Effects of Ginkgo biloba leaf extract on local renin-angiotensin system through TLR4/NF-κB pathway in cardiac myocyte
- Authors:
- Published online on: October 16, 2017 https://doi.org/10.3892/etm.2017.5313
- Pages: 5857-5862
-
Copyright: © Jiang et al. This is an open access article distributed under the terms of Creative Commons Attribution License.
Abstract
Introduction
In 1999, researchers observed that Toll-like receptor 4 (TLR4) was highly expressed in myocardial cells. In normal mice and human myocardium, expression of TLR4 in cardiomyocyte is mainly diffused in cytoplasm. However, expression of TLR4 was upregulated in the myocardium of rats after infarction which is far away from the lesion of ischemic injury and in myocardium of patients with dilated cardiomyopathy. TLR4 staining showed very strong signal at the junction of two or more than two cells (1). It has been shown that lipopolysacchride (LPS) and AngII can synergistically increase intracellular Ca2+ levels at rest, reduce the transient increase of Ca2+ level and damage the function of mitochondria (2). Later on, more studies have shown that TLR4/nuclear factor-κB (NF-κB), a signal transduction pathway closely related to immune, inflammatory and oxidative stress, is closely related to the local renin-angiotensin system (RAS). TLR4/NF-κB is involved in inflammatory response of myocardial injury and physiological processes of myocardial remodeling, and intervention of TLR4/NF-κB signal can attenuate myocarditis and myocardial damage (3–5). Ginkgo biloba extract (GBE) is a traditional Chinese medicine that plays an important role in anti-myocardial injury, and function of GBE is related to its role in scavenging oxygen free radical and suppressing immune response (6–8). However, the involvement of TLR4/NF-κB signaling pathway in myocardial remodeling prevention through GBE is still unknown. In this study, TLR4/NF-κB signaling pathway in rat ventricular myocytes was activated in vitro using LPS, which is a TLR4 specific agonist (9,10). The effects of Ginkgo biloba leaf extract (GBE50) on TLR4/NF-κB signal and local RAS were observed, and the mechanism of GBE50 in the prevention and treatment of myocardial remodeling was also explored.
Materials and methods
Experimental animals and major reagents
Healthy new born Sprague-Dawley rats (6–24 hour old) without restriction on sex were provided by Experimental Animal Center of Dalian Medical University. GBE: Gingko biloba (GBE50) containing 44.1% of Ginkgo biloba total flavonoids and 6.4% of ginkgolides was provided by SPH Xingling Science and Technology Pharmaceutical Co., Ltd. (Dalian, China). LPS (055:B5), caffeic acid phenethyl ester (CAPE), a specific inhibitor of NF-κB, type II collagenase, trypsin, and 5-bromo deoxyuridine (Brdu) were from Sigma (St. Louis, MO, USA). TRIzol and Dulbeccos modified Eagles medium (DMEM) medium were from Gibco (New York, NY, USA). Reverse transcription kit was bought from Takara (Dalian, China). Rabbit anti-rat striated muscle specific sarcomeric α-actin polyclonal antibody (dilution, 1:200; cat. no. PA5-21396) and SP immunohistochemistry kit which included biotinylated secondary antibody, hydrogen peroxide, serum blocker and horseradish peroxidase labeled streptavidin were from Zymed (San Diego, CA, USA). Rabbit anti-rat NF-κB p65 polyclonal antibody (dilution, 1:100; cat. no. SC8008) was from Santa Cruz Biotechnology, Inc. (Dallas, TX, USA). Coomassie Brilliant Blue was from Beijing Zhongshan Golden Bridge Biotechnology Co., Ltd. (Beijing, China).
Primary culture of cardiomyocyte and grouping
Ventricles were collected from Sprague-Dawley rats aged 6–24 h under sterile conditions and were cut into pieces. 0.1% trypsin and 0.1% type II collagenase were added to digest the cells to make single cell suspension. Cardiac fibroblasts and epithelial cells were removed using differential attachment technique. Striated muscle specific sagcomeric α-actin monoclonal antibody was used to identify cardiomyocytes by SABC immunohistochemical staining. In the first 48 h of culture, 0.1 mmol/l Brdu was added to inhibit the proliferation of non-cardiomyocytes. DMEM culture medium without serum was used after culture for 96 h when cells reached sub-cell-fusion state. After culture for another 24 h, cells were divided into the following groups: i) control group: DMEM medium without any intervention factors; ii) LPS group: DMEM medium containing 1 µg/ml LPS; iii) LPS + GBE50 group: GBE50 was added first to the final concentration of 80 µg/ml, then LPS was added to the final concentration of 1 µg/ml 30 min later; iv) LPS + CAPE group: CAPE was added first to the final concentration of 20 µg/ml, 30 min later, LPS was added to the final concentration of 1 µg/ml. After intervention for 24 h, cells were collected.
Immunocytochemical analysis of the activation of NF-κB
Density of neonatal rat ventricular myocytes (NRVMs) was adjusted to 1×106/ml and inoculated on cell culture plate with coverglass in each well. After treatment for 24 h, coverglasses were collected and washed with PBS. Fixation was performed with 1% paraformaldehyde (pH 7.0) at room temperature for 30 min, followed by washing with 0.1% Triton X-100 (pH 7.0) at room temperature for 5 min. Rabbit anti-mouse NF-κB p65 antibody (1:100) was added, followed by incubation at 37°C for 60 min. Biotin-avidin reaction system was used. After color development with DAB and hematoxylin re-staining, slides were sealed. Determination of immunocytochemical results were as follows: because NF-κB is in cytoplasm under normal condition, after activation, it will enter into nucleus, nuclear-positivity was used as the criteria for the determination of the activation of NF-κB. Under light microscope (200-fold; Olympus, Tokyo, Japan), 5 visual fields were randomly selected. The activation of NF-κB was assessed by calculating the percentage of positive cells.
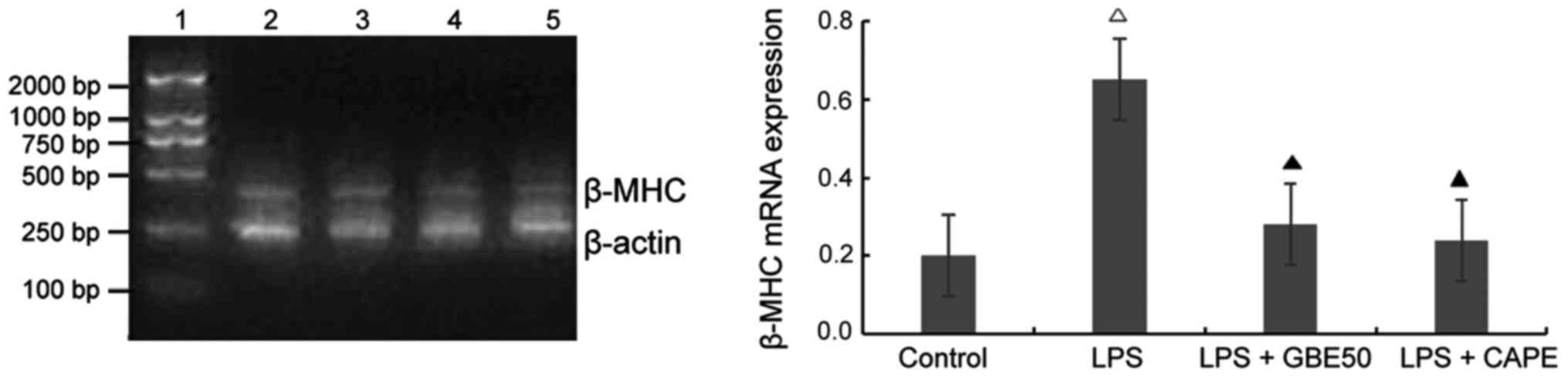
Detection of the expression of TLR4, AT1a receptor, angiotensinogen (ATG) and β-myosin heavy chain (β-MHC) mRNA in cardiomyocytes
Primers were synthesized by Takara
Total RNA was extracted using TRIzol reagent according to the instruction. RNA sample with OD260/OD280=1.6–1.8 were used for reverse transcription. Reverse transcription conditions: 30°C for 10 min, 50°C for 30 min and 99°C 5 min. RT-PCR was performed to detect the expression of TLR4, AT1a receptor, ATG and β-MHC mRNA. TLR4 primer sense, 5′-CGCTTTCAGCTTTGCCTTCATTAC-3′ and antisense, 5′-AGCTACTTCCTTGTGCCCTGTGAG-3′, length of amplified fragment was 555 bp; ATG primer sense, 5′-TTCAGGCCAAGACCTCCC-3′ and antisense, 5′-CCAGCCGGGAGGTGCAGT-3′, length of amplified fragment was 308 bp; AT1a primer sense, 5′-GCACACTGGCAATGTAATGC-3′ and antisense, 5′-GTTGAACAGAACAAGTGACC-3′, length of amplified fragment was 385 bp; β-MHC primer sense, 5′-GTGGACGTTTATTGACTTCGG-3′ and antisense, 5′-TTCTTTGCTTTGCCTTTGC-3′, length of amplified fragment was 399 bp; endogenous control β-actin primer sense, 5′-AACCCTAAGGCCAACCGTGAAAAG-3′ and antisense, 5-TCATGAGGTAGTCTGTCAT-3′, length of amplified fragment was 241 bp. PCR reaction conditions for TLR4, 94°C for 2 min, followed by 30 cycles of 94°C for 30 sec, 60°C for 60 sec and 72°C for 1.5 min, and 72°C for 10 min; PCR reaction conditions for ATG, 94°C for 2 min, followed by 30 cycles of 94°C for 30 sec, 63°C for 60 sec and 72°C for 1.5 min, and 72°C for 10 min; PCR reaction conditions for AT1a receptor, 94°C for 2 min, followed by 30 cycles of 94°C for 30 sec, 58°C for 45 sec and 72°C for 1.5 min, and 72°C for 10 min; PCR reaction conditions for β-MHC, 94°C for 2 min, followed by 35 cycles of 94°C for 30 sec, 55°C for 45 sec and 72°C for 1 min, and 72°C for 7 min. PCR products were subjected to 1.5% agarose gel electrophoresis. A gel imaging system was used to take the images. The ratio of the absorbance of the target gene band to the absorbance of the β-actin band was taken as the relative expression level of target mRNA.
Determination of protein content in cardiomyocytes
Cells were digested with 0.125% trypsin, and 0.1% SDS (0.1 ml) was added to lysis cells, followed by centrifugation at 2,000 × g for 5 min to collect supernatant. Total protein content of cardiomyocytes was measured by Coomassie Brilliant Blue method.
Statistical analysis
Data were processed using SPSS 11.5 statistical software (version X; IBM, Armonk, NY, USA). Each sample was measured three times in parallel, and the data were expressed as mean ± standard deviation (mean ± SD). Comparisons among multiple samples were performed by variance analysis. Comparisons between two samples were performed by Q test. P<0.05 was considered to be statistically significant.
Results
Culture and identification of NRVMS
After fibroblasts were removed by differential attachment technique, cells were cultured for 24 h. Cardiomyocytes were long rod-, polygonal or spindle-shaped with concentric or radial growth. After culture for 48 h, synchronized pulsation (12–80 bpm) was observed in cardiomyocytes. Immunocytochemical identification of striated muscle specific sarcomeric α-actin showed that the purity of cardiomyocytes was >95%.
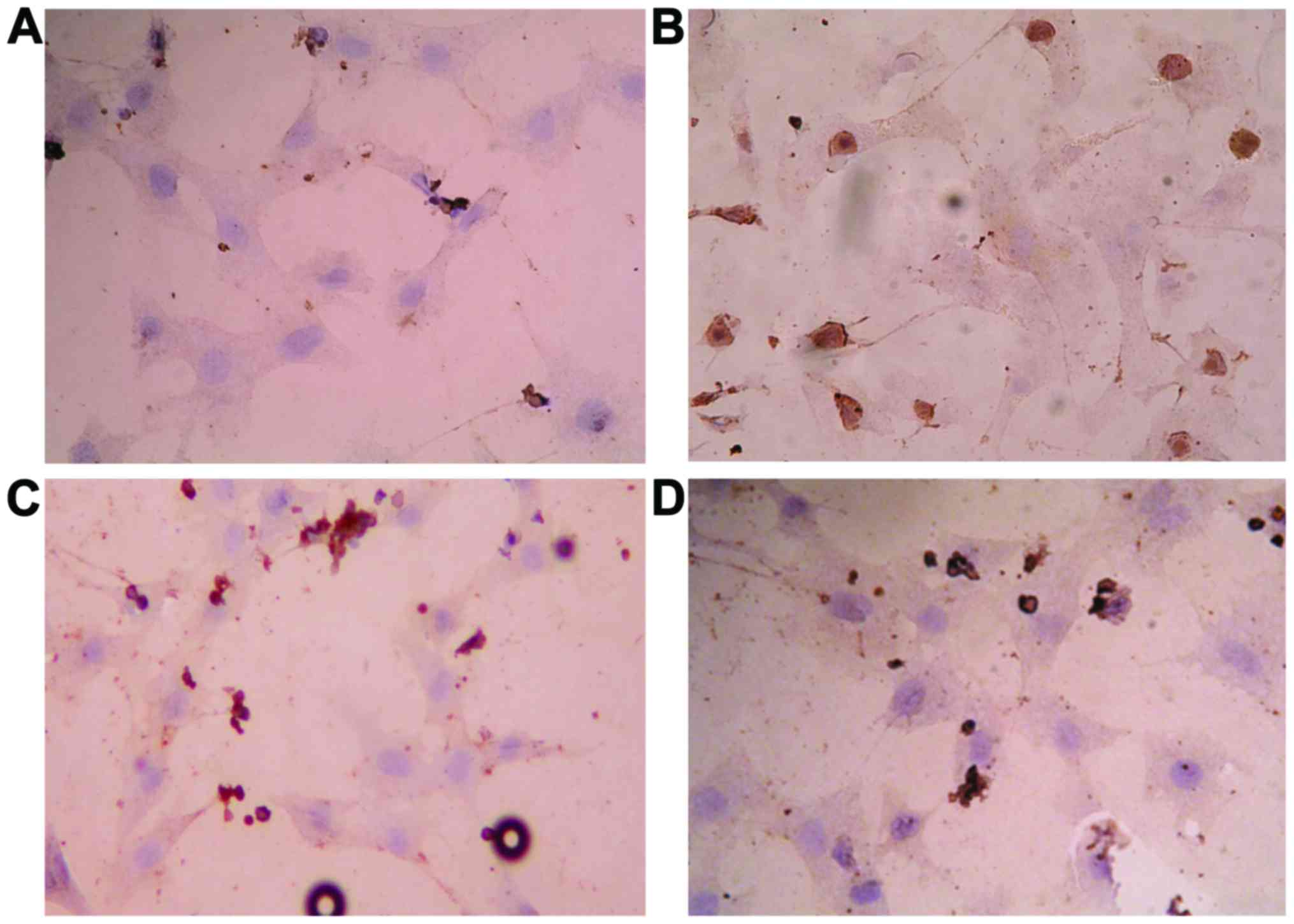
Effects of GBE50 and NF-κB inhibitor CAPE on activation of NF-κB induced by LPS in NRVMs
Results of immunocytochemical analysis showed that NF-κB was activated 24 h after LPS (1 µg/ml) stimulation, and nuclear staining was significantly increased (P<0.01). After treatment with GBE50 (80 µg/ml) and CAPE (20 µg/ml), almost no nuclear staining was observed (P<0.01) (Fig. 1).
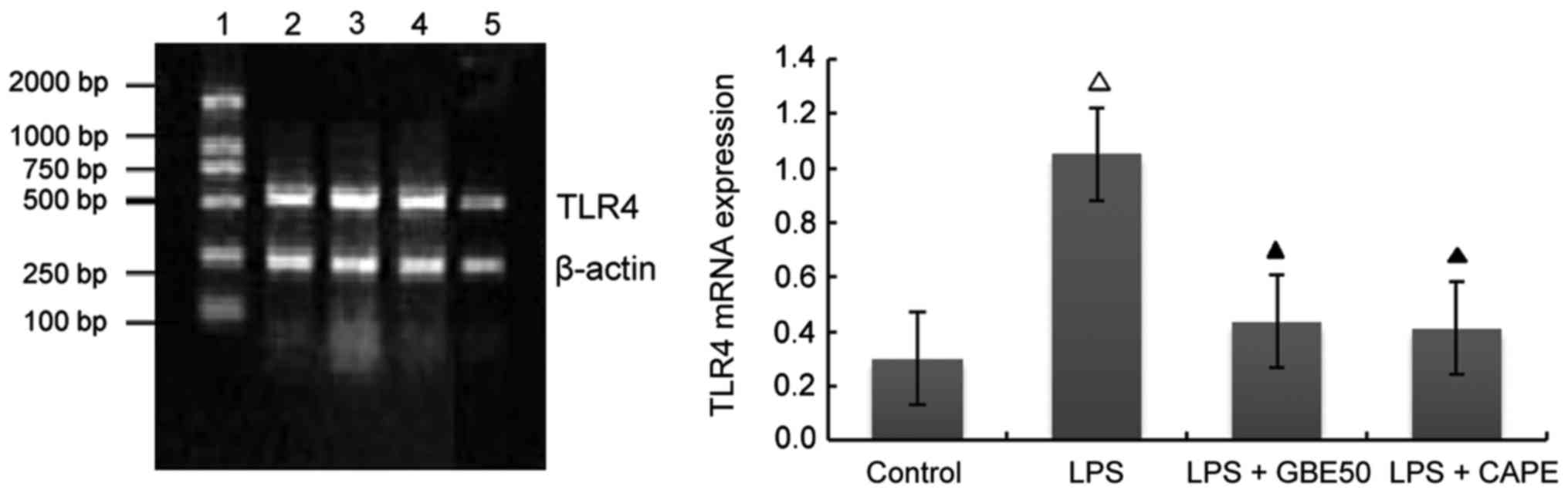
Effects of GBE50 and NF-κB inhibitor CAPE on TLR4 mRNA expression induced by LPS in NRVMs
Compared with control group (expression level of TLR4 mRNA was 0.301±0.112), expression level of TLR4 mRNA in NRVMs were significantly increased at 24 h after LPS (1 µg/ml) stimulation (expression level of TLR4 mRNA was 1.052±0.227, P<0.01); compared with LPS group, GBE50 (80 µg/ml) and CAPE (20 µg/ml) significantly reduced the expression of TLR4 mRNA in NRVMs induced by LPS (expression levels of TLR4 mRNA were 0.436±0.145 and 0.411±0.123, respectively, P<0.01). Compared with LPS + GBE50 group, expression level of TLR4 mRNA was slightly reduced in LPS + CAPE group, but the difference was not significant (Fig. 2).
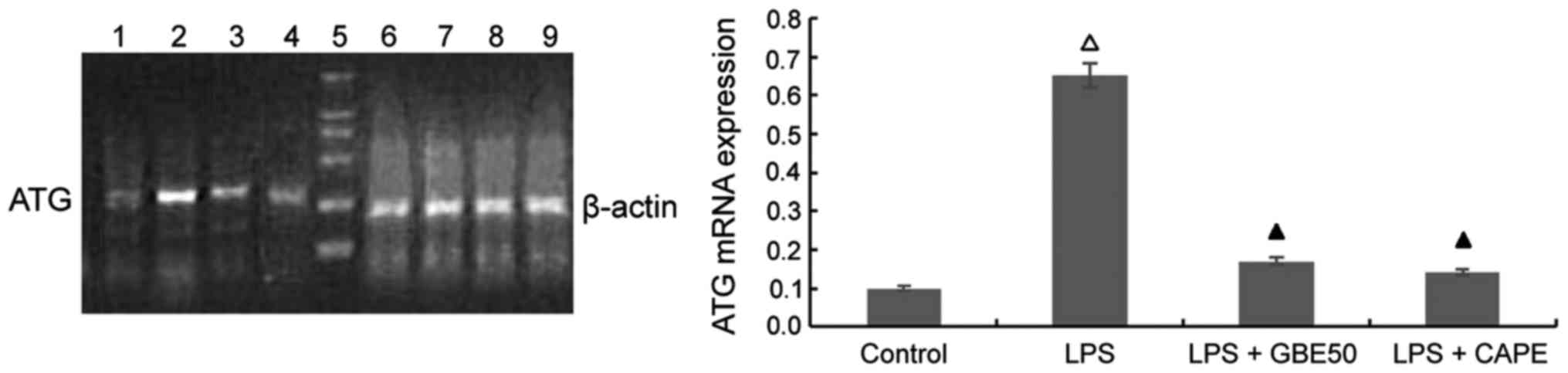
Effects of GBE50 and NF-κB inhibitor CAPE on LPS-induced expression of ATG mRNA in NRVMs
Compared with control group (expression level of ATG mRNA was 0.652±0.227), expression level of ATG mRNA was significantly increased in NRVMs at 24 h after LPS (1 µg/ml) stimulation (expression level of ATG mRNA was 0.652±0.227, P<0.01). GBE50 (80 µg/ml) and CAPE (20 µg/ml) could inhibit the increased expression level of ATG mRNA induced by LPS (expression levels of ATG mRNA were 0.171±0.102 and 0.141±0.097, respectively, P<0.01). No significant differences in the expression level of ATG mRNA were found between LPS + GBE50 group, LPS + CAPE group and control group. Compared with LPS + GBE50 group, expression level of ATG mRNA was slightly reduced in LPS + CAPE group, but the difference was not significant (Fig. 3).
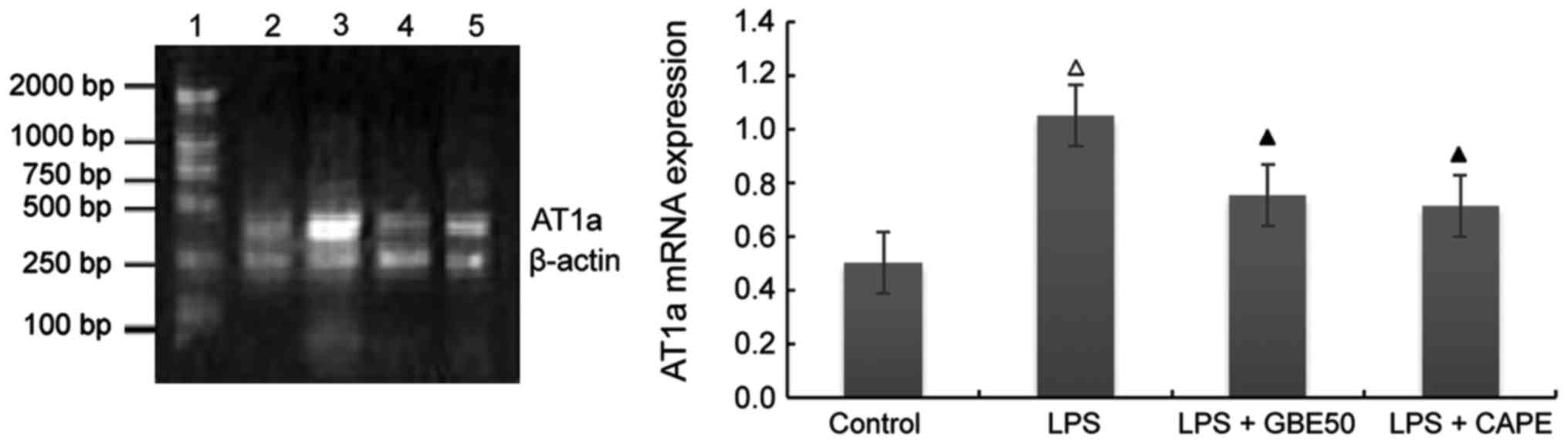
Effect of GBE50 and NF-κB inhibitor CAPE on expression of AT1a receptor mRNA induced by LPS in NRVMs
Compared with control group (expression level of AT1a receptor mRNA was 0.501±0.112), expression level of AT1a receptor mRNA in NRVMs were significantly increased at 24 h after LPS (1 µg/ml) stimulation (expression level of TLR4 mRNA was 1.05±0.227, P<0.01); compared with LPS group, GBE50 (80 µg/ml) and CAPE (20 µg/ml) significantly reduced the expression of AT1a mRNA in NRVMs induced by LPS (expression levels of AT1a receptor mRNA were 0.751±0.257 and 0.711±0.223, respectively, P<0.01). No significant difference in the expression level of AT1a receptor mRNA was found between LPS + GBE50 group, LPS + CAPE group and control group. Compared with LPS + GBE50 group, expression level of AT1a receptor mRNA was slightly reduced in LPS + CAPE group, but the difference was not significant (Fig. 4).
Effect of GBE50 and NF-κB inhibitor CAPE on expression of β-MHC mRNA induced by LPS in NRVMs
Compared with control group (expression level of β-MHC mRNA was 0.201±0.098), expression level of β-MHC mRNA in NRVMs were significantly increased at 24 h after LPS (1 µg/ml) stimulation (expression level of β-MHC mRNA was 0.653±0.228, P<0.01); compared with LPS group, GBE50 (80 µg/ml) and CAPE (20 µg/ml) significantly reduced the expression of β-MHC mRNA in NRVMs induced by LPS (expression levels of β-MHC mRNA were 0.201±0.098 and 0.191±0.089, respectively, P<0.01). No significant difference in the expression level of β-MHC mRNA was found between LPS + GBE50 group, LPS + CAPE group and control group. Compared with LPS + GBE50 group, expression level of β-MHC mRNA was slightly reduced in LPS + CAPE group, but the difference was not significant (Fig. 5).
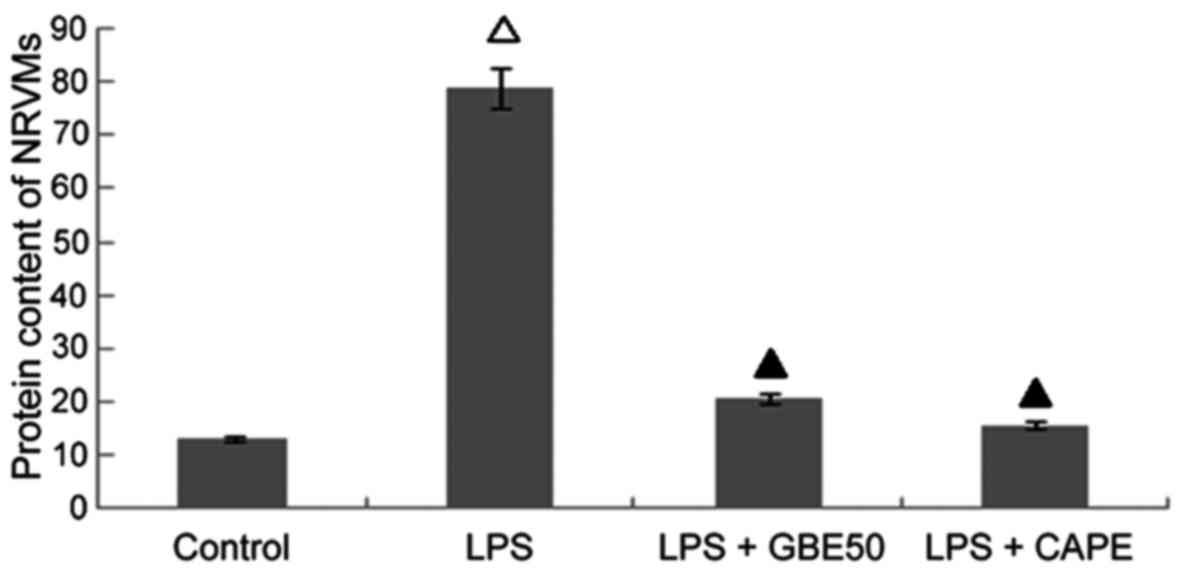
Effects of GBE50 and NF-κB inhibitor CAPE on increased protein content in NRVMs cells induced by LPS
Compared with control group (protein content was 13.18±2.51 mg/l), protein content in NRVMs were significantly increased at 24 h after LPS (1 µg/ml) stimulation (protein content was 78.81±8.51 mg/l, P<0.01); compared with LPS group, GBE50 (80 µg/ml) and CAPE (20 µg/ml) significantly reduced the increased protein content in NRVMs induced by LPS (protein contents were 20.80±3.42 and 15.78±3.02 mg/l, respectively, P<0.01). No significant difference in protein content was found between LPS + GBE50 group, LPS + CAPE group and control group. Compared with LPS + GBE50 group, protein content was slightly reduced in LPS + CAPE group, but the difference was not significant (Fig. 6).
Discussion
In 1997, Medzhitov et al (11) found the first human Toll protein-TLR4, confirming the presence of TLR4/NF-κB signaling pathway in human body. TLR4/NF-κB is the primary pathway that mediates LPS signaling, and LPS from Escherichia coli can specifically activate TLR4 without affecting other members of Toll-like receptor families (9,10). Our results showed that TLR4 was expressed in NRVMs, and LPS increased the expression level of TLR4, ATG and AT1a receptor, and also increased the indexes of cardiomyocyte hypertrophy - protein content in cardiomyocytes and expression level of β-MHC expression, suggesting that TLR4/NF-κB signaling pathway may be involved in the occurrence and development of cardiomyocyte hypertrophy by activating local renin-angiotensin system. NF-κB is a group of multidirectional nuclear transcriptional regulators. Under normal conditions, p50 and p65, which are two subunits of NF-κB, can form heterodimers in cytoplasm, in the inactive form of NF-κB. Inhibitors-κB (IκBs), which can bind to NF-κB dimer to mask the nuclear localization sequence of NF-κB, controls the activation of NF-κB. Phosphorylation and activation of inhibitor-κB kinase (IKK) can be induced by a variety of stimulatory factors, and phosphorylated IκBs will replease and activate NF-κB, then NF-κB will translocate from cytoplasm to nucleus to bind the target genes, which in turn regulate the expression of a series of target genes related to the pathophysiological processes of cardiovascular disease and promote protein synthesis in cardiomyocytes. The target genes include cytokines, angiotensinogen, AT1 receptor and embryonic genes such as β-MHC (12). In vivo and in vitro experiments have shown that NF-κB is the essential nuclear transcriptional regulator for the development of cardiac hypertrophy (13,14). In this study, 24 h after LPS stimulation, NF-κB p65 was activated, while NF-κB inhibitor CAPE inhibited the activation of NF-κB p65 and reduced the expression levels of angiotensinogen, AT1a receptor, β-MHC and increased protein content in cardiomyocytes. Therefore, TLR4/NF-κB is involved in the LPS-induced changes in cardiomyocytes, and NF-κB may play a key role.
GBE50 is the concentrated and purified active ingredient of Ginkgo biloba. GBE50 contains 44.1% of Ginkgo biloba total flavonoids, 26.4% of flavonoid glycosides and 6.4% of ginkgolides. Ginkgolides can effectively inhibit the activation of platelet and flavonoids can remove oxygen free radicals to achieve antioxidant effect. It is reported that GBE can remove oxygen free radicals and reduce the expression levels of p53 and Bcl-2, so GBE is widely used in the treatment of cardiovascular disease (6–8). Myocardial ischemia and reperfusion experiments found that GBE can increase antioxidant activity in plasma, which in turn protects the heart (15). In animal models of doxorubicin-induced chronic cardiotoxicity, Naidu et al (16) showed that GBE could reduce the adipokine-induced increase in myocardial lipid peroxidation and restored the antioxidant activity of superoxide dismutase and reduced glutathione, which in turn protects the heart from adriamycin toxicity and reduces mortality. GBE50 inhibited the activation of TLR4/NF-κB and local RAS in NRVMs induced by LPS, and also reduced the expression of β-MHC and decreased the protein content and reversed cardiac hypertrophy. TLR4/NF-κB and RAS are also closely related to myocardial remodeling, suggesting that GBE50 may have an important role in the prevention and treatment of myocardial remodeling. Results of this study suggest that GBE50 and NF-κB inhibitor CAPE have similar effects, and they both can significantly inhibit the activation of NF-κB. Studies have shown that reactive oxygen species are involved in the activation of NF-κB induced by LPS, and various antioxidants can inhibit NF-κB activation (1,12). So the inhibitory effects of GBE50 on NF-κB activation may be related to the function of GBE50 in scavenging oxygen free radicals.
In this study, LPS upregulated expression level of TLR4 in NRVMs, while GBE50 reduced the increased expression level of TLR4. Compared with LPS + GBE50 group, expression level of TLR4 mRNA was slightly reduced in LPS + CAPE group, but the difference was not significant. In vitro studies performed by Frantz et al (1) suggested that NF-κB activation can not only mediate downstream signal transduction of TLR4, but also is necessary for maintaining TLR4 expression. Results of this study showed that expression level of TLR4 was increased after the activation of NF-κB, while expression level of TLR4 was decreased with the inhibited activation of NF-κB, suggesting that the regulatory role of GBE50 in TLR4 expression may also be associated with the inhibition of NF-κB activation. Through decreasing the expression level of TLR4, GBE50 indirectly inhibited the activation of NF-κB, which in turn reduced TLR4/NF-κB signal, so as to inhibit the activation of RAS, reduce expression level of β-MHC and increase protein content in cardiomyocytes with activated TLR4/NF-κB pathway.
In conclusion, GBE50 can inhibit the activation of NF-κB and expression of TLR4 induced by LPS, which in turn attenuate TLR4/NF-κB signaling pathway and reduce the increased expression levels of angiotensinogen and AT1a receptors. TLR4/NF-κB signaling pathway intervention may be one of the mechanisms of GBE in preventing myocardial remodeling.
Acknowledgements
We are grateful for the support of the National Natural Science Foundation of China (grant no. 30371568).
References
|
Frantz S, Kobzik L, Kim YD, Fukazawa R, Medzhitov R, Lee RT and Kelly RA: Toll4 (TLR4) expression in cardiac myocytes in normal and failing myocardium. J Clin Invest. 104:271–280. 1999. View Article : Google Scholar : PubMed/NCBI | |
|
Yasuda S and Lew WY: Angiotensin II exacerbates lipopolysaccharide-induced contractile depression in rabbit cardiac myocytes. Am J Physiol. 276:H1442–H1449. 1999.PubMed/NCBI | |
|
Li HL, Suzuki J, Bayna E, Zhang FM, Dalle Molle E, Clark A, Engler RL and Lew WY: Lipopolysaccharide induces apoptosis in adult rat ventricular myocytes via cardiac AT(1) receptors. Am J Physiol Heart Circ Physiol. 283:H461–H467. 2002. View Article : Google Scholar : PubMed/NCBI | |
|
Li HB, Li X, Huo CJ, Su Q, Guo J, Yuan ZY, Zhu GQ, Shi XL, Liu JJ and Kang YM: TLR4/MyD88/NF-κB signaling and PPAR-γ within the paraventricular nucleus are involved in the effects of telmisartan in hypertension. Toxicol Appl Pharmacol. 305:93–102. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Dange RB, Agarwal D, Masson GS, Vila J, Wilson B, Nair A and Francis J: Central blockade of TLR4 improves cardiac function and attenuates myocardial inflammation in angiotensin II-induced hypertension. Cardiovasc Res. 103:17–27. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
Liu AH, Bao YM, Wang XY and Zhang ZX: Cardio-protection by Ginkgo biloba extract 50 in rats with acute myocardial infarction is related to Na+-Ca2+ exchanger. Am J Chin Med. 41:789–800. 2013. View Article : Google Scholar : PubMed/NCBI | |
|
Lu S, Guo X and Zhao P: Effect of Ginkgo biloba extract 50 on immunity and antioxidant enzyme activities in ischemia reperfusion rats. Molecules. 16:9194–9206. 2011. View Article : Google Scholar : PubMed/NCBI | |
|
Liu TJ, Yeh YC, Ting CT, Lee WL, Wang LC, Lee HW, Wang KY and Lai HC and Lai HC: Ginkgo biloba extract 761 reduces doxorubicin-induced apoptotic damage in rat hearts and neonatal cardiomyocytes. Cardiovasc Res. 80:227–235. 2008. View Article : Google Scholar : PubMed/NCBI | |
|
Takada H, Yokoyama S and Yang S: Enhancement of endotoxin activity by muramyldipeptide. J Endotoxin Res. 8:337–342. 2002. View Article : Google Scholar : PubMed/NCBI | |
|
Wang J, Zhang Y, Guo LL, Wu GJ and Liu RH: Salvianolic acid B inhibits the TLR4-NFκB-TNFα pathway and attenuates neonatal rat cardiomyocyte injury induced by lipopolysaccharide. Chin J Integr Med. 17:775–779. 2011. View Article : Google Scholar : PubMed/NCBI | |
|
Medzhitov R, Preston-Hurlburt P and Janeway CA Jr: A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature. 388:394–397. 1997. View Article : Google Scholar : PubMed/NCBI | |
|
Jones WK, Brown M, Ren X, He S and McGuinness M: NF-kappaB as an integrator of diverse signaling pathways: The heart of myocardial signaling? Cardiovasc Toxicol. 3:229–254. 2003. View Article : Google Scholar : PubMed/NCBI | |
|
Purcell NH, Tang G, Yu C, Mercurio F, DiDonato JA and Lin A: Activation of NF-kappa B is required for hypertrophic growth of primary rat neonatal ventricular cardiomyocytes. Proc Natl Acad Sci USA. 98:pp. 6668–6673. 2001, View Article : Google Scholar : PubMed/NCBI | |
|
Shi Y, Pan H, Zhang HZ, Zhao XY, Jin J and Wang HY: Lipoxin A4 mitigates experimental autoimmune myocarditis by regulating inflammatory response, NF-κB and PI3K/Akt signaling pathway in mice. Eur Rev Med Pharmacol Sci. 21:1850–1859. 2017.PubMed/NCBI | |
|
Carini M, Aldini G, Rossoni G, Morazzoni P and Facino RM: Complexation of Ginkgo biloba extract with phosphatidylcholine improves cardioprotective activity and increases the plasma antioxidant capacity in the rat. Planta Med. 67:326–330. 2001. View Article : Google Scholar : PubMed/NCBI | |
|
Naidu MU, Kumar KV, Mohan IK, Sundaram C and Singh S: Protective effect of Gingko biloba extract against doxorubicin-induced cardiotoxicity in mice. Indian J Exp Biol. 40:894–900. 2002.PubMed/NCBI |