Antibiotic prophylaxis vs. on‑demand antibiotic treatment in endoscopic therapy for variceal hemorrhage: A meta‑analysis of randomized controlled trials
- Authors:
- Published online on: June 28, 2024 https://doi.org/10.3892/etm.2024.12629
- Article Number: 340
-
Copyright: © Tao et al. This is an open access article distributed under the terms of Creative Commons Attribution License.
Abstract
Introduction
Acute variceal bleeding, including esophageal and gastric variceal bleeding, is the main cause of bleeding in 50-60% of patients with cirrhosis (1). Acute variceal bleeding is usually complicated by ascites (34%), infection (30%), respiratory complications (24%), intensive care unit admission (20%), rebleeding (11%), encephalopathy (7%), acute kidney injury (6%) and failure to control bleeding (4%) (2). In addition, acute variceal bleeding, one of the most fatal complications of cirrhosis, is responsible for 34-50% of cirrhosis-related deaths (3,4). In the last two decades, mortality from acute variceal bleeding has decreased from 40 to 15-20% due to endoscopic therapy, including ligation, sclerotherapy and cyanoacrylate glue injection (4,5). Endoscopy performed within 24 h is recommended for patients with acute upper gastrointestinal bleeding (6,7).
Some studies have reported low or no risk of infection following endoscopic therapy (8,9). However, certain studies have reported that endoscopic therapy can increase the risk of infection (10,11). Therefore, certain studies have recommended the use of antibiotic prophylaxis, leading to the on-demand use of antibiotics following endoscopic therapy (12-15).
Hou et al (12) reported that, in addition to preventing infection, the administration of antibiotic prophylaxis following endoscopic therapy for acute variceal bleeding can decrease early and late rebleeding and mortality. However, Agarwal et al (14) and Liu et al (13) reported conflicting results, stating that antibiotic prophylaxis following endoscopy is not associated with mortality and rebleeding.
Endoscopic therapy is a useful method for the treatment of acute esophageal and gastric variceal bleeding; this therapy can decrease the risk of mortality in patients with acute variceal bleeding. However, it remains unknown whether antibiotic prophylaxis is necessary to decrease infection, rebleeding and mortality. Inconsistent results from different randomized controlled trials (RCTs) (12-15) have resulted in discrepancies in the reported data. Therefore, a systematic review and meta-analysis was performed to integrate the data and provide guidance for gastroenterologists. The aim of the present study was to conduct a meta-analysis that could elucidate the effects of antibiotic prophylaxis on infection, rebleeding and mortality in patients that had undergone endoscopic therapy for variceal hemorrhage.
Materials and methods
Eligibility of included studies
The PubMed (http://www.ncbi.nlm./pubmed/), Embase (http://www.embase.com.) and Cochrane Library (http://www.cochranelibrary.com/www.cochranelibrary.com/) databases were systematically searched between January 1959 and January 2024, to ensure that all relevant literature was covered. The search terms used were: (‘antibiotics’) AND (‘variceal bleeding’) AND (‘endoscopy’ or ‘ligation’ or ‘sclerotherapy’ or ‘glue’).
Inclusion criteria
The comparative studies that explored the efficacy of antibiotic prophylaxis and on-demand treatment for acute variceal bleeding following endoscopic therapy were included. In addition, only RCTs written in English were included. The patients in the prophylactic group received antibiotic treatment immediately following randomization. The antibiotics were used only when infection was suspected or established in the on-demand antibiotic-treatment group of patients. The main outcome was the incidence of infection. The secondary outcomes were the incidence of rebleeding, early rebleeding and mortality. The information on the method of randomization, usage of antibiotics and assessment of infection were necessary for the inclusion of the studies. Infection was defined as fever (>38˚C), hypothermia (<36˚C), unexpected hemodynamic instability, tachypnea, new onset of symptoms, such as cough, dysuria, septicemia, urinary tract infection, spontaneous peritonitis and pneumonia, or positive blood cultures. Rebleeding was defined as rebleeding within 2 months after initial control of bleeding. Early bleeding was defined as rebleeding within 7 days after initial control of bleeding.
Exclusion criteria
The following exclusion criteria were used: i) Non-comparative studies, retrospective studies and studies that were not RCTs; ii) patients with non-acute esophageal or gastric variceal bleeding; iii) studies in which the patients did not receive prophylactic antibiotics following endoscopic therapy; iv) studies that did not include control groups with patients administered on-demand antibiotics; and, v) studies that were not written in English.
Data extraction
Following the initial database search, three reviewers independently screened the titles and abstracts, and excluded irrelevant articles according to the inclusion and exclusion criteria. The number of studies excluded along with the reasons for exclusion were recorded. Following the initial examination of the titles and abstracts, the reviewers performed a thorough evaluation of the included articles and extracted information, including the leading author, country, publication year, baseline characteristics, endoscopic therapeutic methods and outcome indicators involving incidence of infection, rebleeding, early rebleeding and mortality. In case of disagreement among the three reviewers, the final decision was made through discussion and a mutual consensus between the researchers was achieved.
Quality assessment of the included studies
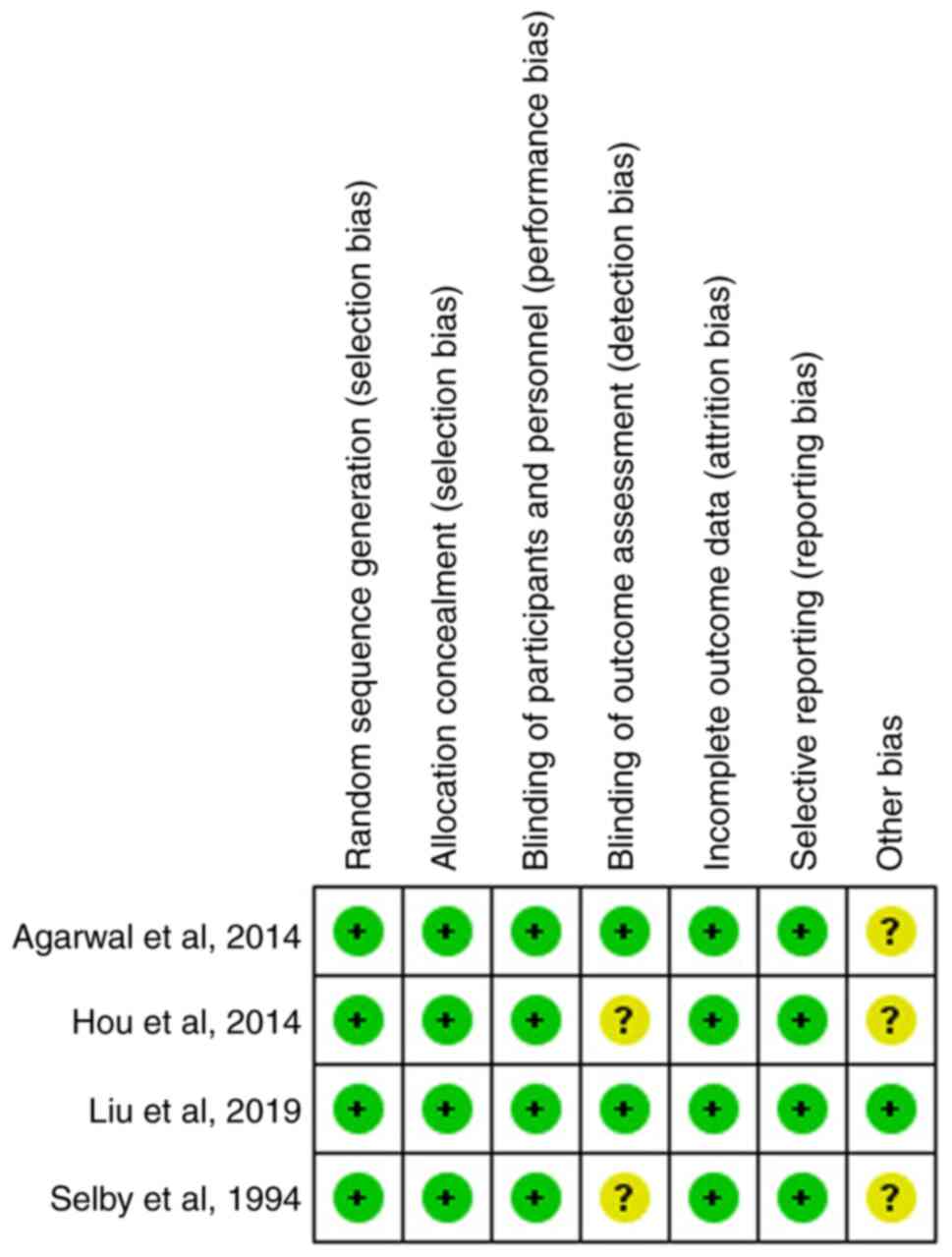
Two researchers were assigned to assess the risk of bias in each trial using the Cochrane risk-of-bias assessment tool (16). The seven domains of the Cochrane risk-of-bias assessment tool were used to assess the bias for each individual study as follows: Randomization, allocation, blind involvement of participants and study personnel, outcome assessors, incomplete outcome data, selection of reporting and other bias (17). Following evaluation, the included studies were graded into three levels, including ‘unclear risk of bias’, ‘low risk of bias’, and ‘high risk of bias’.
Statistical analysis
RevMan software version 5.4.1 (The Nordic Cochrane Center; The Cochrane Collaboration, 2020) was used to analyze the extracted data. The χ2 test was used to qualitatively assess the heterogeneity of the groups (P<0.05 was considered to indicate a statistically significant difference) and the I2 statistic was used to quantitatively evaluate the overall heterogeneity of the studies (18).
A random-effect model was used for all analyses as recommended in the Cochrane Handbook for Systematic Reviews of Interventions version 5.1.0 (https://training.cochrane.org/handbook/archive/v5.1/). Odds ratios (ORs) and 95% confidence intervals (CIs) were used to present the summarized estimates for the dichotomous data (17). The effect estimates and 95% CIs of the individual and overall studies are shown in the figures using forest plots. A funnel plot was not drawn as the total number of studies assessed was <10(19).
Results
Study retrieval and selection
Following the database search, a total of 695 studies were selected for the initial screening. Following removal of the duplicates, 287 studies were excluded and following searching of the titles and abstracts of the remaining 408 studies, 389 were excluded, leaving only 19 studies. Following assessment of the full-text articles for eligibility, 11 retrospective studies were excluded, leaving eight prospective studies for review. The qualitative and quantitative analyses were performed and four prospective RCTs were finally included in the present meta-analysis. A flow diagram of the process of study retrieval and selection is shown in Fig. 1.
Characteristics of the eligible studies
Table I summarizes the basic characteristics of the four studies; they were published from 1994 to 2019, and the study sites were distributed across the following four areas: India, China, Taiwan and Australia. All of the included articles were RCTs. Across the four RCTs, 326 patients were involved. The sample size of each trial ranged from 39 to 120 patients. There were no significant differences in sex ratio, mean age ± standard deviation and the Child-Pugh score (12-15) or grade (A/B/C) between the intervention and control groups in each RCT. All four studies included patients with acute variceal bleeding and those who had undergone endoscopic treatment including endoscopic variceal ligation (EVL), endoscopic variceal sclerotherapy (EVS) and tissue adhesive injection. The intervention methods of the antibiotic prophylaxis group in the included articles were divided into two types. One intervention method was: Intravenous ofloxacin (200 mg) q12H for 2 days, followed by oral ofloxacin (200 mg) q12H for 5 days following endoscopic therapy. The other intervention method was: Intravenous cefotiam (2 g) or intravenous cefotaxime (1 g) administered one day prior to endoscopic therapy. The intervention method for the on-demand group in all four studies was: Antibiotics such as ofloxacin, cefotiam or cefotaxime were used only when infection was suspected or established. The outcomes of all four studies included the incidence of infection and mortality, and three of the included articles reported outcomes of the incidence of rebleeding. The assessments of infection, rebleeding and mortality are described in detail in the previous studies (12-15).
Quality assessment of the included studies
All four studies were randomized using computer-generated numbers and the data were reported in sealed envelopes for allocation concealment. The patients were blinded for intervention in only one of the included studies via the administration of a 100 ml saline solution placebo (13). All four studies had assessed the incidence of infection and mortality (12-15). Three of the included studies reported the incidence of rebleeding (12-14). However, none of the four studies mentioned a blinded assessment of the outcomes, while the follow-up outcomes of all the included patients in the four studies were reported. Publication years, countries and Endoscopic treatment methods are the unclear risks of bias. The quality assessment of four studies is shown in Fig. 2.
Incidence of infection
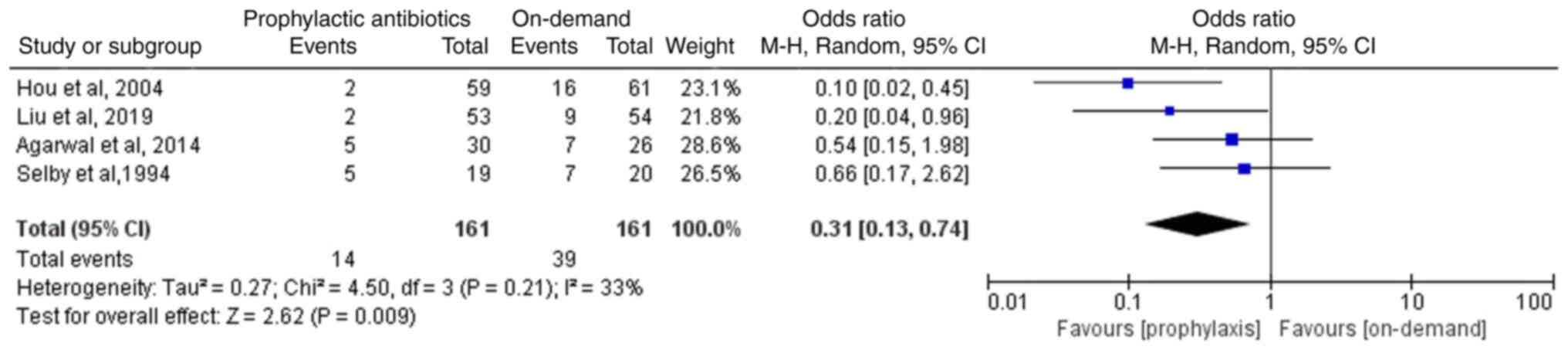
Of the four RCTs (12-15), two studies reported that the comparisons between the on-demand and the prophylactic antibiotics groups indicated lack of statistical significance (14,15). The other two studies reported that the prophylactic antibiotics group exhibited a lower incidence of infection compared with the on-demand group (12,13). The four eligible RCTs involving 322 patients reported the incidence of infection following endoscopic therapy in patients with variceal bleeding. The meta-analysis suggested that the incidence of infection in the prophylactic antibiotics group was significantly lower than that noted in the on-demand group (OR, 0.31; 95% CI, 0.13-0.74; P=0.009; I2, 33%; Fig. 3).
Incidence of rebleeding
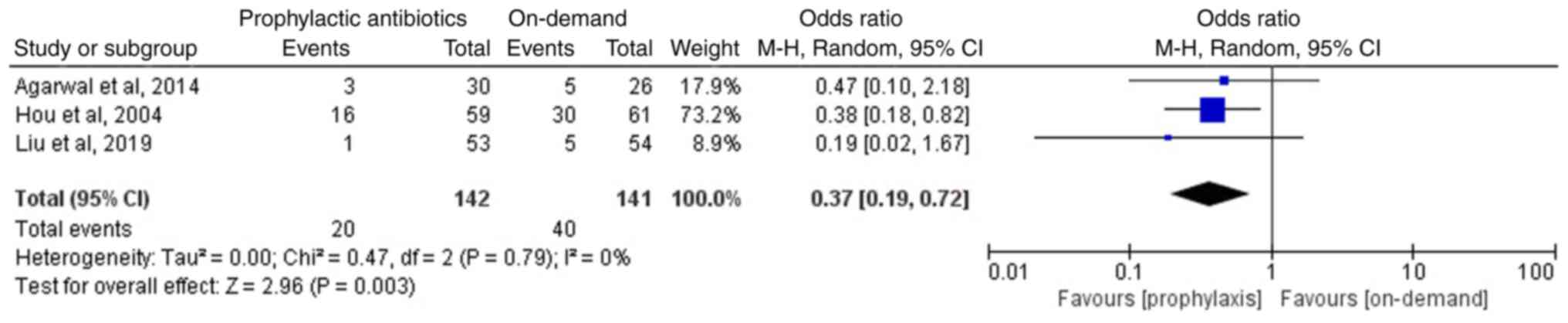
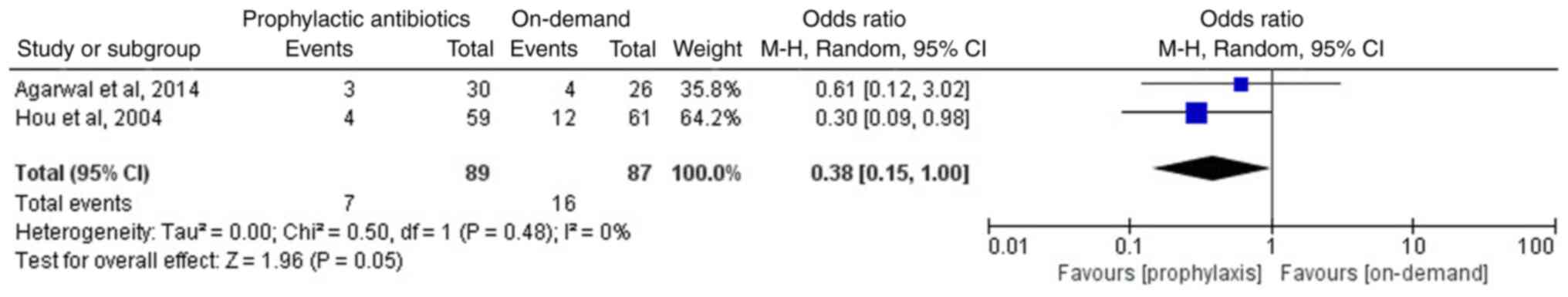
Of the included articles, three studies involving 283 patients reported the incidence of rebleeding in the two groups following endoscopic therapy (12-14); two studies, including those by Agarwal et al (14) and Liu et al (13), reported non-significant differences in the incidence of rebleeding between the prophylactic antibiotics and the on-demand groups. However, Hou et al (12) reported that the prophylactic antibiotics group exhibited a lower incidence of rebleeding than that of the on-demand group. The meta-analysis of the three included studies suggested that the prophylactic antibiotics group exhibited a lower incidence of rebleeding compared with that of the on-demand group (OR, 0.37; 95% CI, 0.19-0.72; P=0.003; I2, 0%; Fig. 4). Furthermore, two studies reported the incidence of early rebleeding (12,14). Early rebleeding was defined as rebleeding within 7 days following the initial control of bleeding (14). A meta-analysis of the two studies, involving 176 patients, suggested that compared with the on-demand group, the prophylactic antibiotics group exhibited a lower incidence of early rebleeding (OR, 0.38; 95% CI, 0.15-1.0; P=0.05; I2, 0%; Fig. 5).
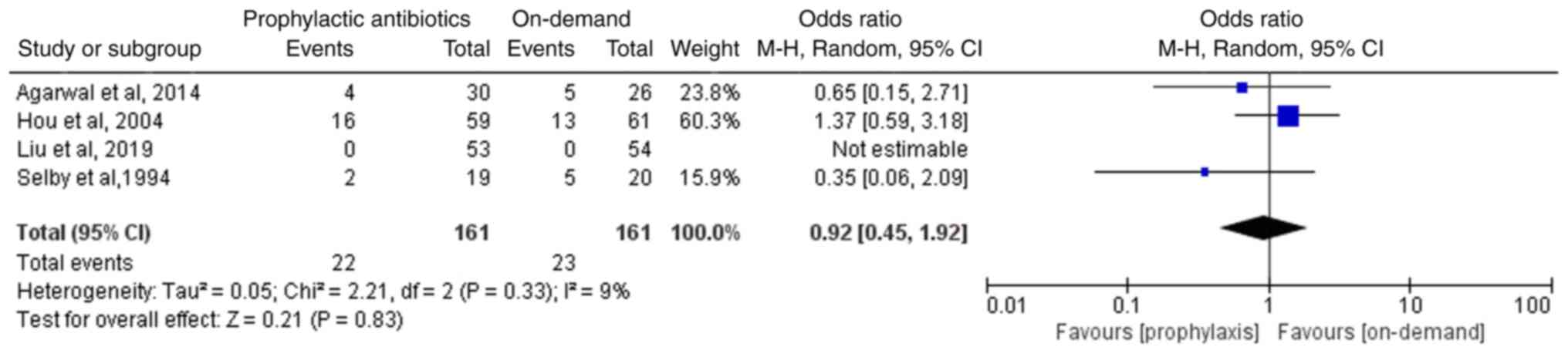
Incidence of mortality
All four included studies, involving 322 patients, reported the incidence of mortality. Liu et al (13) reported no deaths among the included patients, but the three other included studies reported a lack of statistically significant differences in the incidence of mortality between the two groups (12-15). The meta-analysis of the four included RCTs suggested absence of a statistically significant difference in the incidence of mortality between the two groups (OR, 0.92; 95%CI, 0.45-1.92; P=0.83; I2, 9%; Fig. 6).
Publication bias
In the present meta-analysis, <10 articles were included. Therefore, a publication bias test based on a funnel plot was not conducted.
Discussion
Acute variceal bleeding, one of the most fatal complications of cirrhosis, is responsible for 34-50% of cirrhosis-related deaths (3,4,20). The management of acute variceal bleeding requires a multidisciplinary approach, including pharmacological, endoscopic and radiological interventions (21). Endoscopic therapy and antibiotic prophylaxis, as independent factors, improve the survival and decrease the mortality rate of patients with acute variceal bleeding (22,23). Carbonell et al (22) reported that the incidence of mortality in patients with acute variceal bleeding decreased from 42.6% in 1980 to 14.5% in 2000 due to a combination of endoscopic therapy and antibiotic prophylaxis. In addition, for patients with Child-Pugh class A, mortality decreased from 9% in 1980 to 0% in 2000(22). Vuachet et al (24) reported similar results; specifically, endoscopic therapy and antibiotic prophylaxis decreased the 6-week mortality rate and the number of red cell unit transfusions. For esophageal variceal bleeding, endoscopic therapy includes EVL and EVS (25,26), and tissue adhesive injections are recommended (27). Antibiotic use has been reported to reduce the mortality of patients with variceal bleeding (28); morover, it prevented infection in patients following endoscopic therapy. The UK guidelines and the Korean Association for the Study of the Liver clinical practice guidelines have recommended short-term antibiotic use (covering gram-negative antibiotic administration within 1 day) for patients with acute variceal bleeding (7,29). The European Society for Gastrointestinal Endoscopy guidelines have recommended intravenous erythromycin (250 mg) 30-120 min prior to upper gastrointestinal endoscopy in patients with suspected acute variceal bleeding, in the absence of contraindications (30). By contrast, Lee et al (31) reported that early prophylactic antibiotic use increased the risk of early bacterial infections, whereas it did not decrease the risk of infection in cases with acute variceal bleeding and it did not prevent infection in patients following endoscopic therapy. Bacteremia has also been reported in patients with variceal bleeding following endoscopic therapy (10,11). Ueno et al (32) reported that the prophylactic use of antibiotics was not associated with the 30-day mortality rate or the frequency of nosocomial bacterial infections. However, Jia et al (8) reported a low risk of bacteremia in patients with varices following endoscopic therapy. Zuckerman et al (9) reported that bacteremia was not associated with endoscopic therapy in patients with variceal bleeding. Therefore, whether endoscopic therapy for patients with varices increases the rate of infection remains to be elucidated.
Infection is one of the main factors associated with mortality following the cessation of initial variceal bleeding (33). Whether antibiotic prophylaxis is necessary to prevent bacterial infection and to decrease the mortality of patients with variceal bleeding following endoscopic therapy has been debated. Following a search and screening of the databases according to the inclusion and exclusion criteria, four RCTs were included (12-15). All four studies reported different results involving the incidence of infection, rebleeding and mortality; two articles reported that antibiotic prophylaxis was not necessary for patients with variceal bleeding following endoscopic therapy due to the lack of significant difference in the incidence of infection between the antibiotic prophylaxis and the on-demand groups (14,15). By contrast, the other two studies reported opposing results, which indicated that antibiotic prophylaxis was necessary to prevent bacterial infection in patients with variceal bleeding following endoscopic therapy (12,13). The present meta-analysis with 322 patients from the four included studies suggested that antibiotic prophylaxis was necessary to prevent bacterial infection due to the incidence of infection in the prophylactic antibiotic group being significantly lower than that noted in the on-demand antibiotic group. Furthermore, the meta-analysis of three included studies, involving 283 patients, suggested that antibiotic prophylaxis decreased the incidence of rebleeding (12-14). Based on a meta-analysis of two included studies involving 176 patients, antibiotic prophylaxis was also beneficial in decreasing the incidence of early rebleeding (12,14). However, with regard to the incidence of mortality, the present meta-analysis of the four included RCTs, involving 322 patients, suggested that antibiotic prophylaxis did not decrease mortality in patients with variceal bleeding following endoscopic therapy. Inclusion and meta-analysis of the four RCTs suggested that the interventions performed in the antibiotic prophylaxis group aided the prevention of bacterial infection and rebleeding in patients with variceal bleeding following endoscopic therapy compared with those in the on-demand group; however, these interventions did not decrease mortality.
The methods of antibiotic prophylaxis differed among the four RCTs. The two antibiotic prophylaxis methods performed were as follows: Intravenous ofloxacin (200 mg q12H) for 2 days, followed by oral ofloxacin (200 mg q12H) for 5 days after endoscopic therapy (12,14); intravenous cefotiam (2 g) or intravenous cefotaxime (1 g) administered 1 day prior to endoscopic therapy (13,15). The application of prophylaxis for 7 days may influence the outcomes compared with administration of prophylaxis for 1 day. The UK guidelines recommend the 1-day antibiotic prophylaxis program prior to endoscopic therapy (7), while two previous studies have used the 7-day antibiotics prophylaxis program (34,35). The outcomes from antibiotics treatment occurring 7 days following endoscopic therapy and those occurring during prophylaxis antibiotics treatment on the first day prior to endoscopic therapy have not been reported for patients with variceal bleeding; a previous study evaluating these parameters provided certain clarifications, including Child Pugh grade A/B/C, regarding the optimal antibiotic prophylaxis treatment (34). An additional issue that remains to be elucidated is the type of antibiotic; two studies involving the prophylactic intravenous first-generation cephalosporin, cefazolin and the third-generation cephalosporin, ceftriaxone, for patients with variceal bleeding following endoscopic interventions reported contrasting results that third-generation cephalosporin ceftriaxione was not superior to the first-generation cephalosporin cefazolin (34,35). A previous study by Wu et al (34) that included 713 patients reported that the third-generation cephalosporin ceftriaxone exhibited improved efficacy compared with the first-generation cephalosporin cefazolin in preventing infection and reducing rebleeding in patients who underwent endoscopic therapy for acute variceal bleeding. The UK guidelines also recommend the third-generation cephalosporin ceftriaxione as antibiotic prophylaxis following endoscopic therapy for acute variceal bleeding (7). However, a previous study by Lee et al (35) that included 84 patients reported contradictory results, stating that the first-generation cephalosporin cefazolin exhibited improved efficacy than that of the third-generation cephalosporin ceftriaxone; notably, the latter was administered to a small sample size of patients. A clinical RCT or meta-analysis with a larger sample size is required to determine the optimal prophylactic antibiotic following endoscopic intervention for patients with acute variceal bleeding.
The present meta-analysis that included four RCTs with 322 patients suggested that the antibiotic prophylaxis group exhibited a lower incidence of infection and rebleeding following endoscopic therapy in patients with variceal bleeding compared with that of the on-demand group. However, no significant difference was noted in the mortality between the two groups. The current meta-analysis discussed the effectiveness of prophylactic antimicrobial therapy and included a small number of studies, providing limited value for daily clinical practice. Notably, the different intervention methods of the prophylaxis group in the four studies may have affected the outcomes of the current meta-analysis. High-quality evidence obtained from RCTs or meta-analyses with a larger sample size are required to elucidate the optimal prophylactic antibiotic and antibiotic prophylaxis method for patients with variceal bleeding following endoscopic therapy.
Acknowledgements
The authors would like to thank Professor Long Chen from Nanchong Central Hospital (Sichuan, China) who contributed to the statistical guidance.
Funding
Funding: The present study was funded by the Key Program of Science & Technology Department of Sichuan Province (grant no. 2023YFS0473) and the Bureau of Science & Technology Nanchong City (grant no. 22SXQT0401).
Availability of data and materials
The data generated in the present study may be requested from the corresponding author.
Authors' contributions
ZT and WP conceived and designed the study. YG, YZ, XT and YH acquired the data. DH, JC, JY, ZD, SL and SF analyzed and interpretated the data according to the inclusion and exclusion criteria. ZT, WP and YG confirm the authenticity of all the raw data. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
|
van Leerdam ME: Epidemiology of acute upper gastrointestinal bleeding. Best Pract Res Clin Gastroenterol. 22:209–224. 2008.PubMed/NCBI View Article : Google Scholar | |
|
Carneiro de Moura M, Chen S, Kamath BM, Ng VL and Ling SC: Acute variceal bleeding causes significant morbidity. J Pediatr Gastroenterol Nutr. 67:371–376. 2018.PubMed/NCBI View Article : Google Scholar | |
|
Tayyem O, Bilal M, Samuel R and Merwat SK: Evaluation and management of variceal bleeding. Dis Mon. 64:312–320. 2018.PubMed/NCBI View Article : Google Scholar | |
|
Hsu YC, Chung CS and Wang HP: Application of endoscopy in improving survival of cirrhotic patients with acute variceal hemorrhage. Int J Hepatol. 2011(893973)2011.PubMed/NCBI View Article : Google Scholar | |
|
García-Pagán JC, Reverter E, Abraldes JG and Bosch J: Acute variceal bleeding. Semin Respir Crit Care Med. 33:46–54. 2012.PubMed/NCBI View Article : Google Scholar | |
|
Lau JYW, Yu Y, Tang RSY, Chan HCH, Yip HC, Chan SM, Luk SWY, Wong SH, Lau LHS, Lui RN, et al: Timing of endoscopy for acute upper gastrointestinal bleeding. N Engl J Med. 382:1299–1308. 2020.PubMed/NCBI View Article : Google Scholar | |
|
Tripathi D, Stanley AJ, Hayes PC, Patch D, Millson C, Mehrzad H, Austin A, Ferguson JW, Olliff SP, Hudson M, et al: U.K. guidelines on the management of variceal haemorrhage in cirrhotic patients. Gut. 64:1680–1704. 2015.PubMed/NCBI View Article : Google Scholar | |
|
Jia Y, Dwivedi A, Elhanafi S, Ortiz A, Othman M and Zuckerman M: Low risk of bacteremia after endoscopic variceal therapy for esophageal varices: A systematic review and meta-analysis. Endosc Int Open. 3:E409–E417. 2015.PubMed/NCBI View Article : Google Scholar | |
|
Zuckerman MJ, Jia Y, Hernandez JA, Kolli VR, Norte A, Amin H, Casner NA, Dwivedi A and Ho H: A prospective randomized study on the risk of bacteremia in banding versus sclerotherapy of esophageal varices. Front Med (Lausanne). 3(16)2016.PubMed/NCBI View Article : Google Scholar | |
|
Galperine T, Flateau C, Venon MD, Lescure FX, Béraud G, Said Ibrahim T, Delisle F, Durand F, Faure K, Pialoux G and Guery B: Recurrent bacteremia, a complication of cyanoacrylate injection for variceal bleeding: Report of two cases and review of the literature. Case Rep Med. 2009(407053)2009.PubMed/NCBI View Article : Google Scholar | |
|
Randi BA, Ninomiya DA, Nicodemo EL, Lopes BC, Cançado ER and Levin AS: Recurrent bacteremia after injection of N-butyl-2-cyanoacrylate for treatment of bleeding gastric varices: A case report and review of the literature. BMC Res Notes. 8(692)2015.PubMed/NCBI View Article : Google Scholar | |
|
Hou MC, Lin HC, Liu TT, Kuo BI, Lee FY, Chang FY and Lee SD: Antibiotic prophylaxis after endoscopic therapy prevents rebleeding in acute variceal hemorrhage: A randomized trial. Hepatology. 39:746–753. 2004.PubMed/NCBI View Article : Google Scholar | |
|
Liu C, Ma L, Wang J, Li F, Tseng Y, Luo T, Zeng X and Chen S: Prophylactic use of antibiotics in endoscopic injection of tissue adhesive for the elective treatment of gastric varices: A randomized controlled study. J Gastroenterol Hepatol. 32:1486–1491. 2019.PubMed/NCBI View Article : Google Scholar | |
|
Agarwal A, Kumar SS, Sadasivan J and Kate V: Antibiotic prophylaxis in the prevention of rebleeding in acute variceal hemorrhage: A randomized trial. J Pharmacol Pharmacother. 6:24–29. 2015.PubMed/NCBI View Article : Google Scholar | |
|
Selby WS, Norton ID, Pokorny CS and Benn RA: Bacteremia and bacterascites after endoscopic sclerotherapy for bleeding esophageal varices and prevention by intravenous cefotaxime: A randomized trial. Gastrointest Endosc. 40:680–684. 1994.PubMed/NCBI | |
|
Higgins JPT, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA, et al: The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 343(d5928)2011.PubMed/NCBI View Article : Google Scholar | |
|
Higgins JP and Green S: Cochrane handbook for systematic reviews of interventions version 5.0.0. Naunyn-Schmiedebergs Archiv Exp Pathol Pharmackol. 5(S38)2009. | |
|
Bowden J, Tierney JF, Copas AJ and Burdett S: Quantifying, displaying and accounting for heterogeneity in the meta-analysis of RCTs using standard and generalised Q statistics. BMC Med Res Methodol. 11(41)2011.PubMed/NCBI View Article : Google Scholar | |
|
Palma Pérez S and Delgado Rodríguez M: Practical considerations on detection of publication bias. Gac Sanit. 20 (Suppl 3):S10–S16. 2006.PubMed/NCBI View Article : Google Scholar : (In Spanish). | |
|
Zanetto A and Garcia-Tsao G: Management of acute variceal hemorrhage. F1000Res. 8(F1000)2019.PubMed/NCBI View Article : Google Scholar | |
|
Alqahtani SA and Jang S: Pathophysiology and management of variceal bleeding. Drugs. 81:647–667. 2021.PubMed/NCBI View Article : Google Scholar | |
|
Carbonell N, Pauwels A, Serfaty L, Fourdan O, Lévy VG and Poupon R: Improved survival after variceal bleeding in patients with cirrhosis over the past two decades. Hepatology. 40:652–659. 2004.PubMed/NCBI View Article : Google Scholar | |
|
O'Brien J, Triantos C and Burroughs AK: Management of varices in patients with cirrhosis. Nat Rev Gastroenterol Hepatol. 10:402–412. 2013.PubMed/NCBI View Article : Google Scholar | |
|
Vuachet D, Cervoni JP, Vuitton L, Weil D, Dritsas S, Dussaucy A, Koch S, Di Martino V and Thevenot T: Improved survival of cirrhotic patients with variceal bleeding over the decade 2000-2010. Clin Res Hepatol Gastroenterol. 39:59–67. 2015.PubMed/NCBI View Article : Google Scholar | |
|
Hou MC, Lin HC, Kuo BI, Chen CH, Lee FY and Lee SD: Comparison of endoscopic variceal injection sclerotherapy and ligation for the treatment of esophageal variceal hemorrhage: A prospective randomized trial. Hepatology. 21:1517–1522. 1995.PubMed/NCBI | |
|
Dai C, Liu WX, Jiang M and Sun MJ: Endoscopic variceal ligation compared with endoscopic injection sclerotherapy for treatment of esophageal variceal hemorrhage: A meta-analysis. World J Gastroenterol. 21:2534–2541. 2015.PubMed/NCBI View Article : Google Scholar | |
|
Lo GH, Lai KH, Cheng JS, Chen MH and Chiang HT: A prospective, randomized trial of butyl cyanoacrylate injection versus band ligation in the management of bleeding gastric varices. Hepatology. 33:1060–1064. 2001.PubMed/NCBI View Article : Google Scholar | |
|
Moon AM, Dominitz JA, Ioannou GN, Lowy E and Beste LA: Use of antibiotics among patients with cirrhosis and upper gastrointestinal bleeding is associated with reduced mortality. Clin Gastroenterol Hepatol. 14:1629–1637.e1. 2016.PubMed/NCBI View Article : Google Scholar | |
|
Korean Association for the Study of the Liver (KASL). KASL clinical practice guidelines for liver cirrhosis: Varices, hepatic encephalopathy, and related complications. Clin Mol Hepatol. 26:83–127. 2020.PubMed/NCBI View Article : Google Scholar | |
|
Gralnek IM, Camus Duboc M, Garcia-Pagan JC, Fuccio L, Karstensen JG, Hucl T, Jovanovic I, Awadie H, Hernandez-Gea V, Tantau M, et al: Endoscopic diagnosis and management of esophagogastric variceal hemorrhage: European society of gastrointestinal endoscopy (ESGE) guideline. Endoscopy. 54:1094–1120. 2022.PubMed/NCBI View Article : Google Scholar | |
|
Lee S, Saxinger L, Ma M, Prado V, Fernández J, Kumar D, Gonzalez-Abraldes J, Keough A, Bastiampillai R, Carbonneau M, et al: Bacterial infections in acute variceal hemorrhage despite antibiotics-a multicenter study of predictors and clinical impact. United European Gastroenterol J. 5:1090–1099. 2017.PubMed/NCBI View Article : Google Scholar | |
|
Ueno M, Fujiwara T, Tokumasu H, Mano T, Kayahara T, Takabatake H, Morimoto Y, Matsueda K, Fukuoka T and Mizuno M: Real-world efficacy of antibiotic prophylaxis for upper gastrointestinal bleeding in cirrhotic patients in Japan. J Gastroenterol. 58:766–777. 2023.PubMed/NCBI View Article : Google Scholar | |
|
Lee SW, Lee TY, Chang CS, Ko CW, Yeh HZ and Yang SS: Independent factors associated with early outcome in Chinese cirrhotic patients after cessation of initial esophageal variceal hemorrhage. J Clin Gastroenterol. 44:e123–e127. 2010.PubMed/NCBI View Article : Google Scholar | |
|
Wu CK, Wang JH, Lee CH, Wu KL, Tai WC, Lu SN, Hu TH and Chuah SK: The outcome of prophylactic intravenous cefazolin and ceftriaxone in cirrhotic patients at different clinical stages of disease after endoscopic interventions for acute variceal hemorrhage. PLoS One. 8(e61666)2013.PubMed/NCBI View Article : Google Scholar | |
|
Lee J, Xu H, Chuah SK, et al: The effect of intravenous cefazolin and ceftrixions as prophylactic antibiotics in cirrhotic patients with acute variceal hemorrhage after endoscopic interventions-A preliminary report. Hepatol Int. 6(302)2012. |