PI3K/Akt and HIF‑1 signaling pathway in hypoxia‑ischemia (Review)
- Authors:
- Published online on: August 9, 2018 https://doi.org/10.3892/mmr.2018.9375
- Pages: 3547-3554
-
Copyright: © Zhang et al. This is an open access article distributed under the terms of Creative Commons Attribution License.
Abstract
Introduction
Hypoxia-ischemia (H-I) commonly occurs during myocardial infarction, stroke and perinatal asphyxia. It can lead to severe injuries such as cerebral palsy (1), H-I brain damage, chronic neurological and neurodevelopmental disability in children, and even death (2). In addition, H-I may trigger massive cellular malfunction and cell death. On the other hand, the decline of cellular oxygen level during H-I also induces many compensatory responses, such as neovascularization (3), metabolic regulation and production of various neurotrophic mediators, which protect neurons from ischemic death. These processes also form part of an endogenous adaptive response that aims to defend and help tissues recover from ischemic injury (4). The rapid restoration of blood flow in the occluded coronary arteries following H-I is the most important aspect of the protective mechanism. Nevertheless, the early opening of an occluded coronary artery may lead to ischemia/reperfusion (I/R) injury (5,6).
It has been reported that phosphatidylinositol-3 kinase (PI3K)/Akt signaling pathway is involved in H-I. In this review, we have discussed the potential mechanism of PI3K/Akt signaling pathway in cellular responses for resisting H-I.
PI3K/Akt signaling pathway
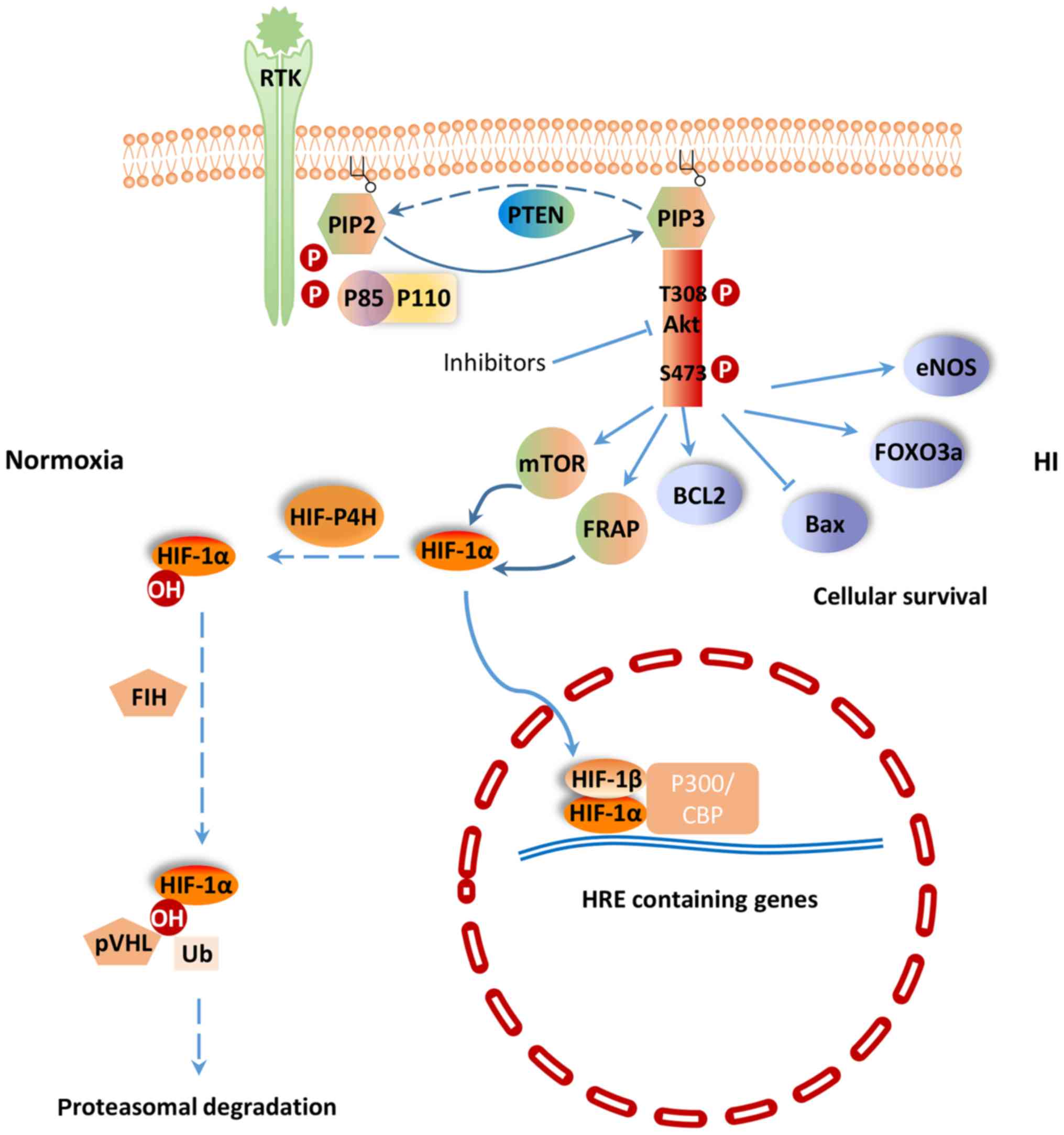
PI3K/Akt signaling pathway regulates a wide range of cellular activities including cell survival, proliferation, metabolism, neuroscience, motility and cancer progression (7). PI3K belongs to a lipid kinase family which is characterized by their ability to phosphorylate inositol ring 3′-OH group in inositol phospholipids in the plasma membrane (8). PI3Ks are divided into two classes: Class-I and II. The function of class-I PI3K is to phosphorylate PIP-2 to generate the second messenger PIP-3 within sec (9). PIP-3 can mediate different cellular functions of PI3K through specific interactions with pleckstrin homology (PH) domain containing proteins such as Akt (10). Akt is considered as the central mediator of the PI3K/Akt signaling pathway, which ultimately leads to the phosphorylation of some vital downstream targets (11). Furthermore, some negative regulators, such as phosphatase and tensin homologue (PTEN) inhibit PI3K/Akt signaling pathway. PTEN is a lipid phosphatase that negatively regulates the PI3K/Akt pathway by hydrolyzing PIP-3 to PIP-2, resulting in a lack of downstream p-Akt (12) (Fig. 1). PI3K and the downstream effector Akt belong to a conserved family of signal transduction enzymes, which are involved in regulating cellular activation, inflammatory responses and apoptosis (13).
PI3K/Akt signaling pathway is involved in H-I
It has been shown that H-I-induced injuries could be treated by certain agents that act on the PI3K/Akt signaling pathway. In cerebral ischemia rats, p-Akt 473 and p-Akt 308 protein expression was significantly increased after treatment with silibinin, a compound of flavonolignan with anti-apoptotic, anti-inflammatory and anti-oxidative functions (14). Phosphorylated Akt promotes the phosphorylation of downstream molecules, including Bcl-2 apoptosis related family members, Forkhead box O3 (FoxO3a) transcription factor, mammalian target of rapamycin (mTOR) and glycogen synthase kinase-3, in order to protect cells from apoptosis. Bcl-2, an inhibitor of neuronal apoptosis, is significantly upregulated, while Bax, which can promote neuronal apoptosis, is significantly downregulated in cerebral ischemia rats treated with silibinin (15). Li et al (16) found that the PI3K/Akt/FoxO3a pathway is involved in neuronal apoptosis in the developing rat brain. Activated Akt phosphorylates FoxO3a, and leads to the cytoplasmic localization of FoxO3a and inhibition of apoptosis (17) (Fig. 1). In addition, sodium tanshinoneIIA sulfonate and bromelain protect the rat heart from I/R injury via the activation of PI3K/Akt/FoxO3a pathway (18). In the cytoplasm, mTOR, a phosphoinositide kinase-related kinase family member, serves as a Ser/Thr protein kinase (19). Previous study found that the regulatory mechanism of mTOR activity is related to the PI3K/Akt signaling pathway (14). Zhong et al (20) were among the first to show that activation of the epidermal growth factor receptor (EGFR)/PI3K/AKT/mTOR pathway could positively regulate hypoxia-induced factor-1α (HIF-1α) at the protein level. Fibroblast growth factor-2 is a signaling molecular in the PI3K/Akt signaling pathway. Activation of PI3K/Akt pathway by fibroblast growth factor-2 prevents reactive oxygen species (ROS)-induced apoptosis and protects heart from I/R injury by decreasing infarct size and improving left ventricular function (21).
Hypoxia-inducible factor-1 (HIF-1)
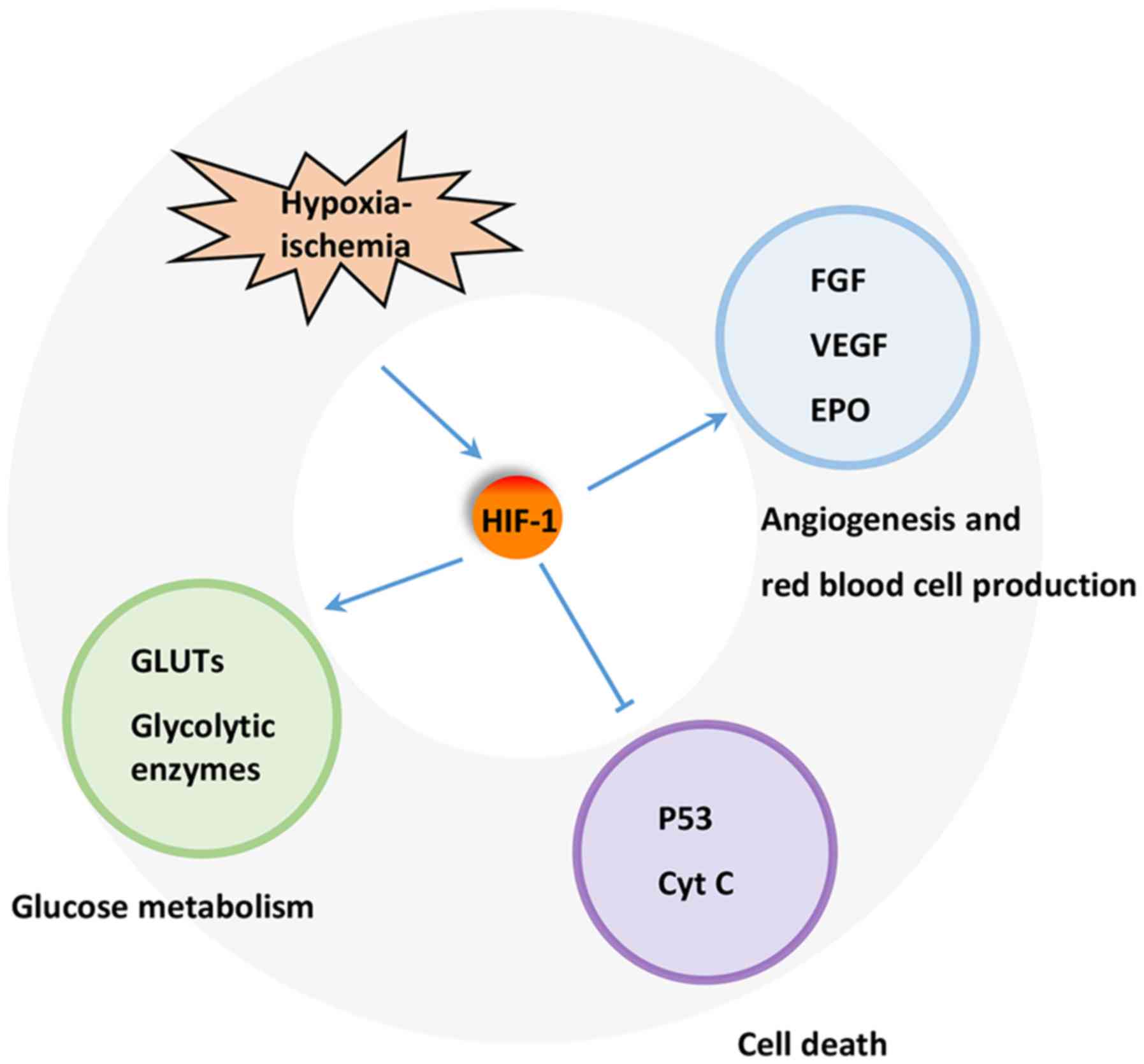
A key regulator of the response to HI is HIF-1. HIF-1 is a heterodimeric transcription factor composed of an oxygen sensitive subunit HIF-1α and an aryl hydrocarbon nuclear translocator HIF-1β. Under normoxic condition, HIF-1α is hydroxylated at prolines 402 and 564 by HIF prolyl-4-hydroxylase, leading to its ubiquitination and proteasomal degradation through the ubiquitin-proteasome (26S) pathway, which can continuously provoke proteasomal degradation. Its destruction is caused by the ubiquitin E3 ligase complex, in which the von Hippel-Lindau tumor suppressor protein (pVHL) is able to bind to the oxygen-dependent destruction domain on the subunit, resulting in a short half-life of the protein under normoxic conditions. In contrast, when HIF prolyl-4-hydroxylase is less active, HIF-1α is more stable. This stabilization allows HIF-1α to translocate to the nucleus and dimerize with its partner HIF-1β (Fig. 1). The HIF-1 dimer subsequently binds to the hypoxia response element site on DNA, initiating the expression of more than 100 genes that participate in hypoxic adaptation (22,23). HIF-1α is involved in pathologic conditions such as hypoxia or ischemia. HIF-1α has also been shown to regulate the expression of vascular endothelial growth factor (VEGF), erythropoietin (EPO) and glycolytic enzymes (24) (Fig. 2).
HIF-1α is regulated by PI3K/Akt signaling pathway
Previous studies have shown that HIF-1α is subjected to regulation by the PI3K/Akt/mTOR (20,25) and PI3K/Akt/FRAP (26) signaling pathways. The p-Akt and HIF-1α protein levels were shown to increase in response to hypoxia in human mesenchymal stem cells. Moreover, p-Akt expression peaked earlier than that of HIF-1α. Interestingly, the PI3K inhibitor LY294002 (27) and Dual PI3K/mTOR inhibitor NVP-BEZ235 (28) could suppress the activation of p-Akt and the expression of HIF-1α and VEGF resulted from H-I. The Akt inhibitor, wortmannin, could also inhibit the expression of HIF-1α at the protein, but not the mRNA level (7). mTOR is a hypoxia/nutrient sensor and a target of Akt during cell cycle regulation, glycogen metabolism and protein synthesis upon phosphorylation of its two main targets, eukaryotic initiation factor 4E-binding protein-1 and ribosomal protein S6 kinase (29). Moreover, mTOR is an upstream mediator of HIF-1α activation (30). Based on these previous findings, the PI3K/Akt signaling pathway could potentially regulates HIF-1α via mTOR, which could alter HIF-1α post-transcriptional protein level, but not at the transcriptional mRNA level.
It has been shown that the pVHL mutant fails to degrade HIF-1α, which implies that pVHL plays an important role in controlling the stability of HIF-1α (31). In another word, the stabilization of HIF-1α could be attributed to failure in pVHL-mediated ubiquitination and proteasomal degradation. The proteasomal degradation process is often controlled by phosphorylation (32). Therefore, it is speculated that HIF-1α activity is under the control of protein kinase phosphorylation, potentially through the universal phosphorylation signal transduction pathway of the PI3K/Akt (33).
PI3K/HIF pathway and H-I
Protective effect
Under H-I condition, PI3K/HIF pathway plays important roles in cardio-protection and neuro-protection. The expression of HIF-1α has been shown to increase significantly in various ischemic organs and tissues, including myocardium, nervous system and retina (34). The protection of HIF-1 has also been widely reported in various H-I models. For example, HIF-1 has been demonstrated to participate in neuroprotection during permanent focal ischemia in vivo (22). Various iron chelators, such as deferoxamine mesylate and mimosine, protect neurons from apoptosis through activating HIF-1 in vitro or in vivo (35,36). These results reveal that induction of HIF-1 by ischemia itself or via pharmacological channels can protect against H-I. Furthermore, HIF-1 can regulate the expression of various genes, including EPO, VEGF, inducible nitric oxide synthase, hemeoxygenase, and cardiotropin as well as those involved in glucose metabolism, mitochondrial function, cell apoptosis, and resistance to oxidative stress that protect or restore cell functions and facilitate cellular adaptation to H-I (37,38).
A number of mechanisms have been proposed for the protective effect of HIF-1. HIF-1 has been found to protect cells from hypoxic injury by promoting nutrient and O2 transport via inducing the expression of downstream proteins such as VEGF and EPO, which promote angiogenesis and erythropoiesis. This induction is partly PI3K/Akt inhibition-dependent, suggesting a close relationship between PI3K/Akt, HIF-1α and the VEGF cascade in HI (7). EPO promotes the production and release of red blood cell into blood, thereby, enhancing the oxygen transport. Meanwhile, the increase in hemoglobin level also affects oxygen transport capacity, and ultimately reduces tissue damage. On the other hand, HIF-1 may prevent apoptotic cell death through inhibiting the release of cytochrome C, PARP cleavage and caspase activation. In addition, HIF-1 may maintain cell survival by suppressing p53 activation. Increased glucose transport and glycolytic flow consequential of HIF-1 activation by H-I has also been implicated in tissue viability and cell survival (39).
PI3K/HIF pathway regulates glucose metabolism
One important function of glucose metabolism is to sustain a reducing environment in cells by generating reducing equivalents through oxidative phosphorylation, glycolysis, and the pentose phosphate pathway (40). The switch from aerobic to anaerobic glucose metabolism by upregulating glucose transporters (GLUTs) and glycolysis-related enzymes, such as phosphofructokinase 1, fructose-bisphosphate aldolase, phosphoglycerate kinase 1, pyruvate dehydrogenase kinase 1, and lactate dehydrogenase, is one of the key mechanisms to maintain cellular energy production and cell survival during ischemia (39). The expression of these proteins is mainly controlled by HIFs. HIF-1 activation leads to increased oxygen and nutrient delivery via enhancing angiogenesis and erythropoiesis (41,42) and improving oxygen utilization in metabolism (43). Activated HIF-1 is either directly or indirectly associated with the upregulation of GLUTs (44) and glycolytic enzymes in glycolysis and lactate production (45,46) (Fig. 2). This effect ultimately leads to the upregulation of aerobic glycolysis in tumor cells, while dampening the oxidative phosphorylation pathway (47). GLUT1 is upregulated by H-RAS, at least in part, via PI3K/HIF (48). Some stimuli, such as insulin, insulin-like growth factor 1, epidermal growth factor and angiotensin II, are able to increase HIF-1α level in cells (49). Other key enzymes involved in metabolism are also upregulated to further ensure cellular survival (50). Other studies have further demonstrated that HIF-1 activation could be attributed to cellular mutations under non-hypoxic conditions. This phenomenon is resulted from inactivation of various tumor suppression genes, along with activation of numerous oncogenes, which then lead to mutations in several growth factor pathways, such as the loss of pVHL. HIF-1α inactivation is caused by a physical interaction with pVHL, which elicits the 26S proteasome response. Studies have also reported that the β domain of pVHL interacts directly with the HIF-1α subunits. Therefore, any mutation that affects the β domain of pVHL may prevent its interaction between HIF-1α and thereby lead to the constitutive activation of HIF-1 (51).
The glycolysis process is an important metabolic pathway in mammals. Similar to GLUT, Hexokinase (HK) acts as a rate-limiting enzyme and is the first glycolytic enzyme which facilitates the irreversible phosphorylation of glucose to glucose-6-phosphate in cells, thereby committing the glucose molecule to the glycolytic cycle. HIF-1 activation can upregulate the expression level of HK1 and HK2 (52). In addition, HIF-1 has been shown to effectively upregulate the expression of many other glycolytic enzymes, leading to enhanced glycolysis. The glycolytic flux triggered by HIF-1α is also related to the kinetic patterns of the expressed isoforms of the key glycolytic enzymes, which can further promote glycolytic energetic capability. Moreover, HIF-1 induces the transcription of pyruvate dehydrogenase kinase 1, which effectively inhibits pyruvate dehydrogenase activity, thereby downregulating acetyl-CoA production and suppressing the TCA cycle (53). The level of Akt directly correlates with the rate of glucose uptake into the cell through the GLUT1 transporter (54). In addition, Akt can further influence glycolysis via HK2. Akt also activates FOXO3a to inhibit apoptosis and increases mitochondrial biogenesis to support a cellular survival (55). PKM2, an isoform of pyruvate kinase, harbors a hormone response element within its first intron, indicating that its transcriptional activity is regulated by HIF-1. PKM2 is also found to interact with HIF-1α in the nucleus and is believed to act as a transcriptional co-activator. It has been shown that activated tyrosine kinase inhibits pyruvate kinase, which further prevents pyruvate from entering into mitochondria and participating in the TCA cycle (56). Other studies on the glycolytic cycle have shown that increased pyruvate and lactate result in an increased expression of the monocarboxylate transporter (MCT) and lactate dehydrogenase (57). Although the mechanism is still unknown, reducing the expression of lactate dehydrogenase may lead to a decrease in production of lactate. On the other hand, MCT provides rapid transportation of monocarboxylate compounds, such as pyruvate and lactate, across plasma membrane, providing essential support for energy metabolism. Furthermore, activation of these transporters is closely related to HIF-1α. Previous studies have revealed that the inhibition of MCT1 can suppress lactate-induced HIF-1 activation. Whereas, the expression of MCT4 is mainly regulated by HIF-1α. Taken together, metabolism via the aerobic glycolytic pathway appears to be favored over the oxidative phosphorylation pathway in the presence of activated HIF. TCA cycle intermediates oxygen molecules and α-ketoglutarate is responsible for facilitating the degradation of HIF-1α (58).
PI3K/HIF pathway regulates angiogenesis
Angiogenesis is a key step in oxygen and nutrient transport. Therapeutic angiogenesis is an attractive approach for curing or alleviating ischemic cardiovascular disease (59). Angiogenesis plays an important role in the repair of tissues subjected to ischemic insult. Neovascularization is expected to reduce ventricular dysfunction and remodeling after myocardial infarction (MI) (60). The PI3K/Akt signaling pathway is crucial to inducing vascularization of heart and inhibiting cardiomyocyte apoptosis after MI (61,62). PI3K has several different isoforms (p110α, p110β, and p110δ), but only p110α is selectively required for angiogenesis (63). Interestingly, the protein kinase, Akt, has also been implicated as a mediator of cardio-protection (64). The activation of Ras and EGFR, a transmembrane receptor tyrosine kinase (RTK) that belongs to the HER family of receptors, upregulates HIF-1α via the PI3K/Akt signaling pathway (65). EGFR/PI3K/AKT/mTOR pathway increases VEGF and endothelial cell NO synthase (eNOS) expression by upregulating HIF-1α. VEGF, an endothelial-specific mitogen and survival factor, is one of the most potent angiogenic factors, and plays key roles in both angiogenesis and vasculogenesis. Hypoxia can increase eNOS phosphorylation by activating the PI3K/AKT pathway (66). HIF-1α can also directly influence the expression of eNOS, which can be activated by phosphorylation of the serine 1177 residue, thereby, triggering migration and angiogenesis (67) (Fig. 2). Accumulating evidence has shown that HIF-1α acts as a potential therapeutic proangiogenic molecule in experimental models (68,69). Furthermore, EGFR amplification and PTEN mutation exert an additive effect on increasing VEGF promoter activity in human glioblastoma cells. A recent study that explored the role of PTEN in hepatocellular carcinoma also found similar inhibition of angiogenesis (70). Elevated levels of VEGF can increase vascular permeability, leading to vessel leakage, sluggish blood flow, and elevated interstitial pressure. One of the potent stimuli for increased VEGF production is hypoxia (71). Binding of both STAT3 and HIF-1α to the VEGF promoter has been demonstrated to be essential for maximum transcription of VEGF mRNA under hypoxia (72). Therefore, therapies that affect HIF-1α expression could potentially induce neoangiogenesis in ischemic heart.
PI3K/HIF and I/R injury
The reintroduction of oxygen after H-I is inevitable, nevertheless, reperfusion is associated with exacerbation of I/R tissue injury caused by inflammatory responses and ROS production. Therefore, the alleviation of I/R injury is a popular strategy for treating diseases associated with H-I. Factors such as high mobility group box 1 (HMGB1) may exert its protective effect by upregulating the protein expression of HIF-1α in the ischemic myocardium via enhancing Akt phosphorylation through the PI3K/Akt signaling pathway. Treatment with LY294002 inhibits HMGB1-induced expression of HIF-1α and eliminates the cardioprotective effects exerted by intravenous HMGB1 in an I/R rat model. ROS can directly damage the cell membrane and cause cell death during I/R. Furthermore, ROS-mediated apoptosis and necrosis can be a determinant of infarct size. HMGB1 reduces the myocardial content of MDA and increases the activity of SOD induced by I/R, whereas LY294002 eliminates these effects (34). Guo et al (73) demonstrated that inhibiting HIF-1α expression by HIF-1α-specific small interfering RNA transfection increases ROS generation and promotes cell death. Cardiomyocyte-specific HIF-1α gene deletion leads to reduced contractility and vascularization, along with altering the expression of multiple genes in normoxic heart. I/R significantly increases the myocardial expression of HIF-1α, while HMGB1 also markedly upregulates the expression of HIF-1α. Furthermore, consistent with the increased expression of HIF-1α, the myocardial injury induced by I/R was inhibited by HMGB1. It was also found that intravenous HMGB1 increases SOD activity in the I/R myocardium, which suggests that these changes may be occurring downstream of its effects on HIF-1α overexpression. Thus, intravenous HMGB1 may exert its cardioprotective effects through increasing the expression of HIF-1α (34). In addition, increasing HIF-1α expression by drugs such as desferrioxamine, can induce a more reducing environment and decrease cell death. These results suggest that maintenance of cellular redox status via HIF-1 can protect cells from H-I mediated injuries (74).
Detrimental effects
Although HIF-1 exerts protective effects, it may also contribute to cellular and tissue damage. It has been reported that HIF-1 may mediate apoptosis in embryonic stem cells under hypoxic conditions (75). Similarly, it has been observed that HIF-1 signaling elicits delayed death via p53 in ischemic primary cortical neurons in vivo (76) and in vitro (77). Chen et al (78) has shown that inhibition of HIF-1 decreases the expression of VEGF and BCL2 interacting protein 3 (BNIP3) and thereby offering protection against delayed cell death. BNIP3 reduces increased levels of ROS via HIF-1-inducible mitochondrial autophagy (79), meanwhile causing mitochondrial dysfunction, opening of the mitochondrial permeability transition pores, membrane depolarization and cell death. Two h of ischemia has been shown to result in damage of brain cortex and blood-brain barrier in the non-infarcted ventromedial striatum and preoptic area. BNIP3 is induced in the brain under H-I condition as a master regulator in hypoxia. Suppression of HIF-1α and VEGF has been shown to reduce acute hyperglycemia-induced HT in the ischemic brain (80). Moreover, the various protective effects through PI3K/AKT and HIF-1 pathways may become reverse in cancer hypoxic microenvironment. Multiple members of the lysyl oxidase family induced in an HIF-1-dependent manner are involved in Metastatic niche formation (81,82). It was shown that HIF-1 is involved in almost every key step of the breast cancer metastatic process including epithelial-mesenchymal transition, invasion, intravasation, extravasation, and metastatic niche formation (83).
Time pattern
Using the same neuron-specific HIF-1α knock-out mice, Baranova et al (84) and Helton et al (85) have reported distinct HIF-1 effects on neuronal injuries following ischemia. Baranova et al (84) found that the neuron-specific knockdown of HIF-1α increases tissue damage and reduces the survival rate of middle cerebral artery occlusion mice, suggesting that HIF-1 is neuroprotective in their ischemic model. On the other hand, Helton et al (85) observed that the knocking out of HIF-1α reduces ischemic injury, indicating that HIF-1 may lead to tissue damage in brain ischemia. Interestingly, the ischemic model that Baranova et al (84) used was subjected to 30 min ischemia with unilateral common carotid artery occlusion (mild ischemia), while Helton's model was exposed to 75 min ischemia with bilateral occlusion (severe ischemia). Studies have demonstrated an enhanced survival and migration capability of dendritic cells (86) and transplanted stem cells in the ischemic myocardium (87,88) via short-term hypoxic preconditioning. Similarly, Jian et al (89) observed that exposure of endothelial progenitor cells to hypoxia for 24 h showed an increase in tube formation and cell motility, while prolonged hypoxia of endothelial progenitor cells for 48 and 72 h were reversed in these effects. Meanwhile, mRNA expressions of Akt and PI3K demonstrated similarly tend in a time-dependent manner. Interestingly, hypoxic preconditioning at 1% O2 in various cell lines accumulated HIF1/2α protein after 4 h followed by a markedly reduce after 24 h to 7 days (90), and the significantly enhancement of HIF2α protein was contrasted by a dramatic reduce of HIF2α under hypoxia within 24 h (91). The varied observations support a notion that hypoxia may induce cell death in severe and prolonged ischemia, while promote cell survival following mild ischemic insults via HIF-1α and PI3K/Akt pathways. Therefore, effects of HIF-1 on ischemic outcomes may be dependent on the duration of H-I, animal age and species (7).
Future directions
Despite the relatively high incidence of ischemic cerebrovascular and cardiovascular disease, limited therapies are currently available for its prevention and treatment (92,93). Although the survival rate for pre-term infants has been increased, neurological conditions such as cerebral palsy still occur in most survivors (94). PI3K/HIF pathway is important for both the mechanistic understanding and therapeutic intervention of diseases associate with H-I such as stroke, cardiovascular disease, cerebral ischemia and perinatal asphyxia. Interestingly, HIF-1 and PI3K/Akt appears to be involved in the cellular responses to H-I, but with a double-edged sword effect, which could possibly be dependent on the degree and duration of H-I. Therefore, therapies for hypoxic injury should be selected with this caveat in mind, and further study is necessary to find the optimal hypoxic pattern of different cell types. Understanding the mechanism of HIF-1 and PI3K/Akt accumulation would undoubtedly provide important insight into its role in H-I and provide potential approaches to regulate its expression.
Acknowledgements
The authors would like to thank Dr Qiang Lin (Department of Rehabilitation, Fifth Affiliated Hospital of Guangzhou Medical University, Guangdong, China) for his linguistic work on this manuscript.
Funding
The present study was supported by the Natural Science Foundation of Guangdong Province (grant no. 2015A030313254) and the Science and Technology Planning Project of Guangdong Province (grant no. 2014A020212493).
Availability of data and materials
All data generated or analyzed during the present study are included in this published article.
Authors' contributions
ZZ, LY and JY designed the study and drafted the manuscript. ZZ and ZW performed the data collection and generated the figures. ZZ and GD conceived the research and were in charge of overall direction and planning. All authors approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Glossary
Abbreviations
Abbreviations:
|
H-I |
hypoxia-ischemia |
|
HIF-1α |
hypoxia-induced factor-1α |
|
MI |
myocardial infarction |
|
I/R |
ischemia/reperfusion |
|
PI3K |
phosphatidylinositol-3 kinase |
|
RTK |
receptor tyrosine kinase |
|
PH |
pleckstrin homology |
|
PTEN |
phosphatase and tensin homologue |
|
FoxO3a |
Forkhead box O3 |
|
mTOR |
mammalian target of rapamycin |
|
FRAP |
FKBP-rapamycin associated protein |
|
pVHL |
von Hippel-Lindau tumor suppressor protein |
|
P300/CBP |
cyclic-adenosine monophosphate-response element-binding protein binding protein |
|
VEGF |
vascular endothelial growth factor |
|
EPO |
erythropoietin |
|
GLUTs |
glucose transporters |
|
HK |
hexokinase |
|
MCT |
monocarboxylate transporter |
|
EGFR |
epidermal growth factor receptor |
|
eNOS |
endothelial cell nitric oxide synthase |
|
ROS |
reactive oxygen species |
|
HMGB1 |
high mobility group box 1 |
|
BNIP3 |
B-cell lymphoma-2 interacting protein 3 |
References
|
Movsas TZ, Weiner RL, Greenberg MB, Holtzman DM and Galindo R: Pretreatment with human chorionic gonadotropin protects the neonatal brain against the effects of hypoxic-ischemic injury. Front Pediatr. 5:2322017. View Article : Google Scholar : PubMed/NCBI | |
|
Ferriero DM: Neonatal brain injury. N Engl J Med. 351:1985–1995. 2004. View Article : Google Scholar : PubMed/NCBI | |
|
Adluri RS, Thirunavukkarasu M, Zhan L, Akita Y, Samuel SM, Otani H, Ho YS, Maulik G and Maulik N: Thioredoxin 1 enhances neovascularization and reduces ventricular remodeling during chronic myocardial infarction: A study using thioredoxin 1 transgenic mice. J Mol Cell Cardiol. 50:239–247. 2011. View Article : Google Scholar : PubMed/NCBI | |
|
Greenberg DA and Jin K: From angiogenesis to neuropathology. Nature. 438:954–959. 2005. View Article : Google Scholar : PubMed/NCBI | |
|
Yellon DM and Hausenloy DJ: Myocardial reperfusion injury. N Engl J Med. 357:1121–1135. 2007. View Article : Google Scholar : PubMed/NCBI | |
|
Eltzschig HK and Eckle T: Ischemia and reperfusion-from mechanism to translation. Nat Med. 17:1391–1401. 2011. View Article : Google Scholar : PubMed/NCBI | |
|
Li L, Qu Y, Mao M, Xiong Y and Mu D: The involvement of phosphoinositid 3-kinase/Akt pathway in the activation of hypoxia-inducible factor-1alpha in the developing rat brain after hypoxia-ischemia. Brain Res. 1197:152–158. 2008. View Article : Google Scholar : PubMed/NCBI | |
|
Fruman DA, Meyers RE and Cantley LC: Phosphoinositide kinases. Annu Rev Biochem. 67:481–507. 1998. View Article : Google Scholar : PubMed/NCBI | |
|
Fresno Vara JA, Casado E, de Castro J, Cejas P, Belda-Iniesta C and González-Barón M: PI3K/Akt signalling pathway and cancer. Cancer Treat Rev. 30:193–204. 2004. View Article : Google Scholar : PubMed/NCBI | |
|
Pawson T and Nash P: Protein-protein interactions define specificity in signal transduction. Genes Dev. 14:1027–1047. 2000.PubMed/NCBI | |
|
Zhang F, Ding T, Yu L, Zhong Y, Dai H and Yan M: Dexmedetomidine protects against oxygen-glucose deprivation-induced injury through the I2 imidazoline receptor-PI3K/AKT pathway in rat C6 glioma cells. J Pharm Pharmacol. 64:120–127. 2012. View Article : Google Scholar : PubMed/NCBI | |
|
Ciuffreda L, Falcone I, Incani UC, Del Curatolo A, Conciatori F, Matteoni S, Vari S, Vaccaro V, Cognetti F and Milella M: PTEN expression and function in adult cancer stem cells and prospects for therapeutic targeting. Adv Biol Regul. 56:66–80. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
Cantley LC: The phosphoinositide 3-kinase pathway. Science. 296:1655–1657. 2002. View Article : Google Scholar : PubMed/NCBI | |
|
Han JQ, Yu KY and He M: Effects of puerarin on the neurocyte apoptosis and p-Akt (Ser473) expressions in rats with cerebral ischemia/reperfusion injury. Zhongguo Zhong Xi Yi Jie He Za Zhi. 32:1069–1072. 2012.(In Chinese). PubMed/NCBI | |
|
Liu BN, Han BX and Liu F: Neuroprotective effect of pAkt and HIF-1α on ischemia rats. Asian Pac J Trop Med. 7:221–225. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
Li D, Qu Y, Mao M, Zhang X, Li J, Ferriero D and Mu D: Involvement of the PTEN-AKT-FOXO3a pathway in neuronal apoptosis in developing rat brain after hypoxia-ischemia. J Cereb Blood Flow Metab. 29:1903–1913. 2009. View Article : Google Scholar : PubMed/NCBI | |
|
Wang Z, Wang Y, Ye J, Lu X, Cheng Y, Xiang L, Chen L, Feng W, Shi H, Yu X, et al: bFGF attenuates endoplasmic reticulum stress and mitochondrial injury on myocardial ischaemia/reperfusion via activation of PI3K/Akt/ERK1/2 pathway. J Cell Mol Med. 19:595–607. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Liu MH, Li GH, Peng LJ, Qu SL, Zhang Y, Peng J, Luo XY, Hu HJ, Ren Z, Liu Y, et al: PI3K/Akt/FoxO3a signaling mediates cardioprotection of FGF-2 against hydrogen peroxide-induced apoptosis in H9c2 cells. Mol Cell Biochem. 414:57–66. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Correia SC, Cardoso S, Santos RX, Carvalho C, Santos MS, Perry G, Smith MA and Moreira PI: New insights into the mechanisms of mitochondrial preconditioning-triggered neuroprotection. Curr Pharm Des. 17:3381–3389. 2011. View Article : Google Scholar : PubMed/NCBI | |
|
Zhong H, Chiles K, Feldser D, Laughner E, Hanrahan C, Georgescu MM, Simons JW and Semenza GL: Modulation of hypoxia-inducible factor 1alpha expression by the epidermal growth factor/phosphatidylinositol 3-kinase/PTEN/AKT/FRAP pathway in human prostate cancer cells: Implications for tumor angiogenesis and therapeutics. Cancer Res 60: 1541–1545, 2000. Cancer Res 60: 1541–1545, 2000. 60: 1541–1545, 2000:1541-1545, 2000–1545, 2000. 2000. | |
|
Wang Z, Zhang H, Xu X, Shi H, Yu X, Wang X, Yan Y, Fu X, Hu H, Li X and Xiao J: bFGF inhibits ER stress induced by ischemic oxidative injury via activation of the PI3K/Akt and ERK1/2 pathways. Toxicol Lett. 212:137–146. 2012. View Article : Google Scholar : PubMed/NCBI | |
|
Kunze R, Zhou W, Veltkamp R, Wielockx B, Breier G and Marti HH: Neuron-specific prolyl-4-hydroxylase domain 2 knockout reduces brain injury after transient cerebral ischemia. Stroke. 43:2748–2756. 2012. View Article : Google Scholar : PubMed/NCBI | |
|
Semenza GL: Hypoxia-inducible factors in physiology and medicine. Cell. 148:399–408. 2012. View Article : Google Scholar : PubMed/NCBI | |
|
Jain T, Nikolopoulou EA, Xu Q and Qu A: Hypoxia inducible factor as a therapeutic target for atherosclerosis. Pharmacol Ther. 183:22–33. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Xiao Y, Peng H, Hong C, Chen Z, Deng X, Wang A, Yang F, Yang L, Chen C and Qin X: PDGF promotes the warburg effect in pulmonary arterial smooth muscle cells via activation of the PI3K/AKT/mTOR/HIF-1α signaling pathway. Cell Physiol Biochem. 42:1603–1613. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Laughner E, Taghavi P, Chiles K, Mahon PC and Semenza GL: HER2 (neu) signaling increases the rate of hypoxia-inducible factor 1alpha (HIF-1alpha) synthesis: Novel mechanism for HIF-1-mediated vascular endothelial growth factor expression. Mol Cell Biol. 21:3995–4004. 2001. View Article : Google Scholar : PubMed/NCBI | |
|
Yang XM, Wang YS, Zhang J, Li Y, Xu JF, Zhu J, Zhao W, Chu DK and Wiedemann P: Role of PI3K/Akt and MEK/ERK in mediating hypoxia-induced expression of HIF-1alpha and VEGF in laser-induced rat choroidal neovascularization. Invest Ophthalmol Vis Sci. 50:1873–1879. 2009. View Article : Google Scholar : PubMed/NCBI | |
|
Karar J, Cerniglia GJ, Lindsten T, Koumenis C and Maity A: Dual PI3K/mTOR inhibitor NVP-BEZ235 suppresses hypoxia-inducible factor (HIF)-1α expression by blocking protein translation and increases cell death under hypoxia. Cancer Biol Ther. 13:1102–1111. 2012. View Article : Google Scholar : PubMed/NCBI | |
|
van den Beucken T, Koritzinsky M and Wouters BG: Translational control of gene expression during hypoxia. Cancer Biol Ther. 5:749–755. 2006. View Article : Google Scholar : PubMed/NCBI | |
|
Hudson CC, Liu M, Chiang GG, Otterness DM, Loomis DC, Kaper F, Giaccia AJ and Abraham RT: Regulation of hypoxia-inducible factor 1alpha expression and function by the mammalian target of rapamycin. Mol Cell Biol. 22:7004–7014. 2002. View Article : Google Scholar : PubMed/NCBI | |
|
Ivan M and Kaelin WG Jr: The von Hippel-Lindau tumor suppressor protein. Curr Opin Genet Dev. 11:27–34. 2001. View Article : Google Scholar : PubMed/NCBI | |
|
Bai S, Datta J, Jacob ST and Ghoshal K: Treatment of PC12 cells with nerve growth factor induces proteasomal degradation of T-cadherin that requires tyrosine phosphorylation of its cadherin domain. J Biol Chem. 282:27171–27180. 2007. View Article : Google Scholar : PubMed/NCBI | |
|
Dimova EY, Michiels C and Kietzmann T: Kinases as upstream regulators of the HIF system: their emerging potential as anti-cancer drug targets. Curr Pharm Des. 15:3867–3877. 2009. View Article : Google Scholar : PubMed/NCBI | |
|
Yao HC, Zhou M, Zhou YH, Wang LH, Zhang DY, Han QF, Liu T, Wu L, Tian KL and Zhang M: Intravenous high mobility group box 1 upregulates the expression of HIF-1α in the myocardium via a protein kinase B-dependent pathway in rats following acute myocardial ischemia. Mol Med Rep. 13:1211–1219. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Zaman K, Ryu H, Hall D, O'Donovan K, Lin KI, Miller MP, Marquis JC, Baraban JM, Semenza GL and Ratan RR: Protection from oxidative stress-induced apoptosis in cortical neuronal cultures by iron chelators is associated with enhanced DNA binding of hypoxia-inducible factor-1 and ATF-1/CREB and increased expression of glycolytic enzymes, p21 (waf1/cip1), and erythropoietin. J Neurosci 19: 9821–9830, 1999. J Neurosci 19: 9821–9830, 1999. 19: 9821–9830, 1999:9821-9830, 1999–9830, 1999. 1999. | |
|
Hamrick SE, McQuillen PS, Jiang X, Mu D, Madan A and Ferriero DM: A role for hypoxia-inducible factor-1alpha in desferoxamine neuroprotection. Neurosci Lett. 379:96–100. 2005. View Article : Google Scholar : PubMed/NCBI | |
|
Sharp FR and Bernaudin M: HIF1 and oxygen sensing in the brain. Nat Rev Neurosci. 5:437–448. 2004. View Article : Google Scholar : PubMed/NCBI | |
|
Semenza GL: Angiogenesis in ischemic and neoplastic disorders. Annu Rev Med. 54:17–28. 2003. View Article : Google Scholar : PubMed/NCBI | |
|
Blanco Pampin J, Garcia Rivero SA, Otero Cepeda XL, Vázquez Boquete A, Forteza Vila J and Hinojal Fonseca R: Immunohistochemical expression of HIF-1alpha in response to early myocardial ischemia. J Forensic Sci. 51:120–124. 2006. View Article : Google Scholar : PubMed/NCBI | |
|
Shi H: Hypoxia inducible factor 1 as a therapeutic target in ischemic stroke. Curr Med Chem. 16:4593–4600. 2009. View Article : Google Scholar : PubMed/NCBI | |
|
Manalo DJ, Rowan A, Lavoie T, Natarajan L, Kelly BD, Ye SQ, Garcia JG and Semenza GL: Transcriptional regulation of vascular endothelial cell responses to hypoxia by HIF-1. Blood. 105:659–669. 2005. View Article : Google Scholar : PubMed/NCBI | |
|
Minet E, Michel G, Remacle J and Michiels C: Role of HIF-1 as a transcription factor involved in embryonic development, cancer progression and apoptosis (review). Int J Mol Med. 5:253–259. 2000.PubMed/NCBI | |
|
Semenza GL: Regulation of cancer cell metabolism by hypoxia-inducible factor 1. Semin Cancer Biol. 19:12–16. 2009. View Article : Google Scholar : PubMed/NCBI | |
|
Adekola K, Rosen ST and Shanmugam M: Glucose transporters in cancer metabolism. Curr Opin Oncol. 24:650–654. 2012. View Article : Google Scholar : PubMed/NCBI | |
|
Semenza GL: Hypoxia-inducible factor 1: Regulator of mitochondrial metabolism and mediator of ischemic preconditioning. Biochim Biophys Acta. 1813:1263–1268. 2011. View Article : Google Scholar : PubMed/NCBI | |
|
Kim JW, Tchernyshyov I, Semenza GL and Dang CV: HIF-1-mediated expression of pyruvate dehydrogenase kinase: a metabolic switch required for cellular adaptation to hypoxia. Cell Metab. 3:177–185. 2006. View Article : Google Scholar : PubMed/NCBI | |
|
Simon MC: Coming up for air: HIF-1 and mitochondrial oxygen consumption. Cell Metab. 3:150–151. 2006. View Article : Google Scholar : PubMed/NCBI | |
|
Chen C, Pore N, Behrooz A, Ismail-Beigi F and Maity A: Regulation of glut1 mRNA by hypoxia-inducible factor-1. Interaction between H-ras and hypoxia. J Biol Chem. 276:9519–9525. 2001. View Article : Google Scholar : PubMed/NCBI | |
|
Lu H, Forbes RA and Verma A: Hypoxia-inducible factor 1 activation by aerobic glycolysis implicates the Warburg effect in carcinogenesis. J Biol Chem. 277:23111–23115. 2002. View Article : Google Scholar : PubMed/NCBI | |
|
Semenza GL: HIF-1 mediates the Warburg effect in clear cell renal carcinoma. J Bioenerg Biomembr. 39:231–234. 2007. View Article : Google Scholar : PubMed/NCBI | |
|
Maxwell PH, Pugh CW and Ratcliffe PJ: Activation of the HIF pathway in cancer. Curr Opin Genet Dev. 11:293–299. 2001. View Article : Google Scholar : PubMed/NCBI | |
|
Semenza GL: HIF-1: Upstream and downstream of cancer metabolism. Curr Opin Genet Dev. 20:51–56. 2010. View Article : Google Scholar : PubMed/NCBI | |
|
Nagy MA: HIF-1 is the commander of gateways to cancer. J Cancer Sei Ther. 3:35–40. 2011. | |
|
Courtnay R, Ngo DC, Malik N, Ververis K, Tortorella SM and Karagiannis TC: Cancer metabolism and the Warburg effect: The role of HIF-1 and PI3K. Mol Biol Rep. 42:841–851. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Dang CV: Links between metabolism and cancer. Genes Dev. 26:877–890. 2012. View Article : Google Scholar : PubMed/NCBI | |
|
Martini M, De Santis MC, Braccini L, Gulluni F and Hirsch E: PI3K/AKT signaling pathway and cancer: an updated review. Ann Med. 46:372–383. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
Koukourakis MI, Giatromanolaki A, Sivridis E, Gatter KC and Harris AL; Tumour Angiogenesis Research Group, : Lactate dehydrogenase 5 expression in operable colorectal cancer: Strong association with survival and activated vascular endothelial growth factor pathway-a report of the Tumour Angiogenesis Research Group. J Clin Oncol. 24:4301–4308. 2006. View Article : Google Scholar : PubMed/NCBI | |
|
Solaini G, Baracca A, Lenaz G and Sgarbi G: Hypoxia and mitochondrial oxidative metabolism. Biochim Biophys Acta. 1797:1171–1177. 2010. View Article : Google Scholar : PubMed/NCBI | |
|
Samuel SM, Akita Y, Paul D, Thirunavukkarasu M, Zhan L, Sudhakaran PR, Li C and Maulik N: Coadministration of adenoviral vascular endothelial growth factor and angiopoietin-1 enhances vascularization and reduces ventricular remodeling in the infarcted myocardium of type 1 diabetic rats. Diabetes. 59:51–60. 2010. View Article : Google Scholar : PubMed/NCBI | |
|
Bai WW, Xing YF, Wang B, Lu XT, Wang YB, Sun YY, Liu XQ, Guo T and Zhao YX: Tongxinluo improves cardiac function and ameliorates ventricular remodeling in mice model of myocardial infarction through enhancing angiogenesis. Evid Based Complement Alternat Med 2013. 8132472013. | |
|
Patten RD, Pourati I, Aronovitz MJ, Baur J, Celestin F, Chen X, Michael A, Haq S, Nuedling S, Grohe C, et al: 17beta-estradiol reduces cardiomyocyte apoptosis in vivo and in vitro via activation of phospho-inositide-3 kinase/Akt signaling. Circ Res. 95:692–699. 2004. View Article : Google Scholar : PubMed/NCBI | |
|
He Z, Opland DM, Way KJ, Ueki K, Bodyak N, Kang PM, Izumo S, Kulkarni RN, Wang B, Liao R, et al: Regulation of vascular endothelial growth factor expression and vascularization in the myocardium by insulin receptor and PI3K/Akt pathways in insulin resistance and ischemia. Arterioscler Thromb Vasc Biol. 26:787–793. 2006. View Article : Google Scholar : PubMed/NCBI | |
|
Graupera M, Guillermet-Guibert J, Foukas LC, Phng LK, Cain RJ, Salpekar A, Pearce W, Meek S, Millan J, Cutillas PR, et al: Angiogenesis selectively requires the p110alpha isoform of PI3K to control endothelial cell migration. Nature. 453:662–666. 2008. View Article : Google Scholar : PubMed/NCBI | |
|
Sumida A, Horiba M, Ishiguro H, Takenaka H, Ueda N, Ooboshi H, Opthof T, Kadomatsu K and Kodama I: Midkine gene transfer after myocardial infarction in rats prevents remodelling and ameliorates cardiac dysfunction. Cardiovasc Res. 86:113–121. 2010. View Article : Google Scholar : PubMed/NCBI | |
|
Dutta PR and Maity A: Cellular responses to EGFR inhibitors and their relevance to cancer therapy. Cancer Lett. 254:165–177. 2007. View Article : Google Scholar : PubMed/NCBI | |
|
Chen JX and Meyrick B: Hypoxia increases Hsp90 binding to eNOS via PI3K-Akt in porcine coronary artery endothelium. Lab Invest. 84:182–190. 2004. View Article : Google Scholar : PubMed/NCBI | |
|
Hirota K and Semenza GL: Regulation of angiogenesis by hypoxia-inducible factor 1. Crit Rev Oncol Hematol. 59:15–26. 2006. View Article : Google Scholar : PubMed/NCBI | |
|
Kido M, Du L, Sullivan CC, Li X, Deutsch R, Jamieson SW and Thistlethwaite PA: Hypoxia-inducible factor 1-alpha reduces infarction and attenuates progression of cardiac dysfunction after myocardial infarction in the mouse. J Am Coll Cardiol. 46:2116–2124. 2005. View Article : Google Scholar : PubMed/NCBI | |
|
Khan M, Varadharaj S, Ganesan LP, Shobha JC, Naidu MU, Parinandi NL, Tridandapani S, Kutala VK and Kuppusamy P: C-phycocyanin protects against ischemia-reperfusion injury of heart through involvement of p38 MAPK and ERK signaling. Am J Physiol Heart Circ Physiol. 290:H2136–H2145. 2006. View Article : Google Scholar : PubMed/NCBI | |
|
Tian T, Nan KJ, Wang SH, Liang X, Lu CX, Guo H, Wang WJ and Ruan ZP: PTEN regulates angiogenesis and VEGF expression through phosphatase-dependent and -independent mechanisms in HepG2 cells. Carcinogenesis. 31:1211–1219. 2010. View Article : Google Scholar : PubMed/NCBI | |
|
Forsythe JA, Jiang BH, Iyer NV, Agani F, Leung SW, Koos RD and Semenza GL: Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol Cell Biol. 16:4604–4613. 1996. View Article : Google Scholar : PubMed/NCBI | |
|
Gray MJ, Zhang J, Ellis LM, Semenza GL, Evans DB, Watowich SS and Gallick GE: HIF-1alpha, STAT3, CBP/p300 and Ref-1/APE are components of a transcriptional complex that regulates Src-dependent hypoxia-induced expression of VEGF in pancreatic and prostate carcinomas. Oncogene. 24:3110–3120. 2005. View Article : Google Scholar : PubMed/NCBI | |
|
Guo S, Miyake M, Liu KJ and Shi H: Specific inhibition of hypoxia inducible factor 1 exaggerates cell injury induced by in vitro ischemia through deteriorating cellular redox environment. J Neurochem. 108:1309–1321. 2009. View Article : Google Scholar : PubMed/NCBI | |
|
Wang Z and Si LY: Hypoxia-inducible factor-1α and vascular endothelial growth factor in the cardioprotective effects of intermittent hypoxia in rats. Ups J Med Sci. 118:65–74. 2013. View Article : Google Scholar : PubMed/NCBI | |
|
Carmeliet P, Dor Y, Herbert JM, Fukumura D, Brusselmans K, Dewerchin M, Neeman M, Bono F, Abramovitch R, Maxwell P, et al: Role of HIF-1alpha in hypoxia-mediated apoptosis, cell proliferation and tumour angiogenesis. Nature. 394:485–490. 1998. View Article : Google Scholar : PubMed/NCBI | |
|
Halterman MW and Federoff HJ: HIF-1alpha and p53 promote hypoxia-induced delayed neuronal death in models of CNS ischemia. Exp Neurol. 159:65–72. 1999. View Article : Google Scholar : PubMed/NCBI | |
|
Halterman MW, Miller CC and Federoff HJ: Hypoxia-inducible factor-1alpha mediates hypoxia-induced delayed neuronal death that involves p53. J Neurosci. 19:6818–6824. 1999. View Article : Google Scholar : PubMed/NCBI | |
|
Chen C, Hu Q, Yan J, Lei J, Qin L, Shi X, Luan L, Yang L, Wang K, Han J, et al: Multiple effects of 2ME2 and D609 on the cortical expression of HIF-1alpha and apoptotic genes in a middle cerebral artery occlusion-induced focal ischemia rat model. J Neurochem. 102:1831–1841. 2007. View Article : Google Scholar : PubMed/NCBI | |
|
Zhang H, Bosch-Marce M, Shimoda LA, Tan YS, Baek JH, Wesley JB, Gonzalez FJ and Semenza GL: Mitochondrial autophagy is an HIF-1-dependent adaptive metabolic response to hypoxia. J Biol Chem. 283:10892–10903. 2008. View Article : Google Scholar : PubMed/NCBI | |
|
Sun Y, Chen X, Zhang X, Shen X, Wang M, Wang X, Liu WC, Liu CF, Liu J, Liu W and Jin X: β2-adrenergic receptor-mediated HIF-1α upregulation mediates blood brain barrier damage in acute cerebral ischemia. Front Mol Neurosci. 10:2572017. View Article : Google Scholar : PubMed/NCBI | |
|
Wong CC, Gilkes DM, Zhang H, Chen J, Wei H, Chaturvedi P, Fraley SI, Wong CM, Khoo US, Ng IO, et al: Hypoxia-inducible factor 1 is a master regulator of breast cancer metastatic niche formation. Proc Natl Acad Sci USA. 108:pp. 16369–16374. 2011; View Article : Google Scholar : PubMed/NCBI | |
|
Wong CC, Zhang H, Gilkes DM, Chen J, Wei H, Chaturvedi P, Hubbi ME and Semenza GL: Inhibitors of hypoxia-inducible factor 1 block breast cancer metastatic niche formation and lung metastasis. J Mol Med (Berl). 90:803–815. 2012. View Article : Google Scholar : PubMed/NCBI | |
|
Liu ZJ, Semenza GL and Zhang HF: Hypoxia-inducible factor 1 and breast cancer metastasis. J Zhejiang Univ Sci B. 16:32–43. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Baranova O, Miranda LF, Pichiule P, Dragatsis I, Johnson RS and Chavez JC: Neuron-specific inactivation of the hypoxia inducible factor 1 alpha increases brain injury in a mouse model of transient focal cerebral ischemia. J Neurosci. 27:6320–6332. 2007. View Article : Google Scholar : PubMed/NCBI | |
|
Helton R, Cui J, Scheel JR, Ellison JA, Ames C, Gibson C, Blouw B, Ouyang L, Dragatsis I, Zeitlin S, et al: Brain-specific knock-out of hypoxia-inducible factor-1alpha reduces rather than increases hypoxic-ischemic damage. J Neurosci. 25:4099–4107. 2005. View Article : Google Scholar : PubMed/NCBI | |
|
Filippi I, Morena E, Aldinucci C, Carraro F, Sozzani S and Naldini A: Short-term hypoxia enhances the migratory capability of dendritic cell through HIF-1α and PI3K/Akt pathway. J Cell Physiol. 229:2067–2076. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
Hu X, Wei L, Taylor TM, Wei J, Zhou X, Wang JA and Yu SP: Hypoxic preconditioning enhances bone marrow mesenchymal stem cell migration via Kv2.1 channel and FAK activation. Am J Physiol Cell Physiol. 301:C362–C372. 2011. View Article : Google Scholar : PubMed/NCBI | |
|
Hu X, Yu SP, Fraser JL, Lu Z, Ogle ME, Wang JA and Wei L: Transplantation of hypoxia-preconditioned mesenchymal stem cells improves infarcted heart function via enhanced survival of implanted cells and angiogenesis. J Thorac Cardiovasc Surg. 135:799–808. 2008. View Article : Google Scholar : PubMed/NCBI | |
|
Jian KT, Shi Y, Zhang Y, Mao YM, Liu JS and Xue FL: Time course effect of hypoxia on bone marrow-derived endothelial progenitor cells and their effects on left ventricular function after transplanted into acute myocardial ischemia rat. Eur Rev Med Pharmacol Sci. 19:1043–1054. 2015.PubMed/NCBI | |
|
Ginouvès A, Ilc K, Macias N, Pouysségur J and Berra E: PHDs overactivation during chronic hypoxia ‘desensitizes’ HIFalpha and protects cells from necrosis. Proc Natl Acad Sci USA. 105:pp. 4745–4750. 2008; View Article : Google Scholar : PubMed/NCBI | |
|
Poitz DM, Augstein A, Hesse K, Christoph M, Ibrahim K, Braun-Dullaeus RC, Strasser RH and Schmeißer A: Regulation of the HIF-system in human macrophages-differential regulation of HIF-α subunits under sustained hypoxia. Mol Immunol. 57:226–235. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
Higgins RD, Raju T, Edwards AD, Azzopardi DV, Bose CL, Clark RH, Ferriero DM, Guillet R, Gunn AJ, Hagberg H, et al: Hypothermia and other treatment options for neonatal encephalopathy: An executive summary of the Eunice Kennedy Shriver NICHD workshop. J Pediatr. 159:851–858.e1. 2011. View Article : Google Scholar : PubMed/NCBI | |
|
Shankaran S, Pappas A, McDonald SA, Vohr BR, Hintz SR, Yolton K, Gustafson KE, Leach TM, Green C, Bara R, et al: Childhood outcomes after hypothermia for neonatal encephalopathy. N Engl J Med. 366:2085–2092. 2012. View Article : Google Scholar : PubMed/NCBI | |
|
Huang J, Zhang L, Qu Y, Zhou Y, Zhu J, Li Y, Zhu T, Zhao F, Tang J and Mu D: Histone acetylation of oligodendrocytes protects against white matter injury induced by inflammation and hypoxia-ischemia through activation of BDNF-TrkB signaling pathway in neonatal rats. Brain Res. 1688:33–46. 2018. View Article : Google Scholar : PubMed/NCBI |