Biological mechanisms underlying depression, epigenetics and their interplay (Review)
- Authors:
- Published online on: June 7, 2023 https://doi.org/10.3892/ije.2023.17
- Article Number: 3
-
Copyright : © Mitsis et al. This is an open access article distributed under the terms of Creative Commons Attribution License [CC BY 4.0].
Abstract
1. Introduction
Depression is a mood disorder characterized by an enduring feeling of sadness and interest loss. According to the Diagnostic and Statistical Manual of Mental Disorders- 5th Edition (DSM-5) (1), depressive disorders can be classified into different groups, including major depressive disorder (MDD) and dysthymia (2). As of 2019, depressive disorders are the leading cause of non-fatal disease worldwide (3). The burden of mental disorders, such as depression is prevalent throughout the entire lifespan in both sexes and across multiple locations (4). Therefore, it is not surprising that depression is of increasing concern, since it negatively affects the quality of life of affected individuals on a global scale (5).
Depression is a multifactorial disease with a relatively complex etiology and great variability in presentation. For this reason, treatment, which includes pharmacotherapy, psychotherapy, or a combination of both, is a complex issue (3,5). Antidepressant drugs, the most widely used and effective form of treatment, still do not lead to complete remission in a considerable percentage of patients, and showcase a delayed clinical onset, which may vary from 2 to 4 weeks (6,7). The variable effects of drugs may be due to several reasons, including but not limited to, drug interactions, disease-related mechanisms, complex pathophysiology and genetics (8). Emerging evidence, though, highlights that epigenetics also play a pivotal role in psychiatric disorders, such as depression and appear to affect the responses of patients to the drugs (9). The present review aims to accumulate information from the currently available literature in order to highlight the mechanisms through which epigenetics may affect depression and the response of patients to antidepressants.
2. Depression
The pathogenesis of mood disorders is not yet fully understood; however, several theories have arisen regarding depression, such as the monoamine and cytokine hypotheses, plus hypotheses based on the dysfunction of the hypothalamus-pituitary-adrenal gland (HPA) axis (10,11).
The most commonly accepted theory regarding the pathogenesis of depression is the monoamine hypothesis, which states that the decrease in monoamines, such as serotonin (5HT), noradrenaline (NA) and dopamine (DA) in synaptic gaps can lead to depression (10). This hypothesis emerged when the anti-hypertensive drug, reserpine, caused the depletion of monoamines and, subsequently, depression in patients who did not suffer from the mentioned disorder prior to drug administration (12). The monoamine hypothesis is supported by the fact that currently used antidepressants, such as tricyclic antidepressants (TCAs), monoamine oxidase inhibitors (MAOIs), selective serotonin reuptake inhibitors (SSRIs), and serotonin and noradrenaline reuptake inhibitors (SNRIs) are considered to function by increasing monoamine levels (13). Moreover, an extensive number of studies have focused on the role of serotonin in depression, with many reporting low 5-HT levels and an altered 5-HT receptor expression in depressed individuals (14). Nevertheless, the response to such antidepressants is extremely varied and several studies have questioned the importance of monoamine dysregulation in depression (15).
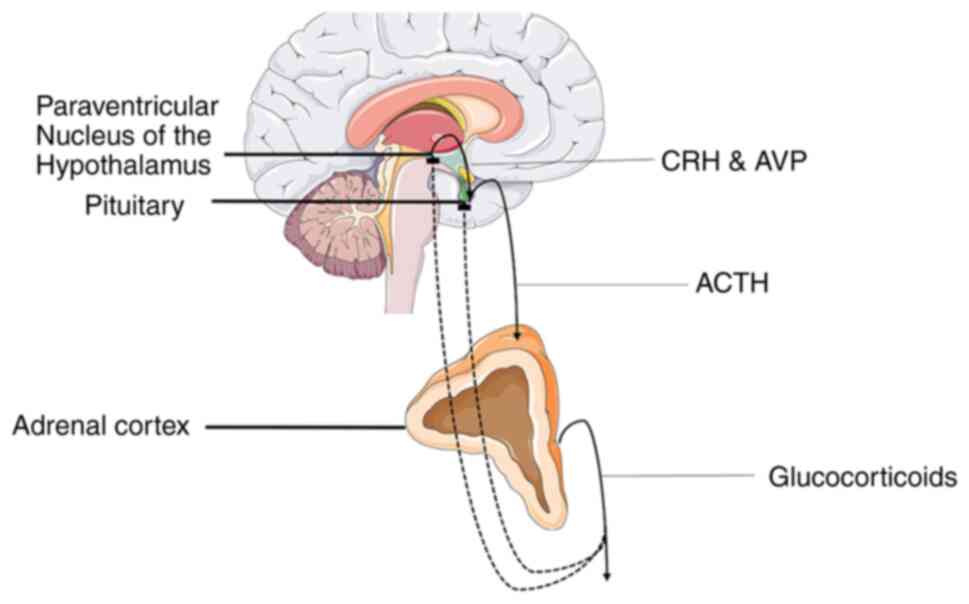
Stress is also known to play a critical role in the emergence of major depressive disorder (16) and the possible role the HPA axis hyperactivity may have on depression pathophysiology has gained an increasing amount of scientific interests (10). All organisms are programmed to maintain an inner equilibrium for optimal organism function termed homeostasis and stress refers to the state of threatened or perceived as such homeostasis. The stress response system is a sophisticated regulatory system, whose role is to maintain or re-establish homeostasis (17). The HPA axis is a vital component of the fight-or-flight response that regulates the production of glucocorticoids (GCs), which are main mediators of the stress response system. Specifically, a stressful stimulus triggers the synthesis and secretion of arginine vasopressin (AVP) and corticotropin-releasing hormone (CRH) by the paraventricular nucleus of the hypothalamus (PVN), with the mentioned hormones eliciting the secretion of adrenocorticotropic hormone (ACTH) in the anterior pituitary, which in turn acts on the adrenal glands to promote the release of GCs (18). Elevated glucocorticoid levels then suppress CRH and ACTH secretion through a negative feedback loop by acting on glucocorticoid receptors (GRs) in the hippocampus and thus reversing their levels to normal (Fig. 1) (19). It is considered that chronic stress leads to the dysfunction of the HPA axis, causing an abnormal increase in GC levels that in turn induce a decrease in the volume of the hippocampus, which is a main characteristic of MDD. These elevated GC levels may promote atrophy in hippocampal mature neurons and/or decrease hippocampal neurogenesis (10). A potential mediator of the effect of stress on hippocampus is the brain-derived neurotrophic factor (BDNF). GR appears to downregulate BDNF expression, an event that may lead to negative morphological changes in hippocampal neurons (10,20). This theory is supported by the fact that depressed patients display lower BDNF serum levels and antidepressants can recover stress-related morphological changes in the hippocampus by increasing BDNF expression (10). The hypothesis of the dysfunction of the HPA axis is reinforced by findings on SSRIs and TCAs, which appear to affect GC signaling (21,22).
The cytokine theory hypothesis claims that depression is dependent on the activation of the inflammatory response system and altered levels of immunomodulatory molecules cause the various symptoms observed in this disorder (11). Specifically, cytokines, which are a category of small proteins that regulate the immune response, have been implicated in the pathogenesis of MDD (23). Cytokines can be grouped into pro-inflammatory and anti-inflammatory cytokines, with pro-inflammatory cytokines, such as interleukin (IL)-1, IL-6 and tumor necrosis factor α (TNFα), being directly or indirectly involved in the inflammatory process and anti-inflammatory cytokines, such as IL-4 and IL-10 suppressing the immune response (11). Research has demonstrated that non-depressed individuals may display symptoms similar to depression following exposure to pro-inflammatory cytokines, an effect that can be ameliorated by antidepressant treatment (24). Moreover, patients with MDD exhibit higher levels of TNFα, which can be significantly decreased following the administration of an SNRI antidepressant, such as venlafaxine. A possible mechanism through which inflammation causes depression involves microglia activation (25). Chronic stress induces microglia activation (26), which in turn causes the production of IL-1 and TNFα. These pro-inflammatory cytokines can hinder long-term potentiation (LTP) induction, which can lead to symptoms characteristic of MDD (25). There exist several other mechanisms in which cytokines may cause depression. One such mechanism includes the activation of indoleamine-2,3-dioxygenase by IL-6 and TNFα, which results in serotonin reduction and changes in monoamine oxidase production (27). Moreover, cytokines such as IL-6 and TNFα may prevent the entry of the GR complex into the neuronal nucleus and inhibit its binding to DNA, thus promoting the hyperactivity of the HPA axis and the loss of its negative feedback loop (28).
3. Antidepressants
As aforementioned, the currently used antidepressants include TCAs, MAOIs, selective SSRIs and SNRIs. These drugs are considered to mainly function through mechanisms supportive of the monoamine hypothesis.
TCAs and MAOIs were the first antidepressant classes discovered and were the sole medication for depression for ~30 years (29). TCAs achieve their effect by acting on distinct neurotransmitter pathways. Specifically, they block the reuptake of serotonin and noradrenaline in the synaptic cleft, increasing the concentrations of mentioned monoamines and exerting an antidepressant effect (30). TCAs function mainly by targeting the serotonin transporter (SERT) and the norepinephrine transporter (NET), but also influence other neurotransmitter systems such as cholinergic, adrenergic, muscarinic and histaminergic receptors (30-32). MAOIs achieve their antidepressant effects by blocking monoamine oxidase function. This enzyme is responsible for breaking down neurotransmitters, such as serotonin, noradrenaline and dopamine in the brain. The use of MAOIs suppresses the breakdown of the aforementioned neurotransmitters, thus increasing their levels and exerting an antidepressant effect (33). Both TCAs and MAOIs are associated with severe side-effects, a fact that has led to their decreased prescription in favor of more modern types of antidepressants, such as SSRIs and SNRIs which are associated with less severe side-effects. In particular, MAOIs may cause a possibly fatal hypertensive crisis after excessive tyramine consumption, while TCAs may cause cardiac sodium channel blockage and arrhythmia (34). Nonetheless, these drugs continue to play a key role in battling depression, particularly in treatment-resistant patients (29).
SSRIs along with SNRIs are among the most commonly prescribed drugs in the USA (35). The function of SSRIs, as their name suggests, is based on the inhibition of serotonin reuptake, and more precisely by inhibiting SERT at the presynaptic axon terminal, therefore increasing the amount of 5-HT in the synaptic cleft. The side-effects of SSRIs are fewer than those of TCAs and MAOIs, since they have a minimal effect on other monoamines, such as dopamine and noradrenaline, and do not affect the functions of histaminergic, adrenergic and cholinergic receptors (36). SNRIs, as their name also suggests, function by inhibiting the presynaptic neuronal uptake of 5-HT and noradrenaline, and act on SERT and NERT in a specific manner (37). These drugs are considered to have a dual effect, though the precise degree of serotonin or adrenaline inhibition is both agent- and dose-dependent (38). Selective serotonin and noradrenaline reuptake inhibitors have been shown to exert their antidepressant effect more rapidly than SSRIs in a clinical setting (39). SNRIs have similar side-effects with SSRIs; however, due to their enhancement of noradrenergic activity, they may also increase blood pressure and heart rate (40).
Lastly, studies published over the last decade have highlighted the potential use of ketamine, an N-methyl-D-aspartate (NMDA) glutamate receptor antagonist, as an antidepressant (41). Chronic stress has been shown to increase glutamate release, and subsequently impair LTP and promote the atrophy of apical dendrites in the hippocampus (42). As an NMDA receptor antagonist, ketamine functions by inhibiting the action of glutamate. In contrast to generally used antidepressants, which require 2 to 4 weeks to ameliorate depressive symptoms, a single intravenous administration of ketamine ameliorates depressive symptoms in 1 to 3 days, and displays a long-lasting effect (10,42). However, the use of ketamine is associated with severe adverse effects, including psychotomimetic effects and dissociative properties, and may lead to drug abuse. Therefore, other research has focused on ketamine enantiomers and metabolites as potential antidepressants (43).
4. Epigenetics
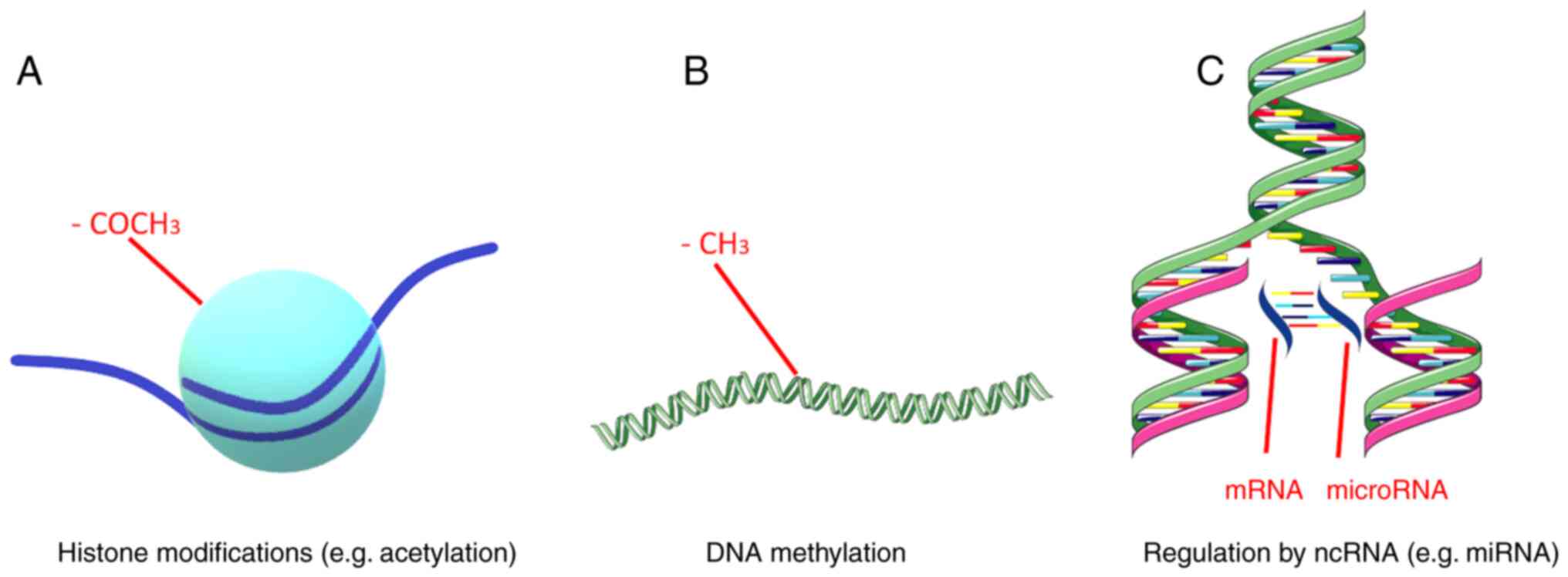
Epigenetics, i.e., heritable and stable structural and biochemical alterations of the chromosome that are not associated with DNA sequence alterations, have been associated with numerous physiological and pathological processes, such as metabolic disorders, autoimmune diseases, cancer and neuropsychiatric disorders (44-50). Epigenetic mechanisms include DNA methylation, regulation by non-coding RNAs (ncRNAs) and histone modifications (Fig. 2). These mechanisms are responsible for fine-tuning gene expression (51). Gene expression, which refers to the production of a functional gene product using the information provided by the DNA sequence, is a quintessential process in all living organisms, since it allows them to adjust the amount and type of gene product in response to different environmental factors (52). A main part of gene expression is achieved at the transcriptional level, although several post-transcriptional events also play a key role, such as the aforementioned histone modifications and regulation by ncRNAs (53,54).
DNA methylation includes the extensively studied attachment of a methyl group to the carbon-5 position of cytosine (m5C) and the lesser-studied linkage of a methyl group to the adenosine base at the nitrogen-6 position of deoxyadenosine (m6dA) (55). The methylation of m5C is considered the predominant form of DNA methylation and occurs on DNA regions known as CpG islands. These genomic regions are at least 500 bp in length and display a high content of cytosine and guanine nucleotides (>55%). The methylation of CpG islands is performed by DNA methyltransferases (DNMTs), while the demethylation of 5-methylcytosine makes use of TET methylcytosine dioxygenases 1, 2, and 3, and leads to the production of m5C oxidative derivatives. The methylation and demethylation of these sites alteres the expression of nearby genes (56). DNA methylation may function either as a repressive or activating mark for gene transcription. Specifically, methylation can make transcription machinery binding more difficult or create a landscape prime for transcription (57).
ncRNAs are RNA molecules that are incapable of protein coding (58). Non-coding RNAs can be classified into two major categories, those with a length of <200 nucleotides, which are termed small ncRNAs, and those with a length >200 nucleotides, which create a distinct category known s long ncRNAs (lncRNAs). Small ncRNAs include RNA types, such as microRNAs (miRNAs/miRs), small interfering RNAs (siRNAs), small nuclear RNAs (snRNAs), small nucleolar RNAs (snoRNAs) and PIWI-interacting RNAs (piRNAs) (59). The majority of ncRNAs have been found to be associated with the regulation of gene expression (60). miRNAs are short single-stranded RNA molecules with a length of ~22 nucleotides (59). Generally, miRNAs silence gene expression by RNA-induced silencing at a post-transcriptional level. lncRNAs can regulate pre-mRNA splicing, inhibit mRNA translation or promote chromatin remodeling. siRNAs are double-stranded RNAs that function by dividing into their single strands and binding to a distinct target mRNA to suppress gene expression (61). snRNAs, which are ~150 nucleotides in length, are primarily found in the splicing regions of eukaryotic cell nuclei, and thus can affect pre-miRNA splicing. snoRNAs are generally located in the nucleoli of eukaryotic cells and play a role in rRNA processing. PIWI-interacting RNAs bind to PIWI proteins, a subfamily of ARGONAUTE proteins, to influence chromatin function (60). Lastly, lncRNAs have the ability to interact with mRNA, DNA, several protein complexes and miRNAs and affect gene expression at multiple regulatory levels (62).
In eukaryotic cells, genomic DNA is organized into chromatin, a polymer whose main structural unit is the nucleosome. The nucleosome core consists of a histone octamer composed of two copies of core histones, H2A, H2B, H3, H4 and 146 DNA base pairs wrapped around it (63). Chromatin condensation influences gene expression, since a highly condensed structure hinders DNA accessibility and thus interferes with gene transcription. Histone modifications can affect chromatin condensation and organize the genome into transcriptionally active or inactive regions (64). These modifications include methylation, acetylation, phosphorylation and the ubiquitination of histone proteins, plus chromatin remodeling and regulation by ncRNAs, piRNAs and lncRNAs (65,66). Histone modifications occur predominantly, although not exclusively, at the N-terminal tails of histone proteins and can alter gene expression (66). Histone methylation usually includes the addition of methyl groups at lysine (K) residues of histones H3 and H4. Histone lysine residues can be mono-, di- and tri-methylated in order to act as repressive or active marks of gene expression, a process mediated by the histone methyltransferase (HMT) group of enzymes (65). Histone acetylation occurs on lysine residues via the addition of an acetyl group from an acetyl-coenzyme A donor to an ε-amino group of a lysine side chain (67). This modification is considered an active gene expression mark and is regulated by the equilibrium between histone acetyltransferases and histone deacetylases (HDACs) (65). Specifically, the addition of an acetyl group weakens the positive charge of a lysine residue, thus reducing the tail's affinity for chromatin and leaving the underlying DNA exposed (67). Histone ubiquitination involves the covalent attachment of ubiquitin, a small 76 amino-acid protein, to a ε-amino group of a lysine residue. This process is catalyzed by E1 ubiquitin activating enzymes, E2 ubiquitin conjugating enzymes, and E3 ubiquitin ligases (68). Histone ubiquitination is a reversible process with deubiquitinating enzymes, a family of proteases and metalloproteases, being able to remove ubiquitin moieties from histones (69). The effect histone ubiquitination has on gene expression depends on the number of ubiquitin moieties being added to the lysine residue and which specific core histone is being altered. Histone phosphorylation is regulated by two enzymes of opposing function, specifically kinases which add phosphate groups and phosphatases which remove phosphate groups. Histone phosphorylation mostly funcionts in conjunction with other histone modifications creating a complex regulating network with varied effects on gene expression (66). Chromatin remodeling is the process of dynamic changes on chromatin structure that alters how condensed or uncondensed a chromatin region is, thereby influencing the exposure of the underlying DNA and subsequently gene expression. This process in undertaken by chromatin-remodeling protein complexes (65). Lastly, as aforementioned, ncRNAs, i.e., RNA molecules that do not code a protein product, can be mediators of histone modification. Specific cases include piRNAs that can bind to PIWI proteins and recruit histone methyltransferases to influence chromatin function and lncRNAs that can change chromatin status by recruiting protein complexes that influence histone methylation and acetylation (65).
5. Epigenetics and depression
Epigenetic mechanisms appear to play a vital role in depression and may help provide a biological framework in which genetic and environmental factors interact and influence disease pathogenesis and pathophysiology (70).
Epigenetics can regulate the expression of antidepressant molecular targets, which are also main participants of monoamine signaling (71). SERT is coded by the SLC6A gene and sustained alterations on its gene expression profile have been implicated in depression. These alterations may emerge due to epigenetic modifications in response to stressful events (72). Indeed, the differential methylation of the SLC6A4 gene has been shown to be associated with a risk of mental illness, including depression (73). A previous study on the SLC6A2 gene, which codes for NET, also demonstrated epigenetic modifications on the mentioned gene, and more specifically DNA acetylation, which may be responsible for a mechanism underlying depression in conjunction with hypertension (74). Lastly, epigenetic alterations on the monoamine oxidase A (MAOA) gene may be a factor associated with the pathogenesis of depression. Specifically, the hypomethylation of the MAOA gene may increase monoamine oxidase expression and subsequently, its activity, thereby reducing monoamine utilization by the brain. This increased monoamine oxidase activity has been detected in patients with depressive symptoms and is in accordance with the monoamine hypothesis and the use of MAOIs as antidepressants (75).
Epigenetic alterations on genes regulating HPA function may play a crucial role in disease pathogenesis and onset age (76). A previous study on mice demonstrated that early-life stress (ELS) affected AVP expression in the PVN through methyl CpG binding protein 2 phosphorylation and AVP enhancer hypomethylation, which in turn promoted neuroendocrine and behavioral features that are present in depression (77). Moreover, animal models which associate ELS with depression have shown that CRH, ACTH are also hypomethylated while the gene encoding GR (NR3C1) is hypermethylated (78). Thus, epigenetics may provide a framework in which HPA axis dysfunction may promote depression.
Genome-wide methylation analysis has identified differentially methylated regions that are associated both with the pathogenesis of depression and immune dysfunction (79). Another study has also proposed that IL-6 methylation may be used as a biomarker for depression (80). Particularly, depressed patients display IL-6 hypomethylation in peripheral tissue. This epigenetic modification possibly leads to a higher IL-6 expression (80) which in turn may play a role in the pathology of depression or even its pathogenesis, through some of the mechanisms mentioned in the previous chapters. These findings give credence to the cytokine hypothesis. Thereby, the role of DNA methylation in depression has both been extensively observed and can be described through the view of all major hypotheses of disease pathogenesis.
Histone methylation has been found to be associated with depression in genome-wide association studies. Studies on mice have demonstrated than chronic social defeat stress can downregulate HMTs, specifically G9α and G9α-like protein, which catalyze the dimethylation of the lysine 9 residue of H3 (H3K9me2). H3k9me2 is known to be a major repressive mark in the NAc of the hypothalamus (81). This brain region is essential in the regulation of reward behavior (82). On the other hand, G9α overexpression in the NAc exerts antidepressant-like effects, and increases in H3K9me2 at distinct gene promoters may be some of the mechanisms of action of fluoxetine, an SSRI. Thus, it is possible that stress leads to maladaptive alterations in the specific brain action via the repression of histone methylation, a process that can be ameliorated by the use of antidepressants (81). Chronic social defeat stress also transiently suppresses histone acetylation in the mouse NAc, with HDAC inhibition resulting in antidepressant effects (82). Studies on the direct association between histone phosphorylation and depression are limited. These studies have focused on the stress response and have detected that H3 phosphorylation is increased in the hippocampus and prefrontal cortex of mice and rats, respectively that have been subjected to stress (83). Research on histone ubiquitination and its role in depression is also limited. A previous study demonstrated that the UBE2A gene, which is an E2 ubiquitin conjugating enzyme is upregulated in the post-mortem dorsolateral prefrontal cortex of MDD patients. Histone ubiquitination by the UBE2A protein is considered a transcriptional activation tag. However, it should be mentioned that this protein also participates in the ubiquitin-proteasome system, which is the main mechanism of protein catabolism, and this process has already been associated with MDD (84). As regards chromatin remodeling, several types of stress have been demonstrated to induce repressive chromatin complexes in the mouse NAc. The same complexes are induced in the NAc of depressed humans (81). The effects of lncRNAs on chromatin have also been associated with MDD. A prime example is the BDNF antisense RNA (BDNF-AS) that functions as a scaffold to recruit chromatin modifiers to act on the BDNF gene promoter and repress its expression, a process implicated in MDD (85).
Multiple studies have highlighted the fact that the dysregulation of ncRNAs is present in depressed patients and in animal models of depression (86,87). miRNAs and lncRNAs are the most extensively studied type of ncRNAs in MDD (88). miRNAs are considered to affect the pathogenesis of depression via the regulation of monoamine and glutamate signaling (86). Numerous miRNAs have displayed altered expression levels in depressed patients. It has been demonstrated the downregulation of several miRNAs in the prefrontal cortex of patients with MDD post-mortem (86). Some of these miRNAs are known to target depression-associated mRNAs. In particular, miR-20α, miR-20b, miR-34α, and miR-34b target VEGF, whose protein levels are elevated in the peripheral blood of patients with MDD, miR-34α targets BCL2, whose protein levels are downregulated in depressed patients, and miR-148b targets DNMT3B, whose protein levels are downregulated in depressed individuals. Additionally, microarray studies on patients with MDD have demonstrated the upregulation of miRNAs, such as let-7d-5p and let-7f-5p, whose expression can be influenced by antidepressant treatment (86). The most well-known depression-associated lncRNA is the aforementioned BDNF-AS. Apart from BDNF-AS, other lncRNAs have also been implicated in the pathogenesis of depression, with a prime example being the growth arrest specific 5 (GAS5). GAS5 is upregulated in the hippocampal tissues of mice with depressive-like behaviors, and its silencing appears to eliminate such behaviors. It appears that GAS5 influences early growth response gene 1 via miR-26α binding, and promotes the release of inflammatory factors and the apoptosis of hippocampal neurons in mice with depressive-like behaviors (89). Additionally, the overexpression of GAS5 may lead to the increased expression of the type 1 receptor of CRH and a subsequent long-term activation of the HPA axis, a common feature of depression (90).
6. Epigenetics and antidepressants
Current antidepressants may exert some of their effects via epigenetic mechanisms, while differences in gene expression in patients due to epigenetic alterations may be one of the underlying causes of variations in drug responses (50,91). Classical antidepressants, such as TCAs and SSRIs have an indirect effect on DNA methylation and chromatin structure (82). Imipramine, a TCA, reverses changes induced by social defeat stress in H3 methylation in the mouse NAc. Furthermore, chronic imipramine treatment decreases histone methylation, increases histone acetylation at BDNF promoters and downregulates HDAC5 in the mouse hippocampus (92). Citalopram, a commonly used SSRI, affects DNA methylation on a large scale and influences the gene expression of a large set of genes that are involved in depression pathogenesis and pathology (93). Moreover, some data suggest that paroxetine, another SSRI, has the ability to affect DNMT activity and thus influence DNA methylation. On the other hand, DNA methylation itself can influence drug response. The promoter methylation of depression-associated genes, such as BDNF, HTR1A, HTR1B, SLC6A4 and IL11 appear to be predictive of an antidepressant response (91). Some examples include the hypermethylation of the SLC6A4 promoter, which is a predictor for an improved SSRI drug response, and the hypomethylation of the BDNF promoter, which is a predictor of non-responsiveness to TCAs, MAOIS, SSRIs and SNRIs (92).
7. Conclusions and future perspectives
Epigenetics appear to play a significant role in the pathology and pathogenesis of depression, while also influencing the response of patients to antidepressants and vice versa. Further research on the epigenetics of depression may help to elucidate the molecular peculiarities of depression, while it may also help to predict the response of patients to antidepressants. The latter is of immense interest, since it can help clinicians tailor therapy to each individual patient.
Acknowledgements
Parts of the figures were drawn using images from Servier Medical Art. Servier Medical Art by Servier is licensed under a Creative Commons Attribution 3.0 Unported License (https://creativecommons.org/licenses/by/3.0/).
Funding
Funding: The authors would like to acknowledge funding from ‘MilkSafe: A novel pipeline to enrich formula milk using omics technologies’, a research co-financed by the European Regional Development Fund of the European Union and Greek national funds through the Operational Program Competitiveness, Entrepreneurship and Innovation, under the call RESEARCH-CREATE-INNOVATE (project code: T2EDK-02222).
Availability of data and materials
Not applicable.
Authors' contributions
All authors (TM, EP and DV) contributed to the conceptualization, design, writing, drafting, revising, editing and reviewing of the manuscript. All authors have read and approved the final manuscript. Data authentication is not applicable.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
DV is an Editor of the journal. However, he had no personal involvement in the reviewing process, or any influence in terms of adjudicating on the final decision, for this article. The other authors declare that they have no competing interests.
References
|
American Psychiatric A, American Psychiatric Association DSMTF (eds): Diagnostic and statistical manual of mental disorders: DSM-5. American Psychiatric Association, Arlington, VA, 2013. | |
|
Chand SP and Arif H: Depression. StatPearls (Internet): StatPearls Publishing, Treasure Island, FL, 2022. | |
|
Mekonen T, Chan GCK, Connor JP, Hides L and Leung J: Estimating the global treatment rates for depression: A systematic review and meta-analysis. J Affect Disord. 295:1234–1242. 2021.PubMed/NCBI View Article : Google Scholar | |
|
Global prevalence and burden of depressive and anxiety disorders in 204 countries and territories in 2020 due to the COVID-19 pandemic. Lancet. 398:1700–1712. 2021.PubMed/NCBI View Article : Google Scholar | |
|
Proudman D, Greenberg P and Nellesen D: The growing burden of major depressive disorders (MDD): Implications for researchers and policy makers. Pharmacoeconomics. 39:619–625. 2021.PubMed/NCBI View Article : Google Scholar | |
|
Marasine NR, Sankhi S, Lamichhane R, Marasini NR and Dangi NB: Use of antidepressants among patients diagnosed with depression: A scoping review. Biomed Res Int. 2021(6699028)2021.PubMed/NCBI View Article : Google Scholar | |
|
Karrouri R, Hammani Z, Benjelloun R and Otheman Y: Major depressive disorder: Validated treatments and future challenges. World J Clin Cases. 9:9350–9367. 2021.PubMed/NCBI View Article : Google Scholar | |
|
Roden DM, McLeod HL, Relling MV, Williams MS, Mensah GA, Peterson JF and Van Driest SL: Pharmacogenomics. Lancet. 394:521–532. 2019.PubMed/NCBI View Article : Google Scholar | |
|
Zhou J, Li M, Wang X, He Y, Xia Y, Sweeney JA, Kopp RF, Liu C and Chen C: Drug response-related DNA methylation changes in schizophrenia, bipolar disorder, and major depressive disorder. Front Neurosci. 15(674273)2021.PubMed/NCBI View Article : Google Scholar | |
|
Boku S, Nakagawa S, Toda H and Hishimoto A: Neural basis of major depressive disorder: Beyond monoamine hypothesis. Psychiatry Clin Neurosci. 72:3–12. 2018.PubMed/NCBI View Article : Google Scholar | |
|
Shadrina M, Bondarenko EA and Slominsky PA: Genetics factors in major depression disease. Front Psychiatry. 9(334)2018.PubMed/NCBI View Article : Google Scholar | |
|
Boas GR, de Lacerda RB, Paes MM, Gubert P, da Cruz AWL, Rescia VC, de Carvalho PMG, de Carvalho AAV and Oesterreich SA: Molecular aspects of depression: A review from neurobiology to treatment. Eur J Pharmacol. 851:99–121. 2019.PubMed/NCBI View Article : Google Scholar | |
|
Marathe SV, D'Almeida PL, Virmani G, Bathini P and Alberi L: Effects of monoamines and antidepressants on astrocyte physiology: Implications for monoamine hypothesis of depression. J Exp Neurosci. 12(1179069518789149)2018.PubMed/NCBI View Article : Google Scholar | |
|
Tian H, Hu Z, Xu J and Wang C: The molecular pathophysiology of depression and the new therapeutics. MedComm (2020). 3(e156)2022.PubMed/NCBI View Article : Google Scholar | |
|
Chávez-Castillo M, Núñez V, Nava M, Ortega Á, Rojas M, Bermúdez V and Rojas-Quintero J: Depression as a neuroendocrine disorder: Emerging neuropsychopharmacological approaches beyond monoamines. Adv Pharmacol Sci. 2019(7943481)2019.PubMed/NCBI View Article : Google Scholar | |
|
Richter-Levin G and Xu L: How could stress lead to major depressive disorder? IBRO Rep. 4:38–43. 2018.PubMed/NCBI View Article : Google Scholar | |
|
Tsigos C, Kyrou I, Kassi E and Chrousos GP: Stress: Endocrine physiology and pathophysiology. In: Feingold KR, Anawalt B, Blackman MR, Boyce A, Chrousos G, Corpas E, et al., (eds). Endotext. South Dartmouth (MA): MDText.com, Inc. Copyright© 2000-2023, MDText.com, Inc.; 2020. | |
|
Menke A: Is the HPA axis as target for depression outdated, or is there a new hope? Front Psychiatry. 10(101)2019.PubMed/NCBI View Article : Google Scholar | |
|
Nicolaides NC, Pavlaki AN, Maria Alexandra MA, Chrousos GP, Feingold KR, Anawalt B, Blackman MR, Boyce A, Chrousos G, Corpas E, et al: Glucocorticoid therapy and adrenal suppression. Copyright © 2000-2023, MDText.com, Inc.; 2018. | |
|
Chen H, Amazit L, Lombès M and Le Menuet D: Crosstalk between glucocorticoid receptor and early-growth response protein 1 accounts for repression of brain-derived neurotrophic factor transcript 4 expression. Neuroscience. 399:12–27. 2019.PubMed/NCBI View Article : Google Scholar | |
|
Budziñski ML, Sokn C, Gobbini R, Ugo B, Antunica-Noguerol M, Senin S, Bajaj T, Gassen NC, Rein T, Schmidt MV, et al: Tricyclic antidepressants target FKBP51 SUMOylation to restore glucocorticoid receptor activity. Mol Psychiatry. 27:2533–2545. 2022.PubMed/NCBI View Article : Google Scholar | |
|
Ronaldson A, Carvalho LA, Kostich K, Lazzarino AI, Urbanova L and Steptoe A: The effects of six-day SSRI administration on diurnal cortisol secretion in healthy volunteers. Psychopharmacology (Berl). 235:3415–3422. 2018.PubMed/NCBI View Article : Google Scholar | |
|
Roohi E, Jaafari N and Hashemian F: On inflammatory hypothesis of depression: What is the role of IL-6 in the middle of the chaos? J Neuroinflammation. 18(45)2021.PubMed/NCBI View Article : Google Scholar | |
|
Miller AH and Raison CL: The role of inflammation in depression: From evolutionary imperative to modern treatment target. Nat Rev Immunol. 16:22–34. 2016.PubMed/NCBI View Article : Google Scholar | |
|
Innes S, Pariante CM and Borsini A: Microglial-driven changes in synaptic plasticity: A possible role in major depressive disorder. Psychoneuroendocrinology. 102:236–247. 2019.PubMed/NCBI View Article : Google Scholar | |
|
Schramm E and Waisman A: Microglia as central protagonists in the chronic stress response. Neurol Neuroimmunol Neuroinflamm. 9(e200023)2022.PubMed/NCBI View Article : Google Scholar | |
|
Arčan IS, Kouter K and Paska AV: Depressive disorder and antidepressants from an epigenetic point of view. World J Psychiatry. 12:1150–1168. 2022.PubMed/NCBI View Article : Google Scholar | |
|
Grygiel-Górniak B, Limphaibool N and Puszczewicz M: Cytokine secretion and the risk of depression development in patients with connective tissue diseases. Psychiatry Clin Neurosci. 73:302–316. 2019.PubMed/NCBI View Article : Google Scholar | |
|
Chockalingam R, Gott BM and Conway CR: Tricyclic antidepressants and monoamine oxidase inhibitors: Are they too old for a new look? Handb Exp Pharmacol. 250:37–48. 2019.PubMed/NCBI View Article : Google Scholar | |
|
Moraczewski J and Aedma KK: Tricyclic Antidepressants. StatPearls. Treasure Island (FL): StatPearls Publishing Copyright©. 2022, StatPearls Publishing LLC.; 2022. | |
|
Andersen J, Stuhr-Hansen N, Zachariassen L, Toubro S, Hansen SM, Eildal JN, Bond AD, Bøgesø KP, Bang-Andersen B, Kristensen AS and Strømgaard K: Molecular determinants for selective recognition of antidepressants in the human serotonin and norepinephrine transporters. Proc Natl Acad Sci USA. 108:12137–12142. 2011.PubMed/NCBI View Article : Google Scholar | |
|
Cottingham C, Percival S, Birky T and Wang Q: Tricyclic antidepressants exhibit variable pharmacological profiles at the α(2A) adrenergic receptor. Biochem Biophys Res Commun. 451:461–466. 2014.PubMed/NCBI View Article : Google Scholar | |
|
Laban TS and Saadabadi A: Monoamine oxidase inhibitors (MAOI). StatPearls. Treasure Island (FL): StatPearls Publishing Copyright ©. 2022, StatPearls Publishing LLC.; 2022. | |
|
Edinoff AN, Akuly HA, Hanna TA, Ochoa CO, Patti SJ, Ghaffar YA, Kaye AD, Viswanath O, Urits I, Boyer AG, et al: Selective serotonin reuptake inhibitors and adverse effects: A narrative review. Neurol Int. 13:387–401. 2021.PubMed/NCBI View Article : Google Scholar | |
|
Fuentes AV, Pineda MD and Venkata KCN: Comprehension of top 200 prescribed drugs in the US as a resource for pharmacy teaching, training and practice. Pharmacy (Basel). 6(43)2018.PubMed/NCBI View Article : Google Scholar | |
|
Chu A and Wadhwa R: Selective serotonin reuptake inhibitors. Statpearls. treasure island (FL): StatPearls publishing copyright©. 2022, StatPearls Publishing LLC.; 2022. | |
|
Takano A, Halldin C and Farde L: SERT and NET occupancy by venlafaxine and milnacipran in nonhuman primates: A PET study. Psychopharmacology (Berl). 226:147–153. 2013.PubMed/NCBI View Article : Google Scholar | |
|
Fanelli D, Weller G and Liu H: New serotonin-norepinephrine reuptake inhibitors and their anesthetic and analgesic considerations. Neurol Int. 13:497–509. 2021.PubMed/NCBI View Article : Google Scholar | |
|
Li J, Lu C, Gao Z, Feng Y, Luo H, Lu T, Sun X, Hu J and Luo Y: SNRIs achieve faster antidepressant effects than SSRIs by elevating the concentrations of dopamine in the forebrain. Neuropharmacology. 177(108237)2020.PubMed/NCBI View Article : Google Scholar | |
|
Haller E, Geier M and Finley P: Antidepressants, pharmacology of. In: Aminoff MJ, Daroff RB, editors. Encyclopedia of the Neurological Sciences (Second Edition). Oxford: Academic Press; 2014. p. 219-23. | |
|
Onaolapo AY and Onaolapo OJ: Glutamate and depression: Reflecting a deepening knowledge of the gut and brain effects of a ubiquitous molecule. World J Psychiatry. 11:297–315. 2021.PubMed/NCBI View Article : Google Scholar | |
|
Pal MM: Glutamate: The master neurotransmitter and its implications in chronic stress and mood disorders. Front Hum Neurosci. 15(722323)2021.PubMed/NCBI View Article : Google Scholar | |
|
Pochwat B, Nowak G and Szewczyk B: An update on NMDA antagonists in depression. Expert Rev Neurother. 19:1055–1067. 2019.PubMed/NCBI View Article : Google Scholar | |
|
Li Y: Modern epigenetics methods in biological research. Methods. 187:104–113. 2021.PubMed/NCBI View Article : Google Scholar | |
|
Sun L, Zhang H and Gao P: Metabolic reprogramming and epigenetic modifications on the path to cancer. Protein Cell. 13:877–919. 2022.PubMed/NCBI View Article : Google Scholar | |
|
Gougousis S, Petanidis S, Poutoglidis A, Tsetsos N, Vrochidis P, Skoumpas I, Argyriou N, Katopodi T and Domvri K: Epigenetic editing and tumor-dependent immunosuppressive signaling in head and neck malignancies. Oncol Lett. 23(196)2022.PubMed/NCBI View Article : Google Scholar | |
|
Dawson MA and Kouzarides T: Cancer epigenetics: From mechanism to therapy. Cell. 150:12–27. 2012.PubMed/NCBI View Article : Google Scholar | |
|
Ling C and Rönn T: Epigenetics in human obesity and type 2 diabetes. Cell Metab. 29:1028–1044. 2019.PubMed/NCBI View Article : Google Scholar | |
|
Surace AEA and Hedrich CM: The role of epigenetics in autoimmune/inflammatory disease. Front Immunol. 10(1525)2019.PubMed/NCBI View Article : Google Scholar | |
|
Menke A, Klengel T and Binder EB: Epigenetics, depression and antidepressant treatment. Curr Pharm Des. 18:5879–5889. 2012.PubMed/NCBI View Article : Google Scholar | |
|
Fardi M, Solali S and Hagh MF: Epigenetic mechanisms as a new approach in cancer treatment: An updated review. Genes Dis. 5:304–311. 2018.PubMed/NCBI View Article : Google Scholar | |
|
Singh KP, Miaskowski C, Dhruva AA, Flowers E and Kober KM: Mechanisms and measurement of changes in gene expression. Biol Res Nurs. 20:369–382. 2018.PubMed/NCBI View Article : Google Scholar | |
|
Corbett AH: Post-transcriptional regulation of gene expression and human disease. Curr Opin Cell Biol. 52:96–104. 2018.PubMed/NCBI View Article : Google Scholar | |
|
Landini A, Trbojević-Akmačić I, Navarro P, Tsepilov YA, Sharapov SZ, Vučković F, Polašek O, Hayward C, Petrović T, Vilaj M, et al: Genetic regulation of post-translational modification of two distinct proteins. Nat Commun. 13(1586)2022.PubMed/NCBI View Article : Google Scholar | |
|
Li X, Zhao Q, Wei W, Lin Q, Magnan C, Emami MR, Wearick-Silva LE, Viola TW, Marshall PR, Yin J, et al: The DNA modification N6-methyl-2'-deoxyadenosine (m6dA) drives activity-induced gene expression and is required for fear extinction. Nat Neurosci. 22:534–544. 2019.PubMed/NCBI View Article : Google Scholar | |
|
Kiselev IS, Kulakova OG, Boyko AN and Favorova OO: DNA methylation as an epigenetic mechanism in the development of multiple sclerosis. Acta Naturae. 13:45–57. 2021.PubMed/NCBI View Article : Google Scholar | |
|
Dhar GA, Saha S, Mitra P and Chaudhuri RN: DNA methylation and regulation of gene expression: Guardian of our health. Nucleus (Calcutta). 64:259–270. 2021.PubMed/NCBI View Article : Google Scholar | |
|
Lee YS: Are we studying non-coding RNAs correctly? Lessons from nc886. Int J Mol Sci. 23(4251)2022.PubMed/NCBI View Article : Google Scholar | |
|
Diamantopoulos MA, Tsiakanikas P and Scorilas A: Non-coding RNAs: The riddle of the transcriptome and their perspectives in cancer. Ann Transl Med. 6(241)2018.PubMed/NCBI View Article : Google Scholar | |
|
Kumar S, Gonzalez EA, Rameshwar P and Etchegaray JP: Non-Coding RNAs as mediators of epigenetic changes in malignancies. Cancers (Basel). 12(3657)2020.PubMed/NCBI View Article : Google Scholar | |
|
Padda IS, Mahtani AU and Parmar M: Small interfering RNA (siRNA) based therapy. StatPearls. Treasure island (FL): StatPearls Publishing Copyright ©. 2022, StatPearls Publishing LLC.; 2022. | |
|
Zhang X, Wang W, Zhu W, Dong J, Cheng Y, Yin Z and Shen F: Mechanisms and functions of long non-coding RNAs at multiple regulatory levels. Int J Mol Sci. 20(5573)2019.PubMed/NCBI View Article : Google Scholar | |
|
Chen JJ, Stermer D and Tanny JC: Decoding histone ubiquitylation. Front Cell Devel Biol. 10(968398)2022.PubMed/NCBI View Article : Google Scholar | |
|
Miller JL and Grant PA: The role of DNA methylation and histone modifications in transcriptional regulation in humans. Subcell Biochem. 61:289–317. 2013.PubMed/NCBI View Article : Google Scholar | |
|
Zhang Y, Sun Z, Jia J, Du T, Zhang N, Tang Y, Fang Y and Fang D: Overview of histone modification. Adv Exp Med Biol. 1283:1–16. 2021.PubMed/NCBI View Article : Google Scholar | |
|
Alhamwe BA, Khalaila R, Wolf J, von Bülow V, Harb H, Alhamdan F, Hii CS, Prescott SL, Ferrante A, Renz H, et al: Histone modifications and their role in epigenetics of atopy and allergic diseases. Allergy Asthma Clin Immunol. 14(39)2018.PubMed/NCBI View Article : Google Scholar | |
|
Barnes CE, English DM and Cowley SM: Acetylation & Co: An expanding repertoire of histone acylations regulates chromatin and transcription. Essays Biochem. 63:97–107. 2019.PubMed/NCBI View Article : Google Scholar | |
|
Sekiguchi M and Matsushita N: DNA damage response regulation by histone ubiquitination. Int J Mol Sci. 23(8187)2022.PubMed/NCBI View Article : Google Scholar | |
|
Wang J, Qiu Z and Wu Y: Ubiquitin regulation: The histone modifying Enzyme's story. Cells. 7(118)2018.PubMed/NCBI View Article : Google Scholar | |
|
Penner-Goeke S and Binder EB: Epigenetics and depression. Dialogues Clin Neurosci. 21:397–405. 2019.PubMed/NCBI View Article : Google Scholar | |
|
Menke A and Binder EB: Epigenetic alterations in depression and antidepressant treatment. Dialogues Clin Neurosci. 16:395–404. 2014.PubMed/NCBI View Article : Google Scholar | |
|
Wankerl M, Miller R, Kirschbaum C, Hennig J, Stalder T and Alexander N: Effects of genetic and early environmental risk factors for depression on serotonin transporter expression and methylation profiles. Transl Psychiatry. 4(e402)2014.PubMed/NCBI View Article : Google Scholar | |
|
Lee JS, Jaini PA and Papa F: An epigenetic perspective on lifestyle medicine for depression: Implications for primary care practice. Am J Lifestyle Med. 16:76–88. 2022.PubMed/NCBI View Article : Google Scholar | |
|
Meng L, Bai X and Zheng Y, Chen D and Zheng Y: Altered expression of norepinephrine transporter participate in hypertension and depression through regulated TNF-α and IL-6. Clin Exp Hypertens. 42:181–189. 2020.PubMed/NCBI View Article : Google Scholar | |
|
Xu Q, Jiang M, Gu S, Wang F and Yuan B: Early life stress induced DNA methylation of monoamine oxidases leads to depressive-like behavior. Front Cell Dev Biol. 8(582247)2020.PubMed/NCBI View Article : Google Scholar | |
|
Humphreys KL, Moore SR, Davis EG, MacIsaac JL, Lin DTS, Kobor MS and Gotlib IH: DNA methylation of HPA-axis genes and the onset of major depressive disorder in adolescent girls: A prospective analysis. Transl Psychiatry. 9(245)2019.PubMed/NCBI View Article : Google Scholar | |
|
Murgatroyd C, Patchev AV, Wu Y, Micale V, Bockmühl Y, Fischer D, Holsboer F, Wotjak CT, Almeida OFX and Spengler D: Dynamic DNA methylation programs persistent adverse effects of early-life stress. Nat Neurosci. 12:1559–1566. 2009.PubMed/NCBI View Article : Google Scholar | |
|
Duan Z and Lu J: DNA methyltransferases in depression: An update. Front Psychiatry. 11(538683)2020.PubMed/NCBI View Article : Google Scholar | |
|
Crawford B, Craig Z, Mansell G, White I, Smith A, Spaull S, Imm J, Hannon E, Wood A, Yaghootkar H, et al: DNA methylation and inflammation marker profiles associated with a history of depression. Hum Mol Genet. 27:2840–2850. 2018.PubMed/NCBI View Article : Google Scholar | |
|
Ryan J, Pilkington L, Neuhaus K, Ritchie K, Ancelin ML and Saffery R: Investigating the epigenetic profile of the inflammatory gene IL-6 in late-life depression. BMC Psychiatry. 17(354)2017.PubMed/NCBI View Article : Google Scholar | |
|
Peña CJ and Nestler EJ: Progress in epigenetics of depression. Prog Mol Biol Transl Sci. 157:41–66. 2018.PubMed/NCBI View Article : Google Scholar | |
|
Park HS, Kim J, Ahn SH and Ryu HY: Epigenetic targeting of histone deacetylases in diagnostics and treatment of depression. Int J Mol Sci. 22(5398)2021.PubMed/NCBI View Article : Google Scholar | |
|
Wu MS, Li XJ, Liu CY, Xu Q, Huang JQ, Gu S and Chen JX: Effects of histone modification in major depressive disorder. Curr Neuropharmacol. 20:1261–1277. 2022.PubMed/NCBI View Article : Google Scholar | |
|
Rey R, Chauvet-Gelinier JC, Suaud-Chagny MF, Ragot S, Bonin B, d'Amato T and Teyssier JR: Distinct expression pattern of epigenetic machinery genes in blood leucocytes and brain cortex of depressive patients. Mol Neurobiol. 56:4697–4707. 2019.PubMed/NCBI View Article : Google Scholar | |
|
Policarpo R, Sierksma A, De Strooper B and d'Ydewalle C: From junk to function: LncRNAs in CNS health and disease. Front Mol Neurosci. 14(714768)2021.PubMed/NCBI View Article : Google Scholar | |
|
Lin R and Turecki G: Noncoding RNAs in depression. Adv Exp Med Biol. 978:197–210. 2017.PubMed/NCBI View Article : Google Scholar | |
|
Shi Y, Wang Q, Song R, Kong Y and Zhang Z: Non-coding RNAs in depression: Promising diagnostic and therapeutic biomarkers. EBioMedicine. 71(103569)2021.PubMed/NCBI View Article : Google Scholar | |
|
Yoshino Y and Dwivedi Y: Non-coding RNAs in psychiatric disorders and suicidal behavior. Front Psychiatry. 11(543893)2020.PubMed/NCBI View Article : Google Scholar | |
|
Wu Y, Rong W, Jiang Q, Wang R and Huang H: Downregulation of lncRNA GAS5 alleviates hippocampal neuronal damage in mice with depression-like behaviors via modulation of MicroRNA-26a/EGR1 axis. J Stroke Cerebrovasc Dis. 30(105550)2021.PubMed/NCBI View Article : Google Scholar | |
|
Zhou Y and Chen B: GAS5-mediated regulation of cell signaling (Review). Mol Med Rep. 22:3049–3056. 2020.PubMed/NCBI View Article : Google Scholar | |
|
Webb LM, Phillips KE, Ho MC, Veldic M and Blacker CJ: The relationship between DNA methylation and antidepressant medications: A systematic review. Int J Mol Sci. 21(826)2020.PubMed/NCBI View Article : Google Scholar | |
|
Czarny P, Białek K, Ziółkowska S, Strycharz J, Barszczewska G and Sliwinski T: The importance of epigenetics in diagnostics and treatment of major depressive disorder. J Person Med. 11(167)2021.PubMed/NCBI View Article : Google Scholar | |
|
Kanherkar RR, Getachew B, Ben-Sheetrit J, Varma S, Heinbockel T, Tizabi Y and Csoka AB: The effect of citalopram on genome-wide DNA methylation of human cells. Int J Genomics. 2018(8929057)2018.PubMed/NCBI View Article : Google Scholar |