Evaluation of the efficacy of the combination of Citrus aurantium, Cistus creticus and Olea europaea leaf extract on the lipid profiles of individuals with marginally elevated lipid levels
- Authors:
- Published online on: October 3, 2023 https://doi.org/10.3892/ijfn.2023.32
- Article Number: 2
-
Copyright : © Tsolakou et al. This is an open access article distributed under the terms of Creative Commons Attribution License [CC BY 4.0].
Abstract
Introduction
Lipids are absorbed in the intestines and are transported throughout the body via lipoproteins for energy, steroid production, or bile acid formation. Cholesterol, low-density lipoprotein (LDL)-cholesterol (LDL-C), triglycerides (TGs) and high-density lipoprotein (HDL)-cholesterol (HDL-C), contribute to these pathways. An imbalance in any of these factors, either from organic or non-organic causes, can lead to dyslipidemia (1). Dyslipidemia may be assessed by evaluating the fasting levels of these lipids in serum. Dyslipidemia includes a large range of lipid abnormalities and may involve a combination of increased total cholesterol [TC; ≥240 mg/dl (6.20 mmol/l)], LDL-C [>160 mg/dl (4.13 mmol/l)] and TG levels [>200 mg/dl (2.25 mmol/l)] or decreased levels of HDL-C (2).
Lipid disorders are frequently encountered in clinical practice and often have implications for cardiovascular risk and general health (3). The main complication of dyslipidemia is cardiovascular diseases, which may include sudden cardiac death, acute myocardial infarction, or stroke. Dyslipidemia is recognized as one of the main risk factors for the development of cardiovascular disease (4) and plays a prominent role in the development of atherosclerosis (5). In addition, non-alcoholic fatty liver disease (NAFLD) is closely linked to dyslipidemia, as previously demonstrated (6). In this sense, many physicians, on the basis of a lot of published data, are turning to natural products to cure/alleviate NAFLD, and there appears to be an increase in the interest in the use of natural products (7). Furthermore, considering the high risk of developing bladder cancer in patients with increased cholesterol levels and hypertriglyceridemia, as well as the association of NAFLD with this type of cancer (8), the use of natural products to lower lipid levels is gaining increasing attention. Recent studies have established that elevated TG levels represent a cardiovascular risk factor, while low HDL levels may contribute less than previously considered (3). It has been demonstrated that statins and appropriate treatments for dyslipidemia have significantly reduced the risk of all-cause mortality, cardiovascular events and cardiovascular mortality (9). In spite the effectiveness of statins, however, there is still a considerable risk for developing atherosclerotic cardiovascular disease, which according to clinical data, is attributed to elevated TG levels, considering these as a marker of residual cardiovascular risk (10,11). Moreover, serum TG levels are biomarkers for TG-rich lipoproteins, which also are independent predictors of atherosclerotic cardiovascular disease (12). In addition, there is evidence to indicate that in certain clinical conditions, such as patients who cannot tolerate statin therapy or the recommended intensities of statin therapy, as well as those with persistent severe elevations in TG levels, or those at a high risk of developing cardiovascular disease, some non-statin therapies may be useful in the reduction of cardiovascular events (13).
The initial management of dyslipidemia comprises of lifestyle changes. This approach should definitely include a diet emphasizing the consumption of vegetables, fruits and whole grains within appropriate caloric requirements. The beneficial role of such a healthy diet and its components, such as flavonoids, on preventing cardiovascular disease has been previously demonstrated (14). In addition, diets rich in polyphenols have been shown to improve cardiovascular risk factors (15) and to have a significant beneficial effect in decrease fasting and post-prandial dyslipidemia by reducing the levels of oxidative stress (16). In a recent meta-analysis of cohort studies, the important protective properties of foods rich in flavonoids against cardiovascular disease mortality was highlighted (17). The ingredients contained in the nutritional supplement that is tested in the present study, for the effects on the lipid profiles of volunteers have been used for similar research separately (18,19). Particularly, the effects of the consumption of citrus fruit and olive leaf extract on lipid metabolism were also previously evaluated on animals by Merola et al (20) and the results revealed a decrease in the symptoms of liver inflammation caused by a high-fat diet.
The aim of the present pilot study was to assess the possible beneficial effects of a dietary supplement on the lipidemic profiles of subjects with mild hyperlipidemia (TC, >190 to <290 mg/dl; TG, >150 mg/dl). The formula of this supplement consists of ingredients rich in polyphenols and flavonoids, naturally included in the plant extracts, with proven efficacy on lipid metabolism when used separately (18,19). The main ingredients of the supplement used in this proof-of-concept study comprised of a blend of Citrus aurantium extract and Olea europaea leaf extract, combined with a Cistus creticus extract standardized in polyphenols, chromium and vitamins B1, B2, B3, B5, B12, contained in a single capsule.
Subjects and methods
Study subjects
A total of 20 individuals who met the inclusion criteria were enrolled. All participants provided consent in writing following extensive debriefing. The inclusion criteria for entering the study were as follows: Males or females >18 years of age with mild dyslipidemia (TC, >190 to <290 mg/dl; TG, >150 mg/dl). Individuals receiving statins, fibrates or any other hypolipidemic treatment were excluded. At the initial visit, the participant's medical history was documented, following a thorough clinical examination. Laboratory biochemical testing included the measurement of TC, LDL-C, HDL and TG serum levels. Sample collection and blood testing began in September, 2021 and the final results were obtained in March, 2022. The study protocol was approved by the Ethics Committee of the ‘Ippokrateion’ General Hospital in Athens, Greece.
In total, 16 of the 20 individuals enrolled completed the study. Of the 20 initial subjects, 4 individuals did not return for the scheduled reassessment visit. Of the included subjects, 12 participants (75%) were females and 4 participants (25%) were males. None of the 16 participants who came to the reassessment visit reported experiencing any adverse events during the administration of the dietary supplement under study. The median age of the study population was 59 years (range, 52-77 years). The mean weight of the subjects was 73.73±10.99 kg and the mean height was equal to 166±7.61, resulting in a mean body mass index of 27.08±4.11. A total of 10 participants (62.5%) were non-smokers, 4 (25.0%) were ex-smokers and 2 (12.5%) were smokers. The mean diastolic blood pressure was 80.06±9.07 and the mean systolic pressure was 122.53±18.55. As regards comorbidities, 1 participant had diabetes mellitus, 1 had hypertension and 1 had hypothyroidism (Table I).
Treatment/supplementation
The nutritional supplement used in the present study was provided by Votaniche S.A. (Athens, Greece) in the form of a capsule, available on the market under the name Elipidio®. All volunteers received two capsules of the supplement daily, before their main meal. Each capsule (548.8 mg) contained the following: 300 mg plant extracts; vitamin B1, 0.55 mg; vitamin B2, 0.7 mg; vitamin B3, 8 mg; vitamin B5, 3 mg; vitamin B12, 0.00125 mg; and chromium, 0.02 mg. In detail, the plant composition of the capsule consisted of 100 mg Cistus creticus, 200 mg Citrus aurantium and Olea europaea blend, at an approximate ratio of 1.5:1. The phenolic compounds included in the Olea/Citrus blend refer to the following: Secoiridoids (oleuropein), phenolics (hydroxytyrosol, tyrosol, vanillic acid and caffeic acid) and polyphenols (verbascoside) with olive leaf origin and with citrus origin: Flavanones (naringin, neohesperidin, neoeriocitrin and hesperidin) and flavones (luteolin-7-glucoside, apigenin-7-glucoside, diosmetin-7-glucoside, luteolin and diosmetin). The composition and content in the bioactive ingredients of the Citrus aurantium and Olea europaea blend have also been previously described in the study by Merola et al (20). All participants were instructed to receive the capsules for 12 weeks and were reassessed after this period, without any instructions for alterations in diet or exercise patterns. Blood samples (5 ml) were drawn at the initial patient visit at the outpatient hypertension clinic and following the end of treatment (week 12, re-evaluation visit).
Statistical analysis
Statistical analyses were conducted using IBM SPSS software (version 29; IBM Corp.). The Kolmogorov-Smirnov test was used to assess the normality of the data. The parametric two-sided paired sample t-test was used to compare the blood results before and after supplement administration. P-values <0.05 were considered to indicate a statistically significant difference.
Results
TC levels
The mean TC levels at the initial visit were measured at 239.3±21 mg/dl, while at the final visit, following the daily administration of two capsules of the supplement, before the main meal for 12 weeks, the levels were 226.2±26.3 mg/dl. The average reduction in TC levels was 5.45%.
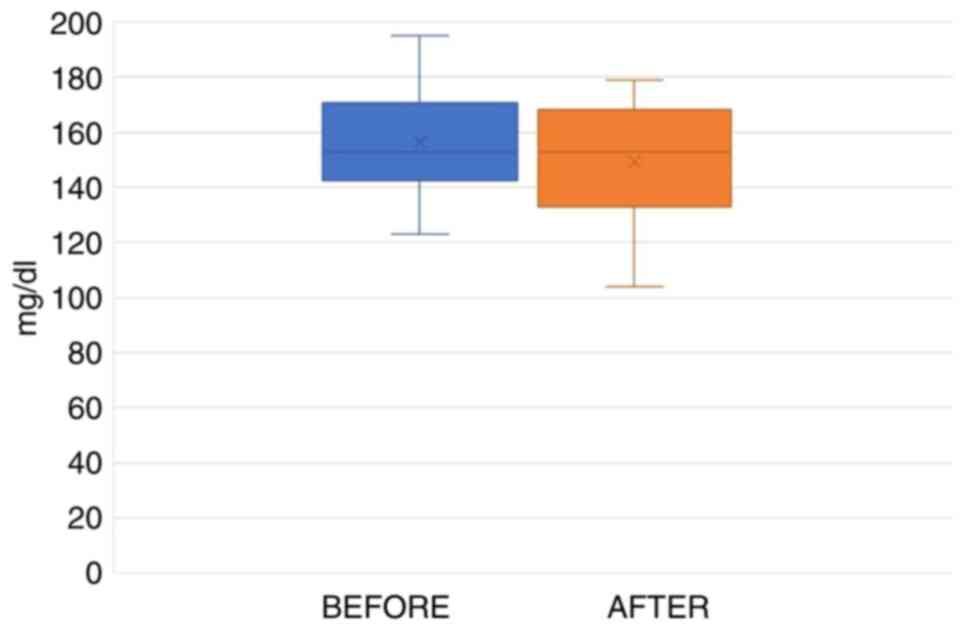
Statistical analysis using the paired t-test indicated that the average TC levels before the administration of the supplement differed from the respective values following treatment. However, the reduction in TC levels was not statistically significant (practical significance=5%, t=1.873, P=0.081). The range of total cholesterol levels in the initial measurement transpired between 205 and 291 mg/dl (marked as an outlier data point), while the final measurement values ranged between 178 and 259 mg/dl (Fig. 1).
LDL levels
The average measured LDL levels at the initial visit were 156.2±18.3 (SD) mg/dl, while at the final visit they were 149.4±22.1 (SD) mg/dl. The mean reduction in LDL levels was 4.36% (Table II).
Table IIVariations in average values after 12 weeks of the daily intake of capsules of the supplement. |
Statistical analysis using the paired t-test indicated that the mean LDL-C levels prior to the administration of the test capsule differed from the levels after the administration of the capsule. However, the reduction in LDL cholesterol was not statistically significant (p.s.=5%, t=1.041, P=0.315). The range in the initial LDL-C levels was between 123 and 195 mg/dl, while the final measurements ranged between 104 and 179 mg/dl (Fig. 2).
HDL levels
The average values of HDL at the initial medical examination were 55.0±13.1 (SD) mg/dl, while final tests results, following the daily consumption of the nutritional supplement, increased to 58.1±11.1 (SD) mg/dl. The average increase in the HDL levels reached 5.78% (Table II).
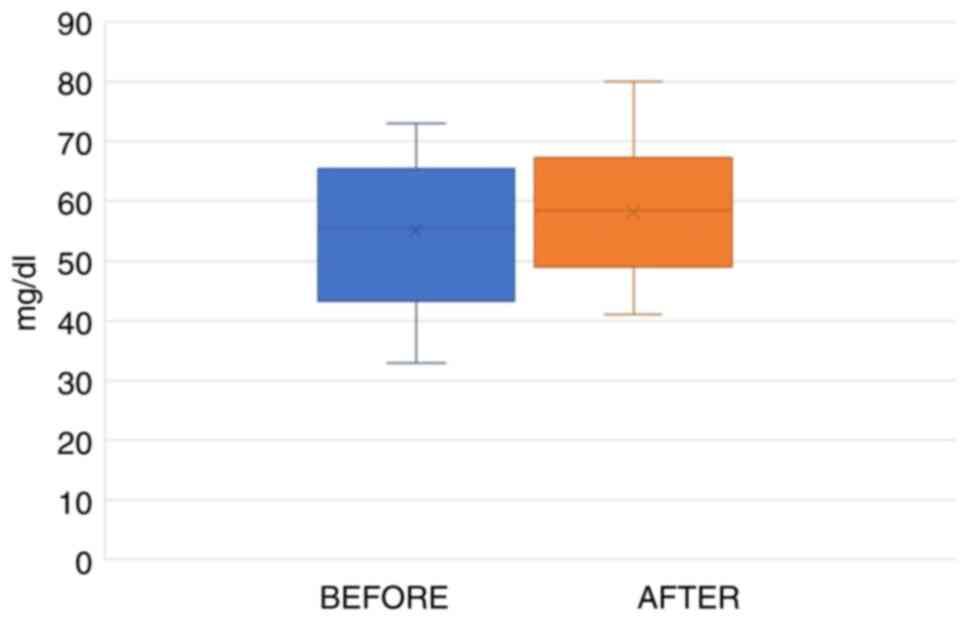
Statistical analysis using the paired t-test indicated that the average HDL levels prior to the intake of the supplement differed from the final test results values. The HDL values increased; however, this increase was not statistically significant (p.s.=5%, t=1.657, P=0.118). The blood test results for HDL of the participants from the first appointment range between 33 and 73 mg/dl, while the final values ranged between 41 and 80 mg/dl (Fig. 3).
TG levels
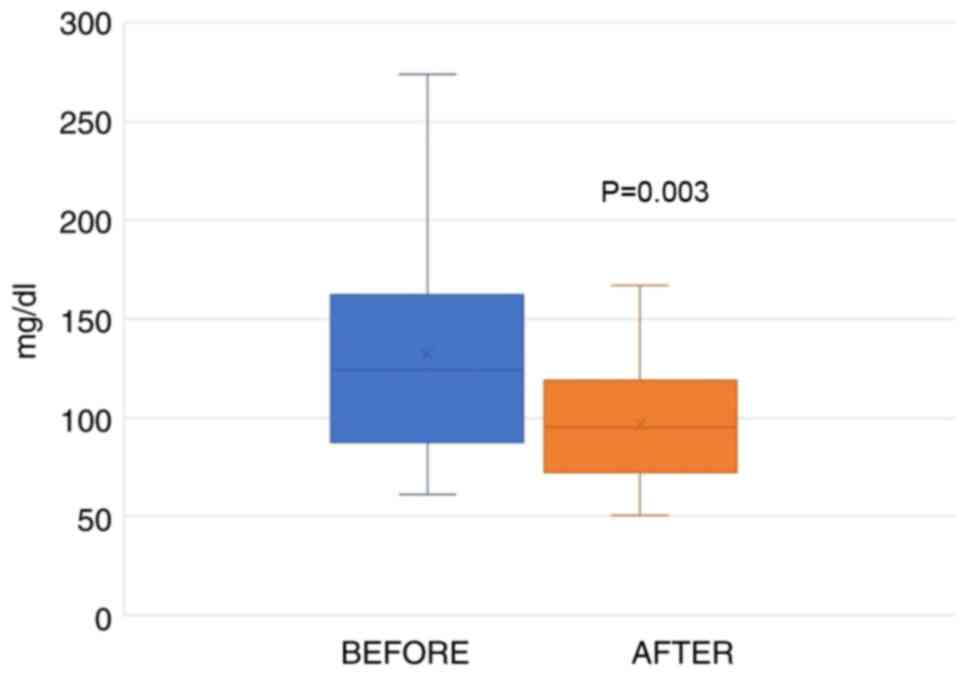
The average values of the TG levels at the initial examination were 132.1±58.1 (SD) mg/dl. Following 12 weeks of the daily consumption of two capsules of the test supplement, the TG values were 97.0±30.2 (SD) mg/dl. The average decrease was 26.72% (Table II). After performing statistical analysis (paired t-test), the results revealed a statistically significant decrease following the administration of the capsules for a 12-week period (p.s.=5%, t=3.501, P=0.003). The values from the first blood draw were between 61 to 274 mg/dl, whereas following the intake of the supplement, these values decreased to 51-175 mg/dl (Fig. 4).
Summary of lipid profiles
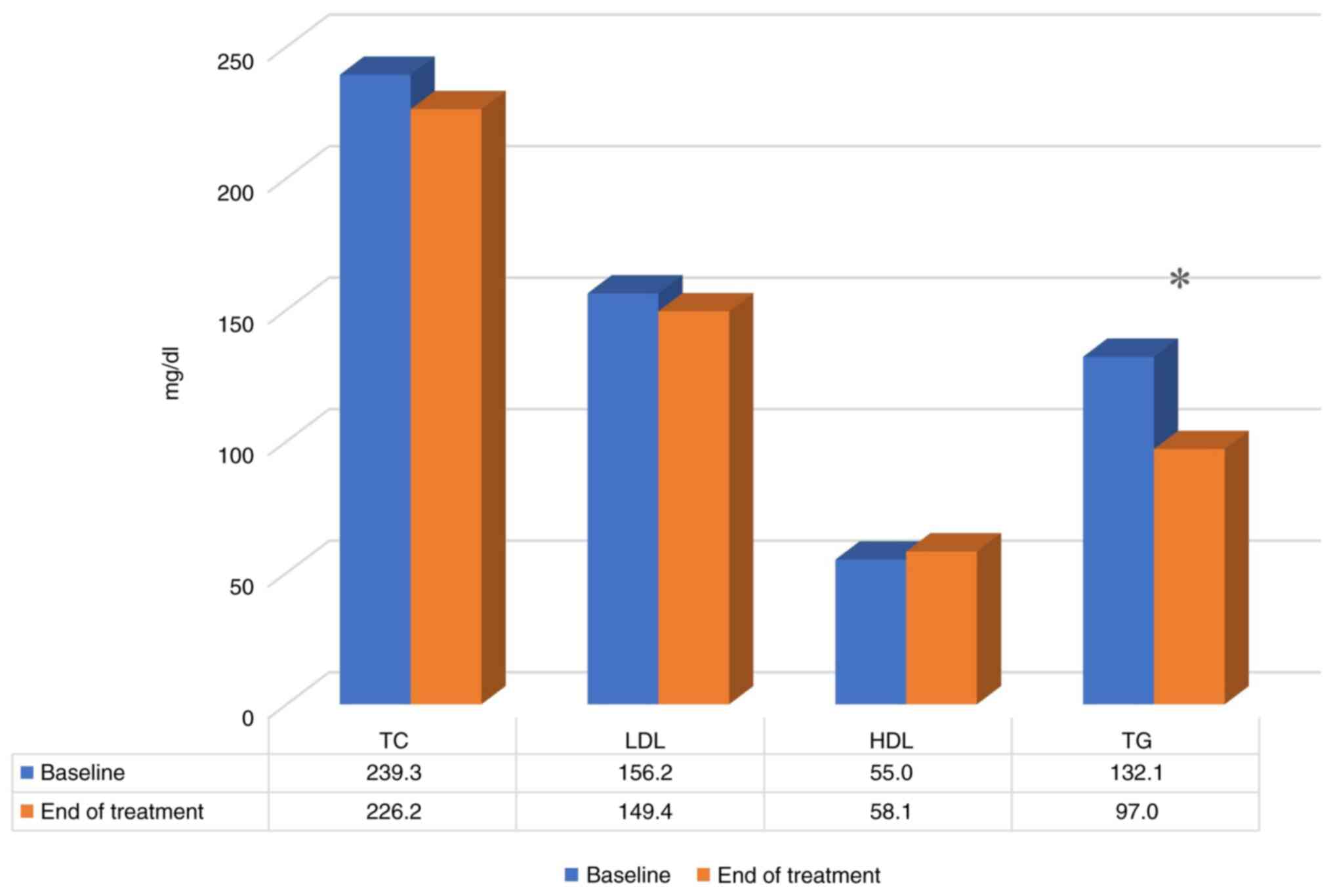
A summary of the final results of the study regarding the efficacy of the nutritional supplement on the lipid profiles of the study participants is presented in Table II and Fig. 5. Following 3 months of receiving the supplement, the TC levels were reduced by 5.45% (from 239.3±21 to 226.2±26.3 mg/dl; P=0.081), the LDL-C cholesterol levels were reduced by 4.36% (from 156.2±18.3 to 149.4±22.1 mg/dl; P=0.31), the HDL-C cholesterol levels increased by 5.78% (from 55.0±13.1 to 58.1±11.1 mg/dl; P=0.31) and the TG levels were reduced by 26.72% (from 132.1±58.1 to 97.0±30.2 mg/dl; P=0.003). Overall, although the changes in the LDL levels are not deemed statistically significant, a tendency towards an improvement was observed in the results for TC, HDL and TG levels.
Discussion
The present open observational prospective study in individuals with mild hyperlipidemia, demonstrated that the nutritional supplement combining plant extracts from Citrus aurantium, Olea europaea leaf and Cistus creticus, administered for a period of 12 weeks, significantly reduced TG levels by -35.31 mg/dl or -26.72% and increased HDL-C levels by +3.18 mg/dl or +5.78%. The results are consistent with those of previous publications (18-20), in which the herbal extracts of the study supplement were studied separately. In a previous study, where the case population was similar to that of the present study, the intervention group that received the blend of extracts of Citrus aurantium and Olea europaea leaf, exhibited a decrease in TG levels by -16 mg/dl or -12.90% (19) compared to the group administered the placebo. In another study, which involved healthy volunteers, the water extract of Cistus incanus decreased the TG levels by -11 mg/dl or -15.49% (18). In both studies, the doses of the extracts used were higher than those used in the present study. Since in the present study, the decrease in TG levels was considerably higher, yet with smaller doses, it can be assumed that this decrease may be attributed to the synergistic effect of the plant extracts.
High TG levels are considered a marker of high cholesterol levels in TG-rich lipoproteins, which are associated with low-grade inflammation (12). Moreover, individuals with TG levels ≥150 mg/dl exhibit subclinical atherosclerosis and vascular inflammation, even when the LDL-C levels are normal (21). Hypertriglyceridemia and high levels of TG-rich lipoproteins are considered an independent risk factor for atherosclerotic cardiovascular disease; therefore, any decrease in TG levels may have a beneficial effect on risk reduction. In another study, fasting TG levels were shown to be associated with short- and long-term cardiovascular risk, even in individuals who were treated effectively with statins (22). Additionally, from a multiple single nucleotide polymorphism Mendelian randomization analysis, the genetic findings support a casual effect of TGs on coronary heart disease events (23). In a meta-regression analysis study, the decrease in TG levels was found to be associated with a reduction in cardiovascular risk and namely for a 1 mmol/l reduction in TG levels, the relative risk reduction was 16% (24), or for a 0.1 mmol/l decrease in TG levels, the risk reduction in coronary events was 5% (25).
Increased levels of TGs are associated with increased levels of remnant cholesterol and with reduced levels of HDL-C (26). On the other hand, HDL-C is important for the lipid transportation and metabolism, due to the removal of the excess cholesterol from peripheral tissues, but also for its anti-inflammatory and antioxidant properties (27,28), attributed to the associated enzyme paraoxonase 1 (PON1) (29). PON1 binds to HDL-C and increases its antioxidant anti-atherogenic effects (30).
In the present study, a marked increase of 3.18 mg/dl or +5.78% in HDL-C levels was observed; however, the difference was not statistically significant. Since it is widely accepted that HDL-C is critical due to its anti-atherogenic properties in mediating cholesterol transport from peripheral tissues to the liver (31), an increase in HDL-C alongside with a significant decrease in TG levels appears to be beneficial for individuals with mild cardiovascular risk factors.
The blend of ingredients which was used in the present study, has been previously suggested to improve PON1 activity in parallel with an increase in HDL-C levels and a decrease in TG levels (19). Moreover, supplementation with a water infusion of Cistus incanus (also known as Cistus criticus) (32), has been demonstrated to exert antioxidant effects by significantly decreasing the malondialdehyde and advanced oxidative protein product concentrations in healthy volunteers (18). Recently, Cistus incanus extract, through its potent antioxidant activity due to its high content of flavonoids, was proven to attenuate the negative effects of high fat-carbohydrates on erythrocytes in animal models (33). It has also been demonstrated in vitro to have the ability to decrease the overproduction of reactive carbonyl species, particularly advanced glycation end products, which function as a prooxidant and pro-inflammatory agent in the organism (34). Cistus creticus is considered to be an excellent source of natural antioxidants and the 2010 European Food Safety Authority included it in its scientific opinion (35).
In previous research, the administration of polyphenol-rich olive leaf extracts was found to significantly lower the serum levels of TC, TGs and LDL-C, and to increase the serum level of HDL-C. These extracts were found to increase the serum antioxidant potential and the hepatic catalase and superoxide dismutase activities (36). Additionally, there are studies that recommend supplementing with vitamin B3 (niacin) or a fibrate as suggested options for the correction of atherogenic dyslipidemia (13,24). Extracts from fruits of the Citrus family, have been mentioned as lipid-lowering components, with statin-like effects (37). More specifically, the use of hesperidin, a flavonoid compound abundantly occurring in Citrus family fruit peel, has been found to lower serum TG levels in hypertriglyceridemic subjects, possibly by reducing very low-density lipoprotein metabolic abnormalities (38). In another study, conducted with the use of Citrus extracts and olive polyphenols, in relation to the lipid profile, there was a significant improvement in the serum levels of TC and LDL (39).
Due to the key antioxidant and anti-inflammatory effects of the plant extracts used in the composition of the nutritional supplement used herein along with vitamins B and chromium, a significant decrease in the TG levels was achieved. The main limitation of the present study was that the findings were obtained from a small group of individuals, as well as in its short duration. The results attained in the present study, even if these are derived from a small sample size, establish a tendency. Although the findings, particularly the suppressive effect on TG levels, appear promising, further larger studies for this nutritional supplement are required. Additionally, possible genetic profiling studies, related to the metabolism of TGs are required to further elucidate the regulatory mechanisms and the individual response after the use of a personalized intervention.
In conclusion, the present study demonstrates that the consumption of a combination of bitter orange flavonoids, olive leaf polyphenols and pink rock-rose rich in polyphenols and flavonoids, for 12 weeks in individuals with mild dyslipidemia (TC, >190 to <290 mg/dl; TG, >150 mg/dl), was associated with a marked reduction in TG and an increase in HDL-C levels.
Acknowledgements
Not applicable.
Funding
Funding: The present study was funded by the National and Kapodistrian University of Athens research grands (research grant no. 8504).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
ND and SB conceived the study. DK and CPT performed the patient medical examinations. EK, AT, SB and ND were involved in the acquisition of data, in the design of the study, and in the writing of the manuscript. VE performed the statistical analyses. All authors have read and approved the final manuscript. AT and VE confirm the authenticity of all the raw data
Ethics approval and consent to participate
The study protocol was approved by the Ethics Committee of the ‘Ippokrateion’ General Hospital in Athens, Greece and every volunteer signed a consent form, following thorough briefing.
Patient consent for publication
Not applicable.
Competing interests
The author SB is affiliated with the company ‘Votaniche S.A’ that provided the supplement used in the present study. The other authors declare that they have no competing interests.
References
|
Rader DJ, Hoeg JM and Brewer HB Jr: Quantitation of plasma apolipoproteins in the primary and secondary prevention of coronary artery disease. Ann Intern Med. 120:1012–1025. 1994.PubMed/NCBI View Article : Google Scholar | |
|
Kopin L and Lowenstein CJ: Dyslipidemia. Ann Intern Med. 167:ITC81–ITC96. 2017.PubMed/NCBI View Article : Google Scholar | |
|
Berberich AJ and Hegele RA: A modern approach to dyslipidemia. Endocr Rev. 43:611–653. 2022.PubMed/NCBI View Article : Google Scholar | |
|
Mascarenhas-Melo F, Sereno J, Teixeira-Lemos E, Marado D, Palavra F, Pinto R, Rocha-Pereira P, Teixeira F and Reis F: Implication of Low HDL-c levels in patients with average LDL-c Levels: A focus on oxidized LDL, Large HDL subpopulation, and adiponectin. Mediators Inflamm. 2013(e612038)2013.PubMed/NCBI View Article : Google Scholar | |
|
Khatana C, Saini NK, Chakrabarti S, Saini V, Sharma A, Saini RV and Saini AK: Mechanistic insights into the oxidized low-density lipoprotein-induced atherosclerosis. Oxid Med Cell Longev. 2020(e5245308)2020.PubMed/NCBI View Article : Google Scholar | |
|
Pei K, Gui T, Kan D, Feng H, Jin Y, Yang Y, Zhang Q, Du Z, Gai Z, Wu J and Li Y: An overview of lipid metabolism and nonalcoholic fatty liver disease. BioMed Res Int. 2020(4020249)2020.PubMed/NCBI View Article : Google Scholar | |
|
Tarantino G, Balsano C, Santini SJ, Brienza G, Clemente I, Cosimini B and Sinatti G: It is high time physicians thought of natural products for alleviating NAFLD. Is there sufficient evidence to use them? Int J Mol Sci. 22(13424)2021.PubMed/NCBI View Article : Google Scholar | |
|
Tarantino G, Crocetto F, Di Vito C, Creta M, Martino R, Pandolfo SD, Pesce S, Napolitano L, Capone D and Imbimbo C: Association of NAFLD and insulin resistance with non metastatic bladder cancer patients: A cross-sectional retrospective study. J Clin Med. 10(346)2021.PubMed/NCBI View Article : Google Scholar | |
|
Chou R, Dana T, Blazina I, Daeges M and Jeanne TL: Statins for prevention of cardiovascular disease in adults: Evidence report and systematic review for the US preventive services task force. JAMA. 316:2008–2024. 2016.PubMed/NCBI View Article : Google Scholar | |
|
Farnier M, Zeller M, Masson D and Cottin Y: Triglycerides and risk of atherosclerotic cardiovascular disease: An update. Arch Cardiovasc Dis. 114:132–139. 2021.PubMed/NCBI View Article : Google Scholar | |
|
Tenenbaum A and Fisman EZ: Fibrates are an essential part of modern anti-dyslipidemic arsenal: Spotlight on atherogenic dyslipidemia and residual risk reduction. Cardiovasc Diabetol. 11(125)2012.PubMed/NCBI View Article : Google Scholar | |
|
Nordestgaard BG: Triglyceride-Rich lipoproteins and atherosclerotic cardiovascular disease: New insights from epidemiology, genetics, and biology. Circ Res. 118:547–563. 2016.PubMed/NCBI View Article : Google Scholar | |
|
Sando KR and Knight M: Nonstatin therapies for management of dyslipidemia: A review. Clin Ther. 37:2153–2179. 2015.PubMed/NCBI View Article : Google Scholar | |
|
Widmer RJ, Flammer AJ, Lerman LO and Lerman A: The mediterranean diet, its components, and cardiovascular disease. Am J Med. 128:229–238. 2015.PubMed/NCBI View Article : Google Scholar | |
|
Medina-Remón A, Casas R, Tressserra-Rimbau A, Ros E, Martínez-González MA, Fitó M, Corella D, Salas-Salvadó J, Lamuela-Raventos RM and Estruch R: PREDIMED Study Investigators. Polyphenol intake from a Mediterranean diet decreases inflammatory biomarkers related to atherosclerosis: A substudy of the PREDIMED trial. Br J Clin Pharmacol. 83:114–128. 2017.PubMed/NCBI View Article : Google Scholar | |
|
Annuzzi G, Bozzetto L, Costabile G, Giacco R, Mangione A, Anniballi G, Vitale M, Vetrani C, Cipriano P, Della Corte G, et al: Diets naturally rich in polyphenols improve fasting and postprandial dyslipidemia and reduce oxidative stress: A randomized controlled trial. Am J Clin Nutr. 99:463–471. 2014.PubMed/NCBI View Article : Google Scholar | |
|
Mazidi M, Katsiki N and Banach M: A greater flavonoid intake is associated with lower total and cause-specific mortality: A meta-analysis of cohort studies. Nutrients. 12(2350)2020.PubMed/NCBI View Article : Google Scholar | |
|
Kuchta A, Konopacka A, Waleron K, Viapiana A, Wesołowski M, Dąbkowski K, Ćwiklińska A, Mickiewicz A, Śledzińska A, Wieczorek E, et al: The effect of Cistus incanus herbal tea supplementation on oxidative stress markers and lipid profile in healthy adults. Cardiol J. 28:534–542. 2021.PubMed/NCBI View Article : Google Scholar | |
|
Victoria-Montesinos D, Abellán Ruiz MS, Luque Rubia AJ, Guillén Martínez D, Pérez-Piñero S, Sánchez Macarro M, García-Muñoz AM, Cánovas García F, Castillo Sánchez J and López-Román FJ: Effectiveness of consumption of a combination of citrus fruit flavonoids and olive leaf polyphenols to reduce oxidation of low-density lipoprotein in treatment-naïve cardiovascular risk subjects: A randomized double-blind controlled study. Antioxidants (Basel). 10(589)2021.PubMed/NCBI View Article : Google Scholar | |
|
Merola N, Castillo J, Benavente-García O, Ros G and Nieto G: The effect of consumption of citrus fruit and olive leaf extract on lipid metabolism. Nutrients. 9(1062)2017.PubMed/NCBI View Article : Google Scholar | |
|
Raposeiras-Roubin S, Rosselló X, Oliva B, Fernández-Friera L, Mendiguren JM, Andrés V, Bueno H, Sanz J, Martínez de Vega V, Abu-Assi E, et al: Triglycerides and residual atherosclerotic risk. J Am Coll Cardiol. 77:3031–3041. 2021.PubMed/NCBI View Article : Google Scholar | |
|
Schwartz GG, Abt M, Bao W, DeMicco D, Kallend D, Miller M, Mundl H and Olsson AG: Fasting triglycerides predict recurrent ischemic events in patients with acute coronary syndrome treated with statins. J Am Coll Cardiol. 65:2267–2275. 2015.PubMed/NCBI View Article : Google Scholar | |
|
Holmes MV, Asselbergs FW, Palmer TM, Drenos F, Lanktree MB, Nelson CP, Dale CE, Padmanabhan S, Finan C, Swerdlow DI, et al: Mendelian randomization of blood lipids for coronary heart disease. Eur Heart J. 36:539–550. 2015.PubMed/NCBI View Article : Google Scholar | |
|
Marston NA, Giugliano RP, Im K, Silverman MG, O'Donoghue ML, Wiviott SD, Ference BA and Sabatine MS: Association between triglyceride lowering and reduction of cardiovascular risk across multiple lipid-lowering therapeutic classes: A Systematic Review and Meta-Regression Analysis of Randomized Controlled Trials. Circulation. 140:1308–1317. 2019.PubMed/NCBI View Article : Google Scholar | |
|
Nordestgaard BG and Varbo A: Triglycerides and cardiovascular disease. Lancet. 384:626–635. 2014.PubMed/NCBI View Article : Google Scholar | |
|
Varbo A, Benn M, Tybjærg-Hansen A, Jørgensen AB, Frikke-Schmidt R and Nordestgaard BG: Remnant Cholesterol as a Causal Risk Factor for Ischemic Heart Disease. J Am Coll Cardiol. 61:427–436. 2013.PubMed/NCBI View Article : Google Scholar | |
|
Nazir S, Jankowski V, Bender G, Zewinger S, Rye KA and van der Vorst EPC: Interaction between high-density lipoproteins and inflammation: Function matters more than concentration! Adv Drug Deliv Rev. 159:94–119. 2020.PubMed/NCBI View Article : Google Scholar | |
|
O'Hagan R, Berg AR, Hong CG, Parel PM, Mehta NN and Teague HL: Systemic consequences of abnormal cholesterol handling: Interdependent pathways of inflammation and dyslipidemia. Front Immunol. 13(972140)2022.PubMed/NCBI View Article : Google Scholar | |
|
Aggarwal G, May-Zhang LS, Yermalitsky V, Dikalov S, Voynov MA, Amarnath V, Kon V, Linton MF, Vickers KC and Davies SS: Myeloperoxidase-induced modification of HDL by isolevuglandins inhibits paraoxonase-1 activity. J Biol Chem. 297(101019)2021.PubMed/NCBI View Article : Google Scholar | |
|
Meneses MJ, Silvestre R, Sousa-Lima I and Macedo MP: Paraoxonase-1 as a regulator of glucose and lipid homeostasis: Impact on the onset and progression of metabolic disorders. Int J Mol Sci. 20(4049)2019.PubMed/NCBI View Article : Google Scholar | |
|
Kudinov VA, Alekseeva OY, Torkhovskaya TI, Baskaev KK, Artyushev RI, Saburina IN and Markin SS: High-Density lipoproteins as homeostatic nanoparticles of blood plasma. Int J Mol Sci. 21(8737)2020.PubMed/NCBI View Article : Google Scholar | |
|
Falchi A, Paolini J, Desjobert JM, Melis A, Costa J and Varesi L: Phylogeography of Cistus creticus L. on Corsica and Sardinia inferred by the TRNL-F and RPL32-TRNL sequences of cpDNA. Mol Phylogenet Evol. 52:538–543. 2009.PubMed/NCBI View Article : Google Scholar | |
|
Cyboran-Mikołajczyk S, Pasławski R, Pasławska U, Nowak K, Płóciennik M, Męczarska K, Oszmiański J, Bonarska-Kujawa D, Kowalczyk P and Wawrzyńska M: Comparison of osmotic resistance, shape and transmembrane potential of erythrocytes collected from healthy and fed with high fat-carbohydrates diet (HF-CD) pigs-protective effect of Cistus incanus L. Extracts. Materials (Basel). 14(1050)2021.PubMed/NCBI View Article : Google Scholar | |
|
Bernacka K, Bednarska K, Starzec A, Mazurek S and Fecka I: Antioxidant and antiglycation effects of Cistus x incanus water infusion, its phenolic components, and respective metabolites. Molecules. 27(2432)2022.PubMed/NCBI View Article : Google Scholar | |
|
EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA): Scientific Opinion on the substantiation of health claims related to various food(s)/food constituent(s) and protection of cells from premature aging, antioxidant activity, antioxidant content and antioxidant properties, and protection of DNA, proteins and lipids from oxidative damage pursuant to Article 13(1) of Regulation (EC) No 1924/2006. EFSA J. 8(1489)2010. | |
|
Jemai H, Bouaziz M, Fki I, El Feki A and Sayadi S: Hypolipidimic and antioxidant activities of oleuropein and its hydrolysis derivative-rich extracts from Chemlali olive leaves. Chem Biol Interact. 176:88–98. 2008.PubMed/NCBI View Article : Google Scholar | |
|
Giglio RV, Patti AM, Nikolic D, Li Volti G, Al-Rasadi K, Katsiki N, Mikhailidis DP, Montalto G, Ivanova E, Orekhov AN and Rizzo M: The effect of bergamot on dyslipidemia. Phytomedicine. 23:1175–1181. 2016.PubMed/NCBI View Article : Google Scholar | |
|
Miwa Y, Mitsuzumi H, Sunayama T, Yamada M, Okada K, Kubota M, Chaen H, Mishima Y and Kibata M: Glucosyl hesperidin lowers serum triglyceride level in hypertriglyceridemic subjects through the improvement of very low-density lipoprotein metabolic abnormality. J Nutr Sci Vitaminol (Tokyo). 51:460–470. 2005.PubMed/NCBI View Article : Google Scholar | |
|
Sánchez Macarro M, Martínez Rodríguez JP, Bernal Morell E, Pérez-Piñero S, Victoria-Montesinos D, García-Muñoz AM, Cánovas García F, Castillo Sánchez J and López-Román FJ: Effect of a combination of citrus flavones and flavanones and olive polyphenols for the reduction of cardiovascular disease risk: An exploratory randomized, double-blind, placebo-controlled study in healthy subjects. Nutrients. 12(1475)2020.PubMed/NCBI View Article : Google Scholar |