Role of vitamins beyond vitamin D3 in bone health and osteoporosis (Review)
- Authors:
- Published online on: December 5, 2023 https://doi.org/10.3892/ijmm.2023.5333
- Article Number: 9
-
Copyright: © Skalny et al. This is an open access article distributed under the terms of Creative Commons Attribution License.
Abstract
1. Introduction
Osteoporosis is as a skeletal disorder characterized by reduced bone mineralization and strength, leading to an increased risk of fractures (1). The overall prevalence of osteoporosis worldwide has been estimated at 18.3%, with an almost 2-fold higher prevalence in females (23.1%) than males (11.7%) (2). Osteoporosis is also characterized by high geographic differences, with the highest prevalence in Africa (26.9%) (3). Yet, even in developed countries, the economic burden of osteoporosis-related fractures is significant, with annual costs of 17.9 billion USD and 4 billion GBP in the USA and UK, respectively (4). The geographic heterogeneity of osteoporosis is mediated by the distinct prevalence of risk factors, including genetic patterns, environmental factors, sedentary lifestyle, smoking, alcohol use, medications (glucocorticoids), morbidities (hyperparathyroidism, rheumatoid arthritis, diabetes mellitus, cancer), as well as nutritional deficiencies (5).
Nutritional factors play a critical role in the prevalence of osteoporosis (6) with Ca2+ and vitamin D considered as critical for bone health (7). The role of vitamin D deficiency in osteoporosis (8) is mediated by the role of its active form, 1,25-dihydroxy vitamin D, in regulation of mineral metabolism and bone remodeling through its effects on osteoblast and osteoclast formation and activity (9). However, increasing evidence demonstrates that other micronutrients, aside from Ca2+ and vitamin D, including minerals and trace elements, vitamins, and polyphenols can modify the risk of developing osteoporosis (10,11). It has been demonstrated that several vitamin groups, including vitamins A, E, K, C and B, are involved in regulation of bone turnover, and that their insufficiency may be considered as a dietary risk factor for osteoporosis (12). However, the existing epidemiological studies are inconsistent and the understanding of molecular mechanisms underlying the role of non-vitamin D vitamins in modulating bone health have yet to be clearly defined. Specifically, the effects of vitamins on bone metabolism and osteoporosis pathogenesis are expected to depend on the particular form of the vitamin (13,14) or exposure dose (15).
The objective of the present review was to highlight the molecular mechanisms of the effects of vitamin groups A, C, E, K and B on bone and their potential role in the development of osteoporosis. To the best of our knowledge, this is the first comprehensive review focusing on the association between the intake of vitamins A, C, E and K, and group B vitamins and osteoporosis since the article by Ahmadieh and Arabi (16) published over than a decade ago and focusing mainly on epidemiological data. Since the publication of the aforementioned study (16) significant progress has been made in understanding the molecular mechanisms of vitamin functions in bone has been achieved, while epidemiological studies provided additional evidence on the association between vitamin status and osteoporosis. Therefore, in the present review, the role of vitamin forms and doses and their biological effects on bone tissue are discussed in detail, with particular focus on the most recent findings. Given the high prevalence of osteoporosis and vitamin deficiency worldwide, the further understanding of the role of vitamins as osteoprotective agents may markedly improve the prevention of and treatment strategies for osteoporosis, as well as prevent adverse effects of excessive supplementation.
2. Vitamin E
Vitamin E (VE) is a fat-soluble vitamin with antioxidant activity that is present in the form of tocopherols (α-, β-, γ- and δ-) and tocotrienols (α-, β-, γ- and δ-) (17). VE is considered as bone-protecting due to its complex effects on bone physiology that are not limited to its antioxidant activity (18). A Mendelian randomization study demonstrated a significant positive association between circulating α-tocopherol levels and bone mineral density (BMD) (19). A low serum VE level has been found to be associated with a reduced BMD, and has therefore been considered a risk factor for osteoporosis in post-menopausal women (20).
Correspondingly, low serum α-tocopherol concentrations have been found to be associated with a 51 and 58% increase in the hazard ratio of hip fractures in older Norwegians (21) and Swedes (22). In turn, supplementation with tocotrienol, a form of VE, for 12 weeks was shown to decrease oxidative stress and bone resorption in post-menopausal women with osteopenia (23,24).
Despite a positive association between serum α-tocopherol and femoral neck BMD observed in the Aberdeen Prospective Osteoporosis Screening Study, the authors considered this association to lack biological significance (25). However, the analysis of NHANES 2005-2006 data demonstrated an inverse association between the serum α-tocopherol levels and femoral neck BMD following adjustment for confounders (26).
Notably, serum α-tocopherol, but not γ-tocopherol, has been found to be inversely associated with bone formation marker, procollagen type 1 amino-terminal propeptide, in post-menopausal women (27). These findings generally corroborate the earlier observed inverse relationship between α-tocopherol intake and γ-tocopherol levels (28).
Experimental studies with in vivo models of osteoporosis have also demonstrated that VE exerts osteoprotective effects. Specifically, VE supplementation has been shown to improve bone histomorphometry, with the most profound effect upon γ-tocotrienol treatment when compared to α-tocopherol and δ-tocotrienol (29). At the same time, Muhammad et al (30) reported similar protective effects of tocotrienol and α-tocopherol against bone loss in ovariectomized rats.
In addition, tocotrienol supplementation has been shown to improve bone calcination in testosterone deficiency-associated osteoporosis (31). In ovariectomy-induced osteoporotic fractures, α-tocopherol supplementation has been found to significantly improve fracture healing, although it does not increase callous bone volume in rats (32), nor does it improve bone strength (33). It has been shown that both an intraperitoneal (34) and intramuscular (35) injection with α-tocopherol significantly increases BMD and osteogenesis, as well as osteoblast activity in a rabbit model of distraction osteogenesis.
Correspondingly, VE deficiency has been shown to alter exercise-induced plasma membrane disruptions, membrane repair and the survival of osteocytes (36). The co-administration of Se and vitamin C (VC) with VE significantly increases its efficiency in the improvement of bone structure (37). In turn, excessive VE intake has failed to induce bone loss in an animal model of ovariectomy-induced osteoporosis (38), as well as in normal female rats (39).
The association between VE intake and bone health established in the aforementioned epidemiological studies is mediated by the influence of tocopherols and tocotrienols on bone physiology.
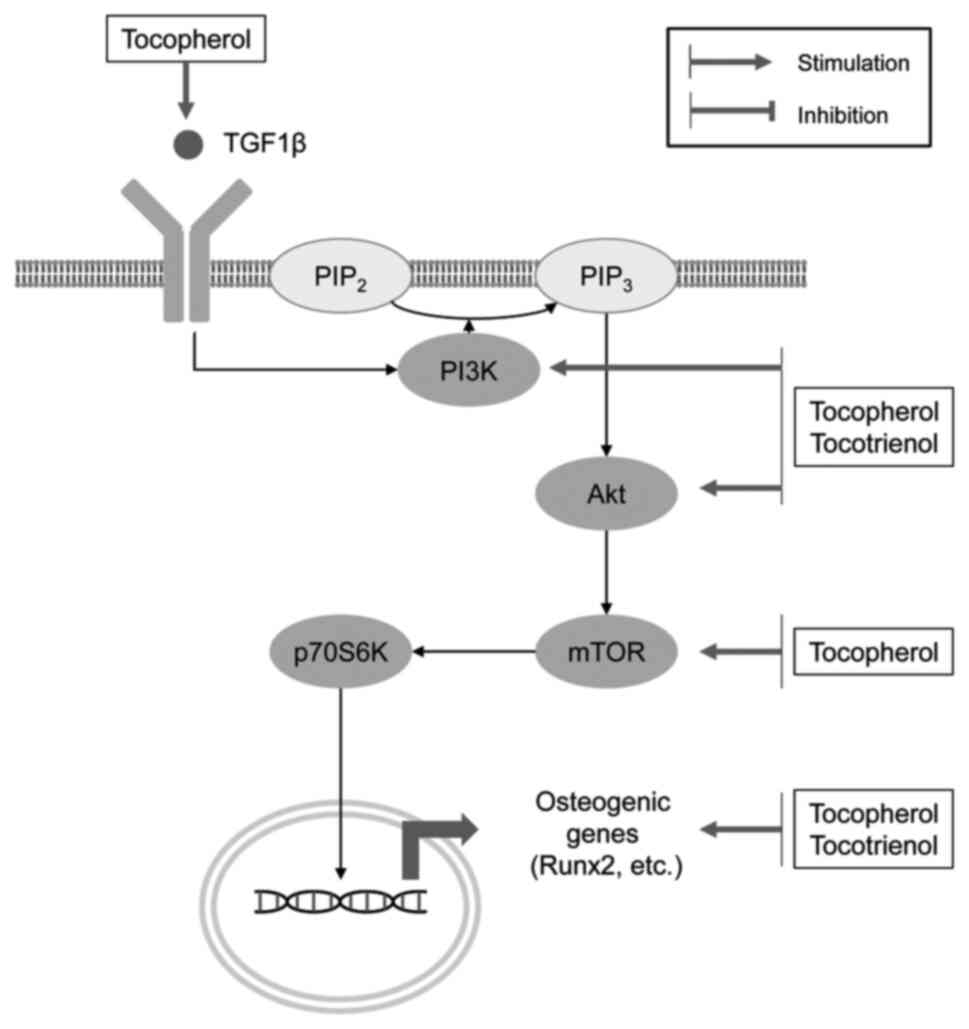
In agreement with the role of VE as an antioxidant, tocopherol has been shown to promote the osteogenic differentiation and oxidative stress resistance of rat bone marrow-derived mesenchymal stem cells by inhibiting H2O2-induced ferroptosis by increasing the phosphorylation of PI3K, Akt and mammalian target of rapamycin (mTOR) (40). α-tocopherol-stimulated osteoblastogenesis has been shown to be associated with the upregulation of alkaline phosphatase (ALP)2, TGF1β, fibroblast growth factor receptor 1, MMP-2, muscle segment homeobox 2, bone morphogenetic protein (BMP)-1, VEGF-B, Runx2, Smad2 and other genes, whereas the expression of osteopetrosis-associated transmembrane protein 1, microphthalmia-associated transcription factor (MITF) and EGFR genes is downregulated (41). VE has been shown to reduce osteocyte apoptosis in a model of steroid-induced osteonecrosis through inhibition of caspase-3 expression and upregulation of Bcl-2 (42). At the same time, α-tocopherol and δ-tocopherol may also inhibit osteoblast differentiation from the early stages of osteogenesis to the osteoid-producing stage (43). At the same time, both α-tocopherol (100 and 200 μM) and δ-tocopherol (2 and 20 μM) significantly reduces osteoblast differentiation (43).
In addition to the promotion of osteoblast differentiation, tocopherol has been shown to inhibit IL-1-induced osteoclastogenesis through the downregulation of receptor activator of nuclear factor kappa-B ligand (RANKL) mRNA expression (44). The VE-induced inhibition of osteoclastogenesis may also be associated with reduced monocyte and lymphocyte production (45). In addition, treatment with 10-20 μM α-tocopherol has been shown to result in reduced bone mass by upregulating osteoclast fusion via p38 MAPK and MITF activation (46).
It has also been demonstrated that another form of VE, tocotrienol, may also significantly modulate bone formation and resorption (47) in a distinct manner of that observed for tocopherols (48). γ-tocotrienol significantly promotes Runx2-dependent osteoblastogenesis with the upregulation of ALP, osteocalcin (OCN) and type I collagen (49). Annatto-derived tocotrienol has been found to significantly increase osteoblast differentiation, as evidenced by increased osterix (OSX), COL1α1, ALP and OCN gene expression, and enhanced mineralization (50).
Tocotrienol also significantly increases mineralization in osteoblasts by increasing BMP-2 protein expression in association with the downregulation of RhoA activation and HMG-CoA reductase gene expression (51). The tocotrienol-induced upregulation of BMP-2 and BMP-4 gene expression has also been shown to be associated with the stimulation of Wnt/β-catenin signaling (52). d-δ-tocotrienol (0-25 μmol/l) has been shown to induce MC3T3-E1 preosteoblast differentiation through the upregulation of BMP-2 and the inhibition of HMG-CoA reductase expression, resulting in mineralized nodule formation (53).
δ-tocotrienol also promotes osteoblast migration through an increase in Akt phosphorylation and Wnt/β-catenin signaling activation (54). Notably, at low doses, γ-tocotrienol has been shown to exert protective effects on osteoblasts against H2O2-induced oxidative stress and apoptosis, whereas high doses are cytotoxic and induce apoptotic cell death (55). It has also been demonstrated that δ-tocotrienol protects osteoblastic MC3T3-E1 and MLO-Y4 cells from oxidative stress and subsequent apoptosis through the upregulation of glutathione production and the upregulation of the PI3K/Akt and nuclear factor-erythroid factor 2-related factor 2 (Nrf2) signaling pathways (56). The osteogenic effects of γ-tocotrienol on human bone marrow-derived mesenchymal stem cells have been shown to be mediated by the promotion of p-AMPK and p-Smad1 phosphorylation (57).
α-Tocotrienol, but not α-tocopherol, has been shown to reduce osteoclastogenesis (58) through the inhibition of RANKL expression along with the downregulation of c-Fos expression (59). Specifically, γ-tocotrienol has been shown to inhibit RANKL mRNA expression, while increasing osteoprotegerin (OPG) mRNA expression in human bone-derived cells, whereas α-tocopherol is capable of only upregulating OPG expression (60). Tocotrienol has also been shown to inhibit IL-17-induced osteoclastogenesis in rheumatoid arthritis fibroblast-like synoviocytes through the downregulation of mTOR, ERK and IκB phosphorylation, and the inhibition of RANKL mRNA expression, while increasing AMPK phosphorylation (61). In a model of metabolic syndrome-associated osteoporosis supplementation with tocotrienol, there was a significant reduction in RANKL and FGF-23 expression, as well as a reduction in Dickkopf-related protein (DKK)-1 levels, being indicative of Wnt pathway activation (62) (Fig. 1).
Annatto bean-derived tocotrienol has also been shown to prevent bone resorption in testosterone-deficiency-associated osteoporosis in rats (63). γ-tocotrienol also reduces ovariectomy-induced bone loss in mice through HMG-CoA reductase inhibition (64). Moreover, palm oil-derived tocotrienols have been shown to prevent bone loss in ovariectomized rats more effectively than Ca2+ (65). The inhibition of skeletal sclerostin expression may be also responsible for the anti-osteoporotic effects of annatto tocotrienol in ovariectomized rats in parallel with the reduction of the RANKL/OPG ratio (66). According to the positive role of tocotrienols in the prevention of bone resorption, these were considered as the potential treatment strategy for menopause-associated osteoporosis (67).
In general, VE may be considered as an osteoprotective agent, although the biological effects are strongly dependent on the specific forms. Epidemiological studies have demonstrated that the serum α-tocopherol level is significantly associated with BMD, whereas its deficiency is related to an increased risk of fractures, although certain inconsistencies exist. Both tocopherol and tocotrienol isomers significantly increase bone quality and promote regeneration in animal models of osteoporosis. α-tocopherol has been shown to exert osteogenic effects due to its antioxidant effects, the inhibition of osteoblast ferroptosis and apoptosis, as well as the activation of the TGF1β/Smad and PI3K/Akt pathways. Even more potent osteogenic effects have been demonstrated for tocotrienol that promote BMP-2 and Wnt/β-catenin signaling, also activating Akt and protecting the cells from oxidative stress and apoptosis. The inhibitory effects of both tocopherol and tocotrienol on osteoclast formation have been shown to be mediated by the inhibition of inflammation-associated RANKL-induced osteoclastogenesis. Therefore, dietary VE as tocotrienol, has been shown to exert osteoprotective effects in laboratory studies, although epidemiological data are available only for tocopherol.
3. Vitamin K
Vitamin K (VK) is a lipid-soluble vitamin that is found in the form of VK1 (phylloquinone), VK2 (menaquinone), VK3 (menadione) and synthetic derivatives (68). VK2, being present most commonly in the form of menaquinone-4, 7 and 10 (indicating the number of isoprenyl groups at C3 position), has been shown to be involved in the regulation of bone remodeling (69).
VK has been shown to be a cost-effective strategy for preventing fractures in older women (70). A recent meta-analysis of 16 randomized controlled trials with 6,425 subjects involved demonstrated that VK2 supplementation significantly improved BMD and reduced the risk of fractures (71), as well as undercarboxylated OCN levels (72) in post-menopausal women. Similarly, other meta-analyses have demonstrated positive impact of vitamin K on BMD and fracture risk (73). Correspondingly, in 10-year follow-up studies, a higher dietary intake of VK was shown to be associated with a 24% decrease in the relative fracture risk (74). Each 1 μg/l increase in serum VK1 (phylloquinone) levels was associated with a 45% reduction in fracture risk in post-menopausal osteoporosis due to an increase in hip strength (75). However, no significant effects of phylloquinone intake on bone turnover or bone mass were observed in adult patients with Crohn's disease (76). In turn, low plasma phylloquinone levels were associated with a higher incidence of vertebral fractures, although no significant difference in BMD in subjects with low and high plasma K1 levels was observed (77). VK intake was also shown to be inversely associated with undercarboxylated OCN that was negatively associated with lumbar BMD and was directly interrelated with urinary type-I collagen cross-linked-N-telopeptide levels, a marker for bone resorption (78).
A previous meta-analysis demonstrated that the combination of vitamin D with VK significantly increased total BMD with the more profound effect observed in VK2 users (79). The co-supplementation of phylloquinone with vitamin D3 and calcium has been shown to increase BMD and bone mineral content (BMC) at the ultradistal radius (80). The combined administration of VK and Ca2+ also possessed positive effect on BMD, as evidenced by a recent meta-analysis (81). Correspondingly, a low dietary Ca2+ and VK intake was considered a risk factor for osteoporotic fractures in women (82). In a previous study, a 3-year low-dose MK-7 supplementation in healthy post-menopausal women significantly reduced the aging-associated decrease in lumbar spine and femoral neck BMD and BMC, vertebral height and bone strength (83). The administration of 375 μg MK-7 for 12 months prevented an increase in trabecular spacing and the reduction of trabecular number in post-menopausal women with osteopenia (84). The results of a 24-month trial demonstrated a significant reduction in the incidence of fractures in patients with osteoporosis supplemented with MK-4 when compared to the control groups (85). Consistently, the results from a meta-analysis demonstrated that MK-4 intake significantly improved BMD and decreased the risk for vertebral fractures as compared to treatment with the placebo (86).
Furthermore, serum VK2 levels are significantly reduced in post-menopausal osteoporotic patients (87). Respectively, the simultaneous assessment of circulating VK levels with other markers of osteoporosis, including pyridinoline and bone alkaline phosphatase, has been shown to significantly increase diagnostic value of the latter in osteoporotic women (88). It has also been demonstrated that the plasma MK-7 level is reduced earlier than the vitamin D concentration in post-menopausal women with osteoporosis (89). However, no significant association of circulating VK1, MK-4 and MK-7 with vertebral or hip fractures has been observed (90).
In animal models of osteoporosis, VK has also been shown to exert osteoprotective effects. Specifically, VK supplementation was even shown to be more effective in the improvement of bone characteristics in a model of immobilization osteoporosis as compared to combined Ca2+ and vitamin D administration (91). A similar protective effect of VK2 (menatetrenone) was observed in a model of glucocorticoid- (92,93) and hyperglycemia-induced (94) bone loss. MK-7 has been shown to promote diaphyseal and metaphyseal Ca2+ deposition due to increased osteoblastic proliferation and differentiation (95). Moreover, MK-7, but not MK-4 intake, has also bees shown to improve bone microstructure characterized by higher trabecular number, improved trabecular architecture and greater bone volume in ovariectomized rats (96).
The results obtained from laboratory studies are generally consistent with those from the epidemiological studies, also demonstrating the osteogenic effects of VK, although the specific effects and underlying mechanisms have been shown to be greatly dependent on the forms and homologues of VK.
MK-7 has been shown to promote MC3T3E1 cell differentiation characterized by an increased OCN, OPG and RANKL mRNA expression (97). Menaquinone-7 treatment also increases osteoblast migration and activity along with the downregulation of Runx2 expression, indicative of promotion of cell maturation (98). MK-7-induced osteogenesis has also been found to be associated with a significant increase in BMP-2 mRNA expression, tenascin C gene expression and increased p-Smad1 levels in MC3T3E1 cells (99). MK-7 promotes vitamin D3-induced osteogenesis that may be at least partially mediated by the enhanced expression of genes, including growth differentiation factor-10 (GDF10), IGF1, VEGFA and fms-related tyrosine kinase 1 (FLT1) (100). Concomitantly, hydrophobins-modified menaquinone-7 has been shown to be more effective in increasing osteoblast differentiation, while reducing osteoclastogenesis in MC3T3-E1 cells, as compared to native MK-7 (101). It has also been demonstrated that MK-7 inhibits basal and cytokine-induced NF-κB signaling through an increase in IκB mRNA expression, and ameliorates TNFα-induced inhibition of SMAD signaling (102). These findings generally resemble the earlier observed amelioration of inhibitory effect of inflammation on osteogenesis through down-regulation of IL-6-induced JAK/STAT signaling upon VK2 treatment (103).
MK-4 has been shown to be the most potent promotor of bone formation compared to estrogen, icariin, lactoferrin and lithium chloride (104). It has been sown that menaquinone 4 inhibits ovariectomy-induced bone loss by increasing osteoblast activity with the stimulation of BMP-2 and Runx2 signaling, and the downregulation of osteoclast differentiation (105). Correspondingly, the osteogenic effect of MK-4 has been shown to be mediated by the activation of the Wnt/β-catenin signaling pathway (106). In addition to increased osteoblast proliferation, the osteogenic effect of MK-4 may be associated with the inhibition of Fas-induced osteoblast apoptosis (107). Correspondingly, MK-4 also prevents osteoblast apoptosis through the upregulation of FoxO signaling and the reduction of reactive oxygen species (ROS) production (108), in agreement with the observed upregulation of SIRT1 signaling and the inhibition of mitochondrial dysfunction and endoplasmic reticulum stress (ERS) (109). At the same time, MK-4 reduces excessive bone mineralization induced by Mg deficiency (110). It is also notable that in vascular smooth muscle cells, MK-4 reduces β-glycerophosphate-induced calcification by downregulating BMP-2 and Smad1 expression (111).
Other mechanisms underlying the osteogenic effects of VK2 have been shown to include the amelioration of hyperglycemia-induced bone loss and ferroptosis through the upregulation of AMPK/SIRT1 signaling (94). Induction of autophagy may also contribute to osteogenic effect of VK2 (112). Correspondingly, VK2 enhances the inhibitory effects of dexamethasone on osteoblast autophagy/mitophagy, thus displaying protective effects on osteoblast differentiation and mineralization (113). The effects of VK on bone may be also dependent on its binding to steroid and xenobiotic receptor (114) with its subsequent activation (115,116). Finally, the osteogenic effect of VK2 in a culture of bone marrow stromal cells has also been shown to be mediated by the nhibition of miR-133a expression (117).
Several studies have demonstrated that VK is capable of inducing osteoblast formation, while inhibiting osteoclast differentiation and bone-resorbing activity. Specifically, in a culture of bone marrow cells, MK-4 was found to significantly inhibit adipogenic and osteoclastogenic differentiation, while promoting osteoblast differentiation (118). It has been demonstrated that, in comparison to VK1 and VK3, MK7 and particularly MK4, are more effective in the promotion of osteoblast activity and the inhibition of osteoclastic bone resorption (119), although another study demonstrated a higher anti-osteoclast activity for MK7 (120). Both phylloquinone (VK1) and menaquinone-4 have been shown to promote osteogenesis, as evidenced by increased OCN and OPG levels in parallel with decreased circulating RANKL levels in a model of high-fat-induced obesity (121). Both MK-4 and VK1 significantly reduce dihydroxyvitamin D3-induced osteoclastogenesis mainly by reducing RANKL expression (observed at 1.0 μM), whereas the upregulation of OPG expression has been observed at higher exposure levels (10 μM) (122). MK-4 also reduced 1,25(OH)2D3-induced formation of multinucleated osteoclasts (123). It has been also demonstrated that MK-7 ameliorated parathyroid hormone (PTH) and prostaglandin E2 (PGE2)-induced bone resorption by osteoclasts (124,125).
The inhibition of RANKL-induced osteoclastogenesis by menaquinone 4 and 7 has been found to be dose-dependent (126). The MK-4-induced inhibition of RANKL signaling has been shown to result in the subsequent reduction of nuclear factor of activated T-cells 1 (NFATc1), osteoclast-associated receptor and cathepsin K mRNA expression (127). In addition to the downregulation of RANKL signaling, menaquinone 4 or VK1 have been shown to inhibit macrophage colony stimulating factor (M-CSF)-induced osteoclast differentiation in a dose-dependent manner (128).
The biological effects of VK on Ca2+ and skeletal homeostasis are dependent on its role as a cofactor of γ-glutamyl carboxylase, which promotes the conversion of specific glutamate (Glu) residues to gamma-carboxyglutamic acid (Gla) residues (129). In addition to hemostatic factors, VK has been shown to be involved in the post-translational carboxylation of OCN and matrix Gla protein, which may also have a significant impact on osteogenesis and systemic metabolism (130).
Although OCN is an abundant protein of bone extracellular matrix, its functioning has been shown to not be responsible for the regulation of bone development; rather, it plays a crucial role in the improvement of bone strength by adjustment of biological apatite parallel to collagen fibrils (131), as well as carbohydrate metabolism regulation in its uncarboxylated form (132). At the same time, the VK-induced decrease in the level of undercarboxylated OCN did not induce insulin resistance, and the change in percentage of undercarboxylated OCN is directly associated with the improvement of glucose sensitivity (133). Moreover, VK treatment has been shown to increase OCN gene expression, resulting in an improvement of β-cell proliferation and adiponectin production, thus exerting a hypoglycemic effect (134). In agreement with this, insulin signaling in osteoblasts has been shown to result in reduced OCN γ-carboxylation, thus increasing its hypoglycemic effect (135).
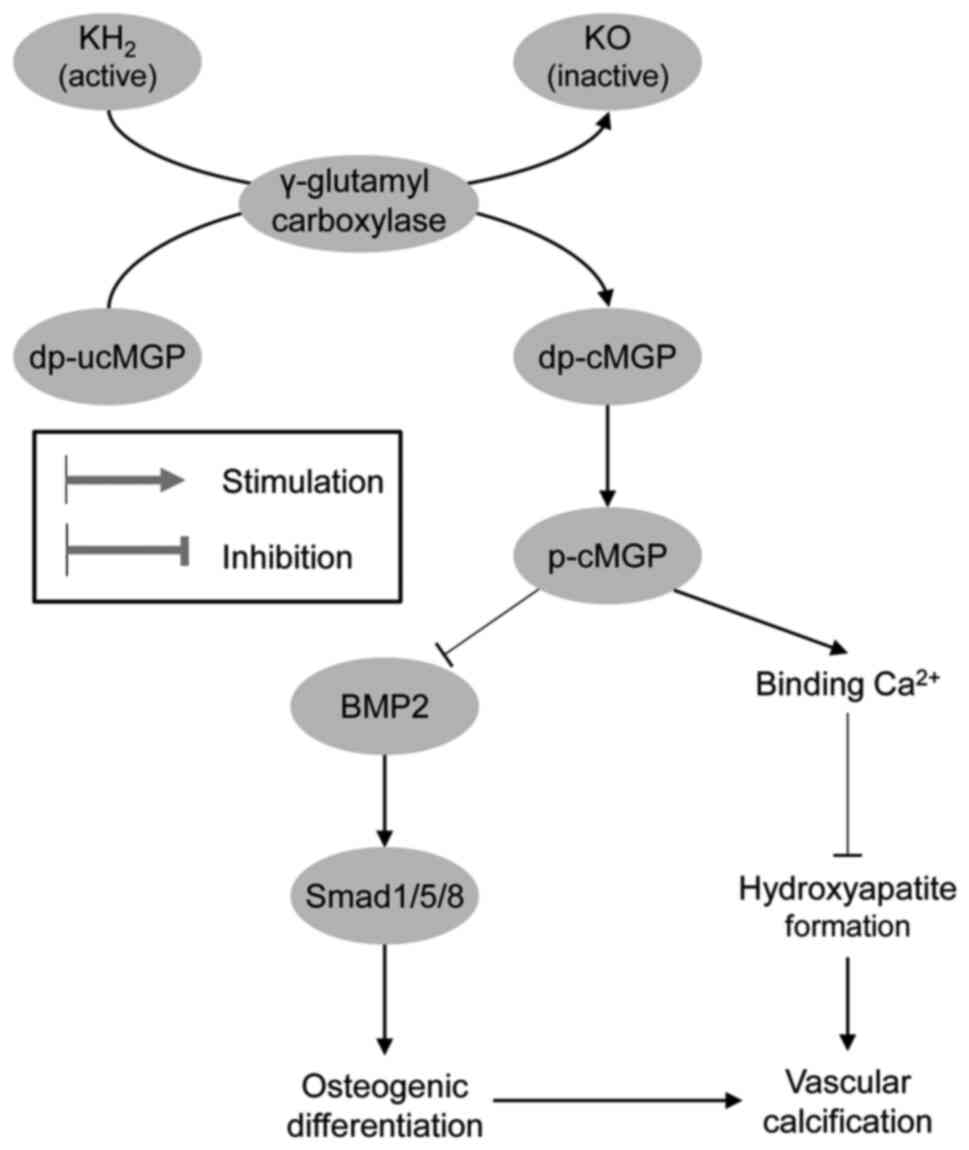
Analogous to OCN, matrix Gla protein has been shown to be activated by VK-dependent carboxylation and phosphorylation, exerting a significant inhibitory effect on vascular calcification (136), and the level of non-phosphorylated uncarboxylated matrix Gla protein (MGP) may be considered as a biomarker of VK status (137). Therefore, VK deficiency is associated with a reduced Ca2+ deposition in bones and an increase in vascular calcification (138). Menaquinone-4 insufficiency is also considered as a predictor of aortic calcification (139). Moreover, the administration of VK antagonists has been shown to significantly increase dephosphorylated and uncarboxylated matrix Gla protein levels, which directly correlated with vascular calcification (140). Correspondingly, MK-4 has been shown to inhibit the osteogenic transdifferentiation of vascular smooth muscle cells and preserve a contractile phenotype in spontaneously hypertensive rats (141). Active MGP has been shown to inhibit osteogenic stimuli by binding to BMP-2 and reducing mineralization, whereas inactive MGP is unable to inhibit the osteogenic differentiation of vascular smooth muscle cells (142). The protective effects of VK on vascular calcification may also be mediated by its influence on Gla-rich protein and growth arrest-specific gene 6 protein expression (143) (Fig. 2).
Taken together, the existing clinical and laboratory data demonstrate that VK supplementation effectively improves BMD and reduces the risk of fractures in post-menopausal women. In addition it enhances the anti-osteoporotic effects of vitamin D and Ca2+ supplementation. The osteogenic effect of VK2 as MK4 and MK7 has been shown to be attributed to the activation of BMP-2 and Wnt/β-catenin signaling, the promotion of autophagy and the amelioration of the inhibitory effects of pro-inflammatory cytokines on SMAD signaling. VK2 also exerts protective effects in osteoblast culture by preventing apoptosis and ferroptosis. In addition to the osteogenic effect, it also inhibits bone resorption by inhibiting osteoclastogenesis and activation by downregulating RANKL signaling with a shift to OPG activation. VK has also been shown to prevent vascular calcification by activating MGP, therefore directing Ca2+ from the vascular wall to its deposition in bones. Therefore, VK may be considered protective, not only against osteoporosis, but also vascular calcification and associated cardiovascular disease.
4. Vitamin A
Vitamin A (VA) has been shown to be involved in the regulation of bone physiology through retinoic acid receptor (RAR) signaling (144), although the existing data on the association between VA intake or accumulation and BMD remain controversial due to the distinct effects of different doses (145).
In osteoporotic untreated post-menopausal women, serum retinol has been shown to be directly associated with the risk of low bone mass at the lumbar spine and femoral neck (146). The association between high retinol levels and osteoporosis is aggravated in subjects with vitamin D deficiency (147). Concomitantly, a U-shaped association between the plasma retinol concentration and BMD has been observed, with both deficiency and excess being associated with a lower BMD in children (148). These findings generally corroborate an observation of improved bone formation following the reduction of VA in children with high vitamin stores (149). The results of a meta-analysis demonstrated that the intake of VA and retinol, but not β-carotene, was associated with the risk of hip fractures, although serum retinol levels were characterized by a U-shaped association with the risk of hip fractures (14).
It is noteworthy that, in non-supplemented subjects with a low dietary VA intake, plasma levels of retinol and carotenoids were inversely associated with osteoporosis (150,151). Moreover, the maternal plasma retinol level is directly associated with adult offspring spine BMD and trabecular bone score following adjustment for multiple covariates, including vitamin D levels (152).
Recent findings have demonstrated that the association between the VA status and the risk of fractures as the outcome of osteoporosis is not significant. Specifically, no association between high serum retinol levels and an increased risk of fractures was observed in the elderly involved in Norwegian Epidemiologic Osteoporosis Studies (153). The results of a meta-analysis demonstrated that an increased VA intake was not associated with a risk of fractures (154). No association between VA intake with BMD or the risk of fractures was observed in pre-menopausal women with a lower baseline VA intake level (155). It is proposed that the association between a high VA intake and an increased risk of fractures may be mediated by an increased body mass index (156).
The VA status is also tightly associated with the dietary intake of provitamin A carotenoids that may also have a significant effect on bone health (157). A high dietary total carotenoid intake (Q1 vs. Q4) has been shown to be associated with a 39% lower risk of hip fractures in males, whereas no association was observed in females (158). Another study also demonstrated reduced odds of hip fractures with a high dietary intake of both total carotenoid and individual β-carotene, β-cryptoxanthin, and lutein/zeaxanthin intake, while the intake of α-carotene and lycopene was not associated with the risk of hip fractures (159). Correspondingly, a meta-analysis of epidemiological studies involving 140,265 subjects demonstrated that a high total carotenoid, as well as β-carotene intake was associated with a 28% lower risk of hip fractures, while no association between circulating carotenoid and fracture risk was shown (160). Correspondingly, the meta-analysis by Gao and Zhao (161) demonstrated a significant association between the dietary β-carotene intake and a reduction in the risk of developing osteoporosis.
Serum β-cryptoxanthin, lycopene and α-carotene levels have been found to be associated with a concentration-dependent increase in BMD in Chinese adults, with a more pronounced association in females (162). Correspondingly, in the European Prospective Investigation into Cancer and Nutrition (EPIC)-Norfolk cohort, plasma α and β-carotene levels were inversely associated with a risk of hip fractures in males (163).
Generally, the results of laboratory in vivo studies correspond to the epidemiological observations, consistent with adverse effects of both VA deficiency and overload on bone physiology. Specifically, VA deficiency has also been shown to be associated with impaired bone regeneration in mice due to the downregulation of BMP-2 (164). At the same time, excessive all-trans retinoic acid exposure (40 mg/kg/day) has been shown to result in reduced longitudinal bone growth in young rats through the alteration of growth hormone (GH)/insulin-like growth factor 1 (IGF)1/IGFBP3 signaling (165). The intraperitoneal administration of 10 mg/kg/day all-trans retinoic acid (ATRA) has been shown to significantly promote testosterone deficiency-induced bone loss (166). In addition, excessive VA intake (60 retinol activity equivalents μg/g chow) inhibits the loading-induced increase in trabecular and cortical bone mass along with the downregulation of osteoblast-specific genes (167).
These findings demonstrate that VA, as well as provitamin A carotenoid intake is associated with bone health, although this association appears to be non-linear. Despite being rather contradictory, the existing epidemiological data demonstrate that both the deficiency and excess of VA may promote the risk of bone loss. The laboratory findings also demonstrate that VA metabolites may possess distinct effect on mechanisms associated with bone formation.
A number of studies have demonstrated that the VA metabolite, ATRA, significantly increases osteoblastogenesis and osteogenesis. Specifically, in rat bone marrow-derived mesenchymal stem cells, exposure to 10 μM ATRA was shown to promote osteogenic differentiation through the pregulation of osteogenic (ALP, BMP-2, OSX, Runx2, OPN and OCN) and angiogenic [VEGF, hypoxia-inducible factor-1, Fms related receptor tyrosine kinase 3, angiotensin (ANG)-2 and ANG-4] gene mRNA expression, while in an in vivo model, ATRA injection (10 μM, 100 μl) into the distraction gap significantly improved bone consolidation and its properties (168). The administration of 10 μm ATRA has been shown to promote the Wnt3a-induced osteogenic differentiation of mesenchymal stem cells through the activation of PI3K/AKT/GSK3β pathway (169). Both ATRA and 9-cis retinoic acid at the concentrations of 5-20 μM have been shown to promote the in vitro osteogenic differentiation induced by BMP-9 in mesenchymal progenitor cells (170). The osteogenic differentiation of retinoic acid-treated murine induced pluripotent stem cells has also shown to be at least partially mediated by Notch signaling (171).
It has also been demonstrated that ATRA promotes a shift from adipogenic to osteogenic differentiation. Specifically, 1 μM retinoic acid-induced osteoblastogenesis and the inhibition of adipogenesis in mesenchymal stem cells have been shown to be dependent on Smad3 upregulation with the subsequent replacement of C/EBPβ from the Runx2 promoter (172), in agreement with earlier observation of the C/EBPβ-induced inhibition of the 1 μM ATRA-induced osteoblastogenesis in C3H10T1/2 cells (173). It has been also demonstrated that 1 μM RA promotes BMP-2-induced osteogenesis, while inhibiting BMP-2-induced adipogenesis with the suppression of adipogenic transcription factors, PPARγ and C/EBPs, thus being a key factor regulating the commitment of mesenchymal stem cells into osteoblasts and adipocytes (174). Moreover, 2.5 μM retinoic acid has been shown to enhance the osteogenic effect of BMP-2 in human adipose-derived stem cells (175). ATRA (1 μM) has been shown to promote the BMP-9-induced osteogenic transdifferentiation of 3T3-L1 preadipocytes through the activation of BMP/Smad and Wnt/β-catenin signaling (176). It has been shown that 1 μM retinoic acid promotes the BMP-2-induced osteoblastic differentiation of preadipocytes through BMP-RIA and BMP-RIB signaling (177). Correspondingly, retinoic acid has been shown to induce the osteogenic differentiation of stromal cells from both visceral and subcutaneous adipose tissue depots (178). Moreover, as previously demonstrated, in mouse embryonic fibroblasts, 0.4 μM ATRA promotes a shift to osteogenesis from rosiglitazone-induced adipogenic differentiation through the upregulation of Smad1/5/8 phosphorylation and Smad6 expression, resulting in the activation of BMP/Smad pathway (179). At the same time, it has also been observed that pharmacological concentrations of 1-10 μM ATRA inhibit osteoblast proliferation, while increasing its differentiation (180). However, it is notable that premature osteoblast-to-preosteocyte transitioning induced by 1 μM ATRA may result in altered bone formation (181).
The osteogenic effects of retinoic acid have also been shown to be associated with RAR activation. Specifically, retinoic acid (1 μM) has been shown to promote the osteogenesis of human induced pluripotent stem cells, a process dependent on RARa and RARb, but not on RARy signaling (182). Correspondingly, it has been demonstrated that treatment with 20 μM ATRA increases the spreading of pre-osteoblasts on bio-inert glass surfaces and its osteogenic activity through RARα and RARβ signaling (183). At the same time, Karakida et al (184) demonstrated that ATRA promoted the osteogenic transdifferentiation of myoblastic C2C12 cells by BMP-2 in a concentration-dependent manner at a range of 8-2,000 nM, while this effect was ameliorated by RARγ, but not RARα or RARβ inhibition.
In contrast to previously discussed observations, several laboratory studies have demonstrated the inhibitory effects of ATRA on osteogenesis. In particular, 1 μM ATRA has been shown to inhibit the osteoblastogenesis of the MC3T3-E1 pre-osteoblast cell line (185). Furthermore, 0.5 μM retinoic acid has been found to significantly inhibit MC-3T3 cell mineralization through the increased expression of the Wnt inhibitors, DKK-1 and DKK-2 (186), resulting in the downregulation of Wnt signaling (187). It has also been shown that 1 μM ATRA inhibits osteoblastogenesis induced by BMP-2, BMP-7 or heterodimer BMP-2/7, with the latter being a more potent activator as compared to homodimers (188). In addition, the inhibition of the osteogenic differentiation of mouse embryonic palate mesenchymal cells by 1 μM ATRA has been shown to be associated with the inhibition of BMPR-IB and Smad5 mRNA expression (189,190).
The inhibitory effect of 1 μM ATRA on BMP-2-induced osteoblastogenesis has also been shown to be dependent on RARα signaling (191). These findings corroborate those of an earlier study by Nuka et al (192), establishing a key role for RARα and RARβ signaling activation in the inhibition of SV HFO osteoblast cell line mineralization in response to 0.1 μM ATRA treatment. In addition, the exposure of C2C12 myotubes to 10-100 nM ATRA has been shown to induce the RAR-dependent production of sclerostin, a protein possessing inhibitory effect on the Wnt/β-catenin pathway (193).
The upregulation of IL-1β expression through NF-κB activation and inflammasome formation may also contribute to the anti-osteogenic effects of 1-10 μM ATRA (194). These findings correspond to the observation that IL-6 overproduction by human osteoblasts occurs even upon exposure to physiological (10 nM) and higher (up to 10 μM) ATRA concentrations (195).
Taken together, the existing studies demonstrate that ATRA at various concentrations can both promote and inhibit osteogenesis, with ATRA at nanomolar concentrations inhibiting, and at micromolar concentrations activating osteoblasts (15). However, it has been suggested that the inhibitory effects on osteogenesis occur at higher exposure levels (196). Therefore, further studies are required to clarify the mode-of-action of ATRA in osteogenesis and to provide a solid rationale for adequate VA intake in vivo.
In addition to its impact on osteoblast physiology, VA is also involved in the regulation of bone resorption through the modulation of osteoclast activity. Specifically, retinoic acid has been shown to increase the proliferation of osteoclast progenitors, while inhibiting osteoclast differentiation by suppressing RANK/RANKL signaling (197) with the downregulation of NFATc1 (198), NFAT2, c-Fos and MafB (199). These effects were shown to de dependent on RAR activation, with RARα signaling being the most effective (198). In another study, 1 μM ATRA significantly inhibited BMP2/7-induced osteoclastogenesis through the downregulation of RANK and Nfatc1 expression (200). It is also notable that not only ATRA, but also 9-cis retinoic acid at a concentration of 1 nM, significantly inhibited calcitriol-induced bone resorption (201).
In another study, retinoic acid was shown to increase periosteal bone resorption by increasing osteoclast differentiation through the RARα-dependent increase in the RANKL/OPG ratio (202). The stimulation of osteoclast activity by retinoic acid was associated with an increased expression of cathepsin K (203). In addition to osteoclast activation, retinoic acid-induced bone damage has been shown to be associated with osteocytic osteolysis, as evidenced by a reduction in mature osteoblast/osteocyte-specific genes (Bglap2 and Ibsp), without any significant alteration of Runx2 mRNA expression (204). Therefore, these findings demonstrate that analogous to osteoblasts, the effects of ATRA on osteoclast proliferation, differentiation and functioning are likely bimodal.
In addition to VA and its metabolites, carotenoids have also been shown to promote osteoblast proliferation and differentiation (157). β-cryptoxanthin has been shown to exert osteoprotective effects by promoting osteoblastogenesis and inhibiting osteoclastic bone resorption (205). It has been shown that β-cryptoxanthin significantly increases the osteoblastic differentiation of MC3T3-E1 cells with a significant increase in Runx2 mRNA expression (206). β-cryptoxanthin-induced osteoblast differentiation has been shown to be mediated by the activation of TGF-β1-induced Smad activation, being independent of BMP2-Smad signaling (207). Both β-cryptoxanthin and p-hydroxycinnamic acid have been shown to inhibit basal NF-κB activity in MC3T3 pre-osteoblasts, whereas only p-hydroxycinnamic acid significantly suppresses TNF-induced NF-κB activity (208). p-Hydroxycinnamic acid ameliorates inhibitory effects of TNF-α-induced NF-κB signaling on Smad-mediated TGF-β and BMP-2 signaling (209).
As previously demonstrated, crocin at a concentration of 40 μM promotes M2 macrophage polarization and increases the osteogenic differentiation of bone mesenchymal stem cells through the inhibition of p38 and c-Jun N-terminal kinase signaling (210). Similar to crocin, crocetin also induces the osteogenic differentiation of mesenchymal stem cells (211).
It has been demonstrated that 10 μM lycopene significantly promotes the osteogenesis of Saos-2 cells through the activation of WNT/β-catenin and ERK1/2 pathways, while inhibiting RANKL mRNA expression (212). In ovariectomized rats, the daily intake of 10 mg/kg lycopene was found to significantly reduce bone loss associated with the upregulation of the osteogenic genes, Sp7, Runx2, Bsp and Bglap (213), whereas the number of osteoclasts was reduced (214). In addition, lycopene derivatives, but not the intact molecule, significantly inhibit NF-κB activation in osteoblastic cells (215) through the inhibition of IκB kinase (IKK) activity and transcriptional activity of p65 through direct interaction with critical thiols (216).
Osteoclastogenesis is also considered as the target for carotenoid effects on bone health. Specifically, it has been shown that 0.1-1 μM β-cryptoxanthin significantly inhibits PTH, PGE2-, 1,25-dihydroxyvitamin D3-, lipopolysaccharide-, or TNFα-induced osteoclastogenesis through the downregulation of RANKL and M-CSF signaling (217). The downregulation of RANKL-mediated osteoclastogenesis by 5 μM β-cryptoxanthin has been shown to be dependent on the suppression of the inhibitor of NF-κB kinase β (IKK β) activity, suppressing NF-κB activation (218). The anti-osteoclastogenic effect of β-cryptoxanthin has also been shown to b associated with the promotion of caspase-3-mediated apoptosis (219). Correspondingly, in another study, dietary β-cryptoxanthin intake prevented osteoclastic bone resorption in ovariectomized mice through interference with the RANKL pathway (220). A similar protective effect was observed against inflammatory bone resorption in a mouse model of periodontitis (221).
In addition to β-cryptoxanthin, other carotenoids have also been shown to modulate osteoclast functioning. It has been shown that β-carotene (0.2 μM) significantly ameliorates RANKL-induced NFATc1, c-Fos and CTSK expression, as well as osteoclastic bone resorption through the inhibition of IκB phosphorylation, whereas ERK, JNK and p38 expression remain unaltered (222). Similarly, 50 μg/ml astaxanthin has been shown to inhibit Nε-carboxymethyllysine-induced osteoclastogenesis through the inhibition of NF-κB activation and subsequent downregulation of NFATc1 expression (223). In bone marrow cells, treatment with 30 μM lutein was shown to inhibit IL-1-induced RANKL-mediated osteoclastogenesis, while promoting osteogenesis in an osteoblast culture by increasing BMP-2 and decreasing sclerostin mRNA expression (224).
In another study, 2.5 μM fucoxanthin was shown to suppress the osteoclastic differentiation of RAW264.7 cells by inducing apoptosis via caspase-3 activation without a decrease in MC3T3-E1 osteoblastic cell viability (225).
The role of VA in osteoporosis thus appears unclear. While multiple studies have demonstrated that the excessive dietary intake of VA and its accumulation in the organism is associated with a reduced BMD and osteoporosis, observations in children and VA-depleted subjects have demonstrated that its deficiency may also exert adverse effects on bone physiology. In vivo studies have demonstrated adverse effects of the excessive VA intake on bone health, while in vitro studies have been inconsistent with the epidemiological findings, indicating positive effects of VA at micromolar doses on osteogenesis, whereas lower nanomolar doses exert inhibitory effects. Specifically, it has been demonstrated that the effects of VA on bone formation are mediated by the modulation of BMP-2 and Wnt/β-catenin-mediated osteogenesis. Other targets for the effects of VA in bones include the GH/IGF-1 axis, RAR and Notch signaling, as well as the modulation of NF-κB-mediated inflammation. Similarly, the effects of VA on osteoclast formation and activity vary significantly from inhibition to stimulation, due to the differential modulation of RANKL signaling. These findings demonstrate that VA intake needs to be carefully monitored in subjects who are at risk in order to avoid the hazardous effects of both hypo- and hypervitaminosis on bone health.
5. Vitamin C
VC plays a crucial role in bone physiology, exerting beneficial effects on trabecular bone formation, thereby being considered as a potential treatment modality for osteoporosis (226).
VC supplementation in post-menopausal women has been shown to be associated with an almost 3% increase in BMD in multiple sites, while the highest BMD was observed in women using VC, estrogen and Ca2+ (227). A higher VC intake has also been shown to be associated with a 33% lower risk of developing osteoporosis (228).
These findings corroborate the results of a more recent meta-analysis, demonstrating that a higher frequency of dietary VC intake was associated with a 34% lower prevalence of hip fractures (229). It has been shown that a 50 mg/day increase in VC intake is associated with a 5% decrease in the risk of hip fractures (230). The results of a 17-year follow-up demonstrated that VC supplementation resulted in lower rates of hip fractures (231). The results from the KNHANES IV (2009) study demonstrated a significant association between dietary VC intake and BMD only in vitamin D-deficient elderly individuals (232).
Epidemiological findings have also demonstrated a positive association between VC intake, circulating ascorbate levels and BMD (233). In turn, a suboptimal plasma VC level is considered as a significant predictor of a low BMD in males (234). Despite the lack of significant effects of dietary VC intake, a normal plasma VC concentration has been shown to be associated with a higher BMD in post-menopausal Puerto Rican women without estrogen therapy (235).
Laboratory in vivo studies have demonstrated that VC deficiency results in impaired osteogenesis in osteogenic disorder Shionogi rats (236) associated with abnormal collagen formation in osteoblasts (237). In turn, VC supplementation improves BMD in vitamin-C-deficient Shionogi rats (238). Moreover, it has been shown that VC supplementation significantly increases bone quality in a model of ovariectomized osteoporotic rats through the stimulation of bone formation and the inhibition of its resorption (239,240). Correspondingly, VC deficiency has been shown to be associated with a risk of spontaneous bone fractures due to the inhibition of osteoblast differentiation and increased PPAR-γ-dependent adipogenic transition (241).
The promotion of bone formation by VC appears to be mediated by the modulatory effects of VC on osteoblast differentiation and activity. Specifically, VC significantly increases osteoblast differentiation in a suspension of mononuclear cells (242), in association with increased type I collagen production and extracellular matrix mineralization (243). VC promotes both the proliferation and osteoblastic differentiation of MC3T3-E1 type pre-osteoblast cells (244). VC-induced osteogenic differentiation has been shown to affect the expression of >15,000 genes that are related to cell growth, morphogenesis, metabolism, cell communication and cell death in addition to osteoblast-specific genes (245). It is also notable that VC increases the phosphate-induced osteoblastic transformation of vascular smooth muscle cells by promoting intracellular Ca2+ deposition (246), thus increasing the risk of vascular calcification.
It has been demonstrated that low doses of VC significantly promote osteoblast differentiation through the upregulation of RUNX2 and SPP1 gene expression in MG-63 cells, whereas high doses of VC induce apoptotic cell death (247). The osteogenic effects of VC have been shown to involve the activation of BMP-2 and Wnt/β-Catenin/ATF4 signaling (248). VC also reduces the number of senescent cells by increasing the proportion of cells with proliferative capacity (249). The activation of casein kinase 2 involved in the regulation of bone formation may also be involved in the osteogenic effects of ascorbate osteoblast-like (MG63) cells (250). Osteoblastogenesis has been shown to be mediated by VC-induced OSX expression through the activation of PHD and subsequent proteasomal degradation of OSX gene transcriptional repressors (251). The activation of osteogenesis by VC has been shown to involve its direct interaction with PHD2 (252).
The osteogenic effects of VC are also dependent on microtubule plus-end-binding protein 1 expression with the subsequent activation of β-catenin expression (253). Of note, VC has been found to exert osteogenic effects at the beginning of bone formation, although at later periods (9 days) it may exert adverse effects (254). It has also been demonstrated that VC induces a shift to osteogenesis and myogenesis from adipogenesis in mesoderm-derived stem cells, at least partially through the p38MAPK/CREB pathway (255). A similar effect mediated by the depletion of the cAMP pool was observed in the OP9 mesenchymal cell line (256).
Ascorbic acid 2-phosphate, a long-acting VC derivative, has been shown to promote osteoblast differentiation, in contrast to the inhibitory effects of VC in a culture of MG-63 cells (257). Correspondingly, ascorbate-2-phosphate has been shown to increase the expression of MMP-2 and MMP-13, whereas the ascorbic acid-induced expression of membrane type1-MMP has been observed only at the early stages of differentiation (258).
Epigenetic mechanisms may also underlie the modulatory effects of VC on osteogenesis. Specifically, VC-induced osteogenic differentiation is tightly associated with H3K9me3 and H3K27me3 demethylation and 5-hydroxy-methyl-cytosine levels (259).
VC also significantly modulates bone resorption through the regulation of osteoclastogenesis and osteoclast activity. Specifically, VC has been shown to reduce RANKL-induced osteoclastogenesis in vitro (260) through the redox-dependent inhibition of NF-κB signaling (261). Correspondingly, it has been shown that VC significantly inhibits the RANKL and NF-κB expression-associated increase in osteoclast differentiation in rats fed a high-cholesterol diet (262). In turn, VC deficiency has been shown to increase bone resorption and osteoclastogenesis via the ERK-dependent upregulation of RANK, c-jun and c-fos expression (263).
VC has also been shown to be essential for appropriate osteoclastogenesis by increasing RANKL mRNA expres- sion (264,265). VC has been shown to be essential for osteoclast differentiation by increasing preosteoclast maturation and improvement in cell viability (266). In addition, VC promotes glycerophosphate-induced osteoclast differentiation by increasing RANKL-induced NFATc1, c-fos and COX-1 expression (267). It is notable that VC promotes osteoclast formation only at earlier stage of osteoclastogenesis, whereas at the late stage, it increases osteoclast death (268).
The existing epidemiological studies demonstrate that a higher VC intake is associated with a lower risk of osteoporosis and fractures, that may be mediated by the osteogenic effects of VC via the activation of BMP-2 and Wnt/β-catenin signaling. Epigenetic effects may also underlie the positive effects of VC on osteoblast differentiation. Despite the observation of inhibitory effects of VC on RANKL and NF-κB-associated osteoclastogenesis, VC has been shown to be essential for appropriate osteoclast formation.
6. Group B vitamins
Group B vitamins represent a group of structurally heterogeneous water-soluble molecules performing cofactor roles for a plethora of enzymes involved in human energy metabolism (269), including bone physiology and protection against osteoporosis (270). However, certain contradictions regarding the protective effects of group B vitamins exist (271).
An analysis of the Framingham Offspring Osteoporosis Study (1996-2001) data demonstrated that males and females with plasma vitamin B12 levels <148 pM are characterized by decreased hip and spine BMD, respectively (271). Correspondingly, an insufficient B12 intake has been considered as a risk factor for osteoporosis in vegans (272). A meta-analysis study by Zhang et al (273) demonstrated that both homocysteine (Hcy) and B12 levels were found to be elevated in post-menopausal osteoporotic women. In addition, in Moroccan women, plasma B12 levels, as well as the circulating Hcy concentration, were inversely associated with hip BMD (274).
A low (<19.2 μg/l) serum B6 level has been found to be associated with a 61% higher risk of developing osteoporosis, while circulating vitamin levels are inversely associated with bone turnover biomarkers (275). A higher dietary B6 intake has been shown to be associated with a 22% lower risk of hip fracture sin the Singapore Chinese Health Study (276). Concomitantly, Li et al (277) demonstrated that increased circulating vitamin B6 levels were associated with a higher risk of ankle fractures in osteoporotic patients.
Folic acid levels have been found to be significantly associated with BMD following adjustment for Hcy concentrations and other confounders (278). It is considered that supplementation with folic acid at a dose of 0.5-5 mg may be useful for the improvement of BMD in patients with low folic acid levels or hyperhomocysteinemia (279).
Several studies have investigated combined group B vitamin supplementation. It was previously demonstrated shown that the 2-year group B vitamin (folic acid, B6, B12, B2) supplementation in subjects with a low B12 status prevented a significant reduction in BMD at the femoral neck and hip (280). In turn, circulating plasma folic acid and B12 levels have been shown to be directly associated with BMD and bone strength in post-menopausal Chinese-Singaporean women, respectively (281). Low serum folic acid and B6, but not B12 levels, have been shown to be associated with lower bone trabecular number and thickness in subjects who underwent hip arthroplasty (282).
The results of a recent meta-analysis demonstrated that severe folic acid, but not B6 or B12 deficiency, was associated with an increased risk of fractures in the elderly (283). Other studies have failed to reveal an association between serum B12 or folic acid levels with BMD (284,285) or the vertebral fracture rate (286), although a reduction in the Hcy concentration has been observed (287).
Hcy affects the efficacy of group B vitamin supplementation. Specifically, although long-term vitamin B12 and folic acid supplementation do not reduce the risk of osteoporotic fractures (288) or improve BMD (289), in the general cohort of the B-PROOF trial, vitamin supplementation reduced the number of fractures in subjects with hyperhomocysteinemia (288). Nonetheless, no effect of folic acid, vitamin B6 and B12 supplementation on fracture risk or bone turnover biomarkers in hyperhomocysteinemic subjects has been observed (290).
Genetic factors also significantly modulate the association between the group B vitamin status and bone health. Ahn et al (291) demonstrated that 3'-UTR polymorphisms of vitamin B-related genes, transcobalamin II, reduced folate carrier protein 1 and thiamine carrier 1, and particularly CD320 (transcobalamin II receptor), were associated with osteoporosis and osteoporotic spinal fractures in post-menopausal women. The association between vitamin B levels and BMD was also shown to be modified by genetic variants in the 1-carbon methylation pathway (292).
Laboratory studies have also demonstrated that group B vitamins have a significant impact on bone physiology and osteoporosis. Specifically, folic acid has been shown to significantly improve bone architecture and prevent bone loss through the reduction of osteoclast number via AMPK activation and the upregulation of Nrf2 signaling in high-fat diet-induced osteoporosis (293). It has been shown that folic acid supplementation significantly reduces the inhibitory effects of dexamethasone on vertebral osteogenesis through the upregulation of the TGF-β signaling pathway, with a subsequent increase in p-Smad2/3, Runx2 and Osterix expression in chick embryos (294). Similar beneficial effect of FA supplementation on bone density was observed in a model of cyclosporine-induced bone loss (295). Folic acid also ameliorated the adverse effect of homocysteine on osteoblast proliferation, differentiation and mineralization through inhibition of PERK-activated ERS (296). Folic acid potentiated osteoblastogenic effect of hydroxyapatite nanoparticles, as evidenced by a more profound RUNX2 expression in human mesenchymal stem cells (297). At the same time, high maternal folic acid intake was shown to reduce BMD in the offspring (298).
The essentiality of B12 for bone physiology was clearly demonstrated in B12-deficient conditions. Specifically, the reversal of B12 deficiency prevented the reduction of cortical and trabecular bone mass loss in a genetic model of B12 deficiency (Gif−/−) in mice (299). It has also been demonstrated that B12-deficiency-induced osteoporosis may be mediated by an altered taurine synthesis and impaired growth hormone/insulin-like growth factor 1 (GH/IGF1) pathway resulting in osteoblast dysfunction (300). In addition, B12 deficiency results in a significant increase in the osteoblastic secretion of Hcy and methylmalonic acid, that exert stimulatory effects on osteoclastogenesis (301). These findings corroborate earlier observations on the stimulatory effects of Hcy on osteoclast activation (302). B12 deficiency has also been shown to be associated with increased osteoclast bone resorption (303).
B6 vitamin deficiency has been shown to be associated with osteoblast dysfunction due to excessive cortisol production (304). At the same time, another study on vitamin B6 deficiency did not note an affect osteoblast mineralization (305).
Vitamin B5 has been shown to promote RANKL-induced osteoclastogenesis at low doses via the upregulation of the PI3K/Akt pathway in pre-osteoclasts, whereas higher vitamin doses decrease osteoclast differentiation, resulting in reduced bone resorption, in association with a decrease in ROS generation and the stimulation of the expression FOXO1/2 and Nrf2 (306), known as one of the key regulators of antioxidant response (307). Vitamin B1 has been shown to exert an inhibitory effect on RANKL-mediated osteoclast differentiation (308).
It has been shown that the reduction of folic acid, B6, and B12 levels significantly increases osteoclast bone resorption activity, as evidenced by the stimulation of tartrate-resistant acid phosphatase and cathepsin K activity (309).
Taken together, although B group vitamins have been shown to play a crucial role in bone physiology, as demonstrated in deficiency models, epidemiological data on the efficiency of vitamin supplementation are inconclusive. However, the beneficial effects of folic acid and B12 supplementation on bone quality have been reported to be critical in subjects with insufficient vitamin intake.
7. Conclusions
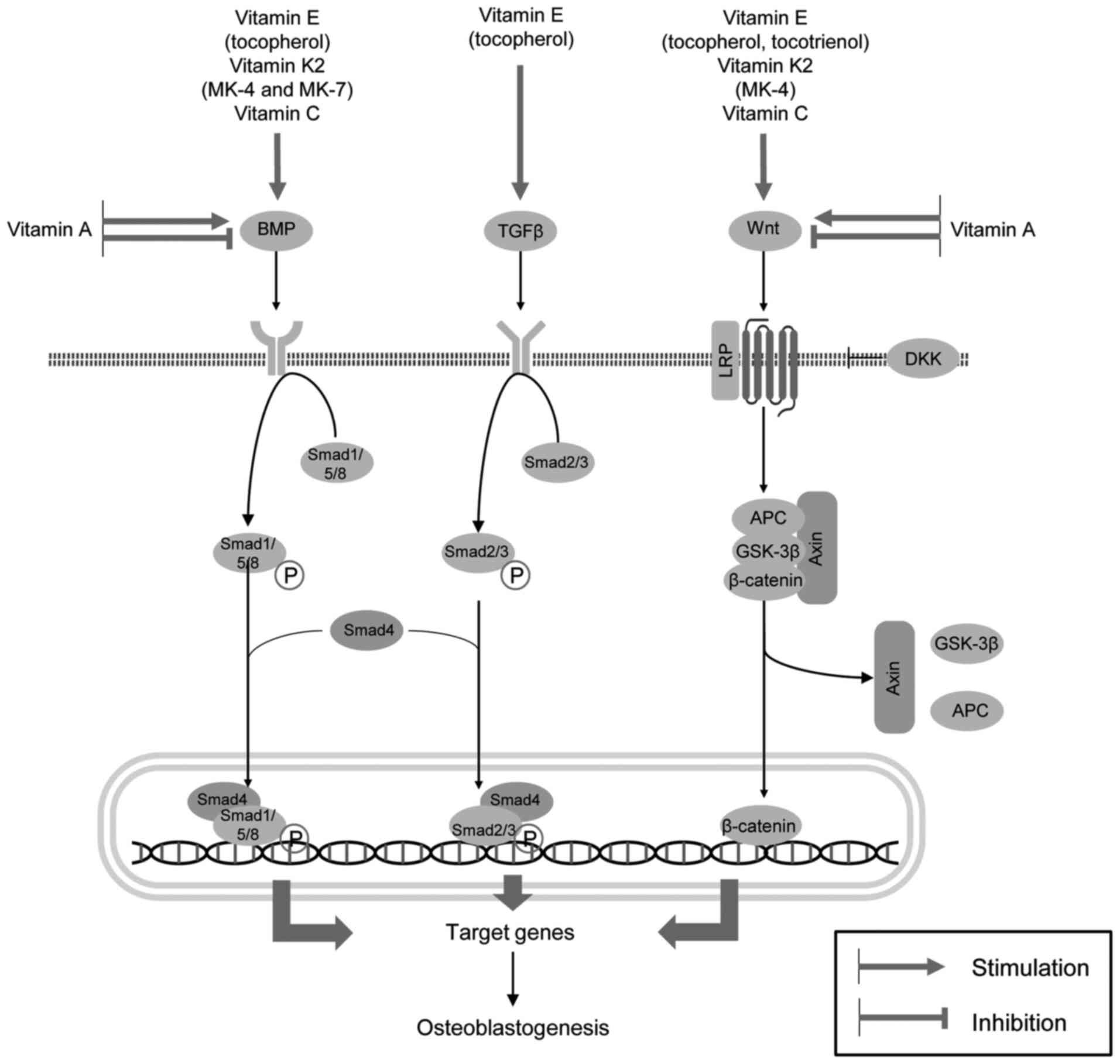
Existing data demonstrate that an adequate vitamin intake is essential for bone health, while vitamin deficiency is associated with an increased risk of developing osteoporosis. Specifically, the intake of vitamins E, K2 and C has been shown to be associated with increased BMD and a reduced risk of fractures. In turn, the excessive intake of vitamins can also have adverse effect on bone health and osteoporosis, as clearly demonstrated for VA. The observed effects of vitamins on the risk of osteoporosis have been shown to be mediated via mechanisms that regulate bone formation and resorption. VE (tocopherols and tocotrienols), VK2 (menaquinones 4 and 7) and VC have been shown to promote osteoblast development via the upregulation of BMP/Smad and Wnt/β-catenin signaling. Tocopherol also contributes to osteoblastogenesis through the stimulation of the TGFβ/Smad pathway. The VA metabolite (ATRA) appears to exert both inhibitory and stimulatory effects on BMP- and Wnt/β-catenin-mediated osteogenesis at nanomolar and micromolar concentrations, respectively (Fig. 3). However, these observations are contradictory to those of epidemiological studies demonstrating adverse effects of the excessive intake of VA on bone health. In addition to these mechanisms, the upregulation of PI3K/Akt/mTOR signaling, the inhibition of osteoblast apoptosis and ferroptosis, the improvement of redox homeostasis through SIRT1/Nrf2 and other pathways, as well as the inhibition of NF-κB signaling, may contribute to higher osteoblast viability and osteogenesis. In addition, the osteogenic effects of certain vitamins have been shown to be mediated by the modulation of the effects of hormones, including insulin, GH and PTH on bone physiology.
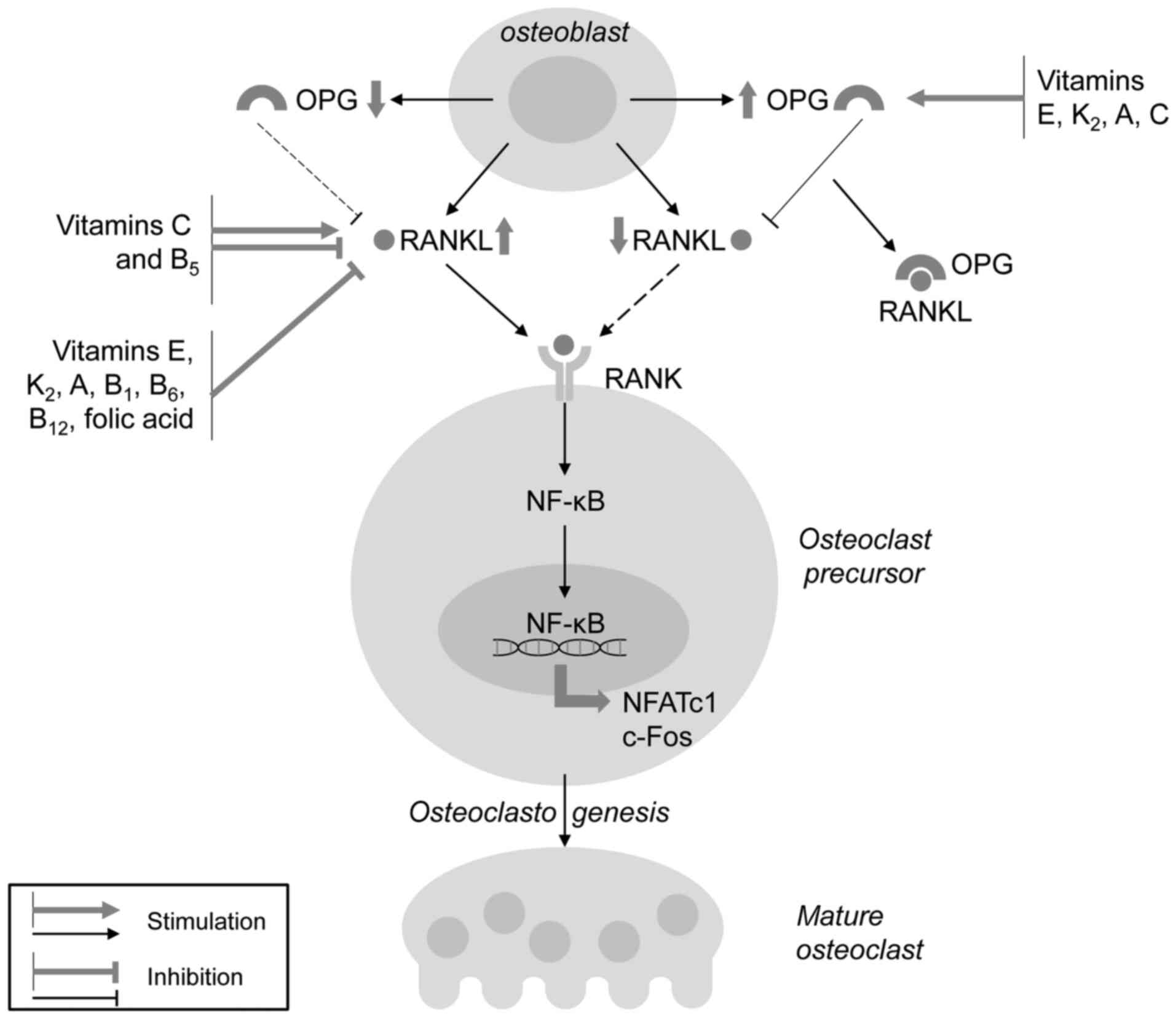
In addition to increased osteoblast proliferation and differentiation, vitamins are involved in the regulation of bone resorption through the modulation of osteoclast development and activity (Fig. 4), thus increasing the ratio between osteoblast and osteoclast activity. Both lipid-soluble vitamins E, K2, A, and water-soluble vitamins B1, B6, B12, C and folic acid significantly reduce RANKL production, thus reducing the RANKL/OPG ratio and RANKL/RANK signaling with a subsequent anti-osteoclastogenic effect. Notably, VC has been shown to be essential for osteoclast development, and its effect on osteoclastogenesis has been shown to be dependent on the dose and the stage of cell development, as also observed for vitamin B5. In addition, VK2 has been shown to prevent vascular calcification by activating MGP through its carboxylation, thereby directing Ca from the vascular wall to its deposition in bones.
In view of the epidemiological and laboratory findings, it appears that antioxidant group E vitamins, particularly in the form of α-tocopherol and VC should be considered as effective micronutrients for the reduction of osteoporosis and to lower the risk of adverse effects. Although VK2 exerts a positive effect on bone formation through the modulation of both osteoblast and osteoclast activity, as well as a reduction in vascular calcification and the promotion of calcium deposition in bones, its intake should be closely monitored in subjects at a higher risk of hypercoagulation due to its role in blood clotting. It appears that the therapeutic window of VA for improved bone health and quality is rather narrow, and both insufficient and excessive VA intake reduces bone quality; thus, it should be supplemented only in subjects with VA deficiency. The beneficial effects of folic acid and B12 supplementation on bone health are also likely to be inherent to subjects with insufficient vitamin intake, thus maintaining optimal B group vitamin dietary intake is also essential for prevention of osteoporosis. In view of the existing data, further studies are required to unravel the effects and mechanisms underlying the impact of various forms and doses of vitamins on bone physiology, as well as dependence of these effects on baseline vitamin status.
Availability of data and materials
Not applicable.
Authors' contributions
AVS, MA and AAT were involved in the conceptualization of the study. MA, AT, JBTR, AS, DAS, ACM, RL, TVK, WC, JSC, JCJC and CL were involved the investigation/search of the literature for the purposes of the review. MA, AT, JBTR, AS, DAS, ACM, RL, TVK, WC, JSC, JCJC, CL and AAT were involved in the data curation. AAT was involved in figure preparation. ACM, RL, TVK, WC, JSC, JCJC, CL and AAT were involved in the writing and preparation of the original draft. AVS, MA, AT, JBTR, AS and DAS were involved in the writing, reviewing and editing of the manuscript. AVS and MA supervised the study. All authors have read and agreed to the published version of the manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
DAS is the Editor-in-Chief for the journal, but had no personal involvement in the reviewing process, or any influence in terms of adjudicating on the final decision, for this article. The other authors declare that they have no competing interests.
Acknowledgments
Not applicable.
Funding
The present study was supported by the RUDN University Strategic Academic Leadership Program (award no. 202713-0-000 'Development of a scientifically based methodology for the ecological adaptation of foreign students to the new environmental conditions').