Overview of carboxyl‑terminal modulator protein 1 and its importance in various metabolic regulations (Review)
- Authors:
- Published online on: July 5, 2024 https://doi.org/10.3892/mmr.2024.13282
- Article Number: 158
-
Copyright: © Nguyen et al. This is an open access article distributed under the terms of Creative Commons Attribution License.
Abstract
Introduction
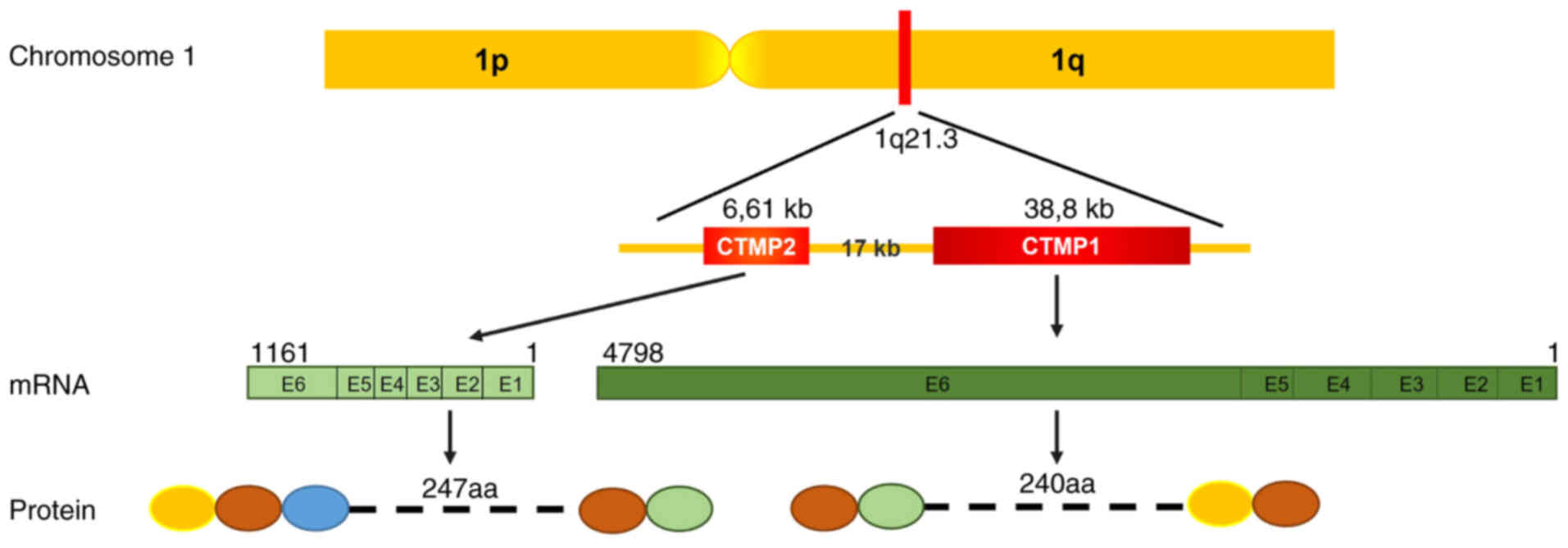
Carboxyl-terminal modulator protein 1 (CTMP1), also known as thioesterase superfamily member 4 (THEM4), was initially identified through yeast two-hybrid analysis as a protein kinase Bα (PKBα)-binding protein that inhibits the phosphorylation of PKBα. CTMP1 reverts the phenotype of viral-PKB-transformed cells (1). It is a crucial component of the PKB signaling pathway, which is involved in a wide range of biological processes including insulin signaling, cell survival, growth and metabolism (2–4). CTMP1, a mitochondrial protein, is synthesized in the nucleus and then translocates to the mitochondria. There, it undergoes maturation through cleavage of its mitochondrial localization signal at the N-terminus by mitochondrial peptidases. In addition, CTMP1 can be phosphorylated at Ser37/Ser38 (5,6). CTMP1 has been linked to several important health-related conditions, including cancer (7–17), drug resistance (18,19), brain injury (20–26), diabetic metabolism (27–29) and fibrosis-related diseases (29,30). CTMP2, also termed THEM5, is a CTMP1 paralog and a component of the mitochondrial proteome. CTMP2 has a vital role in cardiolipin remodeling and the development of fatty liver disease. CTMP1 and CTMP2 are located next to each other in human chromosome 1q21.3 and have six exons in mouse chromosome 3 (Fig. 1). Although CTMP1 is found in lower eukaryotes, including yeast, CTMP2 is only present in mammals (31). The functions of CTMP2 have not been fully revealed and fewer studies have focused on CTMP2 than on CTMP1. Thus, the findings of both are controversial and diverse.
The present review provides an overview of CTMP1 and CTMP2 regulation and discusses how CTMP1 and/or CTMP2 are involved in PKB signaling, mitochondrial function and vital conditions and processes including cancer, brain injury, mitochondrial function and lipid metabolism. The summaries provide additional insight, offer promising avenues for research on CTMP-related subjects and underscore key findings.
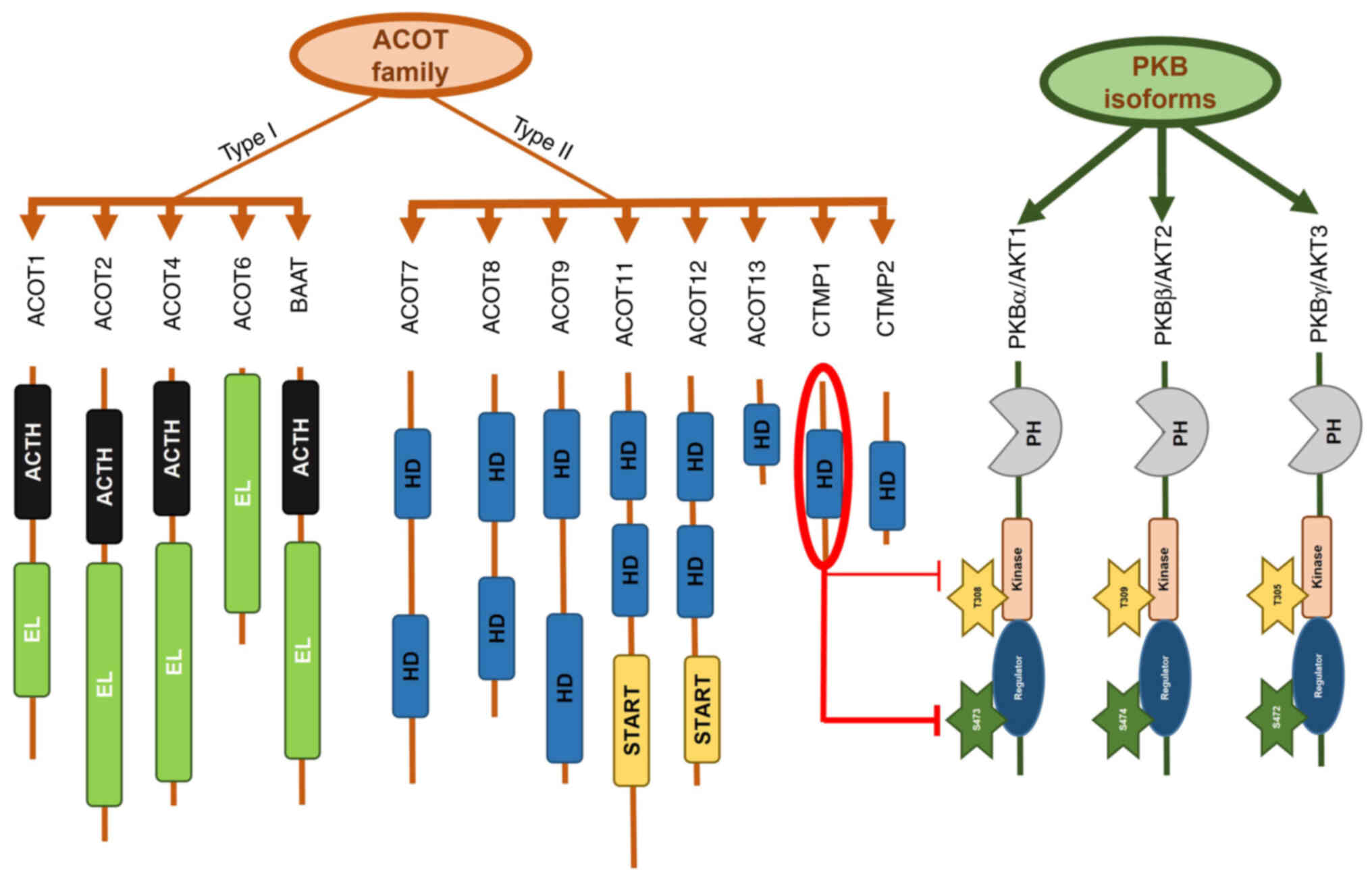
Classification and structural analysis of CTMP
Thioesterases are widely distributed enzymes that can be found in bacteria, archaea and eukaryotes. They catalyze the cleavage of thioester bonds in a variety of substrates, including activated fatty acyl-coenzyme A (CoA) substrates, acyl carrier proteins and glutathione (32). Substrates bonded to CoA are involved in various biosynthetic pathways, such as the synthesis of fatty acids and cholesterol, as well as in catabolic processes, such as fatty acid oxidation and the tricarboxylic acid cycle. Acyl-CoA thioesterases (ACOTs) have a vital role in regulating lipid metabolic functions, including energy expenditure, hepatic gluconeogenesis and neuronal function (33). These enzymes are located within various cellular compartments, including peroxisomes, mitochondria and the cytosol. ACOTs are categorized into two distinct types based on their enzymatic activities: Type I and II. Type I ACOTs belong to the α/β-hydrolase protein family, while type II ACOTs are part of the ‘hotdog’ fold family (34). Among the six human type II gene products, CTMP1 (THEM4) and CTMP2 (THEM5) share identical structural features of type II (Fig. 2) but lack sequence homology with other members of the hotdog superfamily. Research on the structure of CTMP1 has focused on its carboxyl-terminal domain, which consists of ~100 amino acids that include a hotdog-fold thioesterase subunit, conferring thioesterase activity (35,36). More is known about the functional attributes of the C-terminal domain than the N-terminal domain, which remains less explored. X-ray analysis of human CTMP1 revealed its binding with undecan-2-one-CoA, and analysis of the N-terminal domain showed an irregular and flexible secondary structure, suggesting its potential role as a protein-binding domain (36). However, there is no evidence to suggest that CTMP1 inhibits PKB in the regulation of thioesterase activity (35). In their crystal forms, CTMP1 and CTMP2 exhibit the classic hotdog-fold structure and form distinct homodimers (31). However, CTMP1 and CTMP2 may form higher-order oligomers in mitochondria in response to specific stimuli or environmental conditions. Overall, the architecture of CTMP highlights its function as an acyl-CoA thioesterase, whereas its widely acknowledged role as a PKB inhibitor is not supported by current research findings. This indicates that the interaction between CTMP and PKB may depend on the specific subcellular locations or colocalization of each.
Role of CTMP in PKB signaling pathway
PKB, also known as α serine/threonine-protein kinase (AKT), is a serine/threonine kinase that includes three isoforms and belongs to the cyclic adenosine monophosphate (cAMP)-dependent protein kinases A, G and C superfamily. These isoforms share structural homology within their catalytic domains and have similar mechanisms of activation. In mammals, three PKB isoforms have been identified: PKBα or AKT1 (37), PKBβ or AKT2 (38) and PKBγ or AKT3 (39) (Fig. 2). These isoforms are located on chromosomes 14q32, 19q13 and 1q44, respectively (40). PKBα is phosphorylated to regulate a variety of cellular proteins involved in metabolism, apoptosis and proliferation (41). Dysregulation of PKBα is associated with the pathogenesis of cancer, diabetes and multiple ocular diseases (42). PKBα activation occurs through site-specific phosphorylation at Thr308 and Ser473 on the plasma membrane, facilitated by the binding of its pleckstrin homology domain to phosphatidylinositol-3,4,5-trisphosphate (43). Specifically, phosphorylation of PKBα primarily occurs at the activation T-loop on Thr308 by phosphoinositide-dependent kinase 1 (PDK1) via PDK1-directed phosphorylation (44). Another PKBα phosphorylation site is Ser473, located in the C-terminus, a noncatalytic region of the enzyme within the hydrophobic motif (45). In 2005, research showed that the mammalian target of rapamycin (mTOR)/rapamycin-insensitive companion of mTOR complex directly phosphorylates PKB on Ser473 and enables phosphorylation of Thr308 by PDK1 (46).
THEM4 and THEM5 were named based on their structures and original family association. However, understanding why THEM4 is also referred to as CTMP1 requires further exploration into the origin of CTMP1. CTMP1 was initially identified as a PKBα inhibitor in 2001 (1). During this identification, a yeast two-hybrid assay was conducted using the COOH-terminal regulatory domain of PKBα, which includes the hydrophobic motif and residue Ser473, as bait. This led to the discovery of a protein comprising 240 amino acids with a molecular weight of 27 kDa, termed carboxyl-terminal modulator protein 1 (CTMP1). As a result, THEM4 became known as CTMP1. The designations CTMP1 and CTMP2 were then introduced to differentiate between THEM4 and THEM5 (47).
CTMP1 exerts its inhibitory effect on PKB signaling through the carboxyl-terminal regulatory domain of PKBα at the plasma membrane, inhibiting PKBα activity by preventing its phosphorylation at Ser473 and, to a lesser extent, at Thr308 residues (1) (Fig. 2). As PKB is a kinase, a reduction in its active form results in decreased activity and subsequent phosphorylation of downstream substrates, such as glycogen synthase kinase 3β (GSK3β) (1). Colocalization of CTMP1 and PKBα as an endogenous complex is observed in the plasma membrane of serum-starved cells and within cell fractions (48). Conversely, the absence of CTMP1 binding may facilitate PKB phosphorylation by allowing access to the hydrophobic motif at Ser473.
No reports to date have disclosed a relationship between CTMP2 and PKB. In 2013, however, a doctoral thesis by Zhuravleva (47) at the University of Basel demonstrated that in CTMP2(−/-) mice subjected to an insulin challenge, phosphorylation of PKB was increased in the liver and adipose tissues (both white and brown), while no differences were observed in the levels of phosphorylated PKB in muscle, heart or brain tissues. In addition to the increase in PKB phosphorylation, a decrease in the phosphorylation of AMP-activated protein kinase at residue Thr172 was observed (47).
By contrast, a report in 2007 demonstrated an inverse relationship between CTMP1 and PKB, showing that overexpression of CTMP1 increased PKB phosphorylation, while knockdown of CTMP1 decreased PKB phosphorylation (49). In that study, Ono et al (49) conducted experiments on Cos-1, HepG2, HeLa and NIH3T3 cells, yielding comparable results. Although they observed an inhibitory effect of CTMP1 on PKB, consistent with other research, the discrepancies between their study and others may be attributed to the use of different cell lines or sublines. This suggests that the effect of CTMP1 on certain cell lines may be unexpectedly complex. Such findings present both advantages and challenges for researchers focusing on CTMP1 and CTMP more broadly. Thus, given the complexities in the interaction between CTMP and PKB activity, all evidence indicates that CTMP (particularly CTMP1) has a crucial role in regulating PKB and its downstream effects.
Impact of CTMP on metabolic syndrome
In a study from 2013 examining transformed lymphocytes from 190 Caucasian and African-American individuals, researchers aimed to identify functional variants linked to type 2 diabetes susceptibility in the chromosome 1q21-24 region. They found that CTMP1 expression in adipocytes was significantly higher in individuals with the T allele (P=0.005), correlating with glucose homeostasis traits in the MAGIC dataset (28). In addition, in mice fed a high-fat diet, the CTMP1 protein level was higher in white adipose tissue (27) and bone marrow macrophages from mice with diet-induced obesity (50). Conversely, the leucine zipper/EF-hand-containing transmembrane protein 1 (LETM1), known to bind CTMP1 and exhibit anti-cancer effects (51), was downregulated. LETM1 also negatively affects the role of CTMP1 in PKB activation in obese conditions. Specifically, CTMP1 upregulation in obesity enhances its inhibitory effect on PKB activation, contributing to insulin resistance, a hallmark of obesity. Interestingly, the negative impact of LETM1, acting as a CTMP1 inhibitor, is diminished; this leads to increased CTMP1 expression (27).
CTMP1 levels are lower in mice with diabetic kidney disease than in normal mice (29). In the human renal proximal tubular epithelial cell line HKC, CTMP1 expression decreases in response to high-glucose stimuli (29). Following a high-fat diet, CTMP1 expression in the hippocampus is increased while PKB phosphorylation is decreased (52). In addition, CTMP1 has been shown to regulate the synthesis of branched-chain fatty acids in lamb liver (53). Furthermore, CTMP1 is positively associated with the human serum metabolite 3-hydroxyl decanoate, a hydroxyl-saturated medium-chain fatty acid anion (54). The effects of CTMP2 in the regulation of phosphorylated PKB inhibition are limited to adipose tissue and liver; CTMP2 does not impact the heart, muscle or brain in this regard (47). Overall, the influence of CTMP on lipid metabolism, diabetic status or high-glucose conditions varies across different organs, primarily through the modulation of PKB activity.
Regulation of CTMP in apoptosis and mitochondrial function
Both membrane-bound CTMP1 and a free pool of mature CTMP1 are present in the inter-membrane space of mitochondria. Upon apoptosis, CTMP1 is rapidly released from the mitochondria into the cytosol. This release is associated with increased mitochondrial membrane depolarization and enhanced cleavage of caspase-3 and polyADP-ribose polymerase (PARP), all of which are linked to CTMP1 overexpression. Conversely, knockdown of CTMP1 significantly reduces caspase-3 and PARP activation and mitigates loss of the mitochondrial membrane potential, as observed in 293 cells and HeLa cell lines (5). In A549 cells, CTMP1 promotes apoptosis through inhibition of anti-apoptotic heat-shock protein 27 (Hsp27) (55). In HeLa cells, CTMP1 binds to Hsp70, inhibiting the Hsp70-apoptotic protease activating factor-1 complex and thus promoting apoptosis (6). In addition, CTMP1 affects mitochondrial morphology by inhibiting OPA1 mitochondrial dynamin-like GTPase (simply known as OPA1), which is necessary for mitochondrial fusion (55). Furthermore, LETM1, a protein that binds to CTMP1, contributes to mitochondrial fragmentation via OPA1 cleavage (56). A defect in the N-terminus of CTMP1 or loss of the full length of CTMP1 results in clustering of spherical mitochondria, indicating the role of CTMP1 in mitochondrial fission (57).
CTMP2 has also been identified as a mitochondrial protein. Bioinformatics analysis has revealed a mitochondrial targeting sequence at the N-terminal end of the human CTMP2 protein and in the CTMP2 orthologs of other species. Translocase of outer mitochondrial membrane 20 (TOMM20) is a mitochondrial membrane protein. In one study, immunofluorescence staining of CTMP2 and TOMM20 showed overlapping localizations in the cytoplasm of U2OS cells (31). Further analyses indicated that CTMP2 localizes within the mitochondrial matrix. The absence of CTMP2 results in variations in mitochondrial morphology and function (31). Therefore, both CTMP1 and CTMP2 are mitochondrial proteins that may influence apoptosis and mitochondrial functions in various ways.
Various functions of CTMP in cancer and drug resistance
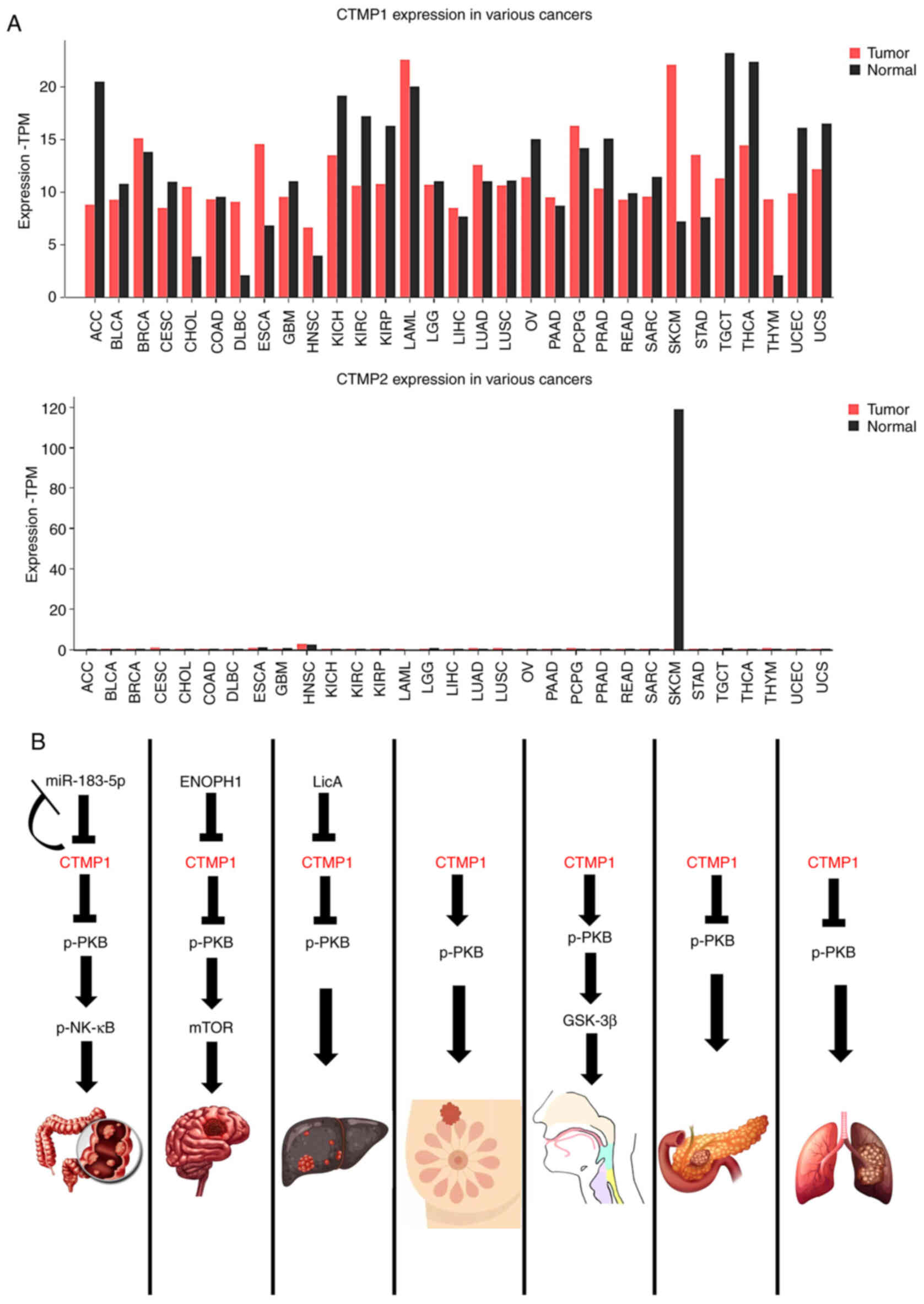
The expressions of CTMP1 and CTMP2 in cancer may vary, being either higher or lower than physiological levels depending on the cancer type (Fig. 3A). The relationship between CTMP1 and cancer is more established than that between CTMP2 and cancer (Fig. 3A). The data were analyzed using Gene Expression Profiling Interactive Analysis (GEPIA2; http://gepia2.cancer-pku.cn/#index). Therefore, the following sections will summarize the latest research on the role of CTMP1 in regulating various types of cancer (Fig. 3B) and on the role of CTMP2 specifically in lung cancer.
Colon cancer
In the context of colon cancer, there is a notable increase in the level of microRNA (miR)-183-5p in M2-polarized tumor-associated macrophages. The subsequent overexpression of CTMP1 reduces the carcinogenic effects mediated by miR-183-5p, and this is accompanied by inactivation of the PKB and NF-κB pathways in colon cancer cells (Fig. 3B). Therefore, CTMP1 may serve as a target for miR-183-5p to modulate the progression of colon cancer (17).
Glioma
Similar to other cancers, in glioma, the different expression between tumor and normal tissues is a foundational finding for a potential biomarker (58). In glioma, CTMP1 interacts with enolase-phosphatase 1, a newly identified enzyme involved in L-methionine biosynthesis. This interaction, proven through immunoprecipitation and western blot analyses, regulates the PI3K/AKT/mTOR signaling pathway (Fig. 3B), thereby affecting glioma cell growth and invasion (59). Furthermore, the level of CTMP1 mRNA was found to be decreased in glioblastoma and six glioma cell lines. This decrease in mRNA is associated with downregulation of CTMP1 transcription and hypermethylation of the CTMP1 promoter in glioblastoma (11,60,61).
Monochemosensitive choriocarcinoma
Analysis of the expression of the 760-gene panel in the PanCancer Pathway, which is related to oncogenesis and immune tolerance in tissue samples of complete hydatidiform moles and gestational choriocarcinoma, showed that CTMP1 expression was higher in monochemoresistant than monochemosensitive choriocarcinoma (15).
Hepatocellular carcinoma (HCC)
To improve the prognostic prediction of HCC, numerous biomarkers have been found (62). A total of 365 samples of HCC were taken from The Cancer Genome Atlas database and least absolute shrinkage and selection operator analysis was conducted to examine HCC mRNA expression; CTMP1 was one of nine mRNAs identified as a prognostic indicator or risk factor (16). In addition, when linking CTMP1 and its LETM1 binding partner and overexpressing them, tumorigenesis in a mouse model of HCC was reduced and mitochondria-mediated apoptosis was induced through morphological changes and defects of mitochondrial function (51). Fenofibrate and activated peroxisome proliferator-activated receptor α can elevate CTMP1 expression in liver cancer cells (Huh7 cell line); additionally, CTMP1 exerts an inhibitory effect on PKB (63). In HepG2 cells, CTMP1 acts downstream of licochalcone A, a novel chemotherapy drug that induces apoptosis by inhibiting Bcl-2. In addition, licochalcone A promotes the generation of reactive oxygen species, leading to induction of autophagy, with CTMP1 playing a role in this process (64) (Fig. 3B).
Breast cancer and triple-negative breast cancer (TNBC)
TNBC accounts for ~15–-20% of all cases of breast cancer (65). CTMP1 is upregulated in both specimens and cell lines of breast cancer (13) and TNBC (14). CTMP1 is overexpressed in breast cancer and knockdown experiments have shown that CTMP1 functions as an oncogene. It enhances cell proliferation and tumorigenic properties by promoting PKB phosphorylation (13). Furthermore, in TNBC metastasis, CTMP1 enhances migration and invasion abilities by elevating PKB activity (14) (Fig. 3B).
PKB activation and loss of CTMP1 occur in tamoxifen-resistant human breast cancer cell lines, indicating that CTMP1 acts as an inhibitory factor for PKB (19). CTMP1 adopts several different roles in regulating PKB phosphorylation due to various genomic aberrations in the tamoxifen resistance model.
Head and neck squamous cell carcinoma
CTMP1 exhibits higher expression at the protein and mRNA levels in head and neck squamous cell carcinoma (both tumor tissue and cell lines) than in normal tissues and is associated with lymph node metastasis (12). CTMP1 is also associated with PKB/GSK3β phosphorylation, increased Snail levels and decreased E-cadherin levels, indicative of epithelial-to-mesenchymal transition. These findings suggest an oncogenic role of CTMP1 in head and neck squamous cell carcinoma (12) (Fig. 3B).
Pancreatic adenocarcinoma
PKB-related genes are rarely mutated in pancreatic adenocarcinoma. When PKB activity was inhibited with a cell-permeable peptide targeting the predicted N-terminal region of CTMP1, both human and murine pancreatic adenocarcinoma cell lines underwent apoptosis. In addition, these cell lines displayed smaller tumors in allograft models (10) (Fig. 3B). In another study, CTMP1 was not detectable in pancreatic cancer cell lines compared with a three-dimensional culture system of pancreatic duct epithelial cells (66).
Endometrial cancer
CTMP1 and its binding partner LETM1 show higher protein expression in endometrial cancer tissues than in atypical hyperplastic tissues and in atypical hyperplastic tissues than in normal tissues. The correlation of CTMP1 and LETM1 serves as an oncogenic factor in endometrial cancer cells (KLE cell line) (9).
Lung cancer
In mice with lung cancer subjected to a diet high in inorganic phosphate, CTMP1 expression was reduced, while PKB kinase activity was increased, leading to enhanced progression of lung tumors (7). In addition, in a mouse model of lung cancer utilizing lentiviral vector-CTMP1 administered as an aerosol, downregulation of PKB phosphorylation resulted in reduced pulmonary tumorigenesis (8) (Fig. 3B). Using short-term interventions (30 min) (8) or long-term interventions (30 min twice a week for 4 weeks) (67) yielded similar results, suggesting that viral delivery of CTMP1 can be a practical tool for lung cancer treatment (67). CTMP2 antisense RNA1 (C2CD4D-AS1) was found to be predominantly upregulated in lung adenocarcinoma tissues and cell lines, and this upregulation was induced by ETS translocation variant 4. In addition, ablation of C2CD4D-AS1 suppressed cell proliferation, migration, invasion and apoptosis of lung adenocarcinoma (68).
In summary, CTMP1 and CTMP2 exhibit different functions and expressions in various cancer types through dissimilar mechanisms, suggesting the high potential for CTMP to serve as a novel component of anti-cancer therapy.
Emerging role of CTMP1 in regulation of fibrosis
CTMP1 protein expression is decreased in the kidneys of diabetic mice. In addition, the kidneys of such mice exhibit an increase in transforming growth factor β1 (TGFβ1) and α-smooth muscle actin (αSMA) (29), both of which are major regulators of extracellular matrix metabolism in various tissues (29,69). An increase in the expression of phospho-PKB (Ser473) has also been observed in the kidneys of diabetic mice, suggesting that CTMP1 mitigates renal extracellular matrix accumulation by modulating phospho-PKB, TGFβ1 and αSMA in these mice (29). In the HKC cell line, high glucose levels were found to reduce CTMP1 protein expression (29). Conversely, enhancement of CTMP1 expression in mice through tail vein injection of the pYr-ads-4-musCTMP vector counteracted the elevations in TGFβ1 and αSMA.
In the heart, CTMP1 serves as a regulator that attenuates cardiac hypertrophy and fibrosis (30). CTMP1 protein expression is lower in human hearts affected by dilated cardiomyopathy and hypertrophic cardiomyopathy than in normal hearts (30). Increased expression of CTMP1 lessens the severity of cardiac hypertrophy and fibrosis induced by pressure overload, whereas the absence of CTMP1 exacerbates these conditions. This is evidenced by mRNA expression of fibrosis markers such as collagen Iα, collagen III and connective tissue growth factor, as well as Sirius red staining for collagen deposition (30). As expected, Ser473 and downstream genes, including mTOR, GSK3β and ribosomal protein S6 kinase β-1 (p70S6K), also showed increased expression (34). Mechanistically, CTMP1 is thought to alleviate pathological cardiac hypertrophy by blocking the PKB pathway (30).
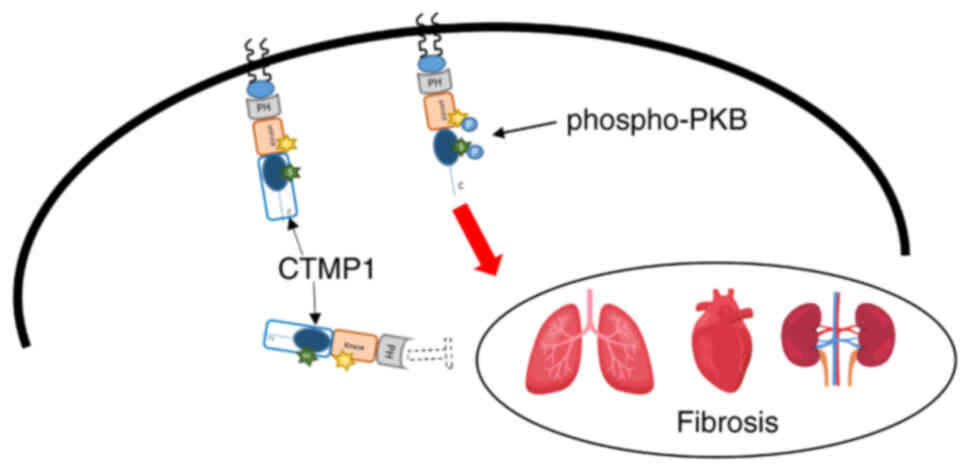
Idiopathic pulmonary fibrosis is a chronic, progressive, fibrotic interstitial lung disease predominantly affecting older individuals (70). Bleomycin is widely recognized for its ability to induce pulmonary fibrosis in mice (71). In a bleomycin-induced lung fibrosis model, CTMP1(−/-) mice exhibited increased collagen deposition and enhanced fibrosis. Specifically, these knock-out mice showed elevated levels of collagen type I α1 chain and α-SMA expression. In addition, epithelial-to-mesenchymal transition was evidenced by a significant increase in mesenchymal markers such as fibronectin and a decrease in the epithelial marker E-cadherin at both the protein and mRNA levels (unpublished data) (Fig. 4). In summary, CTMP1 has been shown to impact fibrosis in the kidneys, heart and lungs by inhibiting PKB phosphorylation. However, the effects of CTMP1 on other organs remain elusive, necessitating further investigation (Fig. 4).
Adverse effects of CTMP on brain injury
Astrocytes from the hippocampi of mice with kainic acid-induced neurodegeneration exhibit an increase in CTMP1 expression and suppression of PKB activity, negatively affecting astrocyte activation (72). CTMP1 inhibits PKB, thereby negatively impacting the mobilization of functional calcium-activated potassium channels in developing parasympathetic neurons. This inhibition hinders the mobilization evoked by β-neuregulin-1 and TGFβ1 (73). In addition, CTMP1 may be related to the mechanism by which isoflurane ameliorates neurological outcomes. This is because isoflurane can prevent neurological complications when administered with a normal diet, but not with a high-fat diet. In addition, a high-fat diet has been shown to increase CTMP1 levels and reduce PKB activity in the hippocampus (52). The CTMP1 mRNA level rapidly increases following ischemic cerebral infarction but only partially recovers after reperfusion. CTMP1 transcription is suppressed by activating transcription factor 3 (ATF3), leading to the protection of neurons from hypoxic insult by indirectly enhancing PKB activity (22). Inhibition of CTMP1 reduces hypoxic neuronal apoptosis by increasing phospho-PKB levels, while upstream ATF3 levels remain constant (23). This reinforces the endogenous neuroprotective ATF3-CTMP1 signaling cascade, representing a potential therapeutic target for ischemic brain injury. Outside the hippocampus, CTMP1 also shows higher expression in type 2 diabetic mice with focal cerebral ischemia (25) and in vulnerable hippocampal neurons (26), leading to suppressed PKB activity and negatively impacting ischemia outcomes (25,26).
Sevoflurane serves as an additional neuroprotective target by enhancing PKB activity and GSK3β. However, the neuroprotective benefits of its preconditioning are diminished in the presence of CTMP1 overexpression. In addition, a correlation has been observed between CTMP1 and reduced PKB expression after ischemic events (21). CTMP1 expression is increased in tissues of the ischemic penumbra, suggesting that the rise in CTMP1 levels with age may have a role in the decreased ischemic tolerance of the brain (20). Furthermore, in mice subjected to traumatic brain injury, phospho-PKB levels initially peak to provide neuroprotection before diminishing, while CTMP1 levels peak and remain stable after injury. This indicates that during traumatic brain injury, CTMP1 activation serves to inhibit PKB phosphorylation. In Parkinson's disease, the second most common neurodegenerative disorder, CTMP2 has been observed to reduce the use of the full-length transcript containing 247 amino acids (Fig. 1) while increasing the use of a shorter transcript containing 119 amino acids (74).
This variation led to an intriguing discovery: The full-length isoform of CTMP2 localizes within mitochondria, whereas the shorter isoform is more likely to be found in the extracellular space than in the mitochondria. This suggests that in Parkinson's disease, mitochondrial CTMP2 is downregulated along with a decrease in the full-length transcript (75). Furthermore, CTMP2 has been found to be significantly associated with cisplatin-induced peripheral neuropathy, in which higher expression of CTMP2 is correlated with this side effect of cisplatin chemotherapy (76). Overall, CTMP appears to exert a negative effect on neuroprotection. Inhibiting CTMP may enhance the recovery of neurological function by increasing phosphorylation of PKB.
Other factors
Age
In a study of autoimmune thyroiditis, one of the most prevalent endocrine autoimmune diseases, the methylation levels of CTMP1 cDNA were analyzed and compared between patients and controls exposed to varying levels of iodine in water. This comparison was performed to assess the impact of genes related to the PI3K-AKT signaling pathway. The study showed a negative correlation between the CTMP1 methylation level and age (77). In addition, in rat brains, an increase in CTMP1 expression was observed alongside a decrease in activated PKB as a function of aging (20).
Vitamin D
The active form of vitamin D, namely 1,25-dihydroxyvitamin D [1,25(OH)2D], alleviates local inflammation by upregulating CTMP1, which in turn reduces the phosphorylation of both PKB and its downstream target, IκBα. Conversely, knockdown of CTMP1 diminishes the inhibitory effect of 1,25(OH)2D on macrophages (78).
Malaria
Malaria is a deadly parasitic disease and its underlying mechanisms are not fully understood. Analysis of the GSE1124 dataset revealed that CTMP1 was positively associated with asymptomatic Plasmodium falciparum infection, a classification of malaria (79).
Muscle atrophy and myogenesis, amyotrophic lateral sclerosis (ALS)
CTMP1 has a negative role in hypertrophy of both skeletal and cardiac muscles. It does so by enhancing muscle atrophy after nerve injury, primarily through inhibition of PKB activity and other downstream factors (80). CTMP1 interacts with N-Myc downstream-regulated gene 4, which hinders its ability to bind to PKB. This interference increases PKB phosphorylation and subsequently enhances cAMP response element-binding protein activity during differentiation of C2C12 myoblasts. This process leads to a boost in the expression of myogenic genes (81). Furthermore, in both ALS model mice and differentiated C2C12 cells, a significant increase in CTMP1 at the protein level was observed in the hindlimb skeletal muscle. This increase was associated with a decrease in the level of phosphorylated PKB (82).
Lung hypertension
CTMP1 ablation enhances PKB activity during the development of pulmonary hypertension. This enhancement is attributed to the ability of asymmetric dimethylarginine to increase peroxynitrite generation and promote the mitochondrial translocation of endothelial nitric oxide synthase. This translocation is essential for regulating mitochondrial function (83).
Acute rejection after transplantation
In cases of acute rejection following renal transplantation, CTMP2 is notably downregulated. It shows a negative correlation with naive B cells but a positive association with M2 macrophages, resting mast cells, memory B cells and resting natural killer cells (84).
Future perspectives
The recent discoveries regarding CTMP1 and CTMP2 have led to several key points, which are summarized as follows and also in Table I.
CTMP1 primarily functions as an inhibitor of PKB, playing a regulatory role in lipid metabolism, cancer, fibrosis and neurodegeneration. However, there are certain exceptions to this. CTMP1 has been shown to increase PKB phosphorylation, leading to opposite conclusions. Therapeutically targeting CTMP1 in cancer and fibrosis presents a promising avenue for future treatment. However, adopting a rigid mindset in researching CTMP1 or CTMP2 is discouraged. In addition, CTMP1 and its binding partner LETM1 are often correlated with apoptosis, lipid metabolism and cancer.
The expression of CTMP1 under obesity-related conditions across different organs remains controversial. However, its effects on organs may be categorized into two groups: i) Adipose tissues and liver, in which lipid metabolism is predominant; and ii) other organs, such as the kidneys, heart and lungs. Further investigation into the effects on these remaining organs is required.
The knowledge of CTMP2 is comparably scarce, particularly in the context of cancer, with no findings related to CTMP2 in this area. The sole functions of CTMP2 identified thus far pertain to fatty liver disease and cardiolipin remodeling. The possibility that CTMP2 may share similar functions with CTMP1 cannot be dismissed. Furthermore, the correlation between CTMP2 and PKB is not well established, suggesting a novel area of exploration.
These findings offer fascinating opportunities for further research. Thorough exploration is required to fully understand the roles of CTMP1 and CTMP2 and their potential for therapeutic applications.
Acknowledgements
Not applicable.
Funding
This work was financially supported by the research fund of Chungnam National University (2022; to SK), by the National Research Foundation of Korea (NRF) grant funded by the Korean Government (MEST; grant nos. NRF-2021R1A2C1008492, NRF-2020R1F1A1049801 and NRF-2018R1A6A1A03023718) and by the Starting growth Technological R&D Program (TIPS Program; grant no. S3198556) funded by the Ministry of Small and Medium-sized Enterprises (SMEs) and Micro Enterprises (Ministry of SMEs and Startups, Korea) in 2021.
Availability of data and materials
Not applicable.
Authors' contributions
HN, SG, UJ, WJ, SHK, SK and JP contributed to the conception and design of the study. HN, UJ, SG, QH, SL, BL and JP were involved in the literature search, selection and analysis. HN, UJ, SG and WJ contributed to the analysis in the GEPIA database. SHK, SK and JP contributed to acquiring funding and performing the final revision of the manuscript. All authors agreed to be accountable for all aspects of the work and all authors read and approved the final manuscript. Data authentication is not applicable.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
|
Maira SM, Galetic I, Brazil DP, Kaech S, Ingley E, Thelen M and Hemmings BA: Carboxyl-terminal modulator protein (CTMP), a negative regulator of PKB/Akt and v-Akt at the plasma membrane. Science. 294:374–380. 2001. View Article : Google Scholar : PubMed/NCBI | |
|
Manning BD and Toker A: AKT/PKB signaling: Navigating the network. Cell. 169:381–405. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Glaviano A, Foo ASC, Lam HY, Yap KCH, Jacot W, Jones RH, Eng H, Nair MG, Makvandi P, Geoerger B, et al: PI3K/AKT/mTOR signaling transduction pathway and targeted therapies in cancer. Mol Cancer. 22:1382023. View Article : Google Scholar : PubMed/NCBI | |
|
Wang Z: Regulation of cell cycle progression by growth factor-induced cell signaling. Cells. 10:33272021. View Article : Google Scholar : PubMed/NCBI | |
|
Parcellier A, Tintignac LA, Zhuravleva E, Cron P, Schenk S, Bozulic L and Hemmings BA: Carboxy-terminal modulator protein (CTMP) is a mitochondrial protein that sensitizes cells to apoptosis. Cell Signal. 21:639–650. 2009. View Article : Google Scholar : PubMed/NCBI | |
|
Piao L, Li Y, Yang KJ, Park KA, Byun HS, Won M, Hong J, Kim JL, Kweon GR, Hur GM, et al: Heat shock protein 70-mediated sensitization of cells to apoptosis by carboxyl-terminal modulator protein. BMC Cell Biol. 10:532009. View Article : Google Scholar : PubMed/NCBI | |
|
Jin H, Xu CX, Lim HT, Park SJ, Shin JY, Chung YS, Park SC, Chang SH, Youn HJ, Lee KH, et al: High dietary inorganic phosphate increases lung tumorigenesis and alters Akt signaling. Am J Respir Crit Care Med. 179:59–68. 2009. View Article : Google Scholar : PubMed/NCBI | |
|
Hwang SK, Kwon JT, Park SJ, Chang SH, Lee ES, Chung YS, Beck GR Jr, Lee KH, Piao L, Park J and Cho MH: Lentivirus-mediated carboxyl-terminal modulator protein gene transfection via aerosol in lungs of K-ras null mice. Gene Ther. 14:1721–1730. 2007. View Article : Google Scholar : PubMed/NCBI | |
|
Niu F, Duan Y, Man Y, Liu W, Dai T, Zhang H, Li C and Wei D: Mitochondrial protein LETM1 and its-mediated CTMP are potential therapeutic targets for endometrial cancer. Anticancer Drugs. 33:632–641. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Simon PO Jr, McDunn JE, Kashiwagi H, Chang K, Goedegebuure PS, Hotchkiss RS and Hawkins WG: Targeting AKT with the proapoptotic peptide, TAT-CTMP: A novel strategy for the treatment of human pancreatic adenocarcinoma. Int J Cancer. 125:942–951. 2009. View Article : Google Scholar : PubMed/NCBI | |
|
Knobbe CB, Reifenberger J, Blaschke B and Reifenberger G: Hypermethylation and transcriptional downregulation of the carboxyl-terminal modulator protein gene in glioblastomas. J Natl Cancer Inst. 96:483–486. 2004. View Article : Google Scholar : PubMed/NCBI | |
|
Chang JW, Jung SN, Kim JH, Shim GA, Park HS, Liu L, Kim JM, Park J and Koo BS: Carboxyl-terminal modulator protein positively acts as an oncogenic driver in head and neck squamous cell carcinoma via regulating Akt phosphorylation. Sci Rep. 6:285032016. View Article : Google Scholar : PubMed/NCBI | |
|
Liu YP, Liao WC, Ger LP, Chen JC, Hsu TI, Lee YC, Chang HT, Chen YC, Jan YH, Lee KH, et al: Carboxyl-terminal modulator protein positively regulates Akt phosphorylation and acts as an oncogenic driver in breast cancer. Cancer Res. 73:6194–6205. 2013. View Article : Google Scholar : PubMed/NCBI | |
|
Lin CH, Lin WD, Huang YC, Chen YC, Loh ZJ, Ger LP, Lin FC, Li HY, Cheng HC, Lee KH, et al: Carboxyl-terminal modulator protein facilitates tumor metastasis in triple-negative breast cancer. Cancer Gene Ther. 30:404–413. 2023.PubMed/NCBI | |
|
Bolze PA, Lopez J, Allias F, Hajri T, Patrier S, Devouassoux-Shisheboran M, Massardier J, You B, Golfier F and Mallet F: Transcriptomic and immunohistochemical approaches identify HLA-G as a predictive biomarker of gestational choriocarcinoma resistance to monochemotherapy. Gynecol Oncol. 158:785–793. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Ni FB, Lin Z, Fan XH, Shi KQ, Ao JY, Wang XD and Chen RC: A novel genomic-clinicopathologic nomogram to improve prognosis prediction of hepatocellular carcinoma. Clin Chim Acta. 504:88–97. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Zhang S, Li D, Zhao M, Yang F, Sang C, Yan C, Wang Z and Li Y: Exosomal miR-183-5p shuttled by M2 polarized tumor-associated macrophage promotes the development of colon cancer via targeting THEM4 mediated PI3K/AKT and NF-κB pathways. Front Oncol. 11:6726842021. View Article : Google Scholar : PubMed/NCBI | |
|
Chen YC, Li HY, Liang JL, Ger LP, Chang HT, Hsiao M, Calkins MJ, Cheng HC, Chuang JH and Lu PJ: CTMP, a predictive biomarker for trastuzumab resistance in HER2-enriched breast cancer patient. Oncotarget. 8:29699–29710. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Block M, Grundker C, Fister S, Kubin J, Wilkens L, Mueller MD, Hemmerlein B, Emons G and Günthert AR: Inhibition of the AKT/mTOR and erbB pathways by gefitinib, perifosine and analogs of gonadotropin-releasing hormone I and II to overcome tamoxifen resistance in breast cancer cells. Int J Oncol. 41:1845–1854. 2012. View Article : Google Scholar : PubMed/NCBI | |
|
Li J, Shan W and Zuo Z: Age-related upregulation of carboxyl terminal modulator protein contributes to the decreased brain ischemic tolerance in older rats. Mol Neurobiol. 55:6145–6154. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Chen Y, Nie H, Tian L, Tong L, Deng J, Zhang Y, Dong H and Xiong L: Sevoflurane preconditioning-induced neuroprotection is associated with Akt activation via carboxy-terminal modulator protein inhibition. Br J Anaesth. 114:327–335. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Kao MH, Huang CY, Cheung WM, Yan YT, Chen JJ, Ho YS, Hsu CY and Lin TN: Activating transcription factor 3 diminishes ischemic cerebral infarct and behavioral deficit by downregulating carboxyl-terminal modulator protein. Int J Mol Sci. 24:23062023. View Article : Google Scholar : PubMed/NCBI | |
|
Huang CY, Chen JJ, Wu JS, Tsai HD, Lin H, Yan YT, Hsu CY, Ho YS and Lin TN: Novel link of anti-apoptotic ATF3 with pro-apoptotic CTMP in the ischemic brain. Mol Neurobiol. 51:543–557. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Zhao S, Fu J, Liu F, Rastogi R, Zhang J and Zhao Y: Small interfering RNA directed against CTMP reduces acute traumatic brain injury in a mouse model by activating Akt. Neurol Res. 36:483–490. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
Chen Y, Cai M, Deng J, Tian L, Wang S, Tong L, Dong H and Xiong L: Elevated expression of carboxy-terminal modulator protein (CTMP) aggravates brain ischemic injury in diabetic db/db Mice. Neurochem Res. 41:2179–2189. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Miyawaki T, Ofengeim D, Noh KM, Latuszek-Barrantes A, Hemmings BA, Follenzi A and Zukin RS: The endogenous inhibitor of Akt, CTMP, is critical to ischemia-induced neuronal death. Nat Neurosci. 12:618–626. 2009. View Article : Google Scholar : PubMed/NCBI | |
|
Park J, Li Y, Kim SH, Yang KJ, Kong G, Shrestha R, Tran Q, Park KA, Jeon J, Hur GM, et al: New players in high fat diet-induced obesity: LETM1 and CTMP. Metabolism. 63:318–327. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
Mondal AK, Sharma NK, Elbein SC and Das SK: Allelic expression imbalance screening of genes in chromosome 1q21-24 region to identify functional variants for Type 2 diabetes susceptibility. Physiol Genomics. 45:509–520. 2013. View Article : Google Scholar : PubMed/NCBI | |
|
Chen N, Hao J, Li L, Li F, Liu S and Duan H: Carboxy-terminal modulator protein attenuated extracellular matrix deposit by inhibiting phospho-Akt, TGF-β1 and α-SMA in kidneys of diabetic mice. Biochem Biophys Res Commun. 474:753–760. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Liu X, Yang Q, Zhu LH, Liu J, Deng KQ, Zhu XY, Liu Y, Gong J, Zhang P, Li S, et al: Carboxyl-terminal modulator protein ameliorates pathological cardiac hypertrophy by suppressing the protein kinase B signaling pathway. J Am Heart Assoc. 7:e0086542018. View Article : Google Scholar : PubMed/NCBI | |
|
Zhuravleva E, Gut H, Hynx D, Marcellin D, Bleck CK, Genoud C, Cron P, Keusch JJ, Dummler B, Esposti MD and Hemmings BA: Acyl coenzyme A thioesterase Them5/Acot15 is involved in cardiolipin remodeling and fatty liver development. Mol Cell Biol. 32:2685–2697. 2012. View Article : Google Scholar : PubMed/NCBI | |
|
Swarbrick CMD, Nanson JD, Patterson EI and Forwood JKL: Structure, function, and regulation of thioesterases. Prog Lipid Res. 79:1010362020. View Article : Google Scholar : PubMed/NCBI | |
|
Tillander V, Alexson SEH and Cohen DE: Deactivating fatty acids: Acyl-CoA thioesterase-mediated control of lipid metabolism. Trends Endocrinol Metab. 28:473–484. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Brocker C, Carpenter C, Nebert DW and Vasiliou V: Evolutionary divergence and functions of the human acyl-CoA thioesterase gene (ACOT) family. Hum Genomics. 4:411–420. 2010. View Article : Google Scholar : PubMed/NCBI | |
|
Zhao H, Martin BM, Bisoffi M and Dunaway-Mariano D: The Akt C-terminal modulator protein is an acyl-CoA thioesterase of the Hotdog-Fold family. Biochemistry. 48:5507–5509. 2009. View Article : Google Scholar : PubMed/NCBI | |
|
Zhao H, Lim K, Choudry A, Latham JA, Pathak MC, Dominguez D, Luo L, Herzberg O and Dunaway-Mariano D: Correlation of structure and function in the human hotdog-fold enzyme hTHEM4. Biochemistry. 51:6490–6492. 2012. View Article : Google Scholar : PubMed/NCBI | |
|
Jones PF, Jakubowicz T and Hemmings BA: Molecular cloning of a second form of rac protein kinase. Cell Regul. 2:1001–1009. 1991. View Article : Google Scholar : PubMed/NCBI | |
|
Cheng JQ, Godwin AK, Bellacosa A, Taguchi T, Franke TF, Hamilton TC, Tsichlis PN and Testa JR: AKT2, a putative oncogene encoding a member of a subfamily of protein-serine/threonine kinases, is amplified in human ovarian carcinomas. Proc Natl Acad Sci USA. 89:9267–9271. 1992. View Article : Google Scholar : PubMed/NCBI | |
|
Brodbeck D, Cron P and Hemmings BA: A human protein kinase Bgamma with regulatory phosphorylation sites in the activation loop and in the C-terminal hydrophobic domain. J Biol Chem. 274:9133–9136. 1999. View Article : Google Scholar : PubMed/NCBI | |
|
Song G, Ouyang G and Bao S: The activation of Akt/PKB signaling pathway and cell survival. J Cell Mol Med. 9:59–71. 2005. View Article : Google Scholar : PubMed/NCBI | |
|
Nicholson KM and Anderson NG: The protein kinase B/Akt signalling pathway in human malignancy. Cell Signal. 14:381–395. 2002. View Article : Google Scholar : PubMed/NCBI | |
|
Ruan GX and Kazlauskas A: Focus on molecules: Akt (PKB). Exp Eye Res. 93:570–571. 2011. View Article : Google Scholar : PubMed/NCBI | |
|
Andjelkovic M, Alessi DR, Meier R, Fernandez A, Lamb NJ, Frech M, Cron P, Cohen P, Lucocq JM and Hemmings BA: Role of translocation in the activation and function of protein kinase B. J Biol Chem. 272:31515–31524. 1997. View Article : Google Scholar : PubMed/NCBI | |
|
Stephens L, Anderson K, Stokoe D, Erdjument-Bromage H, Painter GF, Holmes AB, Gaffney PR, Reese CB, McCormick F, Tempst P, et al: Protein kinase B kinases that mediate phosphatidylinositol 3,4,5-trisphosphate-dependent activation of protein kinase B. Science. 279:710–714. 1998. View Article : Google Scholar : PubMed/NCBI | |
|
Alessi DR, Andjelkovic M, Caudwell B, Cron P, Morrice N, Cohen P, Morrice N, Cohen P and Hemmings BA: Mechanism of activation of protein kinase B by insulin and IGF-1. EMBO J. 15:6541–6551. 1996. View Article : Google Scholar : PubMed/NCBI | |
|
Sarbassov DD, Guertin DA, Ali SM and Sabatini DM: Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 307:1098–1101. 2005. View Article : Google Scholar : PubMed/NCBI | |
|
Zhuravleva E: Structural and functional characterization of novel mitochondrial acyl-CoA thioesterase Them5/CTMP2 (Doctoral Thesis). University of Basel; 2013 | |
|
Brazil DP, Park J and Hemmings BA: PKB binding proteins. Getting in on the Akt. Cell. 111:293–303. 2002. View Article : Google Scholar : PubMed/NCBI | |
|
Ono H, Sakoda H, Fujishiro M, Anai M, Kushiyama A, Fukushima Y, Katagiri H, Ogihara T, Oka Y, Kamata H, et al: Carboxy-terminal modulator protein induces Akt phosphorylation and activation, thereby enhancing antiapoptotic, glycogen synthetic, and glucose uptake pathways. Am J Physiol Cell Physiol. 293:C1576–C1585. 2007. View Article : Google Scholar : PubMed/NCBI | |
|
Zhou Q, Leeman SE and Amar S: Signaling mechanisms involved in altered function of macrophages from diet-induced obese mice affect immune responses. Proc Natl Acad Sci USA. 106:10740–1075. 2009. View Article : Google Scholar : PubMed/NCBI | |
|
Shin JY, Chung YS, Kang B, Jiang HL, Yu DY, Han K, Chae C, Moon JH, Jang G and Cho MH: Co-delivery of LETM1 and CTMP synergistically inhibits tumor growth in H-ras12V liver cancer model mice. Cancer Gene Ther. 20:186–194. 2013. View Article : Google Scholar : PubMed/NCBI | |
|
Yu H, Deng J and Zuo Z: High-fat diet reduces neuroprotection of isoflurane post-treatment: Role of carboxyl-terminal modulator protein-Akt signaling. Obesity (Silver Spring). 22:2396–2405. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
Zhao Y, Zhang Y, Khas E, Bai C, Cao Q and Ao C: Transcriptome analysis reveals candidate genes of the synthesis of branched-chain fatty acids related to mutton flavor in the lamb liver using Allium mongolicum Regel extract. J Anim Sci. 100:skac2562022. View Article : Google Scholar : PubMed/NCBI | |
|
Yu B, Zheng Y, Alexander D, Morrison AC, Coresh J and Boerwinkle E: Genetic determinants influencing human serum metabolome among African Americans. PLoS Genet. 10:e10042122014. View Article : Google Scholar : PubMed/NCBI | |
|
Hwang SK, Minai-Tehrani A, Yu KN, Chang SH, Kim JE, Lee KH, Park J, Beck GR Jr and Cho MH: Carboxyl-terminal modulator protein induces apoptosis by regulating mitochondrial function in lung cancer cells. Int J Oncol. 40:1515–1524. 2012.PubMed/NCBI | |
|
Piao L, Li Y, Kim SJ, Sohn KC, Yang KJ, Park KA, Byun HS, Won M, Hong J, Hur GM, et al: Regulation of OPA1-mediated mitochondrial fusion by leucine zipper/EF-hand-containing transmembrane protein-1 plays a role in apoptosis. Cell Signal. 21:767–777. 2009. View Article : Google Scholar : PubMed/NCBI | |
|
Parcellier A, Tintignac LA, Zhuravleva E, Dummler B, Brazil DP, Hynx D, Cron P, Schenk S, Olivieri V and Hemmings BA: The carboxy-terminal modulator protein (CTMP) regulates mitochondrial dynamics. PLoS One. 4:e54712009. View Article : Google Scholar : PubMed/NCBI | |
|
Vo TT, Kong G, Kim C, Juang U, Gwon S, Jung W, Nguyen H, Kim SH and Park J: Exploring scavenger receptor class F member 2 and the importance of scavenger receptor family in prediagnostic diseases. Toxicol Res. 39:341–353. 2023. View Article : Google Scholar : PubMed/NCBI | |
|
Wang B, Xu X, Liu X, Wang D, Zhuang H, He X, Han T and Hong J: Enolase-phosphatase 1 acts as an oncogenic driver in glioma. J Cell Physiol. 236:1184–1194. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Tews B, Roerig P, Hartmann C, Hahn M, Felsberg J, Blaschke B, Sabel M, Kunitz A, Toedt G, Neben K, et al: Hypermethylation and transcriptional downregulation of the CITED4 gene at 1p34.2 in oligodendroglial tumours with allelic losses on 1p and 19q. Oncogene. 26:5010–5016. 2007. View Article : Google Scholar : PubMed/NCBI | |
|
Knobbe CB, Trampe-Kieslich A and Reifenberger G: Genetic alteration and expression of the phosphoinositol-3-kinase/Akt pathway genes PIK3CA and PIKE in human glioblastomas. Neuropathol Appl Neurobiol. 31:486–490. 2005. View Article : Google Scholar : PubMed/NCBI | |
|
Gao S, Gang J, Yu M, Xin G and Tan H: Computational analysis for identification of early diagnostic biomarkers and prognostic biomarkers of liver cancer based on GEO and TCGA databases and studies on pathways and biological functions affecting the survival time of liver cancer. BMC Cancer. 21:7912021. View Article : Google Scholar : PubMed/NCBI | |
|
Yamasaki D, Kawabe N, Nakamura H, Tachibana K, Ishimoto K, Tanaka T, Aburatani H, Sakai J, Hamakubo T, Kodama T and Doi T: Fenofibrate suppresses growth of the human hepatocellular carcinoma cell via PPARalpha-independent mechanisms. Eur J Cell Biol. 90:657–664. 2011. View Article : Google Scholar : PubMed/NCBI | |
|
Niu Q, Zhao W, Wang J, Li C, Yan T, Lv W, Wang G, Duan W, Zhang T, Wang K and Zhou D: LicA induces autophagy through ULK1/Atg13 and ROS pathway in human hepatocellular carcinoma cells. Int J Mol Med. 41:2601–2608. 2018.PubMed/NCBI | |
|
Garrido-Castro AC, Lin NU and Polyak K: Insights into molecular classifications of triple-negative breast cancer: Improving patient selection for treatment. Cancer Discov. 9:176–198. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Gutierrez-Barrera AM, Menter DG, Abbruzzese JL and Reddy SA: Establishment of three-dimensional cultures of human pancreatic duct epithelial cells. Biochem Biophys Res Commun. 358:698–703. 2007. View Article : Google Scholar : PubMed/NCBI | |
|
Hwang SK, Lim HT, Minai-Tehrani A, Lee ES, Park J, Park SB, Beck GR Jr and Cho MH: Repeated aerosol delivery of carboxyl-terminal modulator protein suppresses tumor in the lungs of K-rasLA1 mice. Am J Respir Crit Care Med. 179:1131–1140. 2009. View Article : Google Scholar : PubMed/NCBI | |
|
Wang B, Cai Y, Li X, Kong Y, Fu H and Zhou J: ETV4 mediated lncRNA C2CD4D-AS1 overexpression contributes to the malignant phenotype of lung adenocarcinoma cells via miR-3681-3p/NEK2 axis. Cell Cycle. 20:2607–2618. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Gao S, Tan H and Li D: Oridonin suppresses gastric cancer SGC-7901 cell proliferation by targeting the TNF-alpha/androgen receptor/TGF-beta signalling pathway axis. J Cell Mol Med. 27:2661–2674. 2023. View Article : Google Scholar : PubMed/NCBI | |
|
Strykowski R and Adegunsoye A: Idiopathic pulmonary fibrosis and progressive pulmonary fibrosis. Immunol Allergy Clin North Am. 43:209–228. 2023. View Article : Google Scholar : PubMed/NCBI | |
|
Gul A, Yang F, Xie C, Du W, Mohammadtursun N, Wang B, Le J and Dong J: Pulmonary fibrosis model of mice induced by different administration methods of bleomycin. BMC Pulm Med. 23:912023. View Article : Google Scholar : PubMed/NCBI | |
|
Shin N, Yi MH, Kim S, Baek H, Triantafillu UL, Park J and Kim DW: Astrocytic expression of CTMP following an excitotoxic lesion in the mouse hippocampus. Exp Neurobiol. 26:25–32. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Chae KS, Martin-Caraballo M, Anderson M and Dryer SE: Akt activation is necessary for growth factor-induced trafficking of functional K(Ca) channels in developing parasympathetic neurons. J Neurophysiol. 93:1174–1182. 2005. View Article : Google Scholar : PubMed/NCBI | |
|
Lampropoulos IC, Malli F, Sinani O, Gourgoulianis KI and Xiromerisiou G: Worldwide trends in mortality related to Parkinson's disease in the period of 1994–2019: Analysis of vital registration data from the WHO mortality database. Front Neurol. 13:9564402022. View Article : Google Scholar : PubMed/NCBI | |
|
Dick F, Nido GS, Alves GW, Tysnes OB, Nilsen GH, Dolle C and Tzoulis C: Differential transcript usage in the Parkinson's disease brain. PLoS Genet. 16:e10091822020. View Article : Google Scholar : PubMed/NCBI | |
|
Dolan ME, El Charif O, Wheeler HE, Gamazon ER, Ardeshir-Rouhani-Fard S, Monahan P, Feldman DR, Hamilton RJ, Vaughn DJ, Beard CJ, et al: Clinical and genome-wide analysis of cisplatin-induced peripheral neuropathy in survivors of adult-onset cancer. Clin Cancer Res. 23:5757–5768. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Ren B, Wan S, Wu H, Qu M, Chen Y, Liu L, Jin M, Zhou Z and Shen H: Effect of different iodine levels on the DNA methylation of PRKAA2, ITGA6, THEM4 and PRL genes in PI3K-AKT signaling pathway and population-based validation from autoimmune thyroiditis patients. Eur J Nutr. 61:3571–3583. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Wang Q, He Y, Shen Y, Zhang Q, Chen D, Zuo C, Qin J, Wang H, Wang J and Yu Y: Vitamin D inhibits COX-2 expression and inflammatory response by targeting thioesterase superfamily member 4. J Biol Chem. 289:11681–1194. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
Nambou K, Nie X, Tong Y and Anakpa M: Weighted gene co-expression network analysis and drug-gene interaction bioinformatics uncover key genes associated with various presentations of malaria infection in African children and major drug candidates. Infect Genet Evol. 89:1047232021. View Article : Google Scholar : PubMed/NCBI | |
|
Wang J, Tierney L, Wilson C, Phillips V, Goldman L, Mumaw C, Muang E and Walker CL: Carboxyl-terminal modulator protein (CTMP) deficiency mitigates denervation-induced skeletal muscle atrophy. Biochem Biophys Res Commun. 644:155–161. 2023. View Article : Google Scholar : PubMed/NCBI | |
|
Zhu M, Zheng R, Guo Y, Zhang Y and Zuo B: NDRG4 promotes myogenesis via Akt/CREB activation. Oncotarget. 8:101720–10134. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Wang J, Fry CME and Walker CL: Carboxyl-terminal modulator protein regulates Akt signaling during skeletal muscle atrophy in vitro and a mouse model of amyotrophic lateral sclerosis. Sci Rep. 9:39202019. View Article : Google Scholar : PubMed/NCBI | |
|
Sun X, Kellner M, Desai AA, Wang T, Lu Q, Kangath A, Qu N, Klinger C, Fratz S, Yuan JX, et al: Asymmetric dimethylarginine stimulates Akt1 phosphorylation via heat shock protein 70-facilitated carboxyl-terminal modulator protein degradation in pulmonary arterial endothelial cells. Am J Respir Cell Mol Biol. 55:275–287. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Lu Z, Tang F, Li Z, Xie Z, Zheng H, Zhang J, Gao Y, Lu Z, Cai Y, Lai Y and He Z: Characteristic genes and immune infiltration analysis for acute rejection after kidney transplantation. Dis Markers. 2022:65750522022. View Article : Google Scholar : PubMed/NCBI |