CD150‑dependent activation of EBV‑transformed B cells induces the differentiation of peripheral blood monocytes via the secretion of multiple cytokines
- Authors:
- Published online on: July 16, 2024 https://doi.org/10.3892/ijmm.2024.5403
- Article Number: 79
-
Copyright: © Kim et al. This is an open access article distributed under the terms of Creative Commons Attribution License.
Abstract
Introduction
CD150, also termed signaling lymphocyte activation molecule, is a type I transmembrane glycoprotein that belongs to the CD2/CD150 family of the immunoglobulin (Ig) superfamily of proteins (1-3). High CD150 expression is frequently associated with B cell malignancies and is implicated in immunodeficiency in X-linked lymphoproliferative disease (XLP) (4-6). CD150 engagement by an anti-CD150 monoclonal antibody (mAb) enhances cell proliferation and cytokine production and plays either stimulatory or inhibitory roles in mediating important regulatory signals (1,7,8).
CD150 is not detected on immature dendritic cells (DCs), granulocytes, monocytes, red blood cells or natural killer cells. However, it is induced on mature DCs after activation with CD40L or IL-1β and on monocytes after activation by lipopolysaccharides (LPS) or Toll-like receptors (9,10). Peripheral blood monocytes can differentiate into DCs depending on the environmental factors encountered during migration from blood to tissues (10). Upon contact with IL-4 and granulocyte-macrophage colony-stimulating factor (GM-CSF), monocytes differentiate into immature DCs, and the addition of IL-6, IL-1β or tumor necrosis factor α (TNF-α) leads to the maturation of DCs (11). DC maturation is characterized by the upregulated expression of costimulatory and accessory molecules, enhanced expression of major histocompatibility complex (MHC) class I and II molecules and neoexpression of CD83 (12).
CD150 also functions as a receptor for the cellular entry of a number of morbilliviruses, including the measles virus (MV) (2,13). CD150-expressing T cells in the human immune system are important target cells for wild-type MV (14,15), and infection of these cells may contribute to immunosuppression through decreased expression of soluble IL-2 receptor, interferon-γ (IFN-γ), IL-1β and TNF-α (16,17).
CD150 is also a critical regulator of immune responses and plays a significant role in the modulation of cytokine production, particularly in the context of Epstein-Barr virus (EBV) infection. The activation of CD150 on EBV-transformed B cells triggers the production of specific cytokines such as IL-1α and GM-CSF, which are essential for immune cell differentiation and activation (6). Despite several studies showing that CD150 has various immunological functions in cytokine production and intracellular signaling in T cells and other cell types (2,5,8), very little is known about the roles and mechanisms that regulate CD150-induced cytokine secretion in virus-infected B cells or in B-cell malignancies. CD4+ helper T cells are critical for germinal center responses, and the activation of these helper T cells depends on T cell-DC interactions (18-20). Subsequent cognate interactions between activated B cells and T cells induce germinal center formation. Serum amyloid P component-deficient mice exhibit marked defects in B-cell proliferation and germinal-center formation (21). However, the relationship between activated B cells and DCs with CD150 still requires investigation. In the present study, EBV-transformed B cells were utilized as a model system to improve the understanding of the effect of cytokines produced by activated B cells on peripheral blood monocytes. The present study aimed not only to elucidate the relationship between CD150 and cytokine expression in EBV-transformed B cells but also to examine the functional role of various cytokines secreted following cross-linking of CD150 in the differentiation of peripheral blood monocytes. Additionally, the complex network of interactions between CD150 activation and cytokine regulation will be explored, providing insights into the potential therapeutic targets for EBV-associated conditions.
Materials and methods
Reagents, cell culture and antibodies
EBV supernatant stock was prepared from an EBV B95-8 marmoset cell line (a gift from Dr B. G. Han, National Genome Research Institute, National Institute of Health, Seoul, Korea). Raji cells (cat. no. CCL-86), an EBV+ human Burkitt's lymphoma cell line, and IM-9 cells (cat. no. CCL-159), an EBV+ human B lymphoblastoid cell line, were obtained from the American Type Culture Collection and were maintained in RPMI-1640 medium (HyClone; Cytiva) supplemented with 10% fetal bovine serum (FBS; HyClone; Cytiva) and antibiotics in the presence of 5% CO2 at 37°C. The authenticity of the IM-9 human B lymphoblastoid cell line utilized in the present study was verified through human short tandem repeat DNA profiling, and mycoplasma contamination was tested via PCR at Cosmogenetech, Co., Ltd.
MV core proteins [MV#1: amino acids (a.a) 399-525 (cat. no. GWB-64F3B4); MV#2: a.a 89-165 (cat. no. GWB-9FDC44] and a recombinant CD150 fusion protein (cat. no. GWB-PPAT45) were generated by GenWay Biotech, Inc. The purity of the MV core proteins and recombinant CD150 fusion protein was >95%. An anti-CD150 mAb (IPO-3; cat. no. MA1-7626) was purchased from Affinity BioReagents, Inc. UPC-10 (mouse IgG2a; cat. no. M9144) was purchased from Sigma-Aldrich (Merck KGaA) and used as an isotype control. PE-conjugated anti-human CD150 (cat. no. 559592), FITC-conjugated anti-CD20 (cat. no. 555622) and FITC-conjugated anti-human CD14 (cat. no. 555397), CD1a (cat. no. 555806), CD80 (cat. no. 555683), CD86 (cat. no. 555657), CD83 (cat. no. 556910), HLA-DR (cat. no. 555811) and CD11c (cat. no. 561355) antibodies were purchased from BD Biosciences.
Generation of EBV-transformed B cells
A total of 10 ml peripheral blood was collected from 5 healthy human donors (written informed consent was obtained from each participant) to establish an EBV-infected B cell line from normal PBMCs. After 2 h of incubation at 37°C, B cells purified from normal PBMCs were added to the EBV stock supernatant, and RPMI-1640 medium was added to achieve 5×105 cells/ml (22). The cultures were incubated for 2-4 weeks until clumps of EBV-infected B cells were visible and the medium turned yellow. Cell phenotypes were monitored using a FACSCalibur flow cytometer (BD Biosciences) equipped with CellQuestpro software (version 5.2.1; BD Biosciences) and confocal laser-scanning microscope (Carl Zeiss AG) after staining with PE-conjugated anti-human CD150 and FITC-conjugated anti-CD20 antibodies. For intracellular staining, cells were fixed with 4% paraformaldehyde at room temperature for 10 min, followed by permeabilization with 0.1% Triton X-100 in PBS for 5 min at room temperature. Human blood samples were collected from healthy donors between January, 2020 and December, 2020 at The Inje University College of Medicine (Busan, South Korea). The donors ranged in age from 20 to 50 years and included 4 male and 2 female participants. Inclusion criteria required that all volunteers be healthy adults without any chronic illnesses or ongoing infections. The exclusion criteria included a history of autoimmune diseases, recent vaccinations or medication use that could affect immune function. All blood donors provided written consent to participate in the study. The present study was approved by The Institutional Bioethics Review Board at the Inje University Busan Paik Hospital (IRB no. 20-0001). For the activation of purified B cells, the cells were treated for 24 h with LPS at a concentration of 50 μg/ml, ionomycin at a concentration of 50 ng/ml, phorbol-12-myristate-13-acetate (PMA) at a concentration of 20 ng/ml, anti-IgM at a concentration of 40 μg/ml or soluble CD40L (sCD40L) at dilutions of 1:50 and 1:100.
alamarBlue assay
To investigate the effect of CD150 expression on the viability of EBV-transformed B cells, an alamarBlue assay (Bio-Rad Laboratories; cat. no. BUF012B) was used to measure cell viability. EBV-transformed B cells were seeded onto 96-well plates at a density of 5×104 cells per well and treated with recombinant CD150 protein at concentrations ranging from 100 ng/ml to 10 μg/ml for 9 h. As a control, cells were treated with the UPC-10 isotype control. After 72 h of incubation, 20 μl alamarBlue solution (10% of the total culture medium) was added to each well. The fluorescence intensity was measured at 570 nm (excitation) and 600 nm (emission) using a Fluorometer (Synergy HT; Bio-Tek Instruments, Inc.) 4 h after the addition of the dye. Each experiment was performed in triplicate.
CD150 stimulation by antibody cross-linking, MV proteins and recombinant proteins
After 4 weeks of infection, EBV-transformed B cells (1×106 cells/ml) were harvested and washed twice with cold PBS. The cells were resuspended in 100 μl PBS and incubated with an anti-CD150 mAb (IPO-3; 1 μg/ml) or an isotype control (UPC-10; 1 μg/ml) at 37°C for 30 min. The cells were washed with PBS, resuspended in 100 μl PBS and then incubated with goat anti-mouse IgG (2 μg/ml; Sigma-Aldrich; Merck KGaA) for 15 min at 37°C. After incubation, the cells were washed and then cultured in RPMI-1640 medium for an additional 24 or 48 h at 37°C. For stimulation with MV core proteins or recombinant CD150, EBV-transformed B cells (4 weeks after infection, 1×106 cells/ml) were resuspended in 100 μl PBS, incubated with MV#1 (1 μg/ml), MV#2 (1 μg/ml) or recombinant CD150 protein (1 μg/ml) at 37°C for 1 h, then cultured in RPMI-1640 medium for an additional 24 or 48 h at 37°C.
Quantification of human cytokines by ELISA
Culture supernatants from CD150-stimulated EBV-transformed B cells or EBV+ lymphoma cell lines were concentrated using Amicon Ultra-15 Centrifugal Filter units (MilliporeSigma). The concentrations of IL-1α, IL-1β, IL-2, IL-4, IL-5, IL-6, IL-8, IL-10, IL-12, IL-13, IL-17α and GM-CSF in the 10-fold concentrated cell-free culture supernatants were determined using multi-cytokine ELISA (Multi-Analyte ELISArray; cat. no. MER-004A; SABiosciences; Qiagen, Inc.) and single cytokine ELISA (Single Analyte ELISArray; cat. no. 336151; SABiosciences; Qiagen, Inc.). The data are presented as the mean of the biological replicates ± standard deviation (SD).
Reverse transcription (RT)-PCR and RT-quantitative (q)PCR
Total RNA from PBMCs, naïve B cells or EBV-transformed B cells was isolated using a RNeasy Mini kit (Qiagen, Inc.). RNA was reverse transcribed into cDNA using TOPscript™ cDNA synthesis kit (Enzynomics Co., Ltd.). To investigate various cytokine levels, PCR amplification was performed using specific primer sets (Bioneer Corporation) for CD150, IL-1α, IL-1β, IL-4, IL-5, IL-6, IL-8, IL-10, IL-12p35, IL-12p40, IL-17α, GM-CSF and β-actin (Table I). PCR was performed using Prime Taq Premix (Genetbio Co., Ltd.). The cDNAs were amplified by PCR under the following conditions: 30 cycles of denaturation at 95°C for 20 sec, annealing at 56°C for 30 sec and extension at 72°C for 30 sec in a thermal cycler. The PCR products were resolved using a 2% agarose gel stained with ethidium bromide and visualized under UV light using the multiple Gel DOC system (FUJIFILM). Band intensity was quantified using ImageJ software (version 1.54g; National Institutes of Health).
qPCR was conducted using a SYBR Green kit (Takara Bio, Inc.), an iCycler thermal real-time PCR system (Bio-Rad Laboratories, Inc.) and specific primer sets (the same primer sets used in the conventional RT-PCR; Table I). The cDNAs were amplified by PCR under the following conditions: Initial denaturation at 95°C for 10 min, 40 cycles of 95°C for 30 sec, 55°C for 30 sec and 72°C for 1 min. Only experiments where a distinct single peak was observed with a melting temperature different than that of the no template control were used. The relative gene expression compared with unstimulated cells was determined by the built in algorithm of the iCycle iQ real-time detection system software (version 3.10; Bio-Rad Laboratories, Inc.) using an adaptive baseline to determine the threshold cycle (Cq). mRNA fold induction values were calculated using the following equations: ΔCq=Cqtarget-Cqβ-actin, Δ(ΔCq)=(ΔCqstimulated-ΔCqcontrol), mRNA fold change=2−Δ(ΔCq) (23). Experiments were performed in triplicate and data are presented as the mean ± SD.
Human monocyte isolation, differentiation and cell surface phenotyping
For monocyte isolation by plastic adherence, 1×107 PBMCs per well were seeded into 6-well plates (BD Biosciences) and allowed to adhere for 4 h in 2 ml complete medium (CM), consisting of RPMI-1640 supplemented with 10% FBS and antibiotics, at 37°C in 5% CO2. The non-adherent cells were removed and the adherent cells were washed three times with prewarmed CM. The differentiation of peripheral blood monocytes was induced by culturing the cells for 7 days in CM supplemented with the concentrated cell culture supernatant from EBV-transformed B cells treated with MV core protein (MV#1 or MV#2) or from cells treated with CD150 protein. The phenotype of the cells was monitored using a FACSCalibur flow cytometer (BD Biosciences) equipped with CellQuestpro software (version 5.2.1; BD Biosciences) and a confocal laser-scanning microscope (Carl Zeiss AG) at a ×400 original magnification, using FITC-conjugated anti-human CD1a, CD14, CD80, CD86, CD83, HLA-DR and CD11c antibodies (BD Biosciences). Images were acquired using confocal microscopy software release 3.0 (Carl Zeiss AG).
Blocking differentiation with neutralizing antibodies
CD14+ monocytes were incubated for 3 days with culture supernatant derived from UPC-10 mock stimulated or CD150-stimulated EBV-transformed B cells with or without neutralizing antibodies against IL-1α (Abcam; cat. no. ab300501) or GM-CSF (Abcam; cat. no. ab300495) to investigate the function of cytokines secreted by CD150-stimulated EBV-transformed B cells. The cells were then harvested and the expression of various cell surface markers was analyzed using flow cytometry, as aforementioned.
Small interfering (si)RNA transfection by electroporation
In total, three different 19 nucleotide CD150 interfering RNA duplexes (with two 3′ end overhanging dT nucleotides) and negative control siRNA duplexes with either low or medium GC contents were obtained from Bioneer Corporation. The three experimentally verified CD150 siRNA sequences were selected from the Bioneer siRNA database, and the target sequences are listed in Table II. A non-specific siRNA labeled with green fluorescence served as a control for validating the transfection efficiency in each experiment. Cells were transiently transfected by electroporation under optimized conditions. Briefly, cells were electroporated with 200 nM siRNA in serum-free medium in a 0.4 cm electroporation cuvette using the Bio-Rad Gene Pulser Xcell system (Bio-Rad Laboratories, Inc.). The electroporation pulse protocol included a single pulse at room temperature for 20 msec, an applied voltage of 250 V, a current measure of 0.1 mA, and a pulse repetition frequency of 1. The pulsing buffer used had a conductivity of 1.0 S/m and an osmolarity of 270 mOsm. Post-electroporation, cells were immediately transferred to a recovery medium composed of RPMI-1640 supplemented with 10% FBS and incubated for 24 h at 37°C with 5% CO2. In the experiments assessing CD150-induced cytokine production, 24 h after transfection, the cells were either left untreated (mock group) or treated with an anti-CD150 mAb for 24 h. For certain experiments, the extracted RNA was analyzed using RT-qPCR.
Statistical analysis
All data are presented as the mean ± SD, and each value represents at least three different experiments. The Shapiro-Wilk test was used to check the normality of the data and Levene's test verified the homogeneity of variance before one-way analysis of variance (ANOVA). ANOVA followed by Scheffe's test was used for the analysis of differences between each treatment condition. P<0.05 was considered to indicate a statistically significant difference. Statistical analyses were performed using SPSS software (version 26.0; IBM Corp.).
Results
CD150 expression on B cells is induced by EBV infection
EBV-infected B cells were harvested every week for 4 weeks after the transformation process was complete to determine CD150 expression patterns using flow cytometry and confocal microscopy. CD150 was minimally expressed on the surface and in the cytoplasm of CD20+ resting B cells. The expression of CD150 slightly increased 1 week after infection and elevated quickly thereafter. After 4 weeks of EBV infection, most of the EBV-transformed B cells strongly expressed CD150 molecules on the cell surface and in the cytoplasm (Fig. 1A and B). We acknowledge that Total Internal Reflection Fluorescence microscopy could offer more precise surface signal imaging; however, we chose confocal microscopy to capture both surface and intracellular details. The images presented in Fig. 1B are single plane images, allowing for detailed visualization of the distribution of the target molecules. This approach complemented the FACS results shown in Fig. 1A, which quantitatively validated the surface expression. CD150 mRNA was weakly expressed until 1 week after infection but was likewise significantly upregulated 4 weeks after EBV infection (Fig. 1C). It was also observed that CD150 expression in resting B cells purified from human blood was induced after treatment with LPS, sCD40L, anti-IgM, PMA and ionomycin (Fig. S1A). Moreover, the other EBV+ lymphoma cell lines, IM-9 and Raji, also expressed high levels of CD150 on their cell surfaces (data not shown). These results suggested that the expression of CD150 was induced on B cells over time after EBV infection and could be upregulated by different immune stimuli, highlighting its potential role in the immune response to EBV.
CD150 stimulation by cross-linking induces the production of various cytokines in EBV-transformed B cells and EBV+ lymphoma cell lines
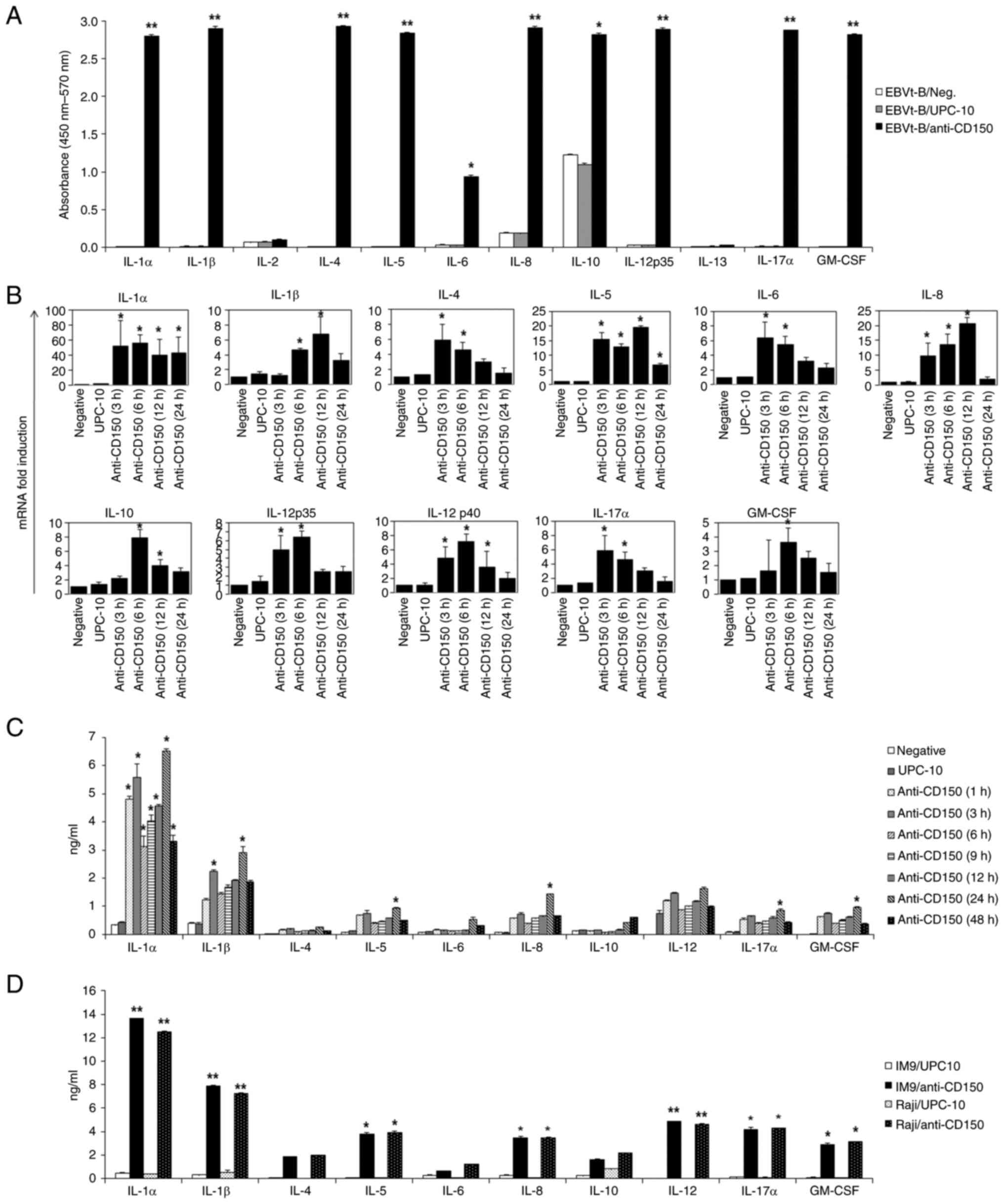
The effect of CD150 stimulation on the viability of EBV-transformed B cells was next investigated. After stimulation with CD150, EBV-transformed B cells were incubated with an anti-CD150 mAb (IPO-3), followed by cross-linking with a secondary antibody. These cells were cultured in the presence of alamarBlue to detect cell viability with a colorimetric analysis. It was observed that CD150 expression had a marked effect on the viability of EBV-transformed B cells (Fig. S1B). Subsequently, it was next examined whether the improved cell viability was related to cytokine production, since numerous cytokines are potent effectors of the maintenance of cell survival (24). EBV-transformed B cells were stimulated with IPO-3 or mock stimulated with the UPC-10 isotype control, followed by cross-linking with a secondary antibody. Multiplex cytokine ELISA, which enabled monitoring of the changes in the levels of multiple cytokines in the culture supernatant, revealed significant increases in the secretion of a number of cytokines, such as IL-1α, IL-1β, IL-4, IL-5, IL-6, IL-8, IL-10, IL-12A, IL-17α and GM-CSF, after stimulation with CD150, but the secreted IL-2 and IL-13 levels did not increase (Fig. 2A).
The changes in the expression of these secreted cytokines were also quantitatively measured using RT-qPCR and single cytokine ELISA. The cytokine mRNA levels increased at 3 or 6 h in EBV-transformed B cells after stimulation with the anti-CD150 mAb (Fig. 2B). Compared with the levels of cytokines secreted by EBV-transformed B cells after mock stimulation with UPC-10, the protein levels of most cytokines were also notably increased at 24 h following CD150 stimulation (P<0.05; Fig. 2C). Notably, the production of IL-1α following CD150 stimulation was markedly greater than that of other cytokines at the mRNA and protein levels. Similarly, the EBV+ lymphoma cell lines, IM-9 and Raji, displayed increased production of various cytokines after 24 h of stimulation with CD150. Although the levels of individual cytokines varied slightly between the cell lines, the overall pattern of cytokine increases in response to CD150 stimulation was consistent with that observed in the EBV-transformed B cells (Fig. 2D). These results suggested that stimulation of CD150 on B cells infected with EBV by antibody cross-linking might result in the production of multiple cytokines, which may affect immune cell proliferation.
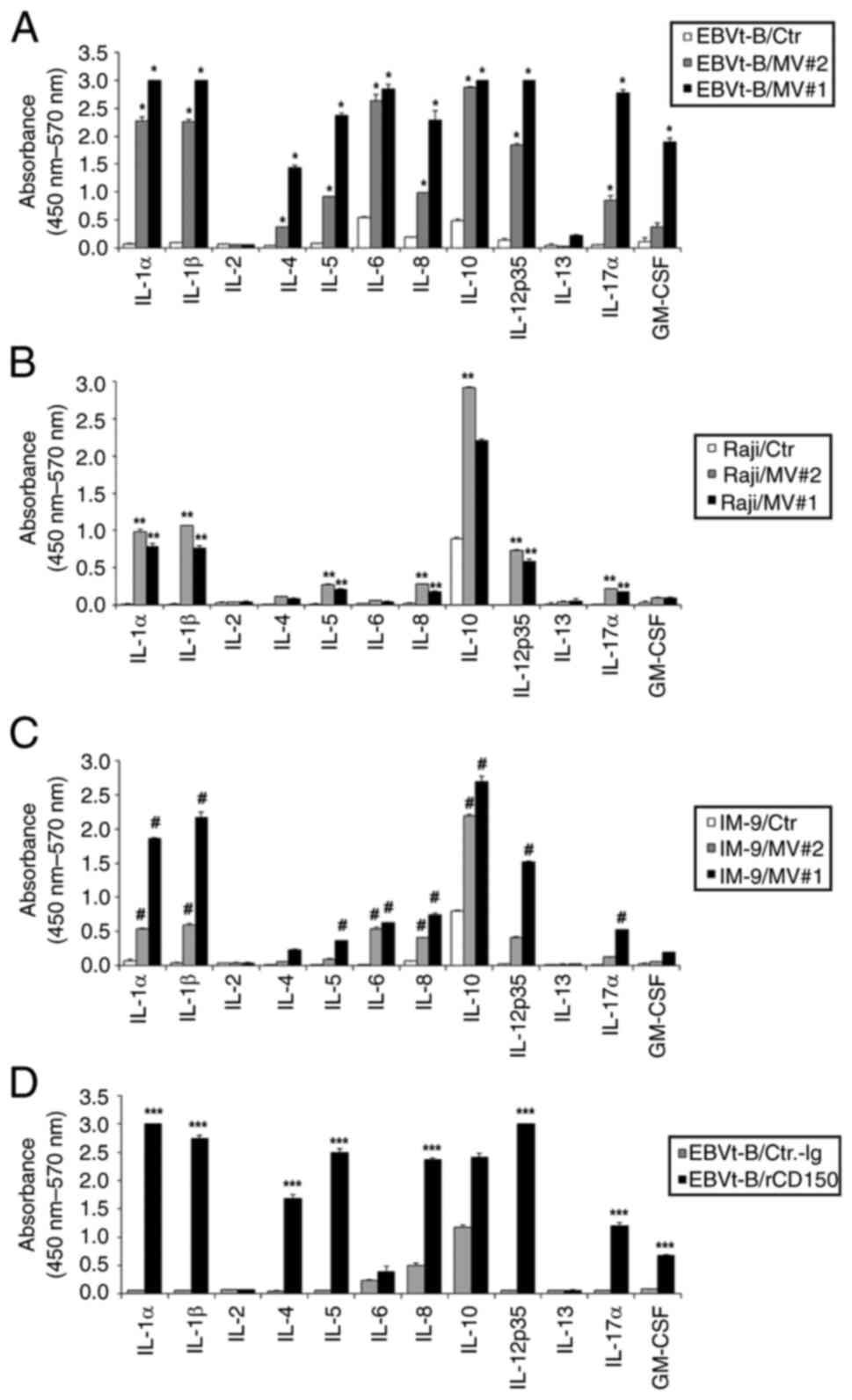
Stimulation with MV core proteins or recombinant CD150 protein induces the production of multiple cytokines in EBV-transformed B cells and EBV+ lymphoma cell lines
CD150 acts as a receptor for MV and mediates virus uptake (14). Therefore, the impact of CD150 activation on cytokine production was explored by treating EBV-transformed B cells, Raji cells and IM-9 cells with MV core protein fragments (MV#1 and #2) or recombinant CD150 protein. After 24 h of treatment, the cytokine levels in the cell culture supernatants were measured using a multiple cytokine ELISA kit. The findings showed that MV#1 (a.a 399-525; 1 μg/ml) and MV#2 (a.a 89-165; 1 μg/ml), as with anti-CD150 mAb, significantly induced the production of various cytokines, with IL-1α, IL-1β, IL-10 and IL-12 being notably elevated (Fig. 3A-C). Additionally, recombinant CD150 protein (1 μg/ml), a natural ligand of CD150, resulted in cytokine profiles like those observed with anti-CD150 mAb treatment (Fig. 3D). These results indicate that CD150 activation, whether by its natural ligand or by MV core protein fragments, can regulate cytokine production. This suggests that CD150 potentially functions as a receptor for MV, mediating similar signaling pathways involved in cytokine regulation (25).
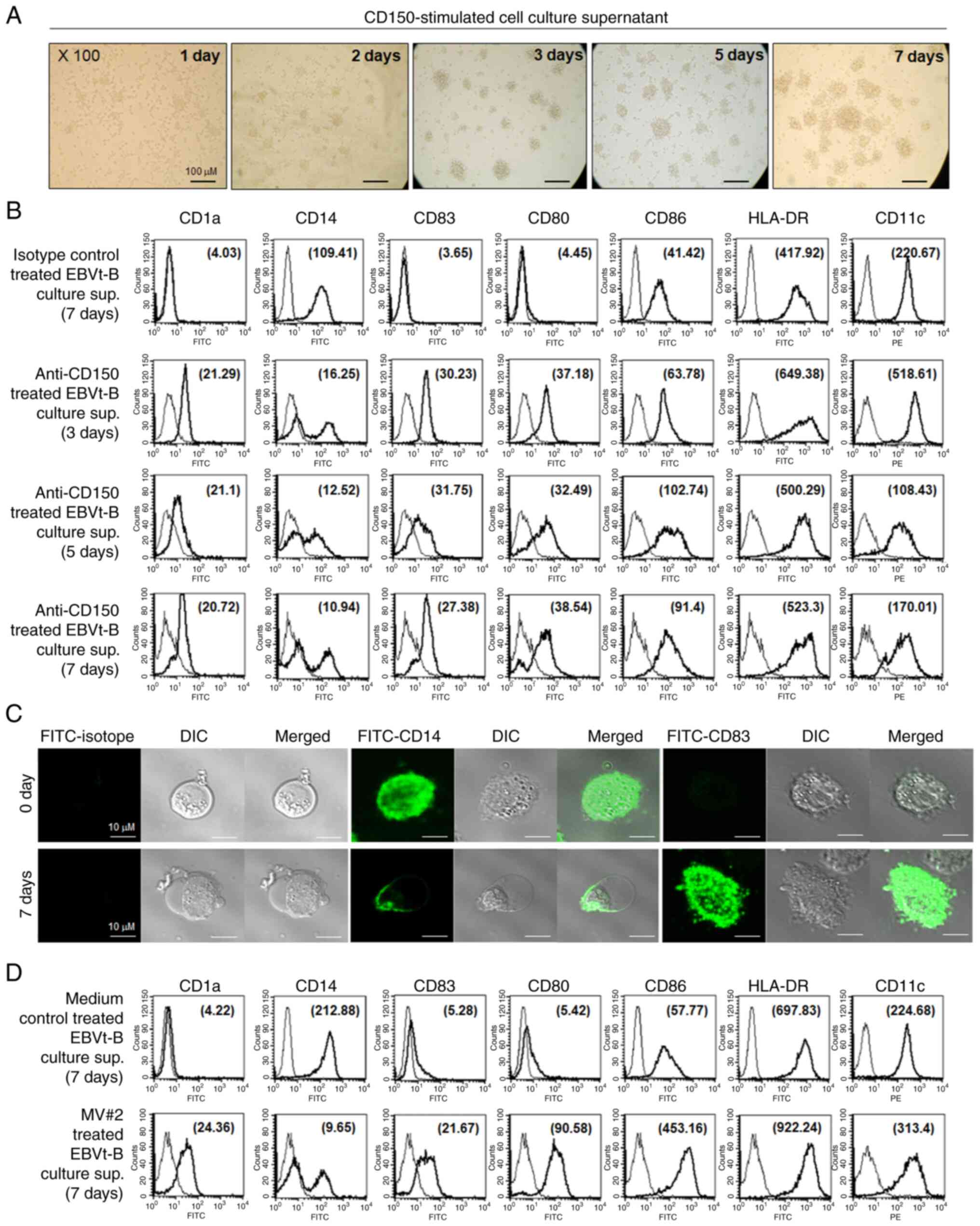
Cytokines produced following CD150 stimulation induce the differentiation of monocytes into DCs
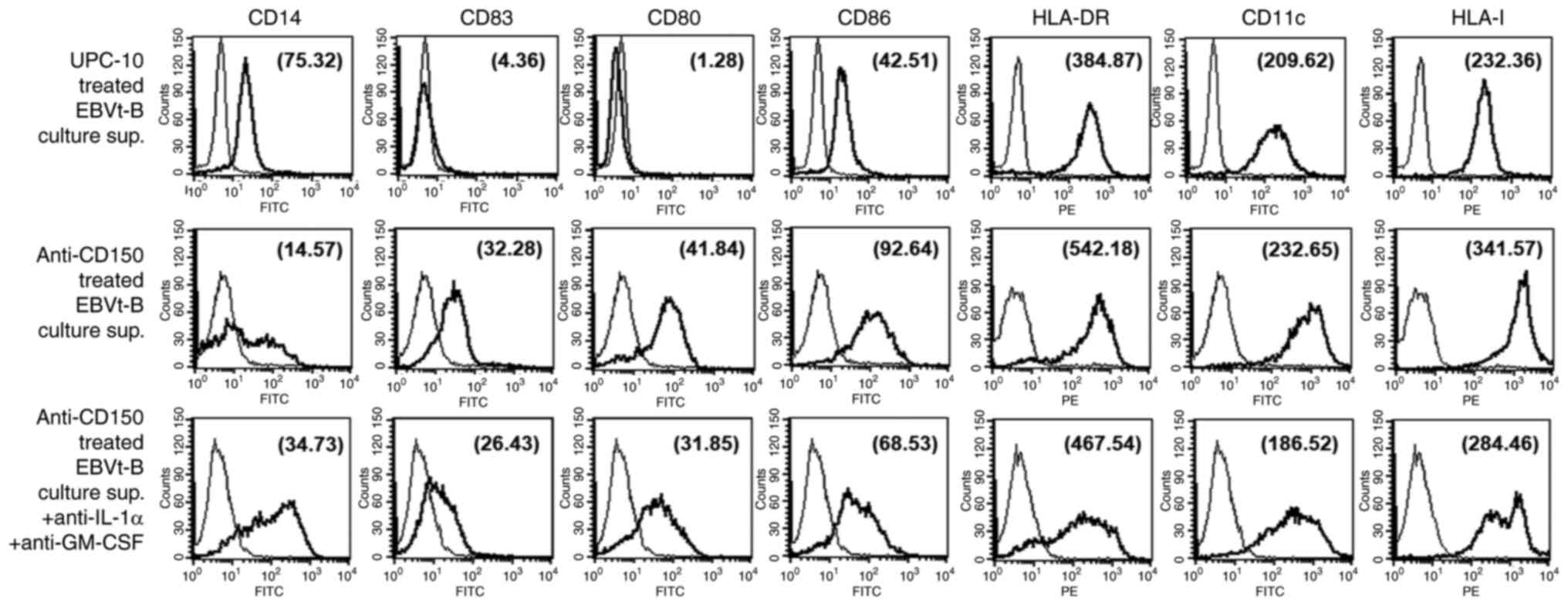
CD150 has been shown to be involved in the regulation of a variety of cellular functions, including proliferation and the expression of certain cytokines (7,8). However, to the best of our knowledge, the ability of CD150 on EBV-transformed B cells to regulate the function of immune cells has not been reported. The effect of the multiple cytokines secreted after CD150 stimulation on the differentiation of peripheral blood monocytes was next examined. DCs generated from monocytes (MoDCs) were used as a model to study the biology of DCs in vitro. Monocytes were isolated from healthy human PBMCs and subsequently incubated with culture supernatant derived from CD150-stimulated EBV-transformed B cells for 7 days to examine MoDC differentiation. Exposure of these monocytes to culture supernatant derived from CD150-stimulated EBV-transformed B cells resulted in cluster formation in a dose-dependent manner (data not shown). The morphology of cell clusters changed to that typical of DCs on day 3, and the cells retained their DC morphology for at least 7 days (Fig. 4A). Peripheral blood monocytes displayed a broad range of elevated surface markers and morphologies consistent with those of fully mature DCs (CD83+CD14−CD80+CD86highHLA-DRhigh) after culture for 3 days in the presence of culture supernatant derived from CD150-stimulated EBV-transformed B cells. By contrast, monocytes incubated with culture supernatant derived from unstimulated EBV-transformed B cells expressed almost no detectable CD83, CD1a or CD80 and retained relatively high levels of CD14, a marker of peripheral blood monocytes (Fig. 4B). The phenotypes and morphology of differentiated monocytes were also observed via fluorescence microscopy, which revealed decreased CD14 expression and high CD83 expression, consistent with mature DC development (Fig. 4C). It was also observed that peripheral blood monocytes exhibited increased expression of CD1a, CD80, CD83, CD86 and HLA-DR and decreased expression of CD14 after incubation with culture supernatant derived from MV#2-treated EBV-transformed B cells for 7 days (Fig. 4D). These results suggested that the cytokines produced by CD150 stimulation may be potent mediators of the transformation of peripheral blood monocytes into DCs with mature phenotypes.
Cytokine-induced differentiation of monocytes into DCs by CD150-stimulated EBV-transformed B cells is partially due to IL-1a and GM-CSF
Rapid DC maturation from monocytes can also be induced by the addition of proinflammatory mediators together with GM-CSF and IL-4 at the initiation of culture (26,27). The functional role of cytokines produced by CD150-stimulated EBV-transformed B cells was investigated using neutralizing antibodies against IL-1α and GM-CSF, which are the cytokines secreted most abundantly by EBV-transformed B cells following stimulation with an anti-CD150 antibody and are essential for the differentiation of monocytes into DCs. The differentiation of monocytes treated with anti-IL-1α and anti-GM-CSF neutralizing antibodies was partially inhibited, as shown by the significant reduction in the expression of the DC surface markers, CD1a, CD83 and CD86 compared with the control (Fig. 5). This result suggested that the differentiation of peripheral blood monocytes into DCs requires IL-1α and GM-CSF as well as other cytokines (such as IL-1β, IL-4 and IL-6).
CD150 knockdown in EBV-transformed B cells results in decreased cytokine production
To determine the role of CD150 in cytokine regulation, siRNAs were used to knockdown CD150 expression in EBV-transformed B cells. The efficacy and specificity of three different siRNAs (si-CD150 #1, #2 and #3) were evaluated and si-CD150 #3 was selected for further experiments due to its more notable effect in reducing CD150 expression (Fig. S2). Following CD150 knockdown, the cells were stimulated with anti-CD150 mAb or isotype control, and the cytokine levels were measured using RT-qPCR (Fig. 6A) and ELISA (Fig. 6B). The results showed that CD150 knockdown significantly suppressed cytokine production compared with the control groups, highlighting the critical role of CD150 in modulating cytokine responses in EBV-transformed B cells (25).
Discussion
The results of the present study revealed that CD150 activation in EBV-transformed B cells triggered the production of cytokines such as IL-1α and GM-CSF, directly impacting immune cell differentiation and activation. CD150 activation also affected the broader cytokine milieu, influencing both pro-inflammatory and anti-inflammatory responses. The elevation of IL-1α and GM-CSF suggested that CD150 enhanced the immune response against EBV, potentially aiding the proliferation and survival of EBV-transformed B cells. This dual role of CD150 in balancing immune responses and contributing to EBV persistence underscores the need for further investigations into targeting this pathway for therapeutic interventions in EBV-associated conditions.
CD150 is a pivotal signaling molecule to produce various cytokines, and the present study provides promising and novel data showing the expression of a variety of cytokine genes and the secretion of multiple cytokines by EBV-transformed B cells, which is likely to contribute to the activation of host immune responses. In humans, CD150 is constitutively expressed on immature thymocytes and CD45RO+ memory T cells and is rapidly induced on naïve B and T cells following activation (28,29). CD150 is a self-ligand considered to have a notable role in adhesion and in signaling in the immune synapse between T cells and antigen-presenting cells (30). Moreover, CD150 may be involved in cancer malignancy and autoimmune disorders, including inflammatory bowel syndrome, rheumatoid arthritis, multiple sclerosis, hepatitis, systemic lupus erythematosus and XLP (3-5,31). The results of the present study demonstrated that CD150 was gradually expressed on the B cell surface during EBV infection and was fully expressed on the surface after 4 weeks of EBV exposure. Therefore, EBV does appear to regulate the expression of CD150. It was also observed that CD150 expression in resting B cells purified from human blood was induced after treatment with LPS, CD40L, anti-IgM, PMA and ionomycin. These results were consistent with the findings of previous studies that indicate that CD150 expression is enhanced by mitogens and is elevated in EBV+ Burkitt's lymphomas and human T-lymphotropic virus type 1-transformed T cell lines (32,33). The findings of the present study suggested that EBV infection triggers CD150 expression, similar to how B cell activation occurs in response to antigens. This insight into the involvement of CD150 in cytokine production and its increased expression during EBV infection highlights CD150 as a potential target for treating EBV-related conditions. Cytokines play a pivotal role in immune responses and inflammation, suggesting that strategies targeting CD150 could be effective in managing EBV-induced B cell transformations and associated cancer types.
In the present study, it was expected that the anti-CD150 mAb would increase the apoptosis of EBV-transformed B cells. This assumption was based on the antiproliferative effects of CD150, which inhibits the proliferation of Hodgkin's lymphoma cell lines and induces cell death following stimulation (34). However, the results of the present study indicated that CD150 stimulation led to an increase in EBV-transformed B cell viability and the secretion of multiple cytokines, including both proinflammatory and anti-inflammatory cytokines, under optimal conditions. Although the expression of some of these genes (such as IL-10) (35) by EBV-associated LCL cells has already been reported in the literature, the results of the present study provide evidence that EBV-transformed B cells potentially produce a variety of regulatory cytokines. Certain cytokines are able to regulate the proliferation of EBV-infected B cells directly (such as IL-1 and IL-10) (35), while others are notable regulators of leukocyte activity (such as IL-4, IL-5 and IL-10) (10) or the differentiation of peripheral blood monocytes (such as IL-4, IL-12 and GM-CSF) (26,27). This unexpected outcome highlights the complexity of the interaction of EBV with host cell signaling pathways, specifically through CD150, suggesting that EBV may exploit these mechanisms to modulate the immune environment.
Several viruses, including MV, human herpesvirus 6 and mouse hepatitis virus, utilize CD150 receptors to maintain equilibrium with their host and evade immune surveillance. These viruses have developed a variety of immune evasion programs based on the regulation of cell surface molecules that serve as viral receptors and on the regulation of cytokine expression (36,37). CD150, which possesses Ig superfamily domains or complement control protein domains, acts as a receptor for MV and mediates virus uptake. In the present study, incubation of EBV-transformed B cells, Raji cells and IM-9 cells with MV core protein #1 or #2 caused the cells to produce multiple cytokines. Specifically, IL-1α, IL-1β, IL-10 and IL-12 levels were highly increased, while the levels of secreted IL-5, IL-8 and IL-17α increased slightly. IL-4 and GM-CSF levels were also slightly elevated, while IL-2 was not detected and the IL-13 level was only slightly increased following stimulation with MV #2. In the present study, it was also determined that inhibition of CD150 using siRNAs might be a potential strategy for blocking the production of multiple cytokines, although further research is needed to fully understand the implications of these interventions for both EBV-associated diseases and normal immune functions.
Certain cancer cells have the capacity to produce multiple cytokines as growth factors, and at least some of these growth factors and their receptors may act in tumor cells via paracrine and/or autocrine loop mechanisms, either by extracellular release of the growth factor or by action of the tumor itself (38). For instance, GM-CSF, a key growth factor for the differentiation of DCs, stimulates the proliferation and growth of some malignant hematopoietic cell types, including acute leukemia and lymphomas (39), and receptors for GM-CSF are present on some leukemic cell lines (40). Typically, DCs used in experimental and clinical fusion trials are obtained primarily from standard in vitro 7-day cultures. The standard 7-day culture protocol yields mature DCs with the ability to stimulate primary antigen-specific Th1-type immune responses, induce the production of IFN-γ and activate autologous naïve T cells (41). Moreover, Hanazawa et al (42) reported that IL-1α and IL-1β potently induce the differentiation of the macrophage-like tumor cell line, P388D1. However, in the present study, typical DC features were observed within 2 days after normal monocytes were exposed to the CD150-stimulated EBV-transformed B cell culture supernatant. In addition, IL-1α was the cytokine secreted at the highest level by EBV-transformed B cells. It was also found that the differentiation of monocytes incubated with anti-IL-1α and anti-GM-CSF neutralizing antibodies was partially inhibited. These results suggest that the differentiation of peripheral blood monocytes into DCs requires IL-1α and GM-CSF, as well as other cytokines (such as IL-1β, IL-4 and IL-6). This accelerated differentiation, driven by cytokines such as IL-1α and GM-CSF, suggests that EBV may exploit CD150 signaling to alter the immune landscape, promoting an environment conducive to its persistence and the proliferation of transformed cells.
In conclusion, the findings of the present study highlight the crucial role of CD150 in regulating cytokine production and immune responses in EBV-transformed B cells. The observed elevation of cytokines such as IL-1α and GM-CSF suggests that targeting CD150 could be a promising strategy for managing EBV-related conditions. Despite these insights, the complexity of the functions of CD150 requires further research to fully explore its therapeutic potential in combating EBV-related diseases. Moreover, the experimental design of the present study did not vary the infection titer of EBV, focusing instead on observing changes over time post-infection. This limitation suggests that future studies should consider varying the infection titer to fully understand its impact on CD150 expression and cytokine regulation.
Supplementary Data
Availability of data and materials
The data generated in the present study may be requested from the corresponding author.
Authors' contributions
All authors made a significant contribution to the present study. IKS performed the investigation and data curation. DYH acquired funding, wrote the original draft of the manuscript and contributed to the conception or design of the study and the acquisition of data. HYK contributed significantly to the conception and design of the study, edited and revised the manuscript and read and approved the final version of the manuscript. All authors read and approved the final version of the manuscript. HYK and DYH confirm the authenticity of all the raw data.
Ethics approval and consent to participate
The present study using human blood was approved by The Institutional Bioethics Review Board at the Inje University Busan Paik Hospitals (Busan, South Korea; IRB no. 20-0001), and all donors gave written informed consent for the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have a competing interest related to the following patent: US patent no. 9845456B2, 'Composition containing complex cytokines derived from EBV-infected B cells for inducing the maturation of dendritic cells', published on December 19, 2017. The patent details methods for using CD150 to modulate immune responses, which are relevant to the findings presented in this manuscript.
Acknowledgements
Not applicable.
Funding
This study was supported by The National Research Foundation of Korea grant funded by the Korea government (MSIT) (grant no. RS-2024-00354069) and supported by Basic Science Research Program through the National Research Foundation of Korea funded by the Ministry of Education (grant no. RS-2023-00248268).
References
|
Cocks BG, Chang CC, Carballido JM, Yssel H, de Vries JE and Aversa G: A novel receptor involved in T-cell activation. Nature. 376:260–263. 1995. View Article : Google Scholar : PubMed/NCBI | |
|
Sidorenko SP and Clark EA: The dual-function CD150 receptor subfamily: The viral attraction. Nat Immunol. 4:19–24. 2003. View Article : Google Scholar | |
|
Farhangnia P, Ghomi SM, Mollazadehghomi S, Nickho H, Akbarpour M and Delbandi AA: SLAM-family receptors come of age as a potential molecular target in cancer immunotherapy. Front Immunol. 14:11741382023. View Article : Google Scholar : PubMed/NCBI | |
|
Kis LL, Nagy N, Klein G and Klein E: Expression of SH2D1A in five classical Hodgkin's disease-derived cell lines. Int J Cancer. 104:658–661. 2003. View Article : Google Scholar : PubMed/NCBI | |
|
Sayos J, Wu C, Morra M, Wang N, Zhang X, Allen D, van Schaik S, Notarangelo L, Geha R, Roncarolo MG, et al: The X-linked lymphoproliferative-disease gene product SAP regulates signals induced through the co-receptor SLAM. Nature. 395:462–469. 1998. View Article : Google Scholar : PubMed/NCBI | |
|
Shcherbina V, Gordiienko I, Shlapatska L, Gluzman D and Sidorenko S: CD150 and CD180 are negative regulators of IL-10 expression and secretion in chronic lymphocytic leukemia B cells. Neoplasma. 68:760–769. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Sidorenko SP and Clark EA: Characterization of a cell surface glycoprotein IPO-3, expressed on activated human B and T lymphocytes. J Immunol. 151:4614–4624. 1993. View Article : Google Scholar : PubMed/NCBI | |
|
Aversa G, Chang CC, Carballido JM, Cocks BG and de Vries JE: Engagement of the signaling lymphocytic activation molecule (SLAM) on activated T cells results in IL-2-independent, cyclosporin A-sensitive T cell proliferation and IFN-gamma production. J Immunol. 158:4036–4044. 1997. View Article : Google Scholar : PubMed/NCBI | |
|
Howie D, Okamoto S, Rietdijk S, Clarke K, Wang N, Gullo C, Bruggeman JP, Manning S, Coyle AJ, Greenfield E, et al: The role of SAP in murine CD150 (SLAM)-mediated T-cell proliferation and interferon gamma production. Blood. 100:2899–2907. 2002. View Article : Google Scholar : PubMed/NCBI | |
|
Farina C, Theil D, Semlinger B, Hohlfeld R and Meinl E: Distinct responses of monocytes to Toll-like receptor ligands and inflammatory cytokines. Int Immunol. 16:799–809. 2004. View Article : Google Scholar : PubMed/NCBI | |
|
Chapuis F, Rosenzwajg M, Yagello M, Ekman M, Biberfeld P and Gluckman JC: Differentiation of human dendritic cells from monocytes in vitro. Eur J Immunol. 27:431–441. 1997. View Article : Google Scholar : PubMed/NCBI | |
|
Randolph GJ, Beaulieu S, Lebecque S, Steinman RM and Muller WA: Differentiation of monocytes into dendritic cells in a model of transendothelial trafficking. Science. 282:480–483. 1998. View Article : Google Scholar : PubMed/NCBI | |
|
Tatsuo H, Ono N and Yanagi Y: Morbilliviruses use signaling lymphocyte activation molecules (CD150) as cellular receptors. J Virol. 75:5842–5850. 2001. View Article : Google Scholar : PubMed/NCBI | |
|
de Swart RL, Ludlow M, de Witte L, Yanagi Y, van Amerongen G, McQuaid S, Yüksel S, Geijtenbeek TB, Duprex WP and Osterhaus AD: Predominant infection of CD150+ lymphocytes and dendritic cells during measles virus infection of macaques. PLoS Pathog. 3:e1782007. View Article : Google Scholar : PubMed/NCBI | |
|
Amurri L, Reynard O, Gerlier D, Horvat B and Iampietro M: Measles virus-induced host immunity and mechanisms of viral evasion. Viruses. 14:26412022. View Article : Google Scholar : PubMed/NCBI | |
|
Hirsch RL, Griffin DE, Johnson RT, Cooper SJ, Lindo de Soriano I, Roedenbeck S and Vaisberg A: Cellular immune responses during complicated and uncomplicated measles virus infections of man. Clin Immunol Immunopathol. 31:1–12. 1984. View Article : Google Scholar : PubMed/NCBI | |
|
Ward BJ, Johnson RT, Vaisberg A, Jauregui E and Griffin DE: Cytokine production in vitro and the lymphoproliferative defect of natural measles virus infection. Clin Immunol Immunopathol. 61:236–248. 1991. View Article : Google Scholar : PubMed/NCBI | |
|
Allen CDC, Okada T and Cyster JG: Germinal-center organization and cellular dynamics. Immunity. 27:190–202. 2007. View Article : Google Scholar : PubMed/NCBI | |
|
Garside P, Ingulli E, Merica RR, Johnson JG, Noelle RJ and Jenkins MK: Visualization of specific B and T lymphocyte interactions in the lymph node. Science. 281:96–99. 1998. View Article : Google Scholar : PubMed/NCBI | |
|
Yin X, Chen S and Eisenbarth SC: Dendritic cell regulation of T helper cells. Annu Rev Immunol. 39:759–790. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Crotty S, Kersh EN, Cannons J, Schwartzberg PL and Ahmed R: SAP is required for generating long-term humoral immunity. Nature. 421:282–287. 2003.PubMed/NCBI | |
|
Park GB, Kim YS, Lee HK, Song H, Kim S, Cho DH and Hur DY: Reactive oxygen species and p38 MAPK regulate Bax translocation and calcium redistribution in salubrinal-induced apoptosis of EBV-transformed B cells. Cancer Lett. 313:235–248. 2011.PubMed/NCBI | |
|
Livak KJ and Schmittgen TD: Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001. | |
|
Ottaviani E, Malagoli D and Franchini A: Invertebrate humoral factors: Cytokines as mediators of cell survival. Prog Mol Subcell Biol. 34:1–25. 2004.PubMed/NCBI | |
|
Hur DY, Park GB, Kim YS, Lee HK and Kim DJ: Composition containing complex cytokines derived from EBV-infected B cells for inducing the maturation of dendritic cells. US Patent US9845456B2. Filed March 20, 2014; issued December 19, 2017. | |
|
Zou GM and Tam YK: Cytokines in the generation and maturation of dendritic cells: Recent advances. Eur Cytokine Netw. 13:186–199. 2002.PubMed/NCBI | |
|
Fricke I, Mitchell D, Petersen F, Böhle A, Bulfone-Paus S and Brandau S: Platelet factor 4 in conjunction with IL-4 directs differentiation of human monocytes into specialized antigen-presenting cells. FASEB J. 18:1588–1590. 2004.PubMed/NCBI | |
|
Isomäki P, Aversa G, Cocks BG, Luukkainen R, Saario R, Toivanen P, de Vries JE and Punnonen J: Increased expression of signaling lymphocytic activation molecule in patients with rheumatoid arthritis and its role in the regulation of cytokine production in rheumatoid synovium. J Immunol. 159:2986–2993. 1997.PubMed/NCBI | |
|
Minagawa H, Tanaka K, Ono N, Tatsuo H and Yanagi Y: Induction of the measles virus receptor SLAM (CD150) on monocytes. J Gen Virol. 82:2913–2917. 2001.PubMed/NCBI | |
|
Bleharski JR, Niazi KR, Sieling PA, Cheng G and Modlin RL: Signaling lymphocytic activation molecule is expressed on CD40 ligand-activated dendritic cells and directly augments production of inflammatory cytokines. J Immunol. 167:3174–3181. 2001.PubMed/NCBI | |
|
Tojjari A, Giles FJ, Vilbert M, Saeed A and Cavalcante L: SLAM modification as an immune-modulatory therapeutic approach in cancer. Cancers (Basel). 15:48082023.PubMed/NCBI | |
|
Mavaddat N, Mason DW, Atkinson PD, Evans EJ, Gilbert RJ, Stuart DI, Fennelly JA, Barclay AN, Davis SJ and Brown MH: Signaling lymphocytic activation molecule (CDw150) is homophilic but self-associates with very low affinity. J Biol Chem. 275:28100–28109. 2000. View Article : Google Scholar : PubMed/NCBI | |
|
Sidorenko SP, Vetrova EP, Yurchenko OV, Berdova AG, Shlapatskaya LN and Gluzman DF: Monoclonal antibodies of IPO series against B cell differentiation antigens in leukemia and lymphoma immunophenotyping. Neoplasma. 39:3–9. 1992.PubMed/NCBI | |
|
Yurchenko MY, Kovalevska LM, Shlapatska LM, Berdova GG, Clark EA and Sidorenko SP: CD150 regulates JNK1/2 activation in normal and Hodgkin's lymphoma B cells. Immunol Cell Biol. 88:565–574. 2010. View Article : Google Scholar : PubMed/NCBI | |
|
Burdin N, Péronne C, Banchereau J and Rousset F: Epstein-Barr virus transformation induces B lymphocytes to produce human interleukin 10. J Exp Med. 177:295–304. 1993. View Article : Google Scholar : PubMed/NCBI | |
|
Tatsuo H, Ono N, Tanaka K and Yanagi Y: SLAM (CDw150) is a cellular receptor for measles virus. Nature. 406:893–897. 2000. View Article : Google Scholar : PubMed/NCBI | |
|
Nédellec P, Dveksler GS, Daniels E, Turbide C, Chow B, Basile AA, Holmes KV and Beauchemin N: Bgp2, a new member of the carcinoembryonic antigen-related gene family, encodes an alternative receptor for mouse hepatitis viruses. J Virol. 68:4525–4537. 1994. View Article : Google Scholar : PubMed/NCBI | |
|
Rowe JM and Rapoport AP: Hemopoietic growth factors: A review. J Clin Pharmacol. 32:486–501. 1992. View Article : Google Scholar : PubMed/NCBI | |
|
Vellenga E, Young DC, Wagner K, Wiper D, Ostapovicz D and Griffin JD: The effects of GM-CSF and G-CSF in promoting growth of clonogenic cells in acute myeloblastic leukemia. Blood. 69:1771–1776. 1987. View Article : Google Scholar : PubMed/NCBI | |
|
Gasson JC, Kaufman SE, Weisbart RH, Tomonaga M and Golde DW: High-affinity binding of granulocyte-macrophage colony-stimulating factor to normal and leukemic human myeloid cells. Proc Natl Acad Sci USA. 83:669–673. 1986. View Article : Google Scholar : PubMed/NCBI | |
|
Dauer M, Obermaier B, Herten J, Haerle C, Pohl K, Rothenfusser S, Schnurr M, Endres S and Eigler A: Mature dendritic cells derived from human monocytes within 48 h: A novel strategy for dendritic cell differentiation from blood precursors. J Immunol. 170:4069–4076. 2003. View Article : Google Scholar : PubMed/NCBI | |
|
Hanazawa S, Hanaizumi C, Amano S, Hirose K, Ohmori Y, Kumegawa M, Yamaura K and Kitano S: Inductive effect of recombinant human interleukin-1 alpha and beta on differentiation of macrophage-like tumor cell line P388D1. J Cell Physiol. 136:543–546. 1988.PubMed/NCBI |