Myeloid‑derived suppressor cells as targets of emerging therapies and nanotherapies (Review)
- Authors:
- Published online on: June 25, 2024 https://doi.org/10.3892/mi.2024.170
- Article Number: 46
-
Copyright : © Kumar et al. This is an open access article distributed under the terms of Creative Commons Attribution License [CC BY 4.0].
Abstract
1. Introduction
Breast cancer (BC) is the most commonly diagnosed type of cancer among women globally, with >2.3 million cases identified in 2020. In that same year, ~685,000 women succumbed due to BC, making it the leading cause of cancer-related death in women (1,2). BC is classified into four subtypes based on the expression of human epidermal growth factor receptor 2 (HER2/neu), progesterone receptor and estrogen receptor triple-negative breast cancer (TNBC), namely as HER2-positive, Luminal A and Luminal B. The treatment options for BC primarily involve surgical interventions, chemotherapy, radiation therapy, hormonal therapy and immunotherapy (3). It is crucial to tailor the treatment approach to the subtype, stage and specific characteristics of the cancer, alongside considering the overall health and preferences of the patient. During BC treatment, patients commonly face challenges, such as side-effects, including nausea, hair loss, fatigue, neuropathy and an elevated susceptibility to infections. Chemotherapy (4,5) and certain treatments, such as trastuzumab (Herceptin), may provoke adverse reactions with potential cardiac toxicity (6,7). Despite the increasing importance of immunotherapy in the treatment of various types of cancer, its efficacy in BC remains limited, benefiting only a small fraction of patients (8). Moreover, there have been reports of immune-related adverse events linked to the use of immune checkpoint inhibitors, often resembling autoimmune diseases, but with a more acute onset and potential for severe, lasting effects. The complexity and heterogeneity of BC pose significant challenges for immunotherapies, particularly immune checkpoint inhibitors (9,10). Without validated targeted therapies, chemotherapy remains the standard treatment strategy for BC (11). However, alongside known adverse effects, the development of drug resistance is a significant issue, leading to disease progression and increased mortality rates. Recent research efforts have focused on overcoming resistance by exploring combinations of cytotoxic, targeted and immune-based therapies (12). Recognizing the crucial role of the tumor microenvironment (TME) in drug resistance underscores the importance of understanding tumor immunity for developing effective immunotherapeutic strategies against BC.
Myeloid-derived suppressor cells (MDSCs) are critical components of the TME, playing a crucial role in suppressing immune responses in cancer, infections and inflammatory diseases. Originating from immature myeloid cells (IMCs), MDSCs exhibit marked heterogeneity, comprising pathologically activated neutrophils and macrophages (13). Recent investigations underscore their dual function in suppressing antitumor immune responses, while stimulating tumor progression. MDSCs promote tumor angiogenesis, facilitate tumor cell invasion and contribute to the formation of premetastatic niches (14,15). MDSC levels are closely associated with clinical outcomes and therapeutic efficacy in patients with BC (16).
In BC, MDSC accumulation in the TME and peripheral circulation is notable, driven by the modulation of immunosuppressive mechanisms, predominantly through T-cell activation inhibition (17), along with the secretion of multiple cytokines and non-immunosuppressive pathways (18). This collective action promotes tumor growth by enabling tumor angiogenesis, enhancing invasion and metastasis, and modifying the TME to favor tumor progression (19). MDSCs play diverse roles in promoting tumor development by impeding the immune system. Given their pivotal role in subverting the body's antitumor defenses, MDSCs are increasingly recognized as promising targets for therapeutic interventions, including innovative approaches such as nanotechnology.
The present review discusses the immunosuppressive functions of MDSCs, their role in BC, and strategies for targeting them in cancer therapy.
2. MDSCs in BC
MDSCs derived from patients with BC exhibit functional and phenotypic similarities to those originating from bone marrow, indicating their myeloid precursor origin (13). These MDSCs are categorized into monocytic (M)-MDSCs (CD11b+ Ly6G- Ly6Chigh) and granulocytic (G)-MDSC (CD11b+ Ly6G+ Ly6Clow) subpopulations (20,21). In humans, M-MDSCs are characterized by CD11b+CD33+HLA-DR-/low CD14+ CD15- markers, while G-MDSCs express CD11b+ CD33+ HLA-DR-/low CD14- CD15+ (or CD66b+) markers (20,22).
MDSC development is regulated by a network of signals that promote the growth of IMCs (23). Various signaling pathways and regulators, such as the signal transducer and activator of transcription (STAT) family, interferon (IFN) regulators, Notch, adenosine receptor A2b and NLRP3, facilitate myelopoiesis, inhibit the maturation and differentiation of progenitor cells, and expand the IMCs. Additionally, signaling pathways and regulators, including NF-κB, STAT1, STAT6, prostaglandin E2 (PGE2), cyclooxygenase-2 and the endoplasmic reticulum stress response contribute to the development of an immunosuppressive phenotype that leads to the pathological activation of immature cells (23,24).
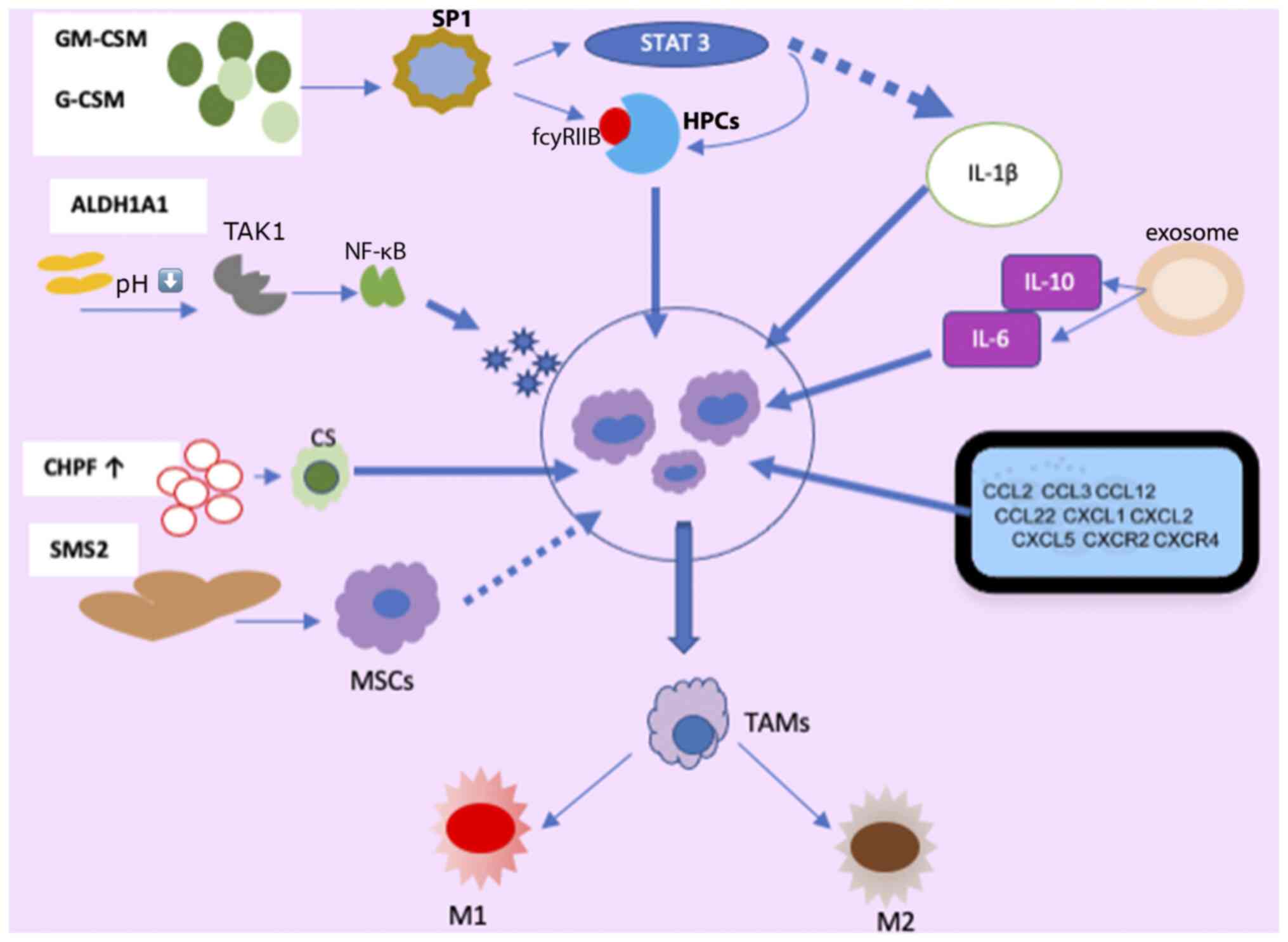
Factors, such as granulocyte colony-stimulating factor (G-CSF), granulocyte-macrophage colony-stimulating factor (GM-CSF) (24-26), vascular endothelial growth factor (VEGF), PGE2, interleukin (ILs)s [IL-1 (27-29), IL-6, IL-13, IL-17, IL-20, IL-33, IL-34 (30-35)], macrophage migration inhibitory factor (MIF) (36), along with microRNAs (miRNAs/miRs), contribute to MDSC amplification and activation in BC (37). Of note, patients with BC with MDSCs often exhibit higher levels of psychological stress, likely influenced by stress-related hormones and cytokines, such as IL-1Ra, IFN-γ-induced protein 10, G-CSF and IL-6, further stimulating MDSC production and accumulation (38) (Fig. 1).
G-CSF and GM-CSF, derived from tumor cells, play pivotal roles in MDSC accumulation (23,25). MDSCs originate from immature, multipotent hematopoietic progenitor cells (HPCs) and respond to signals from the host and tumor cells, particularly through the secretion of GM-CSF, and are subsequently recruited to the TME (39).
Among the receptors implicated in MDSC regulation, FC gamma receptor IIB (FCγRIIB/CD32B) is the sole inhibitory member expressed on B-cells, macrophages, dendritic cells (DCs) and granulocytes, with an upregulation of its expression observed in tumor-infiltrating MDSCs (40). GM-CSF increases FcγRIIB expression in HPCs by activating specificity protein family 1 (SP1) transcription factors, which bind to GC-rich motifs, thereby regulating the expression of genes involved in proliferation, apoptosis, differentiation, and immune responses. The inhibition of SP1 dampens MDSC differentiation and infiltration into the TME. However, when SP1 and FCγRIB are activated, they promote MDSC generation from HPCs via STAT3, a member of the STAT family of transcription factors activated by tyrosine kinases in response to various cytokines and growth factor receptors (41,42). Consequently, tumor cell-induced activation of GM-CSF-driven Sp1 and STAT3 cooperatively trigger the expression of target genes, facilitating the immunosuppressive functions of MDSCs. Moreover, chondroitin polymerase factor, frequently overexpressed in BC tissues, enhances G-CSF binding to cell surface chondroitin sulfate, thereby promoting MDSC accumulation (43).
The enzymatic activity of aldehyde dehydrogenase 1A1 (ALDH1A1) is crucial in reducing intracellular pH in BC cells. This condition triggers increased TGF-β-activated kinase 1 (TAK1) phosphorylation, subsequently activating the NF-κB pathway. Consequently, GM-CSF secretion is induced, amplifying MDSCs and fostering BC progression (44). Exosomes released by 4T1 (syngeneic cell lines derived tumor models) cells contain IL-6 and IL-10, which enhance MDSC stimulation and proliferation by promoting STAT3 phosphorylation in myeloid cells. This diminishes myeloid proliferation and death, expediting differentiation into MDSCs (37).
The study by Jiang et al (45) revealed that tumor exosome-secreted miR-9 and miR-181a targeted suppressor of cytokine signaling protein 3 (SOCS3) and separately activated STAT3 (PIAS3), triggering the JAK/STAT signaling cascade. Prolonged SOCS3 suppression and abnormal JAK/STAT pathway upregulation lead to early-stage MDSC accumulation (46). A number of molecules in signaling pathways form intricate regulatory loops. In BC cells, the mammalian target of the rapamycin pathway induces MDSC accumulation by modulating G-CSF expression. MDSCs reciprocally increase tumor-initiating cell frequency by activating the Notch signaling pathway in tumor cells, which secretes G-CSF, establishing a feed-forward loop promoting MDSC expansion (47). Autocrine secretion of GM-CSF and IL-33 in the TME sustains MDSC viability by inhibiting apoptosis, promoting a positive feedback loop that induces MDSC accumulation (34).
Mechanisms of the recruitment of MDSCs in BC
Several factors, including chemokines, cytokines, and complements produced by both tumor and normal cells, induce the recruitment of MDSCs into tumor tissue (48). Among these, lung fibroblasts secrete chemokine (C-X-C motif) ligand (CXCL)1, which promotes an immunosuppressive lung microenvironment by attracting granulocytic MDSCs and facilitating the formation of BC metastatic niches in the anterior lung (49).
BC exosomes carry elevated levels of miR-200b-3p, which are taken up by alveolar epithelial type II cells, directly impacting phosphatase and tensin homolog (PTEN). PTEN suppression activates the AKT/NF-κB-p65 pathway, increasing chemokine (C-C motif) ligand 2 (CCL2) expression and attracting MDSCs, ultimately promoting lung metastasis in BC (50). Endoplasmic reticulum oxireductin 1a, a disulfide oxidase located in the endoplasmic reticulum and closely associated with tumors, participates in the oxidative folding process, generating and attracting granulocytic MDSCs, and contributes to G-CSF, CXCL1 and CXCL2 production (51). TGF-β1 upregulates miR-494 levels in MDSCs, enhancing MDSC movement via CXCR4(52). The transcription factor, ΔNp63, directly regulates CXCL2 and CCL22, facilitating MDSC recruitment in TNBC (53).
Liver cells contribute to MDSC recruitment at specific sites by producing S100A8, facilitating BC metastasis (54). Chen et al (55) found that VEGF-C produced by breast cancer cells was responsible for increasing the levels of chemokines produced by lymphatic endothelial cells (LECs). This, in turn, helped recruit MDSCs to the TME and lymph nodes (LNs) through the CXCR2 pathway (55). Evidence suggests that in the presence of 4T1 cells (breast cancer cell line derived from the mammary gland tissue of a mouse BALB/c strain), interstitial fluid migration alongside LECs aids MDSC dissemination. Moreover, reduced levels of vascular VEGFR3 decrease the flow response in MDSCs and 4T1 cells (56). The acetylation of the SMAD3 protein, dependent on the epigenetic regulator KAT6A, contributes to MDSC recruitment and TNBC metastasis through epigenetic regulation (57).
The activation of the complement system, particularly through C5a signaling, plays a pivotal role in recruiting MDSCs into the TME and suppressing CD8+ T cell-mediated tumor elimination. Consequently, lung angiogenesis is fostered in tumor-bearing mice, facilitating BC metastasis to this organ (58). Cheng et al (59) demonstrated that periodontal inflammation (PI) activation enhances the expression of chemokines such as CCL5, CXCL12, CCL2 and CCL5, which recruit MDSCs, thereby promoting the establishment of premetastatic niches at sites of inflammation. MDSCs exhibit diverse differentiation pathways regulated by various transcription factors, as depicted in Fig. 1. They can differentiate into tumor-associated macrophages (TAMs) and dendritic cells, which further stimulate the production of inflammatory DCs (60).
During BC progression, MDSCs transition into TAMs. Under environmental pressures, such as hypoxia, M-MDSCs differentiate into TAMs upon migration to specific tissues. TAMs, in turn, can adopt either the M1 phenotype, characterized by pro-inflammatory and antitumor properties, or the M2 phenotype, which exhibits protumor functions, in response to stimuli such as lipopolysaccharide (LPS), TNF-α and IFN-γ (61).
Macrophages and MDSCs are ubiquitous in the majority of solid tumors, driving immune suppression and inflammation (61). Their interaction increases IL-10 production by MDSCs and decreases IL-12 production by macrophages, polarizing the immune system toward a type 2 protumor environment (62). IL-33 is known for its stimulatory effects on myeloid and lymphoid cells, promoting the production of type 2 cytokines. The activation of ST2 in type 2 innate lymphoid cells (ILC2) triggers the release of type 2 cytokines IL-33 and IL-13(63). ILC2 are tissue-resident lymphocytes with various functional roles in mucosal immunity. In tumor immunology, ILC2 is crucial in DC recruitment via CCL5 production and activation through IL-9 and IL-13 secretion (64). IL-33 further stimulates ST2+ regulatory T-cells (Tregs) and amphiregulin (AREG) expression, enhancing immune regulatory functions and tissue repair (65). The activation of ILC2 through IL-33 supports type 2 immune responses and M2 reparative macrophages. ST2 is also expressed in myeloid-derived antigen-presenting cells, such as macrophages and CD11b+ CD11c+ DCs (66). Moreover, IL-33 triggers IL-2 release and fosters Treg cell expansion. The type, density and spatial distribution of these IL-33-modulated immune cells within tumors profoundly influence tumor behavior (67). Thus, the cytokine IL-33 stimulates the upregulation of IL-13 while concurrently suppressing IL-12 levels. This immune profile underlies the negative impact of M2 macrophages and Th2 cell polarization within the TME on antitumor immunity (34).
TAMs are the most abundant immune cells involved in regulating breast cancer progression. The TME contains many immunosuppressive cells (68). Macrophages exhibit heterogeneity, with at least two functionally distinct subtypes responding to different stimuli: classically activated M1 macrophages and alternatively activated M2 macrophages (69). M1 macrophages eliminate tumors directly by recognizing and phagocytizing cancer cells and indirectly by producing pro-inflammatory cytokines, such as IFN-γ and IL-12. Conversely, in the context of tumor development, M2 macrophages are regarded as ‘tumor promoters’. They facilitate cancer progression, promote tumor cell metastasis and angiogenesis, regulate energy metabolism and aid in immune system evasion (70). During tumor progression, M2 macrophages become more prevalent, eventually dominating the TAM population in the TME. The underlying mechanism suggests that TAMs promote and sustain cancer stem cells, thereby supporting tumor growth and self-renewal. Various cytokines and signaling pathways within the TME influence the polarization of macrophages into M1 or M2 types. When IL-4 binds to its receptor, it can promote the phosphorylation of STAT6, leading to the polarization of M2-like macrophages through the JAK/STAT6 signaling pathway (68,71). Phosphorylated STAT6 can bind to Krüppel-like factor 4 (KLF4) and peroxisome proliferator-activated receptor γ, further promoting this polarization. Furthermore, various signals, including TGF-β, IL-10, bone morphogenetic protein-7 and IL-4 itself, induce M2 polarization through the PI3K/Akt signaling pathway. The complex formed by CCAAT/enhancer binding protein α and KLF6 is also associated with the switch from the M1 to the M2 phenotype (71,72). The progression of BC can also be observed through the promotion of monocytic MDSC differentiation into immunosuppressive M2-polarized macrophages, facilitated by both sphingosine synthase 2 and exosomes secreted by mesenchymal stem cells (61,73). Doxorubicin (DOX)-resistant tumor cells release PGE2, activating the EP2-EP4/cAMP/PKA signaling cascade in MDSCs, fostering proliferation and an M2 polarized phenotype shift. This polarization is mediated through miR-10a induction (27). Studies have shown that natural killer (NK) T-cells (NKT cells) help transform CD11b+ HLA-DR MDSCs into CD11b low HLA-DR DCs (74).
Immunosuppressive effects of MDSCs on BC progression
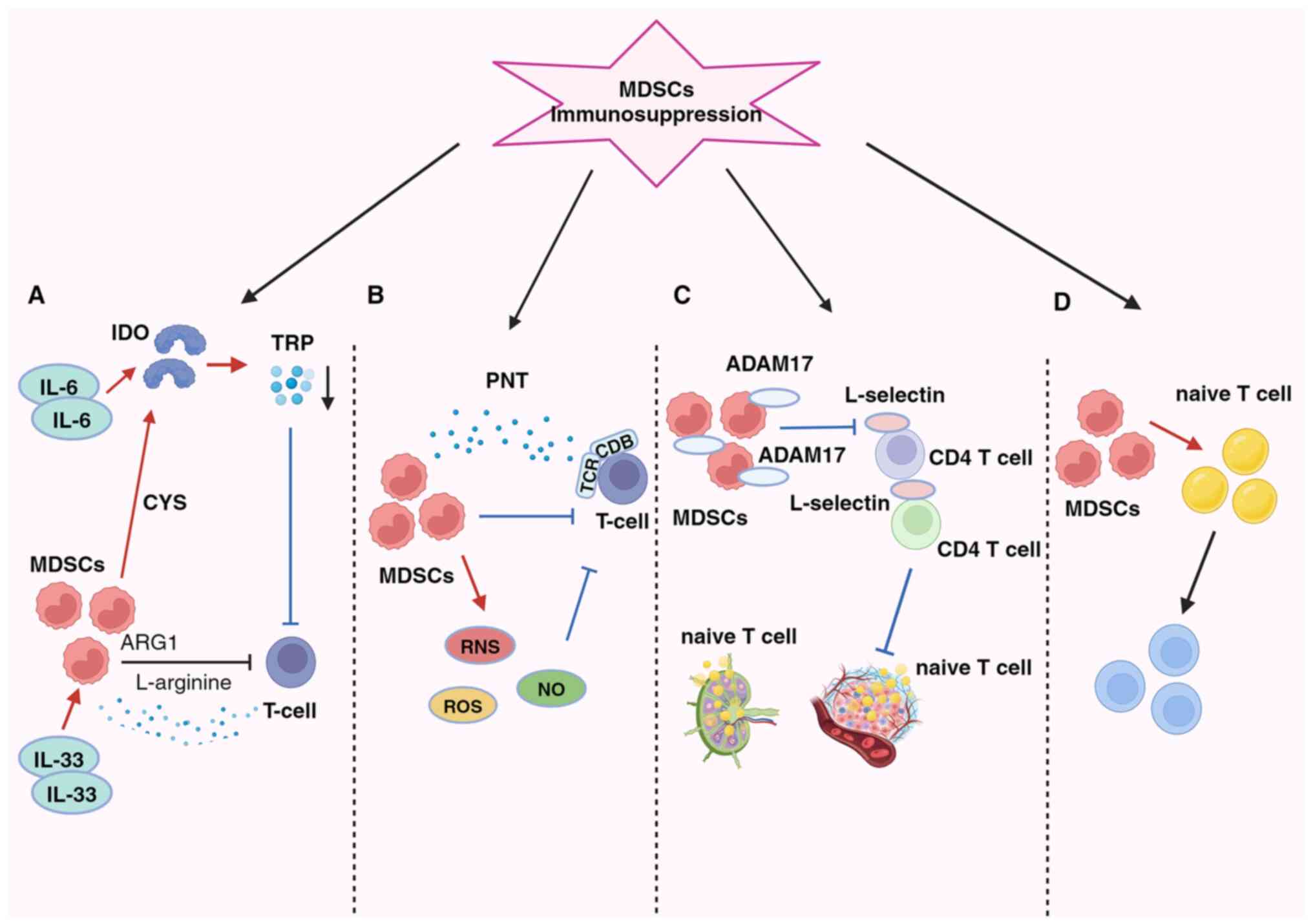
Fig. 2 shows how MDSCs significantly impede the activity of tumor-fighting T- and B-cells within the TME, particularly cytotoxic T-lymphocytes (CTLs) and pro-inflammatory cells, such as NK cells. Additionally, MDSCs can promote cancer progression by inducing the generation of Tregs and T-helper 17 (Th17) cells, thereby altering the local environment to promote tumor growth and enable immune evasion (75).
The primary immunosuppressive mechanism in the BC microenvironment involves the inhibition of T-cell function, which is the main trigger process involving the depletion of vital nutrients (Fig. 2A) (76). MDSCs exert inhibitory effects by activating indoleamine 2,3-dioxygenase (IDO), reducing local tryptophan availability, and generating cytotoxic metabolites, such as kynurenine in the TME and lymphatic drainage regions. This leads to an increase in Tregs, the inhibition of immune responses against antigens and the suppression of tumor-specific CTLs (77). The activation of STAT3-dependent NF-κB by IL-6 is responsible for maintaining IDO overexpression (30,77). Additionally, MDSCs enhance the suppressive action of IL-33 on T-cells by depleting L-arginine via arginase 1 (ARG1) (33,75). Furthermore, MDSCs consume cysteine, essential for T-cell activation and optimal function, leading to its depletion and failure to replenish in their environment (78). The generation of oxidative stress is a key factor in the second mechanism (Fig. 2B).
By producing reactive oxygen species, reactive nitrogen species and nitric oxide (NO), MDSCs suppress T-cells in the TME. MDSCs induce immune tolerance by T-cell receptor and CD8+ surface modifications and generate the free radical peroxynitrite (79). According to Stiff et al (80), MDSC-generated NO also disrupts Fc receptor-mediated NK cell activity, reducing monoclonal antibody efficacy and impeding the immune response against tumors. The third mechanism occurs by preventing lymphocyte migration (Fig. 2C). MDSCs reduce the immune response in peripheral lymphoid organs and accumulate in sentinel LNs, where they impede CD3/CD28-induced T-cell proliferation through contact-dependent mechanisms. This process supports tumor progression and metastasis (80,81). Hanson et al (82) linked decreased L-selectin expression on CD4+ and CD8+ T-cells with a disintegrin and metalloproteinase domain 17 generation on the plasma membrane. Consequently, MDSCs in BC hinder immature T-cell activation and migration into LNs and their subsequent transport to tumors, ultimately compromising the immune system's capacity to combat tumors (82).
The fourth aspect pertains to the expansion and activation of Tregs, facilitated by MDSCs via promoting their proliferation and differentiation of naïve CD4+ T cells (Fig. 2D). Although this pathway mechanism is not yet fully understood, it is known that Tregs can infiltrate tumors and play a crucial role in the antitumor immunosuppressive response. IL-34 triggers the conversion of bone marrow stem cells into monocytic MDSCs, indirectly suppressing the immune response by fostering Treg attraction via CCL22 secretion in the TME, thus contributing to chemotherapy resistance (35). Additionally, BC-induced MDSCs can stimulate effector T-cells to transition into Tregs through the IDO mechanism (77). MDSCs suppress T-cell activation, and once activated, T-cells trigger MDSC apoptosis via the Fas-FasL pathway (83).
MDSCs in BC employ contact-dependent mechanisms and indirect means, releasing NO, ARG and IL-1 to suppress the response of B-cells against tumors (84). Additionally, MDSCs can transform ordinary B-cells into specialized immunomodulatory B-cell (Breg) phenotypes, which efficiently suppress T-cell responses (85). Furthermore, various mediators in the TME, including LPS, can induce programmed cell death protein 1 (PD-1) expression in MDSCs in BC (86). By activating the PI3K/PKB/NF-κB signaling pathway in B-cells, MDSCs can enhance immune evasion mediated by PD-1/PD-L1 Bregs through the PD-1 pathway (87,88).
BC cells cultured under hypoxic conditions secrete various cytokines, including monocyte chemotactic protein-1, which triggers MDSC recruitment and suppresses the cytotoxic activity of NK cells. These mechanisms collectively contribute to cancer metastasis facilitation (89). NKT cells can restore suppressed T-cell function by converting CD11b+ HLA-DR MDSCs into CD11b low HLA-DR DCs through an NKG2D-dependent signaling mechanism (74). The function of MDSCs is further influenced by C5aR signaling, regulating CD4+ T-cell polarization towards the Th2 phenotype in the lungs of tumor-bearing mouse (58). The administration of DOX increases miR-126 exosomes derived from MDSCs, thereby suppressing T-cell functionality, inhibiting Th1 cell activation, and initiating Th2 cell responses in mouse lungs (90).
Clinical aspects of MDSCs related to BC
MDSC levels are associated with the progression of BC, typically showing higher levels in more advanced cancer stages (22). Additionally, surgical stress induced by the excised primary tumor environment can trigger the recruitment of MDSCs to lung and tumor tissues, underscoring the importance of monitoring MDSC levels (91). Data indicate variations in MDSC levels among patients undergoing antitumor treatment, potentially reflecting individual responses to therapy. In neoadjuvant therapy, circulating granulocytic MDSCs may initially increase, decrease with DOX and cyclophosphamide administration, and return close to baseline levels during paclitaxel treatment. Conversely, in metastatic or recurrent BC, monocytic MDSCs undergo significant expansion in the peripheral circulation, being associated with increased degrees of metastases to LNs and other organs (92). Therefore, tracking M-MDSC levels in patients with BC may be a valuable biomarker for monitoring cancer progression and treatment response.
BC treatments targeting MDSCs
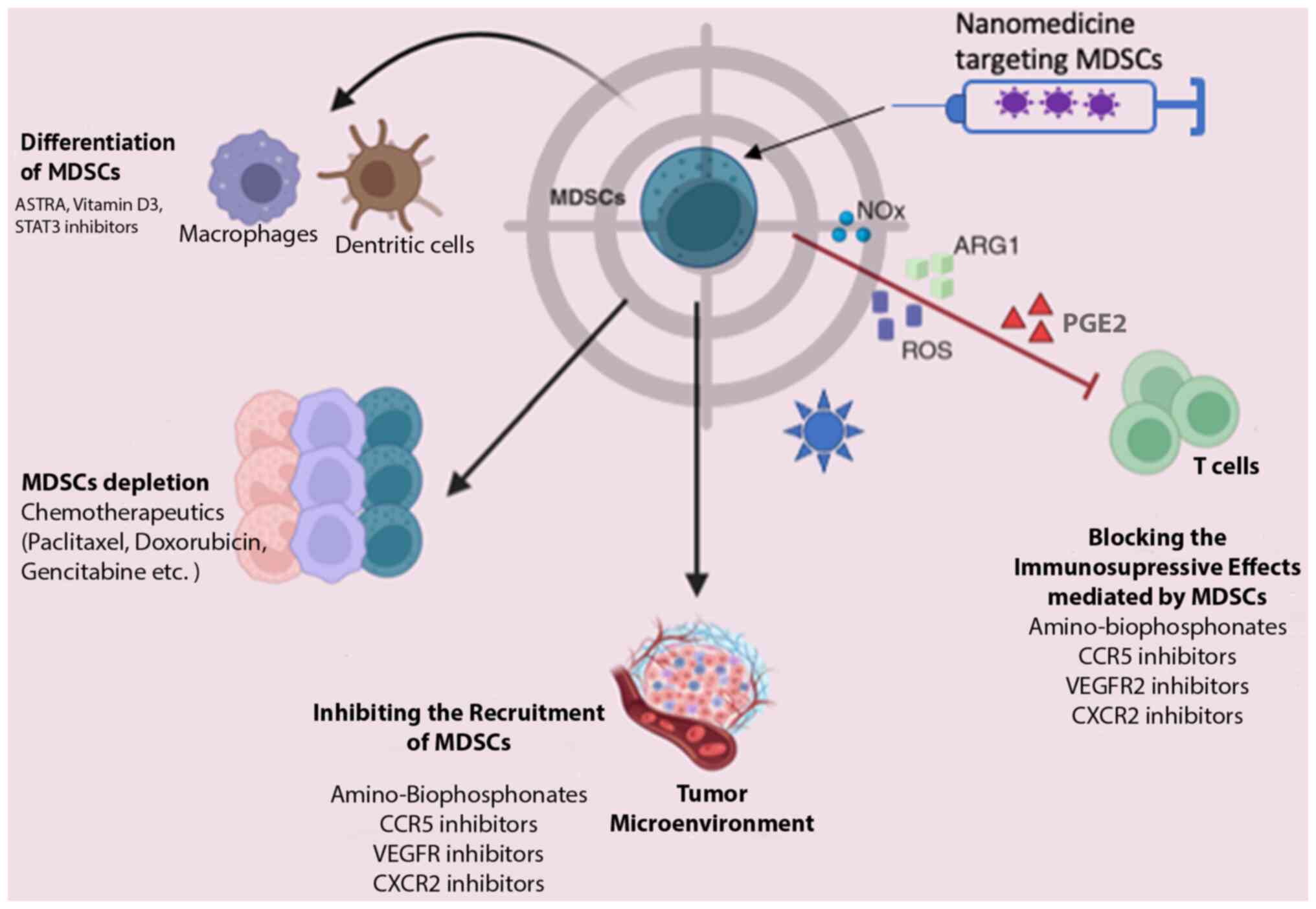
MDSCs play a pivotal role in BC progression and are intricately linked to tumor immune evasion. Consequently, MDSCs are a promising target for tumor immunotherapy, primarily aimed at enhancing host immunity. Currently, therapeutic strategies targeting MDSCs encompass four main approaches, as illustrated in Fig. 3: MDSC depletion, the blockade of MDSC recruitment, the suppression of MDSC immunosuppressive function and the induction of MDSC differentiation into a non-suppressive immune state (93-95).
While no specific selective inhibitors of MDSCs have been identified to date, at least to the best of our knowledge, several existing drugs exert indirect effects on MDSCs. For instance, DNA methyltransferase inhibitors and histone deacetylase (HDAC) inhibitors modulate systemic and intratumoral MDSCs, enhancing the long-term response to immunotherapy (96). Other drugs with the potential to suppress or deplete MDSCs and consequently enhance immunotherapy efficacy include gemcitabine (97), DOX (98) and 5-fluorouracil (99). Additionally, combined therapeutic strategies are under investigation, such as the combination of Romidepsin (an HDAC inhibitor) with cisplatin and nivolumab in TNBC (NCT02393794) (100) and IPI-549 (an inhibitor of PI3Kδ and PI3Kγ isoforms that decreases MDSCs and enhances anti-PD-1 efficacy) with nivolumab in solid tumors (101).
Curcumin is known for its antitumor properties, primarily attributed to its inhibition of IL-6. However, research has revealed that its mechanism also entails the inhibition of MDSCs in both blood and tissues, thus impeding tumor growth (102). Numerous preclinical and clinical studies are also dedicated to exploring strategies for promoting MDSC maturation (103-105). Sulforaphane, an inhibitor of the inflammatory cytokine MIF, disrupts its protumor functions, including the facilitation of MDSC differentiation in the TME. In vitro research has shown that MIF inhibitors, such as sulforaphane inhibit the accumulation of MDSCs in the TME, blocking their differentiation and restoring antitumor immunological activity (36). Silibinin modulates CCR2 expression in MDSCs, resulting in decreased MDSC accumulation in blood and tumor tissue (106). NG-monomethyl-L-arginine acetate, an inducible NO synthase inhibitor, blocks MDSC differentiation into osteoclasts, potentially preventing MDSC-mediated BC bone metastasis and associated bone degradation (107). Activated T-cells (ATCs) combined with bispecific anti-CD3 x anti-Her2/neu antibodies (aATCs) effectively modulate MDSCs via INF and IL-2, suppressing their activity and inhibiting tumor growth (108). According to Thakur et al (109), aATCs suppress the actions and functions of MDSCs (via IFN and IL-2) and effectively inhibit tumor growth and Treg differentiation.
Therapeutic combinations, such as adoptive cell therapy involving sensitized immune and tumor cells reprogrammed with CD25+ NKT, NK and memory T-cells, have achieved the immunosuppression of MDSCs (110). In other strategies tested in preclinical models, a vaccine composed of Listeria monocytogenes has been investigated. When MDSCs are infected with a highly attenuated bacterium, Listeria monocytogenes (Listeriaat), their immunosuppressive function is altered. Moreover, Listeriaat-infected MDSCs, which primarily deliver Listeriaat to the microenvironment of metastases and primary tumors, spread from MDSCs to tumor cells. Consequently, Listeriaat immunotherapy significantly reduces the population of MDSCs and can convert MDSCs into an immunostimulatory phenotype that produces IL-12, while concurrently reducing metastasis and tumor growth (111).
3. Potential nanotechnology-based therapeutics through MDSC targeting
Nanotechnology-based drug delivery systems are the emerging approaches in cancer therapy, characterized by intensive exploration (112,113). Despite the biological and functional barriers posed by the TME, nanoparticles (NPs) have demonstrated efficacy in enhancing intertumoral drug accumulation through passive or active targeting mechanisms. This optimized biodistribution reduces side-effects and increases therapeutic benefits (114,115). While drug delivery platforms targeting MDSCs in cancer treatment (Fig. 3) are relatively new, their potential is promising, particularly in BC.
As examples of successful results, Zhang et al (116) used ursolic acid, a natural pentacyclic triterpenoid known for its antifungal, antibacterial and recently discovered immunomodulatory properties, encapsulated within liposomes to modulate the TME. Following five administrations, treatment with this liposomal formulation led to a significant reduction in biomarker levels across the bloodstream, spleen, and tumor sites. This was coupled with enhanced cytotoxic T-cell activity and consequent reductions in tumor volumes (116). Chen et al (117) used gemcitabine-loaded nanocages in combination with IDO-targeted small interfering RNA and PD-L1 antibody designed on the nanocarrier surface. This combinatorial approach aims to improve immunosuppression and overall treatment outcomes in patients with TNBC. The administration of these tri-loaded nanocarriers (GSZMP) in TNBC mice was shown to result in a significant decrease in MDSC proportions compared to the controls, accompanied by increased T-cell infiltration. Additionally, the GSZMP group exhibited significant tumor volume reduction and increased survival rates compared to the control group (118).
Using this targeted delivery strategy to the tumor site, a nanoparticle formulation comprising c-peptide (RGDfk) in low molecular weight heparin-retinoic acid (LMWH-ATRA) micelles loaded with DOX and the immune adjuvant α-galactosyl ceramide (αGC) (RLA/DOX/αGC NP) was proposed. The hydrophilic segment of LMWH selectively bound to P-selectin present on vascular endothelial cells impedes the recruitment of MDSCs. The hydrophobic ATRA segment facilitated MDSC depletion, inducing their differentiation. This multidimensional approach effectively modulated MDSCs, significantly improving the inflammatory and immunosuppressive microenvironment in the lungs and tumor sites while inhibiting NPM formation. The micelles exhibited synergistic effects with other components in their composition (notably αGC), enhancing overall antitumor immunity. Thus, this formulation is a promising therapeutic avenue for addressing BC and lung metastases (119).
4. Conclusions and future perspectives
There is evidence to suggest a heightened prevalence of MDSCs in patients with BC, indicating their crucial role in the immune-resistant characteristics of the disease. Given the diverse nature of MDSCs, there is a pressing need for assays that can accurately identify MDSC subtypes in patients with BC. It is imperative to evaluate MDSC levels in both peripheral blood and the TME across various stages and subtypes of BC. Such assessments would provide insight into MDSC generation, expansion, and their function in peripheral blood and the TME, thereby elucidating the connection between MDSCs and the advancement of BC stages. The present review aimed to facilitate the practical application of these research findings and lay the foundation for the diagnosis and treatment of MDSC-related aspects of BC. MDSCs are pivotal in advancing tumor growth and metastasis through complex mechanisms.
Immunotherapy is a promising therapeutic approach in cancer treatment, demonstrating increased survival rates in preclinical models and clinical settings. Until recently, immunotherapy was not considered a viable option for BC treatment as BC was long-viewed as a poorly immunogenic tumor type (120). However, increasing evidence in recent years indicating immunogenic activity across various BC subtypes has shifted this paradigm, highlighting immunotherapy as an increasingly important tool in BC treatment (121).
Clinical trials are underway to combine immunotherapy with other therapeutic modalities in BC to target MDSCs (109,110). This approach is justified as MDSCs are essential in the BC microenvironment, promoting tumor growth and metastasis. Thus, MDSC-targeted therapies are promising as potential treatments in clinical settings. Proposed strategies to inhibit MDSCs include promoting differentiation, modulating production flexibility, initiating recruitment in peripheral organs, and direct elimination, aiming to circumvent the strong toxicity and side effects associated with traditional non-specific and sometimes ineffective chemotherapy.
Several therapies targeting MDSCs, either as standalone immunotherapies or combined with standard methods, such as chemotherapy and radiation, are undergoing preclinical trials to enhance their antitumor capabilities. It is hoped that a more in-depth understanding of the clinical significance of MDSCs will prompt the development of MDSC-focused treatments, ultimately improving the outcomes of patients with BC. The TME and MDSCs play pivotal roles in tumor growth and survival, with their influence particularly pronounced in TNBC. Emerging findings suggest that targeting MDSCs may be a promising alternative therapeutic approach, particularly in immunotherapy, reshaping the immunosuppressive microenvironment and enhancing the efficacy of cancer immunotherapy. In this context, nanotechnology is a valuable tool. With its controlled drug release capabilities and increased tumor accumulation, nanoparticles exhibit potential for cancer treatment. Numerous studies on BC mouse models have shown promising results with nanoparticle usage, including significant tumor shrinkage and changes in the TME components. However, the complexity of the microenvironment poses challenges in manipulating it because changes to one cellular component can lead to cascading effects on others. Despite the great promise of incorporating nanotechnology in oncology, its application in clinical settings still lacks successful results in clinical trials. Therefore, comprehensive preclinical evaluations of various nanocarriers and a thorough understanding of the strategies with which to most effectively target MDSCs to alter the TME effectively are crucial before they can be translated into clinical practice.
Acknowledgements
The authors gratefully acknowledge the Department of Genetics and Morphology at the Institute of Biological Sciences, University of Brasilia (GEM-IB-UnB) where the research related to the present study work was conducted, the Coordinating Agency for Advanced Training of Graduate Personnel (CAPES), and the National Council for Technological and Scientific Development of Brazil (CNPq) and the Federal District Research Support Foundation (FAPDF) responsible for the financial support of research and publications.
Funding
Funding: No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
DK conceptualized the study and was involved in the collection of data from the literature for inclusion in the review, in the writing and preparation of the original draft of the manuscript, as well as in the editing of the manuscript, and in figure creation. VCDS was involved in figure editing and in the final reviewing of the manuscript. NLC was involved in the conceptualization of the study, in the collection of data from the literature for inclusion in the review, in the writing and preparation of the original draft of the manuscript, and in the editing and final reviewing of the manuscript. All authors have reviewed and approved the submitted and final versions of the manuscript. Data authentication is not applicable.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
|
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 68:394–424. 2018.PubMed/NCBI View Article : Google Scholar | |
|
Lei S, Zheng R, Zhang S, Wang S, Chen R, Sun K, Zeng H, Zhou J and Wei W: Global patterns of breast cancer incidence and mortality: A population-based cancer registry data analysis from 2000 to 2020. Cancer Commun (Lond). 41:1183–1194. 2021.PubMed/NCBI View Article : Google Scholar | |
|
Gradishar WJ, Moran MS, Abraham J, Aft R, Agnese D, Allison KH, Anderson B, Burstein HJ, Chew H, Dang C, et al: Breast cancer, version 3.2022, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 20:691–722. 2022.PubMed/NCBI View Article : Google Scholar | |
|
Partridge AH, Burstein HJ and Winer EP: Side effects of chemotherapy and combined chemohormonal therapy in women with early-stage breast cancer. J Natl Cancer Inst Monogr. (30):135–142. 2001.PubMed/NCBI View Article : Google Scholar | |
|
Langeh U, Kumar V, Ahuja P, Singh C and Singh A: An update on breast cancer chemotherapy-associated toxicity and their management approaches. Health Sci Re. 9(100119)2023. | |
|
Slamon D, Eiermann W, Robert N, Pienkowski T, Martin M, Press M, Mackey J, Glaspy J, Chan A, Pawlicki M, et al: Adjuvant trastuzumab in HER2-positive breast cancer. N Engl J Med. 365:1273–1283. 2011.PubMed/NCBI View Article : Google Scholar | |
|
Darby SC, Ewertz M, McGale P, Bennet AM, Blom-Goldman U, Brønnum D, Correa C, Cutter D, Gagliardi G, Gigante B, et al: Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med. 368:987–998. 2013.PubMed/NCBI View Article : Google Scholar | |
|
Adams S, Gatti-Mays ME, Kalinsky K, Korde LA, Sharon E, Amiri-Kordestani L, Bear H, McArthur HL, Frank E, Perlmutter J, et al: Current landscape of immunotherapy in breast cancer: A review. JAMA Oncol. 5:1205–1214. 2019.PubMed/NCBI View Article : Google Scholar | |
|
Verheijden RJ, van Eijs MJM, May AM, van Wijk F and Suijkerbuijk KPM: Immunosuppression for immune-related adverse events during checkpoint inhibition: An intricate balance. NPJ Precis Oncol. 7(41)2023.PubMed/NCBI View Article : Google Scholar | |
|
Martins F, Sofiya L, Sykiotis GP, Lamine F, Maillard M, Fraga M, Shabafrouz K, Ribi C, Cairoli A, Guex-Crosier Y, et al: Adverse effects of immune-checkpoint inhibitors: epidemiology, management and surveillance. Nat Rev Clin Oncol. 6:563–580. 2019.PubMed/NCBI View Article : Google Scholar | |
|
Trimboli RM, Giorgi Rossi P, Battisti NML, Cozzi A, Magni V, Zanardo M and Sardanelli F: Do we still need breast cancer screening in the era of targeted therapies and precision medicine? Insights Imaging. 11(105)2020.PubMed/NCBI View Article : Google Scholar | |
|
Elemam NM, Talaat IM, Assal RA and Youness RA: Understanding the crosstalk between immune cells and the tumor microenvironment in cancer and its implications for immunotherapy. Front Med (Lausanne). 10(1202581)2023.PubMed/NCBI View Article : Google Scholar | |
|
Cha YJ and Koo JS: Role of tumor-associated myeloid cells in breast cancer. Cells. 9(1785)2020.PubMed/NCBI View Article : Google Scholar | |
|
Ortiz ML, Lu L, Ramachandran I and Gabrilovich DI: Myeloid-derived suppressor cells in the development of lung cancer. Cancer Immunol Res. 2:50–58. 2014.PubMed/NCBI View Article : Google Scholar | |
|
Srivastava MK, Zhu L, Harris-White M, Kar UK, Huang M, Johnson MF, Lee JM, Elashoff D, Strieter R, Dubinett S and Sharma S: Myeloid suppressor cell depletion augments antitumor activity in lung cancer. PLoS One. 7(e40677)2012.PubMed/NCBI View Article : Google Scholar | |
|
Yang Z, Guo J, Weng L, Tang W, Jin S and Ma W: Myeloid-derived suppressor cells-new and exciting players in lung cancer. J Hematol Oncol. 13(10)2020.PubMed/NCBI View Article : Google Scholar | |
|
Blaye C, Boyer T, Peyraud F, Domblides C and Larmonier N: Beyond immunosuppression: The multifaceted functions of tumor-promoting myeloid cells in breast cancers. Front Immunol. 13(838040)2022.PubMed/NCBI View Article : Google Scholar | |
|
Li L, Li M and Jia Q: Myeloid-derived suppressor cells: Key immunosuppressive regulators and therapeutic targets in cancer. Pathol Res Pract. 248(154711)2023.PubMed/NCBI View Article : Google Scholar | |
|
Parker KH, Beury DW and Ostrand-Rosenberg S: Myeloid-derived suppressor cells: critical cells driving immune suppression in the tumor microenvironment. Adv Cancer Res. 128:95–139. 2015.PubMed/NCBI View Article : Google Scholar | |
|
Bronte V, Brandau S, Chen SH, Colombo MP, Frey AB, Greten TF, Mandruzzato S, Murray PJ, Ochoa A, Ostrand-Rosenberg S, et al: Recommendations for myeloid-derived suppressor cell nomenclature and characterization standards. Nat Commun. 7(12150)2016.PubMed/NCBI View Article : Google Scholar | |
|
Cassetta L, Baekkevold ES, Brandau S, Bujko A, Cassatella MA, Dorhoi A, Krieg C, Lin A, Loré K, Marini O, et al: Deciphering myeloid-derived suppressor cells: isolation and markers in humans, mice, and non-human primates. Cancer Immunol Immunother. 68:687–697. 2019.PubMed/NCBI View Article : Google Scholar | |
|
Wang PF, Song SY, Wang TJ, Ji WJ, Li SW, Liu N and Yan CX: Prognostic role of pretreatment circulating MDSCs in patients with solid malignancies: A meta-analysis of 40 studies. Oncoimmunology. 7(e1494113)2018.PubMed/NCBI View Article : Google Scholar | |
|
Condamine T, Mastio J and Gabrilovich DI: Transcriptional regulation of myeloid-derived suppressor cells. J Leukoc Biol. 98:913–922. 2015.PubMed/NCBI View Article : Google Scholar | |
|
Alshetaiwi H, Pervolarakis N, McIntyre LL, Ma D, Nguyen Q, Rath JA, Nee K, Hernandez G, Evans K, Torosian L, et al: Defining the emergence of myeloid-derived suppressor cells in breast cancer using single-cell transcriptomics. Sci Immunol. 5(eaay6017)2020.PubMed/NCBI View Article : Google Scholar | |
|
Millrud CR, Bergenfelz C and Leandersson K: On the origin of myeloid-derived suppressor cells. Oncotarget. 8:3649–3665. 2017.PubMed/NCBI View Article : Google Scholar | |
|
Sun HW, Wu WC, Chen HT, Xu YT, Yang YY, Chen J, Yu XJ, Wang Z, Shuang ZY and Zheng L: Glutamine deprivation promotes the generation and mobilization of MDSCs by enhancing expression of G-CSF and GM-CSF. Front Immunol. 11(616367)2021.PubMed/NCBI View Article : Google Scholar | |
|
Rong Y, Yuan CH, Qu Z, Zhou H, Guan Q, Yang N, Leng XH, Bu L, Wu K and Wang F: Doxorubicin-resistant cancer cells activate myeloid-derived suppressor cells by releasing PGE2. Sci Rep. 6(23824)2016.PubMed/NCBI View Article : Google Scholar | |
|
Ching MM, Reader J and Fulton AM: Eicosanoids in cancer: prostaglandin E2 receptor 4 in cancer therapeutics and immunotherapy. Front Pharmacol. 11(530199)2020.PubMed/NCBI View Article : Google Scholar | |
|
Pradhan AK, Maji S, Bhoopathi P, Talukdar S, Mannangatti P, Guo C, Wang XY, Cartagena LC, Idowu M, Landry JW, et al: Pharmacological inhibition of MDA-9/Syntenin blocks breast cancer metastasis through suppression of IL-1β. Proc Natl Acad Sci USA. 118(e2103180118)2021.PubMed/NCBI View Article : Google Scholar | |
|
Jiang M, Chen J, Zhang W, Zhang R, Ye Y, Liu P, Yu W, Wei F, Ren X and Yu J: Interleukin-6 trans-signaling pathway promotes immunosuppressive myeloid-derived suppressor cells via suppression of suppressor of cytokine signaling 3 in breast cancer. Front Immunol. 8(1840)2017.PubMed/NCBI View Article : Google Scholar | |
|
Zhao N, Zhu W, Wang J, Liu W, Kang L, Yu R and Liu B: Group 2 innate lymphoid cells promote TNBC lung metastasis via the IL-13-MDSC axis in a murine tumor model. Int Immunopharmacol. 99(107924)2021.PubMed/NCBI View Article : Google Scholar | |
|
Popović M, Dedić Plavetić N, Vrbanec D, Marušić Z, Mijatović D and Kulić A: Interleukin 17 in early invasive breast cancer. Front Oncol. 13(1171254)2023.PubMed/NCBI View Article : Google Scholar | |
|
Gao W, Wen H, Liang L, Dong X, Du R, Zhou W, Zhang X, Zhang C, Xiang R and Li N: IL20RA signaling enhances stemness and promotes the formation of an immunosuppressive microenvironment in breast cancer. Theranostics. 11:2564–2580. 2021.PubMed/NCBI View Article : Google Scholar | |
|
Xiao P, Wan X, Cui B, Liu Y, Qiu C, Rong J, Zheng M, Song Y, Chen L, He J, et al: Interleukin 33 in tumor microenvironment is crucial for the accumulation and function of myeloid-derived suppressor cells. Oncoimmunology. 5(e1063772)2016.PubMed/NCBI View Article : Google Scholar | |
|
Kajihara N, Kobayashi T, Otsuka R, Nio-Kobayashi J, Oshino T, Takahashi M, Imanishi S, Hashimoto A, Wada H and Seino KI: Tumor-derived interleukin-34 creates an immunosuppressive and chemoresistant tumor microenvironment by modulating myeloid-derived suppressor cells in triple-negative breast cancer. Cancer Immunol Immunother. 72:851–864. 2023.PubMed/NCBI View Article : Google Scholar | |
|
Simpson KD, Templeton DJ and Cross JV: Macrophage migration inhibitory factor promotes tumor growth and metastasis by inducing myeloid-derived suppressor cells in the tumor microenvironment. J Immunol. 189:5533–5540. 2012.PubMed/NCBI View Article : Google Scholar | |
|
Liu QW, Chen Y, Li JY, Xiao L, Zhang WJ, Zhao JL, Gu HC, Wu HY, Zuo GS, Deng KY and Xin HB: Bone marrow cells are differentiated into MDSCs by BCC-Ex through down-regulating the expression of CXCR4 and activating the STAT3 signalling pathway. J Cell Mol Med. 25:5497–5510. 2021.PubMed/NCBI View Article : Google Scholar | |
|
Mundy-Bosse BL, Thornton LM, Yang HC, Andersen BL and Carson WE: Psychological stress is associated with altered levels of myeloid-derived suppressor cells in breast cancer patients. Cell Immunol. 270:80–87. 2011.PubMed/NCBI View Article : Google Scholar | |
|
He K, Liu X, Hoffman RD, Shi RZ, Lv GY and Gao JL: G-CSF/GM-CSF-induced hematopoietic dysregulation in the progression of solid tumors. FEBS Open Bio. 12:1268–1285. 2022.PubMed/NCBI View Article : Google Scholar | |
|
Smith KG and Clatworthy MR: FcγRIIB in autoimmunity and infection: Evolutionary and therapeutic implications. Nat Rev Immunol. 10:328–343. 2010.PubMed/NCBI View Article : Google Scholar | |
|
Wu L, Xu Y, Zhao H, Zhou Y, Chen Y, Yang S, Lei J, Zhang J, Wang J, Wu Y and Li Y: FcγRIIB potentiates differentiation of myeloid-derived suppressor cells to mediate tumor immunoescape. Theranostics. 12:842–858. 2022.PubMed/NCBI View Article : Google Scholar | |
|
Hillmer EJ, Zhang H, Li HS and Watowich SS: STAT3 signaling in immunity. Cytokine Growth Factor Rev. 31:1–15. 2016.PubMed/NCBI View Article : Google Scholar | |
|
Liao WC, Yen HR, Chen CH, Chu YH, Song YC, Tseng TJ and Liu CH: CHPF promotes malignancy of breast cancer cells by modifying syndecan-4 and the tumor microenvironment. Am J Cancer Res. 11:812–826. 2021.PubMed/NCBI | |
|
Liu C, Qiang J, Deng Q, Xia J, Deng L, Zhou L, Wang D, He X, Liu Y, Zhao B, et al: ALDH1A1 activity in tumor-initiating cells remodels myeloid-derived suppressor cells to promote breast cancer progression. Cancer Res. 81:5919–5934. 2021.PubMed/NCBI View Article : Google Scholar | |
|
Jiang M, Zhang W, Zhang R, Liu P, Ye Y, Yu W, Guo X and Yu J: Cancer exosome-derived miR-9 and miR-181a promote the development of early-stage MDSCs via interfering with SOCS3 and PIAS3 respectively in breast cancer. Oncogene. 39:4681–4694. 2020.PubMed/NCBI View Article : Google Scholar | |
|
Zhang W, Jiang M, Chen J, Zhang R, Ye Y, Liu P, Yu W and Yu J: SOCS3 suppression promoted the recruitment of CD11b+ Gr-1-F4/80-MHCII-early-stage myeloid-derived suppressor cells and accelerated interleukin-6-related tumor invasion via affecting myeloid differentiation in breast cancer. Front Immunol. 9(1699)2018.PubMed/NCBI View Article : Google Scholar | |
|
Welte T, Kim IS, Tian L, Gao X, Wang H, Li J, Holdman XB, Herschkowitz JI, Pond A, Xie G, et al: Oncogenic mTOR signalling recruits myeloid-derived suppressor cells to promote tumour initiation. Nat Cell Biol. 18:632–644. 2016.PubMed/NCBI View Article : Google Scholar | |
|
Ozga AJ, Chow MT and Luster AD: Chemokines and the immune response to cancer. Immunity. 54:859–874. 2021.PubMed/NCBI View Article : Google Scholar | |
|
Huang YC, Hou MF, Tsai YM, Pan YC, Tsai PH, Lin YS, Chang CY, Tsai EM and Hsu YL: Involvement of ACACA (acetyl-CoA carboxylase α) in the lung pre-metastatic niche formation in breast cancer by senescence phenotypic conversion in fibroblasts. Cell Oncol (Dordr). 46:643–660. 2023.PubMed/NCBI View Article : Google Scholar | |
|
Gu P, Sun M, Li L, Yang Y, Jiang Z, Ge Y, Wang W, Mu W and Wang H: Breast tumor-derived exosomal microRNA-200b-3p promotes specific organ metastasis through regulating CCL2 expression in lung epithelial cells. Front Cell Dev Biol. 9(657158)2021.PubMed/NCBI View Article : Google Scholar | |
|
Tanaka T, Kajiwara T, Torigoe T, Okamoto Y, Sato N and Tamura Y: Cancer-associated oxidoreductase ERO1-α drives the production of tumor-promoting myeloid-derived suppressor cells via oxidative protein folding. J Immunol. 194:2004–2010. 2015.PubMed/NCBI View Article : Google Scholar | |
|
Liu Y, Lai L, Chen Q, Song Y, Xu S, Ma F, Wang X, Wang J, Yu H, Cao X and Wang Q: MicroRNA-494 is required for the accumulation and functions of tumor-expanded myeloid-derived suppressor cells via targeting of PTEN. J Immunol. 188:5500–5510. 2012.PubMed/NCBI View Article : Google Scholar | |
|
Guo L, Kong D, Liu J, Zhan L, Luo L, Zheng W, Zheng Q, Chen C and Sun S: Breast cancer heterogeneity and its implication in personalized precision therapy. Exp Hematol Oncol. 12(3)2023.PubMed/NCBI View Article : Google Scholar | |
|
Vrakas CN, O'Sullivan RM, Evans SE, Ingram DA, Jones CB, Phuong T and Kurt RA: The Measure of DAMPs and a role for S100A8 in recruiting suppressor cells in breast cancer lung metastasis. Immunol Invest. 44:174–188. 2015.PubMed/NCBI View Article : Google Scholar | |
|
Chen JY, Lai YS, Chu PY, Chan SH, Wang LH and Hung WC: Cancer-derived VEGF-C increases chemokine production in lymphatic endothelial cells to promote CXCR2-dependent cancer invasion and MDSC recruitment. Cancers (Basel). 11(1120)2019.PubMed/NCBI View Article : Google Scholar | |
|
Roberts LM, Perez MJ, Balogh KN, Mingledorff G, Cross JV and Munson JM: Myeloid derived suppressor cells migrate in response to flow and lymphatic endothelial cell interaction in the breast tumor microenvironment. Cancers (Basel). 14(3008)2022.PubMed/NCBI View Article : Google Scholar | |
|
Yu B, Luo F, Sun B, Liu W, Shi Q, Cheng SY, Chen C, Chen G, Li Y and Feng H: KAT6A acetylation of SMAD3 regulates myeloid-derived suppressor cell recruitment, metastasis, and immunotherapy in triple-negative breast cancer. Adv Sci (Weinh). 8(e2100014)2021.PubMed/NCBI View Article : Google Scholar | |
|
Vadrevu SK, Chintala NK, Sharma SK, Sharma P, Cleveland C, Riediger L, Manne S, Fairlie DP, Gorczyca W, Almanza O, et al: Complement c5a receptor facilitates cancer metastasis by altering T-cell responses in the metastatic niche. Cancer Res. 74:3454–3465. 2014.PubMed/NCBI View Article : Google Scholar | |
|
Cheng R, Billet S, Liu C, Haldar S, Choudhury D, Tripathi M, Hav M, Merchant A, Hu T, Huang H, et al: Periodontal inflammation recruits distant metastatic breast cancer cells by increasing myeloid-derived suppressor cells. Oncogene. 39:1543–1556. 2020.PubMed/NCBI View Article : Google Scholar | |
|
Tcyganov E, Mastio J, Chen E and Gabrilovich DI: Plasticity of myeloid-derived suppressor cells in cancer. Curr Opin Immunol. 51:76–82. 2018.PubMed/NCBI View Article : Google Scholar | |
|
Mehta AK, Kadel S, Townsend MG, Oliwa M and Guerriero JL: Macrophage biology and mechanisms of immune suppression in breast cancer. Front Immunol. 12(643771)2021.PubMed/NCBI View Article : Google Scholar | |
|
Ostrand-Rosenberg S and Fenselau C: Myeloid-derived suppressor cells: immune-suppressive cells that impair antitumor immunity and are sculpted by their environment. J Immunol. 200:422–431. 2018.PubMed/NCBI View Article : Google Scholar | |
|
Cayrol C and Girard JP: Interleukin-33 (IL-33): A critical review of its biology and the mechanisms involved in its release as a potent extracellular cytokine. Cytokine. 156(155891)2022.PubMed/NCBI View Article : Google Scholar | |
|
Mattiola I and Diefenbach A: Enabling anti-tumor immunity by unleashing ILC2. Cell Res. 30:461–462. 2020.PubMed/NCBI View Article : Google Scholar | |
|
Halvorsen EC, Franks SE, Wadsworth BJ, Harbourne BT, Cederberg RA, Steer CA, Martinez-Gonzalez I, Calder J, Lockwood WW and Bennewith KL: IL-33 increases ST2+ Tregs and promotes metastatic tumour growth in the lungs in an amphiregulin-dependent manner. Oncoimmunology. 8(e1527497)2018.PubMed/NCBI View Article : Google Scholar | |
|
Gurram RK and Zhu J: Orchestration between ILC2s and Th2 cells in shaping type 2 immune responses. Cell Mol Immunol. 16:225–235. 2019.PubMed/NCBI View Article : Google Scholar | |
|
Choi MR, Sosman JA and Zhang B: The janus face of IL-33 signaling in tumor development and immune escape. Cancers (Basel). 13(3281)2021.PubMed/NCBI View Article : Google Scholar | |
|
Huang X, Cao J and Zu X: Tumor-associated macrophages: An important player in breast cancer progression. Thorac Cancer. 13:269–276. 2022.PubMed/NCBI View Article : Google Scholar | |
|
Hao NB, Lü MH, Fan YH, Cao YL, Zhang ZR and Yang SM: Macrophages in tumor microenvironments and the progression of tumors. Clin Dev Immunol. 2012(948098)2012.PubMed/NCBI View Article : Google Scholar | |
|
Boutilier AJ and Elsawa SF: Macrophage polarization states in the tumor microenvironment. Int J Mol Sci. 22(6995)2021.PubMed/NCBI View Article : Google Scholar | |
|
Wang S, Wang J, Chen Z, Luo J, Guo W, Sun L and Lin L: Targeting M2-like tumor-associated macrophages is a potential therapeutic approach to overcome antitumor drug resistance. NPJ Precis Oncol. 8(31)2024.PubMed/NCBI View Article : Google Scholar | |
|
Chen S, Saeed AFUH, Liu Q, Jiang Q, Xu H, Xiao GG, Rao L and Duo Y: Macrophages in immunoregulation and therapeutics. Signal Transduct Target Ther. 8(207)2023.PubMed/NCBI View Article : Google Scholar | |
|
Biswas S, Mandal G, Roy Chowdhury S, Purohit S, Payne KK, Anadon C, Gupta A, Swanson P, Yu X, Conejo-Garcia JR and Bhattacharyya A: Exosomes produced by mesenchymal stem cells drive differentiation of myeloid cells into immunosuppressive M2-polarized macrophages in breast cancer. J Immunol. 203:3447–3460. 2019.PubMed/NCBI View Article : Google Scholar | |
|
Payne KK, Zoon CK, Wan W, Marlar K, Keim RC, Kenari MN, Kazim AL, Bear HD and Manjili MH: Peripheral blood mononuclear cells of patients with breast cancer can be reprogrammed to enhance anti-HER-2/neu reactivity and overcome myeloid-derived suppressor cells. Breast Cancer Res Treat. 142:45–57. 2013.PubMed/NCBI View Article : Google Scholar | |
|
Gabrilovich DI, Ostrand-Rosenberg S and Bronte V: Coordinated regulation of myeloid cells by tumours. Nat Rev Immunol. 12:253–268. 2012.PubMed/NCBI View Article : Google Scholar | |
|
Pansy K, Uhl B, Krstic J, Szmyra M, Fechter K, Santiso A, Thüminger L, Greinix H, Kargl J, Prochazka K, et al: Immune regulatory processes of the tumor microenvironment under malignant conditions. Int J Mol Sci. 22(13311)2021.PubMed/NCBI View Article : Google Scholar | |
|
Li F, Zhao Y, Wei L, Li S and Liu J: Tumor-infiltrating Treg, MDSC, and IDO expression associated with outcomes of neoadjuvant chemotherapy of breast cancer. Cancer Biol Ther. 19:695–705. 2018.PubMed/NCBI View Article : Google Scholar | |
|
Srivastava MK, Sinha P, Clements VK, Rodriguez P and Ostrand-Rosenberg S: Myeloid-derived suppressor cells inhibit T-cell activation by depleting cystine and cysteine. Cancer Res. 70:68–77. 2010.PubMed/NCBI View Article : Google Scholar | |
|
Lu T, Ramakrishnan R, Altiok S, Youn JI, Cheng P, Celis E, Pisarev V, Sherman S, Sporn MB and Gabrilovich D: Tumor-infiltrating myeloid cells induce tumor cell resistance to cytotoxic T cells in mice. J Clin Invest. 121:4015–4029. 2011.PubMed/NCBI View Article : Google Scholar | |
|
Stiff A, Trikha P, Mundy-Bosse B, McMichael E, Mace TA, Benner B, Kendra K, Campbell A, Gautam S and Abood D: , et al: Nitric oxide production by myeloid-derived suppressor cells plays a role in impairing Fc receptor-mediated natural killer cell function. Clin Cancer Res. 24:1891–1904. 2018.PubMed/NCBI View Article : Google Scholar | |
|
Sceneay J, Griessinger CM, Hoffmann SHL, Wen SW, Wong CSF, Krumeich S, Kneilling M, Pichler BJ and Möller A: Tracking the fate of adoptively transferred myeloid-derived suppressor cells in the primary breast tumor microenvironment. PLoS One. 13(e0196040)2018.PubMed/NCBI View Article : Google Scholar | |
|
Hanson EM, Clements VK, Sinha P, Ilkovitch D and Ostrand-Rosenberg S: Myeloid-derived suppressor cells down-regulate L-selectin expression on CD4+ and CD8+ T cells. J Immunol. 183:937–944. 2009.PubMed/NCBI View Article : Google Scholar | |
|
Sinha P, Chornoguz O, Clements VK, Artemenko KA, Zubarev RA and Ostrand-Rosenberg S: Myeloid-derived suppressor cells express the death receptor Fas and apoptose in response to T cell-expressed FasL. Blood. 117:5381–5390. 2011.PubMed/NCBI View Article : Google Scholar | |
|
Lelis FJ, Jaufmann J, Singh A, Fromm K, Teschner AC, Pöschel S, Schäfer I, Beer-Hammer S, Rieber N and Hartl D: Myeloid-derived suppressor cells modulate B-cell responses. Immunol Lett. 188:108–115. 2017.PubMed/NCBI View Article : Google Scholar | |
|
Shen M, Wang J, Yu W, Zhang C, Liu M, Wang K, Yang L, Wei F, Wang SE, Sun Q and Ren X: A novel MDSC-induced PD-1- PD-L1+ B-cell subset in breast tumor microenvironment possesses immuno-suppressive properties. Oncoimmunology. 7(e1413520)2018.PubMed/NCBI View Article : Google Scholar | |
|
Nam S, Lee A, Lim J and Lim JS: Analysis of the expression and regulation of PD-1 protein on the surface of myeloid-derived suppressor cells (MDSCs). Biomol Ther (Seoul). 27:63–70. 2019.PubMed/NCBI View Article : Google Scholar | |
|
Liu M, Wei F, Wang J, Yu W, Shen M, Liu T, Zhang D, Wang Y, Ren X and Sun Q: Myeloid-derived suppressor cells regulate the immunosuppressive functions of PD-1- PD-L1+ Bregs through PD-L1/PI3K/AKT/NF-κB axis in breast cancer. Cell Death Dis. 12(465)2021.PubMed/NCBI View Article : Google Scholar | |
|
Spallanzani RG, Dalotto-Moreno T, Raffo Iraolagoitia XL, Ziblat A, Domaica CI, Avila DE, Rossi LE, Fuertes MB, Battistone MA, Rabinovich GA, et al: Expansion of CD11b+ Ly6G+ Ly6C int cells driven by medroxyprogesterone acetate in mice bearing breast tumors restrains NK cell effector functions. Cancer Immunol Immunother. 62:1781–1795. 2013.PubMed/NCBI View Article : Google Scholar | |
|
Sceneay J, Chow MT, Chen A, Halse HM, Wong CS, Andrews DM, Sloan EK, Parker BS, Bowtell DD, Smyth MJ and Möller A: Primary tumor hypoxia recruits CD11b+/Ly6Cmed/Ly6G+ immune suppressor cells and compromises NK cell cytotoxicity in the premetastatic niche. Cancer Res. 72:3906–3911. 2012.PubMed/NCBI View Article : Google Scholar | |
|
Deng Z, Rong Y, Teng Y, Zhuang X, Samykutty A, Mu J, Zhang L, Cao P, Yan J, Miller D and Zhang HG: Exosomes miR-126a released from MDSC induced by DOX treatment promotes lung metastasis. Oncogene. 36:639–651. 2017.PubMed/NCBI View Article : Google Scholar | |
|
Ma X, Wang M, Yin T, Zhao Y and Wei X: Myeloid-derived suppressor cells promote metastasis in breast cancer after the stress of operative removal of the primary cancer. Front Oncol. 9(855)2019.PubMed/NCBI View Article : Google Scholar | |
|
Bergenfelz C, Roxå A, Mehmeti M, Leandersson K and Larsson AM: Clinical relevance of systemic monocytic-MDSCs in patients with metastatic breast cancer. Cancer Immunol Immunother. 69:435–448. 2020.PubMed/NCBI View Article : Google Scholar | |
|
Liu H, Wang Z, Zhou Y and Yang Y: MDSCs in breast cancer: An important enabler of tumor progression and an emerging therapeutic target. Front Immunol. 14(1199273)2023.PubMed/NCBI View Article : Google Scholar | |
|
Veglia F, Perego M and Gabrilovich D: Myeloid-derived suppressor cells coming of age. Nat Immunol. 19:108–119. 2018.PubMed/NCBI View Article : Google Scholar | |
|
Gatti-Mays ME, Balko JM, Gameiro SR, Bear HD, Prabhakaran S, Fukui J, Disis ML, Nanda R, Gulley JL, Kalinsky K, et al: If we build it they will come: targeting the immune response to breast cancer. NPJ Breast Cancer. 5(37)2019.PubMed/NCBI View Article : Google Scholar | |
|
Kim K, Skora AD, Li Z, Liu Q, Tam AJ, Blosser RL, Diaz LA Jr, Papadopoulos N, Kinzler KW, Vogelstein B and Zhou S: Eradication of metastatic mouse cancers resistant to immune checkpoint blockade by suppression of myeloid-derived cells. Proc Natl Acad Sci USA. 111:11774–11779. 2014.PubMed/NCBI View Article : Google Scholar | |
|
Le HK, Graham L, Cha E, Morales JK, Manjili MH and Bear HD: Gemcitabine directly inhibits myeloid derived suppressor cells in BALB/c mice bearing 4T1 mammary carcinoma and augments expansion of T cells from tumor-bearing mice. Int Immunopharmacol. 9:900–909. 2009.PubMed/NCBI View Article : Google Scholar | |
|
Alizadeh D, Trad M, Hanke NT, Larmonier CB, Janikashvili N, Bonnotte B, Katsanis E and Larmonier N: Doxorubicin eliminates myeloid-derived suppressor cells and enhances the efficacy of adoptive T-cell transfer in breast cancer. Cancer Res. 74:104–118. 2014.PubMed/NCBI View Article : Google Scholar | |
|
Vincent J, Mignot G, Chalmin F, Ladoire S, Bruchard M, Chevriaux A, Martin F, Apetoh L, Rébé C and Ghiringhelli F: 5-Fluorouracil selectively kills tumor-associated myeloid-derived suppressor cells resulting in enhanced T cell-dependent antitumor immunity. Cancer Res. 70:3052–3061. 2010.PubMed/NCBI View Article : Google Scholar | |
|
Sharma P, Abramson V, O’Dea A, Nye L, Mayer I, Crane G, Elia M, Yoder R, Staley J, Schwensen K, et al: Romidepsin (HDACi) plus cisplatin and nivolumab triplet combination in patients with metastatic triple negative breast cancer (mTNBC). J Clin Oncol. 39(10.1200/JCO.2021.39.15_suppl.1076)2021. | |
|
Davis RJ, Moore EC, Clavijo PE, Friedman J, Cash H, Chen Z, Silvin C, Van Waes C and Allen C: Anti-PD-L1 efficacy can be enhanced by inhibition of myeloid-derived suppressor cells with a selective inhibitor of PI3Kδ/γ. Cancer Res. 77:2607–2619. 2017.PubMed/NCBI View Article : Google Scholar | |
|
Tu SP, Jin H, Shi JD, Zhu LM, Suo Y, Lu G, Liu A, Wang TC and Yang CS: Curcumin induces the differentiation of myeloid-derived suppressor cells and inhibits their interaction with cancer cells and related tumor growth. Cancer Prev Res (Phila). 5:205–215. 2012.PubMed/NCBI View Article : Google Scholar | |
|
Sánchez-León ML, Jiménez-Cortegana C, Silva Romeiro S, Garnacho C, de la Cruz-Merino L, García-Domínguez DJ, Hontecillas-Prieto L and Sánchez-Margalet V: Defining the emergence of new immunotherapy approaches in breast cancer: Role of myeloid-derived suppressor cells. Int J Mol Sci. 24(5208)2023.PubMed/NCBI View Article : Google Scholar | |
|
Kusmartsev S, Cheng F, Yu B, Nefedova Y, Sotomayor E, Lush R and Gabrilovich D: All-trans-retinoic acid eliminates immature myeloid cells from tumor-bearing mice and improves the effect of vaccination. Cancer Res. 63:4441–4449. 2003.PubMed/NCBI | |
|
Iclozan C, Antonia S, Chiappori A, Chen DT and Gabrilovich D: Therapeutic regulation of myeloid-derived suppressor cells and immune response to cancer vaccine in patients with extensive stage small cell lung cancer. Cancer Immunol Immunother. 62:909–918. 2013.PubMed/NCBI View Article : Google Scholar | |
|
Forghani P, Khorramizadeh MR and Waller EK: Silibinin inhibits accumulation of myeloid-derived suppressor cells and tumor growth of murine breast cancer. Cancer Med. 3:215–224. 2014.PubMed/NCBI View Article : Google Scholar | |
|
Sawant A, Deshane J, Jules J, Lee CM, Harris BA, Feng X and Ponnazhagan S: Myeloid-derived suppressor cells function as novel osteoclast progenitors enhancing bone loss in breast cancer. Cancer Res. 73:672–682. 2013.PubMed/NCBI View Article : Google Scholar | |
|
Kugler A, Stuhler G, Walden P, Zöller G, Zobywalski A, Brossart P, Trefzer U, Ullrich S, Müller CA, Becker V, et al: Regression of human metastatic renal cell carcinoma after vaccination with tumor cell-dendritic cell hybrids. Nat Med. 6:332–336. 2000.PubMed/NCBI View Article : Google Scholar | |
|
Thakur A, Schalk D, Sarkar SH, Al-Khadimi Z, Sarkar FH and Lum LG: A Th1 cytokine-enriched microenvironment enhances tumor killing by activated T cells armed with bispecific antibodies and inhibits the development of myeloid-derived suppressor cells. Cancer Immunol Immunother. 61:497–509. 2012.PubMed/NCBI View Article : Google Scholar | |
|
Kmieciak M, Basu D, Payne KK, Toor A, Yacoub A, Wang XY, Smith L, Bear HD and Manjili MH: Activated NK T cells and NK cells render T cells resistant to MDSC and result in an effective adoptive cellular therapy against breast cancer in the FVBN202 transgenic mouse. J Immunol. 187:708–717. 2011.PubMed/NCBI View Article : Google Scholar | |
|
Chandra D, Jahangir A, Quispe-Tintaya W, Einstein MH and Gravekamp C: Myeloid-derived suppressor cells have a central role in attenuated Listeria monocytogenes-based immunotherapy against metastatic breast cancer in young and old mice. Br J Cancer. 108:2281–2290. 2013.PubMed/NCBI View Article : Google Scholar | |
|
Chaves NL, Amorim DA, Lopes CAP, Estrela-Lopis I, Böttner J, de Souza AR and Báo SN: Comparison of the effect of rhodium citrate-associated iron oxide nanoparticles on metastatic and non-metastatic breast cancer cells. Cancer Nano. 10:1–12. 2019. | |
|
Yao Y, Zhou Y, Liu L, Xu Y, Chen Q, Wang Y, Wu S, Deng Y, Zhang J and Shao A: Nanoparticle-based drug delivery in cancer therapy and its role in overcoming drug resistance. Front Mol Biosci. 7(193)2020.PubMed/NCBI View Article : Google Scholar | |
|
Chaves NL, Estrela-Lopis I, Böttner J, Lopes CA, Guido BC, de Sousa AR and Báo SN: Exploring cellular uptake of iron oxide nanoparticles associated with rhodium citrate in breast cancer cells. Int J Nanomedicine. 12:5511–5523. 2017.PubMed/NCBI View Article : Google Scholar | |
|
Figueiro Longo JP and Muehlmann LA: Nanomedicine beyond tumor passive targeting: What next? Nanomedicine (Lond). 15:1819–1822. 2020.PubMed/NCBI View Article : Google Scholar | |
|
Zhang N, Liu S, Shi S, Chen Y, Xu F, Wei X and Xu Y: Solubilization and delivery of Ursolic-acid for modulating tumor microenvironment and regulatory T cell activities in cancer immunotherapy. J Control Release. 320:168–178. 2020.PubMed/NCBI View Article : Google Scholar | |
|
Chen C, Li A, Sun P, Xu J, Du W, Zhang J, Liu Y, Zhang R, Zhang S, Yang Z, et al: Efficiently restoring the tumoricidal immunity against resistant malignancies via an immune nanomodulator. J Control Release. 324:574–585. 2020.PubMed/NCBI View Article : Google Scholar | |
|
Ali R, Shao H and Varamini P: Potential Nanotechnology-Based Therapeutics to Prevent Cancer Progression through TME Cell-Driven Populations. Pharmaceutics. 15(112)2022.PubMed/NCBI View Article : Google Scholar | |
|
Lu Z, Liu H, Ma L, Ren K, He Z, Li M and He Q: Micellar nanoparticles inhibit breast cancer and pulmonary metastasis by modulating the recruitment and depletion of myeloid-derived suppressor cells. Nanoscale. 14:17315–17330. 2022.PubMed/NCBI View Article : Google Scholar | |
|
Debien V, De Caluwé A, Wang X, Piccart-Gebhart M, Tuohy VK, Romano E and Buisseret L: Immunotherapy in breast cancer: An overview of current strategies and perspectives. NPJ Breast Cancer. 9(7)2023.PubMed/NCBI View Article : Google Scholar | |
|
Teschendorff AE, Miremadi A, Pinder SE, Ellis IO and Caldas C: An immune response gene expression module identifies a good prognosis subtype in estrogen receptor negative breast cancer. Genome Biol. 8(R157)2007.PubMed/NCBI View Article : Google Scholar |