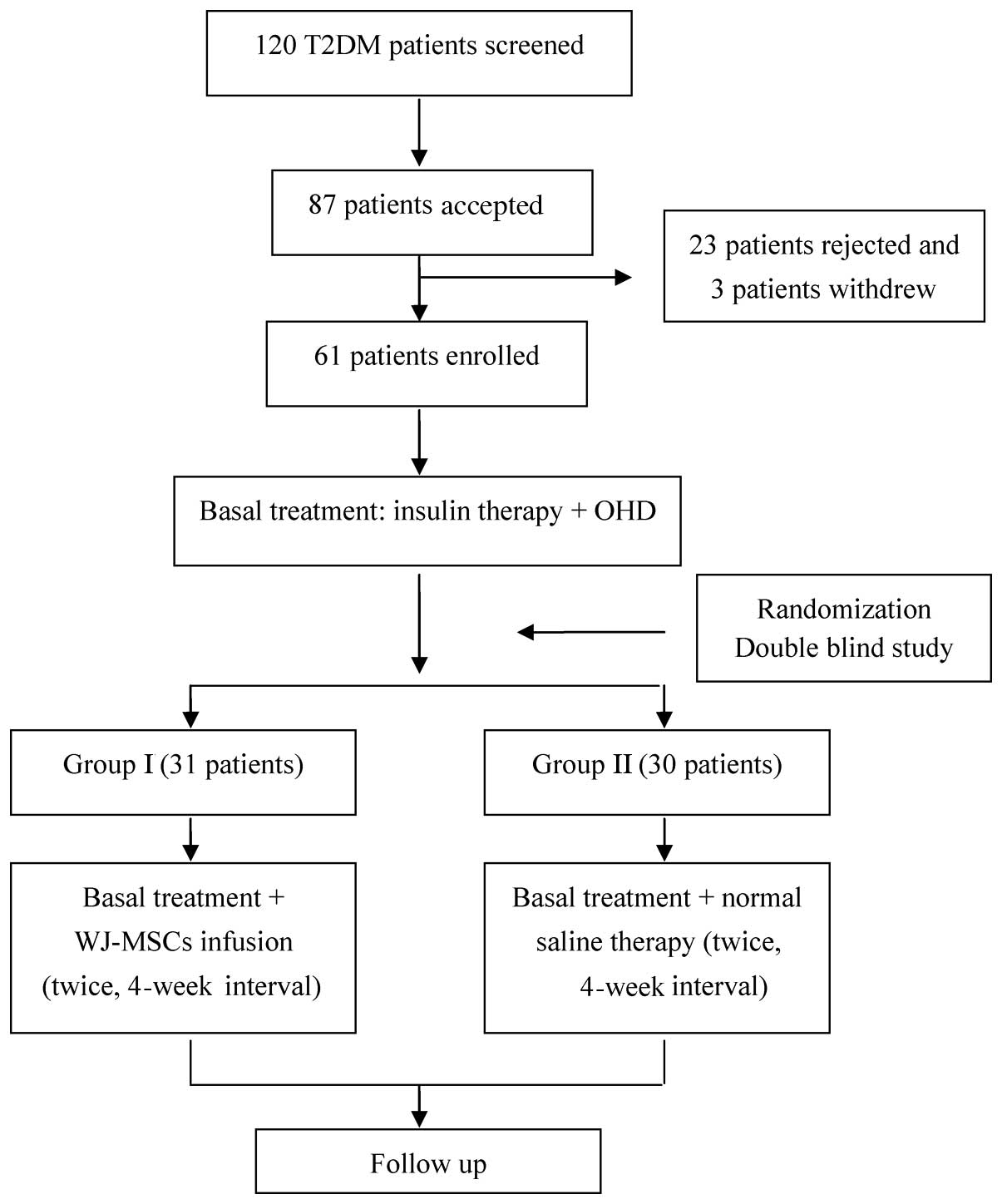
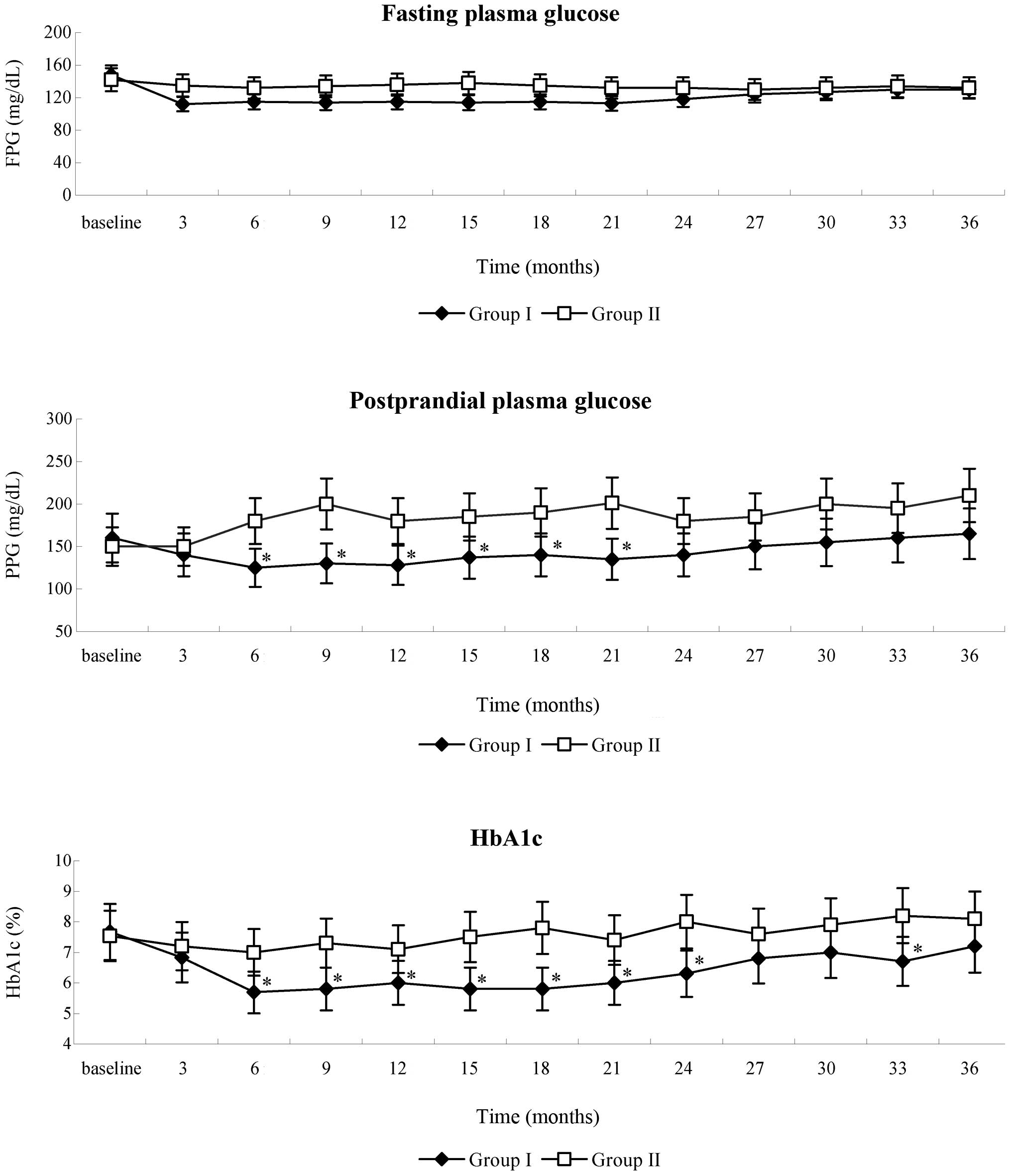
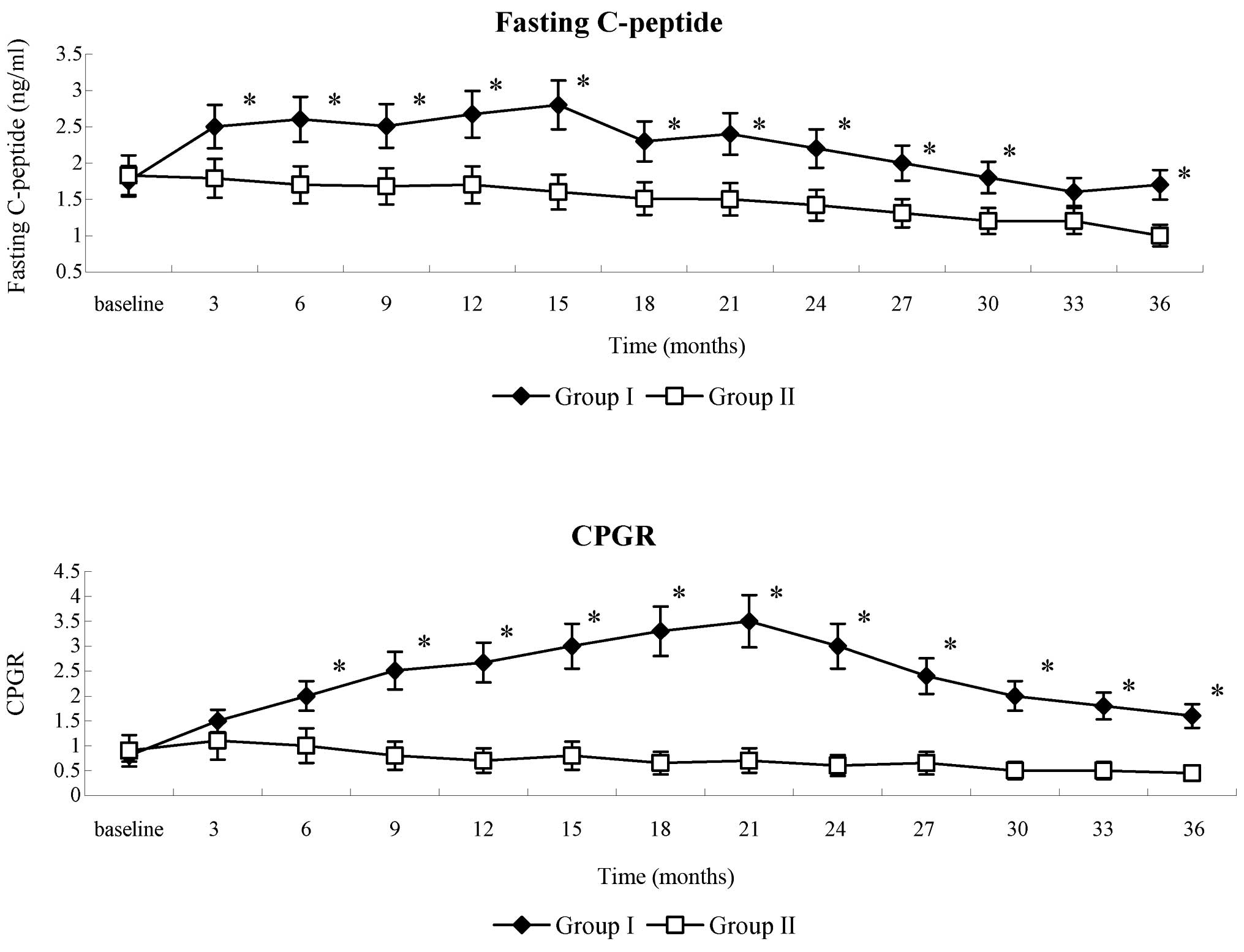
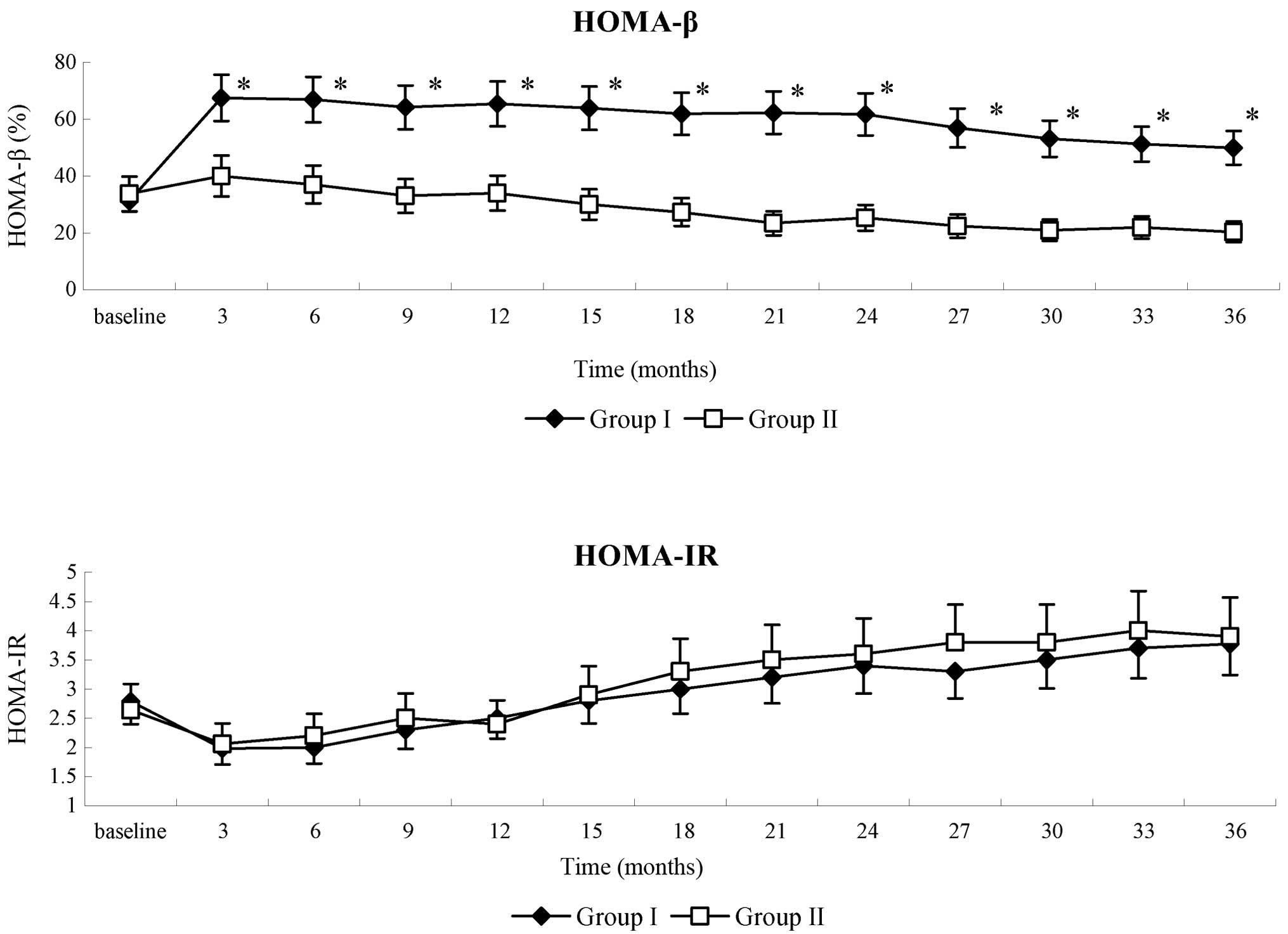
|
1
|
Fukuhara T, Hyogo H, Ochi H, Fujino H, Kan
H, Naeshiro N, Honda Y, Miyaki D, Kawaoka T, Tsuge M, et al:
Efficacy and safety of sitagliptin for the treatment of
nonalcoholic fatty liver disease with type 2 diabetes mellitus.
Hepatogastroenterology. 61:323–328. 2014.PubMed/NCBI
|
|
2
|
Hinnen DA: Therapeutic Options for the
Management of Postprandial Glucose in Patients With Type 2 Diabetes
on Basal Insulin. Clin Diabetes. 33:175–180. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Poole J, Mavromatis K, Binongo JN, Khan A,
Li Q, Khayata M, Rocco E, Topel M, Zhang X, Brown C, et al: Effect
of progenitor cell mobilization with granulocyte-macrophage
colony-stimulating factor in patients with peripheral artery
disease: A randomized clinical trial. JAMA. 310:2631–2639. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhao Y, Jiang Z, Zhao T, Ye M, Hu C, Zhou
H, Yin Z, Chen Y, Zhang Y, Wang S, et al: Targeting insulin
resistance in type 2 diabetes via immune modulation of cord
blood-derived multipotent stem cells (CB-SCs) in stem cell educator
therapy: Phase I/II clinical trial. BMC Med. 11:1602013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Haller MJ, Wasserfall CH, Hulme MA,
Cintron M, Brusko TM, McGrail KM, Wingard JR, Theriaque DW, Shuster
JJ, Ferguson RJ, et al: Autologous umbilical cord blood infusion
followed by oral docosahexaenoic acid and vitamin D supplementation
for C-peptide preservation in children with type 1 diabetes. Biol
Blood Marrow Transplant. 19:1126–1129. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ling W, Zhang J, Yuan Z, Ren G, Zhang L,
Chen X, Rabson AB, Roberts AI, Wang Y and Shi Y: Mesenchymal stem
cells use IDO to regulate immunity in tumor microenvironment.
Cancer Res. 74:1576–1587. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gharibi T, Ahmadi M, Seyfizadeh N,
Jadidi-Niaragh F and Yousefi M: Immunomodulatory characteristics of
mesenchymal stem cells and their role in the treatment of multiple
sclerosis. Cell Immunol. 293:113–121. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pischiutta F, D'Amico G, Dander E, Biondi
A, Biagi E, Citerio G, De Simoni MG and Zanier ER:
Immunosuppression does not affect human bone marrow mesenchymal
stromal cell efficacy after transplantation in traumatized mice
brain. Neuropharmacology. Nov 15–2013.(Epub ahead of print).
PubMed/NCBI
|
|
9
|
Zhang Y, Cai W, Huang Q, Gu Y, Shi Y,
Huang J, Zhao F, Liu Q, Wei X, Jin M, et al: Mesenchymal stem cells
alleviate bacteria-induced liver injury in mice by inducing
regulatory dendritic cells. Hepatology. 59:671–682. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Heldman AW, DiFede DL, Fishman JE,
Zambrano JP, Trachtenberg BH, Karantalis V, Mushtaq M, Williams AR,
Suncion VY, McNiece IK, et al: Transendocardial mesenchymal stem
cells and mononuclear bone marrow cells for ischemic
cardiomyopathy: The TAC-HFT randomized trial. JAMA. 311:62–73.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Conforti A, Biagini S, Del Bufalo F,
Sirleto P, Angioni A, Starc N, Li Pira G, Moretta F, Proia A,
Contoli B, et al: Biological, functional and genetic
characterization of bone marrow-derived mesenchymal stromal cells
from pediatric patients affected by acute lymphoblastic leukemia.
PloS One. 8:e769892013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang X, Cheng H, Hua R, Yang J, Dai G,
Zhang Z, Wang R, Qin C and An Y: Effects of bone marrow mesenchymal
stromal cells on gross motor function measure scores of children
with cerebral palsy: A preliminary clinical study. Cytotherapy.
15:1549–1562. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hao H, Liu J, Shen J, Zhao Y, Liu H, Hou
Q, Tong C, Ti D, Dong L, Cheng Y, et al: Multiple intravenous
infusions of bone marrow mesenchymal stem cells reverse
hyperglycemia in experimental type 2 diabetes rats. Biochem Biophys
Res Commun. 436:418–423. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jiang R, Han Z, Zhuo G, Qu X, Li X, Wang
X, Shao Y, Yang S and Han ZC: Transplantation of placenta-derived
mesenchymal stem cells in type 2 diabetes: A pilot study. Front
Med. 5:94–100. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu X, Zheng P, Wang X, Dai G, Cheng H,
Zhang Z, Hua R, Niu X, Shi J and An Y: A preliminary evaluation of
efficacy and safety of Wharton's jelly mesenchymal stem cell
transplantation in patients with type 2 diabetes mellitus. Stem
Cell Res Ther. 5:572014. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Si Y, Zhao Y, Hao H, Liu J, Guo Y, Mu Y,
Shen J, Cheng Y, Fu X and Han W: Infusion of mesenchymal stem cells
ameliorates hyperglycemia in type 2 diabetic rats: Identification
of a novel role in improving insulin sensitivity. Diabetes.
61:1616–1625. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lambert M: ADA releases revisions to
recommendations for standards of medical care in diabetes. Am Fam
Physician. 85:514–515. 2012.PubMed/NCBI
|
|
18
|
Hu J, Yu X, Wang Z, Wang F, Wang L, Gao H,
Chen Y, Zhao W, Jia Z, Yan S and Wang Y: Long term effects of the
implantation of Wharton's jelly-derived mesenchymal stem cells from
the umbilical cord for newly-onset type 1 diabetes mellitus. Endocr
J. 60:347–357. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hu J, Wang F, Sun R, Wang Z, Yu X, Wang L,
Gao H, Zhao W, Yan S and Wang Y: Effect of combined therapy of
human Wharton's jelly-derived mesenchymal stem cells from umbilical
cord with sitagliptin in type 2 diabetic rats. Endocrine.
45:279–287. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zilliox LA, Ruby SK, Singh S, Zhan M and
Russell JW: Clinical neuropathy scales in neuropathy associated
with impaired glucose tolerance. J Diabetes Complications.
29:372–377. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wu L, Fernandez-Loaiza P, Sauma J,
Hernandez-Bogantes E and Masis M: Classification of diabetic
retinopathy and diabetic macular edema. World J Diabetes.
4:290–294. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hu J, Wang Y, Wang F, Wang L, Yu X, Sun R,
Wang Z, Wang L, Gao H, Fu Z, et al: Effect and mechanisms of human
Wharton's jelly-derived mesenchymal stem cells on type 1 diabetes
in NOD model. Endocrine. 48:124–134. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li XY, Zheng ZH, Li XY, Guo J, Zhang Y, Li
H, Wang YW, Ren J and Wu ZB: Treatment of foot disease in patients
with type 2 diabetes mellitus using human umbilical cord blood
mesenchymal stem cells: Response and correction of immunological
anomalies. Curr Pharm Des. 19:4893–4899. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Josse J, Velard F, Mechiche Alami S, Brun
V, Guillaume C, Kerdjoudj H, Lamkhioued B and Gangloff SC:
Increased internalization of Staphylococcus aureus and cytokine
expression in human Wharton's jelly mesenchymal stem cells. Biomed
Mater Eng. 24(Suppl 1): S27–S35. 2014.
|
|
25
|
Wang Y, Xue M, Xuan YL, Hu HS, Cheng WJ,
Suo F, Li XR, Yan SH and Wang LX: Mesenchymal stem cell therapy
improves diabetic cardiac autonomic neuropathy and decreases the
inducibility of ventricular arrhythmias. Heart Lung Circ.
22:1018–1025. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Rahman MJ, Regn D, Bashratyan R and Dai
YD: Exosomes released by islet-derived mesenchymal stem cells
trigger autoimmune responses in NOD mice. Diabetes. 63:1008–1020.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bassi ÊJ, Moraes-Vieira PM, Moreira-Sá CS,
Almeida DC, Vieira LM, Cunha CS, Hiyane MI, Basso AS, Pacheco-Silva
A and Câmara NO: Immune regulatory properties of allogeneic
adipose-derived mesenchymal stem cells in the treatment of
experimental autoimmune diabetes. Diabetes. 61:2534–2545. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ezquer F, Ezquer M, Simon V and Conget P:
The antidiabetic effect of MSCs is not impaired by insulin
prophylaxis and is not improved by a second dose of cells. PloS
One. 6:e165662011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ho JH, Tseng TC, Ma WH, Ong WK, Chen YF,
Chen MH, Lin MW, Hong CY and Lee OK: Multiple intravenous
transplantations of mesenchymal stem cells effectively restore
long-term blood glucose homeostasis by hepatic engraftment and
β-cell differentiation in streptozocin-induced diabetic mice. Cell
Transplant. 21:997–1009. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hu J, Li C, Wang L, Zhang X, Zhang M, Gao
H, Yu X, Wang F, Zhao W, Yan S and Wang Y: Long term effects of the
implantation of autologous bone marrow mononuclear cells for type 2
diabetes mellitus. Endocr J. 59:1031–1039. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Fan N, Sun H, Wang Y, Zhang L, Xia Z, Peng
L, Hou Y, Shen W, Liu R and Peng Y: Midkine, a potential link
between obesity and insulin resistance. PloS One. 9:e882992014.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lee BC and Lee J: Cellular and molecular
players in adipose tissue inflammation in the development of
obesity-induced insulin resistance. Biochim Biophys Acta.
1842:446–462. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhu Y, Pereira RO, O'Neill BT, Riehle C,
Ilkun O, Wende AR, Rawlings TA, Zhang YC, Zhang Q, Klip A, et al:
Cardiac PI3K-Akt impairs insulin-stimulated glucose uptake
independent of mTORC1 and GLUT4 translocation. Mol Endocrinol.
27:172–184. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Huat TJ, Khan AA, Pati S, Mustafa Z,
Abdullah JM and Jaafar H: IGF-1 enhances cell proliferation and
survival during early differentiation of mesenchymal stem cells to
neural progenitor-like cells. BMC Neurosci. 15:912014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Milanesi A, Lee JW, Li Z, Da Sacco S,
Villani V, Cervantes V, Perin L and Yu JS: β-cell regeneration
mediated by human bone marrow mesenchymal stem cells. PloS One.
7:e421772012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Jin P, Zhang X, Wu Y, Li L, Yin Q, Zheng
L, Zhang H and Sun C: Streptozotocin-induced diabetic rat-derived
bone marrow mesenchymal stem cells have impaired abilities in
proliferation, paracrine, antiapoptosis and myogenic
differentiation. Transplant Proc. 42:2745–2752. 2010. View Article : Google Scholar : PubMed/NCBI
|