Purine metabolite inosine induced by transforming growth factor‑β promotes epithelial‑mesenchymal transition in colorectal cancer
- Authors:
- Published online on: July 1, 2024 https://doi.org/10.3892/ol.2024.14549
- Article Number: 416
-
Copyright: © Hu et al. This is an open access article distributed under the terms of Creative Commons Attribution License.
Abstract
Introduction
Colorectal cancer (CRC) is a prevalent malignant tumor that ranks as the third most frequently diagnosed malignant tumor globally, according to statistical data (1). Despite advancements in available treatment options, the prognosis for CRC remains unfavorable. A total of 50–60% of patients are diagnosed with metastasis, primarily in the liver, making them unsuitable candidates for surgical intervention and resulting in mortality (2). Furthermore, although there is an ongoing introduction of innovative pharmaceuticals and therapeutic approaches, CRC continues to exhibit a high mortality rate worldwide (13.7/100,000) (3). Consequently, CRC presents a substantial risk to human health, emphasizing the crucial and urgent requirement to elucidate the molecular mechanisms that contribute to the onset and progression of CRC (4).
The transforming growth factor-β (TGF-β) signaling pathway serves a crucial role in a multitude of biological processes, including but not limited to proliferation, migration, apoptosis, differentiation and immune responses (5,6). Consequently, the dysregulation of the TGF-β signaling pathway, caused by genetic mutations or abnormal expression, has been implicated in the pathogenesis of several malignancies, including CRC (7–9). Numerous studies have reported that the dysfunction of the TGF-β signaling pathway exhibits a dual functionality, acting as a suppressor during the early stages and as a promoter during the late stages of CRC (10). Additionally, the TGF-β signaling pathway serves a significant role in the initiation and progression of cancer through its regulation of several cellular processes, such as epithelial-mesenchymal transition (EMT) (11,12). EMT is the process by which epithelial cells undergo a transformation into a mesenchymal phenotype, increasing their ability to migrate (13). Several studies have reported that EMT can cause epithelial tumor cells to take on the characteristics of stromal cells, leading to increased invasion and migration (14,15). Therefore, TGF-β-induced EMT is crucial for the TGF-β signaling pathway to promote tumor progression in patients with CRC (16).
Furthermore, accumulating evidence indicates that metabolic reprogramming is prevalent in the majority of cancers, including CRC. It has been established in previous studies that cancer is a metabolic disease characterized by abnormal metabolic alterations (17–19). In recent years, there has been significant progress in the field of tumor metabolism, surpassing the understanding of the Warburg effect and recognizing the intricate metabolic complexity of tumors (20). Increasing evidence suggests that tumor cells have the ability to independently alter their metabolic pathways to fulfill the increased requirements for tumor growth, recurrence, metastasis and resistance to therapy (21).
Recently, there is substantial evidence supporting the role of the TGF-β signaling pathway as a metabolic modulator, capable of remodeling the metabolism of glucose, lipids and amino acids in several cell types, including tumor cells (22–24). Consequently, during the process of TGF-β-induced EMT, tumor cells may modulate their metabolism to meet the increased demand for EMT (25,26). Nevertheless, the precise metabolic reprogramming and essential metabolites which are critical for TGF-β-induced EMT in CRC have not been thoroughly explored. The present study assessed the impact of TGF-β1 on the metabolism of CRC cells and identified the purine metabolite inosine responsible for regulating the EMT induced by the TGF-β1. The present study also provided insights into the regulation of inosine and demonstrated its significance in EMT phenotypes.
Materials and methods
Cell culture and transfection
Human colonic carcinoma SW480 cells (American Type Culture Collection) were maintained in RPMI 1640 medium (Gibco; Thermo Fisher Scientific, Inc.) supplemented with 10% fetal bovine serum (Gibco; Thermo Fisher Scientific, Inc.). The cells were incubated at 37°C in a humidified atmosphere with 5% CO2. Once the cells reached 80–90% confluency, they were subcultured. The SW480 cells were exposed to recombinant human TGF-β1 protein (Abcam) at varying concentrations (10 and 20 ng/ml) for 72 h. The images of cell morphology were captured using a light microscope (Leica Microsystems GmbH).
SW480 cells were transfected with 5 µg pCDNA3.1 plasmids (Invitrogen™; Thermo Fisher Scientific, Inc.) using Lipofectamine™ Transfection Reagent (Invitrogen™; Thermo Fisher Scientific, Inc.) to upregulate the expression levels of LACC1, and SW480 cells were transfected with 5 µg small hairpin (sh)RNAs lentiviral vectors pLKO.1 (Sigma-Aldrich) using Lipofectamine™ Transfection Reagent (Invitrogen™; Thermo Fisher Scientific, Inc.) to downregulate the expression levels of LACC1. Cells were incubated at 37°C for 4 h and harvested 48 h after transfection for analysis. For the lentiviral transfection procedure, pLVpro-MSCV (Takara Biotechnology Co. Ltd.) overexpressing vectors and pLKO.1 (Sigma-Aldrich) shRNA vectors were used for lentivirus packing (lentivirus plasmids, packaging plasmids and envelope plasmids in a ratio of 4:3:1). The lentiviruses were then co-transfected into 293T cells (American Type Culture Collection) using Lipofectamine™ Transfection Reagent (Invitrogen™; Thermo Fisher Scientific, Inc.). The cell culture medium was replaced 12 h post-transfection. After 48 h, the lentiviruses were harvested and centrifuged at 4°C, 250 × g for 5 min to remove cell debris. The resulting supernatant was collected and centrifuged at 4°C, 9,000 × g for 2 h. Subsequently, SW480 cells were infected with the lentiviruses at a multiplicity of infection of 30. The cell culture medium was changed after 12 h post-infection. Following 48 h of infection, the cells were subjected to stable selection with 1 µg/ml puromycin (Invitrogen™; Thermo Fisher Scientific, Inc.). Resistant cells were harvested after 5 days for further cultivation, with a maintenance concentration of 0.5 µg/ml puromycin.
SW480 cells treated with TGF-β1 (20 ng/ml) were utilized for the cloning of LACC1. The total RNA from the cells was isolated using TRIzol™ (Invitrogen; Thermo Fisher Scientific, Inc.), followed by cDNA synthesis employing the PrimeScript™ RT reagent Kit (Takara Biotechnology Co. Ltd.). The reaction was conducted at 37°C for 15 min and 85°C for 5 sec. PCR amplification was carried out using clone PCR primers and PrimeSTAR® HS DNA Polymerase (Takara Biotechnology Co. Ltd.). The thermocycling conditions were as follows: (98°C, 10 sec; 68°C, 5 min) 30 cycles. The resulting PCR product was then analyzed through agarose electrophoresis (1%), and the gel block containing the LACC1 gene fragment was excised for gel recovery. Clone PCR primers were designed using SnapGene 4.1.8 (GSL Biotech LLC.). The sequence used for cloning LACC1 was as follows: Forward, 5′-ATGGCAGAAGCTGTTTTGAT-3′ and reverse, 5′-TCATTCTTTAATTGATATGA-3′. Moreover, sequences for enzyme cleavage sites and protective bases were added. The sequences of the clone PCR primers were as follows: LACC1 clone PCR primers: Forward, 5′-ATGGCGAGCTCATGGCAGAAGCTGTTTTGAT-3′ and reverse, 5′-ATGGCGGGCCCTCATTCTTTAATTGATATGA-3′.
shRNAs were designed using SnapGene 4.1.8 (GSL Biotech LLC.). The sequence used for interfering LACC1 was as follows: 5′-GGAAGACATTGTTGTTGTACT-3′. The designed sequences were as follows: Left, 5′-CCGG-3′; sense, 5′-GGAAGACATTGTTGTTGTACT-3′; loop, 5′-CTCGAG-3′; antisense, 5′-AGTACAACAACAATGTCTTCC-3′; right, 5′-TTTTTG-3′. The sequences of the shRNAs were as follows: LACC1 shRNA, 5′-CCGGGGAAGACATTGTTGTTGTACTCTCGAGAGTACAACAACAATGTCTTCCTTTTTG-3′ and control shRNA, 5′-CCGGCAACAAGATGAAGAGCACCAACTCGAGTTGGTGCTCTTCATCTTGTTGTTTTTG-3′.
Metabolomics assay
The samples (SW480 cells and SW480 cells treated with 20 ng/ml TGF-β1) were thawed on ice and then 100 µl of each sample was transferred into 1.5 ml centrifuge tubes using a pipette. Protein extraction and precipitation were performed by adding 300 µl methanol to all samples, followed by the addition of 10 µl of an internal standard (DL-p-Chlorophenylalanine; Shanghai Aladdin Biochemical Technology Co., Ltd.; 2.9 mg/ml). The samples were then vortexed for 30 sec and centrifuged at 13,400 × g at 4°C for 15 min. Subsequently, 200 µl of the supernatant was transferred to a vial for analysis. Metabolite profiling was performed using a liquid chromatography-mass spectrometry system consisting of a Waters Acquity UPLC (Waters Corporation) and a Q Exactive™ (Thermo Fisher Scientific, Inc.). Column: ACQUITY UPLC HSS T3 (2.1×100 mm; 1.8 µm; Waters Corporation); Column temperature: 40°C; flow rate: 0.3 ml/min; mobile phase A: Water + 0.05% formic acid; mobile phase B: Acetonitrile; injection volume: 5 µl; automatic injector temperature: 4°C. The Q Exactive platform used an electric spray ionization source and operated in both positive ion mode (POS) and negative ion mode (NEG). The following conditions were used for ESI+: Heater Temp 300°C; curtained Air: 30 psi; sheath gas flow rate, 45 arb; aux gas flow rate, 15 arb; sweep gas flow rate, 1 arb; spray voltage, 3.0 KV; capillary temp, 350°C; S-Lens RF level, 30%. The following conditions were used for ESI-: Heater Temp 300°C; curtained air: 30 psi; sheath gas flow rate, 45 arb; aux gas flow rate, 15 arb; sweep gas flow rate, 1 arb; spray voltage, 3.2 KV; capillary temp, 350°C; S-Lens RF level, 60%. The details for the scanning mode included: First level full scan (m/z 70-1,050); resolution, 70,000 (primary mass spectrometry) and 17,500 (secondary mass spectrometry); collision mode: high energy collision dissociation (HCD). In the subsequent data analysis process, the POS and NEG data was analyzed separately, including the retention time, compound molecular weight observations (samples) and peak intensity. The metabolites were characterized by matching their retention times and m/z values with the National Institute of Science and Technology database (https://www.nist.gov). The original metabolite profiling results are deposited in figshare (https://doi.org/10.6084/m9.figshare.25661646).
RNA sequencing (RNA-Seq) assay
In the present study, total RNA was extracted from cells using a RNeasy mini kit (cat. no. 74104; Qiagen GmbH). The concentration and quality of the RNA were assessed using the Qubit 2.0 Fluorometer and the NanoDrop™ One Microvolume UV–Vis spectrophotometer (Thermo Fisher Scientific, Inc.). The integrity of the total RNA was evaluated using the Agilent 2100 Bioanalyzer (Agilent Technologies, Inc.) and only samples with RNA integrity number values >7.0 were included for sequencing. For the RNA sample preparations, 1 µg RNA was used as input material. Strand-specific RNA-seq libraries were constructed using the VAHTS Universal V6 RNA-seq Library Prep Kit for Illumina® (cat. no. NR604-02; Vazyme Biotech Co., Ltd.) following the manufacturer's instructions. The purified libraries were quantified and validated using the Qubit 2.0 Fluorometer and Agilent 2100 Bioanalyzer to confirm the insert size and calculate the mole concentration. The library was diluted to 10 pmol (quantified using the Qubit 2.0 Fluorometer (Invitrogen™; Thermo Fisher Scientific, Inc.) and then subjected to cluster generation using cBot (Illumina, Inc.). Subsequently, the libraries were sequenced on the Illumina NovaSeq 6000 Sequencing System (Illumina, Inc.), and the paired-end 150 bp program was chosen for the purpose of conducting dual end sequencing. The resulting paired RNA reads were mapped to the human GRCh38 genome. Differential expression analysis for mRNA was performed using the R package ‘EdgeR 3.30.3’ (27). Genes showing differential expression with an absolute log2(fold change) >1 and P<0.05 were considered for further analysis. The original sequencing results have been deposited in the Sequence Read Archive under accession number PRJNA1040341.
Integrative pathway analyses
The construction of an integrated pathway analysis involving metabolites and genes was performed using the R package ‘Pathview 1.28.1’ (28). This analysis utilized the Kyoto Encyclopedia of Genes and Genomes database (https://www.kegg.jp) to create a knowledge graph representation, and this representation formed a pathway graph that connected the query of metabolites and genes.
Measurements of metabolites levels
To quantify inosine levels, 5×106 cells were homogenized in ice-cold perchloric acid. The resulting extracts were subsequently centrifuged at 10,000 × g at 4°C for 10 min, and the supernatant was neutralized by the addition of ice-cold potassium carbonate for a duration of 10 min. Following another round of centrifugation, the inosine levels within the cells were assessed using the Inosine Quantification Assay Kit (cat. no. ab126286; Abcam) in accordance with the provided guidelines.
Cell migration assay
The migration of cells was detected using Transwell-chamber culture systems (Becton, Dickinson and Company). After a 48-h incubation at 37°C, the cells were transferred into the upper Transwell chamber (1×105 cells/well in an 8 µm 24-well Transwell insert). Following a 24-h incubation in serum-free RPMI 1640 medium (Gibco; Thermo Fisher Scientific, Inc.; both upper and lower chamber), cells on the upper surface of the upper chamber (non-migrated cells) were removed using cotton swabs and air-dried, and cells on the lower surface of the filters were fixed and stained with Giemsa stain at room temperature for 30 min. A total of three random views were selected and the number of migrated cells were counted using a light microscope (Leica Microsystems GmbH).
Reverse transcription(RT)-quantitative (q)PCR assay
Total RNA of cells were extracted using TRIzol™ (Invitrogen; Thermo Fisher Scientific, Inc.). A total of 1 µg total RNA was used for cDNA synthesis using a PrimeScript™ RT reagent Kit (cat. no. RR037A; Takara Biotechnology Co. Ltd.). The reaction was conducted at 37°C for 15 min and 85°C for 5 sec. Subsequently, the cDNA was used for qPCR using the SYBR® Green Realtime PCR Master Mix (Toyobo Life Science). qPCR was performed by using Applied Biosystems ViiA™ 7 Real-Time PCR System (Thermo Fisher Scientific, Inc.). The thermocycling conditions were as follows: 50°C, 2 min; 95°C, 10 min; (95°C, 15 sec; 60°C, 1 min) 40 cycles. The mRNA expression levels were normalized to GAPDH and quantified using the 2−ΔΔCq method (29).
The mRNA qPCR primers were as follows: E-cadherin: Forward, 5′-GGGGTCTGTCATGGAAGGTG-3′ and reverse, 5′-CAAAATCCAAGCCCGTGGTG-3′; Vimentin: Forward, 5′-AAGTCCGCACATTCGAGCAA-3′ and reverse, 5′-GGTGGACGTAGTCACGTAGC-3′; zonula occludents-1 (ZO-1): Forward, 5′-AGCCATTCCCGAAGGAGTTG-3′ and reverse, 5′-ATCACAGTGTGGTAAGCGCA-3′; N-cadherin: Forward, 5′-GCGTCTGTAGAGGCTTCTGG-3′ and reverse, 5′-GCCACTTGCCACTTTTCCTG-3′; LACC1: Forward, 5′-GCCGAGGCGGGTGATTTATT-3′ and reverse, 5′-CCATGCTGGGACAGCTAACA-3′; and GAPDH: Forward, 5′-TGACTTCAACAGCGACACCCA-3′ and reverse, 5′-CACCCTGTTGCTGTAGCCAAA-3′.
Western blotting assay
The cells were harvested and lysed by RIPA buffer (cat. no. ab288006; Abcam) for 30 min at 4°C. The resulting extracts were subsequently centrifuged at 13,400 × g at 4°C for 20 min, and the supernatant was transferred to a vial for analysis. The protein concentration was determined using a BCA kit (Beyotime Institute of Biotechnology). A total of 50 µg heat-denatured proteins per lane were loaded onto 15% gel for SDS-PAGE, and then transferred to polyvinylidene difluoride membranes for western blotting analysis. After blocking nonspecific binding sites with 5% (wt/vol) nonfat milk at room temperature for 1 h and 0.1% (vol/vol) Tween-20 diluted in Tris (pH 7.8)-buffered saline at room temperature for 15 min, the membranes were incubated overnight at 4°C with the following primary antibodies: Anti-E-cadherin (1:1,000; cat. no. 14472; Cell Signaling Technology, Inc.), anti-Vimentin (1:1,000; cat. no. 3390; Cell Signaling Technology, Inc.), anti-ZO-1 (1:1,000; cat. no. 15652; Cell Signaling Technology, Inc.), anti-N-cadherin (1:1,000; cat. no. 14215; Cell Signaling Technology, Inc.), anti-LACC1 (1:500; cat. no. sc-374553; Santa Cruz Biotechnology, Inc.) and anti-GAPDH (1:1,000; cat. no. 97166; Cell Signaling Technology, Inc.). Subsequently, they were incubated with horseradish peroxidase-conjugated secondary antibodies (1:2,000; cat. no. 7076; Cell Signaling Technology, Inc.) at room temperature for 4 h. The bound antibodies were visualized using the ECL Plus Western Blotting Detection system (Cytiva). GAPDH was used as an internal control. The images were analyzed using ImageJ v1.49 (National Institutes of Health).
Statistical analyses
Data are presented as mean ± standard deviation from at least three separate experiments. A comparison of metabolites between the TGF-β1 and control groups was performed using the Student's unpaired t-test. Other comparisons between two groups were assessed using the Student's unpaired t-test and multiple comparisons between the groups were performed using one-way ANOVA, followed by the Bonferroni method. All statistical analyses were performed using R 4.0.3 (CRAN) software (https://cran.r-project.org). P<0.05 was considered to indicate statistically significant difference.
Results
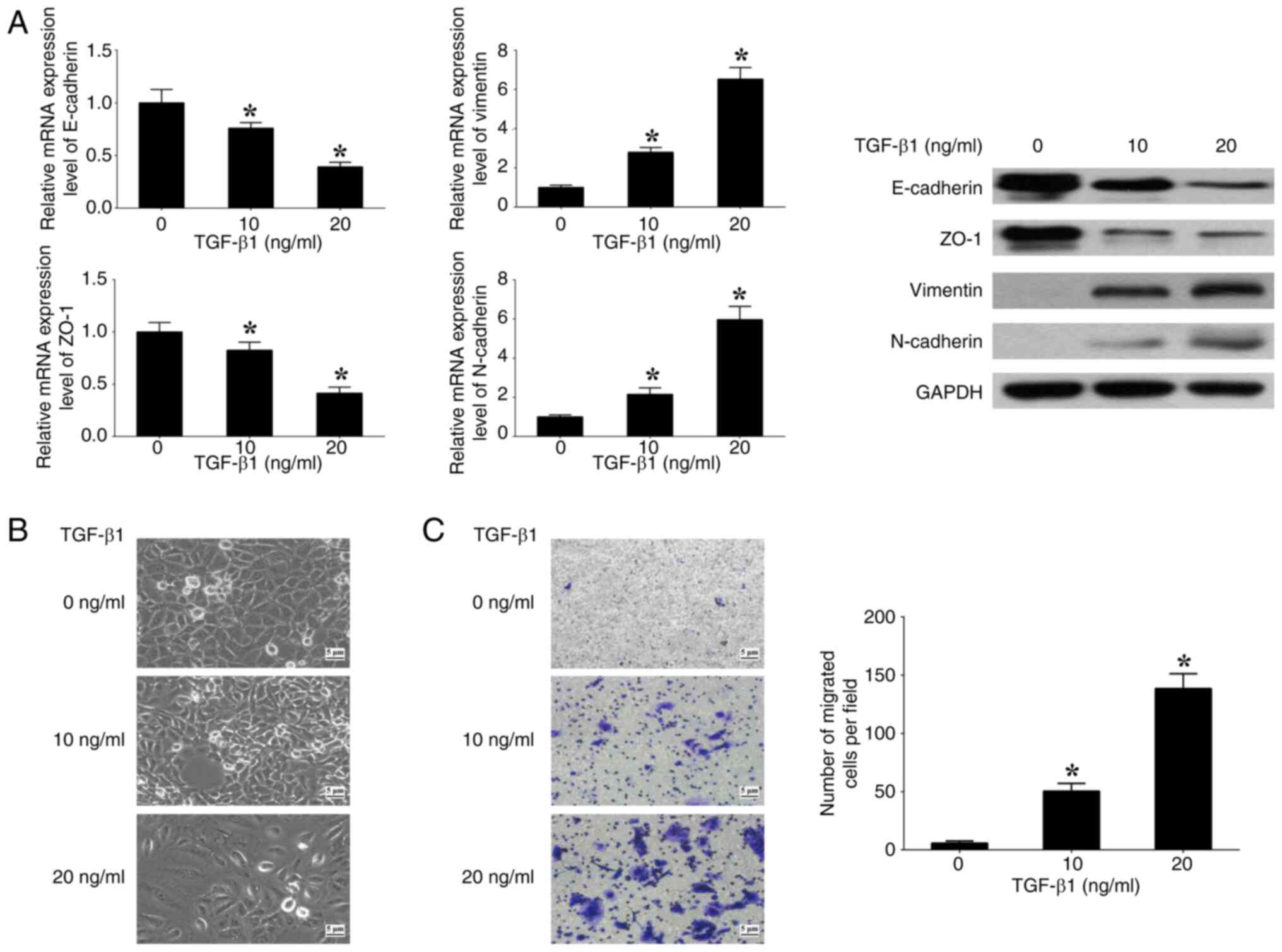
TGF-β1 induces EMT in CRC cells
To assess the impact of the TGF-β signaling pathway on EMT, SW480 cells were cultured with varying concentrations (0, 10 and 20 ng/ml) of human recombinant TGF-β1. At 20 ng/ml TGF-β1, the expression levels of E-cadherin and ZO-1 (epithelial markers) were significantly decreased, whilst the expression levels of N-cadherin and Vimentin (mesenchymal markers) were significantly increased, in comparison with at 0 ng/ml TGF-β1 (Fig. 1A). Spindle-like alterations in morphology and a significant increase in cell migration were also observed at 20 ng/ml TGF-β1 in comparison with at 0 ng/ml TGF-β1 (Fig. 1B and C). The results demonstrate that the induction of EMT in SW480 cells by TGF-β1 is contingent upon the concentration of TGF-β1. Consequently, a concentration of 20 ng/ml TGF-β1 was used for further experiments performed in the present study.
TGF-β1 induces alteration of purine metabolism in CRC cells
To evaluate the influence of the TGF-β signaling pathway on cellular metabolism, exogenous TGF-β1 was introduced to SW480 cells, and subsequent metabolic profiling was performed to identify any differential metabolites (Fig. 2A). Orthogonal projections to latent structures-discriminant analysis (OPLS-DA) plots were generated to visualize the separation of metabolite profiles between the TGF-β1 group and the control group in both the POS and NEG modes (Fig. 2B). By utilizing the variable importance in the projection (VIP) score obtained from the OPLS-DA and Student's t-test analysis, differential metabolites (VIP>1 and P<0.05) were identified between the TGF-β1 group and the control group (Fig. 2C). Furthermore, the differential metabolites were characterized by matching their retention times and m/z values with the National Institute of Science and Technology database, resulting in the identification of 28 and 23 differential metabolites in the POS and NEG modes, respectively (Fig. 2D and Table SI). Among these, 8 metabolites were upregulated and 20 metabolites were downregulated in the POS mode, whilst in the NEG mode, 17 metabolites were upregulated and 6 metabolites were downregulated. Metabolic pathway enrichment analysis was also performed for the differential metabolites induced by TGF-β1, and the bubble plots generated indicated that purine metabolism was the top significant pathway in both the POS and NEG modes (Fig. 2E). These findings indicate that TGF-β1 influences purine metabolism.
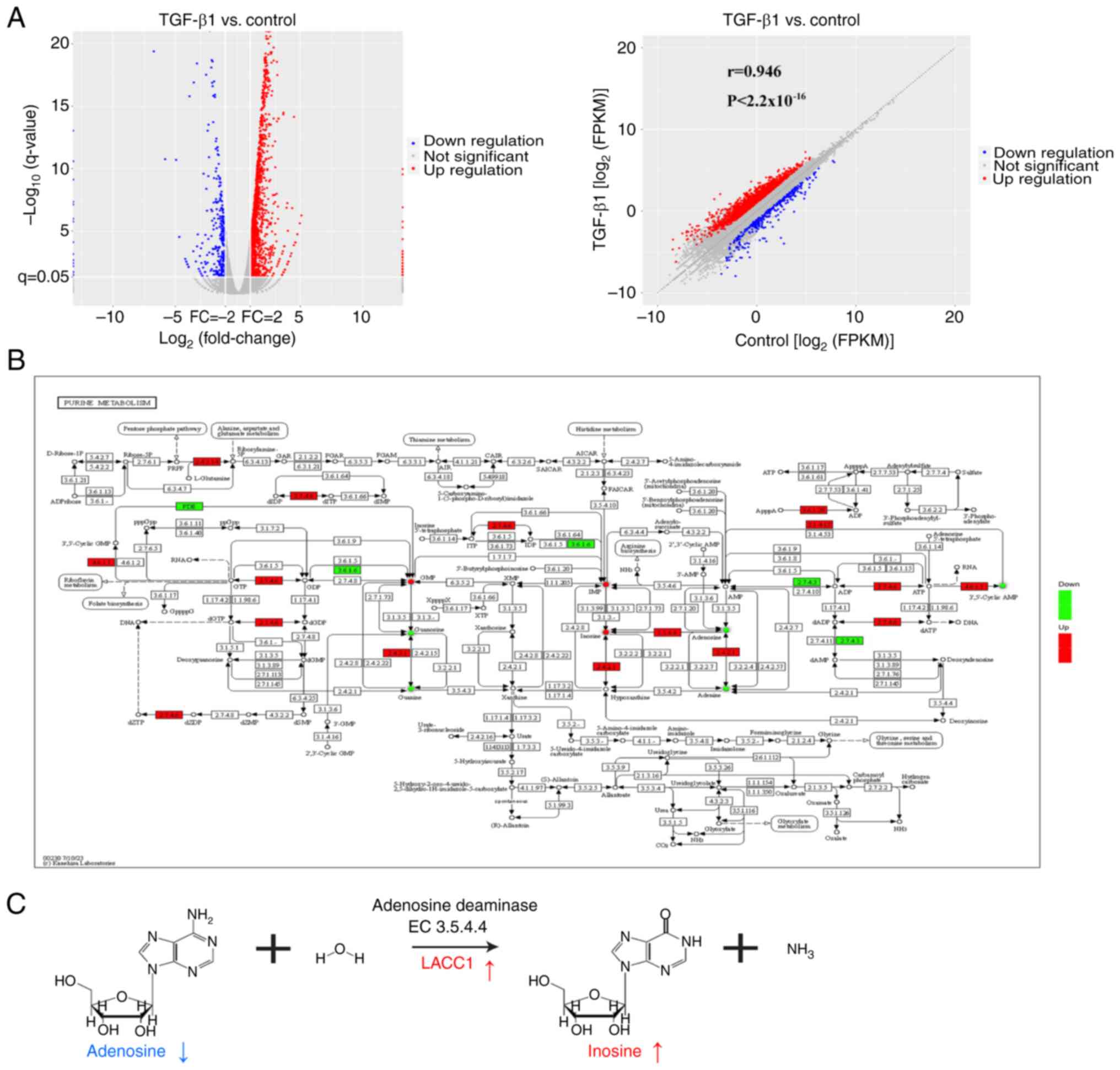
TGF-β1 elevates purine metabolite inosine levels via LACC1
In general, the regulation of metabolite production can be controlled by signaling pathways through the regulation of enzyme gene expression (30). In the present study, integrated analysis of metabolites and transcripts were used to assess the relationship between metabolites and enzyme genes affected by the TGF-β signaling pathway. Firstly, transcriptomic analysis revealed 1,963 differentially expressed genes (log2(fold change)>1 and P<0.05), with 1,640 upregulated and 323 downregulated genes, between the TGF-β1 and control groups (Fig. 3A). To further evaluate the data, the Pathview tool was used to visually represent the differential metabolites and genes involved in purine metabolism between the TGF-β1 and control groups (Fig. 3B). The visualization revealed a notably elevated expression of LACC1, which is an adenosine deaminase (enzyme 3.5.4.4) and is involved in the process of metabolizing adenosine to inosine (31) (Fig. 3C). Additionally, the metabolites inosine and adenosine demonstrated corresponding notable increases and decreases, supporting this finding (Fig. 3C).
Subsequently, the influence of TGF-β1 on the control of LACC1 gene expression and inosine levels was assessed using the introduction of TGF-β1. Moreover, the potential for the reversal of changes instigated by TGF-β1 was evaluated by withdrawing the administration of TGF-β1 following a predetermined period. The findings from RT-qPCR and western blotting indicated that the presence of TGF-β1 led to a significant increase in LACC1 expression, whilst its absence resulted in a significant decrease, in comparison with controls (Fig. 4A). Furthermore, the observed significant increase in inosine levels in cells treated with TGF-β1 was reversed after TGF-β1 was withdrawn (Fig. 4B). To further assess the influence of LACC1 on the accumulation of inosine induced by TGF-β1, overexpression plasmids and shRNAs targeting LACC1 were transfected into SW480 cells to induce overexpression and knockdown of LACC1, respectively (Fig. 4C). The results demonstrated that overexpressing LACC1 significantly increased inosine levels, whilst knockdown of LACC1 expression abolished the TGF-β1-induced increase in inosine levels, in comparison with controls (Fig. 4D). These findings suggest that the upregulation of inosine, a purine metabolite, is mediated by TGF-β1 through the modulation of LACC1 expression.
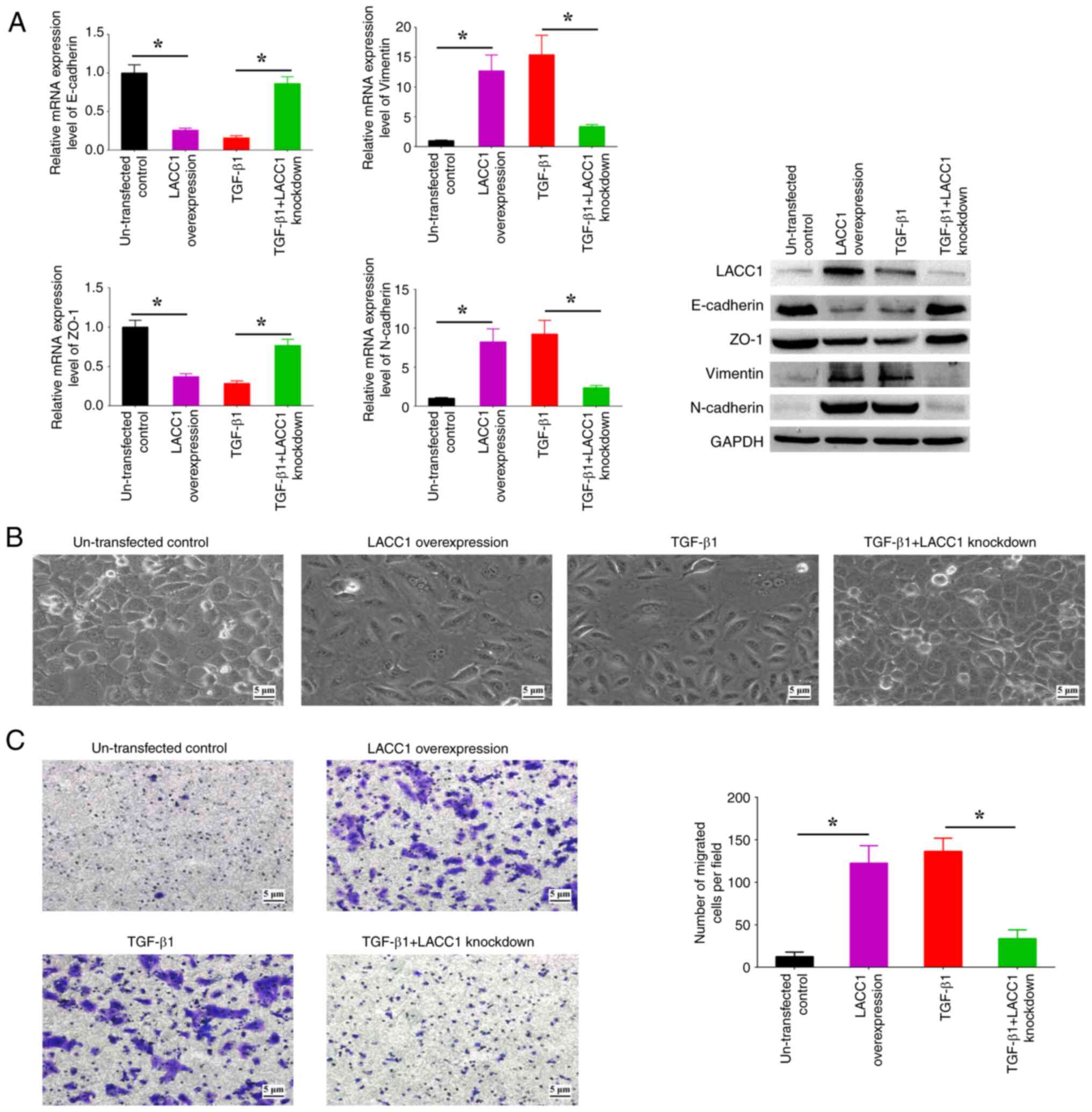
LACC1-controlled inosine accumulation is involved in the regulation of EMT
The present study aimed to assess the role of LACC1 in the EMT process induced by TGF-β1. Upon upregulation of LACC1, there was a significant increase in the expression levels of N-cadherin and Vimentin, and a significant decrease in the expression levels of E-cadherin and ZO-1, in comparison with controls (Fig. 5A). This was accompanied by observable spindle-like alterations in morphology and a significant increase in cell migration in comparison with controls (Fig. 5B and C). Conversely, the suppression of LACC1 expression abolished the increased/decreased EMT marker profiles, morphological alterations and increased cell migration induced by TGF-β1 (Fig. 5A-C). These findings indicate the critical involvement of LACC1 in TGF-β-induced EMT.
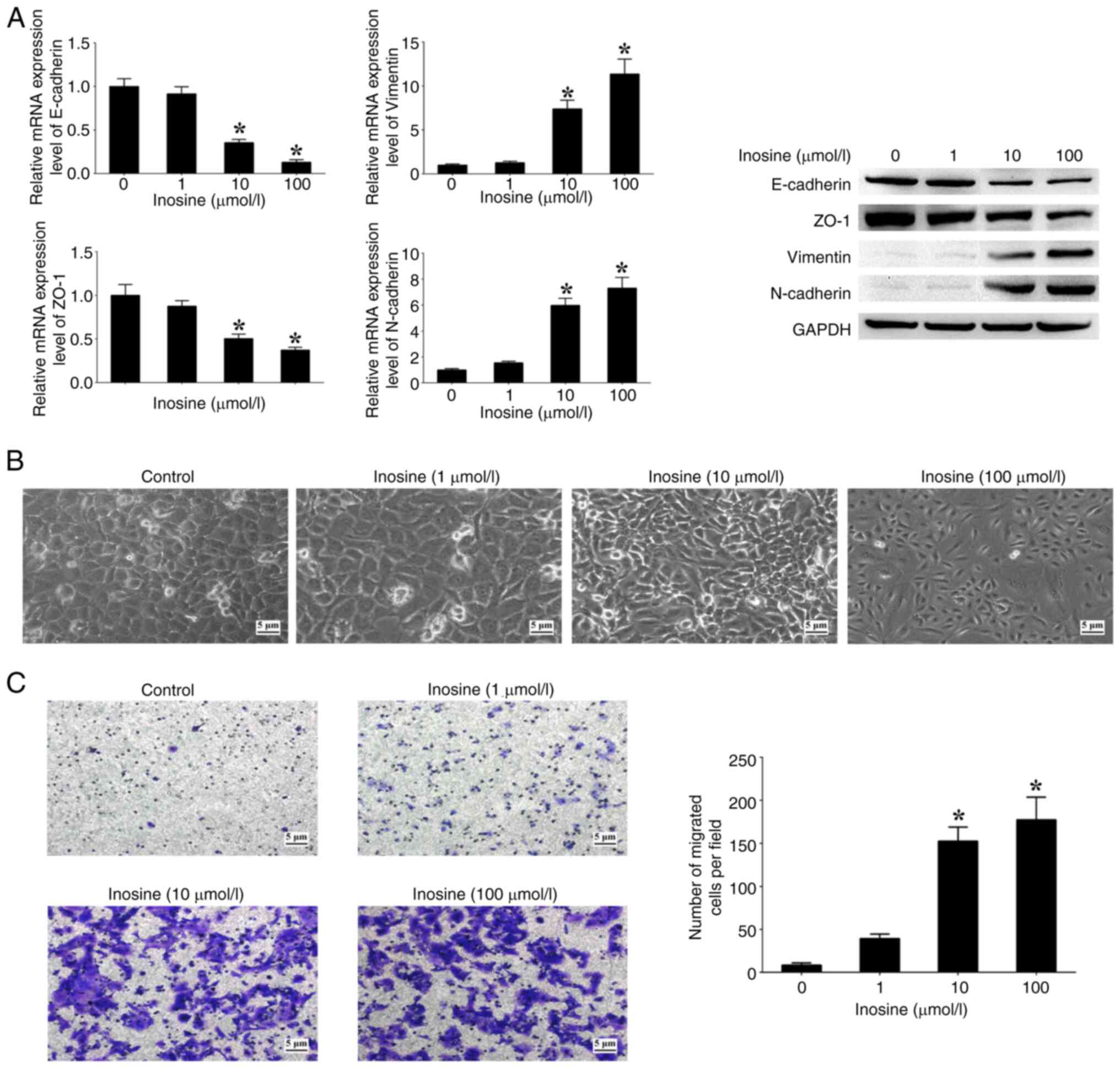
Moreover, the effects of varying concentrations of inosine on patterns of EMT markers, morphological alterations and cell migration were assessed. The analysis revealed an association between the concentration of inosine and the expression levels of N-cadherin and Vimentin, which exhibited a notable increase in a dose-dependent manner when compared with cells not treated with inosine (Fig. 6A). Conversely, the expression levels of E-cadherin and ZO-1 showed a significant decrease with increasing concentrations of inosine in comparison with the untreated cells (Fig. 6A). In addition, an increased concentration of inosine led to a higher proportion of cells displaying a spindle-like morphology, along with a notable increase in the number of migrated cells in comparison with the untreated cells (Fig. 6B and C). These findings suggest a functional association between inosine accumulation regulated by LACC1 and EMT processes in CRC cells.
Discussion
The TGF-β signaling pathway has been extensively studied in relation to its role in several cancers, including CRC (32). Numerous research investigations have shown that the malfunction of the TGF-β signaling pathway is a key factor in the development and advancement of cancer by controlling a range of cellular functions, including EMT, proliferation, angiogenesis and immune evasion (33,34). The present assessed the influence of the TGF-β signaling pathway on EMT through the introduction of human recombinant TGF-β1. As the concentration of TGF-β1 increased, the expression levels of epithelial markers (E-cadherin and ZO-1) decreased, whilst those of mesenchymal markers (N-cadherin and Vimentin) increased, and spindle-like alterations in morphology and an increased in cell migration were also observed. This overall indicated that TGF-β1 triggers EMT in CRC cells when exposed to sufficiently elevated concentrations of TGF-β1.
In general, the TGF-β signaling pathway exerts an influence on the synthesis or functional activity of intracellular metabolites and metabolic proteins through its ability to modulate the expression of genes encoding metabolite-related enzymes, as well as regulate the abundance or post-translational modification of enzyme proteins (35,36). However, there are still numerous unresolved inquiries regarding the intricate relationship between the TGF-β signaling pathway and metabolism, such as a lack of knowledge regarding the number of metabolites and enzyme genes that can act as signaling effectors in response to TGF-β signaling pathway in CRC.
The present research, which combined metabolomics and transcriptomics data, demonstrated that the activation of TGF-β1 led to an increase in the expression of LACC1, which in turn affected the levels of the metabolites inosine and adenosine in CRC cells. LACC1 is a multifunctional purine enzyme found in several organisms, and it serves a role in several purine nucleotide metabolic reactions, including the conversion of adenosine to inosine (37). Mutations in LACC1 have been linked to several human diseases, such as inflammatory bowel disease, Behcet's disease, leprosy, ulcerative colitis, early-onset Crohn's disease and systemic juvenile idiopathic arthritis (38–40). The present study demonstrated that LACC1 has a regulatory function in the accumulation of inosine and is involved in the process of EMT induced by TGF-β1. Therefore, the TGF-β signaling pathway affects purine metabolism by increasing LACC1 expression, potentially contributing to the metastatic properties of CRC.
In recent times, there has been a growing number of studies focusing on the roles of purine metabolites in cell biology and disease, including in cancer (41,42). Several studies have demonstrated that purine metabolites, such as adenosine and inosine, function as modulators to influence cancer development and therapy (43,44). In the present study, it was demonstrated that the TGF-β signaling pathway can trigger the expression of the enzyme LACC1, which in turn activates adenosine deaminase to convert adenosine into inosine. Inosine, a versatile purine compound, acts as a carbon source and facilitates signal transmission with several functional capabilities in different physiological and pathological conditions (45,46). The present research revealed that the accumulation of inosine contributes to EMT and cell migration in CRC cells, indicating that inosine serves a significant role in EMT and the spread of cancer. However, the impact of LACC1 on CRC progression has not been examined, and further investigations are required using in vivo experiments to validate the findings derived from the present study in CRC.
In summary, the TGF-β signaling pathway is significantly involved in regulating the expression of LACC1, thereby influencing the purine metabolism associated with EMT phenotypes by producing the biologically active metabolite inosine. The assessment of the interplay between the TGF-β signaling pathway and purine metabolism in the present study offers valuable understanding of the role of the TGF-β signaling pathway in CRC. Furthermore, LACC1, serving as a link between TGF-β signaling and purine metabolism, holds clinical significance and potential therapeutic implications for CRC treatment.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present work was supported by the Guangdong Basic and Applied Basic Research Foundation (grant nos. 2019A1515010680 and 2022A1515012134) and the Guangzhou Science and Technology Program (grant no. 202102010043).
Availability of data and materials
The original sequencing data generated in the present study may be found in the Sequence Read Archive under accession number PRJNA1040341 or at the following URL: https://www.ncbi.nlm.nih.gov/sra/?term=PRJNA1040341. The original metabolite profiling data generated in the present study may be found in figshare under accession number 10.6084/m9.figshare.25661646 or at the following URL: https://doi.org/10.6084/m9.figshare.25661646. All other data generated in the present study may be requested from the corresponding author.
Authors' contributions
WH, YL and JY conceived and designed the study. WH and LC performed the experiments. WH, YL, JZ and YW collated and analyzed the data. WH and LC drafted the manuscript. YL and JY revised and finalized the manuscript. WH and JY confirm the authenticity of all the raw data. All authors have read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
|
Siegel RL, Miller KD, Wagle NS and Jemal A: Cancer statistics, 2023. CA Cancer J Clin. 73:17–48. 2023. View Article : Google Scholar : PubMed/NCBI | |
|
Dekker E, Tanis PJ, Vleugels JLA, Kasi PM and Wallace MB: Colorectal cancer. Lancet. 394:1467–1480. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Patel SG, Karlitz JJ, Yen T, Lieu CH and Boland CR: The rising tide of early-onset colorectal cancer: A comprehensive review of epidemiology, clinical features, biology, risk factors, prevention, and early detection. Lancet Gastroenterol Hepatol. 7:262–274. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Harada S and Morlote D: Molecular pathology of colorectal cancer. Adv Anat Pathol. 27:20–26. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Derynck R and Budi EH: Specificity, versatility, and control of TGF-β family signaling. Sci Signal. 12:eaav51832019. View Article : Google Scholar : PubMed/NCBI | |
|
Peng D, Fu M, Wang M, Wei Y and Wei X: Targeting TGF-β signal transduction for fibrosis and cancer therapy. Mol Cancer. 21:1042022. View Article : Google Scholar : PubMed/NCBI | |
|
Ali S, Rehman MU, Yatoo AM, Arafah A, Khan A, Rashid S, Majid S, Ali A and Ali MN: TGF-β signaling pathway: Therapeutic targeting and potential for anti-cancer immunity. Eur J Pharmacol. 947:1756782023. View Article : Google Scholar : PubMed/NCBI | |
|
Colak S and Ten Dijke P: Targeting TGF-β signaling in cancer. Trends Cancer. 3:56–71. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Derynck R, Turley SJ and Akhurst RJ: TGFβ biology in cancer progression and immunotherapy. Nat Rev Clin Oncol. 18:9–34. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Itatani Y, Kawada K and Sakai Y: Transforming growth factor-β signaling pathway in colorectal cancer and its tumor microenvironment. Int J Mol Sci. 20:58222019. View Article : Google Scholar : PubMed/NCBI | |
|
Liu A, Yu C, Qiu C, Wu Q, Huang C, Li X, She X, Wan K, Liu L, Li M, et al: PRMT5 methylating SMAD4 activates TGF-β signaling and promotes colorectal cancer metastasis. Oncogene. 42:1572–1584. 2023. View Article : Google Scholar : PubMed/NCBI | |
|
Perez LG, Kempski J, McGee HM, Pelzcar P, Agalioti T, Giannou A, Konczalla L, Brockmann L, Wahib R, Xu H, et al: TGF-β signaling in Th17 cells promotes IL-22 production and colitis-associated colon cancer. Nat Commun. 11:26082020. View Article : Google Scholar : PubMed/NCBI | |
|
Dongre A and Weinberg RA: New insights into the mechanisms of epithelial-mesenchymal transition and implications for cancer. Nat Rev Mol Cell Biol. 20:69–84. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Yang J, Antin P, Berx G, Blanpain C, Brabletz T, Bronner M, Campbell K, Cano A, Casanova J, Christofori G, et al: Guidelines and definitions for research on epithelial-mesenchymal transition. Nat Rev Mol Cell Biol. 21:341–352. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Lambert AW and Weinberg RA: Linking EMT programmes to normal and neoplastic epithelial stem cells. Nat Rev Cancer. 21:325–338. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Lu J, Kornmann M and Traub B: Role of epithelial to mesenchymal transition in colorectal cancer. Int J Mol Sci. 24:148152023. View Article : Google Scholar : PubMed/NCBI | |
|
Stine ZE, Schug ZT, Salvino JM and Dang CV: Targeting cancer metabolism in the era of precision oncology. Nat Rev Drug Discov. 21:141–162. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Gyamfi J, Kim J and Choi J: Cancer as a Metabolic Disorder. Int J Mol Sci. 23:11552022. View Article : Google Scholar : PubMed/NCBI | |
|
La Vecchia S and Sebastián C: Metabolic pathways regulating colorectal cancer initiation and progression. Semin Cell Dev Biol. 98:63–70. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Pan C, Li B and Simon MC: Moonlighting functions of metabolic enzymes and metabolites in cancer. Mol Cell. 81:3760–3774. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Martínez-Reyes I and Chandel NS: Cancer metabolism: Looking forward. Nat Rev Cancer. 21:669–680. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Shi X, Yang J, Deng S, Xu H, Wu D, Zeng Q, Wang S, Hu T, Wu F and Zhou H: TGF-β signaling in the tumor metabolic microenvironment and targeted therapies. J Hematol Oncol. 15:1352022. View Article : Google Scholar : PubMed/NCBI | |
|
Hua W, Ten Dijke P, Kostidis S, Giera M and Hornsveld M: TGFβ-induced metabolic reprogramming during epithelial-to-mesenchymal transition in cancer. Cell Mol Life Sci. 77:2103–2123. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Nakasuka F, Tabata S, Sakamoto T, Hirayama A, Ebi H, Yamada T, Umetsu K, Ohishi M, Ueno A, Goto H, et al: TGF-β-dependent reprogramming of amino acid metabolism induces epithelial-mesenchymal transition in non-small cell lung cancers. Commun Biol. 4:7822021. View Article : Google Scholar : PubMed/NCBI | |
|
Soukupova J, Malfettone A, Bertran E, Hernández-Alvarez MI, Peñuelas-Haro I, Dituri F, Giannelli G, Zorzano A and Fabregat I: Epithelial-Mesenchymal Transition (EMT) Induced by TGF-β in hepatocellular carcinoma cells reprograms lipid metabolism. Int J Mol Sci. 22:55432021. View Article : Google Scholar : PubMed/NCBI | |
|
Corbet C, Bastien E, Santiago de Jesus JP, Dierge E, Martherus R, Vander Linden C, Doix B, Degavre C, Guilbaud C, Petit L, et al: TGFβ2-induced formation of lipid droplets supports acidosis-driven EMT and the metastatic spreading of cancer cells. Nat Commun. 11:4542020. View Article : Google Scholar : PubMed/NCBI | |
|
Robinson MD, McCarthy DJ and Smyth GK: edgeR: A Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics. 26:139–140. 2010. View Article : Google Scholar : PubMed/NCBI | |
|
Luo W and Brouwer C: Pathview: An R/Bioconductor package for pathway-based data integration and visualization. Bioinformatics. 29:1830–1831. 2013. View Article : Google Scholar : PubMed/NCBI | |
|
Livak KJ and Schmittgen TD: Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001. View Article : Google Scholar : PubMed/NCBI | |
|
Vaghari-Tabari M, Ferns GA, Qujeq D, Andevari AN, Sabahi Z and Moein S: Signaling, metabolism, and cancer: An important relationship for therapeutic intervention. J Cell Physiol. 236:5512–5532. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Cristalli G, Costanzi S, Lambertucci C, Lupidi G, Vittori S, Volpini R and Camaioni E: Adenosine deaminase: Functional implications and different classes of inhibitors. Med Res Rev. 21:105–128. 2001. View Article : Google Scholar : PubMed/NCBI | |
|
Tzavlaki K and Moustakas A: TGF-β Signaling. Biomolecules. 10:4872020. View Article : Google Scholar : PubMed/NCBI | |
|
Batlle E and Massagué J: Transforming growth factor-β signaling in immunity and cancer. Immunity. 50:924–940. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Jaykumar AB, Plumber S, Barry DM, Binns D, Wichaidit C, Grzemska M, Earnest S, Goldsmith EJ, Cleaver O and Cobb MH: WNK1 collaborates with TGF-β in endothelial cell junction turnover and angiogenesis. Proc Natl Acad Sci USA. 119:e22037431192022. View Article : Google Scholar : PubMed/NCBI | |
|
Zaiatz-Bittencourt V, Finlay DK and Gardiner CM: Canonical TGF-β signaling pathway represses human NK cell metabolism. J Immunol. 200:3934–3941. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Guido C, Whitaker-Menezes D, Capparelli C, Balliet R, Lin Z, Pestell RG, Howell A, Aquila S, Andò S, Martinez-Outschoorn U, et al: Metabolic reprogramming of cancer-associated fibroblasts by TGF-β drives tumor growth: Connecting TGF-β signaling with ‘Warburg-like’ cancer metabolism and L-lactate production. Cell Cycle. 11:3019–3035. 2012. View Article : Google Scholar : PubMed/NCBI | |
|
Cader MZ, de Almeida Rodrigues RP, West JA, Sewell GW, Md-Ibrahim MN, Reikine S, Sirago G, Unger LW, Iglesias-Romero AB, Ramshorn K, et al: FAMIN is a multifunctional purine enzyme enabling the purine nucleotide cycle. Cell. 180:278–295.e23. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Assadi G, Saleh R, Hadizadeh F, Vesterlund L, Bonfiglio F, Halfvarson J, Törkvist L, Eriksson AS, Harris HE, Sundberg E and D'Amato M: LACC1 polymorphisms in inflammatory bowel disease and juvenile idiopathic arthritis. Genes Immun. 17:261–264. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Wakil SM, Monies DM, Abouelhoda M, Al-Tassan N, Al-Dusery H, Naim EA, Al-Younes B, Shinwari J, Al-Mohanna FA, Meyer BF and Al-Mayouf S: Association of a mutation in LACC1 with a monogenic form of systemic juvenile idiopathic arthritis. Arthritis Rheumatol. 67:288–295. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Kallinich T, Thorwarth A, von Stuckrad SL, Rösen-Wolff A, Luksch H, Hundsdoerfer P, Minden K and Krawitz P: Juvenile arthritis caused by a novel FAMIN (LACC1) mutation in two children with systemic and extended oligoarticular course. Pediatr Rheumatol Online J. 14:632016. View Article : Google Scholar : PubMed/NCBI | |
|
Linden J, Koch-Nolte F and Dahl G: Purine release, metabolism, and signaling in the inflammatory response. Annu Rev Immunol. 37:325–347. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Yin J, Ren W, Huang X, Deng J, Li T and Yin Y: Potential mechanisms connecting purine metabolism and cancer therapy. Front Immunol. 9:16972018. View Article : Google Scholar : PubMed/NCBI | |
|
Zhang C, Wang K and Wang H: Adenosine in cancer immunotherapy: Taking off on a new plane. Biochim Biophys Acta Rev Cancer. 1878:1890052023. View Article : Google Scholar : PubMed/NCBI | |
|
Huo A and Xiong X: PAICS as a potential target for cancer therapy linking purine biosynthesis to cancer progression. Life Sci. 331:1220702023. View Article : Google Scholar : PubMed/NCBI | |
|
Kim IS and Jo EK: Inosine: A bioactive metabolite with multimodal actions in human diseases. Front Pharmacol. 13:10439702022. View Article : Google Scholar : PubMed/NCBI | |
|
Srinivasan S, Torres AG and Ribas de Pouplana L: Inosine in biology and disease. Genes (Basel). 12:6002021. View Article : Google Scholar : PubMed/NCBI |