Bioavailability and safety study of resveratrol 500 mg tablets in healthy male and female volunteers
- Authors:
- Published online on: November 25, 2015 https://doi.org/10.3892/etm.2015.2895
- Pages: 164-170
Abstract
Introduction
Resveratrol (3,4′,5-trihydroxy-trans-stilbene) is a polyphenolic phytoalexin that is naturally produced in plants as a response to injuries or stresses, such as a pathogenic attack or irradiation with ultraviolet light. This compound is found in grapes, peanuts, berries, groundnut, spruce, mulberries and dried roots of Polygonum cuspidatum (also known as kojo-kon in Japanese), which have long been used in traditional oriental medicine (1,2). In plants, resveratrol inhibits the development of an infection (3). In humans, it has several beneficial effects due to its antioxidant, anti-inflammatory, anticarcinogenic, antidiabetic, cardioprotective, estrogenic and anti-aging properties (4–6). Resveratrol is widely available in the human diet, although exposure to this substance is enhanced upon ingestion of grapes, red wine and peanuts (4–13). It has been intensively studied in clinical and nonclinical trials, and was found to act on a number of different targets using various mechanisms of action that can explain its diverse biological activities, including: Free-radical scavenging activity (14), anti-inflammatory activity (5,9,11,12,14,15), prevention of tumour growth and anticancer activity (6–9,16), estrogenic activity (11), inhibition of lipid peroxidation, modulation of lipid metabolism (9), chelation of copper and inhibition of platelet aggregation (9,13).
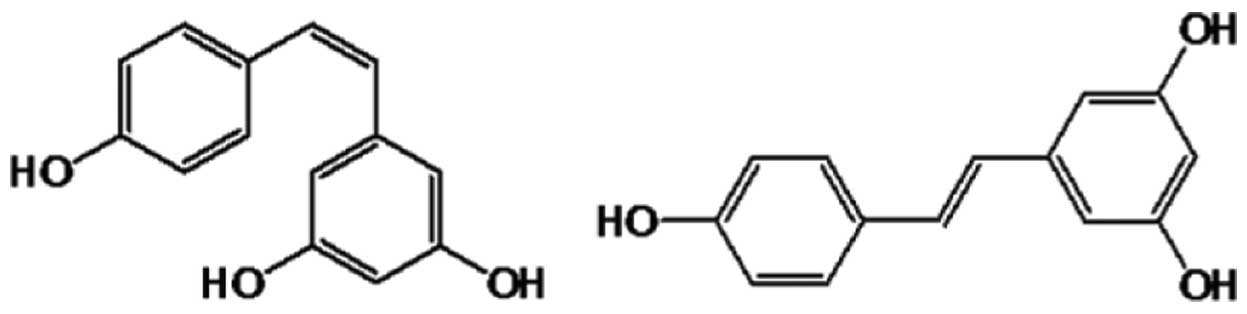
Resveratrol exists as two geometric isomers: Cis and trans (Fig. 1) (8,9). The trans form is able to undergo isomerization into the cis form upon exposure to ultraviolet irradiation (9). Although the two isomers often exist in combination, the trans form is more biologically active and more frequently investigated (8,9,17,18). Red wine is known to contain a high concentration of trans-resveratrol. The levels of trans- and cis-resveratrol in 26 wines were evaluated by Gu et al in 1999 (17), and trans-resveratrol levels ranged between 0.987 and 25.4 µmol/l, while cis-resveratrol levels were considerably lower.
Previous in vitro data have shown that 5 µmol/l resveratrol is the minimum concentration required for the chemopreventive effects of the compound to be elicited (15). Following oral administration in humans, 75% of resveratrol is absorbed, possibly by transepithelial diffusion. However, oral bioavailability is low (<1%) due to rapid and extensive metabolism in the intestine and liver (18,19). These levels are maintained even after repeated or increased dosage administration. The major metabolites identified in the plasma and urine by metabolic studies are resveratrol glucuronides and sulphates (20–22). Other metabolites of resveratrol have been reported in the literature, including reduced dihydro-resveratrol conjugates and other unknown highly polar products (21,23). As already mentioned, the major sites of metabolism are the intestine and liver; however, colonic bacterial metabolism may be an important metabolic pathway (24). The efficacy of resveratrol at the target site may be enhanced by deconjugating enzymes, such as β-glucuronidase and sulphatase, as well as by specific tissue accumulation of resveratrol (24). Lu et al (25) showed that resveratrol is delivered as a stable sulphate conjugated form to the target tissues, where the parent compound is gradually regenerated and provides the beneficial in vivo effects. The activities of the metabolites have also been investigated. The metabolites were found to be pharmacologically active and thus are considered to contribute to the in vivo biological effects of the parent compound (6,25).
Considering all the beneficial effects of resveratrol on human health, drug supplements containing resveratrol have been developed. The aim of the present study was to assess the pharmacokinetic properties and safety of resveratrol following a 500 mg single oral dose (one Evelor 500 mg tablet; Agetis Supplements Ltd., Limassol, Cyprus) administered to fasting healthy male and female subjects. In order to evaluate the bioavailability of resveratrol, plasma concentration-time curves of the parent compound and its metabolites (glucuronides, sulphates and mixed conjugates) were used to assess the rate and extent of absorption.
Materials and methods
Study design
The study was an open label, one period, one sequence, noncontrolled, bioavailability study of 500 mg resveratrol, which was performed on healthy male and female volunteers under fasting conditions. The study was approved by an independent Ethics Committee (County Ethics Committee for Drug Clinical Studies, Suceava, Romania) and was performed respecting the Good Clinical Practice requirements. A written informed consent was obtained from each subject prior to participation in the study.
Study population
A total of 15 healthy male and female subjects, with an age of 18–55 years and body mass index (BMI) of 20–30 kg/m2, were enrolled in the study. Demographic characteristics are presented in Table I. The subjects were selected based on the following eligibility criteria: Normal physical examination, vital signs and laboratory screening results within normal ranges, willingness to abstain from specific aliments and drink beverages, ability to understand the full nature and purpose of the study, and nonpregnant and nonlactating women. The exclusion criteria included the following: History of hypersensitivity to the test substance and to the inactive ingredients, hospitalization for any reason or donation of blood (≥450 ml) within 8 weeks prior to the initiation of the study, intake of any drugs within 2 weeks prior to or during the study, history or presence of any relevant medical condition, history of drug or alcohol abuse, and subjects that were vegetarian or followed a particular diet.
Three days prior to the study and until the end of the study period (24 h post-dose), the volunteers kept a strict diet. The subjects did not consume aliments or beverages with a high content of resveratrol, including red/black fruit, kiwi, red/black grapes, peanuts, nuts and red wine. In addition, the diet was strictly controlled during the entire confinement period. The subjects received standard light meals that avoided aliments containing high quantities of resveratrol.
Resveratrol dose
The test product used in the present study was Evelor 500 mg tablets (containing trans-resveratrol), which were obtained from Agetis Supplements Ltd. These tablets were manufactured under the Good Manufacturing Practice guidelines and according to a patented procedure (26).
Each subject received one 500-mg tablet of Evelor subsequent to overnight fasting. Evelor was administered orally with 200 ml water under the direct observation of the clinical investigator.
Sample preparation
During the treatment day, about 5 ml venous blood samples were drawn in labelled tubes containing lithium heparin as an anticoagulant (Monovettes®; Sarstedt, Nümbrecht, Germany), at the following time points: Prior to resveratrol administration, and at 0.083, 0.167, 0.33, 0.5, 0.75, 1.0, 2.0, 3.0, 4.0, 5.0, 6.0, 7.0, 8.0, 10.0, 12.0, 16.0 and 24.0 h after resveratrol administration.
Each blood sample containing heparin was immediately centrifuged at 1,500 × g for 5 min at 4°C. The plasma was separated and placed in duplicate labelled test tubes (two aliquots per sample). Subsequently, the test tubes were securely closed, and the plasma samples were racked, frozen and stored at −20°C or cooler until tested.
Bioanalytical method
The identification and quantification of resveratrol and its metabolites in plasma was performed by high performance liquid chromatography (HPLC) (27) with detection by tandem mass spectrometry (MS/MS), using a triple quadrupole AB-Sciex model API 4000 mass spectrometer (AB Sciex, Ontario, Canada), equipped with an atmospheric pressure electrospray ionization interface. Analyses were performed on a multiplexing HPLC system controlled by Cohesive Aria software (Thermo Fisher Scientific, San Jose, CA, USA) and composed by two binary chromatographic pumps (Agilent 1100 Series, Agilent Technologies, Santa Clara, CA, USA) and an HTS PAL autosampler (CTC Analytics, Zwingen, Switzerland). Data were acquired in negative ions mode using a multiple reaction monitoring method (27).
Chromatographic separations were performed on Discovery HSC18 chromatographic columns (100 × 2.1 mm, 5 µm; Supelco, Sigma-Aldrich, Bellefonte, PA, USA). For data acquisition and processing, the Analyst software (version 1.6; AB Sciex) was used.
The analytical standard of resveratrol used to prepare the calibration curves and quality control samples was purchased from Sigma-Aldrich. The internal standard added during extraction (resveratrol-13C6) was obtained from TLC Pharmaceutical Standards, Ltd. (Vaughan, Ontario, Canada)
Pharmacokinetic parameters
The parameters calculated for resveratrol and the metabolites using a noncompartmental approach were as follows: AUC0-t, which is the area under the curve that was integrated (by the trapezoidal rule) from the plasma concentrations between time 0 h and the last quantifiable sample; Cmax, which is the maximum plasma concentration, obtained directly from the data without interpolation; Tmax, which is the time to reach the Cmax, obtained directly from the data without interpolation; AUC0-inf, which is the area under the curve that was integrated from the blood concentrations by extrapolation of the terminal elimination period; % extrapolated AUC, which is the ratio of (AUC0-inf - AUC0-t)/AUC0-inf × 100; T1/2, which is the plasma half life, calculated as 0.693/elimination rate constant; MRT, which is the mean residence time, calculated as AUMCinf/AUC0-inf, where AUMCinf is the area under the moment curve.
Safety assessment
Safety assessments were then performed. In particular, adverse events were monitored from the screening visit for the entire duration of the study. Screening and follow-up safety tests included physical examinations, electrocardiograms and investigation of clinical laboratory parameters, including hematology (red cells, hemoglobin, hematocrit, white cells, differential count and platelet count), clinical chemistry (uric acid, total nitrogen, glucose, creatinine, total bilirubine, aspartate transaminase, alanine transaminase, total cholesterol and triglyceride levels) and urinalysis examinations. Vital signs were also evaluated, including the sitting systolic arterial pressure, diastolic arterial pressure and heart rate. Pre-dose safety evaluation included analysis of the blood glucose levels, vital signs (in laying position), oxygen saturation and body temperature.
Statistical analysis
Bioavailability and statistical calculations were performed using the SAS software (version 9.1; SAS Analytical Solutions S.R.L., Bucharest, Romania). Descriptive statistics was performed for all parameters [arithmetic mean, harmonic mean, geometric mean, standard error of mean, standard deviation (SD), median and range]. Mean comparisons for the safety data were conducted using analysis of variance testing, with P<0.05 considered to indicate a statistically significant difference.
Results
Patient characteristics
All volunteers that were enrolled completed the study. The demographic characteristics of the subjects included in the present study are presented in Table I. The mean age of the patients was 31.40±10.80 years (age range, 19–50 years), the mean weight was 69.07±13.22 kg (weight range, 49–98 kg), the mean height was 172.13±9.80 cm (height range, 150–185 cm), and the mean BMI value was 23.13±2.79 (BMI range, 20.2–29.7). Mean values are presented as the mean ± SD.
Pharmacokinetic parameters
The pharmacokinetic parameters for resveratrol and its metabolites (glucuronides, sulphates and mixed conjugates) following oral administration of a single dose of 500 mg resveratrol were determined based on the plasma levels from 15 healthy volunteers.
The results indicated that the pharmacokinetic parameters for free resveratrol were as follows: Cmax, 71.18 ng/ml; Tmax, 1.3 h; and AUC0-inf, 179.1 ng/ml. The mean pharmacokinetic characteristics for resveratrol are presented in the Table II
In the case of sulphated resveratrol, the Cmax of 1,516.01 ng/ml was reached within 2.8 h (Tmax) and the AUC0-inf was found to be 14,441.7 ng/ml. The mean pharmacokinetic characteristics of sulphated resveratrol are listed in the Table III.
With regards to glucuronated resveratrol, the Cmax was 4,083.90 ng/ml and this was reached within 3.0 h (Tmax), while the AUC0-inf value was 39,732.4 ng/ml. The mean pharmacokinetic characteristics of glucuronated resveratrol are presented in the Table IV.
Furthermore, the pharmacokinetic parameters of mixed conjugates (glucuronide and sulphate metabolites) were also considered, and their values were reported as follows: Cmax, 5,440.4 ng/ml; Tmax, 3.2 h; and AUC0-inf, 63,536.6 ng/ml. The mean pharmacokinetic characteristics for mixed metabolites are presented in the Table V.
Table V.The mean pharmacokinetic characteristic of sulphated and glucuronated resveratrol (mixed conjugates). |
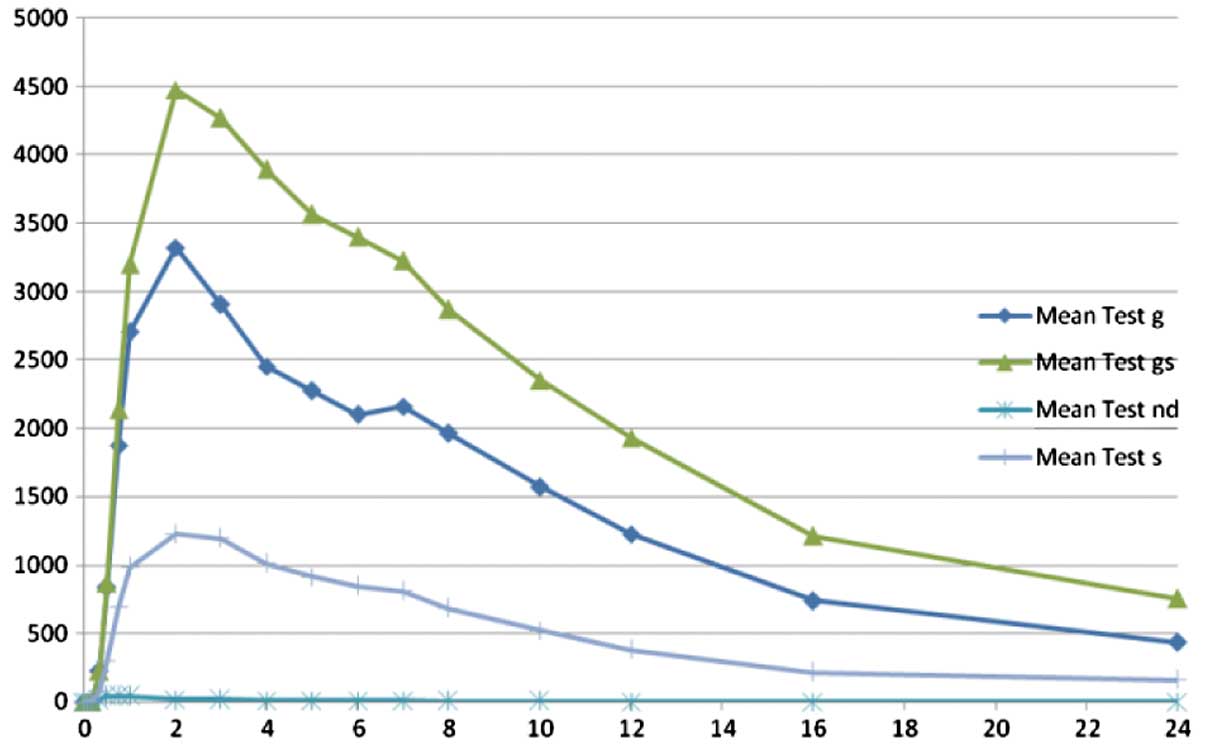
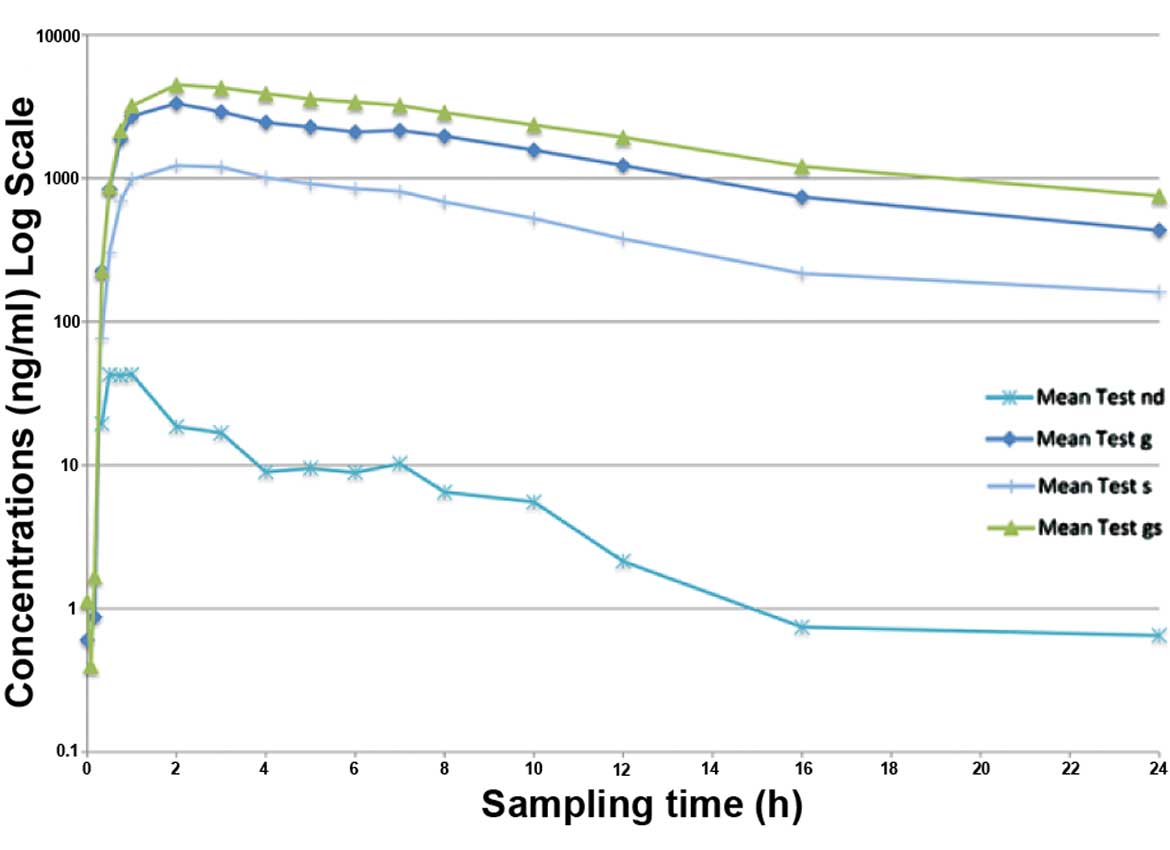
As already mentioned, low levels of resveratrol (parent compound) were identified when compared with the metabolite levels. The plasma concentration versus time curves for resveratrol and its metabolites are depicted in Figs. 2 and 3. In Fig. 2, as the plasma levels of resveratrol were low, the curve for resveratrol is superposing the 0 point. Fig. 3 is a magnified version of Fig. 2 that demonstrates the resveratrol levels, while the plasma levels of metabolites are presented only as a guide to show their high levels.
Safety results
The resveratrol 500 mg tablet was well-tolerated by all study participants. Only one adverse event (traumatic cutaneous wound) of moderate intensity occurred in 1 subject in the present study; however, this was not associated with the medication used in the study. No statistically significant differences in any of the safety parameters considered were observed at follow-up compared with the parameters at screening, with the exception of lower values of red blood cells and hematocrit at follow-up compared with their values at screening; however, these statistical differences are devoid of clinical significance.
Discussion
Previous studies have demonstrated that resveratrol is well-absorbed following oral administration, with ~75% of the dose absorbed. Following absorption, resveratrol undergoes rapid and extensive metabolism leading to low bioavailability (18,19). Resveratrol pharmacokinetics have shown circadian variation, with higher bioavailability after morning administration (18). Resveratrol is mainly metabolized in the intestines and liver, and its major metabolites are resveratrol glucuronides and sulphates (20–22). Colonic bacterial metabolism is also considered to be an important metabolic pathway (24). The plasma levels of the conjugated metabolites were reported to be higher compared with those of the parent compound in animal and in human studies (15,28).
Numerous beneficial effects of resveratrol have been reported, although low levels of this compound are detected in the plasma. According to Biasutto et al (29), up to 76% of resveratrol is not accounted for when only plasma is evaluated; the cellular fraction of resveratrol is missed if analysis of the whole blood is not performed and therefore its bioavailability is not accurately determined (29). According to data in the literature, the conjugate metabolites have relevant biological activities, and the efficacy of resveratrol is also mediated by its metabolites and through accumulation in specific tissues (24,30).
Studies in humans revealed that resveratrol was well-tolerated and the adverse events, if any, were mild in severity (18,19,22). Only high doses of resveratrol (2.5 and 5 g) were associated with mild to moderate gastrointestinal symptoms (22).
The purpose of the present study was to evaluate the rate and extent of absorption, and the safety of resveratrol 500 mg tablets. Plasma levels of resveratrol and its metabolites were determined in 15 healthy volunteers using a validated HPLC-MS/MS method. The plasma levels of resveratrol were lower when compared with those of its conjugated metabolites. The predominant conjugates were the glucuronated resveratrol (62.53% of total resveratrol) and sulphated resveratrol (22.73% of total resveratrol). Free (unconjugated) resveratrol was 0.28% of the total resveratrol. Cmax values for resveratrol, glucuronated resveratrol and sulphated resveratrol were found to be 71.2±42.4, 4,083.9±1,704.4 and 1,516.0±639.0 ng/ml, respectively, while the AUC0-inf values were found to be 179.1±79.1, 39,732.4±16,145.6 and 14,441.7±7,593.2 ng/ml, respectively. In addition, Tmax was 1.3 h for resveratrol, 3.0 h for glucuronated resveratrol and 2.8 h for sulphated resveratrol.
Similar results were reported in studies by Brown et al (22) and Almeida et al (18), in which repeated administration of resveratrol (doses between 0.15 and 5 g) led to lower concentrations of the parent compound and higher levels of glucuronide and sulphate conjugates in the plasma.
The results of the present study were compared with the results reported by Boocock et al (15), and the pharmacokinetics of 500 mg resveratrol treatment in these two studies are presented in Table VI. In the study by Boocock et al (15), 42 volunteers (age, 19–61 years) received a single dose of 0.5, 1.0, 2.5 or 5.0 g resveratrol, but only the data obtained from the 500 mg dose were used for comparison with the present study. The Cmax and AUC0-inf values for resveratrol that were observed in the two studies were similar. In the case of the sulphate conjugate, similar results were observed for Cmax, while for AUC0-inf, the levels registered in the present study were 3.5 times higher than the data obtained by Boocock et al (15). This difference can be justified by the fact that in the study of Boocock et al only a specific sulphate form was measured, while it is possible that other types of sulphates were not detected; however, these were measured in the present study after enzymatic digestion. A similar situation was observed for glucuronated resveratrol, for which the differences between the two studies were even greater, with higher levels observed following the enzymatic degradation. Resveratrol was well-tolerated and no severe adverse reactions were reported in the two studies.
Table VI.Pharmacokinetics parameters reported in the current study and in the study of Boocock et al (15). |
In addition, the present study investigated the doses of resveratrol used in various in vivo and in vitro studies, as well as the concentration required to elicit noteworthy pharmacological activities (8–18,22,23). In vitro data showed that a minimum of 5 µmol/l resveratrol is required for the chemopreventive effects to be elicited (15). In humans, the plasma level of resveratrol following oral administration is very low; however, the metabolite levels are much higher. Considering that back conversion from metabolite to parent compound in the target tissues has been described in the case of resveratrol (30), and also that the metabolites are pharmaceutically active (6,25), the plasma levels of resveratrol along with the plasma levels of the metabolites have to be considered when investigating the concentrations expected to achieve in vivo biological effects. The levels of total plasma resveratrol (free resveratrol and metabolites) reported in the current study are well above the minimum 5 µmol/l level required to promote in vitro biological effects, as described in the literature (15).
In clinical studies, numerous biological effects of resveratrol have been studied and the dose employed varied widely between 10 mg and 5 g (31). Brasnyó et al (32) found that 10 mg oral resveratrol was sufficient to decrease insulin resistance. According to Bhatt et al (33), 250 mg resveratrol improved glycemic control and was proposed as a potential adjuvant in the treatment of diabetes. The study of Patel et al (30) showed that oral doses of 500 mg and 1 g resveratrol administered daily to patients with histologically confirmed colorectal cancer provided plasma levels of resveratrol under the limit of quantification. However, Patel et al (30) were investigating the tissue levels of resveratrol and metabolites in the gastrointestinal tract associated with anticarcinogenic effects. A recent study performed in Germany by Witte et al (34) showed that 200 mg resveratrol administered daily to elderly individuals improved memory performance in correlation with improved glucose metabolism and also increased the hippocampal functional connectivity. Therefore, the proposed 500 mg dose of resveratrol used in the present study is considered to provide adequate plasma and target tissue concentrations to promote various beneficial biological effects.
With regard to the safety profile, 500 mg resveratrol was well-tolerated by all the subjects, with no adverse reactions associated with resveratrol reported during the study. Similarly, good tolerability of 500 mg resveratrol was reported by Brown et al (22), who also found that only 2.5 and 5 g doses caused mild to moderate gastrointestinal symptoms.
In conclusion, the results of the present human pharmacokinetic study proved that resveratrol is well-absorbed following oral administration of Evelor 500 mg tablets. The plasma levels of resveratrol were comparable with those reported in the literature for other formulations of resveratrol. In addition, plasma concentrations of resveratrol and its metabolites were found to be within the range reported to promote noteworthy pharmacological activities in vitro. Finally, resveratrol was well-tolerated by all the participant subjects.
References
|
Arichi H, Kimura Y, Okuda H, Baba K, Kozawa M and Arichi S: Effects of stilbene components of the roots of Polygonum cuspidatum Sieb. et Zucc. on lipid metabolism. Chem Pharm Bull (Tokyo). 30:1766–1770. 1982. View Article : Google Scholar : PubMed/NCBI | |
|
Baur JA and Sinclair DA: Therapeutic potential of resveratrol: The in vivo evidence. Nat Rev Drug Discov. 5:493–506. 2006. View Article : Google Scholar : PubMed/NCBI | |
|
Gambini J, López-Grueso R, Olaso-González G, Inglés M, Abdelazid K, El Alam IM, Bonet-Costa V, Borrás C and Viña J: Resveratrol: Distribution, properties and perspectives. Rev Esp Geriatr Gerontol. 48:79–88. 2013.(In Spanish). View Article : Google Scholar : PubMed/NCBI | |
|
Bertell AA and Das DK: Grapes, wines, resveratrol, and heart health. J Cardiovasc Pharmacol. 54:468–476. 2009. View Article : Google Scholar : PubMed/NCBI | |
|
Bishayee A, Darvesh AS, Politis T and McGory R: Resveratrol and liver disease: From bench to bedside and community. Liver Int. 30:1103–1114. 2010. View Article : Google Scholar : PubMed/NCBI | |
|
Aires V, Limagne E, Cotte AK, Latruffe N, Ghiringhelli F and Delmas D: Resveratrol metabolites inhibit human metastatic colon cancer cells progression and synergize with chemotherapeutic drugs to induce cell death. Mol Nutr Food Res. 57:1170–1181. 2013. View Article : Google Scholar : PubMed/NCBI | |
|
Aziz MH, Kumar R and Ahmad N: Cancer chemoprevention by resveratrol: In vitro and in vivo studies and the underlying mechanisms (review). Int J Oncol. 23:17–28. 2003.PubMed/NCBI | |
|
Asensi M, Medina I, Ortega A, Carretero J, Baño MC, Obrador E and Estrela JM: Inhibition of cancer growth by resveratrol is related to its low bioavailability. Free Radic Biol Med. 33:387–398. 2002. View Article : Google Scholar : PubMed/NCBI | |
|
Frémont L: Biological effects of resveratrol. Life Sci. 66:663–673. 2000. View Article : Google Scholar : PubMed/NCBI | |
|
Hung LM, Chen JK, Huang SS, Lee RS and Su MJ: Cardioprotective effect of resveratrol, a natural antioxidant derived from grapes. Cardiovasc Res. 47:549–555. 2000. View Article : Google Scholar : PubMed/NCBI | |
|
Bhat KPL, Kosmeder JW II and Pezzuto JM: Biological effects of resveratrol. Antioxid Redox Signal. 3:1041–1064. 2001. View Article : Google Scholar : PubMed/NCBI | |
|
de la Lastra CA and Villegas I: Resveratrol as an anti-inflammatory and anti-aging agent: Mechanisms and clinical implications. Mol Nutr Food Res. 49:405–430. 2005. View Article : Google Scholar : PubMed/NCBI | |
|
Ulrich S, Wolter F and Stein JM: Molecular mechanisms of the chemopreventive effects of resveratrol and its analogs in carcinogenesis. Mol Nutr Food Res. 49:452–461. 2005. View Article : Google Scholar : PubMed/NCBI | |
|
Jan-Kan C and Li-man H: Therapeutic use of resveratrol for hyperglycemia. US patent 2006/0034763 A1. Filed. August 11–2004.issued February 16, 2006. | |
|
Boocock DJ, Faust GE, Patel KR, Schinas AM, Brown VA, Ducharme MP, Booth TD, Crowell JA, Perloff M, Gescher AJ, et al: Phase I dose escalation pharmacokinetic study in healthy volunteers of resveratrol, a potential cancer chemopreventive agent. Cancer Epidemiol Biomarkers Prev. 16:1246–1252. 2007. View Article : Google Scholar : PubMed/NCBI | |
|
Agarwal B, Campen MJ, Channell MM, Wherry SJ, Varamini B, Davis JG, Baur JA and Smoliga JM: Resveratrol for primary prevention of atherosclerosis: Clinical trial evidence for improved gene in vascular endothelium. Int J Cardiol. 166:246–248. 2013. View Article : Google Scholar : PubMed/NCBI | |
|
Gu X, Creasy L, Kester A and Zeece M: Capillary electrophoretic determination of resveratrol in wines. J Agric Food Chem. 47:3223–3227. 1999. View Article : Google Scholar : PubMed/NCBI | |
|
Almeida L, Vaz-da-Silva M, Falcão A, Soares E, Costa R, Loureiro AL, Fernandes-Lopes C, Rocha JF, Nunes T, Wright L and Soares-da-Silva P: Pharmacokinetic and safety profile of trans-resveratrol in a rising multiple-dose study in healthy volunteers. Mol Nutr Food Res. 53(Suppl 1): S7–S15. 2009. View Article : Google Scholar : PubMed/NCBI | |
|
Cottart CH, Nivet-Antoine V, Laguillier-Morizot C and Beaudeux JL: Resveratrol bioavailability and toxicity in humans. Mol Nutr Food Res. 54:7–16. 2010. View Article : Google Scholar : PubMed/NCBI | |
|
Kuhnle G, Spencer JP, Chowrimootoo G, Schroeter H, Debnam ES, Srai SK, Rice-Evans C and Hahn U: Resveratrol is absorbed in the small intestine as resveratrol glucuronide. Biochem Biophys Res Commun. 272:212–217. 2000. View Article : Google Scholar : PubMed/NCBI | |
|
Rotches-Ribalta M, Andres-Lacueva C, Estruch R, Escribano E and Urpi-Sarda M: Pharmacokinetics of resveratrol metabolic profile in healthy humans after moderate consumption of red wine and grape extract tablets. Pharmacol Res. 66:375–382. 2012. View Article : Google Scholar : PubMed/NCBI | |
|
Brown A, Patel KR, Viskaduraki M, Crowell JA, Perloff M, Booth TD, Vasilinin G, Sen A, Schinas AM, Piccirilli G, et al: Repeat dose study of the cancer chemopreventive agent resveratrol in healthy volunteers: Safety, pharmacokinetics, and effect on the insulin-like growth factor axis. Cancer Res. 70:9003–9011. 2010. View Article : Google Scholar : PubMed/NCBI | |
|
Wang D, Hang T, Wu C and Liu W: Identification of the major metabolites of resveratrol in rat urine by HPLC-MS/MS. J Chromatogr B Analyt Technol Biomed Life Sci. 829:97–106. 2005. View Article : Google Scholar : PubMed/NCBI | |
|
Walle T: Bioavailability of resveratrol. Ann N Y Acad Sci. 1215:9–15. 2011. View Article : Google Scholar : PubMed/NCBI | |
|
Lu DL, Ding DJ, Yan WJ, Li RR, Dai F, Wang Q, Yu SS, Li Y, Jin XL and Zhou B: Influence of glucuronidation and reduction modifications of resveratrol on its biological activities. Chembiochem. 14:1094–1104. 2013. View Article : Google Scholar : PubMed/NCBI | |
|
Sergides C and Pittas A: Resveratrol compositions for use as dietary supplements. Patent EP2403479 A1. Filed. March 3–2010.issued January 11, 2012. | |
|
Liu X, Teng Z, Zhang Y, Huan M and Zhou S: High performance liquid chromatographytandem mass spectrometric determination of resveratrol and its metabolites in rat tissues. Anal Lett. 43:557–569. 2010. View Article : Google Scholar | |
|
Muzzio M, Huang Z, Hu SC, Johnson WD, McCormick DL and Kapetanovic IM: Determination of resveratrol and its sulfate and glucuronide metabolites in plasma by LC-MS/MS and their pharmacokinetics in dogs. J Pharm Biomed Anal. 59:201–208. 2012. View Article : Google Scholar : PubMed/NCBI | |
|
Biasutto L, Marotta E, Garbisa S, Zoratti M and Paradisi C: Determination of quercetin and resveratrol in whole blood-implications for bioavailability studies. Molecules. 15:6570–6579. 2010. View Article : Google Scholar : PubMed/NCBI | |
|
Patel KR, Brown VA, Jones DJ, Britton RG, Hemingway D, Miller AS, West KP, Booth TD, Perloff M, Crowel JA, et al: Clinical pharmacology of resveratrol and its metabolites in colorectal cancer patients. Cancer Res. 70:7392–7399. 2010. View Article : Google Scholar : PubMed/NCBI | |
|
Tomé-Carneiro J, Larrosa M, González-Sarrías A, Tomás-Barberán FA, García-Conesa MT and Espín JC: Resveratrol and clinical trials: The crossroad from in vitro studies to human evidence. Curr Pharm Des. 19:6064–6093. 2013. View Article : Google Scholar : PubMed/NCBI | |
|
Brasnyó P, Molnár GA, Mohás M, Markó L, Laczy B, Cseh J, Mikolás E, Szijártó IA, Mérei A, Halmai R, et al: Resveratrol improves insulin sensitivity, reduces oxidative stress and activates the Akt pathway in type 2 diabetic patients. Br J Nutr. 106:383–389. 2011. View Article : Google Scholar : PubMed/NCBI | |
|
Bhatt JK, Thomas S and Nanjan MJ: Resveratrol supplementation improves glycemic control in type 2 diabetes mellitus. Nutr Res. 32:537–541. 2012. View Article : Google Scholar : PubMed/NCBI | |
|
Witte AV, Kerti L, Margulies DS and Flöel A: Effects of resveratrol on memory performance, hippocampal functional connectivity, and glucose metabolism in healthy older adults. J Neurosci. 34:7862–7870. 2014. View Article : Google Scholar : PubMed/NCBI |