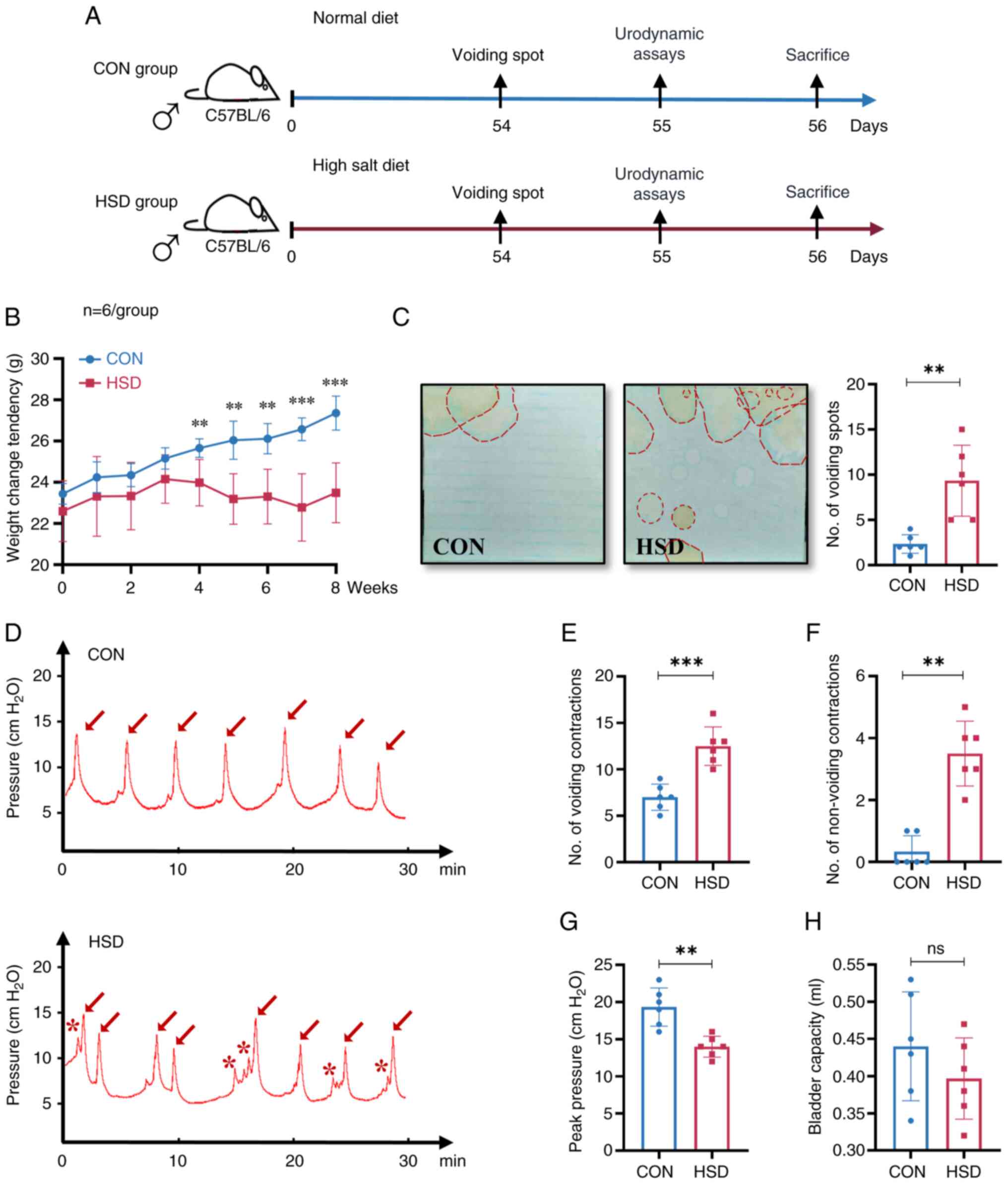
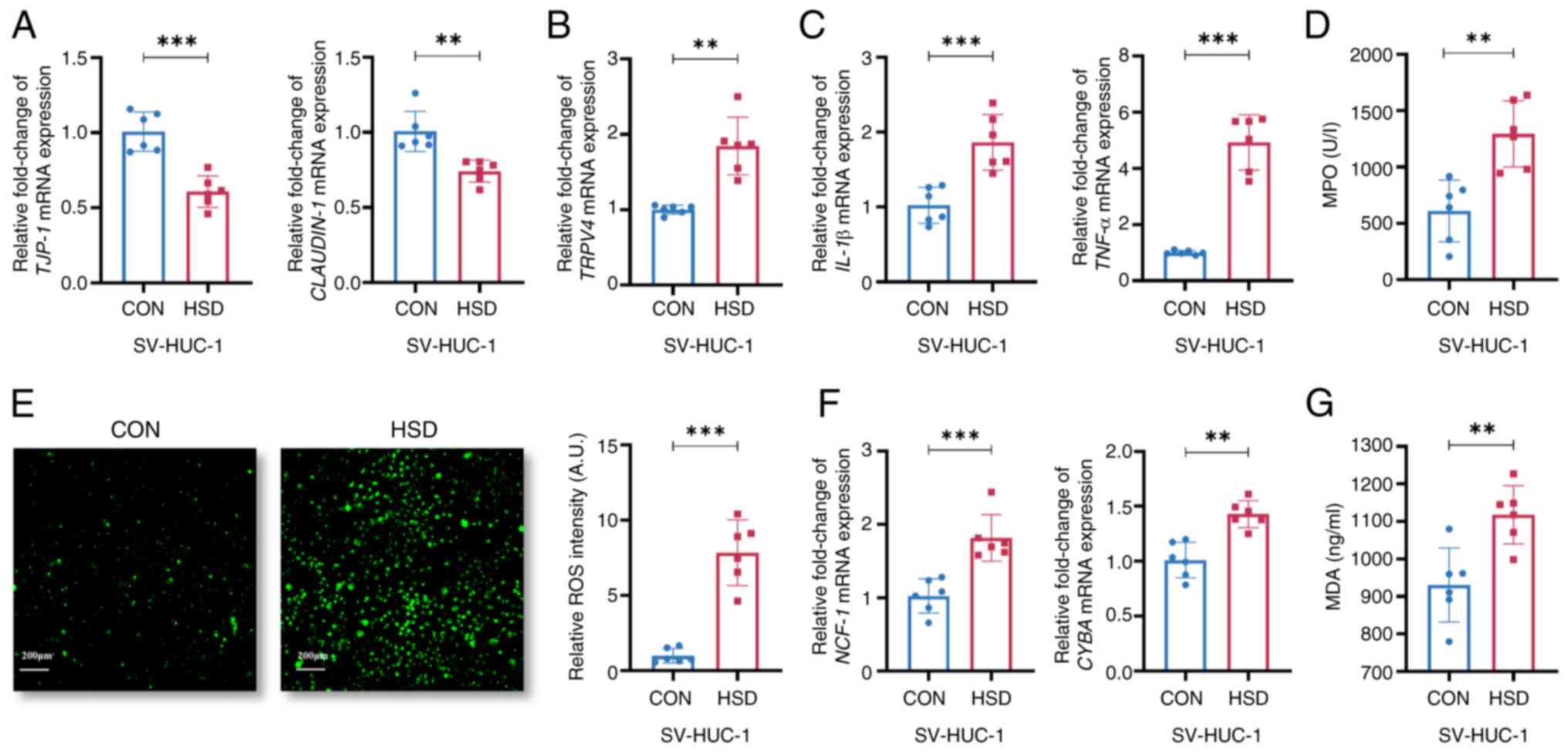
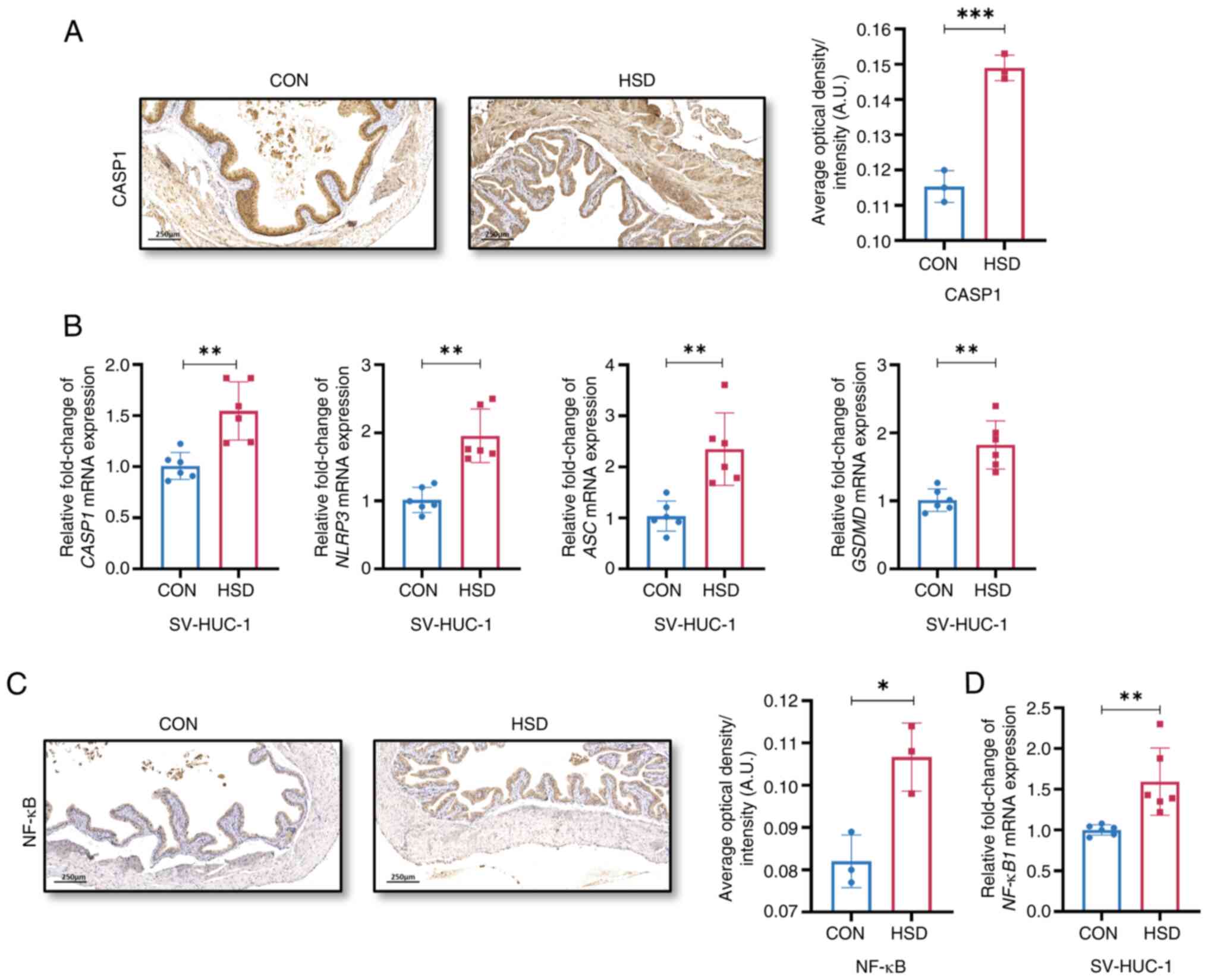
|
1
|
Abrams P, Chapple C, Khoury S, Roehrborn C
and de la Rosette J: International Scientific Committee. Evaluation
and treatment of lower urinary tract symptoms in older men. J Urol.
181:1779–1787. 2009.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Nambiar AK, Arlandis S, Bø K,
Cobussen-Boekhorst H, Costantini E, de Heide M, Farag F, Groen J,
Karavitakis M, Lapitan MC, et al: European association of urology
guidelines on the diagnosis and management of female non-neurogenic
lower urinary tract symptoms. part 1: diagnostics, overactive
bladder, stress urinary incontinence, and mixed urinary
incontinence. Eur Urol. 82:49–59. 2022.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Abrams P, Cardozo L, Fall M, Griffiths D,
Rosier P, Ulmsten U, Van Kerrebroeck P, Victor A and Wein A:
Standardisation Sub-Committee of the International Continence
Society. The standardisation of terminology in lower urinary tract
function: Report from the standardisation sub-committee of the
International Continence Society. Urology. 61:37–49.
2003.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Farag F, Sakalis VI, Arteaga SM, Sihra N,
Karavitakis M, Arlandis S, Bø K, Cobussen-Boekhorst H, Costantini
E, de Heide M, et al: What Are the short-term benefits and
potential harms of therapeutic modalities for the management of
overactive bladder syndrome in women? A review of evidence under
the auspices of the European Association of urology, female
non-neurogenic lower urinary tract symptoms guidelines Panel. Eur
Urol. 84:302–312. 2023.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Chow PM, Liu SP, Chuang YC, Lee KS, Yoo
TK, Liao L, Wang JY, Liu M, Sumarsono B and Jong JJ: The prevalence
and risk factors of nocturia in China, South Korea, and Taiwan:
Results from a cross-sectional, population-based study. World J
Urol. 36:1853–1862. 2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Jeong JB, Lee JH, Choo MS, Ahn DW, Kim SH,
Lee DS, Cho MC, Son H, Jeong H and Yoo S: Association between
life-style, metabolic syndrome and lower urinary tract symptoms and
its impact on quality of life in men ≥ 40 years. Sci Rep.
12(6859)2022.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Yamamoto S, Hotta Y, Maeda K, Kataoka T,
Maeda Y, Hamakawa T, Shibata Y, Sasaki S, Ugawa S, Yasui T and
Kimura K: High salt loading induces urinary storage dysfunction via
upregulation of epithelial sodium channel alpha in the bladder
epithelium in Dahl salt-sensitive rats. J Pharmacol Sci.
135:121–125. 2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Iwamoto T, Torimoto K, Gotoh D, Onishi S,
Hori S, Morizawa Y, Nakai Y, Miyake M and Fujimoto K: Reduced salt
intake partially restores the circadian rhythm of bladder clock
genes in Dahl salt-sensitive rats. Life Sci.
306(120842)2022.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Kawata R, Hotta Y, Maeda K, Kataoka T and
Kimura K: Effects of high salt intake on detrusor muscle
contraction in dahl salt-sensitive rats. Nutrients.
13(539)2021.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Andersson KE: Storage and voiding
symptoms: Pathophysiologic aspects. Urology. 62 (5 Suppl 2):S3–S10.
2003.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Andersson KE: Bladder activation: Afferent
mechanisms. Urology. 59 (5 Suppl 1):S43–S50. 2002.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Birder LA: Urothelial signaling. Auton
Neurosci. 153:33–40. 2010.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Chen YH, Chen CJ, Wang SJ, Lin YN, Chen
WC, Tsai MY and Chen HY: Downregulation of tight junction protein
zonula occludens-2 and urothelium damage in a
cyclophosphamide-induced mouse model of cystitis. Taiwan J Obstet
Gyne. 57:399–406. 2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Chen YH, Chen WC, Liu PL and Chen HY:
Astragalus polysaccharides and astragaloside IV ameliorates
cyclophosphamide-induced mouse model of overactive bladder. Taiwan
J Obstet Gyne. 59:248–255. 2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Montalbetti N, Rued AC, Taiclet SN, Birder
LA, Kullmann FA and Carattino MD: Urothelial tight junction barrier
dysfunction sensitizes bladder afferents. eNeuro.
4(ENEURO.0381-16.2017)2017.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Fredriksson K, Kalimo H, Westergren I,
Kåhrström J and Johansson BB: Blood-brain barrier leakage and brain
edema in stroke-prone spontaneously hypertensive rats. Effect of
chronic sympathectomy and low protein/high salt diet. Acta
Neuropathol. 74:259–268. 1987.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Hu L, Zhu S, Peng X, Li K, Peng W, Zhong
Y, Kang C, Cao X, Liu Z and Zhao B: High salt elicits brain
inflammation and cognitive dysfunction, accompanied by alternations
in the gut microbiota and decreased SCFA production. J Alzheimers
Dis. 77:629–640. 2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Fu J and Wu H: Structural Mechanisms of
NLRP3 inflammasome assembly and activation. Annu Rev Immunol.
41:301–316. 2023.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Liu T, Zhang L, Joo D and Sun SC: NF-κB
signaling in inflammation. Signal Transduct Target Ther.
2(17023)2017.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Pitzer A, Elijovich F, Laffer CL, Ertuglu
LA, Sahinoz M, Saleem M, Krishnan J, Dola T, Aden LA, Sheng Q, et
al: DC ENaC-Dependent Inflammasome Activation Contributes to
Salt-Sensitive Hypertension. Circ Res. 131:328–344. 2022.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Shen S, Duan J, Hu J, Qi Y, Kang L, Wang
K, Chen J, Wu X, Xu B and Gu R: Colchicine alleviates inflammation
and improves diastolic dysfunction in heart failure rats with
preserved ejection fraction. Eur J Pharmacol.
929(175126)2022.PubMed/NCBI View Article : Google Scholar
|
|
22
|
de Oliveira MG, de Medeiros ML, Tavares
EBG, Mónica FZ and Antunes E: Methylglyoxal, a reactive glucose
metabolite, induces bladder overactivity in addition to
inflammation in mice. Front Physiol. 11(290)2020.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Hughes FM Jr, Allkanjari A, Odom MR, Jin H
and Purves JT: Diabetic bladder dysfunction progresses from an
overactive to an underactive phenotype in a type-1 diabetic mouse
model (Akita female mouse) and is dependent on NLRP3. Life Sci.
299(120528)2022.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Chen J, Li Q, Hong Y, Zhou X, Yu C, Tian
X, Zhao J, Long C, Shen L, Wu S and Wei G: Inhibition of the NF-κB
signaling pathway alleviates pyroptosis in bladder epithelial cells
and neurogenic bladder fibrosis. Int J Mol Sci.
24(11160)2023.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Mohanty S, Kamolvit W, Hertting O and
Brauner A: Vitamin D strengthens the bladder epithelial barrier by
inducing tight junction proteins during E. coli urinary tract
infection. Cell Tissue Res. 380:669–673. 2020.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Wu Y, Qi J, Wu C and Rong W: Emerging
roles of the TRPV4 channel in bladder physiology and dysfunction. J
Physiol. 599:39–47. 2021.PubMed/NCBI View
Article : Google Scholar
|
|
27
|
Girard BM, Campbell SE, Perkins M, Hsiang
H, Tooke K, Drescher C, Hennig GW, Heppner TJ, Nelson MT and
Vizzard MA: TRPV4 blockade reduces voiding frequency, ATP release,
and pelvic sensitivity in mice with chronic urothelial
overexpression of NGF. Am J Physiol Renal Physiol. 317:F1695–F1706.
2019.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Gohar EY, De Miguel C, Obi IE, Daugherty
EM, Hyndman KA, Becker BK, Jin C, Sedaka R, Johnston JG, Liu P, et
al: Acclimation to a high-salt diet is sex dependent. J Am Heart
Assoc. 11(e020450)2022.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Hu J, Luo H, Wang J, Tang W, Lu J, Wu S,
Xiong Z, Yang G, Chen Z, Lan T, et al: Enteric dysbiosis-linked gut
barrier disruption triggers early renal injury induced by chronic
high salt feeding in mice. Exp Mol Med. 49(e370)2017.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Dörr W: Cystometry in mice-influence of
bladder filling rate and circadian variations in bladder
compliance. J Urol. 148:183–187. 1992.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Kamiyama Y, Muto S, Masuda H, Ide H,
Ishizuka N, Saito K and Horie S: Inhibitory effects of nicorandil,
a K ATP channel opener and a nitric oxide donor, on overactive
bladder in animal models. BJU Int. 101:360–365. 2008.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Karakus S, Anele UA, Silva FH, Musicki B
and Burnett AL: Urinary dysfunction in transgenic sickle cell mice:
Model of idiopathic overactive bladder syndrome. Am J Physiol Renal
Physiol. 317:F540–F546. 2019.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Wang J, Chen Y, Gu D, Zhang G, Chen J,
Zhao J and Wu P: Ketamine-induced bladder fibrosis involves
epithelial-to-mesenchymal transition mediated by transforming
growth factor-β1. Am J Physiol Renal Physiol. 313:F961–F972.
2017.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Wang Q, Wu Q, Wang J, Chen Y, Zhang G,
Chen J, Zhao J and Wu P: Ketamine analog methoxetamine induced
inflammation and dysfunction of bladder in rats. Int J Mol Sci.
18(117)2017.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Gray KJ, Engelmann UH, Johnson EH and
Fishman IJ: Evaluation of misoprostol cytoprotection of the bladder
with cyclophosphamide (Cytoxan) therapy. J Urol. 136:497–500.
1986.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Chen H, Zhang L, Hill WG and Yu W:
Evaluating the voiding spot assay in mice: A simple method with
complex environmental interactions. Am J Physiol Renal Physiol.
313:F1274–F1280. 2017.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Zhang C, Huang Y, Ouyang F, Su M, Li W,
Chen J, Xiao H, Zhou X and Liu B: Extracellular vesicles derived
from mesenchymal stem cells alleviate neuroinflammation and
mechanical allodynia in interstitial cystitis rats by inhibiting
NLRP3 inflammasome activation. J Neuroinflammation.
19(80)2022.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Obaidul Islam M, Bacchetti T, Berrougui H,
Abdelouahed Khalil and Ferretti G: Effect of glycated HDL on
oxidative stress and cholesterol homeostasis in a human bladder
cancer cell line, J82. Exp Mol Pathol. 126(104777)2022.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Zhao X, Zeng H, Lei L, Tong X, Yang L,
Yang Y, Li S, Zhou Y, Luo L, Huang J, et al: Tight junctions and
their regulation by non-coding RNAs. Int J Biol Sci. 17:712–727.
2021.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Khandelwal P, Abraham SN and Apodaca G:
Cell biology and physiology of the uroepithelium. Am J Physiol
Renal Physiol. 297:F1477–F1501. 2009.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Monastyrskaya K, Sánchez-Freire V, Hashemi
Gheinani A, Klumpp DJ, Babiychuk EB, Draeger A and Burkhard FC:
miR-199a-5p regulates urothelial permeability and may play a role
in bladder pain syndrome. Am J Pathol. 182:431–448. 2013.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Beča KIK, Girard BM, Heppner TJ, Hennig
GW, Herrera GM, Nelson MT and Vizzard MA: The Role of PIEZO1 in
urinary bladder function and dysfunction in a rodent model of
cyclophosphamide-induced cystitis. Front Pain Res (Lausanne).
2(748385)2021.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Perkins ME and Vizzard MA: Transient
receptor potential vanilloid type 4 (TRPV4) in urinary bladder
structure and function. Curr Top Membr. 89:95–138. 2022.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Hughes FM Jr, Hirshman NA, Inouye BM, Jin
H, Stanton EW, Yun CE, Davis LG, Routh JC and Purves JT: NLRP3
promotes diabetic bladder dysfunction and changes in
symptom-specific bladder innervation. Diabetes. 68:430–440.
2019.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Odom MR, Hughes FM Jr, Jin H and Purves
JT: Diabetes causes NLRP3-dependent barrier dysfunction in mice
with detrusor overactivity but not underactivity. Am J Physiol
Renal Physiol. 323:F616–F632. 2022.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Thangavel C, Gomes CM, Zderic SA, Javed E,
Addya S, Singh J, Das S, Birbe R, Den RB, Rattan S, et al: NF-κB
and GATA-Binding factor 6 repress transcription of caveolins in
bladder smooth muscle hypertrophy. Am J Pathol. 189:847–867.
2019.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Birder LA, Wolf-Johnston AS, Sun Y and
Chai TC: Alteration in TRPV1 and Muscarinic (M3) receptor
expression and function in idiopathic overactive bladder urothelial
cells. Acta Physiol (Oxf). 207:123–129. 2013.PubMed/NCBI View Article : Google Scholar
|