Zinc and respiratory tract infections: Perspectives for COVID‑19 (Review)
- Authors:
- Published online on: April 14, 2020 https://doi.org/10.3892/ijmm.2020.4575
- Pages: 17-26
-
Copyright: © Skalny et al. This is an open access article distributed under the terms of Creative Commons Attribution License.
Abstract
1. Introduction
Zinc is an essential metal being involved in a variety of biological processes due to its function as a cofactor, signaling molecule, and structural element. It is involved in the regulation of carbohydrate and lipid metabolism, as well as the functioning of the reproductive, cardiovascular, and nervous system (1). At the same time, the most critical role of zinc is demonstrated for the immune system. Briefly, zinc regulates proliferation, differentiation, maturation, and functioning of leukocytes and lymphocytes (2). Zinc plays a signaling role involved in the modulation of inflammatory responses (3). It is also a component of nutritional immunity (4). Correspondingly, alteration of zinc status significantly affects immune response resulting in increased susceptibility to inflammatory and infectious diseases including acquired immune deficiency syndrome, measles, malaria, tuberculosis, and pneumonia (5). Earlier data demonstrate that populational Zn status is associated with the prevalence of respiratory tract infections in children and adults (6,7).
In view of the high prevalence of zinc deficiency worldwide (up to 17%), its impact on population health is considered as a significant issue (8). Moreover, certain groups of people, including infants, especially preterm ones, and elderly, are considered to be at high risk of zinc deficiency and its adverse effects (9).
Under zinc deficiency condition, organisms are more susceptible to toxin-producing bacteria or enteroviral pathogens that activate guanylate and adenylate cyclases, stimulating chloride secretion, causing diarrhea and diminishing absorption of nutrients, thus exacerbating an already compromised mineral status. In addition, zinc deficiency may impair the absorption of water and electrolytes, delaying the termination of normally self-limiting gastrointestinal disease episodes (10). During chronic deficiency, the production of pro-inflammatory cytokines increases, influencing the outcome of a large number of inflammatory, metabolic, neurodegenerative and immune diseases (11). Diseases such as rheumatoid arthritis, diabetes (12), atherosclerosis and obesity (13), impaired cognitive function (14), as well as age-related macular degeneration (AMD) may be due to zinc deficiency, worsening chronic inflammation and triggering oxidative stress.
Coronaviridae were considered as the etiological agent in 6-29% of respiratory infections (15,16), although the severity of the disease varies significantly on the particular virus and its virulence (17). The viruses from the Coronaviridae family are zoonotic viruses that can be transmitted from animals to humans. The bat is considered the reservoir for these viruses, but other intermediate animals can also transmit the virus to humans (18). COVID-19 is a coronavirus disease caused by the novel 2019-nCoV virus (now called SARS-CoV-2) that appeared for the first time in Wuhan, China at the end of 2019 (19). Despite a close relation other two highly pathogenic coronaviruses, MERS-CoV and SARS-CoV (20), SARS-CoV-2 expanded to the majority of countries (21). On 11 March 2020, WHO characterized COVID-19 as a pandemic (22). Currently, the prevalence of COVID-19 exceeds 1,521,200 cases resulting in 92,700 deaths worldwide (23).
COVID-19 predominantly affects the respiratory system resulting in pneumonia and acute respiratory distress syndrome (24), leading to the requirement of mechanical ventilation (25). In turn, advanced age, acute respiratory distress syndrome (ARDS) and mechanical ventilation are known to be associated with higher COVID-19 mortality (26). The risk is also increased by modern life in which individuals are exposed to a multitude of chemicals, even in low doses that in the long-term predispose to chronic diseases and metabolic disturbances (27-31). Preexisting chronic metabolic diseases including diabetes, cardiovascular diseases (32), and obesity (33) are considered as risk factors for increased COVID-19 susceptibility and mortality. It is proposed that the elderly are at higher risk of COVID-19 due to impaired immune function (34).
Due to the clearly demonstrated role of zinc in immunity (2), and impaired zinc status in ageing (35), metabolic diseases including diabetes, obesity, and cardiovascular diseases (13), it is speculated that zinc compounds may be used as an adjunct therapy in COVID-19 treatment (36) for increasing antiviral resistance (37). Of note, zinc was earlier suggested as the potential agent for immune support and prevention of H1N1 influenza ('swine flu') (38).
In view of lack of clinical data on preventive and/or therapeutic efficiency of zinc in COVID-19, as well as primary involvement of the respiratory system, in this review, we will discuss recent clinical data on the role of zinc in protection against bronchopulmonary infections, as well as the existing indications of the direct impact of zinc on nCoV-2019.
2. Zinc and COVID-19
In view of the global COVID-19 pandemic, potential protective effect of zinc is of particular interest. Zinc is considered as the potential supportive treatment in therapy of COVID-19 infection due to its immune modulatory effect, as well as direct antiviral effect (36). However, the existing data will be only mechanistically discussed in this review, as direct data on anti-COVID-19 effects of zinc are absent to date.
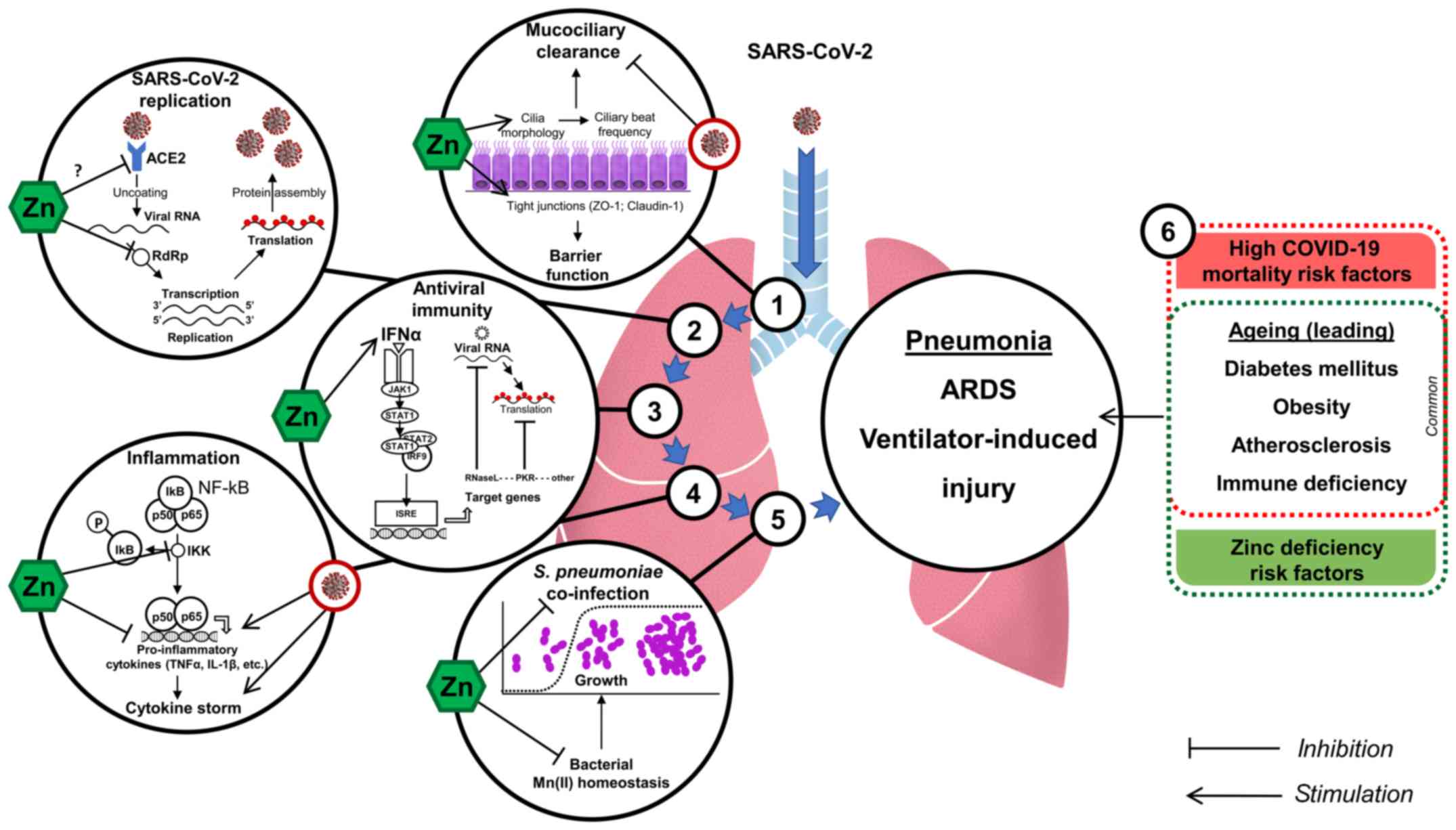
Specifically, Zn2+ cations especially in combination with Zn ionophore pyrithione were shown to inhibit SARS-coronavirus RNA polymerase (RNA dependent RNA polymerase, RdRp) activity by decreasing its replication (39). These important findings demonstrate that Zn2+ may be considered as the particular antiviral agent in COVID-19 treatment. Of note, recent trials have indicated efficiency of chloroquine antiviral activity as a treatment of COVID-19 (40), although the intimate mechanisms of its antiviral activity require further investigation (41). Earlier findings demonstrate that chloroquine is a zinc ionophore increasing Zn2+ flux into the cell (42). Moreover, the authors also propose that chloroquine-mediate zinc influx may underlie anticancer activity of the compound (42). Similarly, it was hypothesized that increasing intracellular Zn2+ concentration by chloroquine may also mediate its antiviral effect against SARS-CoV-2. In this view zinc supplementation without chloroquine might have similar positive effects without adverse side-effects of chloroquine treatment (43). Hypothetically, such an effect may be also observed using other zinc ionophores like quercetin and epigallocatechin-gallate (44) with substantially lower toxicity, although clinical trials supported by experimental in vitro studies are required to support this hypothesis.
Another Zn-related approach to modulation of COVID-19 may include targeting Zn ions in the structure of viral proteins. Particularly, it has been demonstrated that disulfiram-induced Zn2+ release from papain-like protease in MERS-CoV and SARS-CoV resulting in protein destabilization (45). In view of the presence of similar critical Zn-containing sites, Zn-ejector drugs (e.g., disulfiram) may be considered as potential antiviral agents (46) and components of targeted oxidation strategy in anti-SARS-CoV-2 treatment (47).
SARS-CoV-2 similarly to SARS-CoV requires angiotensin-converting enzyme 2 (ACE2) for entry into target cells (48). Therefore, modulation of ACE2 receptor was considered as the potential therapeutic strategy in COVID-19 treatment (49). Speth et al (50) demonstrated that zinc exposure (100 µM) was shown to reduce recombinant human ACE-2 activity in rat lungs. Although this concentration is close to physiological values of total zinc, the modulating effect of zinc on SARS-CoV-2-ACE2 interaction seem to be only hypothetical (51).
Although neither coronavirus HCoV 229E (52) nor HCoV-OC43 (53) infection caused a significant reduction in ciliary beat frequency, HCoV 229E induced ciliary dyskinesia resulting in impaired mucociliary clearance. The latter may not only alter viral particle removal, but also predispose to bacterial co-infection as observed for influenza virus (54). In turn, Zn supplementation was shown to improve ciliary length in bronchial epithelium of Zn-deficient rats (55), as well as increase ciliary beat frequency in vitro (56). Therefore, zinc may hypothetically ameliorate nCoV-2019-induced dysfunction of mucociliary clearance. Generally, zinc was shown to be essential for respiratory epithelium due to antioxidant and anti-inflammatory activity (57), as well as regulation of tight junction proteins ZO-1 and Claudin-1 (58), thus increasing its barrier functions. In turn, downregulation of tight junction protein complexes e.g., ZO-1 and Claudin-1 and reduction in barrier function aggravates viral and bacterial inflammatory processes (59). In addition, loss of TJ perm selectivity in the airways results in an un-controlled leakage of high molecular weight proteins and water into the airways, which results in the formation of alveolar edema and ARDS (60).
3. Zn and respiratory viruses
Despite limited data on the direct effect of zinc on SARS-CoV-2 and COVID-19, its antiviral effects were demonstrated in other viral diseases. Zinc was shown to have a significant impact on viral infections through modulation of viral particle entry, fusion, replication, viral protein translation and further release for a number of viruses including those involved in respiratory system pathology (37,61). Specifically, increasing intracellular Zn levels through application of Zn ionophores such as pyrithione and hinokitiol significantly alters replication of picornavirus, the leading cause of common cold (62). These findings generally correspond to the earlier indications of suppressive effect of zinc on rhinovirus replication originating from the early 1970s (63). In addition, Zn treatment was shown to increase interferon α (IFNα) production by leukocytes (64) and potentiate its antiviral activity in rhinovirus-infected cells (65). As antiviral activity of IFNα is mediated through JAK1/STAT1 downstream signaling and up-regulation of antiviral enzymes [e.g., latent ribonuclease (RNaseL) and protein kinase RNA-activated (PKR)] involved in viral RNA degradation and inhibition of viral RNA translation (66), recent findings allow to propose that these mechanisms may be stimulated by Zn2+.
These findings along with the existing data on the role of zinc in immunity raised interest to the potential use of zinc in prevention and/or treatment of common cold. A systematic review by Singh and Das (67) published in Cochrane database revealed a significant reduction in common cold duration, as well as the incidence rate ratio of developing common cold (IRR=0.64 (95% CI: 0.47-0.88), P=0.006) in response to zinc supplementation. The results of meta-analysis demonstrated that Zn supplementation in the dose >75 mg/day significantly reduced duration of common colds (68), with Zn acetate being the most effective form (69).
Certain studies also revealed the association between Zn status and respiratory syncytial virus (RSV) infection. Particularly, it has been demonstrated that whole blood zinc was significantly lower in children with RSV pneumonia (70). Impaired zinc metabolism in perinatal alcohol exposure is associated with immunosuppression and altered alveolar macrophage activity resulting in increased susceptibility to RSV infection (71). In turn, Zn compounds were shown to inhibit respiratory syncytial virus replication and RSV plaque formation with a more than 1,000-fold reduction at 10 µm Zn preincubation (72).
It is also notable that zinc deficiency was associated with higher mortality and adverse long-term outcome in influenza-MRSA bacterial superinfection (73), also underlining the importance of considering the risk of bacterial coinfection.
Despite the presence of experimental findings on the protective effect of zinc supplementation against respiratory virus infections, clinical and epidemiological data are still to be elaborated and systematized.
4. Pneumonia in adults and the elderly
Zinc is essential for the immune system and elderly people have an increased probability for zinc deficiency (74). Low Zn status was considered as the potential risk factor for pneumonia in elderly. Particularly, subjects with high serum Zn (>70 µg/dl, i.e., approx. 10.8 µmol/l) were characterized by reduced incidence of pneumonia [0.52 (0.36, 0.76), P<0.001], as well as lower disease duration and antibiotic administration as compared to low-Zn (<70 µg/ml) group (75), being also related to all-cause mortality (76). Serum Zn levels were 15% lower in cases of community-acquired pneumonia and advanced age, being also associated with pneumonia severity as evaluated by CURB-65 scores (77). The incidence of severe pneumonia was significantly higher in Irani patients with low Zn status, although the mean duration of fever, tachycardia, and tachypnea only tended to be longer, although not significant (78). Correspondingly, serum Zn levels were found deficient at the onset of acute respiratory failure with the lowest values observed in septic shock patients. However, no association between serum Zn values and day-30 mortality or period of stay in intensive care unit was observed (79).
The results of systematic analysis also confirmed the efficiency of intake of at least 75 mg/day Zn in reduction of pneumonia symptom duration but not severity, with the response being more pronounced in adults than in children (80). At the same time, certain studies failed to reveal any improvement in pneumonia when administered along with standard antibiotic treatment, although the period of supplementation was only 4 days (81).
A detailed study by Boudreault et al (82) demonstrated that low plasma Zn predisposes to ventilator-induced injury in intensive care, being related to the role of metallothionein system in lung protection. These data corroborate the results of the experimental study demonstrating aggravation of ventilation-induced lung injury in Zn deficient rats (83).
In Indian patients high plasma zinc levels were found to be associated with reduced mortality from sepsis as well as lower 48-h SOFA scores (84). Moreover, persistent low serum Zn levels were associated with increased risk of recurrent sepsis in critically ill patients (85).
Altogether, the existing data demonstrate an association between zinc status and pneumonia in adults and elderly, as well as its complications including respiratory failure, ventilator-induced injury, and sepsis.
5. Pediatric respiratory infections
Initial reports have postulated nearly exceptional susceptibility of elderly to SARS-CoV-2 infection allowing to propose natural resistance to COVID-19 in children (86). However, detailed analysis of the pediatric COVID-19 cases (87) and the emerging Russian experience indicate that children may be also severely affected by SARS-CoV-2. In view of high incidence of Zn deficiency in infants, the existing data on the association between Zn status and pneumonia in children is also discussed.
High incidence of pneumonia in developing countries has been considered as the consequence of zinc deficiency in the population (7). The incidence of low serum zinc in children with severe pneumonia was 80% (88). Correspondingly, a 2-fold lower level of serum Zn was observed in pediatric acute lower respiratory infection patients (89). Significantly lower serum zinc levels were observed in children with pneumonia complicated by sepsis, mechanical ventilation, and cases of lethality (90). Generally, indications of low zinc status in children with pneumonia provide a rationale for preventive Zn supplementation.
Particularly, Zn supplementation in developing countries reduced pneumonia morbidity by 19% (RR=0.81; 95% CI: 0.73, 0.90), whereas a 15% decrease in pneumonia-specific mortality was not significant (91). A recent systematic review and meta-analysis published in Cochrane database demonstrated that Zn supplementation significantly reduced the incidence and prevalence of pneumonia in children by 13 and 41% (92).
In contrast to the demonstrated preventive effects of Zn supplementation, data on the therapeutic effect of zinc in treatment of childhood pneumonia are conflicting (93). Despite the earlier observed reduction of treatment failure risk (94) and case fatality [RR=0.67 (95% CI: 0.24-0.85)] (95) in children with severe pneumonia, a more recent study demonstrated that Zn supplementation in 2-24 months old children with radiologically verified pneumonia did not result in significant improvement of risk reduction of treatment failure (96). Moreover, Zn supplementation in Zn-deficient children with pneumonia until achievement of normal serum Zn levels did not improve clinical appearance of the disease (97).
A number of studies revealed the potential efficiency of Zn supplementation in prevention of non-specified acute lower respiratory infections including bronchitis, bronchiolitis, pneumonitis. Specifically, supplementation with 10 mg zinc gluconate in Zn-deficient children resulted in a nearly twofold reduction of the number of episodes of acute lower respiratory infections as well as the time to recovery (98). In addition, Zn supplementation (30 mg/day) in Thai children significantly reduced severity of acute lower respiratory tract infections resulting in faster disease cessation and shorter hospital stay (99). A detailed meta-analysis demonstrated that Zn supplementation significantly decreased the incidence of acute lower respiratory infection defined according to specific clinical criteria in children aged <5 years (100).
In parallel, the impact of Zn supplementation in relation to upper respiratory tract infections was also demonstrated. Particularly, the number of upper respiratory tract infections in Colombian children was reduced by 73% in response to supplementation with 5 mg Zn in a 12-month randomized clinical trial (101). Certain studies also revealed protective effect of zinc supplementation against both acute upper and lower respiratory diseases in children (102,103).
6. Zinc and lung inflammation
Inflammation plays the key role in COVID-19 pathogenesis both at local (pneumonia) and systemic (cytokine storm) levels, and the search for adequate anti-inflammatory agents is of particular importance (104).
Although the role of zinc in regulation of inflammatory response was discussed in detail in a number of reviews (2,5), certain aspects of the regulatory role of zinc in pneumonia pathogenesis and lung inflammation are still to be elucidated. However, the existing data clearly demonstrate that Zn ions may possess anti-inflammatory effects in pneumonia thus limiting tissue damage and systemic effects.
Specifically, Zn deficiency in rats resulted in a significant increase in proinflammatory TNFα and VCAM-1 expression and lung tissue remodeling, being partially reversed by Zn supplementation (105). Zn deficiency also resulted in a significant alteration of lung epithelial cell barrier function through up-regulation of TNFα, IFNγ, and FasR signaling and cellular apoptosis in vitro (106). Zn deficiency was shown to up-regulate acute phase response-related genes through stimulation of JAK-STAT signaling in lungs under septic conditions (107). Zinc and nitric oxide (NO)-metallothioneine (MT)-Zn pathways were shown to mediate lung injury in response to LPS or hyperoxia (108).
In turn, Zn pretreatment significantly reduced LPS-induced pulmonary endothelial cell damage and increased cell viability in vitro, as well as improved respiratory function as assessed by blood oxygen pressure and saturation (109). It has been demonstrated that Zn pretreatment significantly decreases LPS-induced neutrophil recruitment to the lungs thus reducing acute lung injury in mice (110).
It is also notable that zinc deficiency is associated with inflammatory alterations of lung extracellular matrix predisposing to fibrosis (111). This finding is of particular interest in view of the presence of interstitial pulmonary fibrosis in COVID-19 patients (112).
Certain studies revealed protective effect of zinc against lung injury in systemic inflammation including sepsis. Experimental data demonstrate that Zn deficiency increases susceptibility to systemic inflammation and sepsis-induced organ damage including lungs in a murine model of polymicrobial sepsis (113). In a model of polymicrobial sepsis Zn deficiency resulted in increased NF-κB p65 mRNA expression and production in lungs resulting in up-regulation of target genes IL-1β, TNFα, and ICAM-1 (114), whereas Zn supplementation reduced neutrophil infiltration and MPO-mediated oxidative damage (115,116). Modulation of ERK1/2 and NF-κB pathways was shown to be critical for protective effect of zinc in lungs under septic conditions (117).
Correspondingly, patients with sepsis were character-ized by low serum Zn levels that may occur due to increased ZIP8 (SLC39A8) mRNA expression. Moreover, serum Zn concentrations inversely correlated with both disease severity and proinflammatory cytokines IL-6, IL-8, and TNFα (118). Reciprocal regulation of ZIP8 and NF-κB expression in response to TNFα or LPS exposure was demonstrated in lung epithelia and alveolar macrophages (119). In addition, ZIP8-deficient mice were characterized by increased airway neutrophil infiltration and elevated CXCL1 and IL-23 production (120).
Zn-mediated respiratory protection was also demonstrated in models of toxic atmospheric pollutant exposure. Particularly, Zn deficiency in agricultural organic dust-exposed animals aggravated neutrophil migration and proinflammatory cytokine (TNFα, IL-6, CXCL1) overproduction, as well as increased IL-23 and CXCL1 expression by macrophages due to NF-κB activation (121). In turn, Zn supplementation in cigarette smoke exposed mice significantly reduced the number of alveolar macrophages in bronchoalveolar lavage (122).
The observed anti-inflammatory effects of Zn in lung tissue seem to be mainly mediated by inhibition of NF-κB signaling through PKA-induced inhibition of Raf-1 and IκB kinase β (IKKβ) (123,124) or A20-dependent inhibition (125). Moreover, Zn-induced modulation of T-cell activity may also play a significant role in limiting inflammatory response (126,127). Lastly, zinc was shown to normalize the overproduction of proinflammatory cytokines induced by zinc deficiency on the epigenetic level (124,128).
7. Zinc and S. pneumoniae infection
Although COVID-19 is characterized by viral pneumonia caused by SARS-CoV-2 virus, bacterial co-infection may represent a significant issue due its high incidence in H1N1 influenza-associated pneumonia (129). Specifically, human coronavirus NL63 was associated with increased adherence of S. pneumoniae to epithelial cells (130). In turn, Streptococcus pneumoniae infection is considered as the most common cause of pneumonia.
Zinc is an essential component of antibacterial immunity (5). Particularly, Zn deficiency was associated with reduced killing activity of phagocytes in pneumococcal infection (131). In turn, Zn supplementation ameliorated the association between nasopharyngeal S. pneumoniae carriage and acute lower respiratory infection in children (132). Zn deficiency also predisposed to impaired immune response to Pneumococcal surface protein A, increased nasal S. pneumoniae colonization, and severe pneumococcal infection in mice (133) resulting in shorter survival time after infection (134). Correspondingly, patients with better immune response to 23-valent pneumococcal polysaccharide vaccine were characterized by significantly higher serum Zn levels (135). However, no effect (136) or serotype-specific effect (137) of Zn on antibody production in response to polyvalent pneumococcal vaccine was observed. Zn may also exert toxic effect on S. pneumoniae reducing its growth through interference with Mn(II) homeostasis and development of cytoplasmic manganese deficiency (138). The latter, in turn, increases bacterial susceptibility to oxygen-dependent killing by neutrophils (139).
A number of studies demonstrated antibacterial effect of zinc oxide nanoparticles (140). Particularly, ZnO was shown to inhibit both growth and biofilm formation by S. pneumoniae (141). Similar effect was observed for other bacterial agents involved in etiology of pneumonia, including K. pneumoniae (142), methicillin-resistant S. aureus (143), and P. aeruginosa (144). However, the potential antibacterial application of ZnO-(NPs) may be limited due to their toxicity to human lung cells (145), as well as impairment of phagocytic activity of macrophages in bronchi and lungs (146).
When considering the relationship between S. pneumoniae and zinc, one should also note essentiality of Zn ions for bacteria. Specifically, adequate Zn uptake is required for normal bacterial growth and morphology, as well as colonization and virulence (147). Pneumococcal biofilm formation was also shown to be dependent on Zn bioavailability (148).
8. Perspectives and conclusions
The obtained data demonstrate that adequate zinc status of the individual increases immune reactivity. Correspondingly, inadequate zinc supply may predispose to infectious diseases of upper and lower respiratory tract. Although the therapeutic effects of Zn are considered as inconsistent, the existing evidence-based data indicate efficiency of Zn supplementation and improvement of Zn status in prevention of pneumonia and its complications due to anti-inflammatory effect of zinc.
Certain indirect indications of the potential antiviral effect of Zn against nCoV-2019 exist, although their biomedical relevance is yet to be studied. In view of recent data on clinical course of the disease, it appears that adequate Zn status may possess protective effect as adjuvant therapy of COVID-19 through reducing lung inflammation, improvement of mucociliary clearance, prevention of ventilator-induced lung injury, modulation of antibacterial and antiviral immunity especially in elderly (Fig. 1). Further clinical and experimental studies are strongly required to elucidate the potential role of Zn deficiency in COVID-19 susceptibility, as well as effects of Zn supplementation, and the underlying mechanisms.
Funding
The study was partially supported by the Russian Ministry of Science and Higher Education, Project no. 0856-2020-0008. MA was supported by NIH grants nos. NIEHS R0110563, R01ES07331 and NIEHS R01ES020852.
Availability of data and materials
Not applicable.
Authors' contributions
Conceptualization: AVS, LR, MA, JA, AT, AAT; validation, research, resources, data reviewing, and writing: AVS, LR, OPA, MA, VAG, SIA, AAS, DP, DAS, JA, AT, AAT; figure preparation and edition: AAT; review and editing: AVS, LR, MA, JA, AT, AAT. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
DAS is the Editor-in-Chief for the journal, but had no personal involvement in the reviewing process, or any influence in terms of adjudicating on the final decision, for this article. The other authors declare that they have no competing interests.
Acknowledgments
Not applicable.
References
|
Prasad AS: Discovery of Zinc for Human Health and Biomarkers of Zinc Deficiency. Molecular, Genetic, and Nutritional Aspects of Major and Trace Minerals. Collins JF: Academic Press; Cambridge: pp. 241–260. 2017, View Article : Google Scholar | |
|
Wessels I, Maywald M and Rink L: Zinc as a gatekeeper of immune function. Nutrients. 9:12862017. View Article : Google Scholar | |
|
Maywald M, Wessels I and Rink L: Zinc signals and immunity. Int J Mol Sci. 18:22222017. View Article : Google Scholar : | |
|
Haase H and Rink L: Multiple impacts of zinc on immune function. Metallomics. 6:1175–1180. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
Gammoh NZ and Rink L: Zinc in infection and inflammation. Nutrients. 9:6242017. View Article : Google Scholar : | |
|
Aftanas LI, Bonitenko EYu, Varenik VI, Grabeklis AR, Kiselev MF, Lakarova EV, Nechiporenko SP, Nikolaev VA, Skalny AV and Skalnaya MG: Element status of population of Central Federal Region. Element status of population of Russia. Part II. Skalny AV and Kiselev MF: ELBI-SPb; Saint Petersburg: pp. 4302011 | |
|
Walker CLF, Rudan I, Liu L, Nair H, Theodoratou E, Bhutta ZA, O'Brien KL, Campbell H and Black RE: Global burden of childhood pneumonia and diarrhoea. Lancet. 381:1405–1416. 2013. View Article : Google Scholar : PubMed/NCBI | |
|
Bailey RL, West KP Jr and Black RE: The epidemiology of global micronutrient deficiencies. Ann Nutr Metab. 66(Suppl 2): 22–33. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Yasuda H and Tsutsui T: Infants and elderlies are susceptible to zinc deficiency. Sci Rep. 6:218502016. View Article : Google Scholar : PubMed/NCBI | |
|
Wapnir RA: Zinc deficiency, malnutrition and the gastrointestinal tract. J Nutr. 130(Suppl): 1388S–1392S. 2000. View Article : Google Scholar : PubMed/NCBI | |
|
Bonaventura P, Benedetti G, Albarède F and Miossec P: Zinc and its role in immunity and inflammation. Autoimmun Rev. 14:277–285. 2015. View Article : Google Scholar | |
|
Chabosseau P and Rutter GA: Zinc and diabetes. Arch Biochem Biophys. 611:79–85. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Olechnowicz J, Tinkov A, Skalny A and Suliburska J: Zinc status is associated with inflammation, oxidative stress, lipid, and glucose metabolism. J Physiol Sci. 68:19–31. 2018. View Article : Google Scholar : | |
|
Kozlowski H, Luczkowski M, Remelli M and Valensin D: Copper, zinc and iron in neurodegenerative diseases (Alzheimer's, Parkinson's and prion diseases). Coord Chem Rev. 256:2129–2141. 2012. View Article : Google Scholar | |
|
Berry M, Gamieldien J and Fielding BC: Identification of new respiratory viruses in the new millennium. Viruses. 7:996–1019. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Peiris JSM: Coronaviruses. Clinical Virology. Richman DD, Whitley RJ and Hayden FG: 4th edition. ASM Press; Washington: pp. 1244–1265. 2016 | |
|
Docea AO, Tsatsakis A, Albulescu D, Cristea O, Zlatian O, Vinceti M, Moschos SA, Tsoukalas D, Goumenou M, Drakoulis N, et al: A new threat from an old enemy: Re-emergence of coronavirus (Review). Int J Mol Med. 45:1631–1643. 2020.PubMed/NCBI | |
|
Goumenou M, Spandidos DA and Tsatsakis A: [Editorial] Possibility of transmission through dogs being a contributing factor to the extreme Covid 19 outbreak in North Italy. Mol Med Rep. 21:2293–2295. 2020.PubMed/NCBI | |
|
Lai CC, Shih TP, Ko WC, Tang HJ and Hsueh PR: Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): The epidemic and the challenges. Int J Antimicrob Agents. 55:1059242020. View Article : Google Scholar : PubMed/NCBI | |
|
Liu J, Cao R, Xu M, Wang X, Zhang H, Hu H, Li Y, Hu Z, Zhong W and Wang M: Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS-CoV-2 infection in vitro. Cell Discov. 6:162020. View Article : Google Scholar : PubMed/NCBI | |
|
Khachfe HH, Chahrour M, Sammouri J, Salhab H, Makki B and Fares MY: An epidemiological study on COVID-19: A rapidly spreading disease. Cureus. 12:e73132020.PubMed/NCBI | |
|
World Health Organization (WHO): Coronavirus disease 2019. Events as they happen. WHO; Geneva: 2020, https://www.who.int/emergencies/diseases/novel-coronavirus-2019/events-as-they-happen. Updated April 9, 2020. | |
|
World Health Organization (WHO): Coronavirus disease (COVID-2019). Situation report - 81. WHO; Geneva: 2020, https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200410-sitrep-81-covid-19.pdf. Accessed April 10, 2020. | |
|
Rothan HA and Byrareddy SN: The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. J Autoimmun. 109:1024332020. View Article : Google Scholar : PubMed/NCBI | |
|
Ñamendys-Silva SA: Respiratory support for patients with COVID-19 infection. Lancet Respir Med. 8:e182020. View Article : Google Scholar : PubMed/NCBI | |
|
Yang X, Yu Y, Xu J, Shu H, Xia J, Liu H, Wu Y, Zhang L, Yu Z, Fang M, et al: Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. Feb 24–2020.Epub ahead of print. View Article : Google Scholar | |
|
Docea AO, Goumenou M, Calina D, Arsene AL, Dragoi CM, Gofita E, Pisoschi CG, Zlatian O, Stivaktakis PD, Nikolouzakis TK, et al: Adverse and hormetic effects in rats exposed for 12 months to low dose mixture of 13 chemicals: RLRS part III. Toxicol Lett. 310:70–91. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Hernández AF, Docea AO, Goumenou M, Sarigiannis D, Aschner M and Tsatsakis A: Application of novel technologies and mechanistic data for risk assessment under the real-life risk simulation (RLRS) approach. Food Chem Toxicol. 137:1111232020. View Article : Google Scholar : PubMed/NCBI | |
|
Fountoucidou P, Veskoukis AS, Kerasioti E, Docea AO, Taitzoglou IA, Liesivuori J, Tsatsakis A and Kouretas D: A mixture of routinely encountered xenobiotics induces both redox adaptations and perturbations in blood and tissues of rats after a long-term low-dose exposure regimen: The time and dose issue. Toxicol Lett. 317:24–44. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Tsatsakis AM, Kouretas D, Tzatzarakis MN, Stivaktakis P, Tsarouhas K, Golokhvast KS, Rakitskii VN, Tutelyan VA, Hernandez AF, Rezaee R, et al: Simulating real-life exposures to uncover possible risks to human health: A proposed consensus for a novel methodological approach. Hum Exp Toxicol. 36:554–564. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Tsatsakis A, Tyshko NV, Docea AO, Shestakova SI, Sidorova YS, Petrov NA, Zlatian O, Mach M, Hartung T and Tutelyan VA: The effect of chronic vitamin deficiency and long term very low dose exposure to 6 pesticides mixture on neurological outcomes - A real-life risk simulation approach. Toxicol Lett. 315:96–106. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Wu C, Chen X, Cai Y, Zhou X, Xu S, Huang H, Wu C, Chen X, Cai Y, Zhou X, et al: Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan. JAMA Intern Med. Mar 13–2020.Epub ahead of print. View Article : Google Scholar | |
|
Huang R, Zhu L, Xue L, Liu L, Yan X, Wang J, Zhang B, Xu T, Ji F, et al: Clinical findings of patients with coronavirus disease 2019 in Jiangsu province, China: A retrospective, multi-center study. SSRN, 2020. https://ssrn.com/abstract=3548785. Accessed Febryary 28, 2020. | |
|
Jiang F, Deng L, Zhang L, Cai Y, Cheung CW and Xia Z: Review of the clinical characteristics of coronavirus disease 2019 (COVID-19). J Gen Intern Med. Mar 4–2020.Epub ahead of print. View Article : Google Scholar : PubMed/NCBI | |
|
Haase H and Rink L: The immune system and the impact of zinc during aging. Immun Ageing. 6:92009. View Article : Google Scholar : PubMed/NCBI | |
|
Zhang L and Liu Y: Potential interventions for novel coronavirus in China: A systematic review. J Med Virol. 92:479–490. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Read SA, Obeid S, Ahlenstiel C and Ahlenstiel G: The role of zinc in antiviral immunity. Adv Nutr. 10:696–710. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Sandstead HH and Prasad AS: Zinc intake and resistance to H1N1 influenza. Am J Public Health. 100:970–971. 2010. View Article : Google Scholar : PubMed/NCBI | |
|
te Velthuis AJ, van den Worm SH, Sims AC, Baric RS, Snijder EJ and van Hemert MJ: Zn(2+) inhibits coronavirus and arterivirus RNA polymerase activity in vitro and zinc ionophores block the replication of these viruses in cell culture. PLoS Pathog. 6:e10011762010. View Article : Google Scholar : PubMed/NCBI | |
|
Wang M, Cao R, Zhang L, Yang X, Liu J, Xu M, Shi Z, Hu Z, Zhong W and Xiao G: Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 30:269–271. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Liu J, Zheng X, Tong Q, Li W, Wang B, Sutter K, Trilling M, Lu M, Dittmer U and Yang D: Overlapping and discrete aspects of the pathology and pathogenesis of the emerging human pathogenic coronaviruses SARS-CoV, MERS-CoV, and 2019-nCoV. J Med Virol. 92:491–494. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Xue J, Moyer A, Peng B, Wu J, Hannafon BN and Ding WQ: Chloroquine is a zinc ionophore. PLoS One. 9:e1091802014. View Article : Google Scholar : PubMed/NCBI | |
|
Guastalegname M and Vallone A: Could chloroquine/hydroxy-chloroquine be harmful in Coronavirus disease 2019 (COVID-19) treatment? Clin Infect Dis. Mar 24–2020.Epub ahead of print. View Article : Google Scholar | |
|
Dabbagh-Bazarbachi H, Clergeaud G, Quesada IM, Ortiz M, O'Sullivan CK and Fernández-Larrea JB: Zinc ionophore activity of quercetin and epigallocatechin-gallate: From Hepa 1-6 cells to a liposome model. J Agric Food Chem. 62:8085–8093. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
Lin MH, Moses DC, Hsieh CH, Cheng SC, Chen YH, Sun CY and Chou CY: Disulfiram can inhibit MERS and SARS coro-navirus papain-like proteases via different modes. Antiviral Res. 150:155–163. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Sargsyan K, Chen T, Grauffel C and Lim C: Identifying COVID-19 drug-sites susceptible to clinically safe Zn-ejector drugs using evolutionary/physical principles. OSF Preprints, 2020. https://osf.io/snuqf/. Accessed February 13, 2020. | |
|
Xu L, Tong J, Wu Y, Zhao S and Lin BL: Targeted oxidation strategy (TOS) for potential inhibition of Coronaviruses by disulfiram - a 70-year old anti-alcoholism drug. ChemRxiv. In Press. | |
|
Hoffmann M, Kleine-Weber H, Krüger N, Mueller MA, Drosten C and Pöhlmann S: The novel coronavirus 2019 (2019-nCoV) uses the SARS-coronavirus receptor ACE2 and the cellular protease TMPRSS2 for entry into target cells. bioRxiv. In Press. | |
|
Zhang H, Penninger JM, Li Y, Zhong N and Slutsky AS: Angiotensin-converting enzyme 2 (ACE2) as a SARS-CoV-2 receptor: Molecular mechanisms and potential therapeutic target. Intensive Care Med. 46:586–590. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Speth R, Carrera E, Jean-Baptiste M, Joachim A and Linares A: Concentration-dependent effects of zinc on angiotensin-converting enzyme-2 activity (1067.4). FASEB J. 28(Suppl 1): 1067.42014. | |
|
Chilvers MA, McKean M, Rutman A, Myint BS, Silverman M and O'Callaghan C: The effects of coronavirus on human nasal ciliated respiratory epithelium. Eur Respir J. 18:965–970. 2001. View Article : Google Scholar | |
|
Maret W: Analyzing free zinc(II) ion concentrations in cell biology with fluorescent chelating molecules. Metallomics. 7:202–211. 2015. View Article : Google Scholar | |
|
Essaidi-Laziosi M, Brito F, Benaoudia S, Royston L, Cagno V, Fernandes-Rocha M, Piuz I, Zdobnov E, Huang S, Constant S, et al: Propagation of respiratory viruses in human airway epithelia reveals persistent virus-specific signatures. J Allergy Clin Immunol. 141:2074–2084. 2018. View Article : Google Scholar | |
|
Pittet LA, Hall-Stoodley L, Rutkowski MR and Harmsen AG: Influenza virus infection decreases tracheal mucociliary velocity and clearance of Streptococcus pneumoniae. Am J Respir Cell Mol Biol. 42:450–460. 2010. View Article : Google Scholar : | |
|
Darma A, Ranuh RG, Merbawani W, Setyoningrum RA, Hidajat B, Hidayati SN, Andaryanto A and Sudarmo SM: Zinc supplementation effect on the bronchial cilia length, the number of cilia, and the number of intact bronchial cell in zinc deficiency rats. Indones Biomed J. 12:78–84. 2020. View Article : Google Scholar | |
|
Woodworth BA, Zhang S, Tamashiro E, Bhargave G, Palmer JN and Cohen NA: Zinc increases ciliary beat frequency in a calcium-dependent manner. Am J Rhinol Allergy. 24:6–10. 2010. View Article : Google Scholar : PubMed/NCBI | |
|
Truong-Tran AQ, Carter J, Ruffin R and Zalewski PD: New insights into the role of zinc in the respiratory epithelium. Immunol Cell Biol. 79:170–177. 2001. View Article : Google Scholar : PubMed/NCBI | |
|
Roscioli E, Jersmann HP, Lester S, Badiei A, Fon A, Zalewski P and Hodge S: Zinc deficiency as a codeterminant for airway epithelial barrier dysfunction in an ex vivo model of COPD. Int J Chron Obstruct Pulmon Dis. 12:3503–3510. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Wittekindt OH: Tight junctions in pulmonary epithelia during lung inflammation. Pflugers Arch. 469:135–147. 2017. View Article : Google Scholar : | |
|
Günzel D and Yu AS: Claudins and the modulation of tight junction permeability. Physiol Rev. 93:525–569. 2013. View Article : Google Scholar : PubMed/NCBI | |
|
Ishida T: Review on the role of Zn2+ ions in viral pathogenesis and the effect of Zn2+ ions for host cell-virus growth inhibition. Am J Biomed Sci Res. 2:AJBSR.MS.ID.000566. 2019. View Article : Google Scholar | |
|
Krenn BM, Gaudernak E, Holzer B, Lanke K, Van Kuppeveld FJM and Seipelt J: Antiviral activity of the zinc ionophores pyrithione and hinokitiol against picornavirus infections. J Virol. 83:58–64. 2009. View Article : Google Scholar : | |
|
Korant BD, Kauer JC and Butterworth BE: Zinc ions inhibit replication of rhinoviruses. Nature. 248:588–590. 1974. View Article : Google Scholar : PubMed/NCBI | |
|
Cakman I, Kirchner H and Rink L: Zinc supplementation reconstitutes the production of interferon-α by leukocytes from elderly persons. J Interferon Cytokine Res. 17:469–472. 1997. View Article : Google Scholar : PubMed/NCBI | |
|
Berg K, Bolt G, Andersen H and Owen TC: Zinc potentiates the antiviral action of human IFN-α tenfold. J Interferon Cytokine Res. 21:471–474. 2001. View Article : Google Scholar : PubMed/NCBI | |
|
Lin FC and Young HA: Interferons: Success in anti-viral immunotherapy. Cytokine Growth Factor Rev. 25:369–376. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
Singh M and Das RR: Zinc for the common cold. Cochrane Database Syst Rev. 2013:CD0013642013. | |
|
Hemilä H: Zinc lozenges and the common cold: A meta-analysis comparing zinc acetate and zinc gluconate, and the role of zinc dosage. JRSM Open. 8:20542704176942912017. View Article : Google Scholar : PubMed/NCBI | |
|
Hemilä H: Zinc lozenges may shorten the duration of colds: A systematic review. Open Respir Med J. 5:51–58. 2011. View Article : Google Scholar : PubMed/NCBI | |
|
Che Z and Sun J: Investigation on relationship between whole blood zinc and Fe elements with children pneumonia caused by respiratory syncytial virus. Int J Lab Med. 37:2401–2402. 2016. | |
|
Johnson JK, Harris FL, Ping XD, Gauthier TW and Brown LAS: Role of zinc insufficiency in fetal alveolar macrophage dysfunction and RSV exacerbation associated with fetal ethanol exposure. Alcohol. 80:5–16. 2019. View Article : Google Scholar | |
|
Suara RO and Crowe JE Jr: Effect of zinc salts on respiratory syncytial virus replication. Antimicrob Agents Chemother. 48:783–790. 2004. View Article : Google Scholar : PubMed/NCBI | |
|
Kaynar AM, Andreas A, Maloy A, Austin W, Pitt BR, Gopal R and Alcorn JF: Zinc deficiency worsens the long-term outcome and exacerbates inflammation in a murine model of influenza-MRSA superinfection. Am J Respir Crit Care Med. 199:A41302019. | |
|
Haase H, Mocchegiani E and Rink L: Correlation between zinc status and immune function in the elderly. Biogerontology. 7:421–428. 2006. View Article : Google Scholar : PubMed/NCBI | |
|
Barnett JB, Hamer DH and Meydani SN: Low zinc status: A new risk factor for pneumonia in the elderly? Nutr Rev. 68:30–37. 2010. View Article : Google Scholar : PubMed/NCBI | |
|
Meydani SN, Barnett JB, Dallal GE, Fine BC, Jacques PF, Leka LS and Hamer DH: Serum zinc and pneumonia in nursing home elderly. Am J Clin Nutr. 86:1167–1173. 2007. View Article : Google Scholar : PubMed/NCBI | |
|
Bhat MH, Rather AB, Dhobi GN, Koul AN, Bhat FA and Hussain A: Zinc levels in community acquired pneumonia in hospitalized patients; a case control study. Egypt J Chest Dis Tuberc. 65:485–489. 2016. View Article : Google Scholar | |
|
Saleh P, Sadeghpour A, Mirza-Aghazadeh-Attari M, Hatampour M, Naghavi-Behzad M and Tabrizi A: Relationship between plasma levels of zinc and clinical course of pneumonia. Tanaffos. 16:40–45. 2017.PubMed/NCBI | |
|
Linko R, Karlsson S, Pettilä V, Varpula T, Okkonen M, Lund V, Ala-Kokko T and Ruokonen E; FINNALI Study Group: Serum zinc in critically ill adult patients with acute respiratory failure. Acta Anaesthesiol Scand. 55:615–621. 2011. View Article : Google Scholar : PubMed/NCBI | |
|
Saigal P and Hanekom D: Does zinc improve symptoms of viral upper respiratory tract infection? EBP. 23:37–39. 2020. | |
|
Sharafi S and Allami A: Efficacy of zinc sulphate on in-hospital outcome of community-acquired pneumonia in people aged 50 years and over. Int J Tuberc Lung Dis. 20:685–688. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Boudreault F, Pinilla-Vera M, Englert JA, Kho AT, Isabelle C, Arciniegas AJ, Barragan-Bradford D, Quintana C, Amador-Munoz D, Guan J, et al: MICU Registry: Zinc deficiency primes the lung for ventilator-induced injury. JCI Insight. 2:e865072017. View Article : Google Scholar | |
|
Chen X, Bian J and Ge Y: Zinc-deficient diet aggravates ventilation-induced lung injury in rats. J Biomed Res. 26:59–65. 2012. View Article : Google Scholar : PubMed/NCBI | |
|
Visalakshy J, Surendran S, Pillai MPG, Rajendran A and Sherif AA: Could plasma zinc be a predictor for mortality and severity in sepsis syndrome? Int J Res Med Sci. 5:3929–3934. 2017. View Article : Google Scholar | |
|
Hoeger J, Simon TP, Beeker T, Marx G, Haase H and Schuerholz T: Persistent low serum zinc is associated with recurrent sepsis in critically ill patients - A pilot study. PLoS One. 12:e01760692017. View Article : Google Scholar : PubMed/NCBI | |
|
Lee PI, Hu YL, Chen PY, Huang YC and Hsueh PR: Are children less susceptible to COVID-19? J Microbiol Immunol Infect. Feb 25–2020.Epub ahead of print. View Article : Google Scholar | |
|
Dong Y, Mo X, Hu Y, Qi X, Jiang F, Jiang Z and Tong S: Epidemiological characteristics of 2143 pediatric patients with 2019 coronavirus disease in China. Pediatrics. In Press. | |
|
Kumar N, Jayaprakash S and Kavitha D: Low serum zinc level - a possible marker of severe pneumonia. JMSCR. 5:21554–21570. 2017. View Article : Google Scholar | |
|
Islam SN, Kamal MM, Rahmatullah R, Sadi SKS and Ahsan M: Serum zinc levels in children with acute respiratory infections: Association with sociodemography and nutritional status. Clin Nutr Exp. 22:11–18. 2018. View Article : Google Scholar | |
|
Saleh NY and Abo El Fotoh WMM: Low serum zinc level: The relationship with severe pneumonia and survival in critically ill children. Int J Clin Pract. 72:e132112018. View Article : Google Scholar : PubMed/NCBI | |
|
Yakoob MY, Theodoratou E, Jabeen A, Imdad A, Eisele TP, Ferguson J, Jhass A, Rudan I, Campbell H, Black RE, et al: Preventive zinc supplementation in developing countries: Impact on mortality and morbidity due to diarrhea, pneumonia and malaria. BMC Public Health. 11(Suppl 3): S232011. View Article : Google Scholar : PubMed/NCBI | |
|
Lassi ZS, Moin A and Bhutta ZA: Zinc supplementation for the prevention of pneumonia in children aged 2 months to 59 months. Cochrane Database Syst Rev. 12:CD0059782016.PubMed/NCBI | |
|
Das RR, Singh M and Shafiq N: Short-term therapeutic role of zinc in children <5 years of age hospitalised for severe acute lower respiratory tract infection. Paediatr Respir Rev. 13:184–191. 2012. View Article : Google Scholar : PubMed/NCBI | |
|
Basnet S, Shrestha PS, Sharma A, Mathisen M, Prasai R, Bhandari N, Adhikari RK, Sommerfelt H, Valentiner-Branth P, Strand TA, Zinc Severe Pneumonia and Study Group: A randomized controlled trial of zinc as adjuvant therapy for severe pneumonia in young children. Pediatrics. 129:701–708. 2012. View Article : Google Scholar : PubMed/NCBI | |
|
Srinivasan MG, Ndeezi G, Mboijana CK, Kiguli S, Bimenya GS, Nankabirwa V and Tumwine JK: Zinc adjunct therapy reduces case fatality in severe childhood pneumonia: A randomized double blind placebo-controlled trial. BMC Med. 10:142012. View Article : Google Scholar : PubMed/NCBI | |
|
Bagri NK, Bagri N, Jana M, Gupta AK, Wadhwa N, Lodha R, Kabra SK, Chandran A, Aneja S, Chaturvedi MK, et al: Efficacy of oral zinc supplementation in radiologically confirmed pneumonia: Secondary analysis of a randomized controlled trial. J Trop Pediatr. 64:110–117. 2018. View Article : Google Scholar | |
|
Yuan X, Qian SY, Li Z and Zhang ZZ: Effect of zinc supplementation on infants with severe pneumonia. World J Pediatr. 12:166–169. 2016. View Article : Google Scholar | |
|
Shah UH, Abu-Shaheen AK, Malik MA, Alam S, Riaz M and Al-Tannir MA: The efficacy of zinc supplementation in young children with acute lower respiratory infections: A randomized double-blind controlled trial. Clin Nutr. 32:193–199. 2013. View Article : Google Scholar | |
|
Rerksuppaphol S and Rerksuppaphol L: A randomized controlled trial of zinc supplementation in the treatment of acute respiratory tract infection in Thai children. Pediatr Rep. 11:79542019. View Article : Google Scholar : PubMed/NCBI | |
|
Roth DE, Richard SA and Black RE: Zinc supplementation for the prevention of acute lower respiratory infection in children in developing countries: Meta-analysis and meta-regression of randomized trials. Int J Epidemiol. 39:795–808. 2010. View Article : Google Scholar : PubMed/NCBI | |
|
Martinez-Estevez NS, Alvarez-Guevara AN and Rodriguez-Martinez CE: Effects of zinc supplementation in the prevention of respiratory tract infections and diarrheal disease in Colombian children: A 12-month randomised controlled trial. Allergol Immunopathol (Madr). 44:368–375. 2016. View Article : Google Scholar | |
|
Aggarwal R, Sentz J and Miller MA: Role of zinc administration in prevention of childhood diarrhea and respiratory illnesses: A meta-analysis. Pediatrics. 119:1120–1130. 2007. View Article : Google Scholar : PubMed/NCBI | |
|
Khera D, Singh S, Purohit P, Sharma P and Singh K: Prevalence of Zinc deficiency and effect of Zinc supplementation on prevention of acute respiratory infections: A non randomized open label study. SSRN. 2018, https://ssrn.com/abstract=3273670. Accessed October 26, 2018. View Article : Google Scholar | |
|
Mehta P, McAuley DF, Brown M, Sanchez E and Tattersall RS: COVID-19: Consider cytokine storm syndromes and immuno-suppression. Lancet. 395:1033–1034. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Biaggio VS, Pérez Chaca MV, Valdéz SR, Gómez NN and Gimenez MS: Alteration in the expression of inflammatory parameters as a result of oxidative stress produced by moderate zinc deficiency in rat lung. Exp Lung Res. 36:31–44. 2010. View Article : Google Scholar : PubMed/NCBI | |
|
Bao S and Knoell DL: Zinc modulates cytokine-induced lung epithelial cell barrier permeability. Am J Physiol Lung Cell Mol Physiol. 291:L1132–L1141. 2006. View Article : Google Scholar : PubMed/NCBI | |
|
Liu MJ, Bao S, Napolitano JR, Burris DL, Yu L, Tridandapani S and Knoell DL: Zinc regulates the acute phase response and serum amyloid A production in response to sepsis through JAK-STAT3 signaling. PLoS One. 9:e94934PubMed/NCBI | |
|
St Croix CM, Leelavaninchkul K, Watkins SC, Kagan VE and Pitt BR: Nitric oxide and zinc homeostasis in acute lung injury. Proc Am Thorac Soc. 2:236–242. 2005. View Article : Google Scholar : PubMed/NCBI | |
|
Krones CJ, Klosterhalfen B, Butz N, Hoelzl F, Junge K, Stumpf M, Peiper C, Klinge U and Schumpelick V: Effect of zinc pretreatment on pulmonary endothelial cells in vitro and pulmonary function in a porcine model of endotoxemia. J Surg Res. 123:251–256. 2005. View Article : Google Scholar : PubMed/NCBI | |
|
Wessels I, Pupke JT, von Trotha KT, Gombert A, Himmelsbach A, Fischer HJ, Jacobs MJ, Rink L and Grommes J: Zinc supplementation ameliorates lung injury by reducing neutrophil recruitment and activity. Thorax. 75:253–261. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Biaggio VS, Salvetti NR, Pérez Chaca MV, Valdez SR, Ortega HH, Gimenez MS and Gomez NN: Alterations of the extracellular matrix of lung during zinc deficiency. Br J Nutr. 108:62–70. 2012. View Article : Google Scholar | |
|
Luo W, Yu H, Gou J, Li X, Sun Y, Li J and Liu L: Clinical pathology of critical patient with novel Coronavirus pneumonia (COVID-19). Preprints. 2020:20200204072020. | |
|
Knoell DL, Julian MW, Bao S, Besecker B, Macre JE, Leikauf GD, DiSilvestro RA and Crouser ED: Zinc deficiency increases organ damage and mortality in a murine model of polymicrobial sepsis. Crit Care Med. 37:1380–1388. 2009. View Article : Google Scholar : PubMed/NCBI | |
|
Bao S, Liu MJ, Lee B, Besecker B, Lai JP, Guttridge DC and Knoell DL: Zinc modulates the innate immune response in vivo to polymicrobial sepsis through regulation of NF-kappaB. Am J Physiol Lung Cell Mol Physiol. 298:L744–L754. 2010. View Article : Google Scholar : PubMed/NCBI | |
|
Nowak JE, Harmon K, Caldwell CC and Wong HR: Prophylactic zinc supplementation reduces bacterial load and improves survival in a murine model of sepsis. Pediatr Crit Care Med. 13:e323–e329. 2012. View Article : Google Scholar : PubMed/NCBI | |
|
Ganatra HA, Varisco BM, Harmon K, Lahni P, Opoka A and Wong HR: Zinc supplementation leads to immune modulation and improved survival in a juvenile model of murine sepsis. Innate Immun. 23:67–76. 2017. View Article : Google Scholar | |
|
Slinko S, Piraino G, Hake PW, Ledford JR, O'Connor M, Lahni P, Solan PD, Wong HR and Zingarelli B: Combined zinc supplementation with proinsulin C-peptide treatment decreases the inflammatory response and mortality in murine polymicrobial sepsis. Shock. 41:292–300. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
Besecker BY, Exline MC, Hollyfield J, Phillips G, Disilvestro RA, Wewers MD and Knoell DL: A comparison of zinc metabolism, inflammation, and disease severity in critically ill infected and noninfected adults early after intensive care unit admission. Am J Clin Nutr. 93:1356–1364. 2011. View Article : Google Scholar : PubMed/NCBI | |
|
Liu MJ, Bao S, Gálvez-Peralta M, Pyle CJ, Rudawsky AC, Pavlovicz RE, Killilea DW, Li C, Nebert DW, Wewers MD, et al: ZIP8 regulates host defense through zinc-mediated inhibition of NF-κB. Cell Rep. 3:386–400. 2013. View Article : Google Scholar : PubMed/NCBI | |
|
Hall SC, Smith DR, Katafiasz DM, Bailey KL and Knoell DL: Novel role of zinc homeostasis in IL-23 regulation and host defense following bacterial infection. J Immunol. 202(Suppl 1): 62–6. 2019. | |
|
Knoell DL, Smith DA, Sapkota M, Heires AJ, Hanson CK, Smith LM, Poole JA, Wyatt TA and Romberger DJ: Insufficient zinc intake enhances lung inflammation in response to agricultural organic dust exposure. J Nutr Biochem. 70:56–64. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Lang CJ, Hansen M, Roscioli E, Jones J, Murgia C, Leigh Ackland M, Zalewski P, Anderson G and Ruffin R: Dietary zinc mediates inflammation and protects against wasting and metabolic derangement caused by sustained cigarette smoke exposure in mice. Biometals. 24:23–39. 2011. View Article : Google Scholar | |
|
von Bülow V, Dubben S, Engelhardt G, Hebel S, Plümäkers B, Heine H, Rink L and Haase H: Zinc-dependent suppression of TNF-α production is mediated by protein kinase A-induced inhibition of Raf-1, IκB kinase β, and NF-κB. J Immunol. 179:4180–4186. 2007. View Article : Google Scholar | |
|
Wessels I, Haase H, Engelhardt G, Rink L and Uciechowski P: Zinc deficiency induces production of the proinflammatory cytokines IL-1β and TNFα in promyeloid cells via epigenetic and redox-dependent mechanisms. J Nutr Biochem. 24:289–297. 2013. View Article : Google Scholar | |
|
Prasad AS, Bao B, Beck FW and Sarkar FH: Zinc-suppressed inflammatory cytokines by induction of A20-mediated inhibition of nuclear factor-κB. Nutrition. 27:816–823. 2011. View Article : Google Scholar | |
|
Wellinghausen N, Martin M and Rink L: Zinc inhibits interleukin-1-dependent T cell stimulation. Eur J Immunol. 27:2529–2535. 1997. View Article : Google Scholar : PubMed/NCBI | |
|
Rosenkranz E, Metz CH, Maywald M, Hilgers RD, Weßels I, Senff T, Haase H, Jäger M, Ott M, Aspinall R, et al: Zinc supplementation induces regulatory T cells by inhibition of Sirt-1 deacetylase in mixed lymphocyte cultures. Mol Nutr Food Res. 60:661–671. 2016. View Article : Google Scholar | |
|
Kahmann L, Uciechowski P, Warmuth S, Plümäkers B, Gressner AM, Malavolta M, Mocchegiani E and Rink L: Zinc supplementation in the elderly reduces spontaneous inflammatory cytokine release and restores T cell functions. Rejuvenation Res. 11:227–237. 2008. View Article : Google Scholar : PubMed/NCBI | |
|
Kim H: Outbreak of novel coronavirus (COVID-19): What is the role of radiologists? Eur Radiol. Feb 18–2020.Epub ahead of print. View Article : Google Scholar : | |
|
Golda A, Malek N, Dudek B, Zeglen S, Wojarski J, Ochman M, Kucewicz E, Zembala M, Potempa J and Pyrc K: Infection with human coronavirus NL63 enhances streptococcal adherence to epithelial cells. J Gen Virol. 92:1358–1368. 2011. View Article : Google Scholar : PubMed/NCBI | |
|
Eijkelkamp BA, Morey JR, Neville SL, Tan A, Pederick VG, Cole N, Singh PP, Ong CY, Gonzalez de Vega R, Clases D, et al: Dietary zinc and the control of Streptococcus pneumoniae infection. PLoS Pathog. 15:e10079572019. View Article : Google Scholar : PubMed/NCBI | |
|
Coles CL, Sherchand JB, Khatry SK, Katz J, Leclerq SC, Mullany LC and Tielsch JM: Zinc modifies the association between nasopharyngeal Streptococcus pneumoniae carriage and risk of acute lower respiratory infection among young children in rural Nepal. J Nutr. 138:2462–2467. 2008. View Article : Google Scholar : PubMed/NCBI | |
|
Strand TA, Hollingshead SK, Julshamn K, Briles DE, Blomberg B and Sommerfelt H: Effects of zinc deficiency and pneumococcal surface protein A immunization on zinc status and the risk of severe infection in mice. Infect Immun. 71:2009–2013. 2003. View Article : Google Scholar : PubMed/NCBI | |
|
Strand TA, Briles DE, Gjessing HK, Maage A, Bhan MK and Sommerfelt H: Pneumococcal pulmonary infection, septicaemia and survival in young zinc-depleted mice. Br J Nutr. 86:301–306. 2001. View Article : Google Scholar : PubMed/NCBI | |
|
Hamza SA, Mousa SM, Taha SE, Adel LA, Samaha HE and Hussein DA: Immune response of 23-valent pneumococcal poly-saccharide vaccinated elderly and its relation to frailty indices, nutritional status, and serum zinc levels. Geriatr Gerontol Int. 12:223–229. 2012. View Article : Google Scholar | |
|
Mansouri F, Vaziri S, Janbakhsh A, Sayad B, Najafi F, Karimivafa SM, Kashef M and Azizi M: The effect of zinc on the Immune responses of pneumococcal vaccination in elderly. Int J Med Microbiol. 10:67–73. 2016. | |
|
Osendarp SJ, Prabhakar H, Fuchs GJ, van Raaij JM, Mahmud H, Tofail F, Santosham M and Black RE: Immunization with the heptavalent pneumococcal conjugate vaccine in Bangladeshi infants and effects of zinc supplementation. Vaccine. 25:3347–3354. 2007. View Article : Google Scholar : PubMed/NCBI | |
|
Jacobsen FE, Kazmierczak KM, Lisher JP, Winkler ME and Giedroc DP: Interplay between manganese and zinc homeostasis in the human pathogen Streptococcus pneumoniae. Metallomics. 3:38–41. 2011. View Article : Google Scholar : PubMed/NCBI | |
|
McDevitt CA, Ogunniyi AD, Valkov E, Lawrence MC, Kobe B, McEwan AG and Paton JC: A molecular mechanism for bacterial susceptibility to zinc. PLoS Pathog. 7:e10023572011. View Article : Google Scholar : PubMed/NCBI | |
|
Pasquet J, Chevalier Y, Pelletier J, Couval E, Bouvier D and Bolzinger MA: The contribution of zinc ions to the antimicrobial activity of zinc oxide. Colloids Surf A Physicochem Eng Asp. 457:263–274. 2014. View Article : Google Scholar | |
|
Bhattacharyya P, Agarwal B, Goswami M, Maiti D, Baruah S and Tribedi P: Zinc oxide nanoparticle inhibits the biofilm formation of Streptococcus pneumoniae. Antonie van Leeuwenhoek. 111:89–99. 2018. View Article : Google Scholar | |
|
Reddy LS, Nisha MM, Joice M and Shilpa PN: Antimicrobial activity of zinc oxide (ZnO) nanoparticle against Klebsiella pneumoniae. Pharm Biol. 52:1388–1397. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
Kadiyala U, Turali-Emre ES, Bahng JH, Kotov NA and VanEpps JS: Unexpected insights into antibacterial activity of zinc oxide nanoparticles against methicillin resistant Staphylococcus aureus (MRSA). Nanoscale. 10:4927–4939. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Ann LC, Mahmud S, Bakhori SKM, Sirelkhatim A, Mohamad D, Hasan H, Seeni A and Rahman RA: Antibacterial responses of zinc oxide structures against Staphylococcus aureus, Pseudomonas aeruginosa and Streptococcus pyogenes. Ceram Int. 40:2993–3001. 2014. View Article : Google Scholar | |
|
Sahu D, Kannan GM, Vijayaraghavan R, Anand T and Khanum F: Nanosized zinc oxide induces toxicity in human lung cells. ISRN Toxicol. 2013:3160752013. View Article : Google Scholar : PubMed/NCBI | |
|
Lin CD, Kou YY, Liao CY, Li CH, Huang SP, Cheng YW, Liao WC, Chen HX, Wu PL, Kang JJ, et al: Zinc oxide nanoparticles impair bacterial clearance by macrophages. Nanomedicine (Lond). 9:1327–1339. 2014. View Article : Google Scholar | |
|
Bayle L, Chimalapati S, Schoehn G, Brown J, Vernet T and Durmort C: Zinc uptake by Streptococcus pneumoniae depends on both AdcA and AdcAII and is essential for normal bacterial morphology and virulence. Mol Microbiol. 82:904–916. 2011. View Article : Google Scholar : PubMed/NCBI | |
|
Brown LR, Caulkins RC, Schartel TE, Rosch JW, Honsa ES, Schultz-Cherry S, Meliopoulos VA, Cherry S and Thornton JA: Increased zinc availability enhances initial aggregation and biofilm formation of Streptococcus pneumoniae. Front Cell Infect Microbiol. 7:2332017. View Article : Google Scholar : | |
|
Skalnaya MG and Skalny AV: Essential trace elements in human health: a physician's view. Publishing House of Tomsk State University; Tomsk: 2018 |