Current updates on the association between celiac disease and cancer, and the effects of the gluten‑free diet for modifying the risk (Review)
- Authors:
- Published online on: January 24, 2022 https://doi.org/10.3892/ijfn.2022.25
- Article Number: 2
-
Copyright: © Kalra et al. This is an open access article distributed under the terms of Creative Commons Attribution License.
Abstract
1. Introduction
Celiac disease (CD) is a chronic T-cell mediated intestinal inflammatory condition, caused by the ingestion of gluten in genetically susceptible individuals who carry the human leukocyte antigen (HLA)-DQ2 and HLA-DQ8 haplotypes (1,2). Gluten is a storage protein of wheat and is also found in related grains, such as rye, barley and spelt. CD affects ~1% of the population worldwide. The HLA-DQ haplotype is strongly associated with the development of CD. CD is an interplay between the adaptive and innate immune systems. The gluten peptide is deamidated by the tissue transglutaminase (tTG) enzyme on gluten consumption in patients with CD. Deamidated gluten protein is recognized by the HLA-DQ molecules that activate CD4+ T lymphocytes that secrete interferon (IFN)-γ, causing the destruction of the small intestinal mucosa. Gliadin peptide can also trigger innate immune components (e.g., IL-15), leading to intestinal tissue damage. Hence, the elimination of gluten is strongly recommended in CD, and a strict and life-long gluten-free diet (GFD) is the only accepted treatment (3). Complete adherence to a GFD heals the mucosal injury and leads to the remission of celiac-related symptoms (e.g., frequent diarrhoea) (4) Daily exposure to even a minor amount of gluten traces is able to restore chronic inflammation and CD-related symptoms (5). Hence, complete gluten elimination is strictly required to heal the damaged intestinal mucosa. As gluten is a pervasive molecule, it is challenging to eliminate gluten from the diet. Several other factors, such as availability, cost and product labelling prevent the adherence to a GFD (6). Continuous exposure to gluten severely affects CD management and may cause a complex condition known as a refractory CD, in which patients do not respond to a GFD (7).
Cancer is a life-threatening disease marked by uncontrollable, disorganized and undesired cell division. Cancer encompasses >200 diseases, and according to the World Health Organization (WHO), cancer is one of the most severe medical issues confronting humanity in the 21st century. In 2019, the WHO placed cancer in the first or second position as the cause of mortality for individuals <70 years of age in 112 countries (8). Cancer is likely to become the most prevalent cause of mortality due to increased diagnostics, insufficient health care, and poor lifestyle habits and diet (9). Based on a study involving over 7,000 tumour samples and 29 cancer types, it was found that 1 to 10 mutations are required for cancer to grow (10). Hereditary cancers are caused by a prior family history of the disease. By contrast, sporadic cancer is caused by environmental factors, such as comorbidities, lifestyle habits, or the use of specific medicines, such as immunosuppressants (10).
In recent decades, several studies have confirmed that the long-term constant exposure to gluten traces is associated with an increased risk of developing certain types of cancer. In the first set of studies, Green et al (11) in 2003 reported a connection between cancer and CD. Later on, various studies demonstrated that the risk of developing certain types of cancer, such as lymphoma and oropharyngeal cancer increased at alarming rates in patients with CD (12-14). There is also evidence to indicate an association between CD and cancer development, primarily intestinal cancer (13-16). In a recent nationwide cohort study, Lebwohl et al (17) reported that patients with CD were at an increased risk of developing cancer, particularly those diagnosed with CD after the age of 40.
The present review article addresses the prominent link between CD and certain types of cancer, and the role of a GFD. Furthermore, the present review also discusses whether a strict GFD increases or decreases the risk of developing CD-associated cancer.
2. Celiac disease: Pathophysiology
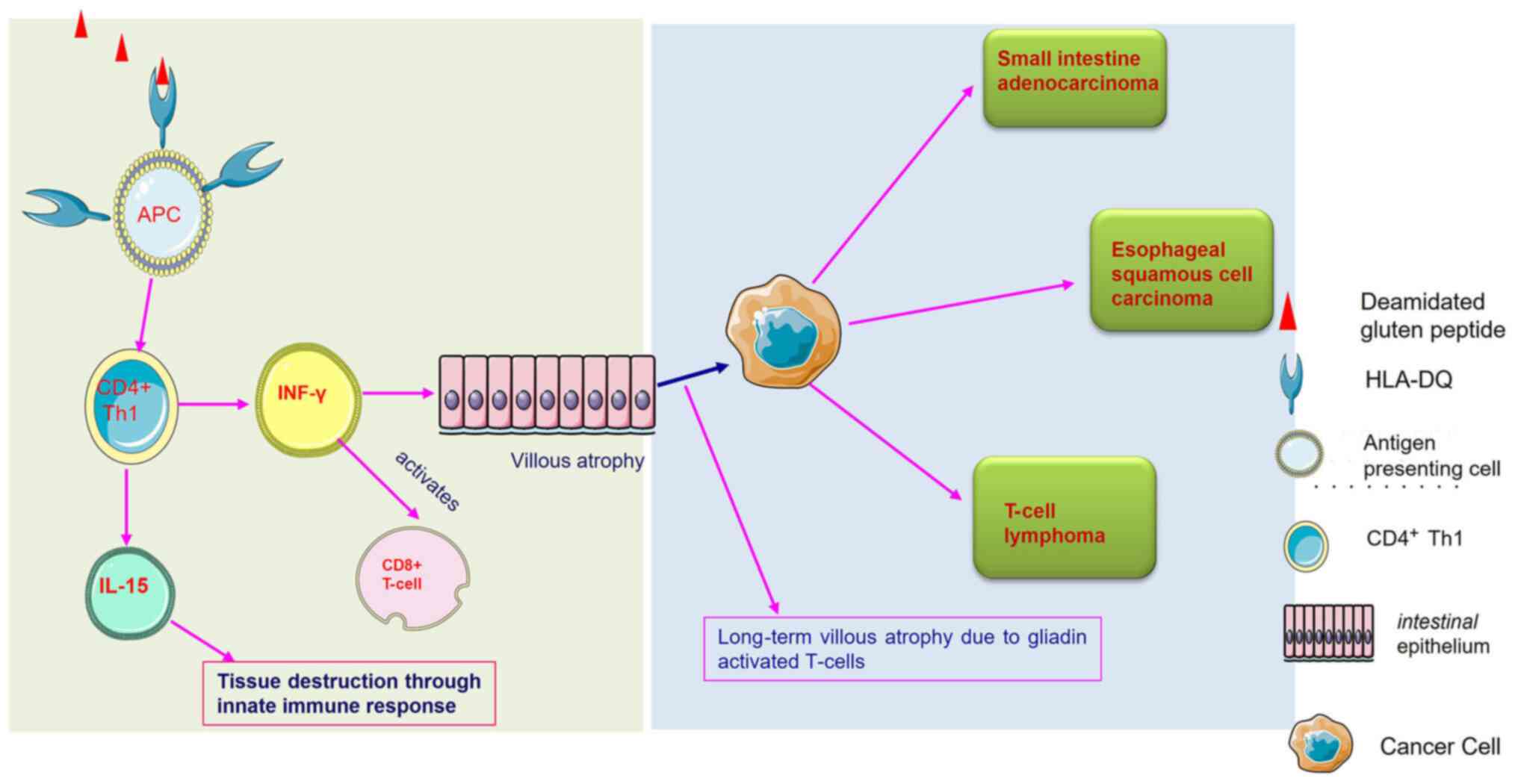
Celiac disease occurs upon exposure to gluten in susceptible individuals. Gluten protein contains a 33-mer protein sequence rich in the repetitive sequence of proline and glutamine, which develops due to the partial digestion of wheat molecules; this specific sequence remains highly stable toward the breakdown by all gastric, pancreatic and intestinal brush border membrane endoprotease (18). Hence, when a CD-affected individual consumes wheat, the wheat peptides are partially digested, and relatively large gluten peptides are transported across the mucosal epithelium (19), where tTG enzymes are entangled with processed gluten peptides and deamidated glutamine into negatively charged glutamic acid. These negatively charged glutamic acid residues increase the binding affinity for the disease-relevant HLA-DQ2/-DQ8 molecules. Once bound to the HLA-DQ2/8 molecule, this gluten-HLA-DQ complex activates the CD4 T-helper 1 (Th1) cells (20). The gluten-reactive CD4 T-cells produce IFN-γ upon activation. T-cells also stimulate the production of IFN-γ in the epithelium. T-cells migrate to the epithelium and facilitate the killing of enterocytes that eventually damages the intestinal mucosa, leading to partial/total villous atrophy (21).
CD is a systemic disease. It is associated with other similar autoimmune diseases. HLA-DQ molecules that cause susceptibility to CD also play a role in type 1 diabetes mellitus (T1DM), the pathophysiology of which is identical to CD (22). HLA-DQ molecules bind and present β-cell autoantigen derived peptides in T1DM. CD exhibits genetic similarity with another autoimmune diseases, such as rheumatoid arthritis (RA). Hence, patients with CD remain at risk of developing T1DM and RA (23,24). Furthermore, gluten has been shown to be frequently associated with neurological disorders, such as cerebellar ataxia, peripheral neuropathy, seizures, headaches, cognitive impairment and neuropsychiatric diseases (25). However, a relief in the symptoms has been reported upon the implementation of a GFD in such conditions (26).
3. Role of the gut microbiota in celiac disease
According to increasing evidence, changes in gut microbiome composition and function are linked to various chronic inflammatory diseases, including obesity, diabetes, inflammatory bowel disease and cancer (27). This may also be the case with CD. Gluten metabolism is aided by the gut bacteria found in the human colon. Lactobacilli and Bifidobacterium spp. may play a role in modifying the immunogenic potential of gluten and its peptides (28). Patients implementing a long-term GFD who have persistent symptoms of CD have an altered microbial gut composition, with significant differences between patients with classic gastrointestinal symptoms (such as weight loss and diarrhoea) and patients with extraintestinal manifestations (such as anaemia, malabsorption of iron, folate, Vitamin D, calcium and short stature) (29). Intestinal dysbiosis has been documented in patients with CD, whether untreated or treated with a GFD, compared with healthy participants. Recent investigations on patients with CD have found an increase in the relative amounts of Gram-negative bacterial genera, including Bacteroides, Prevotella and Escherichia, and a decrease in the relative amounts of beneficial anti-inflammatory bacteria, such as Bifidobacteria and lactobacilli. Dysbiotic microbiota can cause a dysregulated immunological response, contributing to CD pathogenesis. Antibiotic usage and certain feeding habits during infancy may also lead to changes in the developing gut microbiota, affecting immunological maturation and predisposing the individual to CD. De Palma et al (30) found that the milk-feeding style, in combination with the HLA-DQ genotype, affected the gut microbiota of newborns in a study involving 164 healthy newborns with one first-degree relative with CD. The discovery of the role of intestinal bacteria in the development of CD opens up new avenues for treatment with probiotics. However, further research is required in this field (28).
4. Association between celiac disease and an increased risk of cancer
Chronic inflammation is one of the hallmarks of cancer, and chronic intestinal inflammation remains associated with the development of CD (16,31,32). Therefore, it can be considered that CD can lead to the development of certain types of cancer, particularly cancers related to the gastrointestinal tract (Fig. 1).
Cancer is a manifestation of immune system abnormalities, since malignant cells avoid detection and removal by the immune system. Tumour growth is further aided by chronic infections and inflammation associated with polarised or limited immune responses. The tumour immune environment refers to the conditions and factors that enable malignant cells to survive (9). In the case of CD, it is most likely related to how the human body reacts to the inflammation, intestinal damage and nutritional deficits caused by villous atrophy (33). T-cells in the bowel are generally ‘tissue-healing’ cells that protect the body. T-cells, on the other hand, generate inflammation in patients with CD when they are exposed to gluten. This inflammation damages the gut lining, resulting in acute symptoms in patients with CD following the consumption of gluten (34). Patients with CD primarily develop an adaptive T-cell-mediated immune response. However, in cancer development, apart from T-cells, natural killer (NK) T-cells also play an essential role. These cells are a subset of T-cells involved indirectly, inducing the death of tumour cells when activated. In patients with CD, the NK T-cell count remains low compared with healthy subjects. The loss of these immunoregulatory cells may lead to the improper activation of gluten-sensitive T-cells, resulting in intestinal damage (35). The association between different types of cancer and CD is summarised in the following sections, and a summary of their association is presented in Table I and Fig. 1.
5. Celiac disease and its association with various types of cancer
CD and oesophageal cancer
Oesophageal cancer is the seventh most common cause of cancer-related mortality among males, thus exhibiting a strong male predominance attributed to environmental factors, such as alcohol consumption, cigarette smoking, nutrition and socioeconomic status (36). Apart from the environmental factors, the risk of developing this type of cancer can increase due to underlying disorders, such as gastroesophageal reflux disease (GERD), CD, Barrett's oesophagus, achalasia and obesity. Cancer generally develops in the inner layer of the oesophageal wall and continues to spread to the outer layers. According to the cancer statistics, the prevalence of oesophageal cancer is more common in Iran, India, Northern China and South Africa than in Western countries, such as the USA (37).
In GERD, the acid from the stomach rises to the oesophagus and damages the oesophageal tissue, causing a scar in the oesophagus. This constant insult and injury to the lower oesophageal cell lining and tissues can ultimately increase the proliferation of cells (38). A change in cell shape from squamous to columnar following GERD indicates the disruption of cell polarity, which marks the onset of cancer (39).
Patients with CD have been observed to have a high risk of developing oesophageal malignancies. It has been found that in patients with untreated CD, the delayed emptying of the stomach is quite common, and this may be an essential factor that can be linked to gastroesophageal reflux (23). The abnormal concentrations of a number of hormones related to the GIT have also been observed in untreated patients with CD. This causes a disbalance in upper gastrointestinal function controlled by neuroendocrine factors (40). Constant acid reflux from the stomach manifests Barrett's oesophagus. GFD has been shown as a valuable approach to reduce GERD symptoms (41).
CD and colorectal cancer
Colorectal cancer is a cancer of the colon or rectum. It is the third most common type of cancer in males and the second most prevalent among females in the USA (42). The risk of developing colorectal cancer increase with age, diet and lifestyle. Apart from age, a family history also contributes to an increased risk of this type of cancer. It begins with the loss of the adenomatous polyposis coli (APC) gene present on chromosome 5q21 and the subsequent activation of the KRAS oncogene and mutations in the TP53 gene (42,43).
A previous study by Lasa et al (42) demonstrated that patients with CD have an increased risk of developing colorectal cancer. Consistent gluten exposure in individuals with CD may increase their risk of developing colorectal neoplasia (42). Golfetto et al (43) compared the count of Bifidobacterium in faecal matter and reported that patients with CD had a lower Bifidobacterium count. Bifidobacterium metabolites exhibit anticancer activity, as previously observed using the SW742 colon cancer cell line (44). This may be one of the probable causes of the increased incidence of colon cancer among patients with CD. Contrary to previous reports, other studies have not found any direct link between CD and an increased risk of colorectal neoplasia (45,46). It is crucial to note that these studies all involved patients with CD on a GFD and were compared to non-celiac, otherwise healthy controls (42,45,46). The simple fact may explain this as a high-fat, low-fibre diet has been associated with an increased risk of colorectal cancer, and celiac-associated intestinal damage may aid to reduce that risk by preventing the body from absorbing fat. Alternatively, immunological changes in the small intestine may prevent colon cancer from developing (46). Body mass index (BMI) is also a crucial factor when assessing the risk of developing colorectal cancer. Patients with CD are generally found to have a low BMI compared to healthy individuals (47,48), which acts as a protective factor when measuring the risk of CD (48). In addition, the lack of an increased risk of colorectal cancer in population research is due to a genuine average risk of colorectal neoplasia, not to greater colonoscopy and concomitant polypectomies in the celiac community (46).
CD and small intestine adenocarcinoma (SBA)
SBA, also known as small bowel adenocarcinoma, is a rare form of neoplasm that accounts for <5% of all gastrointestinal malignancies (15). This type of cancer can affect all three parts of the small intestine, i.e., the duodenum, jejunum and ileum (49). Often, SBA is attributed as the ‘sibling’ of colorectal cancer due to epidemiological and clinical similarities between both types of cancers (49). This type of cancer arises first from the glandular cells on the small intestine lining and then spreads to deeper layers. Genes commonly mutated in this type of cancer include TP53, KRAS, APC, SMAD4, BRAF, SOX9, ATM, ARID2, ACVR2A, ACVR1B, BRCA2 and SMARCA4 (50). The risk factors for SBA can range from the consumption of sugar, red meat, alcohol, smoking and underlying diseases, such as Crohn's disease and CD. The main trigger factor for SBA arises due to inflammation in the small intestinal walls, which occurs following gluten consumption. This leads to damage in the villi of the small intestine. It has been reported that during the course of CD, γ-δ- and T-cell expansion increases in the small intestinal epithelium, which plays an active role in pathogenic inflammation (51).
Inflammation promotes the development of tumours. Gliadin peptides trigger the excessive release of zonulin (the protein that modulates tight intercellular junctions), leading to the disruption of the gut barrier and the disassembly of tight junctions (51). Tight junction proteins play an essential role in establishing and maintaining the apicobasal cell polarity (52), leading to alterations in cell polarity, a major driver and hallmark of oncogenesis (53). Zona occludens 1 (ZO-1) expression has been shown to be significantly reduced in patients with CD (54). ZO-1 is a tight junction protein that, along with occludin, helps maintain the barrier function. However, this downregulation has been shown to be reversed by following a GFD (54). Thus, persistent inflammation and alterations in cell morphology due to severe insults to cells increases the risk of SBA.
CD and lymphoma
Lymphomas begin in the lymphatic system, where white blood cells proliferate uncontrollably. Although the disease is rare in children <5 years of age, it is the most commonly diagnosed type of cancer in teens aged between 15 to 19 years. The disease accounts for 12% of all cancer cases in this age group (55).
This is a rare type of aggressive-cell lymphoma that accounts for <5% of all GIT lymphomas, with an annual incidence rate of 0.5-1 per million subjects in Western countries (13). The incidence and temporal trend patterns differ by age, sex, race/ethnicity and geography, implying that infectious agents, environmental variables and lifestyle factors, in addition to the genetic status of the host, may play a role in the development of lymphoma. Immune modulation and persistent antigen activation are two critical pathogenetic pathways (56).
Patients with CD have a high risk of developing lymphoma. Patients with untreated CD often have a high risk of developing enteropathy-associated T-cell lymphoma (EATL) (57). Based on appearance, immunohistochemistry and genetic profile, it is divided into two types. However, type I is more frequent and strongly linked to CD, particularly when the condition becomes refractory to gluten withdrawal. The molecular mechanisms that cause EATL in the gluten-sensitive atrophic intestinal mucosa have been thoroughly investigated. Classic EATL frequently exhibits chromosomal gains at 9q33-34, 7q, 5q34-35 and 1q21-23, and losses at 8p, 13q21 and 9p21(58). Cellier et al (14) proposed that cryptic enteropathy-related T-cell lymphoma represents a transitory stage of gluten refractory disease with aberrant intraepithelial T-lymphocyte clones. The adjacent small bowel usually shows villous atrophy associated with CD (14). The use of a GFD to treat CD effectively inhibits the development of EATL. The mechanisms underlying the strong link between EATL and CD are unknown; antigen-driven T-cell proliferation and less specific pro-proliferative effects of chronic inflammation are both potential candidates (57).
CD and primary liver cell cancer
Hepatocellular carcinoma is the most common form of primary liver cell cancer due to liver disease. It is the fifth most common type of cancer in males and seventh most common cancer in females, and ranks second as regards cancer-related mortality (59). The risk factors include heavy alcohol consumption, hepatitis B and C infection, diabetes mellitus and fatty liver disease (60). Celiac hepatitis is the most common form of liver abnormality in patients with CD (61). Untreated CD can lead to a disbalance between liver enzyme levels, ultimately increasing the risk of liver cancer. The mechanisms through which patients with CD develop liver cancer remain unknown. One proposition may be gut microbiota alterations (62). CD causes a decrease in the numbers of Bifidobacterium; as a result, the Bifidobacterium/Enterobacteriaceae ratio is altered, which then causes liver disease (62). Liver disease can be a threat that can lead to cirrhosis of the liver and, ultimately, cancer and gluten consumption by patients with CD can cause an inflammatory response, leading to liver scarring. The scar tissues hinder the flow of blood through that region of the liver, and eventually, the scars lead to cirrhosis of the liver, leading to cancer onset. Hence, untreated CD can increase the risk of primary liver cell carcinoma (61).
CD and breast cancer
According to the WHO and the statistics released by the International Agency for Research on Cancer (IARC), as of December 2020, breast cancer is the most commonly diagnosed cancer among females (8). It has surpassed lung cancer, which used to have the highest incidence worldwide. Breast cancer remains the most common form of cancer in females in the USA, with 1 out of 8 women bearing the risk of developing breast cancer at some point in their lives (63). In breast cancer, the epithelial cells residing in the breast (preferably in the lobules) lead to the formation of a tumour. The risk factors for breast cancer include age, sex, lifestyle, food, sleep patterns, hormone status (oestrogen and progesterone), and inherited genetic mutations from family members. Breast cancer comprises four types, namely invasive ductal carcinoma, invasive lobular carcinoma, Paget's disease and inflammatory breast cancer. The genes associated with breast cancer include BRCA1 and BRCA2. Other than these two genes, BRCA3, located on chromosome 8p11-21, exhibits mutations that give rise to sporadic disease. Inactivating mutations in TP53 are also associated with breast cancer. The inactivation of TP53 occurs not only in breast cancer, but also in all cancer types, as this tumour suppressor gene is mainly involved in the suppression of cancer cell growth (64). Other genetic mutations associated with breast cancer include the PTEN, CDH1, PALB2, CHEK2 and STK11 genes (65).
As demonstrated in the study by Askling et al (12), patients with CD have a low risk of developing breast cancer. Patients with CD often have lactose intolerance, which occurs due to a deficiency in the lactase enzyme. Lactase deficiency can lead to CD demonstrated by the H2 lactose breath test, as previously demonstrated in a retrospective study involving 54 subjects (66). Milk products contain insulin-like growth factor 1 (IGF-1) (43), which facilitates tumour growth through angiogenesis, low rates of apoptosis (a process where the damaged cells destroy themselves and macrophages eat away the debris), and a high proliferative potential leading to a higher risk of breast cancer (43). Therefore, the possible reason for the low risk of breast cancer in patients with CD is that they consume fewer dairy products due to lactose intolerance.
Additionally, women with CD have a low nutritional status due to malabsorption by villi, leading to weight loss. This can be another factor for the reduced risk of breast cancer (67). Ugalde-Morales et al (68) demonstrated the association between common shared single nucleotide polymorphisms between the two diseases mapped onto genes enriched for immunoregulatory and apoptotic processes behind the decreased risk of breast cancer in celiac disease. Another study suggested that the reduced risk may be attributed to the lower BMI in individuals with CD, as reported in anorexia nervosa (69).
CD and oropharyngeal cancer
According to the American Cancer Society statistics, oropharyngeal cancer, commonly known as head and neck cancer, is the eighth most common type of cancer among males (70). The majority of oropharyngeal cancers arise in the squamous. Risk factors include human papilloma virus infection, smoking and alcohol consumption. In a previous study on Swedish patients, it was found that the risk of developing oropharyngeal cancer increased to a moderate amount in patients with CD (12). Another study reported several oral health issues, such as canker sores, xerostomia (dry mouth), dental discoloration and enamel defects in patients with CD (71). The study by Askling et al (12) employed a population cohort of 11,019 patients with CD. They reported that patients with CD were at a higher risk of developing both oropharyngeal and oesophageal cancers by 2.3- and 4.2-fold, respectively (12). Thus, one answer for the risk of oropharyngeal cancer in CD is oesophageal cancer manifestation. The radiotherapy involved in treating cancer (preferably oesophageal cancer in this case) damages the salivary glands and thickens saliva, thus leading to dry mouth disease. However, the exact reason for the increased risk remains unknown. A two-fold relative risk of cancer was found due to an increased risk of cancer of the mouth and pharynx. However, the risk was increased in those taking reduced gluten, or a normal diet, with an excess of cancers of the mouth, pharynx, and oesophagus (12). Holmes et al (72) reported a significant decrease in the morbidity rate of patients with CD adhering to the GFD diet; the results of their study suggest a protective role for a GFD against malignancy in CD (72).
CD and endometrial cancer
Endometrial cancer is a type of cancer that forms in the tissues of the endometrium (lining of the uterus). Female-related cancer begins in the hollow, pear-shaped baby-bearing organ, and the uterus is the fourth most common type of cancer among females in the USA (73). Endometrial cancers are of two types, i.e., type I, which exhibits a dependency on oestrogen, and type II, which is oestrogen-independent. (73) Type I is most common, with an occurrence rate of 85% and generally harbours mutations in KRAS, PTEN and β-catenin (73). It also exhibits microsatellite instability. Type II endometrial cancer exhibits p53 mutation and HER-2 amplification (73). The risk factors for endometrial cancer include age, tamoxifen consumption, oestrogen treatment, obesity, menstrual factors and underlying disorders, such as diabetes mellitus (73).
This type of has been found to have an inverse association with CD. The risk for female hormone-related cancers is generally reported to be low in patients with CD (67). One reason for this may be the low exposure to oestrogen, which acts as a protective shield for these types of cancer in women with CD (67). A previous study reported that untreated CD can lead to early menopause in women, which eventually leads to low oestrogen levels in the body as ovaries begin to lose their ability to produce this hormone (74). Endometrial cancer generally occurs in females >50 years of age, i.e., after menopause. The risk increases if oestrogen pills are administered post-menopause without maintaining a balance with progesterone (75). Hence, a burst in oestrogen hormone levels following menopause may be the primary reason for developing this type of cancer.
Thus, untreated CD clearly explains the negative association with endometrial cancer. However, with a proper GFD, the timing of menopause becomes normal compared to celiac-free subjects (74).
CD and ovarian cancer
In ovarian cancer, ovarian cells divide uncontrollably, leading to the formation of tumours. Ovarian cancer is the seventh most common type of cancer among females. This cancer has a high mortality rate (76). Mutations in the BRCA1 and BRCA2 genes are responsible for this type of cancer. Apart from these genes, mutations in other genes, such as CHEK2, RAD51, BRIP1 and PIK3CA have also been observed. The risk of developing ovarian cancer increases if an individual has a family history of breast or ovarian cancer (77).
Women with CD have a lower risk of developing ovarian cancer, as reported by a study carried out in Sweden, in which 17,825 women with CD were diagnosed between the years 1969 and 2007(67). It has been observed that patients with CD have a reduced risk of developing female hormone-related cancer. A possible explanation for the reduction in ovarian cancer is that women with CD may be less exposed to oestrogen in their lifetime (67). Oestrogen displays a close association with ovarian cancer along with endometrial and breast cancer (67). The inflammation that generally arises due to this disease can cause hormonal disbalance. This condition later gives rise to issues regarding pregnancy and menopause.
6. The gluten-free diet
A GFD implies avoiding any gluten sources containing wheat, rye and barley, as well as their derivatives in any form (3). Other foods that do not contain gluten naturally, such as vegetables, fruits and animal-derived food (fish, poultry and meats) are permitted in their natural state. Naturally, gluten-free grains, such as rice, corn and potatoes are widely used to substitute gluten-containing grains.
Of note, even though grains naturally lack gluten, they are considered suspicious. Oats are such a grain that has been excluded from CD dietary advice for several years, as avenin (the storage protein found in oats) is also toxic to patients with CD. However, it has been demonstrated that the oat grain is safe for patients with CD. In a previous study, Gatti et al (78) reported that gluten-uncontaminated oats were safe.
7. Role of a gluten-free diet in the prevention of cancer risk
Several studies have explored the effects of a GFD on the healing of CD-related mucosal tissue damage. It has been found that following a strict GFD causes complete mucosal healing in a large number of patients with CD (79-82). However, only a limited number of studies have explored the effects of a GFD in reducing celiac-associated cancer types. In a critical study conducted in 1989, Holmes et al (72) investigated the effects of GD adherence in patients with CD related to cancers of the mouth, pharynx and oesophagus. They found that patients with CD following a GFD ≥5 five years had a reduced risk of developing cancer compared to the general population. The study concluded that the GFD plays a protective role; hence, a strict GFD must be followed by patients with CD (72). Another study found that the GFD was indeed helpful for preventing certain aggressive forms of cancer (13). Pereyra et al (45) suggested that patients with CD with a poor GFD compliance had a higher risk of colorectal adenomas. Even though the proportion of such patients who did not adhere to a GFD was small, this finding suggests that untreated CD patients may have a higher risk of colorectal neoplasia (45). They further investigated whether the lack of a GFD among patients with CD could be a trigger for the development of colorectal lesions. This theory was supported by evidence indicating an increased risk of mortality among patients with CD. On the other hand, in refractory CD, continuous gluten exposure causes histopathological lesions (83). Gluten exposure induces a toxic and immunological response in patients with CD (84). Hence, it is possible that a nutritional element plays a role in the development of neoplasms in patients with CD, including colorectal neoplasia. It may also emphasize the need of adhering to a GFD as a possible preventive factor against the development of colon cancer (45). In a recent study, Um et al investigated an association between whole-grain intake with lower risk of colorectal cancer among older US men (84). The authors of that study found that men who ate more whole grains had a decreased risk of colorectal cancer, while women did not (85). As per a recent report, there has been an increase in cancer risk among individuals diagnosed with CD >40 year of age, and this risk is predominantly prevalent within the first year of diagnosis (17). Thus far, the majority of studies investigating the risk of cancer in patients with CD have been conducted before CD serologic testing, and a GFD was generally available. Thus, the GFD may reduce cancer risk in CD (17). Silano et al (86) emphasized the early diagnosis of CD and the importance of a GFD and its protection against the development of malignancies in patients with CD. A GFD has been demonstrated to reduce inflammatory markers, such as antibody levels (87). Hence, it is possible that a GFD minimizes the risk of cancer where inflammation is a major trigger. The number of prospective studies investigating the effects of a GFD on cancer in patients with CD is still limited in the literature (72,88). The effects of a GFD in preventing/reducing the risk of developing malignancies in patients with CD is still under debate. As non-adherence and/or non-responsiveness to a GFD can result in chronic inflammation of the small bowel, it is tempting to speculate that a gluten-containing diet in patients with CD may promote the activation of immune/inflammatory signals, eventually leading to the onset/progression of lymphomas and other cancer types (16).
8. Conclusions
A long-term failure to adherence to a GFD increases the risk of developing different types of malignancies. It has been found that a strict GFD reduces the risk of developing colon cancer, oropharyngeal cancer and intestine adenocarcinoma in patients with CD. Although a GFD minimizes the risk of certain types of cancer, this fact has not yet been fully explored however. Thus, there is a need for studies investigating the role of a GFD in decreasing cancer risk in CD.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
NK was involved in the study design, analysis and interpretation of the data to be included in the review (literature search), as well as in the writing and preparation of the original draft, in the drafting of the manuscript, and in the critical revision of the manuscript for important intellectual content. AM contributed to acquiring the data for inclusion in the review (literature search), as well as in the writing and preparation of the original draft, and in designing the figure. SS and VM contributed to the acquisition of data for inclusion in the review (literature search), and in the writing and preparation of the original draft. YS was involved in the writing and reviewing of the manuscript, as well as in the drafting the manuscript. AT contributed to the critical revision of the manuscript. AKV contributed to the conception and design of the study, and in the critical revision of the manuscript. All authors have read and approved the final manuscript. Data authentication is not applicable.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
|
Verma AK, Singh A, Gatti S, Lionetti E, Galeazzi T, Monachesi C, Franceschini E, Ahuja V, Catassi C and Makharia GK: Validation of a novel single-drop rapid human leukocyte antigen-DQ2/-DQ8 typing method to identify subjects susceptible to celiac disease. JGH Open. 2:311–316. 2018.PubMed/NCBI View Article : Google Scholar | |
|
Megiorni F, Mora B, Bonamico M, Barbato M, Nenna R, Maiella G, Lulli P and Mazzilli MC: HLA-DQ and risk gradient for celiac disease. Hum Immunol. 70:55–59. 2009.PubMed/NCBI View Article : Google Scholar | |
|
Ciacci C, Ciclitira P, Hadjivassiliou M, Kaukinen K, Ludvigsson JF, McGough N, Sanders DS, Woodward J, Leonard JN and Swift GL: The gluten-free diet and its current application in coeliac disease and dermatitis herpetiformis. United European Gastroenterol J. 3:121–135. 2015.PubMed/NCBI View Article : Google Scholar | |
|
Parzanese I, Qehajaj D, Patrinicola F, Aralica M, Chiriva-Internati M, Stifter S, Elli L and Grizzi F: Celiac disease: From pathophysiology to treatment. World J Gastrointest Pathophysiol. 8:27–38. 2017.PubMed/NCBI View Article : Google Scholar | |
|
Catassi C, Fabiani E, Iacono G, D'Agate C, Francavilla R, Biagi F, Volta U, Accomando S, Picarelli A, De Vitis I, et al: A prospective, double-blind, placebo-controlled trial to establish a safe gluten threshold for patients with celiac disease. Am J Clin Nutr. 85:160–166. 2007.PubMed/NCBI View Article : Google Scholar | |
|
MacCulloch K and Rashid M: Factors affecting adherence to a gluten-free diet in children with celiac disease. Paediatr Child Health. 19:305–309. 2014.PubMed/NCBI View Article : Google Scholar | |
|
Soldera J, Salgado K and Pêgas KL: Refractory celiac disease type 2: How to diagnose and treat? Rev Assoc Med Bras (1992). 67:168–172. 2021.PubMed/NCBI View Article : Google Scholar | |
|
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J Clin. 71:209–249. 2021.PubMed/NCBI View Article : Google Scholar | |
|
Shurin MR: Cancer as an immune-mediated disease. Immunotargets Ther. 1:1–6. 2012.PubMed/NCBI View Article : Google Scholar | |
|
Martincorena I, Raine KM, Gerstung M, Dawson KJ, Haase K, Van Loo P, Davies H, Stratton MR and Campbell PJ: Universal patterns of selection in cancer and somatic tissues. Cell. 171:1029–1041.e21. 2017.PubMed/NCBI View Article : Google Scholar | |
|
Green PH, Fleischauer AT, Bhagat G, Goyal R, Jabri B and Neugut AI: Risk of malignancy in patients with celiac disease. Am J Med. 115:191–195. 2003.PubMed/NCBI View Article : Google Scholar | |
|
Askling J, Linet M, Gridley G, Halstensen TS, Ekström K and Ekbom A: Cancer incidence in a population-based cohort of individuals hospitalized with celiac disease or dermatitis herpetiformis. Gastroenterology. 123:1428–1435. 2002.PubMed/NCBI View Article : Google Scholar | |
|
Catassi C, Bearzi I and Holmes GKT: Association of celiac disease and intestinal lymphomas and other cancers. Gastroenterology. 128 (4 Suppl 1):S79–S86. 2005.PubMed/NCBI View Article : Google Scholar | |
|
Cellier C, Delabesse E, Helmer C, Patey N, Matuchansky C, Jabri B, Macintyre E, Cerf-Bensussan N and Brousse N: Refractory sprue, coeliac disease, and enteropathy-associated T-cell lymphoma. Lancet. 356:203–208. 2000.PubMed/NCBI View Article : Google Scholar | |
|
Barsouk A, Rawla P, Barsouk A and Thandra KC: Epidemiology of cancers of the small intestine: Trends, risk factors, and prevention. Med Sci (Basel). 7(46)2019.PubMed/NCBI View Article : Google Scholar | |
|
Marafini I, Monteleone G and Stolfi C: Association between celiac disease and cancer. Int J Mol Sci. 21(4155)2020.PubMed/NCBI View Article : Google Scholar | |
|
Lebwohl B, Green PHR, Emilsson L, Mårild K, Söderling J, Roelstraete B and Ludvigsson JF: Cancer risk in 47,241 individuals with celiac disease: A nationwide cohort study. Clin Gastroenterol Hepatol. 20:e111–e131. 2022.PubMed/NCBI View Article : Google Scholar | |
|
Sharma N, Bhatia S, Chunduri V, Kaur S, Sharma S, Kapoor P, Kumari A and Garg M: Pathogenesis of celiac disease and other gluten related disorders in wheat and strategies for mitigating them. Front Nutr. 7(6)2020.PubMed/NCBI View Article : Google Scholar | |
|
Balakireva AV and Zamyatnin AA: Properties of gluten intolerance: Gluten structure, evolution, pathogenicity and detoxification capabilities. Nutrients. 8(644)2016.PubMed/NCBI View Article : Google Scholar | |
|
Meresse B, Malamut G and Cerf-Bensussan N: Celiac disease: An immunological jigsaw. Immunity. 36:907–919. 2012.PubMed/NCBI View Article : Google Scholar | |
|
Jabri B and Sollid LM: T cells in celiac disease. J Immunol. 198:3005–3014. 2017.PubMed/NCBI View Article : Google Scholar | |
|
Bhatia E: Celiac disease & type 1 diabetes: A double burden. Indian J Med Res. 149:5–7. 2019.PubMed/NCBI View Article : Google Scholar | |
|
Lerner A and Matthias T: Rheumatoid arthritis-celiac disease relationship: Joints get that gut feeling. Autoimmun Rev. 14:1038–1047. 2015.PubMed/NCBI View Article : Google Scholar | |
|
Kostopoulou E, Lagadinou M, Avgeri A and Varvarigou A: Delayed presentation of seropositivity in pre-existent coeliac disease in patients with Type 1 diabetes mellitus: A possible co-occurrence? Eur Rev Med Pharmacol Sci. 25:7093–7096. 2021.PubMed/NCBI View Article : Google Scholar | |
|
Casella G, Bordo BM, Schalling R, Villanacci V, Salemme M, Di Bella C, Baldini V and Bassotti G: Neurological disorders and celiac disease. Minerva Gastroenterol Dietol. 62:197–206. 2016.PubMed/NCBI | |
|
Campagna G, Pesce M, Tatangelo R, Rizzuto A, La Fratta I and Grilli A: The progression of coeliac disease: Its neurological and psychiatric implications. Nutr Res Rev. 30:25–35. 2017.PubMed/NCBI View Article : Google Scholar | |
|
Valitutti F, Cucchiara S and Fasano A: Celiac disease and the microbiome. Nutrients. 11(2403)2019.PubMed/NCBI View Article : Google Scholar | |
|
Pecora F, Persico F, Gismondi P, Fornaroli F, Iuliano S, de'Angelis Gl and Esposito S: Gut microbiota in celiac disease: Is there any role for probiotics? Front Immunol. 11(957)2020.PubMed/NCBI View Article : Google Scholar | |
|
Akobeng AK, Singh P, Kumar M and Al Khodor S: Role of the gut microbiota in the pathogenesis of coeliac disease and potential therapeutic implications. Eur J Nutr. 59:3369–3390. 2020.PubMed/NCBI View Article : Google Scholar | |
|
Palma GD, Capilla A, Nova E, Castillejo G, Varea V, Pozo T, Garrote JA, Polanco I, López A, Ribes-Koninckx C, et al: Influence of milk-feeding type and genetic risk of developing coeliac disease on intestinal microbiota of infants: The PROFICEL study. PLoS One. 7(e30791)2012.PubMed/NCBI View Article : Google Scholar | |
|
Hanahan D and Weinberg RA: Hallmarks of cancer: The next generation. Cell. 144:646–674. 2011.PubMed/NCBI View Article : Google Scholar | |
|
Maluf SW, Wilhelm Filho D, Parisotto EB, Medeiros GDS, Pereira CHJ, Maraslis FT, Dornelles Schoeller CC, Rosa JSD and Fröde TS: DNA damage, oxidative stress, and inflammation in children with celiac disease. Genet Mol Biol. 43(e20180390)2020.PubMed/NCBI View Article : Google Scholar | |
|
Lebwohl B, Blaser MJ, Ludvigsson JF, Green PH, Rundle A, Sonnenberg A and Genta RM: Decreased risk of Celiac disease in patients with Helicobacter Pylori Colonization. Am J Epidemiol. 178:1721–1730. 2013.PubMed/NCBI View Article : Google Scholar | |
|
Mayassi T, Ladell K, Gudjonson H, McLaren JE, Shaw DG, Tran MT, Rokicka JJ, Lawrence I, Grenier JC, van Unen V, et al: Chronic inflammation permanently reshapes tissue-resident immunity in celiac disease. Cell. 176:967–981.e19. 2019.PubMed/NCBI View Article : Google Scholar | |
|
Grose RH, Cummins AG and Thompson FM: Deficiency of invariant natural killer T cells in coeliac disease. Gut. 56:790–795. 2007.PubMed/NCBI View Article : Google Scholar | |
|
Zhang Y: Epidemiology of esophageal cancer. World J Gastroenterol. 19:5598–5606. 2013.PubMed/NCBI View Article : Google Scholar | |
|
Howlader N, Noone AM, Krapcho M, Miller D, Brest A, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis DR (eds), et al: SEER Cancer Statistics Review, 1975-2018. National Cancer Institute. Bethesda, MD, 2021. https://seer.cancer.gov/csr/1975_2018/. Based on November 2020 SEER data submission, posted to the SEER web site, April 2021. | |
|
Souza RF: The role of acid and bile reflux in oesophagitis and Barrett's metaplasia. Biochem Soc Trans. 38:348–352. 2010.PubMed/NCBI View Article : Google Scholar | |
|
Daum O, Kokošková B and Švajdler M: Morphology of the gastroesophageal reflux disease. Cesk Patol. 52:15–22. 2016.PubMed/NCBI(In Czech). | |
|
Reimer C, Søndergaard B, Hilsted L and Bytzer P: Proton-pump inhibitor therapy induces acid-related symptoms in healthy volunteers after withdrawal of therapy. Gastroenterology. 137:80–87, 87.e1. 2009.PubMed/NCBI View Article : Google Scholar | |
|
Usai P, Manca R, Cuomo R, Lai MA, Russo L and Boi MF: Effect of gluten-free diet on preventing recurrence of gastroesophageal reflux disease-related symptoms in adult celiac patients with nonerosive reflux disease. J Gastroenterol Hepatol. 23:1368–1372. 2008.PubMed/NCBI View Article : Google Scholar | |
|
Lasa J, Rausch A, Bracho LF, Altamirano J, Speisky D, de Dávila MTG, Iotti A and Zubiaurre I: Colorectal adenoma risk is increased among recently diagnosed adult celiac disease patients. Gastroenterol Res Pract. 2018(6150145)2018.PubMed/NCBI View Article : Google Scholar | |
|
Golfetto L, de Senna FD, Hermes J, Beserra BT, França Fda S and Martinello F: Lower bifidobacteria counts in adult patients with celiac disease on a gluten-free diet. Arq Gastroenterol. 51:139–143. 2014.PubMed/NCBI View Article : Google Scholar | |
|
Bahmani S, Azarpira N and Moazamian E: Anti-colon cancer activity of Bifidobacterium metabolites on colon cancer cell line SW742. Turk J Gastroenterol. 30:835–842. 2019.PubMed/NCBI View Article : Google Scholar | |
|
Pereyra L, Gonzalez R, Mohaidle A, Fischer C, Mella JM, Panigadi GN, Manazzoni D, Matoso MD, Lasa JS, Novillo A, et al: Risk of colorectal neoplasia in patients with celiac disease: A multicenter study. J Crohns Colitis. 7:e672–e677. 2013.PubMed/NCBI View Article : Google Scholar | |
|
Lebwohl B, Stavsky E, Neugut AI and Green PH: Risk of colorectal adenomas in patients with coeliac disease: Aliment Pharmacol. Ther. 32:1037–1043. 2010.PubMed/NCBI View Article : Google Scholar | |
|
Kabbani TA, Goldberg A, Kelly CP, Pallav K, Tariq S, Peer A, Hansen J, Dennis M and Leffler DA: Body mass index and the risk of obesity in coeliac disease treated with the gluten-free diet. Aliment Pharmacol Ther. 35:723–729. 2012.PubMed/NCBI View Article : Google Scholar | |
|
Volta U, Vincentini O, Quintarelli F, Felli C and Silano M: Collaborating Centres of the Italian Registry of the Complications of Celiac Disease. Low risk of colon cancer in patients with celiac disease. Scand J Gastroenterol. 49:564–568. 2014.PubMed/NCBI View Article : Google Scholar | |
|
Delaunoit T, Neczyporenko F, Limburg PJ and Erlichman C: Pathogenesis and risk factors of small bowel adenocarcinoma: A colorectal cancer sibling? Am J Gastroenterol. 100:703–710. 2005.PubMed/NCBI View Article : Google Scholar | |
|
Hänninen UA, Katainen R, Tanskanen T, Plaketti RM, Laine R, Hamberg J, Ristimäki A, Pukkala E, Taipale M, Mecklin JP, et al: Exome-wide somatic mutation characterization of small bowel adenocarcinoma. PLoS Genet. 14(e1007200)2018.PubMed/NCBI View Article : Google Scholar | |
|
Dunne MR, Byrne G, Chirdo FG and Feighery C: Coeliac Disease Pathogenesis: The uncertainties of a well-known immune mediated disorder. Front Immunol. 11(1374)2020.PubMed/NCBI View Article : Google Scholar | |
|
Shin K, Fogg VC and Margolis B: Tight junctions and cell polarity. Annu Rev Cell Dev Biol. 22:207–235. 2006.PubMed/NCBI View Article : Google Scholar | |
|
Lee M and Vasioukhin V: Cell polarity and cancer-cell and tissue polarity as a non-canonical tumor suppressor. J Cell Sci. 121 (Pt 8):1141–1150. 2008.PubMed/NCBI View Article : Google Scholar | |
|
Pizzuti D, Bortolami M, Mazzon E, Buda A, Guariso G, D'Odorico A, Chiarelli S, D'Incà R, De Lazzari F and Martines D: Transcriptional downregulation of tight junction protein ZO-1 in active coeliac disease is reversed after a gluten-free diet. Dig Liver Dis. 36:337–341. 2004.PubMed/NCBI View Article : Google Scholar | |
|
Siegel RL, Miller KD, Fuchs HE and Jemal A: Cancer statistics, 2021. CA A Cancer J Clin. 71:7–33. 2021.PubMed/NCBI View Article : Google Scholar | |
|
Chiu BC and Hou N: Epidemiology and etiology of non-hodgkin lymphoma. Cancer Treat Res. 165:1–25. 2015.PubMed/NCBI View Article : Google Scholar | |
|
Marušić M, Gulić S, Gašparov S, Bilić A, Jurčić D, Vučković B, Stanić G, Luetić K, Dominković A and Sučić T: Celiac disease and fulminant T lymphoma detected too late in a 35-year-old female patient: Case report. Bosn J Basic Med Sci. 11:190–193. 2011.PubMed/NCBI View Article : Google Scholar | |
|
Zettl A, Lee SS, Rüdiger T, Starostik P, Marino M, Kirchner T, Ott M, Müller-Hermelink HK and Ott G: Epstein-Barr virus-associated B-cell lymphoproliferative disorders in angioimmunoblastic T-cell lymphoma and peripheral T-cell lymphoma, unspecified. Am J Clin Pathol. 117:368–379. 2002.PubMed/NCBI View Article : Google Scholar | |
|
Mittal S and El-Serag HB: Epidemiology of hepatocellular carcinoma: Consider the population. J Clin Gastroenterol. 47 (Suppl):S2–S6. 2013.PubMed/NCBI View Article : Google Scholar | |
|
Janevska D, Chaloska-Ivanova V and Janevski V: Hepatocellular carcinoma: Risk factors, diagnosis and treatment. Open Access Maced J Med Sci. 3:732–736. 2015.PubMed/NCBI View Article : Google Scholar | |
|
Rubio-Tapia A and Murray JA: Liver involvement in celiac disease. Minerva Med. 99:595–604. 2008.PubMed/NCBI | |
|
Fang D, Shi D, Lv L, Gu S, Wu W, Chen Y, Guo J, Li A, Hu X, Guo F, et al: Bifidobacterium pseudocatenulatum LI09 and Bifidobacterium catenulatum LI10 attenuate D-galactosamine-induced liver injury by modifying the gut microbiota. Sci Rep. 7(8770)2017.PubMed/NCBI View Article : Google Scholar | |
|
Feuer EJ, Wun LM, Boring CC, Flanders WD, Timmel MJ and Tong T: The lifetime risk of developing breast cancer. J Natl Cancer Inst. 85:892–897. 1993.PubMed/NCBI View Article : Google Scholar | |
|
de Jong MM, Nolte IM, te Meerman GJ, van der Graaf WT, Oosterwijk JC, Kleibeuker JH, Schaapveld M and de Vries EG: Genes other than BRCA1 and BRCA2 involved in breast cancer susceptibility. J Med Genet. 39:225–242. 2002.PubMed/NCBI View Article : Google Scholar | |
|
Wood ME, McKinnon W and Garber J: Risk for breast cancer and management of unaffected individuals with non-BRCA hereditary breast cancer. Breast J. 26:1528–1534. 2020.PubMed/NCBI View Article : Google Scholar | |
|
Furnari M, Bonfanti D, Parodi A, Franzè J, Savarino E, Bruzzone L, Moscatelli A, Di Mario F, Dulbecco P and Savarino V: A comparison between lactose breath test and quick test on duodenal biopsies for diagnosing lactase deficiency in patients with self-reported lactose intolerance. J Clin Gastroenterol. 47:148–152. 2013.PubMed/NCBI View Article : Google Scholar | |
|
Ludvigsson JF, West J, Ekbom A and Stephansson O: Reduced risk of breast, endometrial and ovarian cancer in women with celiac disease. Int J Cancer. 131:E244–E250. 2012.PubMed/NCBI View Article : Google Scholar | |
|
Ugalde-Morales E, Li J, Humphreys K, Ludvigsson JF, Yang H, Hall P and Czene K: Common shared genetic variation behind decreased risk of breast cancer in celiac disease. Sci Rep. 7(5942)2017.PubMed/NCBI View Article : Google Scholar | |
|
Olén O, Montgomery SM, Marcus C, Ekbom A and Ludvigsson JF: Coeliac disease and body mass index: A study of two Swedish general population-based registers. Scand J Gastroenterol. 44:1198–1206. 2009.PubMed/NCBI View Article : Google Scholar | |
|
Siegel R, Naishadham D and Jemal A: Cancer statistics, 2013. CA Cancer J Clin. 63:11–30. 2013.PubMed/NCBI View Article : Google Scholar | |
|
de Carvalho FK, de Queiroz AM, Bezerra da Silva RA, Sawamura R, Bachmann L, Bezerra da Silva LA and Nelson-Filho P: Oral aspects in celiac disease children: Clinical and dental enamel chemical evaluation. Oral Surg Oral Med Oral Pathol Oral Radiol. 119:636–643. 2015.PubMed/NCBI View Article : Google Scholar | |
|
Holmes GK, Prior P, Lane MR, Pope D and Allan RN: Malignancy in coeliac disease-effect of a gluten free diet. Gut. 30:333–338. 1989.PubMed/NCBI View Article : Google Scholar | |
|
Lucas WE and Yen SS: A study of endocrine and metabolic variables in postmenopausal women with endometrial carcinoma. Am J Obstet Gynecol. 134:180–186. 1979.PubMed/NCBI | |
|
Santonicola A, Iovino P, Cappello C, Capone P, Andreozzi P and Ciacci C: From menarche to menopause: The fertile life span of celiac women. Menopause. 18:1125–1130. 2011.PubMed/NCBI View Article : Google Scholar | |
|
Bharatnur S, Kustagi P and Krishnamohan D: Endometrial carcinoma in a young woman: ‘30 is not immune.’. J Obstet Gynecol India. 61:686–688. 2011.PubMed/NCBI View Article : Google Scholar | |
|
Momenimovahed Z, Tiznobaik A, Taheri S and Salehiniya H: Ovarian cancer in the world: Epidemiology and risk factors. Int J Womens Health. 11:287–299. 2019.PubMed/NCBI View Article : Google Scholar | |
|
Toss A, Tomasello C, Razzaboni E, Contu G, Grandi G, Cagnacci A, Schilder RJ and Cortesi L: Hereditary ovarian cancer: Not only BRCA 1 and 2 genes. Biomed Res Int. 2015(341723)2015.PubMed/NCBI View Article : Google Scholar | |
|
Gatti S, Caporelli N, Galeazzi T, Francavilla R, Barbato M, Roggero P, Malamisura B, Iacono G, Budelli A, Gesuita R, et al: Oats in the diet of children with celiac disease: Preliminary results of a double-blind, randomized, placebo-controlled multicenter Italian study. Nutrients. 5:4653–4664. 2013.PubMed/NCBI View Article : Google Scholar | |
|
Rubio-Tapia A, Rahim MW, See JA, Lahr BD, Wu TT and Murray JA: Mucosal recovery and mortality in adults with celiac disease after treatment with a gluten-free diet. Am J Gastroenterol. 105:1412–1420. 2010.PubMed/NCBI View Article : Google Scholar | |
|
Wahab PJ, Meijer JW and Mulder CJ: Histologic follow-up of people with celiac disease on a gluten-free diet: Slow and incomplete recovery. Am J Clin Pathol. 118:459–463. 2002.PubMed/NCBI View Article : Google Scholar | |
|
Collin P, Mäki M and Kaukinen K: Complete small intestinal mucosal recovery is obtainable in the treatment of celiac disease. Gastrointest Endosc. 59:158–159; author reply 159-60. 2004.PubMed/NCBI View Article : Google Scholar | |
|
Szakács Z, Mátrai P, Hegyi P, Szabó I, Vincze Á, Balaskó M, Mosdósi B, Sarlós P, Simon M, Márta K, et al: Younger age at diagnosis predisposes to mucosal recovery in celiac disease on a gluten-free diet: A meta-analysis. PLoS One. 12(e0187526)2017.PubMed/NCBI View Article : Google Scholar | |
|
Ciccocioppo R, Di Sabatino A and Corazza GR: The immune recognition of gluten in coeliac disease. Clin Exp Immunol. 140:408–416. 2005.PubMed/NCBI View Article : Google Scholar | |
|
Silano M, Vincentini O and De Vincenzi M: Toxic, immunostimulatory and antagonist gluten peptides in celiac disease. Curr Med Chem. 16:1489–1498. 2009.PubMed/NCBI View Article : Google Scholar | |
|
Um CY, Campbell PT, Carter B, Wang Y, Gapstur SM and McCullough ML: Association between grains, gluten and the risk of colorectal cancer in the cancer prevention study-II nutrition cohort. Eur J Nutr. 59:1739–1749. 2020.PubMed/NCBI View Article : Google Scholar | |
|
Silano M, Volta U, Mecchia AM, Dessì M, Di Benedetto R and De Vincenzi M: Collaborating centers of the Italian registry of the complications of coeliac disease. Delayed diagnosis of coeliac disease increases cancer risk. BMC Gastroenterol. 7(8)2007.PubMed/NCBI View Article : Google Scholar | |
|
Midhagen G, Aberg AK, Olcén P, Jarnerot G, Valdimarsson T, Dahlbom T, Hansson T and Ström M: Antibody levels in adult patients with coeliac disease during gluten-free diet: A rapid initial decrease of clinical importance. J Intern Med. 256:519–524. 2004.PubMed/NCBI View Article : Google Scholar | |
|
Elfström P, Granath F, Ye W and Ludvigsson JF: Low risk of gastrointestinal cancer among patients with celiac disease, inflammation, or latent celiac disease. Clin Gastroenterol Hepatol. 10:30–36. 2012.PubMed/NCBI View Article : Google Scholar |