Triptonide protects retinal cells from oxidative damage via activation of Nrf2 signaling
- Authors:
- Published online on: July 8, 2024 https://doi.org/10.3892/ijmm.2024.5400
- Article Number: 76
-
Copyright: © Li et al. This is an open access article distributed under the terms of Creative Commons Attribution License.
Abstract
Introduction
Age-related macular degeneration (AMD) is a major cause of visual impairment worldwide. As the world population ages, the number of patients with AMD is expected to increase to 288 million by 2040, which will impose a heavy economic and social burden on modern society (1). Age, high blood pressure, atherosclerosis, diabetic retinopathy, smoking, alcohol abuse and genetics are factors that increase AMD risk. However, the oxidative stress, inflammation and choroidal vascular dysfunction induced by these high-risk factors are considered key pathological events in AMD pathogenesis (2). Retinal pigment epithelium (RPE) and Müller cells are susceptible to oxidative damage due to their high oxygen consumption during retinal metabolism (3-5). Oxidative stress is the main cause of RPE cell senescence, RPE-Bruch's membrane-choroid complex dysfunction, and ultimately drusen formation (6). Neovascularization, a typical sign of exudative AMD, may also be due to RPE-Bruch's membrane-choroid complex dysfunction and drusen formation, such that dry AMD can eventually progress to wet AMD (7). Most patients with early AMD have mild symptoms that progress relatively slowly; by the time severe visual impairment has occurred, most patients have progressed to having wet AMD. Uniform treatment protocols have been developed for wet AMD, and a wide range of treatment options is available with favorable results (8). Unfortunately, there is no unified treatment protocol for early AMD, highlighting the growing demand for personalized treatment of early AMD (9). Therefore, it is particularly important to identify new personalized drugs or protocols for early AMD intervention. Existing evidence indicates that early AMD treatment should focus on rescuing retinal cells from oxidative stress and inflammatory damage, especially RPE and Müller cells (10,11). Several groups, including the authors', have been focusing on the underlying mechanisms of reactive oxygen species (ROS)-induced retinal cell damage and developing drugs or therapeutic targets to inhibit or even reverse this process (12,13) to achieve precise and personalized treatment.
Research on personalized medicine in the field of ophthalmology has progressed recently. Chemical compounds originating from traditional Chinese herbal medicines are drawing increasing attention in personalized medicine because of their ability to influence certain pathways without systemic toxic effects (14). Additionally, compounds from traditional Chinese medicine can provide a broader range of options for individualized treatment. Tripterygium wilfordii is a traditional Chinese medicine widely used to treat various inflammatory and autoimmune diseases such as rheumatoid arthritis, Behçet's disease and multiple sclerosis (15). Triptolide (Tl) and triptonide (Tn) are active derivatives of Tripterygium wilfordii that have attracted marked attention in recent years (16). Tl has demonstrated anti-inflammatory effects in the treatment of immune diseases by activating the Kelch-like ECh-associated protein 1/nuclear factor erythrocyte 2-related factor 2/antioxidant response element (Keap1/Nrf2/ARE) signaling pathway. However, toxicity and side effects have limited its clinical application (15). Previous studies did not find toxic effects in mice treated with 20-fold the effective dose of Tn, and subsequent studies confirmed Tn as a potential agent with powerful anti-inflammatory and antioxidant effects (17,18). Although understanding of the role of Tn in certain diseases has increased recently, its potential activity against retinal oxidative damage remains unexplored.
The Keap1/Nrf2/ARE pathway is one of the most critical defense mechanisms against oxidative stresses (19). Oxidative damage caused by various factors can cause conformational changes in the Keap1-Cul3-E3 ubiquitin ligase and interfere with Nrf2 ubiquitination. Subsequently, Nrf2 translocates to the nucleus by heterodimerizing with the sMAF protein and binding to the ARE/electrophilic reaction element, inducing a series of cell protective genes, such as heme oxygenase-1 (HO1), NAD(P)H: quinone oxidoreductase 1 (NQO1), and gamma-glutamyl-cysteine ligase catalytic subunits (20). Thus, the activation of Nrf2 may lead to a remarkable antioxidant response and protect the cells from oxidative stress damage (21).
Using in vitro and in vivo experiments, the present study intended to verify whether a low Tn concentration could effectively protect retinal cells from oxidative damage, inhibit retinal inflammation, stabilize retinal structure, and promote functional recovery; it was also aimed to confirm Tn as a potential highly efficient Nrf2 signaling pathway activator, thus providing strong evidence for AMD treatment, and to some extent, address the clinical need for personalized treatments.
Materials and methods
Chemicals and reagents
Tn and N-Methyl-D-aspartic acid (NMDA) were obtained from Sigma-Aldrich; Merck KGaA. All antibodies used in the present study were acquired from Cell Signaling Technology, Inc., Asbcam and Thermo Fisher Scientific, Inc., as detailed in Table SI. All primers used in the present study were provided by Sangon Biotech Co., Ltd., and their sequences are listed in Table SII.
Cell cultures
Human umbilical vein endothelial cells (HUVECs, AC337632), human retinal capillary endothelial cells (HRCECs, AC340334) and Müller cells (MIO-M1, YS1695C) were obtained from Nanjing Saiyan Biotechnology Co., Ltd. and stably cultured in Dulbecco's Modified Eagle's Medium (DMEM) (Gibco; Thermo Fisher Scientific, Inc.) supplemented with 10% fetal bovine serum (Gibco; Thermo Fisher Scientific, Inc.) in a humidified incubator containing 5% CO2 at 37°C. ARPE-19 (cat. no. CL-0026) cells were purchased from ProCell Life Science & Technology Co., Ltd. and maintained in Ham's F12 nutrient medium (Gibco; Thermo Fisher Scientific, Inc.) supplemented with 10% fetal bovine serum in the same environment.
Model establishment and mice grouping
In total, 50 C57BL/6 male mice (6-8 weeks old; weight, 20-25 g) provided by Nanjing Junke Bioengineering Co., Ltd. were used for the in vivo studies. All animals received ad libitum access to food and water in a pathogen-free environment with a constant temperature (22±2°C) and (50±10%) humidity, and maintained in a 12/12-h light/dark cycle during the experiment. The animal experimental procedures used in the present study were approved (approval no. 2303048) by the Ethics Committee of Nanjing Medical University (Nanjing, China).
In the establishment of the animal model, the methods and criteria in the previous authoritative literature were strictly followed (22-25). The animals were randomly assigned to one of five groups: i) control; ii) light-induced retinal neurodegeneration model: Mice were acclimatized to a 12-h dark environment before the experiments, and they were exposed to 8000 lX of white light for 12 h/day for 7 days after mydriasis; iii) Tn + light damage: 2 μl of Tn (1 mg/ml) was intravitreally injected 24 h before exposure to light; iv) NMDA: 20 nmol of NMDA was intravitreally injected 2 days before the experiments; and v) Tn + NMDA: 2 μl of Tn (1 mg/ml) was intravitreally injected 24 h before the NMDA treatment. All mice were euthanized by cervical dislocation after anesthesia with 90 mg/kg ketamine and 7.5 mg/kg xylazine injected intraperitoneally, and the retinal tissue was subsequently removed.
Cell viability and cytotoxicity assays
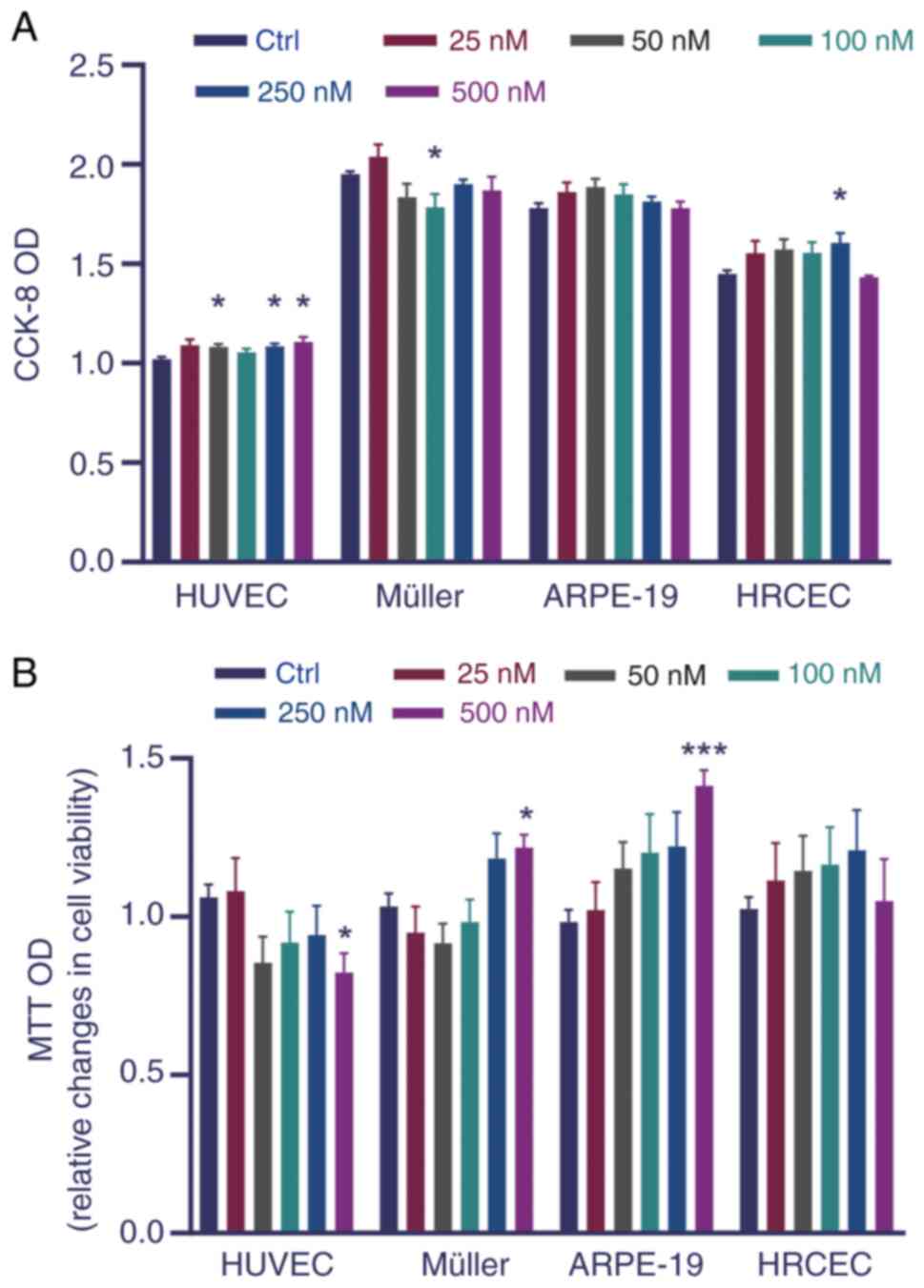
Cell viability tests were performed using MTT and CCK-8 assays (Biosharp Life Sciences). Cells were seeded in 96-well plates, with 100 μl of cell suspension containing 5,000 cells injected into each well, and five parallel replicates were prepared. Cells were incubated with 25, 50, 100, 250, or 500 nM Tn for 24 h at 37°C. For the CCK-8 assay, post-incubation, a precise volume of 10 μl of CCK-8 reagent was dispensed into each designated well, followed by a subsequent incubation period of 1 h at 37°C. This was succeeded by spectrophotometric measurement of the absorbance at a wavelength of 450 nm to assess cell viability. For the MTT assay, upon completion of the initial incubation period, 50 μl of MTT solution were administered to each well. The plates were then incubated at 37°C for another 4 h to reduce the MTT to formazan. Subsequently, the supernatant was aspirated without disturbing the cell monolayer, and 150 μl of DMSO was added to each well to solubilize the crystals. The plates were then shaken for 10 min on a shaker. The absorbance was spectrophotometrically measured at 490 nm to assess cytotoxicity.
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
The Müller and ARPE-19 cells were treated with different Tn concentration levels 24 h before RT-qPCR assays. RNA was extracted from cells and tissues using Trizol™ reagent (Thermo Fisher Scientific, Inc.), and followed the manufacturer's protocol for reverse transcription using PrimeScript RT Reagent kit (cat. no. RR037A; Takara Bio USA, Inc.) at 37°C for 15 min and 85°C for 5 sec. RT-qPCR was performed using the SYBR Green qPCR SuperMix kit (Invitrogen; Thermo Fisher Scientific, Inc.) with a PikoReal Real-Time PCR System (Thermo Fisher Scientific, Inc.). The thermocycling conditions were as follows: Initial denaturation at 95°C for 2 min, followed by 35 cycles at 94°C for 45 sec, 56°C for 30 sec, and 72°C for 45 sec. Glyceraldehyde-3-phosphate dehydrogenase was used as a control. All data were analyzed using the 2−ΔΔCq method (26).
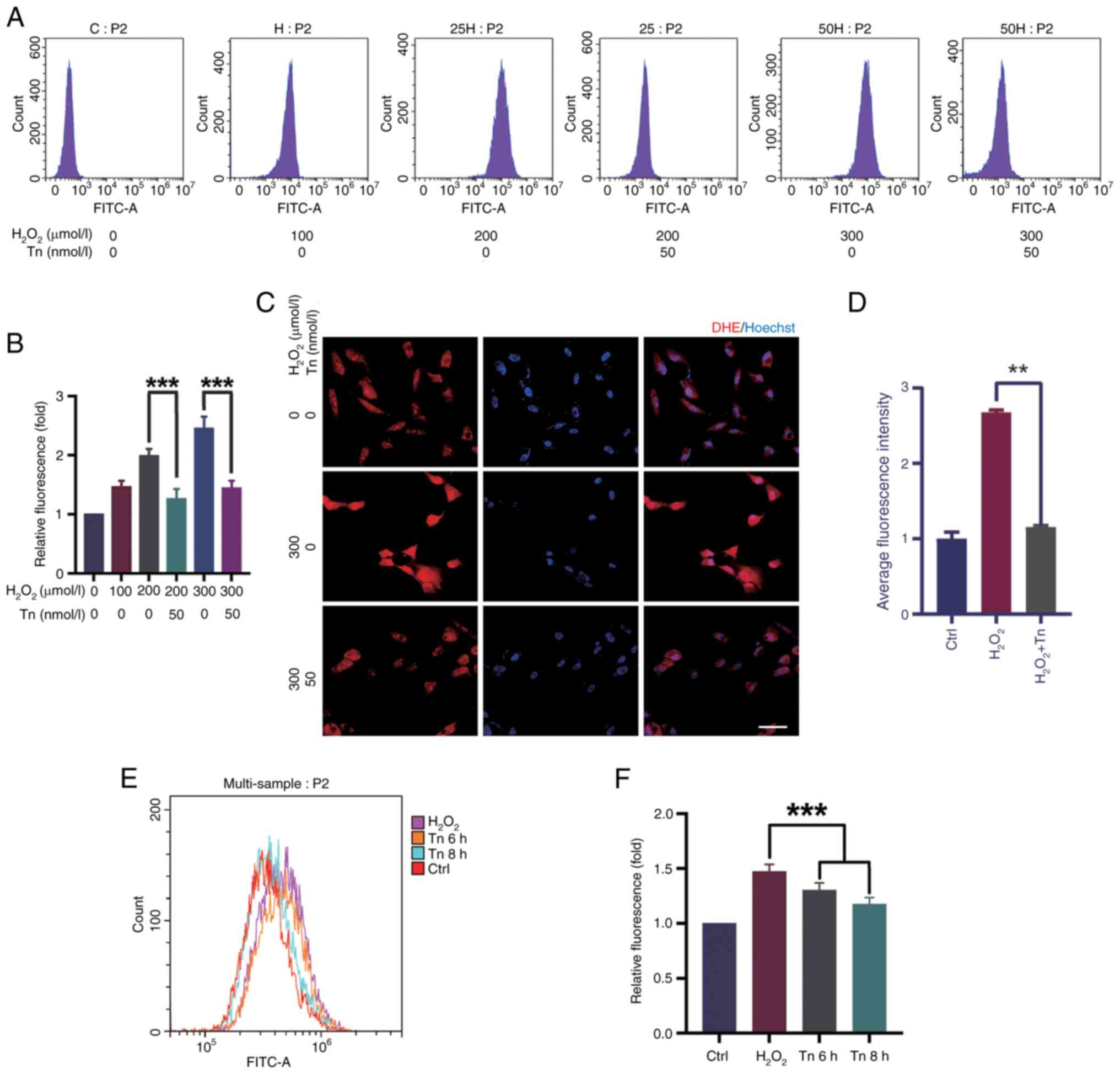
ROS estimation
ROS levels were measured using a ROS Assay kit (cat. no. S0033S; Beyotime Institute of Biotechnology) according to the manufacturer's protocol. Cells were treated with 50 nM Tn at 37°C for 6 h or 8 h at a density of 5×105 cells/ml, and then with H2O2, followed by incubation of cells with DCFH-DA (10 μM) for 30 min at 37°C. Finally, flow cytometry was used to detect fluorescence intensity. Dihydroethidium (DHE) staining was used to determine the level of super oxidation in ARPE-19 cells using a DHE staining kit (cat. no. ab145360; Abcam), according to the manufacturer's protocols.
Flow cytometry and apoptosis detection
ARPE-19 and Müller cells were incubated with Tn at 10, 25, 50, and 100 nM for 6 h at 37°C at a density of 5×105 cells/ml before stimulation with H2O2 (300 μM/ml). The cell suspension was counter-stained with fluorescein isothiocyanate-labeled Annexin V and propidium iodide (PI) for 10 min at 37°C in the dark. Apoptosis was measured using a CytoFLEX flow cytometer (Beckman Coulter, Inc.) and analyzed using CytExpert 2.2 (Beckman Coulter, Inc.). Early apoptosis was detected using the JC-1 staining kit (cat. no. C2006; Beyotime Institute of Biotechnology) according to the manufacturer's protocol.
Western blotting
Western blot assay protocols were applied as previously described (12). Following treatment, cells were collected and lysed using RIPA lysis buffer (Beyotime Institute of Biotechnology) to extract proteins. Protein concentration was determined using the BCA assay to ensure consistent protein loading across samples. Protein samples were mixed with SDS-PAGE loading buffer and denatured by boiling. During the electrophoresis phase, equal quantities of proteins (20 μg/lane) were separated using a 10% SDS-PAGE gel. Subsequently, the proteins on the gel were transferred onto a polyvinylidene fluoride membranes. After the transfer, the membrane was blocked with 5% non-fat milk for 2 h at room temperature on a shaking platform. It was then incubated with primary antibodies overnight at 4°C with gentle agitation. On the following day, the primary antibodies were removed, and the membrane was incubated with secondary antibodies for 1 h at room temperature. According to the instructions of the ECL kit (Biosharp Life Sciences), the luminescent substrates A and B were mixed in equal volumes under light-protected conditions to prepare the luminescent working solution. The solution was then evenly applied to the membrane and allowed to incubate for at least 1 min. Finally, the membrane was scanned using the Tannon 5200 (Tanon Science and Technology Co., Ltd.) equipment to observe the protein bands, and ImageJ software (https://imagej.net; National Institutes of Health) was used for quantitative densitometric analysis of the protein bands.
Immunofluorescence staining of retinal tissues
Retinal tissues from the different groups were fixed with 4% paraformaldehyde at 4°C overnight and dehydrated in 30% sucrose for 24 h. The retinal tissues were then embedded in the compound at the optimal cutting temperature and stored at −80°C after being cut into 10-μm sections at −25°C. The sections were incubated for 45 min at 37°C in 5% bovine serum albumin (neoFroxx GmbH) in phosphate buffered saline containing 0.5% Triton X-100 and then incubated with anti-NeuN primary antibodies overnight at 4°C and then washed with phosphate-buffered saline containing 0.05% Tween, and incubated with the Alexa Fluor™ 647 secondary antibody (1:1,000; Thermo Fisher Scientific, Inc.) for 4 h at room temperature and shielded from light. Finally, the sections were observed and images were captured under a fluorescence microscope (Olympus Corporation).
Hematoxylin and eosin (H&E) staining of retinal tissues
H&E staining kit (cat. no. C0105S; Beyotime Institute of Biotechnology) was used to examine the retinal layers in light-damaged and NMDA-treated mice. The eyes were fixed in 4% paraformaldehyde at 4°C overnight. They were then embedded in paraffin and cut into 5 μm-thick slices, which were securely adhered to glass slides. After dewaxing and hydration, the sections were stained in hematoxylin solution for 10 min at room temperature, followed by eosin staining for 1 min. The stained sections were dehydrated, cleared, and mounted with neutral balsam (Biosharp Life Sciences). The sections were observed and photographed under a fluorescence microscope.
Terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL) assay
The TUNEL Apoptosis Assay kit (cat. no. C1091; Beyotime Institute of Biotechnology) was used to detect retinal cell apoptosis in light-damaged and NMDA-treated mice. Eyes were preserved in 4% paraformaldehyde and fixed overnight at 4°C. Following this, the specimens were embedded in paraffin, sectioned into 5 μm-thick slices and firmly adhered to the glass slides. Post-dewaxing and hydration, the sections underwent protease K (Beyotime Institute of Biotechnology) treatment at 37°C for 30 min. Subsequently, they were incubated with the TUNEL reaction mixture at 37°C for 1 h in a light-protected environment. To visualize the nuclei, cells were stained with 5 μg/ml of 4',6-diamidino-2-phenylindole (DAPI) (Beyotime Institute of Biotechnology) for 10 min at room temperature, maintaining light protection throughout. Sections were observed under a fluorescence microscope with a 1.1-mm field of view, and images were captured from three randomly selected fields of view for each section. Apoptotic retinal cells were clearly labeled with green fluorescence, while the nuclei showed blue fluorescence due to DAPI staining.
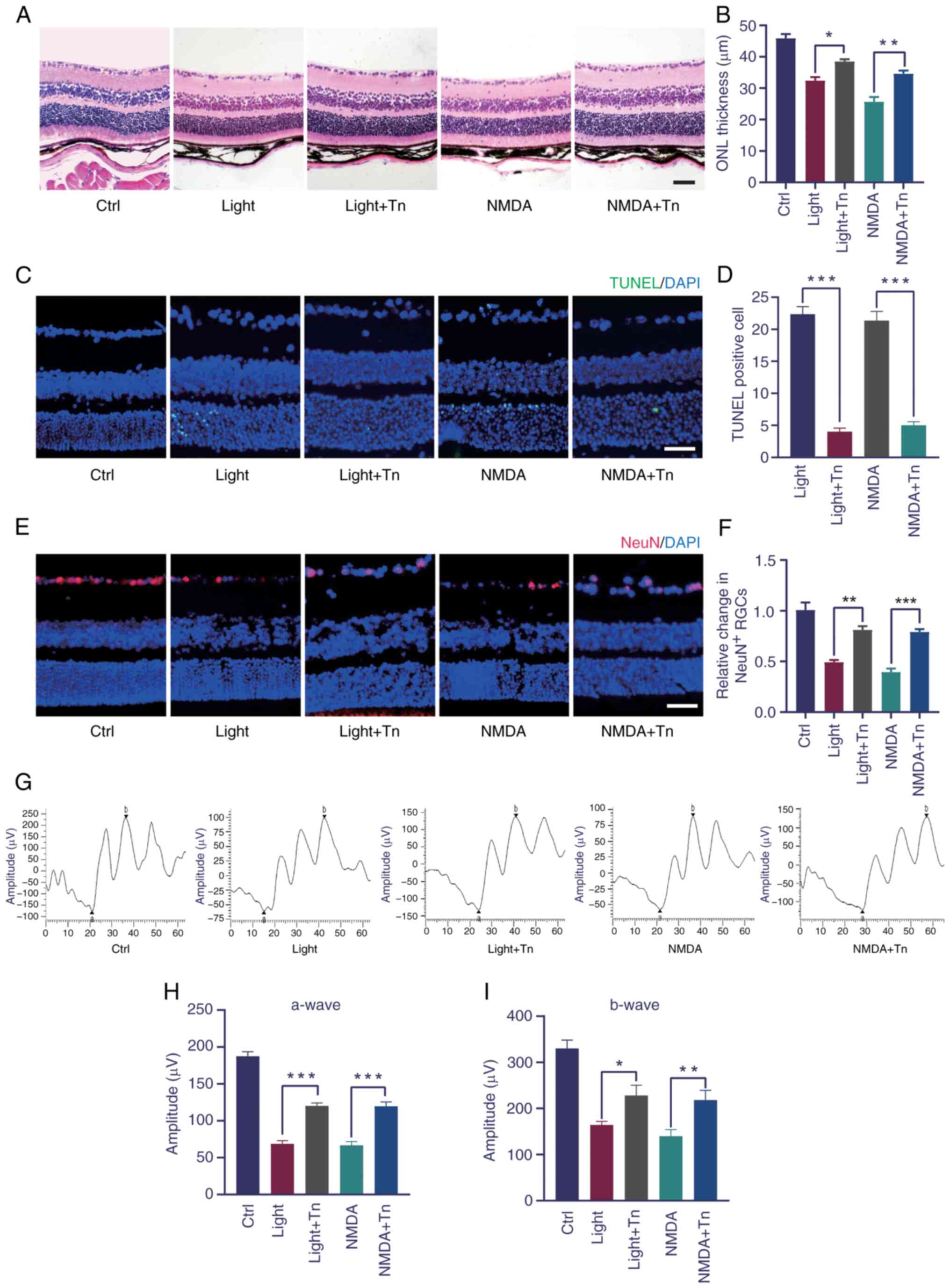
Electroretinography (ERG)
The experimental mice were acclimated to the dark for at least 12 h before changes in retinal electrical activity were evaluated. The pupils of the mice were dilated using a 1% tropicamide solution (Alcon). ERGs were recorded in both eyes by placing a wire electrode (Nihon Kohden) contacting the cornea with the reference electrode in the forehead and ground electrode in the tail. Data were analyzed for a- and b-waves in each group.
Cell functional assays
The 5-ethynyl-2'-deoxyuridine (EdU) staining, Transwell migration, invasion and tube formation assays were performed to observe the proliferative ability of endothelial cells. HUVECs were pretreated with Tn at 250 and 500 nM, followed by stimulation with 10 ng/ml vascular endothelial growth factor (VEGF) in 24-well plates for 24 h. Endothelial cells were incubated with EdU (Beyotime Institute of Biotechnology) at 37°C for 2 h and images were captured under a fluorescence microscope after fixation and rinsing. Transwell migration and invasion assays were performed to evaluate their influence on endothelial cell migration and invasion. For the migration assay, no Matrigel (Corning, Inc.) was added to the upper chamber of the Transwell inserts (Corning, Inc.). For the invasion assay, the Matrigel was diluted with DMEM medium at a ratio of 1:8, and 100 μl of the diluted Matrigel was evenly spread onto the upper chamber of the Transwell inserts, which was then incubated at 37°C for 3 h. Subsequently, treated cells were suspended in serum-free DMEM medium and adjusted to a density of 1×105 cells, and then seeded onto the upper chamber of Transwell inserts of 8.0-μm pore size treated or untreated with Matrigel. The lower chamber was filled with 600 μl of DMEM medium containing 10% serum to support cell migration. After incubation, the migratory cells were fixed with methanol for 10 min at room temperature, stained with 0.5% crystal violet solution for 30 min, and counted under a light microscope. Tube formation assays were designed to evaluate the angiogenic ability of endothelial cells. After the same treatment as aforementioned, the cells were added to Matrigel for 6 h. The capillary network was observed using a bright-field microscope. All results were quantified using the ImageJ software (https://imagej.net).
Laser-induced choroidal neovascularization (CNV)
A total of 3 days before laser irradiation, Tn and normal saline injections were performed in the vitreous cavity of mice. CNV was induced using a 532-nm laser, as previously described (27). The mice were anesthetized with ketamine (90 mg/kg) and xylazine (7.5 mg/kg) by intraperitoneal injection, followed by pupil dilation. Laser photocoagulation was then performed using a laser photocoagulator (75 μm spot size, 100 msec duration, and 100 mW) to rupture the Bruch's membrane. A 10-mm coverslip was used to view the retinal vessels. Choroids were harvested for immunohistochemical staining 7 days after photocoagulation. Mouse eyes of different groups were fixed in 4% paraformaldehyde at 4°C for 1 h. The choroids were isolated by microsurgery and flat laid on slides. The flat mounts were incubated in 5% bovine serum albumin in phosphate buffered saline containing 1% Triton X-100 at 37°C for 45 min. Subsequently, flat mounts were incubated with isolin B4 (iB4; cat. no. I21411; 1:50; Thermo Fisher Scientific, Inc.) overnight at 4°C, protected from light. The next day, the flat mounts were washed with phosphate-buffered saline containing 0.05% Tween and viewed under the fluorescence microscope and images were captured.
Statistical analyses
All data for the present study was obtained from at least three independent experiments and expressed as the mean ± standard error of the mean. In conducting statistical difference analysis, the unpaired Student's t-test was used to evaluate the disparity between two data groups. For comparisons involving multiple data groups, ANOVA was first used to examine the overall variability among the groups, followed by Tukey's multiple comparisons test to further identify the groups that exhibited significant differences. Data analyses were conducted using GraphPad Prism 8.0.0 for Windows (GraphPad Software; Dotmatics). P<0.05 was considered to indicate a statistically significant difference.
Results
Tn: Novel and efficient activator of Nrf2 signaling
The potential effects of Tn on the viability of HUVECs, HRCECs and Müller and ARPE-19 cells were first analyzed to evaluate its influence on cytotoxicity. No pronounced reduction in cell viability or significant cytotoxicity were observed in vitro (Fig. 1A and B). Hence, Tn 10-100 nM was used in subsequent experiments.
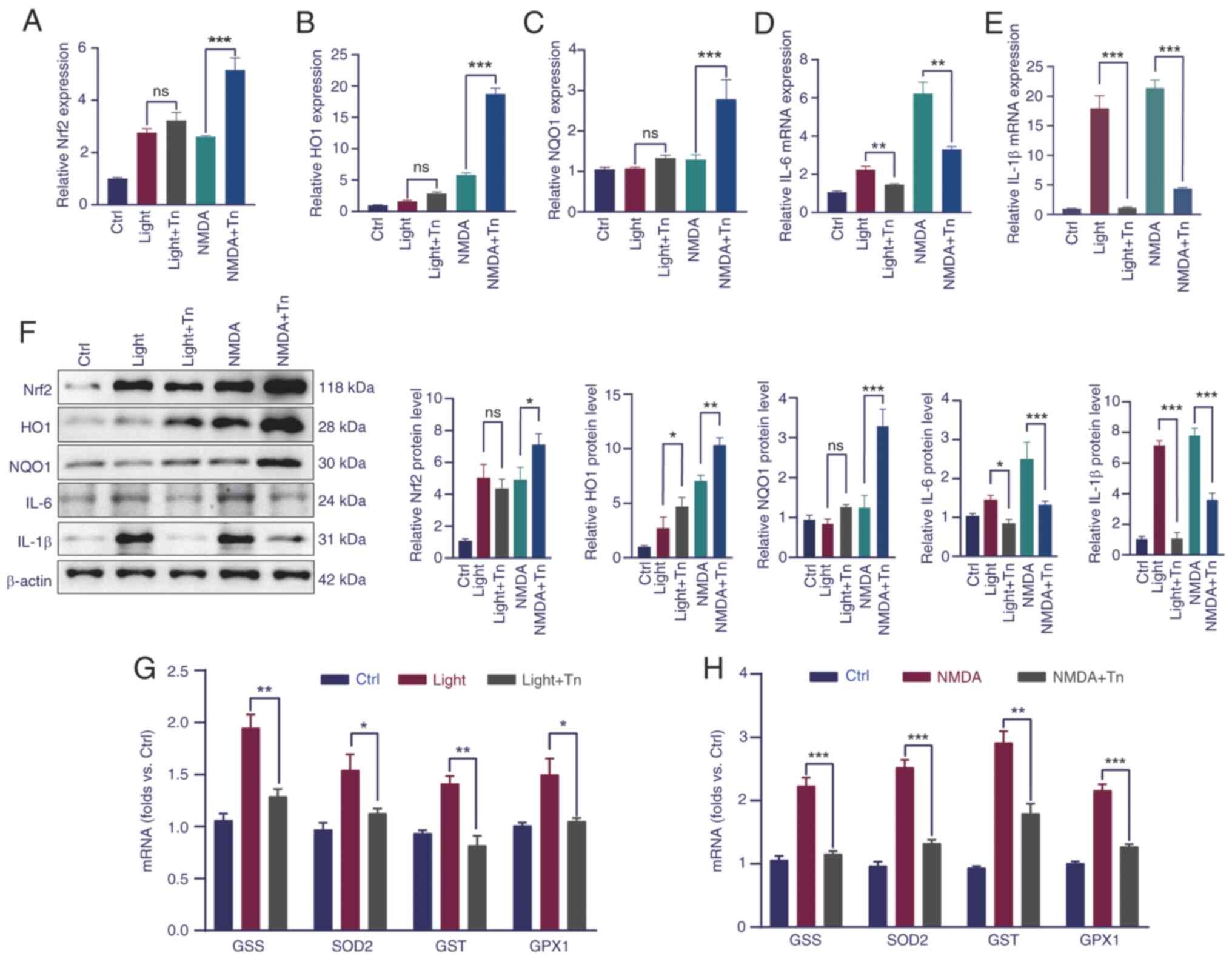
Nrf2/Keap1/ARE signaling is widely acknowledged as one of the most critical antioxidant signaling pathways. The Nrf2 cascade protects against oxidative injury in vitro (19). To investigate whether Tn activates the Nrf2/Keap1 signaling pathway, the mRNA expression levels of Nrf2, Keap1 and Nrf2 downstream genes, including HO1 and NQO1, were measured in Müller (Fig. 2A-D) and ARPE-19 cells (Fig. 2E-H). The RT-qPCR results showed that following Tn administration, the mRNA levels of Nrf2, HO1 and NQO1 increased significantly in Müller and ARPE-19 cells. By contrast, the mRNA level of Keap1 was significantly reduced in Tn-treated cells.
Western blotting was used to evaluate the protein expression of the Nrf2/Keap1 cascade in ARPE-19 cells. As expected, in Tn-treated ARPE-19 cells, the protein expression levels of Nrf2 and its downstream effectors, HO1 and NQO1, exhibited a significant concentration-dependent increase compared with the control group (Fig. 2I-N). Concurrently, the protein expression level of Keap1 also demonstrated a corresponding concentration-dependent decrease (Fig. 2O and P). These results indicated that Tn may have been an efficient activator of the Nrf2/Keap1 signaling cascade.
Tn significantly alleviates apoptosis caused by oxidative injury in vitro
Oxidative stress is an important pathogenic factor in the development of retinal injury and diseases, especially AMD (28). To confirm the protective effects of Tn against H2O2-induced oxidative stress, the mitochondrial potential of ARPE-19 cells was examined using the JC-1 assay. The change in fluorescence from red to green indicates a marked reduction in mitochondrial potential (29). The fluorescence analysis using confocal microscopy and flow cytometry revealed that Tn effectively ameliorated the reduction in mitochondrial membrane potential (Fig. 3A-D), indicating that the Tn cytoprotective effect was correlated with oxidative stress and apoptosis. Furthermore, double labeling of Müller cells was performed with PI and Annexin V to verify the Tn cytoprotective effect. Flow cytometric assays showed that the percentage of PI-and Annexin V-positive cells in the Tn-pretreated group was significantly lower than that in the H2O2-stimulated group (Fig. 3E and F). These results suggested that Tn substantially reduced the mitochondrial membrane potential and thus protected retinal cells from H2O2-induced apoptosis in ARPE-19 and Müller cells.
Tn decreases H2O2-induced ROS production in vitro
It was investigated whether the decrease in apoptosis in ARPE-19 and Müller cells was due to a decrease in ROS production induced by Tn. To examine the effect of Tn on antioxidants, the ROS expression was evaluated in Müller cells and ARPE-19 cells. Flow cytometry revealed that exposure to H2O2 at a concentration of 300 μM for 18 h resulted in high ROS levels. Simultaneously, Tn significantly suppressed ROS levels in Müller and ARPE-19 cells under stressed conditions. Tn pretreatment decreased ROS optical density compared with the H2O2-induced group, indicating that Tn enhanced the antioxidant capacity of Müller and ARPE-19 cells (Fig. 4A, B, E and F). DHE staining, a sensitive probe for detecting ROS generation in cells, showed similar results. After H2O2 stimulation, ARPE-19 cells had a substantial increase in DHE staining intensity, which was significantly decreased in the Tn pretreatment group (Fig. 4C and D). These results illustrated that Tn pretreatment significantly alleviated ROS production induced by H2O2 in ARPE-19 and Müller cells.
Tn significantly reduces overall retinal inflammatory response and oxidative stress
In vivo experiments were performed to verify the protective effects of Tn injection into the mouse retina against oxidative stress injury. Firstly, the concentration of Tn was determined by referring to existing literature (30) and through calculations based on the molecular weight of Tn (358.39 Da) and anticipated in vivo bioavailability. At this concentration, Tn can generate significant biological effects without inducing notable toxic effects on the retina (Fig. S1). As previously described, the mouse retinal photodamage and NMDA injury models are relatively effective to evaluate retinal oxidative stress injury (22,31).
First, RT-qPCR analysis of the genes involved in the Nrf2 pathway was performed. The results showed that in the light- and NMDA-induced retinal models, the mRNA levels of Nrf2 pathway-related genes increased, which was more elevated in the Tn pretreatment group, although the mRNA changes in the light-induced model were not significant (Fig. 5A-C). To further investigate the effect of Tn on oxidative protection, the relative mRNA levels of inflammatory cytokines and genes related to oxidative stress were measured. The results of RT-qPCR demonstrated that the mRNA expression of inflammatory factors, such as interleukin (IL)-6 and IL-1β, significantly increased in the NMDA-treated group; in the Tn pretreatment these mRNA expression levels decreased (Fig. 5D and E). Western blotting further validated the aforementioned results (Fig. 5F). Genes related to the oxidative stress response, including glutathione synthetase (GSS), glutathione S-transferase (GST), superoxide dismutase 2 (SOD2), and glutamine peroxidase 1 (GPX1), almost doubled in the light-induced group and tripled in the NMDA-induced group, which plunged to a level equivalent to that of the control group after Tn intravitreal injection (Fig. 5G and H). The RT-qPCR results indicated that Tn promoted anti-inflammatory and anti-oxidative activities and protected against light- and NMDA-induced damage in vivo.
Tn ameliorates retina function in vivo
Using the light- and NMDA-induced oxidative stress, it was tested whether intravitreally injected Tn could ameliorate the retinal structure and function. H&E staining revealed that light- and NMDA-induced retinal injury significantly decreased the outer nuclear layer thickness, which was alleviated by Tn pretreatment (Fig. 6A and B). The TUNEL assay demonstrated that light exposure and NMDA treatment significantly increased the number of apoptotic retinal cells. Tn pretreatment significantly decreased the number of apoptotic retinal cells (Fig. 6C and D). Immunohistochemistry was performed to determine whether Tn affected the number of retinal ganglion cells. The number of NeuN-positive cells decreased in the light-damaged and NMDA-treated groups and increased in the Tn-pretreated group (Fig. 6E and F). These results identified that Tn pretreatment protected against light- and NMDA-induced retinal degeneration in vivo. To determine the protective effect of Tn on retinal function, ERGs were performed in the mice. A decrease was found in the a- and b-wave amplitudes in the retinas of the light-damaged and NMDA-induced groups. However, this dysfunction was partially reversed in the treatment group, as demonstrated by the ERG a- and b-wave amplitudes (Fig. 6G-I).
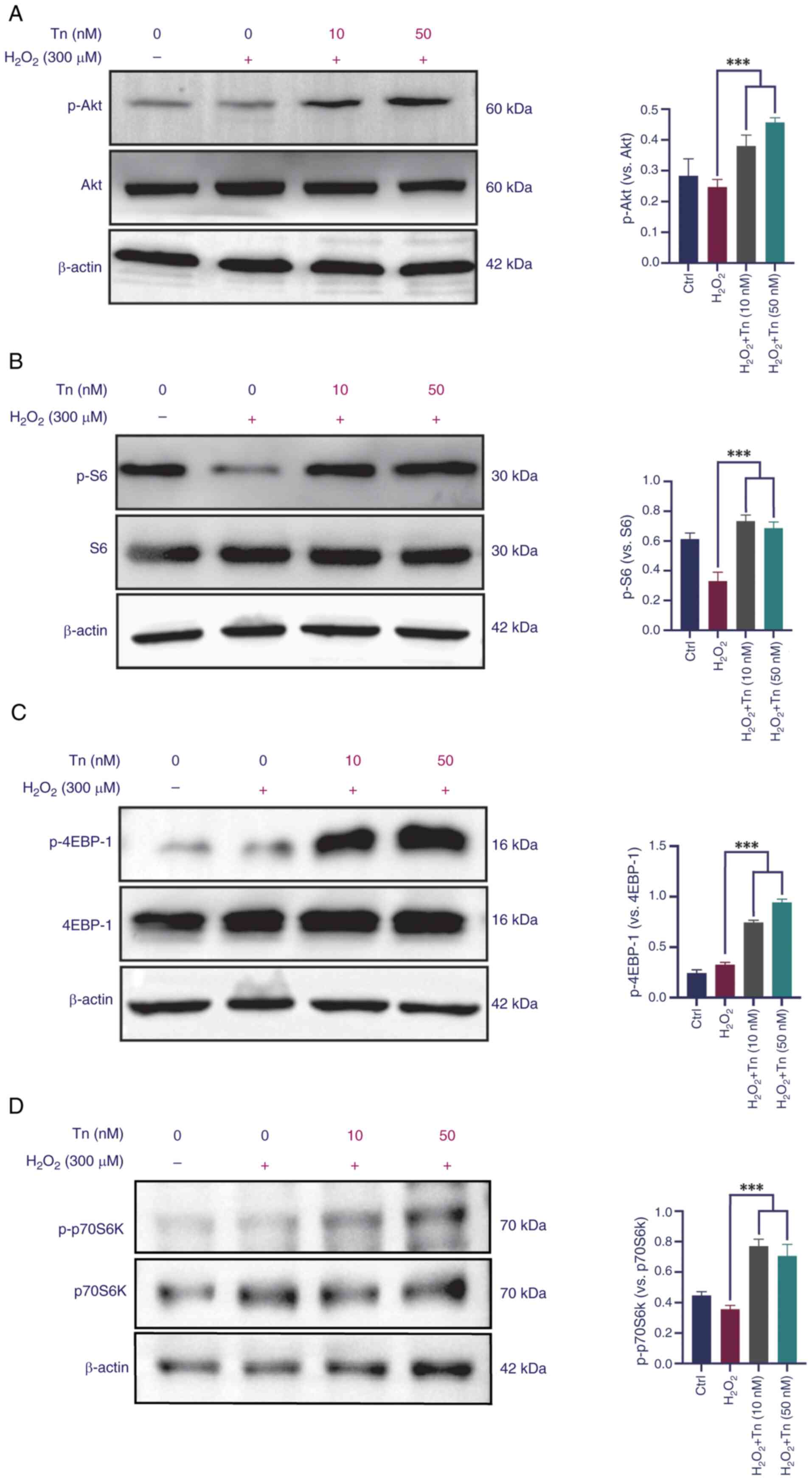
Tn effects on the expression of the PI3K/Akt/mTOR signaling pathway
PI3K/Akt/mTOR signaling is vital for Nrf2 phosphorylation and nuclear translocation, as confirmed in a previous study by the authors (12). Western blotting was performed to investigate whether Tn pretreatment affected activation of the Akt signaling pathway. The present results demonstrated that Tn phosphorylated Akt and its downstream target, S6, in ARPE-19 cells (Fig. 7A and B). Furthermore, mTOR downstream proteins 4EBP-1 and p70S6K1 were significantly phosphorylated in the Tn-pretreated groups (Fig. 7C and D). Increased expression of the signaling proteins revealed that Tn pretreatment greatly enhanced Akt pathway activation in ARPE-19 cells when treated with H2O2. Hence, it was deduced that the Tn-mediated effects on Nrf2 activation likely depend on the PI3K/Akt/mTOR signaling pathway. Thus, it was reported that Tn protects ARPE-19 cells from oxidative stress by activating the PI3K/Akt/mTOR signaling axis.
Tn regulates angiogenic function in endothelial cells
VEGF is involved in angiogenesis, which is a key pathological change in exudative AMD (32). HUVECs were pretreated with Tn (250 or 500 nM) and stimulated with VEGF (10 ng/ml). The VEGF-treated group had increased viability and endothelial cell proliferation compared with the control group. However, EdU staining demonstrated that pretreatment with Tn reduced endothelial cell proliferation and rescued the effects of VEGF (Fig. S2A). Further investigation using Transwell migration and invasion assays showed that VEGF-mediated migration and infiltration were interrupted after Tn administration (Fig. S2B and C). Matrigel tube formation assay results indicated that Tn decreased the ability of HUVECs to form tubes (Fig. S2D). In addition, in vivo experiments using laser-induced CNV models revealed that the neovascular area in the Tn-pretreated group was significantly smaller than that in the saline-treated control group (Fig. S3). Therefore, it was concluded that Tn reduced the angiogenic function of endothelial cells.
Discussion
It has been shown that retinal tissue is highly sensitive to oxidative stress due to high oxygen consumption during phototransduction (33). Therefore, oxidative stress is a key factor in accelerating the pathological progression of ocular diseases. The AMD pathophysiological course involves various changes, including RPE injury, Bruch's membrane lesions, neurodegenerative changes, and abnormal elevation of proinflammatory and proangiogenic cytokines (34,35). RPE cell senescence was previously considered to initiate AMD; although, senescence and degeneration of RPE cells involve pathological processes such as oxidative stress damage and apoptosis, which are considered important causes of AMD (6). However, Müller cells are the first cell type to show metabolic abnormalities during retinal degeneration due to their high aerobic metabolism (36-38). The peaks of glutamine and glutathione are more than double the normal levels in the Müller cells of patients with early dry and wet AMD (38). Müller cells activated under hypoxic or high-glucose conditions secrete angiogenic factors such as VEGF and basic fibroblast growth factor, which promote endothelial cell proliferation (39,40). Therefore, future studies on the pathological changes of RPE and Müller cells in AMD are expected to reveal therapeutic targets for maintaining or restoring healthy retinal function.
Previously, numerous edible plants (cauliflowers, melons, blueberries and legumes) and traditional Chinese herbs have been extensively studied for their powerful antioxidant capacity and low toxicity, and are expected to be antioxidant therapeutics (41,42). Tn is a key bioactive small molecule extracted from Tripterygium wilfordii, a plant utilized in traditional Chinese medicine, which has a molecular structure similar to that of Tl, but is significantly less toxic than Tl (43,44). Tn can play a neuroprotective role by regulating MAPKs and NF-κB pathways, inhibiting microglia activation, inflammatory factor release, oxidative stress and calcium overload, antagonizing excitatory toxicity, and promoting the synthesis of neurotrophic factors (16,17). The results of the present study revealed that Tn was an efficient Nrf2 activator with the potential for use in the treatment of diseases such as AMD. An oxidative stress model of Müller and ARPE-19 cells induced by H2O2 was constructed, and the results showed that Tn pretreatment at different concentrations had no obvious cytotoxicity in retinal cells. Mitochondria are the main source of ROS in AMD and mitochondrial DNA is a sensitive target of oxidative stress. Therefore, early oxidative damage is often accompanied by the destruction of mitochondrial transmembrane potential (3,45,46). It was found that nanomolar levels of Tn effectively ameliorated the reduction in mitochondrial membrane potential; flow cytometry and JC-1 staining also confirmed the anti-oxidative stress and anti-apoptotic effects of Tn. To further investigate the antioxidant effect of Tn, ROS production in ARPE-19 and Müller cells was detected by flow cytometry and DHE staining. As previously described, light-induced retinal oxidative stress and NMDA-induced neuro-excitotoxicity models have been used to study oxidative stress conditions in AMD and other ocular diseases because of their proposed ability to induce oxidative stress, mitochondrial dysfunction and retinal inflammation (22-25). Tn pretreatment significantly decreased the expression of oxidative stress-related genes and inflammatory factors in the retinal tissues of the two models; and the results obtained by both western blotting and RT-qPCR methods were highly consistent with each other. In addition, H&E staining and immunofluorescence results indicated that Tn improved retinal function and sustained the integrity of retinal morphology, which was further verified by ERG.
The transcription factor Nrf2 is a key signaling molecule that regulates cell survival and maintains redox homeostasis. It mediates the expression of antioxidant genes and phase II detoxification enzymes by activating ARE signals. It is considered as a potential intervention target for numerous oxidative stress-related ocular diseases (47,48). Oxidative stress stimulates Nrf2 dissociation from Keap1 and subsequent nuclear translocation. Gene transcription related to antioxidant protection, such as HO1, NQO1 and SOD (49), is activated by Nrf2 when it interacts with downstream AREs. Some natural plant components (such as quercetin, ginsenosides, astaxanthin and curcumin) have been shown to inhibit oxidative damage in ARPE-19 cells by activating Nrf2 signaling (50-53). Previous studies by the authors have found that salvianolic acid A, ginsenosides Rg1, Rg3, 3h-1, 2-dimercaptiol-3-thione, and other Chinese herbal extracts or small molecular compounds are effective Nrf2 activators, inducing phosphorylation of Nrf2 dependent on Akt-mTORC1, with potential therapeutic value for oxidative stress-related retinal degenerative diseases (12,13,54). Based on these results, the mRNA expression levels of Nrf2, Keap1 and the transcription levels of the downstream target genes HO1 and NQO1 were determined. The RT-qPCR results showed that the mRNA levels of Nrf2, HO1 and NQO1 significantly increased after Tn administration in ARPE-19 and Müller cells. This indicates that Tn can indeed activate the Nrf2 signaling pathway and promote the expression of these antioxidant genes. Notably, the mRNA level of Keap1 decreased slightly after Tn treatment. This may be due to the direct modification of Keap1 by Tn, leading to decreased stability or transcriptional inhibition. However, this decrease was not significant, suggesting that Tn mainly exerts its therapeutic effects by promoting the activation of Nrf2, rather than by directly inhibiting Keap1 expression. Considering that the core pathological process in the early stage of AMD mainly affects RPE cells and photoreceptor cells (while Müller cells may play a relatively indirect role, primarily providing nutritional support and neuroprotective functions as glial cells within the retina and are not the direct targets of damage), the protein expression levels of Nrf2, Keap1 and their downstream targets HO1 and NQO1 were further measured in ARPE-19 cells using western blotting. The results indicated that Tn not only promotes the transcription of these genes except Keap1, but also effectively facilitates their protein translation and accumulation.
Although Tn was confirmed to be an effective activator of Nrf2 in the present study, promoting the expression of downstream antioxidant proteins, the underlying signal transduction mechanism has not been fully elucidated. Akt-mTORC1 is a key signal for cell proliferation and survival (55). Existing evidence suggests that the phosphorylation of Nrf2 is dependent on the activation of Akt-mTORC1 and that the PI3K/Akt/mTORC1 pathway mediates activation of the Keap1/Nrf2/ARE antioxidant pathway (56,57). Specifically, the activation of PI3K triggers the phosphorylation of phosphatidylinositol (PI) to generate 3-phosphoinositide (PIP3). PIP3, as a second messenger, recruits and activates Akt, leading to its phosphorylation. Phosphorylated Akt not only further activates downstream mTORC1 but also stabilizes Nrf2 by phosphorylating and inhibiting GSK3-β, a kinase that negatively regulates Nrf2. By inhibiting GSK3-β, Akt protects Nrf2 from degradation, promoting its nuclear translocation and transcriptional activity (58,59). Therefore, it was attempted to observe whether Tn activated the PI3K/Akt/mTORC1 pathway and its downstream target genes. It was found that Tn pretreatment significantly increases the phosphorylation levels of Akt, S6, as well as the mTORC1 downstream target proteins 4EBP-1 and p70S6K after H2O2 exposure, indicating that the Tn effect in rescuing the antioxidant and anti-inflammatory pathways of Nrf2/HO1 may have arisen from reactivation of the PI3K/Akt/mTORC1 axis (Fig. 8).
CNV is the core pathological change in wet AMD (60,61). Interestingly, it was found that Tn significantly inhibited pathological neovascularization, both in vitro and in vivo. Specifically, Tn attenuated VEGF-induced vascular endothelial cell proliferation and migration, and a significantly decreased level of pathological angiogenesis was observed in laser-induced CNV (animal models of exudative AMD) (62).
The present study indicated that Tn can induce the activation of Nrf2 signaling by activating the PI3K/Akt signaling pathway, thereby improving the structure and function of the retina. However, in addition to this core mechanism, there may be other signaling mechanisms that interact with the effects of Tn. There is a close interaction between the PI3K/Akt and NF-κB signaling pathways. Akt, as a key molecule downstream of PI3K, can inhibit the nuclear translocation and transcriptional activity of NF-κB by phosphorylating the IκB kinase (IKK) complex, thereby achieving negative regulation (63). Therefore, Tn may indirectly inhibit the excessive activation of NF-κB by activating the PI3K/Akt signaling pathway. Furthermore, autophagy, a highly conserved cellular self-degradation process, is crucial for clearing damaged organelles and protein aggregates and maintaining intracellular homeostasis. The activation of autophagy is closely related to the regulation of the PI3K/Akt signaling pathway. Akt, as a key molecule in this pathway, can regulate autophagic activity by influencing the expression of autophagy-related genes and the initiation of the autophagic process (64). Tn may promote the clearance of harmful substances within retinal cells by activating the autophagic signaling pathway.
Tn, as a potent Nrf2 activator, exhibits significant antioxidant effects on the retina. In AMD, retinal cells are chronically exposed to high oxidative stress, leading to cellular dysfunction and death. Therefore, by activating the Nrf2 signaling pathway, Tn holds promise as an effective antioxidant protective agent for patients with AMD. Furthermore, Tn is capable of modulating inflammatory signaling pathways, such as NF-κB, and suppressing the expression of inflammation-related genes, thereby attenuating retinal inflammation. This mechanism contributes to the protection of retinal cells from inflammatory damage and the slowing of AMD progression. Notably, Tn also demonstrates remarkable anti-angiogenic properties. It can inhibit the proliferation and migration of vascular endothelial cells, thus suppressing the formation of pathological neovascularization. This mechanism provides a potential therapeutic strategy for patients with wet AMD.
However, AMD is a complex retinal disease; combining Tn with other drugs or therapies may lead to more comprehensive therapeutic outcomes. In the treatment of AMD, high doses of antioxidant vitamins and minerals, such as vitamin C, vitamin E, beta-carotene and zinc, have been extensively studied and applied (65). These substances effectively scavenge free radicals and reduce retinal oxidative stress. When combined with Tn, they can provide additional antioxidant protection by synergistically activating the Nrf2 pathway, thereby delaying the progression of AMD. Furthermore, the combination of Tn with anti-VEGF drugs, such as ranibizumab and aflibercept, may also demonstrate promising results. This combination not only synergistically controls the condition of AMD, reduces the growth and leakage of abnormal blood vessels, but also provides additional antioxidant protection. In some severe cases of AMD, glucocorticoids are used to control retinal inflammation (66). However, long-term or high-dose steroid use may increase the risk of side effects such as cataracts and glaucoma. Therefore, combining low-dose steroids with Tn may be a strategy to balance efficacy and safety. Finally, cell therapy, particularly the transplantation of stem cells or RPE cells, offers promise in restoring the structure and function of the retina (67). When combined with Tn, this therapeutic approach can be further optimized as Tn promotes the survival and integration of transplanted cells through its antioxidant and anti-inflammatory actions, thereby enhancing the overall treatment outcome.
The present study also has some limitations. Firstly, further studies are needed to investigate the specific mechanism of Tn on the Nrf2 signaling pathway by in vivo and silencing experiments. Secondly, the reactivation of the PI3K/AKT/mTOR signaling pathway by Tn could be further verified using specific inhibitors or activators. Additionally, to improve evaluation of the protective effects of Tn, it can be compared with other known retinal protective strategies such as antioxidants and anti-inflammatory agents to improve identification of the superiority or uniqueness of Tn. Finally, the current study did not address the evaluation of the long-term efficacy of Tn in retinal diseases. In the future, rigorous long-term follow-up trials will be conducted by the authors to systematically assess both the sustained efficacy and safety profile of Tn in the treatment of retinal diseases.
In conclusion, the results of the present study suggest that nanomolar concentrations of Tn protected retinal cells against oxidative damage and inflammation. Activation of Tn-induced Nrf2 signaling may be realized by activating PI3K/Akt/mTOR signaling, thus enhancing the protective effect on cells. The in vivo studies demonstrated that intravitreal injection of Tn protected mice from light- and NMDA-induced retinal damage and dysfunction. Consequently, it is reasonable to hypothesize that Tn is a highly potent Nrf2 activator that could become a new therapeutic agent for retinal oxidative stress injury and pathological neovascular ocular diseases such as AMD.
Supplementary Data
Availability of data and materials
The data generated in the present study may be requested from the corresponding author.
Authors' contributions
KL designed and supervised the study. JinL, JiaL and YC performed the experiments, analyzed data, followed-up experimental supplement and wrote the manuscript. JY, YS and LL participated in parts of the experiments. JiaL, YC and KL confirm the authenticity of all the raw data. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The animal experimental procedures in the present study were approved (approval no. 2303048) by the Ethics Committee of Nanjing Medical University (Nanjing, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National Natural Science Foundation of China (grant nos. 82171080 and 82101156).
References
|
Thomas CJ, Mirza RG and Gill MK: Age-related macular degeneration. Med Clin North Am. 105:473–491. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Hanus J, Anderson C and Wang S: RPE necroptosis in response to oxidative stress and in AMD. Ageing Res Rev. 24:286–298. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Liang FQ and Godley BF: Oxidative stress-induced mitochondrial DNA damage in human retinal pigment epithelial cells: A possible mechanism for RPE aging and age-related macular degeneration. Exp Eye Res. 76:397–403. 2003. View Article : Google Scholar : PubMed/NCBI | |
|
Tu W, Wang H, Li S, Liu Q and Sha H: The anti-inflammatory and anti-oxidant mechanisms of the keap1/Nrf2/ARE signaling pathway in chronic diseases. Aging Dis. 10:637–651. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Ulyanova T, Szél A, Kutty RK, Wiggert B, Caffé AR, Chader GJ and van Veen T: Oxidative stress induces heme oxygenase-1 immunoreactivity in Müller cells of mouse retina in organ culture. Invest Ophthalmol Vis Sci. 42:1370–1374. 2001.PubMed/NCBI | |
|
Flores R, Carneiro Â, Vieira M, Tenreiro S and Seabra MC: Age-related macular degeneration: Pathophysiology, management, and future perspectives. Ophthalmologica. 244:495–511. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Zhang X and Sivaprasad S: Drusen and pachydrusen: The definition, pathogenesis, and clinical significance. Eye (Lond). 35:121–133. 2021. View Article : Google Scholar | |
|
Hernández-Zimbrón LF, Zamora-Alvarado R, Ochoa-De la Paz L, Velez-Montoya R, Zenteno E, Gulias-Cañizo R, Quiroz-Mercado H and Gonzalez-Salinas R: Age-related macular degeneration: New paradigms for treatment and management of AMD. Oxid Med Cell Longev. 2018:83746472018. View Article : Google Scholar : PubMed/NCBI | |
|
García-Layana A, Cabrera-López F, García-Arumí J, Arias-Barquet L and Ruiz-Moreno JM: Early and intermediate age-related macular degeneration: Update and clinical review. Clin Interv Aging. 12:1579–1587. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Cabral de Guimaraes TA, Daich Varela M, Georgiou M and Michaelides M: Treatments for dry age-related macular degeneration: Therapeutic avenues, clinical trials and future directions. Br J Ophthalmol. 106:297–304. 2022. View Article : Google Scholar | |
|
Damico FM, Gasparin F, Scolari MR, Pedral LS and Takahashi BS: New approaches and potential treatments for dry age-related macular degeneration. Arq Bras Oftalmol. 75:71–76. 2012. View Article : Google Scholar : PubMed/NCBI | |
|
Li KR, Yang SQ, Gong YQ, Yang H, Li XM, Zhao YX, Yao J, Jiang Q and Cao C: 3H-1,2-dithiole-3-thione protects retinal pigment epithelium cells against Ultra-violet radiation via activation of Akt-mTORC1-dependent Nrf2-HO-1 signaling. Sci Rep. 6:255252016. View Article : Google Scholar : PubMed/NCBI | |
|
Li KR, Zhang ZQ, Yao J, Zhao YX, Duan J, Cao C and Jiang Q: Ginsenoside Rg-1 protects retinal pigment epithelium (RPE) cells from cobalt chloride (CoCl2) and hypoxia assaults. PLoS One. 8:e841712013. View Article : Google Scholar | |
|
Ong FS, Kuo JZ, Wu WC, Cheng CY, Blackwell WLB, Taylor BL, Grody WW, Rotter JI, Lai CC and Wong TY: Personalized medicine in ophthalmology: From pharmacogenetic biomarkers to therapeutic and dosage optimization. J Pers Med. 3:40–69. 2013. View Article : Google Scholar : PubMed/NCBI | |
|
Liu R, Li X, Huang N, Fan M and Sun R: Toxicity of traditional Chinese medicine herbal and mineral products. Adv Pharmacol. 87:301–346. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Tang B, Zhu J, Zhang B, Wu F, Wang Y, Weng Q, Fang S, Zheng L, Yang Y, Qiu R, et al: Therapeutic potential of triptolide as an anti-inflammatory agent in dextran sulfate sodium-induced murine experimental colitis. Front Immunol. 11:5920842020. View Article : Google Scholar : PubMed/NCBI | |
|
Liu Q: Triptolide and its expanding multiple pharmacological functions. Int Immunopharmacol. 11:377–383. 2011. View Article : Google Scholar : PubMed/NCBI | |
|
Zheng YL, Lin JF, Lin CC and Xu Y: Anti-inflammatory effect of triptolide. Zhongguo Yao Li Xue Bao. 15:540–543. 1994.In Chinese. PubMed/NCBI | |
|
Buendia I, Michalska P, Navarro E, Gameiro I, Egea J and León R: Nrf2-ARE pathway: An emerging target against oxidative stress and neuroinflammation in neurodegenerative diseases. Pharmacol Ther. 157:84–104. 2016. View Article : Google Scholar | |
|
Baird L and Yamamoto M: The molecular mechanisms regulating the KEAP1-NRF2 pathway. Mol Cell Biol. 40:e00099–20. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Hui Q, Karlstetter M, Xu Z, Yang J, Zhou L, Eilken HM, Terjung C, Cho H, Gong J, Lai MJ, et al: Inhibition of the Keap1-Nrf2 protein-protein interaction protects retinal cells and ameliorates retinal ischemia-reperfusion injury. Free Radic Biol Med. 146:181–188. 2020. View Article : Google Scholar : | |
|
Han S, Chen J, Hua J, Hu X, Jian S, Zheng G, Wang J, Li H, Yang J, Hejtmancik JF, et al: MITF protects against oxidative damage-induced retinal degeneration by regulating the NRF2 pathway in the retinal pigment epithelium. Redox Biol. 34:1015372020. View Article : Google Scholar : PubMed/NCBI | |
|
Dai S, Wang C, Feng L, Zhang C, Zhang W, He Y, Zhou X, Xia X, Chen B and Song W: Protective activity of tert-butylhydroquinone against oxidative stress and apoptosis induced by glutamate agonizts in R28 cells and mice retina. Biomed Pharmacother. 152:1131172022. View Article : Google Scholar : PubMed/NCBI | |
|
Ozawa Y: Oxidative stress in the light-exposed retina and its implication in age-related macular degeneration. Redox Biol. 37:1017792020. View Article : Google Scholar : PubMed/NCBI | |
|
Wu T, Handa JT and Gottsch JD: Light-induced oxidative stress in choroidal endothelial cells in mice. Invest Ophthalmol Vis Sci. 46:1117–1123. 2005. View Article : Google Scholar : PubMed/NCBI | |
|
Livak KJ and Schmittgen TD: Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001. View Article : Google Scholar | |
|
Lambert V, Lecomte J, Hansen S, Blacher S, Gonzalez ML, Struman I, Sounni NE, Rozet E, de Tullio P, Foidart JM, et al: Laser-induced choroidal neovascularization model to study age-related macular degeneration in mice. Nat Protoc. 8:2197–2211. 2013. View Article : Google Scholar : PubMed/NCBI | |
|
Beatty S, Koh H, Phil M, Henson D and Boulton M: The role of oxidative stress in the pathogenesis of age-related macular degeneration. Surv Ophthalmol. 45:115–134. 2000. View Article : Google Scholar : PubMed/NCBI | |
|
Perelman A, Wachtel C, Cohen M, Haupt S, Shapiro H and Tzur A: JC-1: Alternative excitation wavelengths facilitate mitochondrial membrane potential cytometry. Cell Death Dis. 3:e4302012. View Article : Google Scholar : PubMed/NCBI | |
|
Yang F, Wu L, Guo X, Wang D and Li Y: Improved retinal ganglion cell survival through retinal microglia suppression by a chinese herb extract, triptolide, in the DBA/2J mouse model of glaucoma. Ocul Immunol Inflamm. 21:378–389. 2013. View Article : Google Scholar : PubMed/NCBI | |
|
Sakamoto K, Suzuki T, Takahashi K, Koguchi T, Hirayama T, Mori A, Nakahara T, Nagasawa H and Ishii K: Iron-chelating agents attenuate NMDA-Induced neuronal injury via reduction of oxidative stress in the rat retina. Exp Eye Res. 171:30–36. 2018.PubMed/NCBI | |
|
Ferrara N: VEGF and intraocular neovascularization: From discovery to therapy. Transl Vis Sci Technol. 5:102016.PubMed/NCBI | |
|
Bruninx R, Betz P and Lepièce G: Functionnal revalidation of patients with age-related macular degeneration. Rev Med Liege. 75:711–716. 2020.In French. PubMed/NCBI | |
|
Mitchell P, Liew G, Gopinath B and Wong TY: Age-related macular degeneration. Lancet. 392:1147–1159. 2018.PubMed/NCBI | |
|
Telander DG: Inflammation and age-related macular degeneration (AMD). Semin Ophthalmol. 26:192–197. 2011.PubMed/NCBI | |
|
Jones BW, Watt CB, Frederick JM, Baehr W, Chen CK, Levine EM, Milam AH, Lavail MM and Marc RE: Retinal remodeling triggered by photoreceptor degenerations. J Comp Neurol. 464:1–16. 2003.PubMed/NCBI | |
|
Marc RE, Jones BW, Watt CB and Strettoi E: Neural remodeling in retinal degeneration. Prog Retin Eye Res. 22:607–655. 2003.PubMed/NCBI | |
|
Pfeiffer RL, Marc RE, Kondo M, Terasaki H and Jones BW: Müller cell metabolic chaos during retinal degeneration. Exp Eye Res. 150:62–70. 2016.PubMed/NCBI | |
|
Amin RH, Frank RN, Kennedy A, Eliott D, Puklin JE and Abrams GW: Vascular endothelial growth factor is present in glial cells of the retina and optic nerve of human subjects with nonproliferative diabetic retinopathy. Invest Ophthalmol Vis Sci. 38:36–47. 1997.PubMed/NCBI | |
|
Yafai Y, Iandiev I, Lange J, Yang XM, Wiedemann P, Bringmann A and Eichler W: Basic fibroblast growth factor contributes to a shift in the angioregulatory activity of retinal glial (Müller) cells. PLoS One. 8:e687732013. | |
|
Baby B, Antony P and Vijayan R: Antioxidant and anticancer properties of berries. Crit Rev Food Sci Nutr. 58:2491–2507. 2018. | |
|
Durazzo A, Lucarini M, Novellino E, Daliu P and Santini A: Fruit-based juices: Focus on antioxidant properties-Study approach and update. Phytother Res. 33:1754–1769. 2019.PubMed/NCBI | |
|
Chang Z, Qin W, Zheng H, Schegg K, Han L, Liu X, Wang Y, Wang Z, McSwiggin H, Peng H, et al: Triptonide is a reversible non-hormonal male contraceptive agent in mice and non-human primates. Nat Commun. 12:12532021.PubMed/NCBI | |
|
Dong F, Yang P, Wang R, Sun W, Zhang Y, Wang A, Chen M, Chen L, Zhang C and Jiang M: Triptonide acts as a novel antiprostate cancer agent mainly through inhibition of mTOR signaling pathway. Prostate. 79:1284–1293. 2019.PubMed/NCBI | |
|
Estaquier J, Vallette F, Vayssiere JL and Mignotte B: The mitochondrial pathways of apoptosis. Adv Exp Med Biol. 942:157–183. 2012.PubMed/NCBI | |
|
Green DR: The mitochondrial pathway of apoptosis: Part I: MOMP and beyond. Cold Spring Harb Perspect Biol. 14:a0410382022.PubMed/NCBI | |
|
Nakagami Y: Nrf2 is an attractive therapeutic target for retinal diseases. Oxid Med Cell Longev. 2016:74693262016.PubMed/NCBI | |
|
Nam LB and Keum YS: Binding partners of NRF2: Functions and regulatory mechanisms. Arch Biochem Biophys. 678:1081842019.PubMed/NCBI | |
|
Ma Q: Role of nrf2 in oxidative stress and toxicity. Annu Rev Pharmacol Toxicol. 53:401–426. 2013.PubMed/NCBI | |
|
Alhasani RH, Biswas L, Tohari AM, Zhou X, Reilly J, He JF and Shu X: Gypenosides protect retinal pigment epithelium cells from oxidative stress. Food Chem Toxicol. 112:76–85. 2018. | |
|
Du W, An Y, He X, Zhang D and He W: Protection of kaempferol on oxidative stress-induced retinal pigment epithelial cell damage. Oxid Med Cell Longev. 2018:16107512018. View Article : Google Scholar : PubMed/NCBI | |
|
Li Z, Dong X, Liu H, Chen X, Shi H, Fan Y, Hou D and Zhang X: Astaxanthin protects ARPE-19 cells from oxidative stress via upregulation of Nrf2-regulated phase II enzymes through activation of PI3K/Akt. Mol Vis. 19:1656–1666. 2013.PubMed/NCBI | |
|
Zhao B, Wang Z, Han J, Wei G, Yi B and Li Z: Rhizoma Paridis total saponins alleviate H2O2-induced oxidative stress injury by upregulating the Nrf2 pathway. Mol Med Rep. 21:220–228. 2020. | |
|
Zhang H, Liu YY, Jiang Q, Li KR, Zhao YX, Cao C and Yao J: Salvianolic acid A protects RPE cells against oxidative stress through activation of Nrf2/HO-1 signaling. Free Radic Biol Med. 69:219–228. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
Manning BD and Toker A: AKT/PKB signaling: Navigating the network. Cell. 169:381–405. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Hu H, Hao L, Tang C, Zhu Y, Jiang Q and Yao J: Activation of KGFR-Akt-mTOR-Nrf2 signaling protects human retinal pigment epithelium cells from Ultra-violet. Biochem Biophys Res Commun. 495:2171–2177. 2018. View Article : Google Scholar | |
|
Portelli SS, Hambly BD, Jeremy RW and Robertson EN: Oxidative stress in genetically triggered thoracic aortic aneurysm: Role in pathogenesis and therapeutic opportunities. Redox Rep. 26:45–52. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Koundouros N and Poulogiannis G: Phosphoinositide 3-kinase/Akt signaling and redox metabolism in cancer. Front Oncol. 8:1602018. View Article : Google Scholar : PubMed/NCBI | |
|
Wang L, Chen Y, Sternberg P and Cai J: Essential roles of the PI3 kinase/Akt pathway in regulating Nrf2-dependent antioxidant functions in the RPE. Invest Ophthalmol Vis Sci. 49:1671–1678. 2008. View Article : Google Scholar : PubMed/NCBI | |
|
Deng Y, Qiao L, Du M, Qu C, Wan L, Li J and Huang L: Age-related macular degeneration: Epidemiology, genetics, pathophysiology, diagnosis, and targeted therapy. Genes Dis. 9:62–79. 2021. View Article : Google Scholar | |
|
Fleckenstein M, Keenan TDL, Guymer RH, Chakravarthy U, Schmitz-Valckenberg S, Klaver CC, Wong WT and Chew EY: Age-related macular degeneration. Nat Rev Dis Primers. 7:312021. View Article : Google Scholar : PubMed/NCBI | |
|
Yang TJ, Yao MD, Sun YN, Li XM, Jiang Q and Yan B: Suppression of choroidal neovascularization by silencing of long non-coding RNA IPW. Aging (Albany NY). 13:10584–10602. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Ozes ON, Mayo LD, Gustin JA, Pfeffer SR, Pfeffer LM and Donner DB: NF-kappaB activation by tumour necrosis factor requires the Akt serine-threonine kinase. Nature. 401:82–85. 1999. View Article : Google Scholar : PubMed/NCBI | |
|
Wang RC, Wei Y, An Z, Zou Z, Xiao G, Bhagat G, White M, Reichelt J and Levine B: Akt-mediated regulation of autophagy and tumorigenesis through beclin 1 phosphorylation. Science. 338:956–959. 2012. View Article : Google Scholar : PubMed/NCBI | |
|
Agrón E, Mares J, Clemons TE, Swaroop A, Chew EY and Keenan TDL; AREDS and AREDS2 Research Groups: Dietary nutrient intake and progression to late age-related macular degeneration in the age-related eye disease studies 1 and 2. Ophthalmology. 128:425–442. 2021. View Article : Google Scholar | |
|
Narayanan R and Kuppermann BD: Corticosteroids and anti-complement therapy in retinal diseases. Handb Exp Pharmacol. 242:309–320. 2017. View Article : Google Scholar | |
|
O'Neill HC, Limnios IJ and Barnett NL: Advancing a stem cell therapy for age-related macular degeneration. Curr Stem Cell Res Ther. 15:89–97. 2020. View Article : Google Scholar |