Ubiquitination of p21 by E3 ligase RNF135 promotes the proliferation of human glioblastoma cells
- Authors:
- Published online on: June 27, 2024 https://doi.org/10.3892/ol.2024.14539
- Article Number: 406
-
Copyright: © Gu et al. This is an open access article distributed under the terms of Creative Commons Attribution License.
Abstract
Introduction
Glioblastoma multiforme (GBM), also known simply as glioblastoma, is one of the most prevalent tumors of the central nervous system in adults. It originates from glial brain cells and is associated with an unfavorable prognosis (1,2). Glioblastoma is the most prevalent and lethal primary brain tumor in adults, with an estimated annual incidence of approximately three cases per 100,000 individuals (1,3). These tumors can be classified into two main categories based on the presence or absence of specific genetic mutations: Primary glioblastomas arise de novo, without a history of low-grade gliomas, while secondary glioblastomas result from the progression of low-grade gliomas to higher-grade tumors. In contrast to primary GBMs, TP53 mutations associated with methylation of the MGMT promoter are observed in most secondary GBMs, along with partial loss of heterozygosity of 10q, 13q, 19q, and 22q (4).
Despite the administration of optimal treatments, including radical surgical resection in conjunction with standard radiotherapy and chemotherapy, patients with GBM have a median survival duration of only ~16 months following diagnosis (5). In contrast to extracranial cancers, GBM has the capacity to infiltrate deeply into the surrounding brain tissue, with a low propensity for metastasis outside of the brain (6). The recurrence patterns and diffuse infiltration of GBM are in part attributable to the tortuous blood vessels of these tumors, which provide migration routes for tumor cells (7). At present, surgical intervention remains the primary treatment for GBM, with only a limited number of specific therapeutic agents being employed. Consequently, there is a pressing need for the identification of novel and efficacious targets.
GBM is highly heterogeneous, as evidenced by diverse histological features and alterations in epigenetics, genetics and transcriptomics (1). A number of biomarkers and therapeutic targets have been identified for GBM, including TP53 mutations, loss of heterozygosity 10q, PTEN mutations, EGFR amplification and the aberrant expression of E3 ubiquitin (Ub) ligases (E3s) (8–10). E3s are proteins that regulate the turnover and activity of numerous target proteins (10). Among them, RING finger E3s are crucial in maintaining the equilibrium between cell proliferation and apoptosis (11,12).
RING finger protein 135 (RNF135) is a member of the E3 family, which is characterized by an N-terminal RING finger domain and C-terminal SPRY and PRY motifs (13). A study of Schwann tumor cells from malignant peripheral nerve sheath tumors revealed the downregulation of RNF135, and postulated that RNF135 may contribute to the heightened malignant risk observed in patients with neurofibromatosis 1 gene microdeletion (14). In addition, another study identified that RNF135 is upregulated in glioblastoma tissue and demonstrated that RNF135 promotes the proliferation of human glioblastoma cells, primarily via the ERK pathway (10). However, the specific substrate targeted by RNF135 was not investigated in that study. In general, E3 ligases exert their biological functions mainly through the ubiquitination of substrates, so the underlying mechanism requires further elucidation. In the present study, the substrate of RNF135 was identified and its functions in GBM were extensively studied.
Materials and methods
Data analysis using public databases
Gene Expression Profiling Interactive Analysis 2 (GEPIA2; http://gepia2.cancer-pku.cn) is a tool for the analysis of gene expression data from The Cancer Genome Atlas (TCGA) and the Tissue Genotype Expression database (15). GEPIA2 was used to compare the mRNA expression of RNF135 in human GBM with that in normal tissue, and the P-value was calculated using an unpaired Student's t-test. A significant difference was defined as an absolute log2 fold change of ≥1 and P<0.05. GEPIA2 was also used to perform a prognostic value analysis of RNF135 by calculation of the overall survival rate of patients with GBM. The survival graph was generated directly by GEPIA2, using the log-rank test as the sole option for analysis. Hazard ratios with 95% confidence intervals and log-rank P-values were calculated.
Plasmid construction
Plasmids containing RNF135 or p21 with Myc or Flag tag were inserted into empty vectors, including pCDH, pCADNA3.0, pGEX4T-1, pET22b, pDEST32 or pDEST22 were purchased from Shanghai Cell Researcher Biotech Co., Ltd. The pGEX4T-1-RNF135 and pET22b-p21 vectors were employed to express recombinant proteins for the purpose of conducting a GST pull-down and in vitro ubiquitination assay. Mutations of p21 were generated using site-directed mutagenesis, as previously described (16). Table I presents the specific sequences of short hairpin RNAs (shRNAs) targeting RNF135 in pLKO.1 vector and p21 in pGPU6/Hygro vectors, which were also purchased from Shanghai Cell Researcher Biotech Co., Ltd.
Cell culture, transfection and reagents
The human U87 glioblastoma of unknown origin (cat. no. TCHu138) and U251 GBM cell lines, as well as the human 293T cell line, were purchased from The Cell Bank of Type Culture Collection of The Chinese Academy of Sciences. The authenticity of all three cell lines was validated through the use of short tandem repeat profiling, as detailed on the National Collection of Authenticated Cell Cultures website (https://www.cellbank.org.cn/). The cell lines were cultured in high-glucose Dulbecco's Modified Eagle Medium supplemented with 10% fetal bovine serum and 100 µg/l streptomycin/penicillin. The cells were transfected with the overexpression vectors and shRNA using Lipofectamine® 2000 (Thermo Fisher Scientific, Inc.) according to the manufacturer's instructions. The ratio of plasmid mass to Lipofectamine® 2000 volume was 1:1 (2 µg:2 µl). The two components were mixed together at room temperature for 20 min, after which they were added to cells for continued culture at 37°C. Stable cell lines were generated by selecting with puromycin (2 µg/ml; Beyotime Institute of Biotechnology) or hygromycin B (400 µg/ml; Beyotime Institute of Biotechnology) for ≥7 days.
Cycloheximide (CHX; Selleck Chemicals) was dissolved in dimethyl sulfoxide (Sigma-Aldrich; Merck KGaA) at a concentration of 100 mM and stored at −40°C. The U87 and U251 cells were treated with 100 µM CHX for 3 or 6 h at 37°C in an incubator before immunoblotting (IB) to assess protein degradation. For analysis of the p21 degradation pathway, U87 and U251 cells were treated with 1 µM proteasomal inhibitor bortezomib (BTZ; Selleck Chemicals) or 20 nM autophagy inhibitor bafilomycin (BAF; Selleck Chemicals) in combination with 100 µM CHX for 6 h at 37°C prior to IB analysis.
Cell proliferation assay
Stably transfected U87 and U251 cells were seeded into 96-well plates at a density of 5,000 cells per well. At the 0, 24, 48 and 72 h time points, where the 0 h time point was 6 h after seeding, cells were incubated with Cell Counting Kit-8 (CCK-8) solution (Beyotime Institute of Biotechnology) for 3 h. The absorbance was then measured using a microplate reader (Bio-Rad Laboratories, Inc.) at a wavelength of 450 nm. The experiments were conducted with six replicates and repeated at least three times.
Colony formation assay
The transfected U87 and U251 cells were seeded into 6-well plates at a density of 1,000 cells per well. After 7 days of incubation at 37°C, the cells were fixed with 4% paraformaldehyde (Sigma-Aldrich; Merck KGaA) for 15 min at room temperature, stained with 0.2% crystal violet (Beyotime Institute of Biotechnology) for 10 min at room temperature. The number of colonies was manually counted using a light microscope (CKX53; Olympus Corporation). A colony was considered to comprise ≥50 cells.
Recombinant protein purification
Recombinant proteins tagged with glutathione S-transferase (GST) or hexahistidine (His6) were purified from the BL21 Escherichia coli (E. coli) system, following previously described methods (16). Following induction with isopropyl-β-D-mercapto-galactopyranoside (Sangon Biotech Co., Ltd.) overnight at 4°C, the cells transfected with protein-encoding plasmids were centrifuged and lysed in phosphate-buffered saline (PBS; 137 mM NaCl, 2.7 mM KCl, 8 mM Na2HPO4 and 2 mM KH2PO4, pH 7.6). The samples were incubated with glutathione or Ni2+ affinity gels (Sangon Biotech Co., Ltd.), and eluted with 20 mM reduced L-glutathione solution (pH 8.0) or 400 mM imidazole solution (pH 8.0). The eluate was subsequently dialyzed in PBS buffer with 20% glycerol overnight at 4°C, after which aliquots were taken and stored at −80°C.
GST pull-down assay
GST-tagged protein (20 µg), His6-tagged protein (20 µg) and 50 µl Glutathione Sepharose™ 4B (Sangon Biotech Co., Ltd.) were incubated in 600 µl GST pull-down buffer [20 mM Tris-Cl, 1 mM EDTA, 5 mM MgCl2, 100 mM NaCl, 1% NP-40 and 1 mM dithiothreitol (DTT), pH 7. 6] overnight at 4°C, with the addition of 10 mg/ml of fresh bovine serum albumin (Beyotime Institute of Biotechnology). The samples were then centrifuged at 2,000 × g for 2 min at 4°C, and washed three times with GST pull-down buffer. The immunoprecipitates were denatured in 40 µl 2X SDS protein loading buffer at 100°C for 10 min before analysis by IB.
Yeast two-hybrid (Y2H) screening
The Y2H assay was performed as described previously (17). Briefly, empty vectors or pDEST32-RNF135 were co-transformed with pDEST22-p21/cyclin-dependent kinase inhibitor 1A (CDKN1A) into yeast strain MaV203 (Thermo Fisher Scientific, Inc.). The positive colonies were selected and tested for their ability to survive in SD-2 medium (deficient in leucine and tryptophan) and SD-4 medium (deficient in uracil, histidine, leucine and tryptophan) which were purchased from Shanghai Cell Researcher Biotech Co., Ltd. This was followed by staining with 5-bromo-4-chloro-3-indolyl-b-D-galactopyranoside (X-gal; Sangon Biotech Co., Ltd.).
Co-immunoprecipitation (Co-IP), immunoprecipitation (IP) and IB
For the Co-IP assay, transfected U87 and U251 cells were lysed in 600 µl Co-IP buffer (50 mM Tris-HCl, 5 mM EDTA, 150 mM NaCl and 1% NP-40, pH 7.5) supplemented with a fresh protease inhibitor cocktail (Roche Diagnostics GmbH). The cell lysates (500 µl) were then incubated overnight at 4°C with an anti-p21 antibody (1:100 dilution; 10355-1-AP; Wuhan Sanying Biotechnology; Proteintech Group, Inc.) and 25 µl Protein G magnetic beads (L-1002; Biolinkedin). For the IP assay, transfected cells were lysed in 600 µl RIPA buffer (150 mM NaCl, 50 mM Tris-HCl, 5 mM EDTA, 0.1% SDS and 1% NP-40, pH 7.5) containing a fresh protease inhibitor cocktail. Subsequently, the cell lysates (500 µl) were incubated with either the anti-p21 antibody or anti-Flag antibody (1:100 dilution; 66008-4-Ig; Proteintech Group, Inc.) and 25 µl Protein G magnetic beads overnight at 4°C. The immunoprecipitates were pelleted and washed three times with Co-IP or RIPA buffer as aforementioned, followed by denaturation for 10 min at 100°C in 40 µl 2X SDS protein loading buffer. Subsequently, the immunoprecipitates, along with inputs and other lysates, were subjected to 10% SDS-PAGE and transferred to polyvinylidene fluoride membranes (MilliporeSigma). After blocking with 5% non-fat milk at room temperature for 1 h, the membranes were incubated with the following primary antibodies overnight at 4°C: Anti-RNF135 (1:1,000 dilution; 25061-1-AP), anti-GAPDH (1:5,000 dilution; 60004-1-Ig), anti-p21 (1:1,000 dilution; 10355-1-AP), anti-p53 (1:1,000 dilution; 10442-1-AP), anti-Myc (1:5,000 dilution; 16286-1-AP), anti-His (1:5,000 dilution; 66005-1-Ig), anti-GST (1:10,000 dilution; HRP-66001), anti-Flag (1:2,000 dilution; 20543-1-AP), anti-hemagglutinin (1:2,000 dilution; 81290-1-RR) or anti-Ub (1:2,000, 10201-2-AP), all from Proteintech Group, Inc. The membranes were then washed three times with Tris-buffered saline with Tween-20 (50 mM Tris-HCl, 150 mM NaCl and 0.2% Tween-20, pH 8.0). The next day, the membranes were incubated with goat anti-mouse IgG (1:5,000 dilution; SA00001-1; Proteintech Group, Inc.) or goat anti-rabbit IgG (1:5,000 dilution; SA00001-2; Proteintech Group, Inc.) at room temperature for 2 h. High-signal ECL western blotting substrate (180–5001; Tanon Science & Technology Co., Ltd.) was used to visualize the signals. Images were captured using a Tanon 5200 imaging system (Tanon Science & Technology Co., Ltd.).
In vitro ubiquitination assay
An in vitro ubiquitination assay was conducted following the previously described method (16). Briefly, 20 ng His6-UBA1 (E1), 50 ng His6-UBCH5A (E2), 100 ng GST-RNF135 (E3), 100 ng His6-p21 and 20 ng His6-Ub were added to in vitro ubiquitination buffer (25 mM Tris-Cl, 5 mM MgCl2 and 100 mM NaCl, pH 7.6, supplemented with 0.5 mM DTT and 1 mM fresh ATP), brought to a final volume of 25 µl and incubated for 1 h at 37°C. Then, 100 ng Ub carboxyl-terminal hydrolase 2 catalytic core (Usp2cc; Shanghai Cell Researcher Biotech Co., Ltd.) was added to the mixture, which was then incubated at 37°C for 30 min. The ubiquitination of p21 was detected through IB analysis using an anti-p21 antibody.
Statistical analysis
Data were analyzed using GraphPad Prism 5 (GraphPad; Dotmatics) and expressed as the mean ± SD. Statistical significance was determined using unpaired Student's t-test or one-way ANOVA with Tukey's post hoc test. P<0.05 was considered to indicate a statistically significant difference.
Results
RNF135 promotes the proliferation of glioblastoma cells
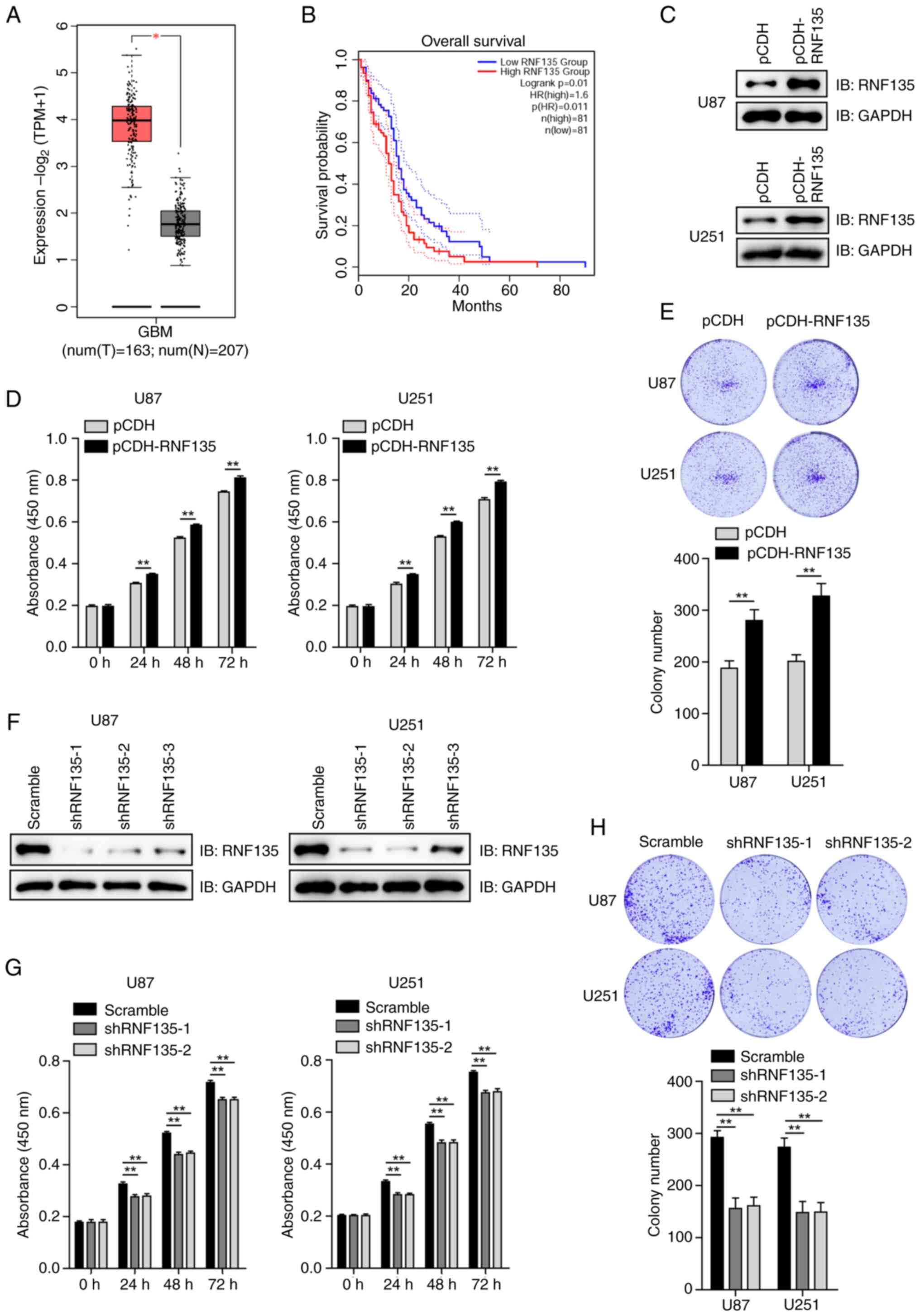
To investigate the potential role of RNF135 in GBM, TCGA data was analyzed using GEPIA2. At the mRNA level, the expression of RNF135 was found to be significantly upregulated in GBM tissues compared with relevant normal tissues (Fig. 1A). The association between RNF135 and the clinical outcome of GBM was also analyzed using GEPIA2. It was found that patients with GBM who exhibited high mRNA expression levels of RNF135 had poorer overall survival (Fig. 1B).
U87 and U251 cells were transfected with either an empty vector (pCDH) or pCDH-RNF135. The expression of RNF135 was detected using IB analysis, which confirmed that pCDH-RNF135 increased the expression of RNF135 (Fig. 1C). CCK-8 assays conducted at 24, 48 and 72 h revealed that cell growth was promoted in the U87 and U251 cells transfected with pCDH-RNF135 in comparison with that in the pCDH groups (Fig. 1D). Colony formation assays were also conducted, and the results were found to be consistent with those of the CCK-8 assays, showing that the overexpression of RNF135 increased colony formation (Fig. 1E).
Three shRNAs targeting RNF135 were designed and tested in U87 and U251 cells. The knockdown efficiencies were determined by IB analysis (Fig. 1F). shRNF135-1 and shRNF135-2 exhibited high knockdown efficacy and were therefore selected for use in further experiments. CCK-8 and colony formation assays demonstrated that cell growth and colony formation was inhibited in the RNF135-knockdown cells transfected with shRNF135-1 and shRNF135-2 in comparison with those in the negative control (scramble) groups (Fig. 1G and H). The results suggest that RNF135 plays a regulatory role in the proliferation of GBM cells.
RNF135 interacts with and mediates the degradation of p21
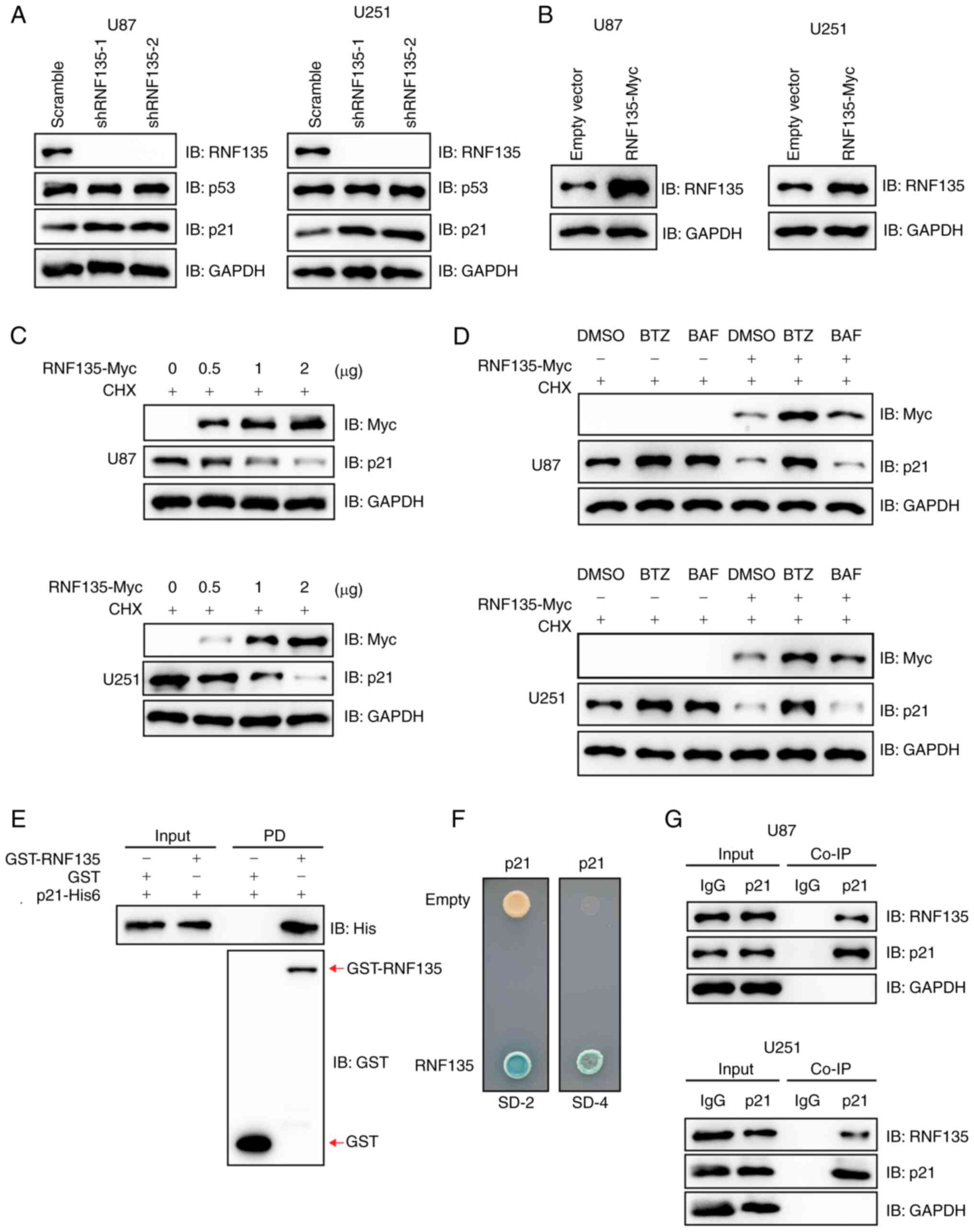
The mechanism by which RNF135 controls the proliferation of GBM cells is unknown. As the p53-p21 signaling pathway is the most classic cell cycle regulatory pathway (18), the effect of RNF135 on p53 and p21 was investigated. In the RNF135-knockdown U87 and U251 cells, the protein levels of cell cycle-related p21 were markedly increased compared with those in the scramble groups, whereas p53 protein levels did not show any clear changes following RNF135 knockdown (Fig. 2A). The impact of RNF135 on the stability of p21 protein was then examined. A pCDNA3.0-RNF135-Myc plasmid was constructed and transiently transfected into U87 and U251 cells. The overexpression of RNF135 was then confirmed by IB analysis by comparison with cells transfected with empty vector (Fig. 2B). Subsequently, U87 and U251 cells were transiently transfected with varying amounts of the RNF135-Myc plasmid and incubated with CHX. The results of IB analysis indicated that RNF135 promoted the degradation of p21 in a concentration-dependent manner (Fig. 2C). These findings indicate that RNF135 facilitates the degradation of p21.
RNF135-mediated degradation of p21 proceeds primarily through the proteasomal pathway
U87 and U251 cells transfected with plasmids encoding RNF135 or with empty vector were treated with the CHX and either proteasomal inhibitor BTZ or the autophagy inhibitor BAF for 6 h, and then subjected to IB analysis. The results in Fig. 2D demonstrate that RNF135-mediated p21 degradation occurs primarily via the proteasomal pathway.
Recombinant RNF135 and p21 proteins were purified from an E. coli system. The GST pull-down assay demonstrated that GST-tagged RNF135 directly interacted with His6-tagged p21 in vitro (Fig. 2E). The Y2H technology employs nutrient deficiency to monitor gene expression and protein interactions. The RNF135 gene was cloned into pDEST32, which has a fused activating domain (AD), while the p21/CDKN1A gene was cloned into pDEST22, which has a fused DNA binding domain (BD). It is only when the RNF135 and p21 genes are both expressed and AD and BC interact that downstream gene expression is initiated, allowing the yeast to grow on SD-4 plates and be stained by X-gal. The results confirmed the interaction between RNF135 and p21 (Fig. 2F). Furthermore, Co-IP assays utilizing anti-p21 antibodies demonstrated that endogenous RNF135 formed complexes with p21 in both U87 and U251 cells (Fig. 2G). These findings indicate that RNF135 is an interacting partner for p21 and promotes its degradation primarily through the proteasomal pathway.
RNF135 mediates the ubiquitination of p21
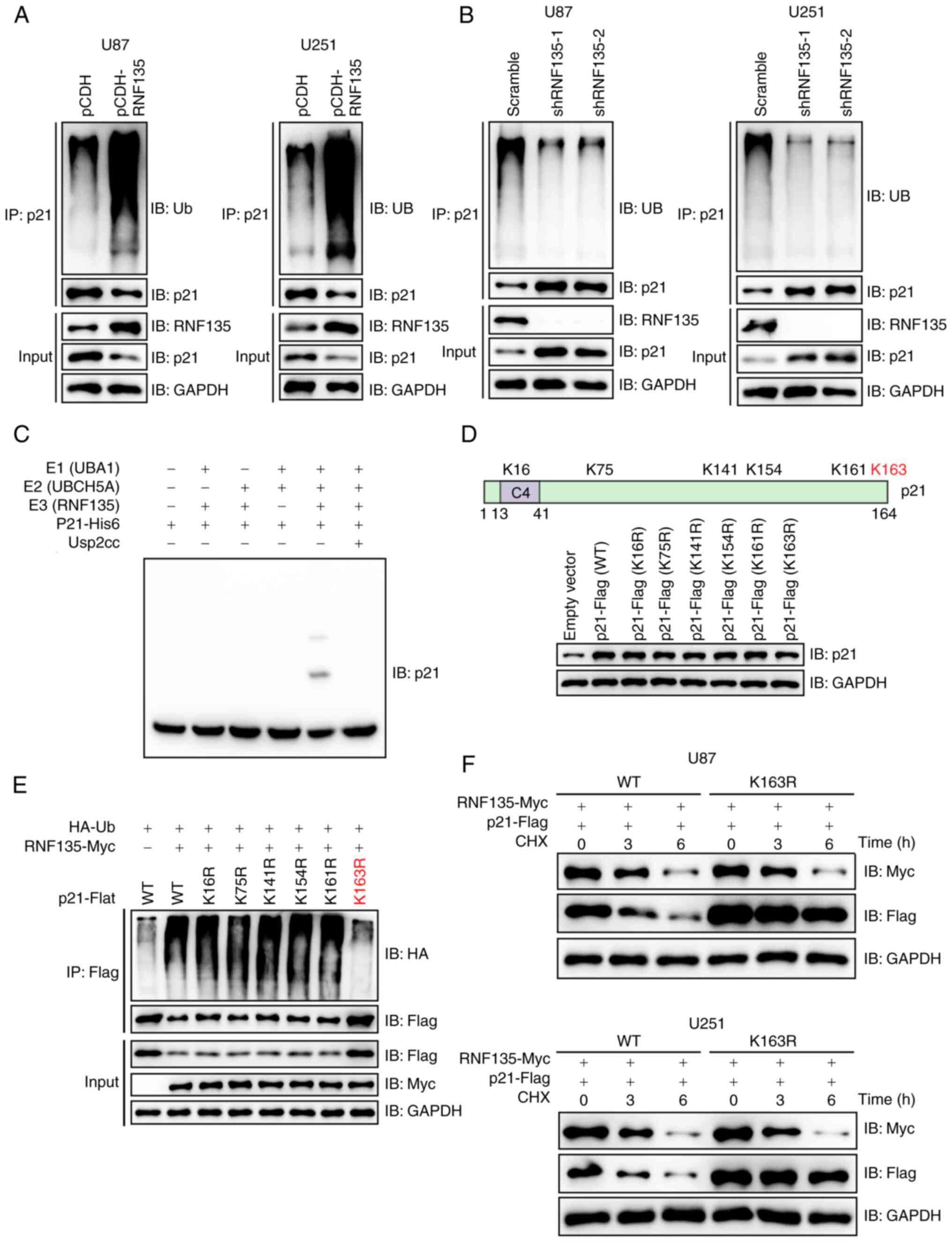
Several experiments were conducted to ascertain whether RNF135 is an E3 ligase for p21. In U87 and U251 cells stably expressing pCDH-RNF135, there was a clear increase in the ubiquitination of p21 compared with that in control cells stably transfected with the empty vector pCDH (Fig. 3A). Conversely, RNF135 knockdown resulted in a reduction in the ubiquitination of p21 and an increase in p21 protein levels in both U87 and U251 cells (Fig. 3B). An in vitro ubiquitination assay was then performed to detect the ubiquitination of p21 in the presence of E1 (UBA1), E2 (UBCH5A) and E3 (RNF135) by IB. The modification induced by the combination of these three enzymes was efficiently eliminated by Usp2cc, the catalytic core of the human deubiquitinase enzyme USP2 (Fig. 3C). The p21 protein contains six lysine (K) residues (Fig. 3D). To map the RNF135-mediated ubiquitination site on p21, pCDNA3.0-p21-Flag and several mutant plasmids were constructed, transiently transfected into 293T cells, and the p21 expression levels of the cells were detected by IB analysis (Fig. 3D). K163 was identified as the primary site for RNF135-mediated ubiquitination. This was confirmed by the p21 mutant K163R, with a lysine-to-arginine (K-to-R) substitution, which showed almost complete resistance to RNF135-mediated ubiquitination on p21 (Fig. 3E). Further investigation revealed that RNF135 facilitated the degradation of wild-type p21 protein, but had a minimal effect on K163R mutant p21 protein in both U87 and U251 cells (Fig. 3F), which corroborates the importance of this site. In conclusion, the results demonstrate that p21 is a substrate of RNF135.
RNF135 promotes the proliferation of GBM cells mainly though p21
Further experiments were performed to investigate whether RNF135 promotes the proliferation of GBM cells in a p21-dependent manner. Three shRNAs were designed to target p21 and transfected into U87 and U251 cells. The knockdown efficiencies were detected through IB analysis, as shown in Fig. 4A. Two of the shRNAs, shp21-2 and shp21-3, exhibited high knockdown efficacy and were thus selected for use in further experiments. U87 and U251 cells stably expressing scramble shRNA, shp21-2 or shp21-3 were stably transfected with either empty vector (pCDH) or pCDH-RNF135. The protein levels of RNF135 and p21 were then detected by IB analysis (Fig. 4B). The results of CCK-8 assays conducted at 24, 48 or 72 h demonstrated that RNF135 suppressed the proliferation of U87 and U251 cells with intact p21, but not that of p21-knockdown cells (Fig. 4C). Colony formation assays were also conducted, and the results were consistent with those of the CCK-8 assays, showing that RNF135 inhibited the colony formation only of cells with intact p21 (Fig. 4D). These data suggests that RNF135 promotes the proliferation of GBM cells in a p21-dependent manner.
Discussion
In the present study, analyses performed using the GEPIA2 public database revealed that RNF135 was upregulated in human GBM and served as a prognostic marker for overall survival. Furthermore, in vitro, the overexpression of RNF135 was found to promote the proliferation of GBM cells, while the knockdown of RNF135 inhibited it, which is consistent with a previous study (10). These findings provide evidence to suggest that RNF135 may act as an oncogene in human GBM.
The Ub-proteasome pathway is a selective protein degradation pathway that breaks down intracellular proteins. It plays a crucial role in cell proliferation, differentiation, apoptosis, autophagy and other cellular processes (19–21). Dysfunctions in ubiquitination have been found to be associated with a range of issues, including cancers and neurodegenerative diseases (17,22,23). RNF135 is a RING-type E3 ubiquitin ligase with an N-terminal RING domain. It has been reported that RNF135 ubiquitinates the retinoic acid-inducible gene-I protein, and increases its ability to transmit signals, resulting in the production of antiviral IFN (13). It has also been identified that RNF135 is upregulated in glioblastoma tissue and promotes the proliferation of human glioblastoma cells via ERK (10). However, the underlying mechanism remains incompletely understood. In the present study, the cell cycle inhibitor CDKN1A/p21, which is involved in the tumor suppressor p53-associated signaling pathway, was identified as a novel substrate for RNF135. The results of the present study also suggest that RNF135 promotes the proliferation of GBM cells mainly via p21.
The cyclin-dependent kinase inhibitor CDKN1A/p21 is a pivotal downstream effector of p53, which acts as a cell cycle inhibitor and anti-proliferative effector in normal cells (24). Dysregulation of this protein has been found to be common in numerous types of cancer, and it has been shown to affect several cellular processes including apoptosis, DNA damage response and actin cytoskeleton remodeling (25,26). In response to p53 transcription factor activity, p21 induction may result in tumor growth arrest through the inhibition of cyclin-dependent kinase complexes, proliferation cell nuclear antigen, transcription factors and coactivators (25). The overexpression of p21 has been demonstrated to promote cell death and induce senescence in human glioblastoma (27). Consistent with this, the present study has confirmed that p21-knockdown promotes the proliferation of GBM cells.
To date, >10 E3 ligases for p21 have been reported, including MDM2, CHIP, makorin-1 and TRIM21 (28). The ubiquitination and degradation of p21 play an important role in regulation of the cell cycle and tumorigenesis (29). The findings of the present study indicate that the RNF135-p21 axis contributes to GBM cell proliferation, suggesting that RNF135 may serve as a therapeutic target for the treatment of GBM. The present study demonstrated that the knockdown of RNF135 significantly inhibited the proliferation of GBM cells. Consequently, it is possible that agents that inhibit RNF135 activity may have the potential to be developed for the treatment of GBM.
It must be noted that there are certain limitations to the present study. For example, the absence of data from animal experiments and clinical data limits the translatability of the research. In addition, the construction of RNF135 knockout cell lines was unsuccessful using a CRISPR-based method; therefore, only shRNAs for RNF135 were used and rescue experiments were omitted. Furthermore, the RNF135 antibody used in the study is not compatible with immunofluorescence assay, so it was not possible to perform immunofluorescence experiments. Despite these limitations, the present study elucidated the molecular mechanism by which RNF135 regulates GBM cell proliferation and identified a potential therapeutic target for the treatment of GBM.
Acknowledgements
Not applicable.
Funding
The present study was funded by the National Natural Science Foundation of China (grant no. 81972339) and the Shanghai Municipal Science and Technology Commission (grant no. 18XD1403400).
Data availability
The data generated in the present study may be requested from the corresponding author.
Authors' contributions
YC and ZW designed and supervised the project. WG and MW performed experiments and data analysis. WG, YC and ZW wrote the manuscript. YC and ZW confirm the authenticity of all the raw data. All authors read and approved the manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
|
Isachesku E, Braicu C, Pirlog R, Kocijancic A, Busuioc C, Pruteanu LL, Pandey DP and Berindan-Neagoe I: The role of non-coding RNAs in epigenetic dysregulation in glioblastoma development. Int J Mol Sci. 24:163202023. View Article : Google Scholar : PubMed/NCBI | |
|
Tu W, Zheng H, Li L, Zhou C, Feng M, Chen L, Li D, Chen X, Hao B, Sun H, et al: Secreted phosphoprotein 1 promotes angiogenesis of glioblastoma through upregulating PSMA expression via transcription factor HIF1α. Acta Biochim Biophys Sin (Shanghai). 55:417–425. 2022.PubMed/NCBI | |
|
Chen B, Chen C, Zhang Y and Xu J: Recent incidence trend of elderly patients with glioblastoma in the United States, 2000–2017. BMC Cancer. 21:542021. View Article : Google Scholar : PubMed/NCBI | |
|
Delgado-Martin B and Medina MÁ: Advances in the knowledge of the molecular biology of glioblastoma and its impact in patient diagnosis, stratification, and treatment. Adv Sci (Weinh). 7:19029712020. View Article : Google Scholar : PubMed/NCBI | |
|
Khaddour K, Johanns TM and Ansstas G: The landscape of novel therapeutics and challenges in glioblastoma multiforme: Contemporary state and future directions. Pharmaceuticals (Basel). 13:3892020. View Article : Google Scholar : PubMed/NCBI | |
|
Li C, Wang S, Yan JL, Torheim T, Boonzaier NR, Sinha R, Matys T, Markowetz F and Price SJ: Characterizing tumor invasiveness of glioblastoma using multiparametric magnetic resonance imaging. J Neurosurg. 132:1465–1472. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Cuddapah VA, Robel S, Watkins S and Sontheimer H: A neurocentric perspective on glioma invasion. Nat Rev Neurosci. 15:455–465. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
Verhaak RG, Hoadley KA, Purdom E, Wang V, Qi Y, Wilkerson MD, Miller CR, Ding L, Golub T, Mesirov JP, et al: Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell. 17:98–110. 2010. View Article : Google Scholar : PubMed/NCBI | |
|
Sun Y: E3 ubiquitin ligases as cancer targets and biomarkers. Neoplasia. 8:645–654. 2006. View Article : Google Scholar : PubMed/NCBI | |
|
Liu Y, Wang F, Liu Y, Yao Y, Lv X, Dong B, Li J, Ren S, Yao Y and Xu Y: RNF135, RING finger protein, promotes the proliferation of human glioblastoma cells in vivo and in vitro via the ERK pathway. Sci Rep. 6:206422016. View Article : Google Scholar : PubMed/NCBI | |
|
Feng X, Song D, Liu X, Liang Y, Jiang P, Wu S and Liu F: RNF125-mediated ubiquitination of MCM6 regulates the proliferation of human liver hepatocellular carcinoma cells. Oncol Lett. 27:1052024. View Article : Google Scholar : PubMed/NCBI | |
|
Jia C, Tang H, Yang Y, Yuan S, Han T, Fang M, Huang S, Hu R, Li C and Geng W: Ubiquitination of IGF2BP3 by E3 ligase MKRN2 regulates the proliferation and migration of human neuroblastoma SHSY5Y cells. Biochem Biophys Res Commun. 529:43–50. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Oshiumi H, Matsumoto M, Hatakeyama S and Seya T: Riplet/RNF135, a RING finger protein, ubiquitinates RIG-I to promote interferon-beta induction during the early phase of viral infection. J Biol Chem. 284:807–817. 2009. View Article : Google Scholar : PubMed/NCBI | |
|
Pasmant E, Masliah-Planchon J, Lévy P, Laurendeau I, Ortonne N, Parfait B, Valeyrie-Allanore L, Leroy K, Wolkenstein P, Vidaud M, et al: Identification of genes potentially involved in the increased risk of malignancy in NF1-microdeleted patients. Mol Med. 17:79–87. 2011. View Article : Google Scholar : PubMed/NCBI | |
|
Tang Z, Li C, Kang B, Gao G, Li C and Zhang Z: GEPIA: A web server for cancer and normal gene expression profiling and interactive analyses. Nucleic Acids Res. 45 (W1):W98–W102. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Li C, Han T, Li Q, Zhang M, Guo R, Yang Y, Lu W, Li Z, Peng C, Wu P, et al: MKRN3-mediated ubiquitination of Poly(A)-binding proteins modulates the stability and translation of GNRH1 mRNA in mammalian puberty. Nucleic Acids Res. 49:3796–3813. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Xu X, Li C, Gao X, Xia K, Guo H, Li Y, Hao Z, Zhang L, Gao D, Xu C, et al: Excessive UBE3A dosage impairs retinoic acid signaling and synaptic plasticity in autism spectrum disorders. Cell Res. 28:48–68. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Engeland K: Cell cycle regulation: p53-p21-RB signaling. Cell Death Differ. 29:946–960. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Mansour MA: Ubiquitination: Friend and foe in cancer. Int J Biochem Cell Biol. 101:80–93. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Wang CC, Peng H, Wang Z, Yang J, Hu RG, Li CY and Geng WJ: TRIM72-mediated degradation of the short form of p62/SQSTM1 rheostatically controls selective autophagy in human cells. Mil Med Res. 9:352022.PubMed/NCBI | |
|
Yang Y, Luo Y, Yang C, Hu R, Qin X and Li C: TRIM25-mediated ubiquitination of G3BP1 regulates the proliferation and migration of human neuroblastoma cells. Biochim Biophys Acta Gene Regul Mech. 1866:1949542023. View Article : Google Scholar : PubMed/NCBI | |
|
Liu Z, Chen P, Gao H, Gu Y, Yang J, Peng H, Xu X, Wang H, Yang M, Liu X, et al: Ubiquitylation of autophagy receptor Optineurin by HACE1 activates selective autophagy for tumor suppression. Cancer Cell. 26:106–120. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
Li C, Lu W, Yang L, Li Z, Zhou X, Guo R, Wang J, Wu Z, Dong Z, Ning G, et al: MKRN3 regulates the epigenetic switch of mammalian puberty via ubiquitination of MBD3. Natl Sci Rev. 7:671–685. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Abbas T and Dutta A: p21 in cancer: Intricate networks and multiple activities. Nat Rev Cancer. 9:400–414. 2009. View Article : Google Scholar : PubMed/NCBI | |
|
Shamloo B and Usluer S: p21 in cancer research. Cancers (Basel). 11:11782019. View Article : Google Scholar : PubMed/NCBI | |
|
Wu L, Yang Z, Dai G, Fan B, Yuan J, Liu Y, Liu P and Ou Z: SOX5 promotes cell growth and migration through modulating the DNMT1/p21 pathway in bladder cancer. Acta Biochim Biophys Sin (Shanghai). 54:987–998. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Mansour MA, Rahman M, Ayad AA, Warrington AE and Burns TC: P21 overexpression promotes cell death and induces senescence in human glioblastoma. Cancers (Basel). 15:12792023. View Article : Google Scholar : PubMed/NCBI | |
|
Wang F, Wu Z, Li Q, Ni Z, Wang C and Lu J: Ubiquitination of p21 by E3 ligase TRIM21 promotes the proliferation of human neuroblastoma cells. Neuromolecular Med. 23:549–560. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Dang F, Nie L and Wei W: Ubiquitin signaling in cell cycle control and tumorigenesis. Cell Death Differ. 28:427–438. 2021. View Article : Google Scholar : PubMed/NCBI |