Imaging characteristics and endovascular treatment strategy for cerebellar arteriovenous malformations
- Authors:
- Published online on: June 23, 2021 https://doi.org/10.3892/mi.2021.6
- Article Number: 5
-
Copyright: © Piao et al. This is an open access article distributed under the terms of Creative Commons Attribution License.
Abstract
Introduction
Cerebellar arteriovenous malformations (CAVMs) account for 7-15% of all intracranial arteriovenous malformations (1,2). The angioarchitecture of CAVMs mainly involves the feeding artery, nidus, draining vein, aneurysm on the feeding artery and the aneurysm in the nidus, which all affect the clinical characteristics and treatment prognosis of patients with CAVMs (2,3).
As CAVMs are located in the posterior cranial fossa with a relatively deep position and close to the brainstem, traditional microsurgery is difficult, as the depth of CAVMs can hinder surgical manipulation, and is associated with increased risks for various complications (4). In recent years, an increasing number of CAVMs have been treated with endovascular treatment (EVT), which can also be utilized as a pre-adjuvant therapy to reduce the risk of craniotomy or radiotherapy (5). The present study retrospectively analyzed patients with CAVMs who underwent EVT to investigate the imaging characteristics and EVT strategy of CAVMs at the First Hospital of Jilin University.
Patients and methods
Patient information
A total of 33 cases from the Department of Neurosurgery of the First Hospital of Jilin University from January, 2015 to January, 2020 were continuously collected and retrospectively analyzed. All the patients had arteriovenous malformations (AVMs) located in the cerebellum and underwent EVT. The present study was approved by the Ethics Committee of the First Hospital of Jilin University (approval no. 2021-429). Written informed consent was obtained from the patients >18 years old; for patients who were <18 years old, consent was provided by their parents The pre-operative state was evaluated with the Hunt-Hess grade, as follows, Grade I: Asymptomatic, or minimal headache and slight nuchal rigidity; grade II: Moderate to severe headache, nuchal rigidity, no neurological deficit other than cranial nerve palsy; grade III: Drowsiness, confusion, or mild focal deficit; grade IV: Stupor, moderate to severe hemiparesis, possibly early decerebrate rigidity and vegetative disturbances; and grade V: Deep coma, decerebrate rigidity, moribund appearance (6).
Positions and main observation indexes of CAVM
According to the blood supply area of the cerebellar arteries, the CAVM locations were divided into the upper (Figs. 1 and 2), middle (Fig. 3) and lower areas of the cerebellum (Fig. 4). The location, size, feeding artery and venous drainage (such as superficial and deep vein drainage, which involved a deep vein and included the vein around the brainstem and vein of Galen, as shown in Fig. 2) of the CAVM were recorded in detail. The Spetzler-Martin grade was recorded, and the existence of aneurysms was observed (7). Aneurysms were divided into two types as follows: Prenidal aneurysms located on the feeding artery (Figs. 5 and 6) and intranidal aneurysms located in the CAVM (Fig. 7) (8).
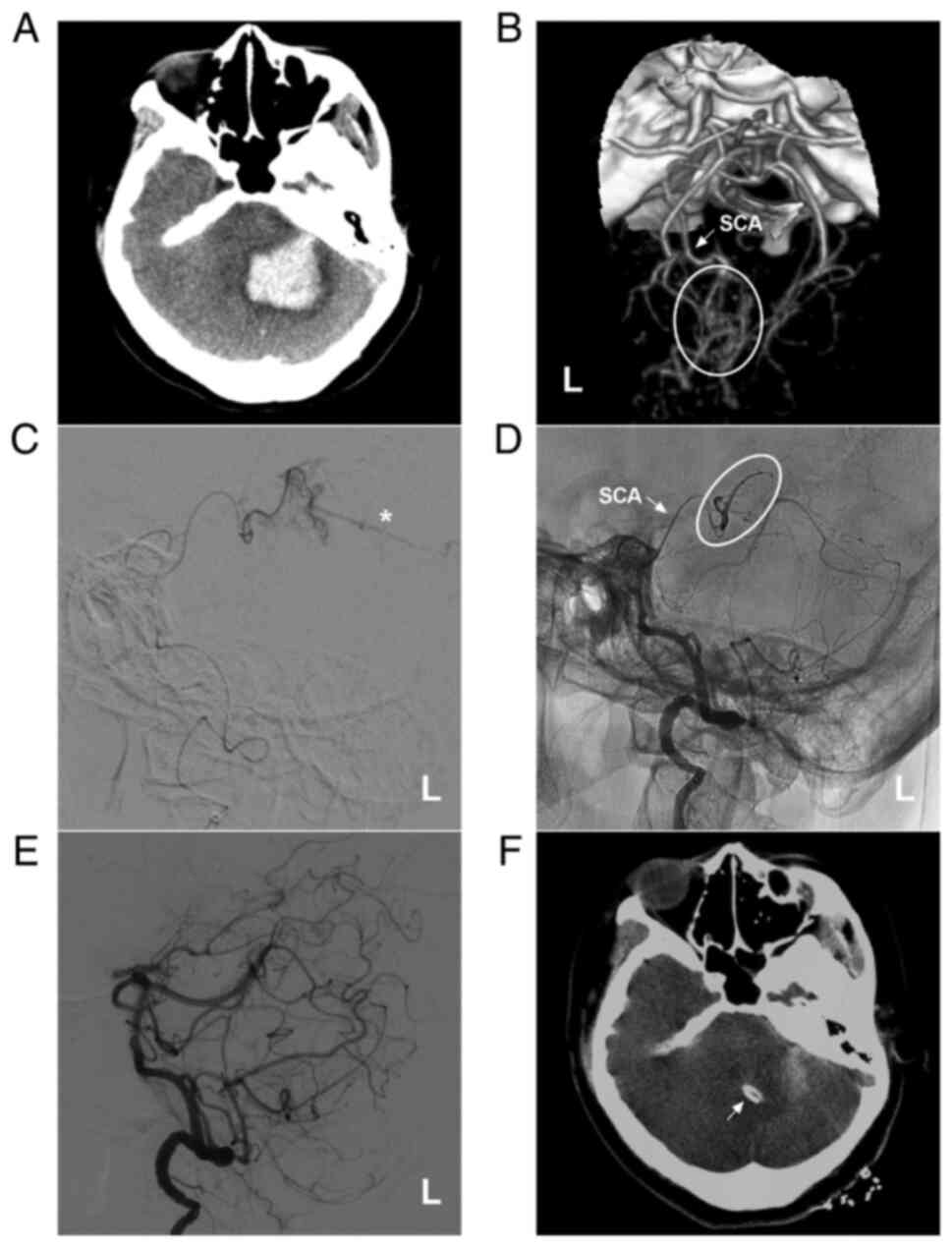 |
Figure 1AVM in the upper cerebellum fed by the SCA alone. (A) Brain CT illustrating a left cerebellar hemispheric hemorrhage. (B) Brain CTA illustrating the left SCA (arrow) feeding the AVM (ellipse). (C) Microcatheter angiography via the SCA showing that the diffuse AVM drained through a superficial vein (star). (D) Microcatheter through the SCA (arrow) Onyx™ casting (ellipse). (E) DSA showing no imaging of the AVM following embolization. (F) Cerebellar hematoma drainage was performed following embolization, and the drainage tube (arrow) was observed. The case presented in this figure was a 30-year-old female. She had a sudden a headache and vomiting, and she was awake after onset. Following EVT, hematoma puncture and aspiration were performed. She recovered well. AVM, arteriovenous malformation; CT, computed tomography; CTA, computed tomography angiography; L, left; SCA, superior cerebellar artery. |
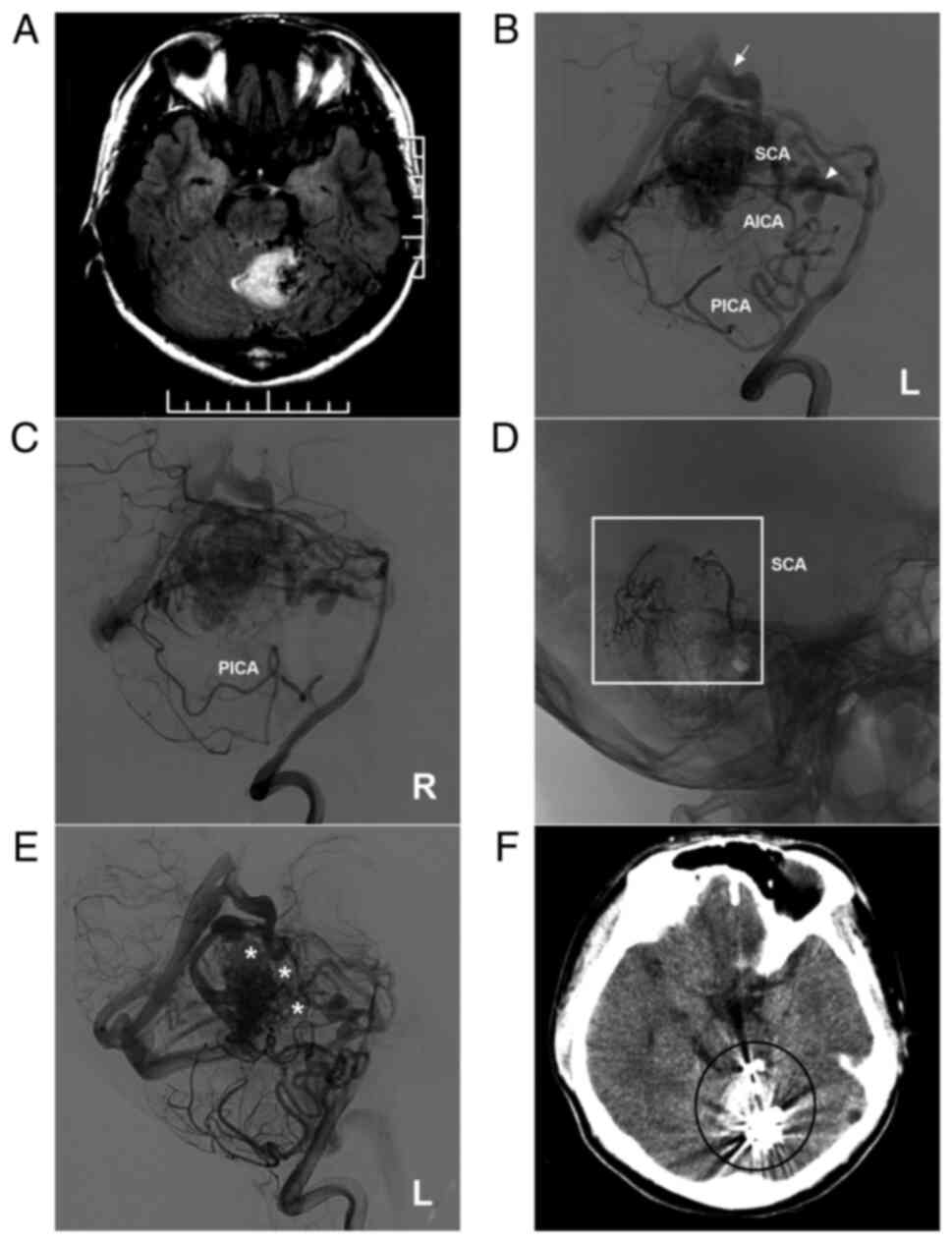 |
Figure 2AVM located in the upper cerebellum with a mixed blood supply from the SCA, AICA and PICA. (A) Brain MRI showing a cerebellar vermis hemorrhage and the absence of flow voids beside the hematoma. (B and C) Lateral DSA of the left (B) and right (C) vertebral arteries showing that the AVM is widely supplied by the SCA, AICA and bilateral PICA and mainly drained by deep veins [in (B), the arrow noted the vein of Galen, and the triangle indicates the vein around the brainstem]. (D) X-ray showing the Onyx™ casting (square box) through the two branches of the SCA. (E) DSA of the left vertebral artery in the arterial phase after embolization showing that the volume of the AVM nidus decreased, and the stars denote the part of Onyx casting. (F) Post-operative CT scan showing an artifact (ellipse) of Onyx™. The case presented in this figure was a 17-year-old male who developed a sudden headache; he was awake after onset. EVT was performed. No EVT complication was observed. Currently, the boy is of normal health and attends university. However, further EVT was suggested. AICA, anterior inferior cerebellar artery; AVM, arteriovenous malformation; CT, computed tomography; DSA, digital subtraction angiography; L, left; MRI, magnetic resonance imaging, PICA, posterior inferior cerebellar artery; R, right; SCA, superior cerebellar artery. |
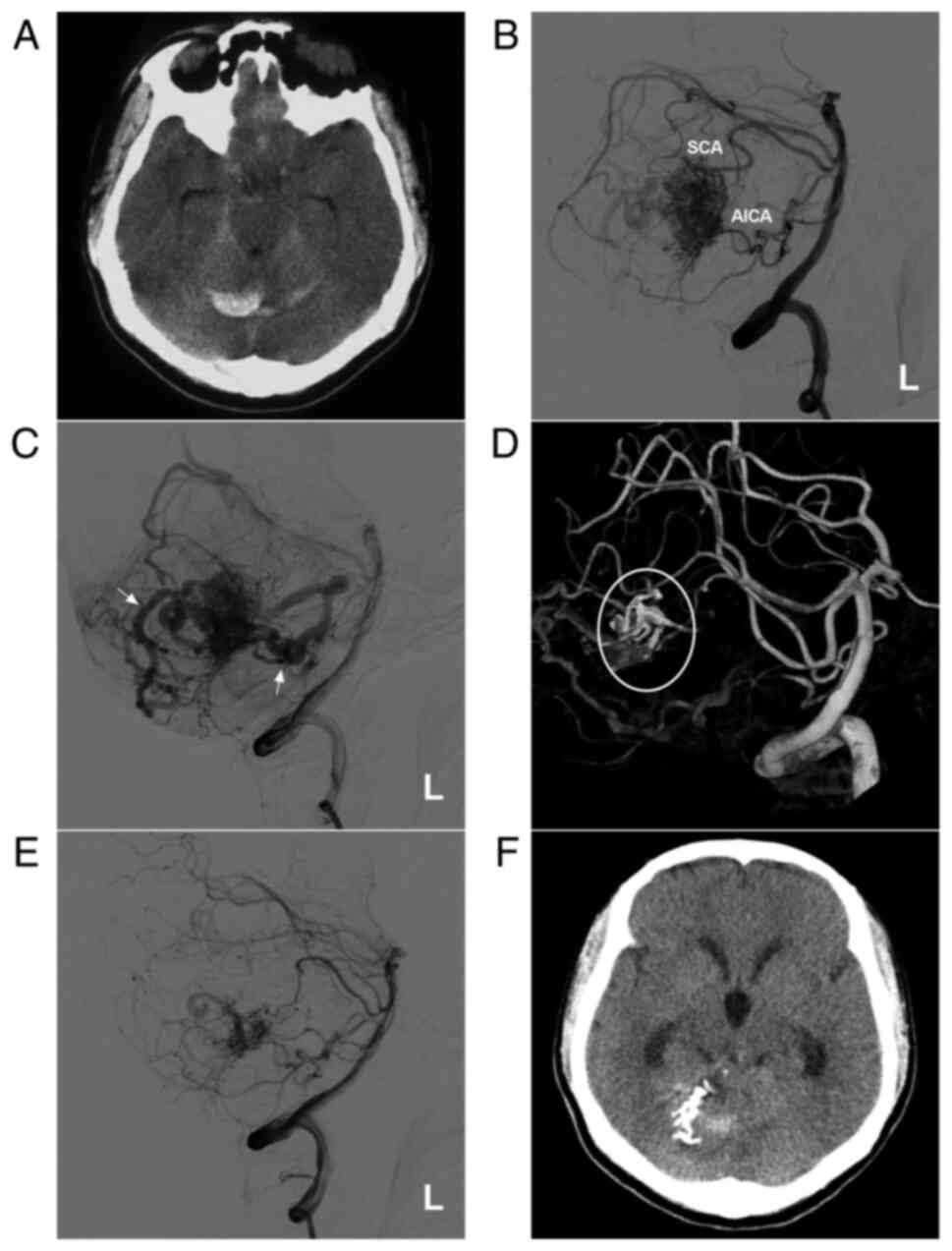 |
Figure 3AVM in the middle cerebellum with a mixed blood supply from the SCA and AICA. (A) CT scan showing a subarachnoid hemorrhage and right cerebellar vermis hematoma. (B) DSA of the left vertebral artery shows the AVM supplied by the SCA and AICA. (C) DSA of the late arterial stage shows the draining veins (arrows). (D) Three-dimensional reconstruction DSA showing Onyx™ casting (ellipse) through the SCA. (E) DSA after embolization showing a small amount of residual AVM nidus. (F) Post-operative CT scan showing that the hemorrhage was absorbed, but had a dilated ventricle. The case presented in this figure was a 20-year-old female who had a sudden headache. EVT was performed. No EVT complication was observed. Currently, the woman exhibits normal healthy features and attends university. Radiotherapy was suggested. AICA, anterior superior cerebellar artery; AVM, arteriovenous malformation; CT, computed tomography; DSA, digital subtraction angiography; L, left; SCA, superior cerebellar artery. |
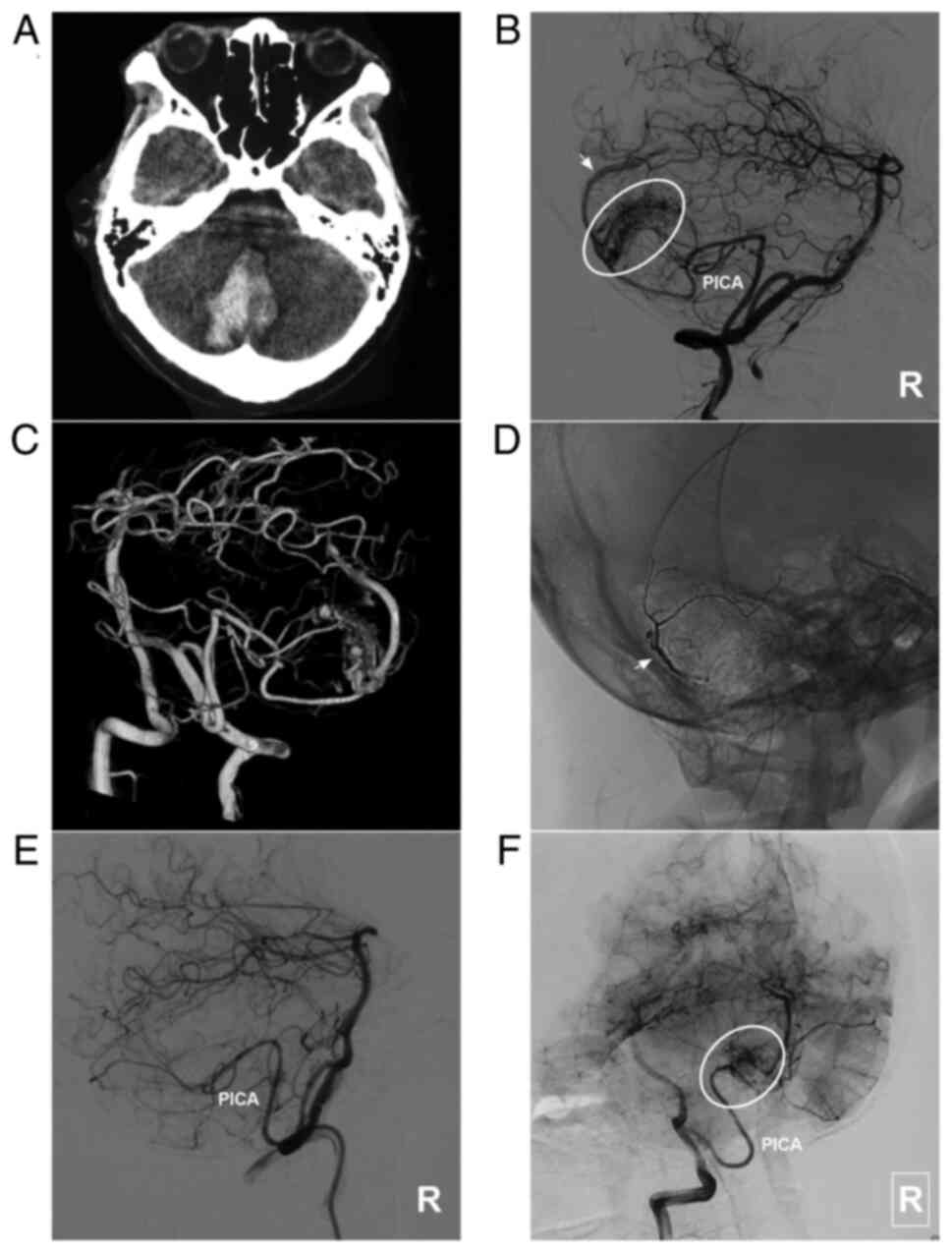 |
Figure 4AVM located in the lower cerebellum supplied by the PICA alone. (A) Brain CT scan showing a cerebellar vermis hematoma. (B) Lateral DSA of the right vertebral artery in the arterial phase showing that the AVM was supplied by the PICA (ellipse), and the arrow indicates superficial vein drainage. (C) Three-dimensional DSA showing the AVM more clearly. (D) X-ray film showing the Onyx™ casting (arrow). (E) DSA in the arterial phase after EVT showing that the AVM is not visualized. (F) DSA at the 6-month follow-up indicates a small residual AVM (ellipse). The case presented in this figure was a 19-year-old female. She experienced sudden headaches, and she was awake after their onset. EVT was performed. No EVT complication was observed. The woman currently exhibits a normal health status. AVM, arteriovenous malformation; CT, computed tomography; DSA, digital subtraction angiography; PICA, posterior inferior cerebellar artery; R, right. |
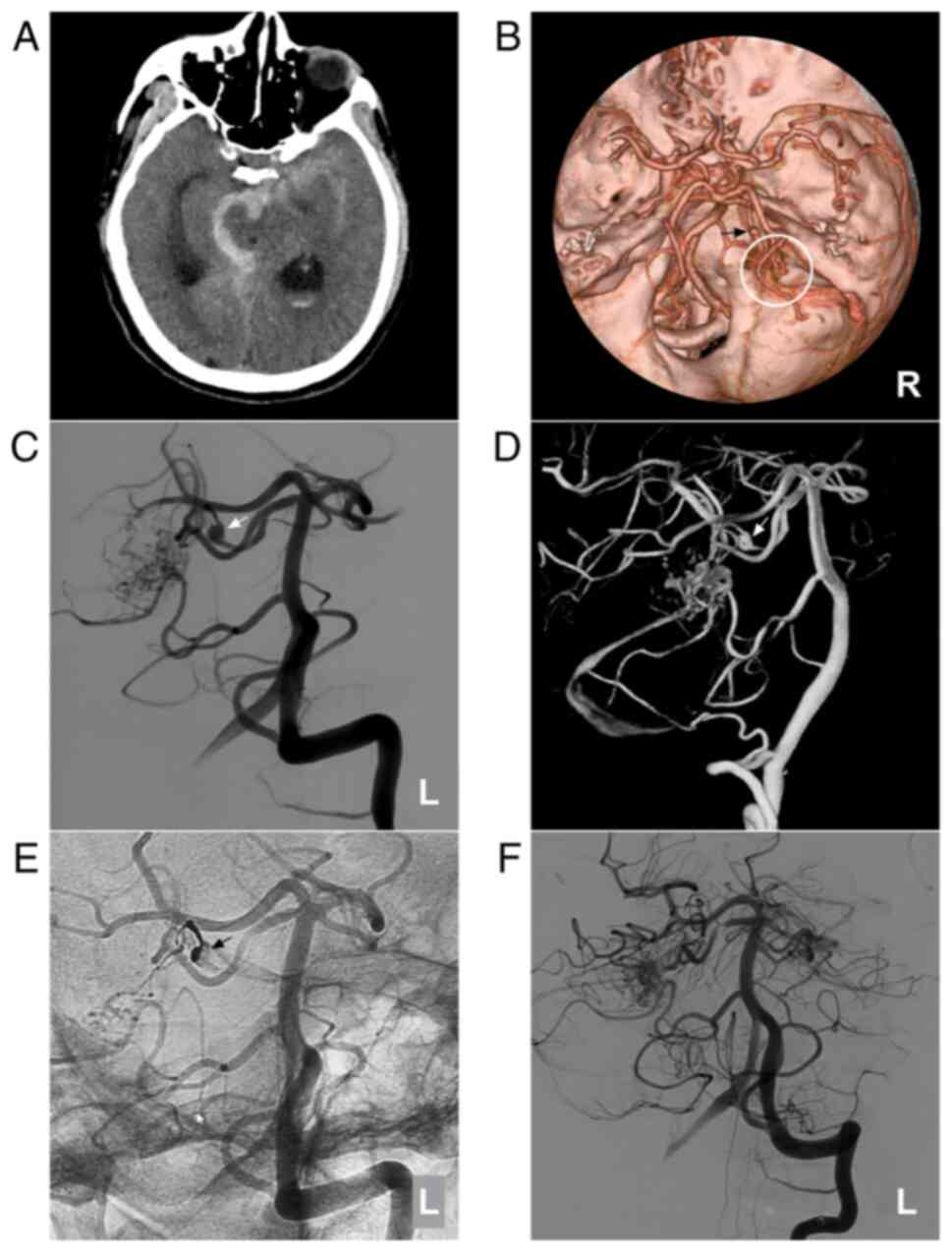 |
Figure 5AVM combined with a prenidal aneurysm on the SCA. (A) Brain CT scan showing subarachnoid hemorrhage in the right ambient cistern. (B) Brain CTA showing the right SCA feeding the AVM (circle), and an aneurysm (arrow) is observed. (C) Two-dimensional and (D) three-dimensional DSA showing that the aneurysm (arrow) can be observed. (E) Unsubtracted DSA showing that the prenidal aneurysm was occluded with Onyx™ casting (arrow). (F) Following embolization, the aneurysm was not observed. The case presented in this figure was a 67-year-old male with a sudden headache; he was awake after its onset. Following EVT, conservative treatment was administered, and no EVT complications were observed. His health status is currently classified as normal. AVM, arteriovenous malformation; CT, computed tomography; CTA, computed tomography angiography; DSA, digital subtraction angiography; L, left; R, right; SCA, superior cerebellar artery. |
 |
Figure 6AVM combined with multiple prenidal aneurysms on the SCA and PICA. (A and B) Brain CT scan showing (A) fourth ventricular hemorrhage and (B) lateral ventricular hemorrhage. (C) Two-dimensional and (D) three-dimensional DSA of the vertebral artery showing that multiple aneurysms (arrows) can be observed on the SCA and PICA. (E) X-ray film showing Onyx™ casting (square box) in the aneurysms. (F) DSA of the right vertebral artery showing that the aneurysms were not observed. The case presented in this figure was a 38-year-old male. He developed a headache and then fell into a coma. Following external ventricular drainage, he recovered well and underwent EVT. No EVT complication was observed. The patient currently appears almost normal. AVM, arteriovenous malformation; CT, computed tomography; DSA, digital subtraction angiography; PICA, posterior inferior cerebellar artery; R, right; SCA, superior cerebellar artery. |
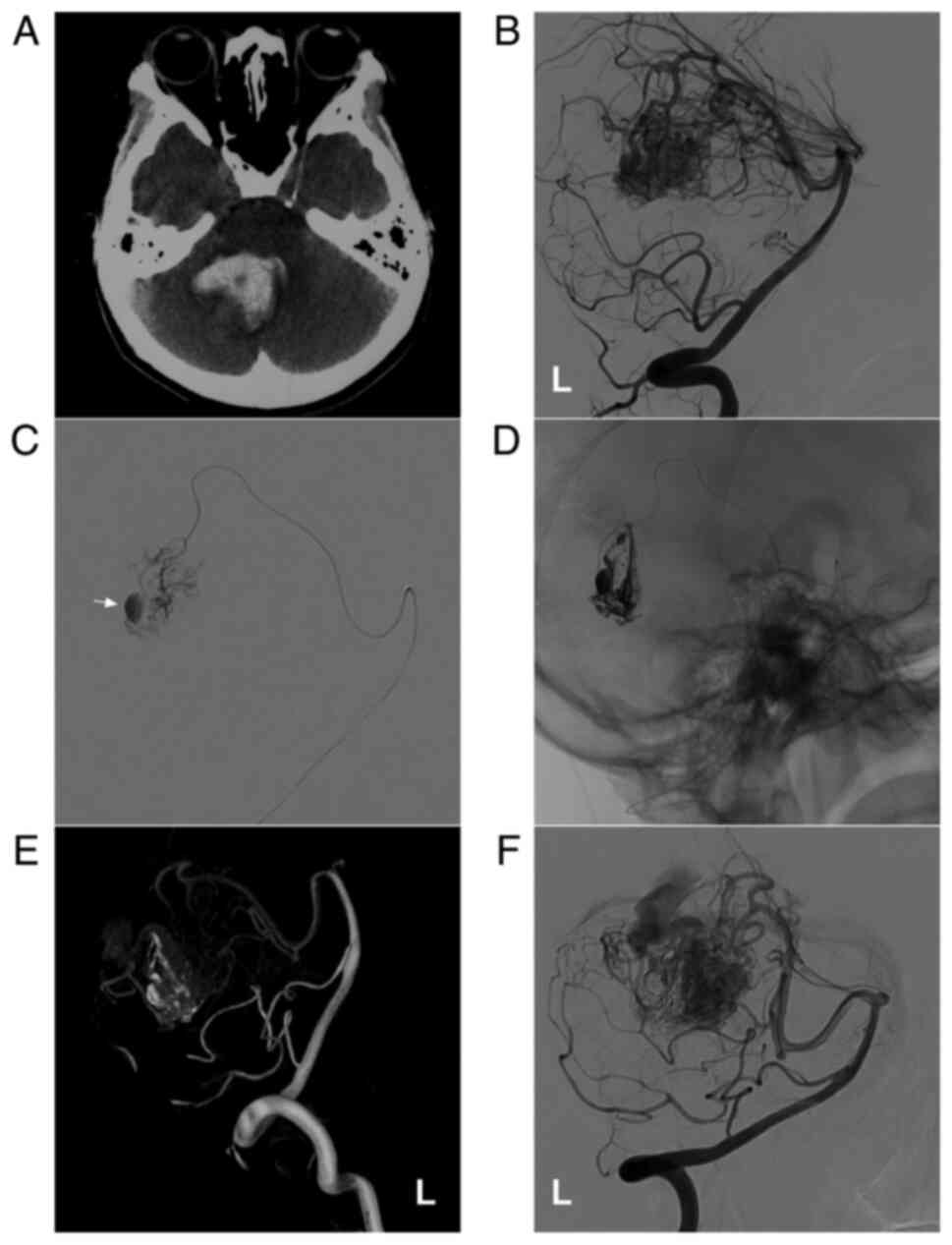 |
Figure 7AVM combined with an intranidal aneurysm. (A) Brain CT scan showing a cerebellar vermis hematoma. (B) DSA of the left vertebral artery showing that the AVM was supplied by the SCA. (C) The microcatheter reached the intranidal aneurysm through the SCA, clearly revealing the aneurysm (arrow). (D) Onyx™ casting through a microcatheter. (E and F) Follow-up (E) three-dimensional DSA and (F) two-dimensional DSA showing no recurrence of the intranidal aneurysm. The case presented in this figure was an 11-year-old girl who had a sudden headache and vomiting, and she was awake after onset. Following EVT, conservative treatment was administered, and no EVT complications were observed. Currently, the girl exhibits normal health and has returned to school. Further EVT was suggested. AVM, arteriovenous malformation; CT, computed tomography; DSA, digital subtraction angiography; L, left; SCA, superior cerebellar artery. |
Scheme and strategy of EVT
Ruptured CAVMs were treated according to the ruptured structures. For aneurysms on the feeding artery, selective coiling with/without stent assistance was preferred. Parent artery occlusion was preferred when the aneurysm was near the CAVM. For the intranidal aneurysm, EVT targeted the AVM compartment harboring the intranidal aneurysm. If there were no clear risk factors, the aim of EVT was to reduce the blood flow of the ruptured CAVM (9).
For unruptured CAVMs, the identification and management of weak structures, such as aneurysms on the feeding artery or dilated structures in the brain AVM (BAVM), are of utmost importance. These weak structures require treatment. If no weak structures were identified or the venous drainage was patent, a ‘wait and see’ strategy was adopted (8).
For combined aneurysms, Onyx™ (Medtronic Neurovascular) was used to embolize aneurysms without retaining the parent artery when aneurysms were located in the CAVM or close to the CAVM. However, the parent artery was preserved when it was located at the origin of the feeding artery away from the CAVM; at this time, coils were the preferred choice.
Treatment evaluation
The EVT process of the patients was recorded, including the embolization degree of the AVM nidus by Onyx™ casting. According to the proportion of the embolization degree accounting for the nidus, the patients were classified based on embolization volumes of <1/3, 1/3-2/3 and >2/3 of the nidus. Aneurysm treatment, complications, the management of cerebellar hemorrhage, the length of hospital stay and the Glasgow Outcome Scale (GOS) (10) of the patients at discharge were all recorded.
Results
General patient data
In the present study, the 33 patients were aged from 8 to 73 years (mean age, 40.4±17.8 years). In total, 21 patients were female (63%, 21/33), and 12 patients were male (37%, 12/33). Among the 33 cases, four were unruptured (12.1%, 4/33), including 3 cases of headaches and 1 case of dizziness; 29 cases had bleeding (87.9%, 29/33), including 7 cases of subarachnoid hemorrhage, 3 cases of subarachnoid hemorrhage + intracerebral hematoma, 11 cases of intracerebral hematoma, 4 cases of intraventricular hemorrhage, 2 cases of intracerebral hematoma + intraventricular hemorrhage and 2 cases of subarachnoid hemorrhage + intraventricular hemorrhage. Among the 29 cases of bleeding, 4 were Hunt-Hess grade I, 22 were grade II and 3 were grade III (Table I).
Table IClinical data of the patients in the present study. |
Imaging data i) Position of the AVM
Among the 33 cases, 18 were in the upper area (54.5%, 18/33), 13 were in the middle (39.4%, 13/33), and 2 were in the lower area of the cerebellum (6.1%, 2/33).
ii) Size of the AVM and SM classification
The size of the AVM in the 33 cases ranged from 1.5 to 7 cm (3.3±1.6 cm). As regards the Spetzler-Martin grade, 17 cases were grade 1 (51.5%, 17/33), 10 cases were grade 2 (30.3%, 10/33), 4 cases were grade 3 (12.1%, 4/33) and 2 cases were grade 4 (6.1%, 2/33).
iii) Feeding arteries
Among the 33 cases, 33 CAVM cases, 15 (45.5%, 15/33) were fed by a single artery, and 18 (54.5%, 18/33) were fed by multiple arteries. Specifically, 6 (18.2%, 6/33) were supplied by the superior cerebellar artery alone, 5 (15.2%, 5/33) by the anterior inferior cerebellar artery alone, 2 (6.1%, 2/33) by the posterior inferior cerebellar artery alone, 2 (6.1%, 2/33) by perforating branches of the basilar artery, 7 (21.2%, 7/33) by the superior cerebellar artery + anterior inferior cerebellar artery, 3 (9.1%, 3/33) by the anterior posterior cerebellar artery + posterior inferior cerebellar artery, 1 (3.0%, 1/33) by the superior cerebellar artery + occipital artery, 3 (9.1%, 3/33) by the superior cerebellar artery + posterior inferior cerebellar artery and 4 (12.0%, 4/33) by the superior cerebellar artery + anterior inferior artery + posterior inferior cerebellar artery (Table II).
Table IIDistribution of feeding arteries of cerebellar arteriovenous malformations. |
iv) Draining vein
In total, 27 cases (81.8%, 27/33) had superficial vein drainage, 2 (6.1%, 2/33) had deep vein drainage, and 4 (12.1%, 4/33) had both superficial and deep vein drainage.
v) Combined with aneurysms
A total of 15 (45.5% 15/33) cases were complicated with aneurysms, of which 13 had aneurysms on the feeding arteries. Given that 1 case had 2 aneurysms, there were a total of 14 prenidal aneurysms (7 in the anterior inferior cerebellar artery, 5 in the superior cerebellar artery, 1 in the posterior inferior cerebellar artery, and 1 in the perforating branch of the basilar artery). There were 2 intranidal aneurysms located in the AVM nidus. Thus, a total of 16 aneurysms were found.
Results of EVT
Among the 33 cases, the nidus of 29 (87.9%, 29/33) patients underwent Onyx™ casting, including 17 cases via superior cerebellar artery embolization, 6 cases via anterior inferior cerebellar artery embolization, 4 cases via posterior inferior cerebellar artery embolization, 1 case via superior cerebellar artery + posterior inferior cerebellar artery embolization and 1 case via basilar artery perforating branch. Among the 29 cases, 8 (27.6%, 8/29) had an embolization degree of <1/3 of the nidus, 11 (37.9%, 11/29) had a volume of 1/3-2/3 of the nidus and 10 cases (34.5%, 10/29) had a volume >2/3 of the nidus.
Of the 14 prenidal aneurysms, 8 were embolized with coils and 6 were embolized with Onyx™. Two complicated cases of intranidal aneurysm were embolized with Onyx™, no complications occurred in these 15 patients Among the complications associated with EVT, there were 3 cases of intraoperative or post-operative bleeding (9.1%, 3/33). Following EVT, hemorrhage removal with/without decompression and external ventricular drainage was performed. Of these cases, the complications resulted in 2 deaths (on the 1st and 7th days after EVT). The other patient recovered well. Cases with typical EVT treatment are shown in Fig. 1, Fig. 2, Fig. 3, Fig. 4, Fig. 5, Fig. 6 and Fig. 7. The clinical data for the patients are summarized in Table I.
Length of hospital stay and the GOS
The length of hospital stay was 2-24 days (10.7±5.4 days). Upon discharge, 27 patients (81.7, 27/33) had GOS scores of 5, 2 patients (6.1%, 2/33) had GOS scores of 4, 2 patients (6.1%, 2/33) had GOS scores of 3, and 2 patients (6.1%, 2/33) had GOS scores of 1 (Table I).
Discussion
CAVMs are located in the posterior cranial fossa, accounting for 7-15% of all intracranial AVMs (1-3). The angioarchitecture of CAVM consists of the feeding artery, nidus, draining vein, aneurysm on the feeding artery and aneurysm in the nidus. For CAVMs, the feeding artery mainly includes the superior cerebellar artery, anterior inferior cerebellar artery, posterior inferior cerebellar artery and perforating branch of the basilar artery, and there are often multiple feeding arteries (11).
In the present study, >50% of CAVMs had multiple feeding arteries, including dural branches of the external carotid artery (occipital artery). The drainage vein is a part of CAVM, and deep vein drainage may affect patient prognosis. Compared with superficial vein drainage, the post-operative prognosis of patients with CAVMs involving deep vein drainage is relatively poor (12). In the present study, 6 patients had deep vein drainage with an incidence rate of 18.2% (6/33), including 2 (6.1%, 2/33) with deep vein drainage and 4 (12.1%, 4/33) with both superficial and deep vein drainage. AVM can be associated with aneurysms, and the incidence of CAVM-related aneurysms is as high as 20.8-47% (13). The incidence of aneurysms in the present study was 45.5%, which was similar to the aforementioned study (13).
Supratentorial AVM exhibits relatively diverse clinical manifestations, including intracranial hemorrhage, recurrent headache, epilepsy, progressive neurological defects, etc. By contrast, CAVM exhibits relatively simple clinical manifestations (mainly hemorrhage) (14,15). For example, among the 33 cases of CAVM summarized in the present study, hemorrhage accounted for 87.9% as CAVMs may be related to the annual bleeding rate of 8.6% of CAVM, which is higher than AVM apart from the cerebellum (the bleeding rate of 2.4%) (16,17).
A nidus is a risk factor for hemorrhage in CAVMs; however, aneurysms are present in a number of cases. Aneurysms on the feeding artery are an independent predictor of adverse consequences, can directly cause intracranial hemorrhage and can be the primary clinical manifestation, such as in subarachnoid hemorrhage (13,18). According to previous findings, aneurysms on the feeding artery have a larger probability of rupture and bleeding than the AVM nidus itself, which may trigger even more serious symptoms and secondary edema due to the small space of the posterior cranial fossa close to the brainstem region (10,20).
The treatment of CAVMs includes craniotomy, EVT, radiotherapy, or a combination of multiple methods (21). In recent years, EVT has been used in an increasing number of CAVM cases, and EVT can also be used as a pre-adjuvant therapy to reduce the risk of craniotomy or radiotherapy (22,23).
EVT should be performed for CAVMs for the structure when rupture and bleeding occur. For unruptured CAVMs, EVT should be used to treat structures at risk, such as weak points, aneurysms on the feeding artery or dilated structures in the CAVM. However, for unruptured aneurysms, EVT is controversial, as embolization of the AVM nidus reduces aneurysmal dilatation and blood flow, thus promoting the formation of thrombi in the aneurysm cavity and finally resulting in aneurysm self-atrophy or even disappearance (22,23). Regardless, for aneurysms in CAVMs with a high grade (Spetzler-Martin grade 4 or 5), it is not recommended to prioritize the CAVM nidus as early embolization of the CAVM nidus can increase the risk of aneurysm rupture by increasing intralesional perfusion pressure (24).
In the present study, the embolization of the aneurysms was prioritized in 14 cases of CAVMs combined with prenidal aneurysms followed by embolization of the nidus to varying degrees. For 2 cases of AVM combined with intranidal aneurysms, embolization with Onyx™ was mainly performed on aneurysms. The outcome is safe and satisfactory, as no complications occurred.
As previously reported, 67-80% of patients with CAVMs treated with EVT can achieve ideal outcomes (5,11). In the present study, 27 patients (81.7%, 27/33) had a GOS score of 5 at discharge. EVT is thus a feasible treatment for CAVM and may be used to obtain acceptable therapeutic effects.
EVT is safer and has less complications than craniotomy (8.8-18.7% vs. 25-40%) (5,11,13,18). In the present study, the complication rate was 9.1%, including intraoperative and post-operative bleeding. The cause of this hemorrhagic complication was unclear; during EVT, the rapid and high pressure of Onyx™ casting in the weak or ruptured compartment of the nidus can result in intraoperative bleeding (25). In addition, excessive Onyx™ casting in one stage, particularly the occlusion of the draining vein, can result in post-operative bleeding based on the normal perfusion pressure breakthrough theory (26). Therefore, hemorrhagic complications of EVT should be considered.
In conclusion, the findings of the present study suggest that EVT is a feasible treatment for CAVM and that EVT should focus on weak or ruptured structures. Excessive Onyx™ casting and the occlusion of the draining vein should be avoided to reduce the risk of hemorrhagic complications.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
JY and JP designed the study and drafted the manuscript. YW and TL collected the data. JY and JP confirm the authenticity of all the raw data. All authors have read and approved the final manuscript.
Ethics approval and consent to participate
The present study was approved by the Ethics Committee of the First Hospital of Jilin University (approval no. 2021-429). Written informed consent was obtained from the patients.
Patient consent for publication
The patients or their parents/guardians provided consent and agreed for their data (shown in the figures) to be published.
Competing interests
The authors declare that they have no competing interests.
References
|
Drake CG, Friedman AH and Peerless SJ: Posterior fossa arteriovenous malformations. J Neurosurg. 64:1–10. 1986.PubMed/NCBI View Article : Google Scholar |
|
|
Bowden G, Kano H, Tonetti D, Niranjan A, Flickinger J and Lunsford LD: Stereotactic radiosurgery for arteriovenous malformations of the cerebellum. J Neurosurg. 120:583–590. 2014.PubMed/NCBI View Article : Google Scholar |
|
|
Batjer H and Samson D: Arteriovenous malformations of the posterior fossa. Clinical presentation, diagnostic evaluation, and surgical treatment. J Neurosurg. 64:849–856. 1986.PubMed/NCBI View Article : Google Scholar |
|
|
Neacsu A and Ciurea AV: General considerations on posterior fossa arteriovenous malformations (clinics, imaging and therapy). Actual concepts and literature review. J Med Life. 3:26–35. 2010.PubMed/NCBI |
|
|
da Costa L, Wallace MC, Ter Brugge KG, O'Kelly C, Willinsky RA and Tymianski M: The natural history and predictive features of hemorrhage from brain arteriovenous malformations. Stroke. 40:100–105. 2009.PubMed/NCBI View Article : Google Scholar |
|
|
Hunt WE and Hess RM: Surgical risk as related to time of intervention in the repair of intracranial aneurysms. J Neurosurg. 28:14–20. 1968.PubMed/NCBI View Article : Google Scholar |
|
|
Pollock BE, Kondziolka D, Lunsford LD, Bissonette D and Flickinger JC: Repeat stereotactic radiosurgery of arteriovenous malformations: Factors associated with incomplete obliteration. Neurosurgery. 38:318–324. 1996.PubMed/NCBI View Article : Google Scholar |
|
|
Rammos SK, Gardenghi B, Bortolotti C, Cloft HJ and Lanzino G: Aneurysms associated with brain arteriovenous malformations. AJNR Am J Neuroradiol. 37:1966–1971. 2016.PubMed/NCBI View Article : Google Scholar |
|
|
Hou K, Xu K, Chen X, Ji T, Guo Y and Yu J: Targeted endovascular treatment for ruptured brain arteriovenous malformations. Neurosurg Rev. 43:1509–1518. 2020.PubMed/NCBI View Article : Google Scholar |
|
|
Teasdale G and Jennett B: Assessment and prognosis of coma after head injury. Acta Neurochir (Wien). 34:45–55. 1976.PubMed/NCBI View Article : Google Scholar |
|
|
Robert T, Blanc R, Ciccio G, Gilboa B, Fahed R, Boissonnet H, Redjem H, Pistocchi S, Bartolini B and Piotin M: Endovascular treatment of posterior fossa arteriovenous malformations. J Clin Neurosci. 25:65–68. 2016.PubMed/NCBI View Article : Google Scholar |
|
|
Lefevre E, Robert T, Escalard S, Fahed R, Smajda S, Ciccio G, Desilles JP, Mazighi M, Blanc R and Piotin M: Presence of direct vertebrobasilar perforator feeders in posterior fossa arteriovenous malformations and association with poor outcomes after endovascular treatment. J Neurosurg: Nov 8, 2019 (Epub ahead of print). |
|
|
Orning J, Amin-Hanjani S, Hamade Y, Du X, Hage ZA, Aletich V, Charbel F and Alaraj A: Increased prevalence and rupture status of feeder vessel aneurysms in posterior fossa arteriovenous malformations. J Neurointerv Surg. 8:1021–1024. 2016.PubMed/NCBI View Article : Google Scholar |
|
|
Brown RD Jr, Wiebers DO, Torner JC and O'Fallon WM: Frequency of intracranial hemorrhage as a presenting symptom and subtype analysis: A population-based study of intracranial vascular malformations in Olmsted Country, Minnesota. J Neurosurg. 85:29–32. 1996.PubMed/NCBI View Article : Google Scholar |
|
|
Khaw AV, Mohr JP, Sciacca RR, Schumacher HC, Hartmann A, Pile-Spellman J, Mast H and Stapf C: Association of infratentorial brain arteriovenous malformations with hemorrhage at initial presentation. Stroke. 35:660–663. 2004.PubMed/NCBI View Article : Google Scholar |
|
|
Symon L, Tacconi L, Mendoza N and Nakaji P: Arteriovenous malformations of the posterior fossa: A report on 28 cases and review of the literature. Br J Neurosurg. 9:721–732. 1995.PubMed/NCBI View Article : Google Scholar |
|
|
Tong X, Wu J, Lin F, Cao Y, Zhao Y, Wang S and Zhao J: Risk factors for subsequent hemorrhage in patients with cerebellar arteriovenous malformations. World Neurosurg. 92:47–57. 2016.PubMed/NCBI View Article : Google Scholar |
|
|
Kouznetsov E, Weill A, Ghostine JS, Gentric JC, Raymond J and Roy D: Association between posterior fossa arteriovenous malformations and prenidal aneurysm rupture: Potential impact on management. Neurosurg Focus. 37(E4)2014.PubMed/NCBI View Article : Google Scholar |
|
|
Westphal M and Grzyska U: Clinical significance of pedicle aneurysms on feeding vessels, especially those located in infratentorial arteriovenous malformations. J Neurosurg. 92:995–1001. 2000.PubMed/NCBI View Article : Google Scholar |
|
|
Abla AA, Nelson J, Rutledge WC, Young WL, Kim H and Lawton MT: The natural history of AVM hemorrhage in the posterior fossa: Comparison of hematoma volumes and neurological outcomes in patients with ruptured infra- and supratentorial AVMs. Neurosurg Focus. 37(E6)2014.PubMed/NCBI View Article : Google Scholar |
|
|
Magro E, Darsaut TE, Mezui EDO, Bojanowski MW, Ziegler D, Gentric JC, Roy D and Raymond J: Arteriovenous malformations of the posterior fossa: A systematic review. Acta Neurochir (Wien). 162:905–910. 2020.PubMed/NCBI View Article : Google Scholar |
|
|
Azzam CJ: Growth of multiple peripheral high flow aneurysms of the posterior inferior cerebellar artery associated with a cerebellar arteriovenous malformation. Neurosurgery. 21:934–939. 1987.PubMed/NCBI View Article : Google Scholar |
|
|
Cunha E, Sa MJ, Stein BM, Solomon RA and McCormick PC: The treatment of associated intracranial aneurysms and arteriovenous malformations. J Neurosurg. 77:853–859. 1992.PubMed/NCBI View Article : Google Scholar |
|
|
Alexander MD, Connolly ES and Meyers PM: Revisiting normal perfusion pressure breakthrough in light of hemorrhage-induced vasospasm. World J Radiol. 2:230–232. 2010.PubMed/NCBI View Article : Google Scholar |
|
|
Carvi Y, Nievas M, Haas E and Hollerhage HG: Severe intracranial bleedings during endovascular procedures: Outcome of surgically treated patients. Neurol Res. 29:81–90. 2007.PubMed/NCBI View Article : Google Scholar |
|
|
Rangel-Castilla L, Spetzler RF and Nakaji P: Normal perfusion pressure breakthrough theory: A reappraisal after 35 years. Neurosurg Rev. 38:399–405. 2015.PubMed/NCBI View Article : Google Scholar |








