as a separate disease state due to its less aggressive clinical course and lower dissemination capacity than its widely metastatic counterpart (57–59). A significant number (20–50%) of patients with NSCLC are diagnosed with OM-NSCLC at presentation, and although life expectancy has traditionally been considered short for patients with metastatic NSCLC, treatments were always reserved for symptom palliation, but incremental research is now indicating that the prognosis for OM-NSCLC may be better than previously thought.
From observational study to population-based hypothesis-generating analysis, patients with NSCLC with single-organ metastases (even if it is to the brain or the bone) have been shown to have better prognosis than those with multi-organ metastases (60,61), and in view of this, long-term control could be possible and as such, systemic chemotherapy alone is no longer optimal, so more aggressive local therapies are warranted. Indeed, both a phase II multi-national study by Gomez et al (62) and a randomized phase II study by Iyengar et al (63) found aggressive local treatments to have a significant PFS benefit over maintenance chemotherapy alone for patients with OM-NSCLC; so much that both trials had to be closed early. This survival benefit could also be applied to patients with OM-NSCLC with limited cerebral metastasis, which will be discussed later.
With treatment intent shifting from palliative to curative, there is a potential role for immunotherapy + SBRT synergy in OM-NSCLC. So far, only one published trial has specifically investigated the OM-NSCLC subgroup (57,64), being a single-arm, phase 2 trial that enrolled 51 patients with OM-NSCLC who completed local ablative RT to receive pembrolizumab therapy with their PFS compared to historical data (65), revealing improved median PFS in the study (19.1 months vs. historical data of 6.6 months). Another published study worth discussing is Watanabe et al (66)'s analysis of two patients with oligometastatic melanoma treated with anti-PD1 + SBRT, where an intriguing part of the study was that one of the patients had only low levels of cytotoxic T-cell infiltration and high expression levels of immunosuppressive arginase; still, the patient produced an abscopal response that lasted >2.5 years. Although Watanabe et al (66)'s study was not on NSCLC, it may, along with the results of PEMBRO-RT (where PD-L1-negative patients benefited from SBRT + ICI synergy), imply an expanded indication for SBRT + immunotherapy for patients with an unfavorable pre-treatment immune signature. Furthermore, another case report recently demonstrated that even after oligoprogression, SBRT + ICI could still be a reasonable treatment option, as a delayed treatment response may occur (67). In short, SBRT and immunotherapy synergy are currently pushing NSCLC treatment boundaries.
Among the ongoing OM-NSCLC trials, CHESS (NCT03965468) and CHEERS (NCT03511391) are two much-anticipated studies. Details on the two trials will be addressed later in this review. On the other hand, most of the other ongoing OM-NSCLC trials involve unapproved agents: A phase Ib single arm study (NCT03275597) with SBRT followed by durvalumab and tremelimumab, an unapproved CTLA-4 inhibitor for one; IMMUNOs-SBRT (NCT05259319) being another study aiming to establish a safe scheme of administration for tiragolumab + atezolizumab + SBRT in OM-NSCLC where tiragolumab is a novel anti-T cell immunoreceptor with immunoglobulin and ITIM domain inhibitory immune checkpoint agent, although its combination with atezolizumab has recently been shown to be safe and more effective than atezolizumab + placebo in metastatic NSCLC in the CITYSCAPE trial (68).
Regarding non-ICI immunotherapy, there is a study that looks at combining SBRT with a targeted form of IL-2, Darleukin, a potent immune-activating cytokine. By itself, IL-2 can cause serious toxicity throughout the body, but when conjugated to L19, it can selectively accumulate at the tumor site, thereby boosting anti-tumor response selectively (69). Darleukin plus SBRT has been shown to be safe and promising in oligometastatic solid tumors in a small phase I study (NCT02086721), where three patients (50% of the studied population) had OM-NSCLC (70). Now, a multicenter, randomized phase II study, ImmunoSABR (NCT03705403), is taking place, stratifying patients according to their metastatic load and comparing PFS between a SABR + Darleukin group and the standard of care (69). Watanabe et al (66)'s analysis mentioned earlier also found an increase in tumor epitope-specific T cells after SBRT, suggesting additional epitope-based immunotherapy, such as a tumor vaccine, may have added benefit. All in all, OM-NSCLC is a budding area of research for SBRT + immunotherapy synergy (Table II).
Locally advanced NSCLC (LA-NSCLC)
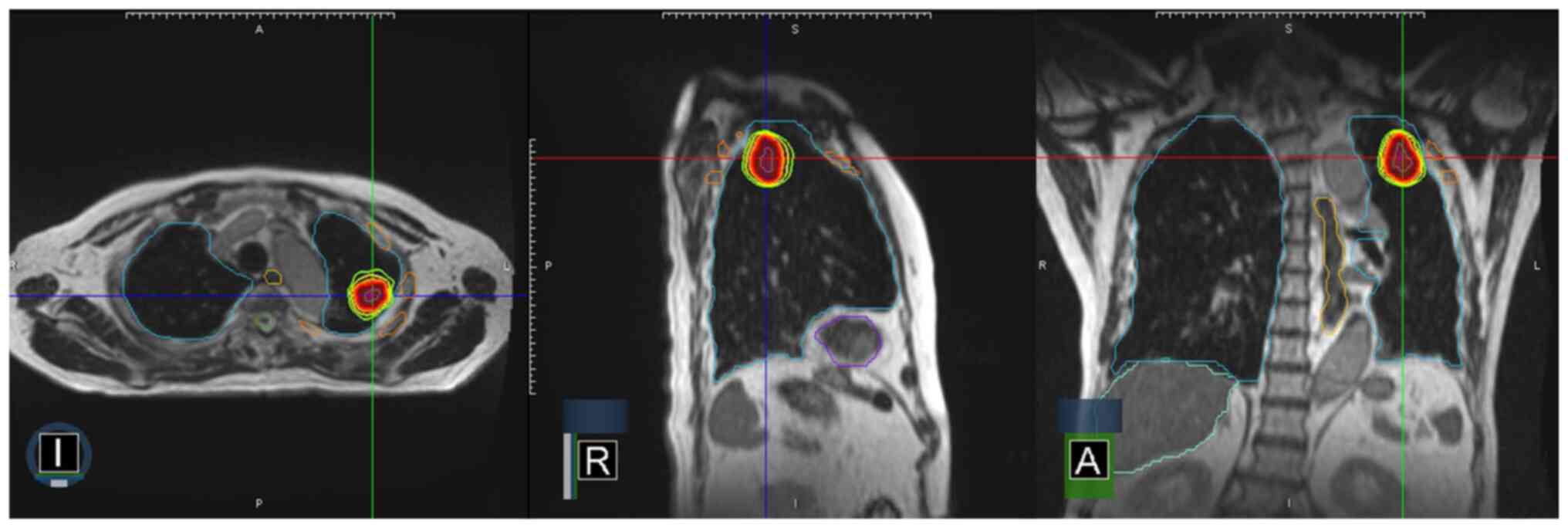
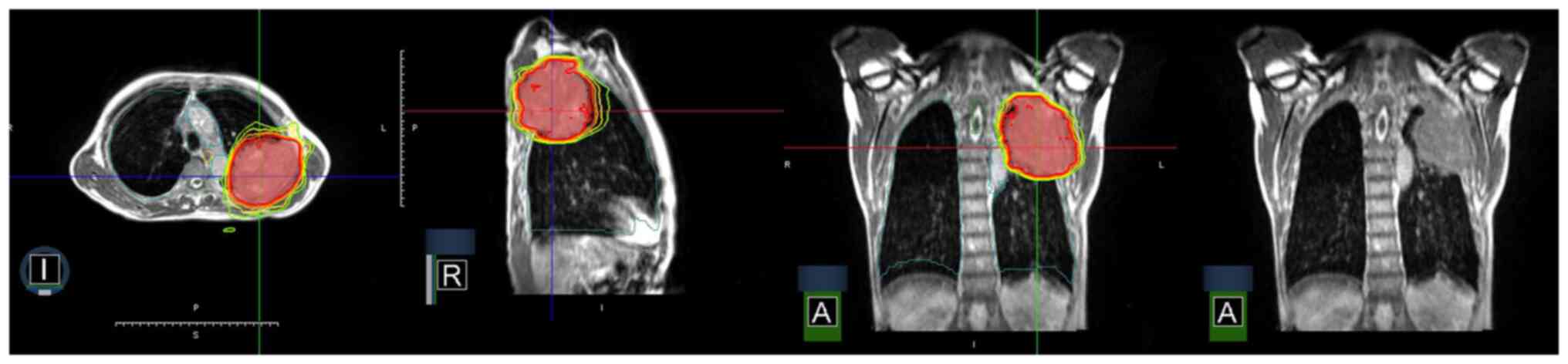
According to the AJCC TNM staging 8th edition (45), stage III lung cancer describes LA-NSCLC with N1-N3 nodal involvement and/or primary tumor of T3 and T4 classifications, without evidence of distant metastasis (34). The mainstay of treatment for LA-NSCLC has been CCRT for numerous years, yet even after CCRT, the OS rate was only 15–20%, with disease recurrence as the main contributor to the low OS rate (33,34). Fig. 3 demonstrates high conformality and fast dose-falloff in an SBRT plan with in-room magnetic resonance image guidance for squamous cell carcinoma lung cancer invading the ribs. The resulting partial response is shown in Fig. 4′s serial computed tomography scans, which were taken over a span of 6 months.
A major issue with chemo-RT is its toxicity associated with dose escalation, which severely limits its tumoricidal effect (33,71). This is best demonstrated by the result of RTOG 0617, which showed that the 74 Gy arm has shorter survival compared to the 60 Gy arm (20 vs. 29 months) due to increased toxicity and no benefit to the local control (LC) rate (33,71), so the trial had to be terminated early, and since then, the research focus has shifted to the optimization of chemo-RT. After ICI and SBRT each showing a survival benefit and tolerable toxicity profile when added to existing CCRT, the idea of a ‘quad-modality’ approach to LA-NSCLC treatment was proposed (33,72,73). Kumar et al (33) suggested that patients with LA-NSCLC undergo CCRT to a dosage of 44–60 Gy, followed by a two-week break to allow for treatment planning and recovery from leukopenia; then, an SBRT boost should be administered to stimulate the immune system, which is immediately (within 1 week) followed by immunotherapy. As a theory in its infancy, numerous issues, such as its risk of autoimmune toxicity, remain to be explored, as do other integrated approaches. Wang et al (74) reported a case series of resectable LA-NSCLC who were given neoadjuvant ICI + SBRT before surgery. Only one patient had 20% residual tumor, with the rest either achieving complete response or major pathologic response (74). Although differing in the specific treatment modalities involved, a multimodality treatment model comprising SBRT and ICI appears to be the trend in LA-NSCLC treatment.
At present, none of the completed clinical trials on SBRT and immunotherapy combination are specifically for LA-NSCLC (15), although the Brown University is conducting a small phase II single arm study (NCT03589547) of durvalumab with consolidation SBRT following chemo-RT. This is the first study to recruit patients with stage III NSCLC only (Table II), and is set to complete in 2026.
Early-stage NSCLC
Roughly 16% of patients with NSCLC present with early-stage disease (AJCC stage I or II) (75), and that number is expected to increase with the implementation of routine lung cancer screening programs (75). SBRT is the standard treatment for inoperable, peripheral stage I or II NSCLC owing to numerous trials, including a recent randomized phase III study, showing a lower local recurrence rate and better OS in the SBRT group compared to conventional RT (34,75,76). When it comes to the synergy between SBRT and immunotherapy, there is only a small number of published trials that focus on stage I or II NSCLC. Atezolizumab (PD-L1 inhibitor) + SBRT was recently studied in a phase I single-arm trial (NCT02599454) for inoperable stage I or II NSCLC, with the combination revealed to be both feasible and tolerable (15). Accordingly, a randomized phase III trial (NCT04214262) is undergoing that is evaluating Atezolizumab + SBRT vs. SBRT alone in high-risk stage I or II NSCLC (77).
A recent rise in interest in applying SBRT + immunotherapy synergy to early-stage NSCLC has been observed (Table II) (15). A phase II Canadian study (NCT04944173), SCION, is investigating the effect of combining SBRT with durvalumab in stage I NSCLC. ASTEROID (NCT03446547) is another ongoing multi-center, phase II study that randomized patients with stage I NSCLC to receive SBRT or SBRT + durvalumab, where its latest toxicity report showed the combination to be well-tolerated with only 2 out of 47 patients experiencing pneumonitis of ≥ grade 3 (78). KEYNOTE-867 (NCT03924869) is a phase III randomized, placebo-controlled study that evaluates SBRT + pembrolizumab in unresectable stage I or II NSCLC. The results will add valuable data to SBRT + immunotherapy synergy in early-stage NSCLC.
As discussed, central and ultra-central locations are at increased risk of SBRT toxicity, although it was also observed that the LC rate was significantly better with higher RT doses (79). This dilemma presents an opportunity for SBRT + immunotherapy synergy, and whereas SBRT primes the immune system for immunotherapy, the synergistic effect means lower SBRT doses can be used for tumors located in central and ultra-central locations, effectively decreasing the risk of toxicity, although evidence in this area is naturally required. Likewise, although large (defined as ≥5 cm in diameter by RTOG0236 and other phase II trials) (80,81), node-negative tumors are found to be an independent risk factor for SBRT toxicity, these too have the potential to benefit from SBRT + immunotherapy synergy.
Surgical series have shown that, as the NSCLC lesion size increases, the rate of pathological nodal-positive disease also increases, hence the risk of distant metastasis (82). It naturally follows that the use of systemic therapy to safeguard against nodal and metastatic diseases is a reasonable next step, and indeed, trials have shown an OS benefit in large tumors with postoperative chemotherapy (83,84). The 2023 National Comprehensive Cancer Network guideline continues to endorse post-SBRT systemic therapy in NSCLC with high-risk features, one of these being tumors of ≥4 cm in diameter (85). Of the systemic therapies, immunotherapy has been found to be better-tolerated than cytotoxic chemotherapy (86), so SBRT + immunotherapy may be worth exploring as a treatment for large, node-negative NSCLC. The MD Anderson Cancer Center's ongoing I-SABR clinical trial (NCT03110978) will shed some light on this issue, since it includes tumors sized ≥5 cm (Table II), with the results expected later in 2025.
Multiple primary lung cancers (MPLC)
As diagnostic technology and treatment improve, the lifespan of cancer patients is also lengthening, meaning the incidence rate of second primary lung cancer will also increase (87,88). Since most patients with MPLC are diagnosed at early stages of the disease, retrospective studies have demonstrated SBRT as a safe and feasible alternative in those not suitable for surgery (87), while in contrast, the role of immunotherapy in MPLC treatment remains unknown. The homing tendency of immune cells makes immunotherapy appealing to patients with MPLC whose lesions are disperse and inoperable; however, the inconsistent and generally low PD-L1 expression across the independent MPLC lesions in addition to the unknown characteristics of MPLCs' TME make it difficult to predict response to immunotherapy (87). SBRT's immune priming effect has the potential to help overcome these difficulties, making SBRT + immunotherapy synergy a topic worth exploring in MPLCs, although no trial has yet been designed specifically for SBRT + immunotherapy synergy in MPLCs (87).
Practical issues to be addressed
Sequencing/timing of SBRT and ICI or non-ICI immunotherapy
Encouraging results from studies on the sequential use of immunotherapy and SBRT in NSCLC have led researchers to wonder what the effect would be if immunotherapy is administered earlier in the treatment course. Compared to sequential therapy, concurrent therapy has the practical advantages of starting all treatment earlier and that such immunotherapy may mitigate the immunosuppressive effect of higher-dosage RT, as previously discussed. In a preclinical study, it was found that concurrent RT + anti-PD1 therapy in a mouse model led to improved LC and survival, as well as decreased recurrence rates, but not in subjects who received the combination treatment sequentially (89); however, clinical studies on the optimal sequencing and timing of SBRT + immunotherapy are lacking (90,91). Of the available studies, there is also no consensus regarding the definition of ‘sequential’ and ‘concurrent’ therapy, making it difficult to directly compare the results across the various studies.
A recent retrospective chart review by Woody et al (90) found that OS was significantly better in concurrent and sequential therapy where immunotherapy was completed after SBRT compared to completing immunotherapy before SBRT in metastatic cancers. This is in contrast with their preliminary data suggesting concurrent therapy should have a better outcome than sequential therapy (90). In a study on ipilimumab + SBRT for non-melanoma metastatic cancer, the patients were non-randomized into sequential or concurrent groups with liver or lung metastasis (92), and in the phase I portion of the study, it was found that the sequence of SBRT and ICI was irrelevant to peripheral T-cell response. By contrast, in the phase II study, the sequential lung group had the highest rate of clinical benefit with no difference in treatment-related toxicity, although the authors admitted that their study was not powered to compare sequential vs. concurrent therapy (92).
COSINR, a randomized phase I trial that compared sequential to concurrent multisite SBRT with nivolumab and ipilimumab in stage IV NSCLC, found no statistical significance between the 2 arms in terms of global response rate, PFS and time to second progression or death (91). The study, however, found that concurrent therapy was not more toxic than sequential therapy and that no RT dose reduction was needed for concurrent therapy (91). More definitive conclusions await further research, including the aforementioned CHESS study, which is a multicentre phase II single-arm study that will combine and alternate the use of immunotherapy and chemotherapy with SBRT to metastases followed by definitive surgery or RT to the primary tumor (64), yielding important data on the optimal integration of various treatment strategies for OM-NSCLC that includes the optimal timing and sequencing of SBRT + immunotherapy (93).
On the other hand, there is no study that directly compares sequential to concurrent non-ICI immunotherapy with SBRT. However, based on the aforementioned START trial, we may speculate that the sequencing of combination treatment involving non-ICI immunotherapy matters. The START trial was a phase III RCT on chemo-RT and tecemotide in patients with unresectable stage III NSCLC (32). In its subgroup analysis, those who were previously on concurrent chemo-RT responded better to subsequent tecemotide therapy than those on sequential chemo-RT. The survival benefit was kept at the 20-month follow-up, with exploratory biomarker analysis showing prolonged survival among those with high soluble mucin-1 and antinuclear antibody levels (94). Although follow-up studies did not support this observation and the development of tecemotide was eventually terminated by the manufacturer (95), the finding of the START trial still underscored the potential importance of sequencing/timing in combination treatment involving non-ICI immunotherapy. As such, future investigation on the optimal sequencing of non-ICI immunotherapy + SBRT is warranted.
Corticosteroid premedication
The importance of investigating optimal scheduling for SBRT + immunotherapy is underscored by research on corticosteroid premedication, which is common practice among radiation oncologists to prevent acute lung toxicity when a high RT dosage is used. Corticosteroids such as dexamethasone and prednisone are at times administered 15–60 min prior to each fraction of RT (96); however, as a potent immunosuppressor, corticosteroid prophylaxis may blunt the action of ICI during SBRT + ICI therapy. Arbour et al (97) have shown that corticosteroid can decrease the efficacy of ICIs in patients with NSCLC on baseline corticosteroid treated with PDL1 blockers. The mechanism may be through the dampening of the CD8+ T-cell response to ICI (96,98). On the other hand, corticosteroid administration before and after SBRT treatment seems to neither change the toxicity rate of SBRT nor confer a worsening of the cancer recurrence rate (96,99). However, both of these studies were limited by their retrospective nature, and the role of corticosteroid in the context of SBRT + ICI therapy remains indeterminate; meanwhile, Alite et al (96) concluded that investigation into optimal SBRT + immunotherapy dosing and scheduling to prevent treatment-related toxicity may be more worthwhile than relying on corticosteroid prophylaxis.
Patients with limited cerebral metastasis
The concept of oligometastatic tumor has led researchers to re-evaluate our expectations for metastatic cancer patients, specifically those with brain metastases. Traditionally, these patients are excluded from clinical trials due to presumed dismal prognoses, although recent evidence has demonstrated that patients with limited metastases, even those with metastases to the brain, may fare better than those with widely metastatic diseases (60,61). The benefit of aggressive local therapy for OM-NSCLC is also seen in those with limited brain metastases. As an example, Nikitas et al (100) demonstrated that when treated aggressively with synchronous thoracic SBRT and brain SRS, the 1-year rate for thoracic LC was 100 and 80% for central nervous system LC, respectively, with the OS being 67% at 1 year and only 1 case of grade 2 chest wall toxicity was recorded.
A published systemic review investigated the SBRT + ICI treatment prognoses among patients with OM-NSCLC or melanoma with metastases to various organs and found that the treatment benefit of ICI + SRS in cerebral metastases was less clear than that of ICI + SBRT in extracerebral metastases (101). However, COSINR, one of the few RCTs on metastatic NSCLC that included patients with controlled brain metastasis, showed that patients with treated or limited brain metastases did not fare worse than those without (91). More data on cerebral metastases could have been added should clinical trials such as MIGRAINE (NCT04427228) and STICk-IM-NSCLC (NCT04650490) recruit enough participants to procced (101). MIGRAINE was a study that directly compares single vs. multiple-fraction SRS in patients with brain cancers and brain metastases who were also on immunotherapy, while STICk-IM-NSCLC planned to look at the timing of SRS relative to ICI to determine whether it has any effect on patients with NSCLC with untreated brain metastases. Thus far, it is indicated that SBRT + ICI may be promising for OM-NSCLC, but future analysis needs to take the site of metastases into consideration when stratifying patients.
Predictive biomarkers for SBRT + immunotherapy synergy
In the era of precision/personalized medicine, there is increasing emphasis on identifying predictive biomarkers that could spot populations who may potentially benefit the most from a treatment (15,102) Although several biomarkers have been identified for ICI or SBRT treatment, their effects in the combined treatment remain unknown. Subsequently, Chen et al (15) suggested integrated biomarkers consisting of multiple parameters such as PD-L1 expression level, tumor mutation burden and cytotoxic T lymphocyte counts; in other words, a liquid biopsy approach to inform clinical decisions. Watanabe et al (66)'s analysis, as previously discussed, is an example where a liquid biopsy predicts the prognosis of two patients with the same clinical staging, where in contrast to Patient 2, Patient 1 showed strong lymphocyte tumor infiltration and high expression of lymphocyte-attracting chemokines with a rapid complete response to SBRT + ICI treatment that was ongoing for >4.5 years, revealing patient 1′s prognosis was significantly better than that of Patient 2, despite both having been diagnosed with oligometastatic melanoma with liver metastasis. This suggests a clinical value for liquid biopsy and an implication for clinical decision-making. Chen et al (15) further suggested that, based on the result of PEMBRO-RT, which found a statistically significant OS difference in the PD-L1-negative group compared to the PD-L1-positive group, PD-L1 negativity could be a candidate for predictive biomarkers, although further investigations are needed to confirm this speculation.
As mentioned, CHEERS (NCT03511391) is a study on OM-NSCLC that will be particularly interesting, being the first randomized phase II study that assesses ICI + SBRT to a maximum of three lesion sites and, more importantly, it also contains a translational endpoint that will inform us of the potential biomarkers for treatment response (103). The Canadian SCION study also contains a translational component but aims to investigate if levels of circulating tumor DNA can predict which patient will most likely respond to prolonged ICI treatment after SBRT (Table II) (102).
On a side note, the duration of immunotherapy after SBRT is also an area with a paucity of research. A Chinese case study reported on a patient with 5 years of PFS following 52 months of extended pembrolizumab monotherapy after SBRT, representing the most prolonged medication duration with the best efficacy among Chinese patients in the literature (104), suggesting that the duration of ICI treatment may influence survival. The advent of precision medicine for NSCLC means translational research will now have a more important role to play in clinical trials than ever before.
Future research trends and direction
There are several key areas in the integration of ICI and non-ICI immunotherapy with SBRT for lung cancer (Table III).
Optimal timing and sequencing
Further research is needed to determine the most effective timing and sequencing of immunotherapy and SBRT. This includes investigating whether concurrent therapy offers superior outcomes compared to sequential therapy, as well as defining clear guidelines for treatment protocols.
Biomarker identification
The identification of predictive biomarkers for treatment response is essential for personalized medicine approaches. Future studies should focus on integrating multiple parameters such as PD-L1 expression, tumor mutation burden and lymphocyte counts to identify patients who are most likely to benefit from combination therapy.
Clinical trial design
Future clinical trials should incorporate translational endpoints to elucidate potential biomarkers for treatment response. Additionally, studies should aim to include diverse patient populations, e.g. those with limited cerebral metastases, to better understand the efficacy of combination therapy across different disease presentations.
Duration of immunotherapy
Investigating the optimal duration of immunotherapy following SBRT is crucial for maximizing treatment efficacy while minimizing toxicity. Research should aim to determine whether extended immunotherapy regimens offer improved long-term outcomes for patients with NSCLC.
Precision medicine
The advent of precision medicine underscores the importance of translational research in guiding clinical decision-making. Future trends should prioritize the integration of translational endpoints into clinical trials to advance our understanding of the underlying mechanisms of treatment response and resistance.
Overall, future trends in the integration of immunotherapy with SBRT for NSCLC should focus on refining treatment protocols, identifying predictive biomarkers and incorporating translational research to optimize patient outcomes in the era of precision medicine.
Concluding remarks
The dynamic duo of SBRT and immunotherapy has maximized the therapeutic potential of lung cancer treatment to the historical record. The addition of SBRT allows more patients to respond to immunotherapy, which correspondingly lowers the risk of toxicity that comes with higher ablative RT doses. Yet, much more about the combined treatment of SBRT + immunotherapy remains to be elucidated. For instance, as evident in Table II, various SBRT regimens exist, but it remains undetermined which are the most suitable for harnessing the synergistic power of RT and immunotherapy, and it is elusive which ICI agent has the best survival outcome when combined with SBRT. Furthermore, now that more and more positive trials support SBRT in up to three metastatic sites, it needs to be determined how many sites should be irradiated to achieve the best immune priming effect for synergy with immunotherapy. Finally, other immunotherapies that can surpass ICI as an adjunct treatment with SBRT should be developed. To resolve these practical issues and meet these research needs, knowledge gained from basic scientific research is required. As cancer treatment becomes increasingly personalized, research in preclinical and translational studies has great potential in advancing our knowledge on SBRT + immunotherapy synergy, particularly in the areas that are discussed in this review. A potential limitation of the present review is that very few clinical studies on non-ICI immunotherapies and SBRT synergy exist. Therefore, despite our best effort, the discussion still mostly centers on ICIs.
The discovery of SBRT + immunotherapy synergy has offered tremendous hope to those who suffer from lung cancer; still, much more remains to be explored. Further research in cell biology, molecular biology and biophysics could aid in the understanding of the many practical aspects of this novel, yet promising treatment. With the many ongoing preclinical and clinical trials discussed here, it will be exciting to see how treatments evolve in the future.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
Conceptualization, HHL and SC; writing-original draft preparation, SC; writing-review and editing, SC, HHL and KYT; data curation, KYT; visualization, KYT and SC; supervision, HHL. All authors have read and agreed to the published version of the manuscript. Data authentication is not applicable.
Ethics approval and consent to participate
The medical images in the figures of the present review are from the database of a retrospective study, which was approved by the Ethical and Research Committee at Kaohsiung Medical University Hospital [Kaohsiung, Taiwan; approval no. KMUHIRB-E(I)-20220101]. It was conducted in compliance with the Institutional Review Board regulations in accordance with the Helsinki Declaration of 1975 as revised in 1983. Patient informed consent was waived due to the retrospective design.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Glossary
Abbreviations
Abbreviations:
|
NSCLC |
non-small-cell lung cancer |
|
RT |
radiation therapy |
|
SBRT |
stereotactic body radiation therapy |
|
SABR |
stereotactic ablative radiotherapy |
|
SRS |
stereotactic radiosurgery |
|
ICI |
immune checkpoint inhibitor |
|
TME |
tumor microenvironment |
|
TIME |
tumor immune microenvironment |
|
Tregs |
regulatory T cells |
|
TAA |
tumor-associated antigen |
|
OS |
overall survival |
|
PFS |
progression-free survival |
|
PD-L1 |
programmed death-ligand 1 |
|
PD-1 |
programmed death-1 |
|
CTLA-4 |
cytotoxic T-lymphocyte antigen-4 |
|
CCRT |
concurrent chemoradiation therapy |
|
AE |
adverse effect |
|
TNM |
tumor-node-metastasis |
|
VALSG |
Veterans Administration Lung Study Group |
|
AJCC |
American Joint Committee on Cancer |
|
LS |
limited stage |
|
ES |
extensive stage |
|
ORR |
objective response rate |
|
OM |
oligometastatic |
|
LC |
local control |
References
|
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Xiao Y and Zhuang H: Effect of stereotactic radiotherapy on immune microenvironment of lung cancer. Front Immunol. 13:10258722022. View Article : Google Scholar : PubMed/NCBI | |
|
Billing DL and Rimner A: Results of radiation therapy as local ablative therapy for oligometastatic non-small cell lung cancer. Cancers (Basel). 13:57732021. View Article : Google Scholar : PubMed/NCBI | |
|
Chakraborty M, Abrams SI, Camphausen K, Liu K, Scott T, Coleman CN and Hodge JW: Irradiation of tumor cells up-regulates Fas and enhances CTL lytic activity and CTL adoptive immunotherapy. J Immunol. 170:6338–6347. 2003. View Article : Google Scholar : PubMed/NCBI | |
|
Garnett CT, Palena C, Chakraborty M, Tsang KY, Schlom J and Hodge JW: Sublethal irradiation of human tumor cells modulates phenotype resulting in enhanced killing by cytotoxic T lymphocytes. Cancer Res. 64:7985–7994. 2004. View Article : Google Scholar : PubMed/NCBI | |
|
Lugade AA, Moran JP, Gerber SA, Rose RC, Frelinger JG and Lord EM: Local radiation therapy of B16 melanoma tumors increases the generation of tumor antigen-specific effector cells that traffic to the tumor. J Immunol. 174:7516–7523. 2005. View Article : Google Scholar : PubMed/NCBI | |
|
Reits EA, Hodge JW, Herberts CA, Groothuis TA, Chakraborty M, Wansley EK, Camphausen K, Luiten RM, de Ru AH, Neijssen J, et al: Radiation modulates the peptide repertoire, enhances MHC class I expression, and induces successful antitumor immunotherapy. J Exp Med. 203:1259–1271. 2006. View Article : Google Scholar : PubMed/NCBI | |
|
Schaue D, Ratikan JA, Iwamoto KS and McBride WH: Maximizing tumor immunity with fractionated radiation. Int J Radiat Oncol Biol Phys. 83:1306–1310. 2012. View Article : Google Scholar : PubMed/NCBI | |
|
Formenti SC, Rudqvist NP, Golden E, Cooper B, Wennerberg E, Lhuillier C, Vanpouille-Box C, Friedman K, Ferrari de Andrade L, Wucherpfennig KW, et al: Radiotherapy induces responses of lung cancer to CTLA-4 blockade. Nat Med. 24:1845–1851. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Navarro-Martín A, Galiana IL, Berenguer Frances MA, Cacicedo J, Cañas Cortés R, Comas Anton S, Padrones Sánchez S, Bolívar Cuevas S, Parry R and Guedea Edo F: Preliminary study of the effect of stereotactic body radiotherapy (SBRT) on the immune system in lung cancer patients unfit for surgery: Immunophenotyping analysis. Int J Mol Sci. 19:39632018. View Article : Google Scholar : PubMed/NCBI | |
|
Zhou P, Chen D, Zhu B, Chen W, Xie Q, Wang Y, Tan Q, Yuan B, Zuo X, Huang C, et al: Stereotactic body radiotherapy is effective in modifying the tumor genome and tumor immune microenvironment in non-small cell lung cancer or lung metastatic carcinoma. Front Immunol. 11:5942122021. View Article : Google Scholar : PubMed/NCBI | |
|
Zhang T, Yu H, Ni C, Zhang T, Liu L, Lv Q, Zhang Z, Wang Z, Wu D, Wu P, et al: Hypofractionated stereotactic radiation therapy activates the peripheral immune response in operable stage I non-small-cell lung cancer. Sci Rep. 7:48662017. View Article : Google Scholar : PubMed/NCBI | |
|
Lockney NA, Zhang M, Morris CG, Nichols RC, Okunieff P, Swarts S, Zhang Z, Zhang B, Zhang A and Hoppe BS: Radiation-induced tumor immunity in patients with non-small cell lung cancer. Thorac Cancer. 10:1605–1611. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Lei QQ, Sui JD, Jin F, Luo HL, Shan JJ, Tang L, Wang Y and Wu YZ: Impact of high-dose rate radiotherapy on B and natural killer (NK) cell polarization in peripheral blood mononuclear cells (PBMCs) via inducing non-small cell lung cancer (NSCLC)-derived exosomes. Transl Cancer Res. 10:3538–3547. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Chen Y, Gao M, Huang Z, Yu J and Meng X: SBRT combined with PD-1/PD-L1 inhibitors in NSCLC treatment: A focus on the mechanisms, advances, and future challenges. J Hematol Oncol. 13:1052020. View Article : Google Scholar : PubMed/NCBI | |
|
Breen WG, Leventakos K, Dong H and Merrell KW: Radiation and immunotherapy: Emerging mechanisms of synergy. J Thorac Dis. 12:7011–7023. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Heinzerling JH, Mileham KF and Simone CB II: The utilization of immunotherapy with radiation therapy in lung cancer: A narrative review. Transl Cancer Res. 10:2596–2608. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
He J and Hu Q: Progress in the clinical application of immune checkpoint inhibitors in small cell lung cancer. Front Immunol. 14:11265822023. View Article : Google Scholar : PubMed/NCBI | |
|
Luna J, Zafra J, Areses Manrique MC, Rodríguez A, Sotoca A, Fírvida JL, Chicas-Sett R, Mielgo X, Reyes JCT and Couñago F: New challenges in the combination of radiotherapy and immunotherapy in non-small cell lung cancer. World J Clin Oncol. 12:983–999. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Schlick B, Shields MD, Marin-Acevedo JA, Patel I and Pellini B: Immune checkpoint inhibitors and chemoradiation for limited-stage small cell lung cancer. Curr Treat Options Oncol. 23:1104–1120. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Azghadi S and Daly ME: Radiation and immunotherapy combinations in non-small cell lung cancer. Cancer Treat Res Commun. 26:1002982021. View Article : Google Scholar : PubMed/NCBI | |
|
Chicas-Sett R, Zafra-Martin J, Morales-Orue I, Castilla-Martinez J, Berenguer-Frances MA, Gonzalez-Rodriguez E, Rodriguez-Abreu D and Couñago F: Immunoradiotherapy as an effective therapeutic strategy in lung cancer: From palliative care to curative intent. Cancers (Basel). 12:21782020. View Article : Google Scholar : PubMed/NCBI | |
|
Oweida A, Hararah MK, Phan A, Binder D, Bhatia S, Lennon S, Bukkapatnam S, Van Court B, Uyanga N, Darragh L, et al: Resistance to radiotherapy and PD-L1 blockade is mediated by TIM-3 upregulation and regulatory T-cell infiltration. Clin Cancer Res. 24:5368–5380. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Della Corte CM, Ciaramella V, Ramkumar K, Vicidomini G, Fiorelli A, Nardone V, Cappabianca S, Cozzolino I, Zito Marino F, Di Guida G, et al: Triple blockade of Ido-1, PD-L1 and MEK as a potential therapeutic strategy in NSCLC. J Transl Med. 20:5412022. View Article : Google Scholar : PubMed/NCBI | |
|
Han MG, Wee CW, Kang MH, Kim MJ, Jeon SH and Kim IA: Combination of OX40 co-stimulation, radiotherapy, and PD-1 inhibition in a syngeneic murine triple-negative breast cancer model. Cancers (Basel). 14:26922022. View Article : Google Scholar : PubMed/NCBI | |
|
Pieper AA, Zangl LM, Speigelman DV, Feils AS, Hoefges A, Jagodinsky JC, Felder MA, Tsarovsky NW, Arthur IS, Brown RJ, et al: Radiation augments the local anti-tumor effect of in situ vaccine with CpG-oligodeoxynucleotides and anti-OX40 in immunologically cold tumor models. Front Immunol. 12:7638882021. View Article : Google Scholar : PubMed/NCBI | |
|
Giaccone G, Bazhenova LA, Nemunaitis J, Tan M, Juhász E, Ramlau R, van den Heuvel MM, Lal R, Kloecker GH, Eaton KD, et al: A phase III study of belagenpumatucel-L, an allogeneic tumour cell vaccine, as maintenance therapy for non-small cell lung cancer. Eur J Cancer. 51:2321–2329. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
O'Brien MER, Anderson H, Kaukel E, O'Byrne K, Pawlicki M, Von Pawel J and Reck M; SR-ON-12 Study Group, : SRL172 (killed Mycobacterium vaccae) in addition to standard chemotherapy improves quality of life without affecting survival, in patients with advanced non-small-cell lung cancer: Phase III results. Ann Oncol. 15:906–914. 2004. View Article : Google Scholar : PubMed/NCBI | |
|
Zhou L, Wang XL, Deng QL, Du YQ and Zhao NQ: The efficacy and safety of immunotherapy in patients with advanced NSCLC: A systematic review and meta-analysis. Sci Rep. 6:320202016. View Article : Google Scholar : PubMed/NCBI | |
|
Xiong W, Zhao Y, Du H and Guo X: Current status of immune checkpoint inhibitor immunotherapy for lung cancer. Front Oncol. 11:7043362021. View Article : Google Scholar : PubMed/NCBI | |
|
Schild SE, Wang X, Bestvina CM, Williams T, Masters G, Singh AK, Stinchcombe TE, Salama JK, Wolf S, Zemla T, et al: Alliance A082002-a randomized phase II/III trial of modern immunotherapy-based systemic therapy with or without SBRT for PD-L1-negative, advanced non-small cell lung cancer. Clin Lung Cancer. 23:e317–e320. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Butts C, Socinski MA, Mitchell PL, Thatcher N, Havel L, Krzakowski M, Nawrocki S, Ciuleanu TE, Bosquée L, Trigo JM, et al: Tecemotide (L-BLP25) versus placebo after chemoradiotherapy for stage III non-small-cell lung cancer (START): A randomised, double-blind, phase 3 trial. Lancet Oncol. 15:59–68. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
Kumar SS, Higgins KA and McGarry RC: Emerging therapies for stage III non-small cell lung cancer: stereotactic body radiation therapy and immunotherapy. Front Oncol. 7:1972017. View Article : Google Scholar : PubMed/NCBI | |
|
Modi C, Berim L, Isserow L, Malhotra J, Patel M, Langenfeld J, Aisner J, Almeldin D and Jabbour SK: Combining radiation therapy and immunotherapy for lung cancers: A narrative review. Shanghai Chest. 5:102021. View Article : Google Scholar : PubMed/NCBI | |
|
Shaverdian N, Lisberg AE, Bornazyan K, Veruttipong D, Goldman JW, Formenti SC, Garon EB and Lee P: Previous radiotherapy and the clinical activity and toxicity of pembrolizumab in the treatment of non-small-cell lung cancer: A secondary analysis of the KEYNOTE-001 phase 1 trial. Lancet Oncol. 18:895–903. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Thompson M and Rosenzweig KE: The evolving toxicity profile of SBRT for lung cancer. Transl Lung Cancer Res. 8:48–57. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Murray L, Karakaya E, Hinsley S, Naisbitt M, Lilley J, Snee M, Clarke K, Musunuru HB, Ramasamy S, Turner R and Franks K: Lung stereotactic ablative radiotherapy (SABR): Dosimetric considerations for chest wall toxicity. Br J Radiol. 89:201506282016. View Article : Google Scholar : PubMed/NCBI | |
|
Welsh J, Thomas J, Shah D, Allen PK, Wei X, Mitchell K, Gao S, Balter P, Komaki R and Chang JY: Obesity increases the risk of chest wall pain from thoracic stereotactic body radiation therapy. Int J Radiat Oncol Biol Phys. 81:91–96. 2011. View Article : Google Scholar : PubMed/NCBI | |
|
Owen D and Sio TT: Stereotactic body radiotherapy (SBRT) for central and ultracentral node-negative lung tumors. J Thorac Dis. 12:7024–7031. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Schneider BJ, Daly ME, Kennedy EB, Antonoff MB, Broderick S, Feldman J, Jolly S, Meyers B, Rocco G, Rusthoven C, et al: Stereotactic body radiotherapy for early-stage non-small-cell lung cancer: American society of clinical oncology endorsement of the American society for radiation oncology evidence-based guideline. J Clin Oncol. 36:710–719. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Naidoo J, Wang X, Woo KM, Iyriboz T, Halpenny D, Cunningham J, Chaft JE, Segal NH, Callahan MK, Lesokhin AM, et al: Pneumonitis in patients treated with anti-programmed death-1/programmed death ligand 1 therapy. J Clin Oncol. 35:709–717. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Tian S, Switchenko JM, Buchwald ZS, Patel PR, Shelton JW, Kahn SE, Pillai RN, Steuer CE, Owonikoko TK, Behera M, et al: Lung stereotactic body radiation therapy and concurrent immunotherapy: A multicenter safety and toxicity analysis. Int J Radiat Oncol Biol Phys. 108:304–313. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Korpics MC, Katipally RR, Partouche J, Cutright D, Pointer KB, Bestvina CM, Luke JJ, Pitroda SP, Dignam JJ, Chmura SJ and Juloori A: Predictors of pneumonitis in combined thoracic stereotactic body radiation therapy and immunotherapy. Int J Radiat Oncol Biol Phys. 114:645–654. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Simone CB II, Bogart JA, Cabrera AR, Daly ME, DeNunzio NJ, Detterbeck F, Faivre-Finn C, Gatschet N, Gore E, Jabbour SK, et al: Radiation therapy for small cell lung cancer: An ASTRO clinical practice guideline. Pract Radiat Oncol. 10:158–173. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Amin MB, Greene FL, Edge SB, Compton CC, Gershenwald JE, Brookland RK, Meyer L, Gress DM, Byrd DR and Winchester DP: The eighth edition AJCC cancer staging manual: Continuing to build a bridge from a population-based to a more ‘personalized’ approach to cancer staging. CA Cancer J Clin. 67:93–99. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Stahel RA, Ginsberg R, Havemann K, Hirsch FR, Ihde DC, Jassem J, Karrer K, Maurer LH, Osterlind K and van Houtte P: Staging and prognostic factors in small cell lung cancer: A consensus report. Lung Cancer. 5:119–126. 1989. View Article : Google Scholar | |
|
Jones GS, Elimian K, Baldwin DR, Hubbard RB and McKeever TM: A systematic review of survival following anti-cancer treatment for small cell lung cancer. Lung Cancer. 141:44–55. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Verma V, Simone CB II, Allen PK, Gajjar SR, Shah C, Zhen W, Harkenrider MM, Hallemeier CL, Jabbour SK, Matthiesen CL, et al: Multi-institutional experience of stereotactic ablative radiation therapy for stage I small cell lung cancer. Int J Radiat Oncol Biol Phys. 97:362–371. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Pakkala S, Higgins K, Chen Z, Sica G, Steuer C, Zhang C, Zhang G, Wang S, Hossain MS, Nazha B, et al: Durvalumab and tremelimumab with or without stereotactic body radiation therapy in relapsed small cell lung cancer: A randomized phase II study. J Immunother Cancer. 8:e0013022020. View Article : Google Scholar : PubMed/NCBI | |
|
Luke JJ, Lemons JM, Karrison TG, Pitroda SP, Melotek JM, Zha Y, Al-Hallaq HA, Arina A, Khodarev NN, Janisch L, et al: Safety and clinical activity of pembrolizumab and multisite stereotactic body radiotherapy in patients with advanced solid tumors. J Clin Oncol. 36:1611–1618. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Theelen WSEM, Peulen HMU, Lalezari F, van der Noort V, de Vries JF, Aerts JGJV, Dumoulin DW, Bahce I, Niemeijer AN, de Langen AJ, et al: Effect of pembrolizumab after stereotactic body radiotherapy vs pembrolizumab alone on tumor response in patients with advanced non-small cell lung cancer: Results of the PEMBRO-RT phase 2 randomized clinical trial. JAMA Oncol. 5:1276–1282. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Chen D, Patel RR, Verma V, Ramapriyan R, Barsoumian HB, Cortez MA and Welsh JW: Interaction between lymphopenia, radiotherapy technique, dosimetry, and survival outcomes in lung cancer patients receiving combined immunotherapy and radiotherapy. Radiother Oncol. 150:114–120. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD, Schadendorf D, Dummer R, Smylie M, Rutkowski P, et al: Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 373:23–34. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Huang YS, Li Z, Xiao ZF, Li D and Liu WY: Case report: Radiotherapy plus pneumococcal conjugate vaccine stimulates abscopal immune response in a patient with ALK+ NSCLC. Front Immunol. 13:9502522022. View Article : Google Scholar : PubMed/NCBI | |
|
de Sousa LG, McGrail DJ, Li K, Marques-Piubelli ML, Gonzalez C, Dai H, Ferri-Borgogno S, Godoy M, Burks J, Lin SY, et al: Spontaneous tumor regression following COVID-19 vaccination. J Immunother Cancer. 10:e0043712022. View Article : Google Scholar | |
|
Papachristofilou A, Hipp MM, Klinkhardt U, Früh M, Sebastian M, Weiss C, Pless M, Cathomas R, Hilbe W, Pall G, et al: Phase Ib evaluation of a self-adjuvanted protamine formulated mRNA-based active cancer immunotherapy, BI1361849 (CV9202), combined with local radiation treatment in patients with stage IV non-small cell lung cancer. J Immunother Cancer. 7:382019. View Article : Google Scholar : PubMed/NCBI | |
|
Román-Jobacho A, Hernández-Miguel M, García-Anaya MJ, Gómez-Millán J, Medina-Carmona JA and Otero-Romero A: Oligometastatic non-small cell lung cancer: Current management. J Clin Transl Res. 7:311–319. 2021.PubMed/NCBI | |
|
Al-Shafa F, Arifin AJ, Rodrigues GB, Palma DA and Louie AV: A Review of ongoing trials of stereotactic ablative radiotherapy for oligometastatic cancers: Where will the evidence lead? Front Oncol. 9:5432019. View Article : Google Scholar : PubMed/NCBI | |
|
Milano MT, Biswas T, Simone CB II and Lo SS: Oligometastases: History of a hypothesis. Ann Palliat Med. 10:5923–5930. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Bates JE and Milano MT: Prognostic significance of sites of extrathoracic metastasis in patients with non-small cell lung cancer. J Thorac Dis. 9:1903–1910. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Hendriks LE, Derks JL, Postmus PE, Damhuis RA, Houben RM, Troost EG, Hochstenbag MM, Smit EF and Dingemans AM: Single organ metastatic disease and local disease status, prognostic factors for overall survival in stage IV non-small cell lung cancer: Results from a population-based study. Eur J Cancer. 51:2534–2544. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Gomez DR, Tang C, Zhang J, Blumenschein GR Jr, Hernandez M, Lee JJ, Ye R, Palma DA, Louie AV, Camidge DR, et al: Local consolidative therapy vs maintenance therapy or observation for patients with oligometastatic non-small-cell lung cancer: Long-term results of a multi-institutional, phase II, randomized study. J Clin Oncol. 37:1558–1565. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Iyengar P, Wardak Z, Gerber DE, Tumati V, Ahn C, Hughes RS, Dowell JE, Cheedella N, Nedzi L, Westover KD, et al: Consolidative radiotherapy for limited metastatic non-small-cell lung cancer: A phase 2 randomized clinical trial. JAMA Oncol. 4:e1735012018. View Article : Google Scholar : PubMed/NCBI | |
|
Brandão M, Durieux V and Berghmans T: Current and future research efforts in oligometastatic non-small cell lung cancer-a systematic review. Transl Lung Cancer Res. 10:3473–3485. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Bauml JM, Mick R, Ciunci C, Aggarwal C, Davis C, Evans T, Deshpande C, Miller L, Patel P, Alley E, et al: Pembrolizumab after completion of locally ablative therapy for oligometastatic non-small cell lung cancer: A phase 2 trial. JAMA Oncol. 5:1283–1290. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Watanabe T, Firat E, Scholber J, Gaedicke S, Heinrich C, Luo R, Ehrat N, Multhoff G, Schmitt-Graeff A, Grosu AL, et al: Deep abscopal response to radiotherapy and anti-PD-1 in an oligometastatic melanoma patient with unfavorable pretreatment immune signature. Cancer Immunol Immunother. 69:1823–1832. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Griswold CR, Kerrigan K and Patel SB: Combination of local ablative therapy and continuation of immune checkpoint inhibitor (ICI) therapy provides durable treatment response past oligometastatic progression in NSCLC: A case report. Case Rep Oncol. 12:866–871. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Cho BC, Abreu DR, Hussein M, Cobo M, Patel AJ, Secen N, Lee KH, Massuti B, Hiret S, Yang JCH, et al: Tiragolumab plus atezolizumab versus placebo plus atezolizumab as a first-line treatment for PD-L1-selected non-small-cell lung cancer (CITYSCAPE): Primary and follow-up analyses of a randomised, double-blind, phase 2 study. Lancet Oncol. 23:781–792. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Lieverse RIY, Van Limbergen EJ, Oberije CJG, Troost EGC, Hadrup SR, Dingemans AC, Hendriks LEL, Eckert F, Hiley C, Dooms C, et al: Stereotactic ablative body radiotherapy (SABR) combined with immunotherapy (L19-IL2) versus standard of care in stage IV NSCLC patients, ImmunoSABR: A multicentre, randomised controlled open-label phase II trial. BMC Cancer. 20:5572020. View Article : Google Scholar : PubMed/NCBI | |
|
Van Limbergen EJ, Hoeben A, Lieverse RIY, Houben R, Overhof C, Postma A, Zindler J, Verhelst F, Dubois LJ, De Ruysscher D, et al: Toxicity of L19-interleukin 2 combined with stereotactic body radiation therapy: A phase 1 study. Int J Radiat Oncol Biol Phys. 109:1421–1430. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Liu Y, Jiang S, Lin Y, Yu H, Yu L and Zhang X: Research landscape and trends of lung cancer radiotherapy: A bibliometric analysis. Front Oncol. 12:10665572022. View Article : Google Scholar : PubMed/NCBI | |
|
Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, Yokoi T, Chiappori A, Lee KH, de Wit M, et al: Durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. N Engl J Med. 377:1919–1929. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Feddock J, Arnold SM, Shelton BJ, Sinha P, Conrad G, Chen L, Rinehart J and McGarry RC: Stereotactic body radiation therapy can be used safely to boost residual disease in locally advanced non-small cell lung cancer: A prospective study. Int J Radiat Oncol Biol Phys. 85:1325–1331. 2013. View Article : Google Scholar : PubMed/NCBI | |
|
Wang Z, Qiang Y, Shen Q, Zhu XX and Song Y: Neoadjuvant programmed cell death protein 1 blockade combined with stereotactic body radiation therapy for stage III(N2) non-small cell lung cancer: A case series. Front Oncol. 12:7792512022. View Article : Google Scholar : PubMed/NCBI | |
|
Luna J, Sotoca A, Fernández P, Miralles C and Rodríguez A: Recent advances in early stage lung cancer. J Clin Transl Res. 7:163–174. 2021.PubMed/NCBI | |
|
Ball D, Mai GT, Vinod S, Babington S, Ruben J, Kron T, Chesson B, Herschtal A, Vanevski M, Rezo A, et al: Stereotactic ablative radiotherapy versus standard radiotherapy in stage 1 non-small-cell lung cancer (TROG 09.02 CHISEL): A phase 3, open-label, randomised controlled trial. Lancet Oncol. 20:494–503. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Daly ME, Redman M, Simone CB, Monjazeb AM, Bauman JR, Hesketh P, Feliciano J, Kashani R, Steuer C, Ganti AK, et al: SWOG/NRG S1914: A randomized phase III trial of induction/consolidation atezolizumab + SBRT vs SBRT alone in high risk, early-stage NSCLC (NCT#04214262). Int J Radiat Oncol Biol Phys. 114:e4142022. View Article : Google Scholar | |
|
Hallqvist A, Koyi H, de Petris L, Lindberg K, Farooqi S, Helland Å, Wikström A, Johansson M, Planck M, Lindberg L, et al: 63MO Safety analysis of durvalumab following stereotactic body radiotherapy (SBRT) in early-stage non-small cell lung cancer (NSCLC) patients: A first report of a randomized phase II trial (ASTEROID). J Thorac Oncol. 16 (Suppl):S729–S730. 2021. View Article : Google Scholar | |
|
Bezjak A, Paulus R, Gaspar LE, Timmerman RD, Straube WL, Ryan WF, Garces YI, Pu AT, Singh AK, Videtic GM, et al: Safety and efficacy of a five-fraction stereotactic body radiotherapy schedule for centrally located non-small-cell lung cancer: NRG oncology/RTOG 0813 trial. J Clin Oncol. 37:1316–1325. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Sun B, Brooks ED, Komaki RU, Liao Z, Jeter MD, McAleer MF, Allen PK, Balter PA, Welsh JD, O'Reilly MS, et al: 7-year follow-up after stereotactic ablative radiotherapy for patients with stage I non-small cell lung cancer: Results of a phase 2 clinical trial. Cancer. 123:3031–3039. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Verma V and Simone CB II: Approaches to stereotactic body radiation therapy for large (≥5 centimeter) non-small cell lung cancer. Transl Lung Cancer Res. 8:70–77. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Ichinose Y, Yano T, Yokoyama H, Inoue T, Asoh H and Katsuda Y: The correlation between tumor size and lymphatic vessel invasion in resected peripheral stage I non-small-cell lung cancer. A potential risk of limited resection. J Thorac Cardiovasc Surg. 108:684–686. 1994. View Article : Google Scholar : PubMed/NCBI | |
|
Strauss GM, Herndon JE II, Maddaus MA, Johnstone DW, Johnson EA, Harpole DH, Gillenwater HH, Watson DM, Sugarbaker DJ, Schilsky RL, et al: Adjuvant paclitaxel plus carboplatin compared with observation in stage IB non-small-cell lung cancer: CALGB 9633 with the cancer and leukemia group B, radiation therapy oncology group, and north central cancer treatment group study groups. J Clin Oncol. 26:5043–5051. 2008. View Article : Google Scholar : PubMed/NCBI | |
|
Pignon JP, Tribodet H, Scagliotti GV, Douillard JY, Shepherd FA, Stephens RJ, Dunant A, Torri V, Rosell R, Seymour L, et al: Lung adjuvant cisplatin evaluation: A pooled analysis by the LACE collaborative group. J Clin Oncol. 26:3552–3559. 2008. View Article : Google Scholar : PubMed/NCBI | |
|
National Comprehensive Cancer Network, . Non-small cell lung cancer. (version 2.2024). 2024. | |
|
Brahmer J, Reckamp KL, Baas P, Crinò L, Eberhardt WE, Poddubskaya E, Antonia S, Pluzanski A, Vokes EE, Holgado E, et al: Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 373:123–135. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Zhao L, Liu C, Xie G, Wu F and Hu C: Multiple primary lung cancers: A new challenge in the era of precision medicine. Cancer Manag Res. 12:10361–10374. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Boyle JM, Tandberg DJ, Chino JP, D'Amico TA, Ready NE and Kelsey CR: Smoking history predicts for increased risk of second primary lung cancer: A comprehensive analysis. Cancer. 121:598–604. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Dovedi SJ, Adlard AL, Lipowska-Bhalla G, McKenna C, Jones S, Cheadle EJ, Stratford IJ, Poon E, Morrow M, Stewart R, et al: Acquired resistance to fractionated radiotherapy can be overcome by concurrent PD-L1 blockade. Cancer Res. 74:5458–5468. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
Woody S, Hegde A, Arastu H, Peach MS, Sharma N, Walker P and Ju AW: Survival is worse in patients completing immunotherapy prior to SBRT/SRS compared to those receiving it concurrently or after. Front Oncol. 12:7853502022. View Article : Google Scholar : PubMed/NCBI | |
|
Bestvina CM, Pointer KB, Karrison T, Al-Hallaq H, Hoffman PC, Jelinek MJ, Juloori A, Melotek JM, Murgu S, Partouche J, et al: A phase 1 trial of concurrent or sequential ipilimumab, nivolumab, and stereotactic body radiotherapy in patients with stage IV NSCLC study. J Thorac Oncol. 17:130–140. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Welsh JW, Tang C, de Groot P, Naing A, Hess KR, Heymach JV, Papadimitrakopoulou VA, Cushman TR, Subbiah V, Chang JY, et al: Phase II trial of ipilimumab with stereotactic radiation therapy for metastatic disease: Outcomes, toxicities, and low-dose radiation-related abscopal responses. Cancer Immunol Res. 7:1903–1909. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Windisch P and Guckenberger M: Randomized phase II trial reporting overall survival advantage by adding local consolidative therapy to systemic therapy for oligometastatic non-small cell lung cancer: Another step forward on the long road of evidence-based medicine for oligometastatic disease. J Thorac Dis. 11 (Suppl 15):S1869–S1873. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Mitchell P, Thatcher N, Socinski MA, Wasilewska-Tesluk E, Horwood K, Szczesna A, Martín C, Ragulin Y, Zukin M, Helwig C, et al: Tecemotide in unresectable stage III non-small-cell lung cancer in the phase III START study: updated overall survival and biomarker analyses. Ann Oncol. 26:1134–1142. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Patel JD, Lee JW, Carbone DP, Wagner H, Shanker A, de Aquino MTP, Horn L, Johnson ML, Gerber DE, Liu JJ, et al: P Phase II study of immunotherapy with tecemotide and bevacizumab after chemoradiation in patients with unresectable stage III non-squamous non-small-cell lung cancer (NS-NSCLC): A trial of the ECOG-ACRIN cancer research group (E6508). Clin Lung Cancer. 21:520–526. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Alite F, Shaikh PM and Mahadevan A: Influence of dexamethasone premedication on acute lung toxicity in lung SBRT. Front Oncol. 12:8375772022. View Article : Google Scholar : PubMed/NCBI | |
|
Arbour KC, Mezquita L, Long N, Rizvi H, Auclin E, Ni A, Martínez-Bernal G, Ferrara R, Lai WV, Hendriks LEL, et al: Impact of baseline steroids on efficacy of programmed cell death-1 and programmed death-ligand 1 blockade in patients with non-small-cell lung cancer. J Clin Oncol. 36:2872–2878. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Im SJ, Hashimoto M, Gerner MY, Lee J, Kissick HT, Burger MC, Shan Q, Hale JS, Lee J, Nasti TH, et al: Defining CD8+ T cells that provide the proliferative burst after PD-1 therapy. Nature. 537:417–421. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Floudas CS, Brar G, Mabry-Hrones D, Duffy AG, Wood B, Levy E, Krishnasamy V, Fioravanti S, Bonilla CM, Walker M, et al: A pilot study of the PD-1 targeting agent AMP-224 used with low-dose cyclophosphamide and stereotactic body radiation therapy in patients with metastatic colorectal cancer. Clin Colorectal Cancer. 18:e349–e360. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Nikitas J, Roach M, Robinson C, Bradley J, Huang J, Perkins S, Tsien C and Abraham C: Treatment of oligometastatic lung cancer with brain metastases using stereotactic radiosurgery (SRS) and stereotactic body radiation therapy (SBRT). Clin Transl Radiat Oncol. 21:32–35. 2019.PubMed/NCBI | |
|
Rodríguez Plá M, Dualde Beltrán D and Ferrer Albiach E: Immune checkpoints inhibitors and SRS/SBRT synergy in metastatic non-small-cell lung cancer and melanoma: A systematic review. Int J Mol Sci. 22:116212021. View Article : Google Scholar : PubMed/NCBI | |
|
Shields MD, Chen K, Dutcher G, Patel I and Pellini B: Making the rounds: Exploring the role of circulating tumor DNA (ctDNA) in non-small cell lung cancer. Int J Mol Sci. 23:90062022. View Article : Google Scholar : PubMed/NCBI | |
|
Spaas M, Sundahl N, Hulstaert E, Kruse V, Rottey S, De Maeseneer D, Surmont V, Meireson A, Brochez L, Reynders D, et al: Checkpoint inhibition in combination with an immunoboost of external beam radiotherapy in solid tumors (CHEERS): Study protocol for a phase 2, open-label, randomized controlled trial. BMC Cancer. 21:5142021. View Article : Google Scholar : PubMed/NCBI | |
|
Ni J, Yang L, Zhu H, Chu M, Zhang C, Zhao W, Yang M, Xu X, Zheng E, Jiang X, et al: A patient with metastatic non-small cell lung cancer who received pembrolizumab monotherapy after stereotactic body radiotherapy had progression-free survival of nearly 5 years: A case report. Ann Palliat Med. 10:4999–5009. 2021. View Article : Google Scholar : PubMed/NCBI |