Solanum nigrum Linne improves DNCB‑induced atopic dermatitis‑like skin disease in BALB/c mice
- Authors:
- Published online on: July 28, 2020 https://doi.org/10.3892/mmr.2020.11381
- Pages: 2878-2886
-
Copyright: © Hong et al. This is an open access article distributed under the terms of Creative Commons Attribution License.
Abstract
Introduction
Atopic dermatitis (AD) is a chronic inflammatory skin disease characterized by erythema, edema, thickening of the skin and pruritic, eczematous skin lesions (1,2). The prevalence of AD is increasing worldwide (3). It generally occurs in infancy and childhood, but can also appear in adults. When AD begins in childhood, it is usually followed by other allergic diseases, such as allergic rhinitis and asthma (4). A number of remedies for AD involve the topical or systemic administration of steroids and antihistamines (5). Corticosteroids, the most commonly used steroids, are the main anti-inflammatory therapy and they are effective at inhibiting both acute and chronic skin inflammation (6). Steroids are most commonly used for anti-inflammatory therapy; however, their long-term use is limited due to side-effects such as osteoporosis, brittle skin, muscle weaknesses and diabetes (7). Therefore, alternative treatment strategies are required for AD.
AD is caused by inflammatory reactions to environmental factors and allergen stimulation (8). The inflammatory reaction begins when allergens bind to mast cells, which play an important role in early inflammatory responses (9). When mast cells are activated, they express inflammatory cytokine, such as granulocyte-macrophage colony-stimulating factor (GM-CSF) (10). Subsequently, activated dendritic cells also express cytokines, such as thymus and activation regulated chemokine (TARC/CCL17) and C-X-C motif chemokine ligand 8 (CXCL-8/IL-8) (11). These actions cause the thickening of the skin, erythema and itching.
1-Chloro-2,4-dinitrobenzene (DNCB) is a chemical substance that causes chronic contact dermatitis and is widely used in human studies of AD (12). When DNCB is topically applied to mice, it causes dermatopathy similar to symptoms of AD, such as an increased IgE expression, inflammatory cytokines, edema and itching (13). Human adult low calcium, high temperature (HaCaT) cells are human keratinocytes and have also been widely used in dermatological studies (14). TNF-α/IFN-γ activate the phagocytosis of granulocytes and macrophages, causing them to secrete various cytokines. All these cytokines strongly influence the recruitment of inflammatory cells, neutrophils and monocyte chemotactic activity (15).
Traditionally, Solanum nigrum Linne (SNL) has been used for decoding, urination, swelling, and heat treatment in a number of traditional medicine systems worldwide, including traditional Chinese medicine (16). Recently, SNL has been reported to exert several positive biotic effects, such as anti-inflammatory, tumor growth inhibitory and hepatoprotective effects (17–19). However, the inhibitory effects of SNL on AD have not yet been proven, at least to the best of our knowledge.
The present study aimed to investigate the anti-inflammatory response by inhibiting mast cell and keratinocyte activation during SNL treatment. For this purpose, BALB/c mice with DNCB-induced AD and human keratinocyte lineage cell line HaCaT cells were used as models of AD. The thickness of the epidermis and dermis, the infiltration of inflammatory mediators, and the serum expression levels of IgE were evaluated in BALB/c mice with DNCB-induced AD. Moreover, the expression of the inflammatory cytokines TARC, GM-CSF and CXCL-8, were investigated in TNF-α/IFN-γ-stimulated HaCaT cells.
Materials and methods
Reagents
HaCaT cells were obtained from the cell line service (CLS, Eppelheim, Germany). Penicillin-streptomycin (P/S), fetal bovine serum (FBS) and goat serum (16210-064) were obtained from Gibco; Thermo fisher Scientific, Inc. Dulbecco's modified Eagle's medium (DMEM) was purchased form Welgene. CD4 (ab183685) and CD8 (ab209775) antibodies were obtained from Abcam. Antibodies to phosphorylated-extracellular signal-regulated kinase (p-ERK, #4370), extracellular signal-regulated kinase (ERK, #4695), phosphorylated-p38 (p-p38, #4511), p38 (#9212), phosphorylated-Jun N-terminal kinase (p-JNK, #4668), Jun N-terminal kinase (JNK, #9258) and phosphorylated-NF-κB (p-NF-κB, #3033) were purchased from Cell Signaling Technology, Inc. Lamin B was purchased from Santa Cruz Biotechnology, Inc. Protease inhibitor cocktail (#P8340) and phosphatase inhibitor cocktail (#P0044, #P5726) were purchased from Sigma-Aldrich; Merck KGaA. ECL solution (RPN2106) was obtained from GE Healthcare Life Sciences. PCR primers glyceraldehyde 3-phosphate dehydrogenase (GAPDH), TARC/CCL17, GM-CSF, CXCL-8 were obtained from Genotech. Taq polymerase was obtained from KaPa BioSystems. TNF-α (#285-IF), IFN-γ (#210-TA) were purchased from R&D Systems.
Preparation of SNL
SNL was purchased from Omniherb. SNL was prepared as a dried powder with lyophilization following sonication in 80% ethanol for 2 h. The extract was concentrated in a rotary evaporator and lyophilized (yield ratio 13.88%). The extracts were stored at −20°C until use.
High-performance liquid chromatography (HPLC) analysis of SNL
The separation and determination of SNL were performed on an HPLC instrument (waters 2695 Alliance system with a 2996 UV detector) connected to a Photodiode Array detector (PDA). The SNL extraction was separated by an Xbridge C18 column (4.6×250 mm, 5 µm). The temperature was maintained at 30°C, the injection volume was 10 µl, and the flow rate was 1 ml/min. The mobile phase consisted of acetonitrile (sol A) and 1% acetic acid/H2O (sol B). The mobile phase solvent was used at a 9:1 ratio of sol A and sol B. The PDA detector wavelength was set at 192 nm and the running time was 20 min.
Animals
All animal experiments were approved by the Kyung Hee University Animal Care and Use Committee (KHMC-IACUC-18-016) and were performed for 50 days in accordance with the guidelines of Kyung Hee University Institutional Animal Care and Use Committee. (KHMC-IACUC-18-016). BALB/c mice (6 weeks old) male were obtained from KOATECH. All mice were bred in a controlled room (22±2°C temperature, 50±10% humidity, 12-h light/dark cycle). They were also stabilized for 7 days in a controlled room. All animals were checked the daily for the health and sighs of morbidity. The criteria for determining the animal's euthanasia period (endpoint) are rapid weight loss, lethargy, debilitating diarrhea, sizable abdominal enlargement and ascites. No animals died during the experiment period.
AD model and drug treatment in mice
The mice were anesthetized by the use of 5% isoflurane of inhalant anesthetics in 100% oxygen and anesthesia was maintained at 2~2.5% isoflurane inhalation. After anesthesia, the dorsal skin of the mice was shaved with a clipper. The mice were divided into 4 groups (n=8/group) as follows: The untreated group (Normal), the DNCB-sensitized group (Control), the SNL 1 mg/ml-treated group (SNL_L), and the SNL 10 mg/ml-treated group (SNL_H). The mice in the Normal group were treated with 9:1 phosphate-buffered saline (PBS)/olive oil. To induce AD, 1-chloro-2,4-dinitrobenzene (DNCB) was diluted at 0.5 and 1%. The DNCB solvent was used at a 3:1 ratio of olive oil and acetone. The mice in the Control, SNL_L and SNL_H groups were subjected to initial sensitization with 1% DNCB. After 5 days, in the mice in the Control, SNL_L and SNL_H groups, were 0.5% DNCB was dorsally applied 3 times a week for 4 weeks. Following 2 h of the second sensitization, SNL preparations of 1 and 10 mg/ml were applied on the dorsal skin of the mice in the SNL_L and SNL_H groups once every day for 35 days. The SNL solvent was used a 9:1 ratio of PBS/olive oil. On day 36 after SNL treatments, all animals were sacrificed. When the mouse was sacrificed, the isoflurane concentration was adjusted to 5% to expose it, and the isoflurane was exposed until one minute after breathing stopped. After confirming that the heart and breathing has stopped, either 0.8 or 1 ml blood was collected and body weight was 30–35 g at the time of sacrifice. According to the existing research, herbal medicines were improved atopy dermatitis when the concentration of 1 to 10 mg/ml was applied to 200 µl. Therefore, we decided on the concentration of SNL referring to the results of previous studies (20–22).
Histological analysis
The skin tissues were fixed in 10% neutral buffered formalin (NBF) for one day. After fixation, the skin tissues were washed under running water for 24 h, then embedded with paraffin. The paraffin block was sectioned at 5 µm thickness using a microtome (ZEISS). The skin tissues were stained with hematoxylin and eosin (H&E), Masson trichrome and toluidine blue to measure the infiltration of eosinophils and mast cells and thickness of epidermis and dermis.
Immunohistochemistry (IHC)
The skin tissue was deparaffinized and hydrated in xylene and ethanol series. Skin tissues were heated with a 0.01 M sodium citrate buffer (0.1 M citric acid, 0.1 M sodium citrate) using an Electric Pressure Cooker (CPC-600; Cuisinart). After being washed three times with tris-buffered saline (TBS), the skin tissue was reacted with 0.3% H2O2 in methanol at room temperature for 30 min to inhibit the activity of peroxidase. The tissues were blocked with 10% goat serum in PBS for 10 min. They were then incubated in CD4 and CD8 antibodies at 4°C for 24 h. After 24 h, CD4- and CD8-positive cells were colored red using the Polink-2 Plus AP rabbit kit (D70-18, GBI Labs) according to the manufacturer's protocol, and were then counterstained with H&E. CD8- and CD4-positive cells were counted in 1- fields using a light microscope at ×400 magnification (BX51, Olympus Corp.).
Enzyme-linked immunosorbent assay
Blood samples were collected from the mice by cardiac puncture following anesthesia, and the serum was separated by centrifugation at 2,000 rpm for 10 min. IgE levels were determined using a mouse enzyme-linked immunosorbent assay (ELISA) kit, according to the manufacturer's protocol.
Cell culture and assessment of cell viability
HaCaT cells were cultured in DMEM with 10% FBS and 1% P/S. They were then incubated at 37°C, 5% CO2 and 95% humidity. HaCaT cells were seeded in a 96-well plate with 1.5×104 cells/well. After 24 h, the cells were treated with SNL at 6.25, 12.5, 25, 50 and 100 µg/ml for 24 h. A 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, inner salt (MTS) solution was then added at 20 µl per well and the HaCaT cells were incubated for 2 h at 37°C. Cell viability was measured at a wavelength of 490 nm using an ELISA reader (Versamax; Molecular Devices, LLC).
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
HaCaT cells were seeded in a 6-well plate at 1×106 cells/well. After 24 h, the HaCaT cells were pretreated with various concentrations of SNL (6.25, 12.5, 25 and 50 µg/ml) for 1 h and stimulated with 10 ng/ml TNF-α/IFN-γ for 24 h. Total RNA was extracted using TRIzol reagent (TAKARA BIO) according to the manufacturer's protocol. cDNA was prepared using SuperScript II reverse transcriptase (Invitrogen; Thermo Fisher Scientific, Inc.). The primer sequences for TARC, GM-CSF, CXCL-8, and GAPDH are presented in Table I. The cDNA samples were separated on a 1.2% agarose gel and determined using NαBI (Neoscience). mRNA expression was measured using ImageJ software (Ver. 1.52a, National Institutes of Health).
Western blot analysis
The phosphorylation of MAPK and the activation of NF-κB were examined by western blot analysis. The HaCaT cells, incubated with diverse concentrations of SNL, were washed in cold Dulbecco's phosphate-buffered saline (DPBS). The HaCaT cells were lysed in a RIPA buffer (0.1% SDS, 150 mM NaCl, 50 mM Tris-Cl, 1% NP-40, 0.5% Na-deoxycholate, a protease inhibitor cocktail, and a phosphatase inhibitor cocktail) and incubated in ice for 30 min. Following centrifugation at 13,200 rpm for 20 min at 4°C, the protein concentration was calculated by bicinchoninic acid (BCA) assay. Protein samples (40 µg) were separated by 10% SDS-PAGE. The protein was transferred to a nitrocellulose membrane and then blocked with 5% skim milk for 1 h. After blocking, the membrane was incubated with primary antibodies, such as p-ERK, t-ERK, p-JNK, t-JNK, p-p38, t-p38, p-NF-κB and Lamin B in a 1% bovine serum albumin (BSA) solution at 4°C overnight. After the membrane was incubated with secondary antibodies, the protein was detected using an ECL solution.
Statistical analysis
Each experiment was repeated at least 3 times. The data are presented as the mean ± standard error of the mean (SEM). All data were analyzed using the Graph Pad PRISM software (GraphPad Software, Inc.). One-way ANOVA was used to evaluate the treatment effect, followed by Tukey's post-hoc test. P<0.05 was considered to indicate a statistically significant difference.
Results
HPLC analysis of SNL
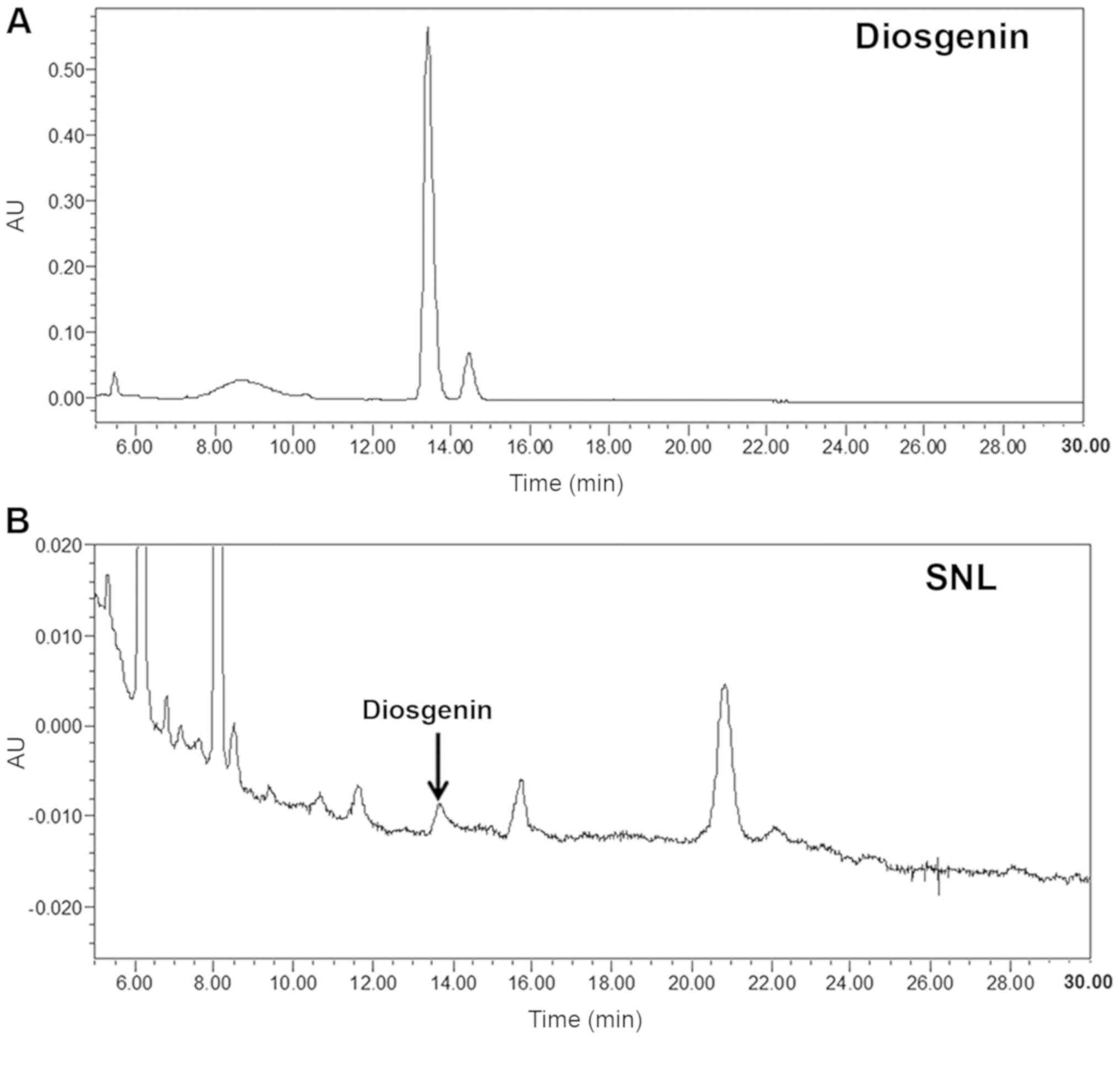
According to previous studies, the diosgenin is one of the ingredients of SNL, which has excellent anti-inflammatory and anticancer effects and is detected in SNL to 0.16–3% (23,24). In the present study, the quality and purity of SNL were measured using a HPLC analyzer. The typical chromatography profile of the diosgenin standard and SNL extract are depicted in Fig. 1. The retention time of the diosgenin standard and SNL extract was 13.439 min. Therefore, SNL was identified as diosgenin based on the HPLC-PDA data.
Effects of SNL on DNCB-induced AD-like symptoms in BALB/c mice
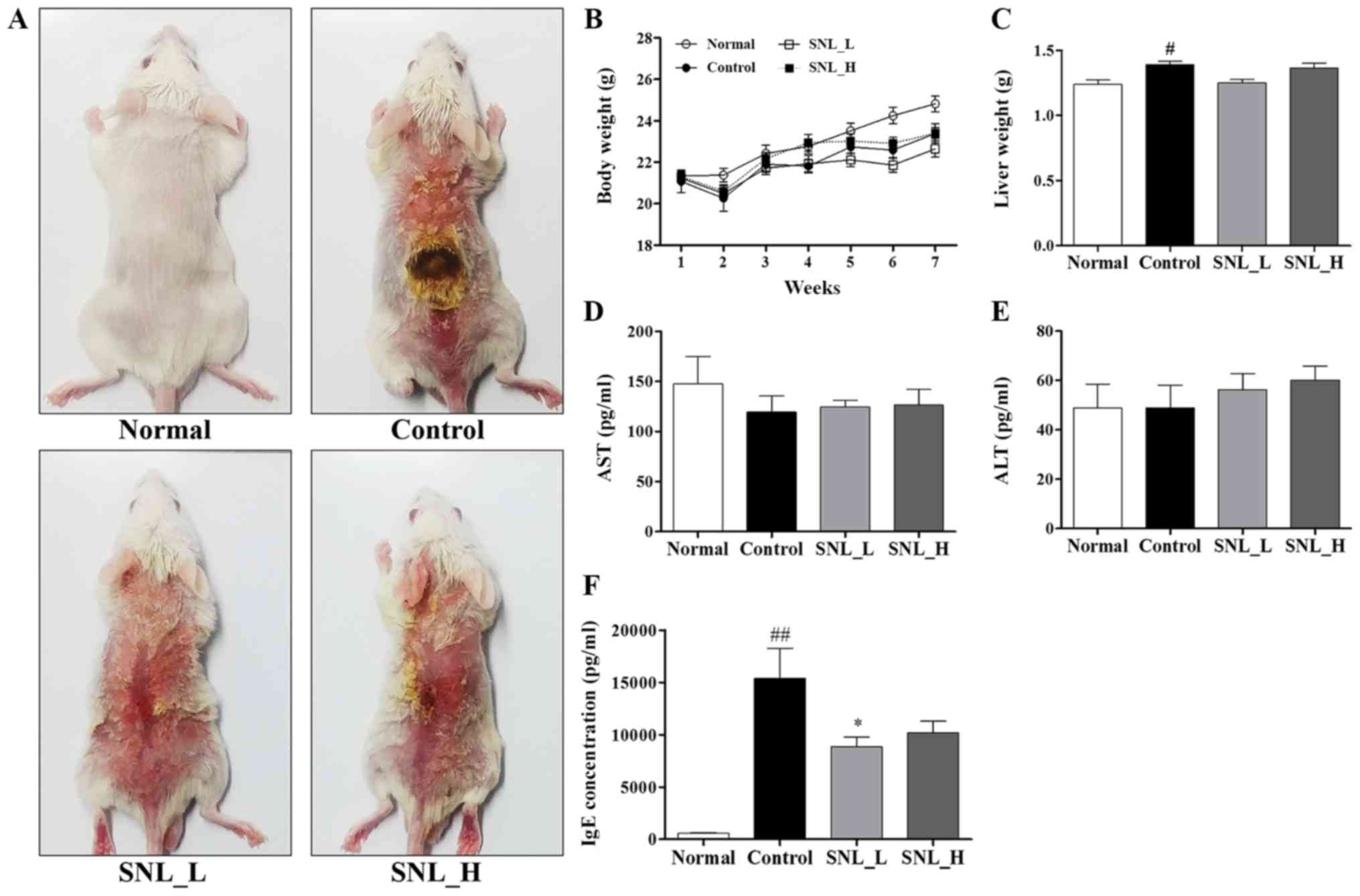
All animal experiments were based on the schedule illustrated in Fig. 2. The dorsal skin on which the SNL was applied for 5 weeks in the model of DNCB-induced atopic dermatitis is illustrated in Fig. 3A. Body weight, liver weight and aspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels were measured to determine the toxicity of SNL in the mouse model of AD (Fig. 3B-E). The body weight of the mice did not exhibit any marked differences between the Control, SNL_L and SNL_H groups were compared. Following sacrifice, the livers were rapidly removed and weighed. The liver weight was measured to evaluate the toxicity of SNL. The liver weight of the mice in the Control group was significantly increased compared with the mice in the Normal group. The mice in the SNL-L group exhibited a decreased liver weight compared with the mice in the Control and the SNL-H groups. Total AST and ALT levels are indicators of hepatic function. No significant changes in the levels of AST and ALT were observed in the SNL_L and SNL_H compared to the Control group. This result indicated that SNL was an effective sample for atopic dermatitis at a non-toxic concentration. The levels of serum IgE were measured by ELISA (Fig. 3F). The serum IgE levels were increased in the Control group compared with the Normal group. However, the serum IgE levels were significantly decreased in the SNL_L compared with the Control group.
Effects of SNL on epidermal and dermal thickness, and mast cells in mice with DNCB-induced AD
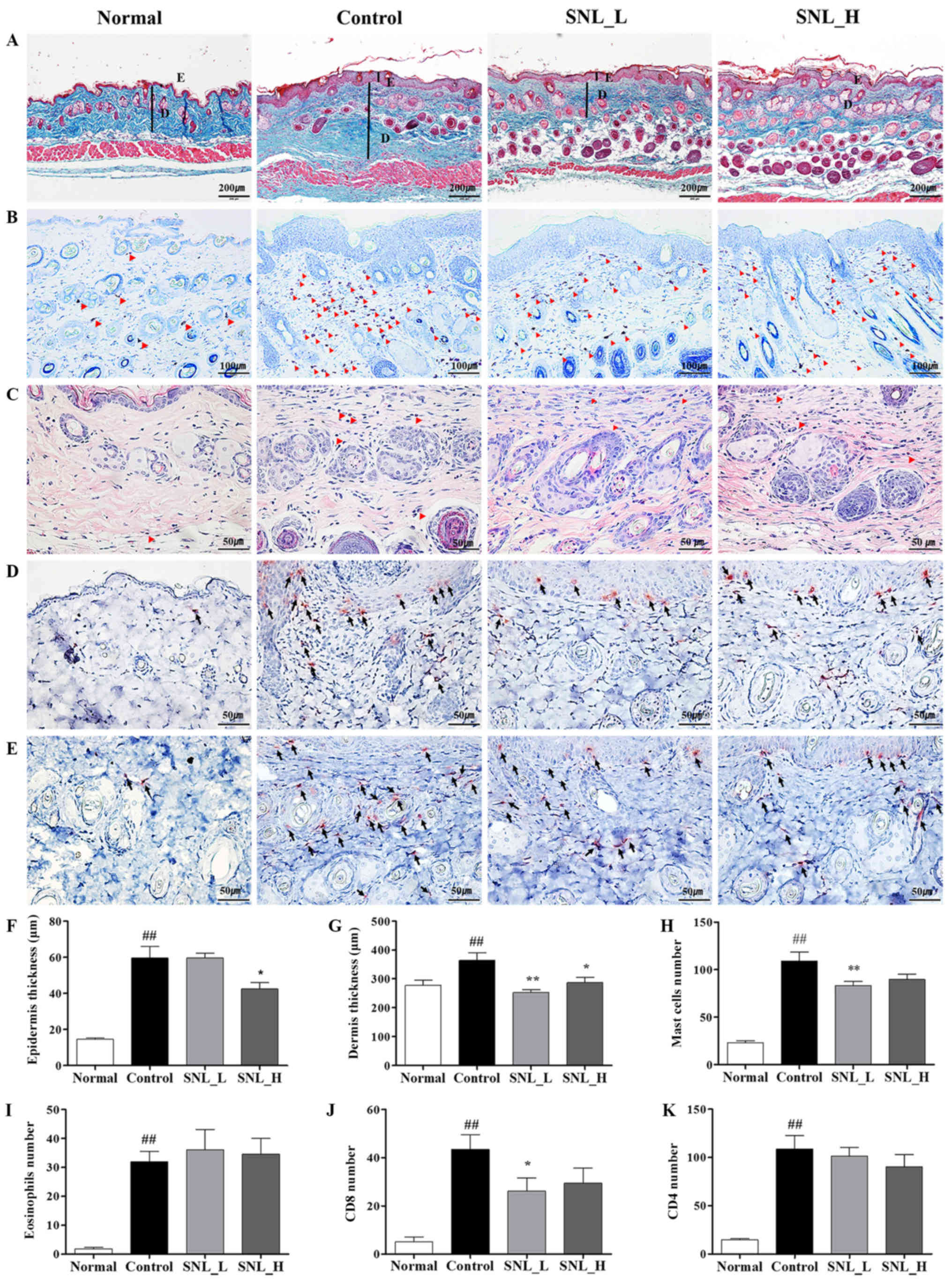
The mouse skin was stained with Masson's trichrome stain to evaluate the effects of SNL on epidermal and dermal thickness (Fig. 4A). The thicknesses of the epidermis and dermis were measured using ImageJ software. The thickness of the epidermis and dermis increased in the Control group compared to the Normal group. The thickness of the epidermis was significantly decreased in the SNL_H compared with the Control group. The thickness of the dermis was significantly decreased in both the SNL_L and SNL_H groups compared to the Control group (Fig. 4F and G). To measure the degree of mast cell infiltration, skin tissues were stained with toluidine blue (Fig. 4B). The infiltration of mast cells increased in the Control group compared with the Normal group. The numbers of mast cells were decreased in the SNL_L compared with the Control group (Fig. 4H). To measure the degree of eosinophils infiltration, skin tissues were stained with H&E (Fig. 4C). The infiltration of eosinophils increased in the Control group compared with the Normal group. However, eosinophils did not affect the SNL_L and SNL_H compared to the Control group (Fig. 4I). IHC staining was performed on the skin with atopic dermatitis to observe the extent of CD4 and CD8 infiltration. CD4 and CD8 infiltration increased in the Control group compared with the Normal group (Fig. 4D and E). However, the number of CD8-positive cells decreased in the SNL_L compared with the Control group (Fig. 4J). No significant difference was observed in the number of CD4-positive cells between the Control, SNL_L and SNL_H groups (Fig. 4K).
Cytotoxicity of SNL in HaCaT cells
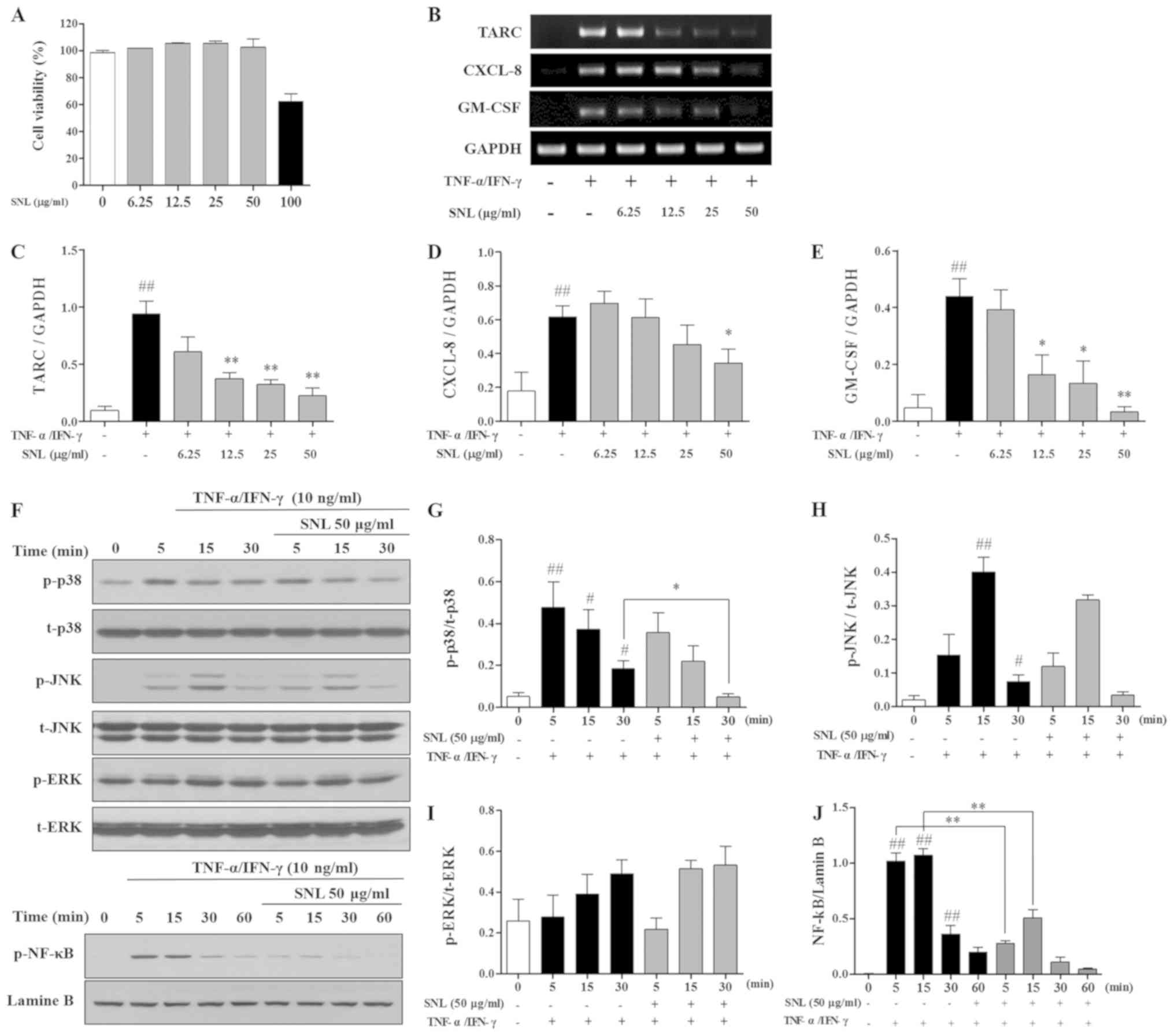
MTS assay was used to measure the cytotoxicity of SNL in HaCaT cells. HaCaT cells were treated with various concentrations of SNL (0–100 µg/ml). SNL was found to be cytotoxic at 100 µg/ml (Fig. 5A). Therefore, SNL was used at a concentration <50 µg/ml in following experiments, which was not cytotoxic to HaCaT cells.
Effects of SNL on mRNA expression in TNF-α/IFN-γ stimulated HaCaT cells
mRNA expression in TNF-α/IFN-γ-stimulated HaCaT cells was measured by RT-qPCR. SNL at concentrations of 12.5, 25, and 50 µg/ml significantly decreased the level of TARC in the HaCaT cells. The expression of the inflammatory cytokine, CXCL-8, was inhibited with SNL at 50 µg/ml. In addition, SNL at 12.5, 25 and 50 µg/ml decreased the GM-CSF level in the TNF-α/IFN-γ-stimulated HaCaT cells (Fig. 5B-E).
Effects of SNL on phosphorylation of MAPKs and NF-κB in TNF-α/IFN-γ stimulated HaCaT cells
The present study examined the mechanisms through which SNL affects the expression of the MAPK and NF-κB pathway. As shown in Fig. 5F-J, the expression of p-p38 was significantly inhibited at 30 min of TNF-α/IFN-γ stimulation; however, SNL treatment increased p-p38 expression after 5 min. However, the levels of JNK and ERK were not affected by SNL. The expression of p-NF-κB was significantly inhibited at 5, 15 min compared with the TNF-α/IFN-γ-stimulated HaCaT cells.
Discussion
The present study aimed to investigate the effects of SNL on a model of DNCB-induced AD and TNF-α/IFN-γ-stimulated HaCaT cells. Symptoms of AD include the thickening of the epidermis and dermis, the infiltration of mast cells and eosinophils, the overexpression of Th2 cytokines, and increased IgE production (12,13,25,26). In the present study, BALB/c mice with DNCB-induced AD and human keratinocyte HaCaT cells were used, which are widely used as models of AD, to determine whether SNL decreases the inflammatory response.
Skin thickening is a well-known symptom of AD, both clinically and historically (27). Continuous inflammation and allergic reactions can cause the skin to become thick and hard (28). In the present study, it was found that SNL inhibited the thickening of the epidermis and dermis in the model of DNCB-induced AD. As shown Fig. 4, the skin of the mice in the Control group thickened compared to that of the Normal group, and the skin of the mice in the SNL_L and SNL_H groups was thinner than that of the Control group. These results indicate that SNL reduces the hyperkeratosis of AD.
The expression of IgE is known to cause acute and chronic skin inflammation (8). In particular, the increase in IgE levels has been reported as a characteristic of AD. IgE binds to high-affinity receptors on the surface of mast cells to activate them (29). In the present study, the serum IgE levels were significantly increased in mice with DNCB-induced AD. SNL decreased the level of IgE in the serum of mice with DNCB-induced AD. Mast cells are an early indicator of the inflammatory response (30). Mast cells are activated by the stimulation of IgE molecules, and activated mast cells increase the production of inflammatory cytokines, such as IL-5, IL-6, IL-13 and GM-CSF (31). The findings of the present study demonstrated that the infiltration of mast cells was decreased in the SNL_L group. This suggests that SNL inhibits mast cell infiltration and may improve tissue-related changes, such as characteristic edema and skin thickening in AD. CD8 is a transmembrane glycoprotein that serves as a co-receptor for the T cell receptor (32). Cytotoxic T cells that express CD8 on the cell surface are termed CD8+ T cells. CD8+ T cells play an immune defense against intracellular pathogens, including viruses and bacteria (33). Previous studies have demonstrated that CD8+ T cells are essential for eczema formation in a mouse model, they appear in human skin (even prior to Th2 cells) following allergen exposure, and are a source of inflammatory cytokines (34). Hijnen et al reported that inflammatory cytokines are overexpressed by CD8+ T cells in AD-affected skin (35). The results of the present study confirmed the increase production of CD8 in the model of DNCB-induced AD. The infiltration of CD8+ T cells was significantly increased in DNCB-exposed mice, and SNL treatment of the DNCB-exposed mice significantly reduced the infiltration CD8+ T cells compared to the Control group. The expression of IgE is known to cause acute and chronic skin inflammation.
The inflammatory cytokine, GM-CSF, is secreted by mast cells and induces the infiltration of eosinophils (36). This causes the skin to thicken as a result of swelling and edema. TARC secreted from dendritic cells plays an important role in recruiting and activating Th2 cells, which, in turn, play an important in the inflammatory response (18). CXCL-8 is an eosinophil-activating cytokine. CXCL-8 is expressed by various tissue and phagocytic cells when exposed to inflammatory stimulants (37). CXCL-8 recruits and activates eosinophils from inflammatory lesions (38). These eosinophils amplify the inflammatory response (39). In the present study, SNL significantly decreased the levels of inflammatory cytokines, such as TARC, CXCL-8 and GM-CSF in the TNF-α/IFN-γ-stimulated HaCaT cells. This result suggests that SNL decreased the inflammatory reaction in BALB/c mice by inhibiting TARC, CXCL-8, and GM-CSF in keratinocytes. MAPKs are signal transduction pathways, and they are important mediators of transcriptional responses to extracellular signals, including hormones, cytokines, and environmental stress. In particular, p38 MAPK is activated by cellular stress and modulates the expression of inflammatory cytokines, such as CXCL-8, TARC and GM-CSF (40,41). NF-κB is an important transcription factor activated by various stimuli, such as TNF-α and IFN-γ (42). In the present study, p38 MAPK and NF-κB signaling in the TNF-α/IFN-γ-stimulated HaCaT cells were decreased by SNL treatment.
In conclusion, the findings of the present study suggest that p38 MAPK induces the activity of NF-κB and activated NF-κB produces inflammatory cytokines. Immune cell infiltration, as well as cytokine and keratinocyte production were confirmed in the skins of mice with AD. However, SNL relieved the symptoms of AD.
Acknowledgements
Not applicable.
Funding
The current study was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (grant no. 2020R1A2C2005836).
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Authors' contributions
YS and HSJ conceptualized the study. SH was been involved in drafting the manuscript. SH, BL, BK and HC performed all experiments and verified the analytical data. BK, BL and HC involved in critically revising the manuscript. EYK, JHK and MK contributed to the statistical analysis and helped interpret the results. EYK supervised the experiments in discussion with SH and BL. All authors read and approved the final manuscript.
Ethics approval and consent to participate
All animal experiments were approved by the Kyung Hee University Animal Care and Use Committee (approval no. KHMC-IACUC-18-016).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
|
Jung M, Lee TH, Oh HJ, Kim H, Son Y, Lee EH and Kim J: Inhibitory effect of 5,6-dihydroergosteol-glucoside on atopic dermatitis-like skin lesions via suppression of NF-kappaB and STAT activation. J Dermatol Sci. 79:2878–261. 2015. View Article : Google Scholar | |
|
Lim SJ, Kim M, Randy A, Nam EJ and Nho CW: Effects of Hovenia dulcis Thunb. Extract and methyl vanillate on atopic dermatitis-like skin lesions and TNF-α/IFN-γ-induced chemokines production in HaCaT cells. J Pharm Pharmacol. 68:1465–1479. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Spergel JM and Paller AS: Atopic dermatitis and the atopic march. J Allergy Clin Immunol. 112 (Suppl 6):S118–S127. 2003. View Article : Google Scholar : PubMed/NCBI | |
|
Nutten S: Atopic dermatitis: Global epidemiology and risk factors. Ann Nutr Metab. 66 (Suppl 1):S8–S16. 2015. View Article : Google Scholar | |
|
Charman CR, Morris AD and Williams HC: Topical corticosteroid phobia in patients with atopic eczema. Br J Dermatol. 142:931–936. 2000. View Article : Google Scholar : PubMed/NCBI | |
|
Leung DYM, Boguniewicz M, Howell MD, Nomura I and Hamid OA: New insights into atopic dermatitis. J Clin Invest. 113:651–657. 2004. View Article : Google Scholar : PubMed/NCBI | |
|
Kleiman A and Tuckermann JP: Glucocorticoid receptor action in beneficial and side effects of steroid therapy: Lessons from conditional knockout mice. Mol Cell Endocrinol. 275:98–108. 2007. View Article : Google Scholar : PubMed/NCBI | |
|
Galli SJ, Tsai M and Piliponsky AM: The development of allergic inflammation. Nature. 454:445–454. 2008. View Article : Google Scholar : PubMed/NCBI | |
|
Kraneveld AD, Sagar S, Garssen J and Folkerts G: The two faces of mast cells in food allergy and allergic asthma: The possible concept of Yin Yang. Biochim Biophys Acta. 1822:93–99. 2012. View Article : Google Scholar : PubMed/NCBI | |
|
Dahl C, Hoffmann HJ, Saito H and Schiotz PO: Human mast cells express receptors for IL-3, IL-5 and GM-CSF; a partial map of receptors on human mast cells cultured in vitro. Allergy. 59:1087–1096. 2004. View Article : Google Scholar : PubMed/NCBI | |
|
Liu YJ: Thymic stromal lymphopoietin: Master switch for allergic inflammation. J Exp Med. 203:269–273. 2006. View Article : Google Scholar : PubMed/NCBI | |
|
Fujii Y, Takeuchi H, Sakuma S, Sengoku T and Takakura S: Characterization of a 2,4-dinitrochlorobenzene-induced chronic dermatitis model in rats. Skin Pharmacol Physiol. 22:240–247. 2009. View Article : Google Scholar : PubMed/NCBI | |
|
Inagaki N, Shiraishi N, Igeta K, Itoh T, Chikumoto T, Nagao M, Kim JF and Nagai H: Inhibition of scratching behavior associated with allergic dermatitis in mice by tacrolimus, but not by dexamethasone. Eur J Pharmacol. 546:189–196. 2006. View Article : Google Scholar : PubMed/NCBI | |
|
Yang CT, Yang ZL, Zhang MF, Dong Q, Wang XY, Lan AP, Zeng FQ, Chen PX, Wang CH and Feng JQ: Hydrogen sulfide protects against chemical hypoxia-induced cytotoxicity and inflammation in HaCaT cells through inhibition of ROS/NF-κB/COX-2 pathway. PLoS One. 6:e219712011. View Article : Google Scholar : PubMed/NCBI | |
|
Chodorowska G: Plasma concentrations of IFN-gamma and TNF-alpha in psoriatic patients before and after local treatment with dithranol ointment. J Eur Acad Dermatol Venereol. 10:147–151. 1998. View Article : Google Scholar : PubMed/NCBI | |
|
Loganayaki N, Siddhuraju P and Manian S: Antioxidant activity of two traditional Indian vegetables: Solanum nigrum L. and Solanum torvum L. Food Sci Biotechnol. 19:121–127. 2010. View Article : Google Scholar | |
|
Proksch E, Folster-Holst R and Jensen JM: Skin barrier function, epidermal proliferation and differentiation in eczema. J Dermatol Sci. 43:159–169. 2006. View Article : Google Scholar : PubMed/NCBI | |
|
Lambrecht BN and Hammad H: The role of dendritic and epithelial cells as master regulators of allergic airway inflammation. Lancet. 376:835–843. 2010. View Article : Google Scholar : PubMed/NCBI | |
|
Galli SJ: The Mast Cell-IgE Paradox: From homeostasis to anaphylaxis. Am J Pathol. 186:212–224. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Kim SY, Yohannes SB, Damte D, Lee SJ, Hossain MA, Kim JY, Rhee MH, Suh JW and Park SC: Effect of fermented rhus verniciflua extract on DNCB induced-atopy like dermatitis in BALB/c mice. Pak Vet J. 34:333–336. 2014. | |
|
Choi YY, Kim MH, Ahn KS, Um JY, Lee SG and Yang WM: Immunomodulatory effects of Pseudostellaria heterophylla (Miquel) Pax on regulation of Th1/Th2 levels in mice with atopic dermatitis. Mol Med Rep. 15:649–656. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Lee HG, Cho NC, Jeong AJ, Li YC, Rhie SJ, Choi JS, Lee KH, Kim Y, Kim YN, Kim MH, et al: Immunomodulatory activities of the benzoxathiole derivative BOT-4-one ameliorate pathogenic skin inflammation in mice. J Invest Dermatol. 136:107–116. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Suthar AC and Mulani RM: A high performance thin layer chromatography method for quantitative estimation of Diosgenin in Solanum nigrum Linn. Pharmacog Magazine. 4:112–115. 2008. | |
|
Desai S, Tatke P and Gabhe SY: Quantification of diosgenin in extracts and formulations containing Solanum Nigrum. Int J Pharm Sci Res. 6:676–681. 2015. | |
|
Matsuda H, Watanabe N, Geba GP, Sperl J, Tsudzuki M, Hiroi J, Matsumoto M, Ushio H, Saito S, Askenase PW and Ra C: Development of atopic dermatitis-like skin lesion with IgE hyperproduction in NC/Nga mice. Int Immunol. 9:461–466. 1997. View Article : Google Scholar : PubMed/NCBI | |
|
Kabashima K: New concept of the pathogenesis of atopic dermatitis: Interplay among the barrier, allergy, and pruritus as a trinity. J Dermatol Sci. 70:3–11. 2013. View Article : Google Scholar : PubMed/NCBI | |
|
Wang G, Savinko T, Wolff H, Dieu-Nosjean MC, Kemeny L, Homey B, Lauerma AI and Alenius H: Repeated epicutaneous exposures to ovalbumin progressively induce atopic dermatitis-like skin lesions in mice. Clin Exp Allergy. 37:151–161. 2007. View Article : Google Scholar : PubMed/NCBI | |
|
Skoner DR: Allergic rhinitis: Definition, epidemiology, detection, and pathophysiology, diagnosis. J Allergy Clin Immun. 108 (Suppl 1):S2–S8. 2001. View Article : Google Scholar : PubMed/NCBI | |
|
Amarasekera M: Immunoglobulin E in health and disease. Asia Pac Allergy. 1:12–15. 2011. View Article : Google Scholar : PubMed/NCBI | |
|
De Filippo K, Dudeck A, Hasenberg M, Nye E, van Rooijen N, Hartmann K, Gunzer M, Roers A and Hogg N: Mast cell and macrophage chemokines CXCL1/CXCL2 control the early stage of neutrophil recruitment during tissue inflammation. Blood. 121:4930–4937. 2013. View Article : Google Scholar : PubMed/NCBI | |
|
Stone KD, Prussin C and Metcalfe DD: IgE, mast cells, basophils, and eosinophils. J Allergy Clin Immun. 125 (2 Suppl 2):S73–S80. 2010. View Article : Google Scholar : PubMed/NCBI | |
|
Janeway CA Jr: The T cell receptor as a multicomponent signalling machine: CD4/CD8 coreceptors and CD45 in T cell activation. Annu Rev Immunol. 10:645–674. 1992. View Article : Google Scholar : PubMed/NCBI | |
|
Nagata T and Koide Y: Induction of specific CD8(+) T cells against intracellular bacteria by CD8(+) T-cell-oriented immunization approaches. J Biomed Biotechnol. 2010:7645422010. View Article : Google Scholar : PubMed/NCBI | |
|
Roesner LM, Heratizadeh A, Wieschowski S, Mittermann I, Valenta R, Eiz-Vesper B, Hennig C, Hansen G, Falk CS and Werfel T: α-NAC-specific autoreactive CD8+ T cells in atopic dermatitis are of an effector memory type and secrete IL-4 and IFN-γ. J Immunol. 196:3245–3252. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Hijnen D, Knol EF, Gent YY, Giovannone B, Beijn SJ, Kupper TS, Bruijnzeel-Koomen CA and Clark RA: CD8(+) T cells in the lesional skin of atopic dermatitis and psoriasis patients are an important source of IFN-γ, IL-13, IL-17, and IL-22. J Invest Dermatol. 133:973–979. 2013. View Article : Google Scholar : PubMed/NCBI | |
|
Gordon JR, Burd PR and Galli SJ: Mast cells as a source of multifunctional cytokines. Immunol Today. 11:458–464. 1990. View Article : Google Scholar : PubMed/NCBI | |
|
Baggiolini M and Clark-Lewis I: Interleukin-8, a chemotactic and inflammatory cytokine. FEBS Lett. 307:97–101. 1992. View Article : Google Scholar : PubMed/NCBI | |
|
Hedges JC, Singer CA and Gerthoffer WT: Mitogen-activated protein kinases regulate cytokine gene expression in human airway myocytes. Am J Respir Cell Mol Biol. 23:86–94. 2000. View Article : Google Scholar : PubMed/NCBI | |
|
Jacobsen EA, Lee NA and Lee JJ: Re-defining the unique roles for eosinophils in allergic respiratory inflammation. Clin Exp Allergy. 44:1119–1136. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
Barnes PJ and Stockley RA: COPD: Current therapeutic interventions and future approaches. Eur Respir J. 25:1084–1106. 2005. View Article : Google Scholar : PubMed/NCBI | |
|
Kwon DJ, Bae YS, Ju SM, Goh AR, Youn GS, Choi SY and Park J: Casuarinin suppresses TARC/CCL17 and MDC/CCL22 production via blockade of NF-κB and STAT1 activation in HaCaT cells. Biochem Biophys Res Commun. 417:1254–1259. 2012. View Article : Google Scholar : PubMed/NCBI | |
|
Ju SM, Song HY, Lee SJ, Seo WY, Sin DH, Goh AR, Kang YH, Kang IJ, Won MH, Yi JS, et al: Suppression of thymus- and activation-regulated chemokine (TARC/CCL17) production by 1,2,3,4,6-penta-O-galloyl-beta-D-glucose via blockade of NF-kappaB and STAT1 activation in the HaCaT cells. Biochem Biophys Res Commun. 387:115–120. 2009. View Article : Google Scholar : PubMed/NCBI |