Temozolomide based treatment in glioblastoma: 6 vs. 12 months
- Authors:
- Published online on: July 2, 2024 https://doi.org/10.3892/ol.2024.14551
- Article Number: 418
-
Copyright: © Fasano et al. This is an open access article distributed under the terms of Creative Commons Attribution License.
Abstract
Introduction
Glioblastoma is the most aggressive and the most frequent brain neoplasia: its incidence is about 5–8 cases per 100,000 inhabitants and represents 54% of all the diagnosed gliomas (1,2). Recent data show a stable incidence in the US or Canada (3), while English and European reports indicate that the incidence is increasing (4).
These tumors are believed to origin from neuroglial or progenitor stem cells and are molecularly heterogeneous (5). The brain tissue microenvironment, including stem cells niches and blood-brain barrier, strongly affects the low rate of glioblastoma metastasis out of the brain, but better promotes brain-invading cancer cells (6).
Molecular profiling has identified three subgroups associated with TERT promoter mutation (7,8): a molecule that plays an important role in cancer formation and in safeguarding chromosomal steadiness by maintaining telomeres' length and has also a role in cellular aging (9). These molecular elements impact tumor growth, avoiding senescence and enabling immortal growth. None of the above-mentioned subtypes are predictive for pharmacological response to present therapies, besides the assignment to subtypes can be difficult be-cause of the intra-tumoral heterogeneity and also the switching subtype is possible through the evolution of disease. Despite the advantages made in our understanding of glioblastoma biology and the current treatment of glioblastoma, including chemotherapy, radiotherapy and surgical approaches, the outcome remains dismal: the median overall survival (mOS) ranging from 14.6 to 20 months (10) and the 5-year survival is less than 10% (11). The treatments fail mainly for the unique molecular features of GBM, particularly due to the presence of a population of stem-like cells called glioma stem cells (GSCs) with ability of self-renewal, making it resistant to current treatments, but also to the presence of blood-brain barrier (BBB) and the privileged immune status (12). For this reason, even a little surgical residue after resection can lead to a lethal recurrence (13). The main weapon following surgery, is the use of the temozolomide (TMZ)-based treatment: this drug is an alkylating agent that better works in methylguanine-DNA-methyltransferase (MGMT)-methylated glioblastomas (14,15). Because of the absence of approved healing treatments, the National Comprehensive Cancer Network (NCCN) recommends clinical trials for eligible patients (16) in order to administer tailored treatments basing on age, functional status, goals of care, etc. and to present palliative care earlier in the course of disease (3). If the patient cannot be entered into any clinical trial, the Stupp protocol is the approved standard treatment but roughly 70% of patients will progress within a year and only approximately 27% will be alive at two years (17,18). In Stupp protocol, TMZ can be administered in its conventional schedule (6 cycles) or in its extended schedule (more than 6 cycles). Extended duration of TMZ has been found to be well tolerated, with a low number of major toxicities. Many studies have demonstrated a survival benefit in the extended schedule (mOS 24–31 months) compared to the conventional schedule (mOS 8–16.5 months) (19–22). However, the Spanish Group of Research in Neuro-Oncology (GEINO group) investigated in a phase 2 prospective trial (GEINO 14–01) the optimal duration of TMZ treatment, finding out that extending TMZ after the sixth cycle gave more toxicities and no benefit in 6 months progression free survival (PFS) (23).
Because of these contrasting data, we decided to conduct a bi-centric retrospective analysis to highlight the efficacy of extending adjuvant treatment with temozolomide in patients with glioblastoma.
Patients and methods
Study design and participants
Our study analyzed the effectiveness of extended temozolomide as adjuvant therapy after a first phase of concomitant chemo-radiation in 87 patients diagnosed with glioblastoma. All data were collected retrospectively from two institutions, Azienda Ospedaliera Universitaria Luigi Vanvitelli (Napoli, Italy) and Ospedale Civile ‘San Giovanni di Dio’ (Frattamaggiore, Italy). Inclusion criteria were those of clinical practice: patients should be 18 years or older, histologically confirmed glioblastoma diagnosis, adequate bone marrow, liver and renal function, stable dose of glucocorticoids with a performance status according to the Eastern Cooperative Oncology Group (ECOG) between 0 and 2. Exclusion criteria were recurrent disease, other metachronous malignancies, need for antiviral treatment for active hepatitis B and C, contemporary use of strong cytochrome P3A4 inhibitors or inducers, treatment discontinuation due to toxicity. We collected data on Isocitrate Dehydrogenase (IDH) mutational status, although the newer WHO classification of CNS (24) tumors define glioblastomas as strictly IDH wild type. We decided to include also these patients based on the initial histological report made at the time of first diagnosis. MGMT methylation was also collected. Both were analyzed on archived tumor tissue, stored in separate laboratories for each center. MGMT methylation status was assessed by methylation array by EPIC array Illumina 850k (25) or Methylation Specific PCR (MSP/PCR) (26), while IDH mutation status was assessed by methylation array by EPIC array Illumina 850k (25) or immunohistochemistry (27). Molecular analysis was not available for all patients as some patients underwent surgery in different centers and, due to the retrospective nature of our study, information were difficult to retrieve.
Procedures
All patients underwent surgical resection or biopsy followed by radiotherapy with concomitant temozolomide (75 mg/m2/day). After concurrent chemoradiation, treatment was temporarily suspended for the duration of one month and then reprised with adjuvant temozolomide as monotherapy, five days every 28 days: first cycle was administered as 150 mg/m2/day, following cycles as 200 mg/m2/day. The choice to administer six or more cycles was taken by the neurooncologist responsible for the patient based on her/his experience. Brain MRI evaluation was conducted firstly after 40–60 days the last day of chemoradiation and then every three months since the start of temozolomide monotherapy; in case of clinical signs suggestive of progressive disease, brain MRI could be anticipated based on clinician's decision. Tumor progression was defined according to Response Assessment in Neuro-Oncology (RANO criteria). Data were collected until 17th April 2023.
Outcomes
Primary endpoint was OS, defined as time from treatment start to death from any cause, whereas secondary endpoint was PFS, defined as time from treatment start to disease progression or death. PFS2, time from second line start to disease progression or death, was also analyzed. OS, PFS and PFS2 were estimated with Kaplan-Meier methods. Survival data were also stratified according to MGMT methylation status and then excluding IDH mutant tumors. We evaluated the outcomes between 45 patients who discontinued temozolomide therapy at 6 cycles in accordance with the protocol outlined by Stupp et al (17) (6C group) and 42 patients wherein TMZ therapy was continued until 12 cycles (12C group). Accordingly, patients who stopped temozolomide before 6 cycles of therapy because of tumor progression or death were excluded from analysis.
Statistical analysis
Patient data were accounted as median with range of minimum and maximum values between parentheses for continuous variables and only percentages for categorical variables. Kaplan Meier estimates were used to help computing survival curves, while survival differences were analyses using the log-rank test, significance level of P=0.05. Statistical analyses were performed using IBM SPSS statistics v.23.0.
Results
Patient's characteristics are summarized in Table I. We included 87 patients with glioblastoma, who received 6 or 12 cycles of temozolomide therapy between 2012 and 2022. Around sixty-five percent (n=56) were male. Median age was 61.6 years (range 31–75). The majority of the patients (83.9%) presented with Eastern Cooperative Oncology Group (ECOG) PS 0–1. Forty-five patients were in the 6C group and forty-two patients in the 12C group. In these 87 patients, MGMT promoter status was known in 56 patients. MGMT promoter was methylated in 44.4% (20/45) and 23.8% (10/42) in the 6C and 12C group respectively. In the remaining 26 patients, MGMT promoter was unmethylated. There was no association between MGMT promoter methylation status and the number of cycles given. As anticipated, we included both IDH wild type and IDH mutant tumors based on initial report made at time of first diagnosis. As expected, the majority (70.1%) were IDH wild type tumors. In only 6 patients IDH was mutated and in 23% the mutational status was instead unknown.
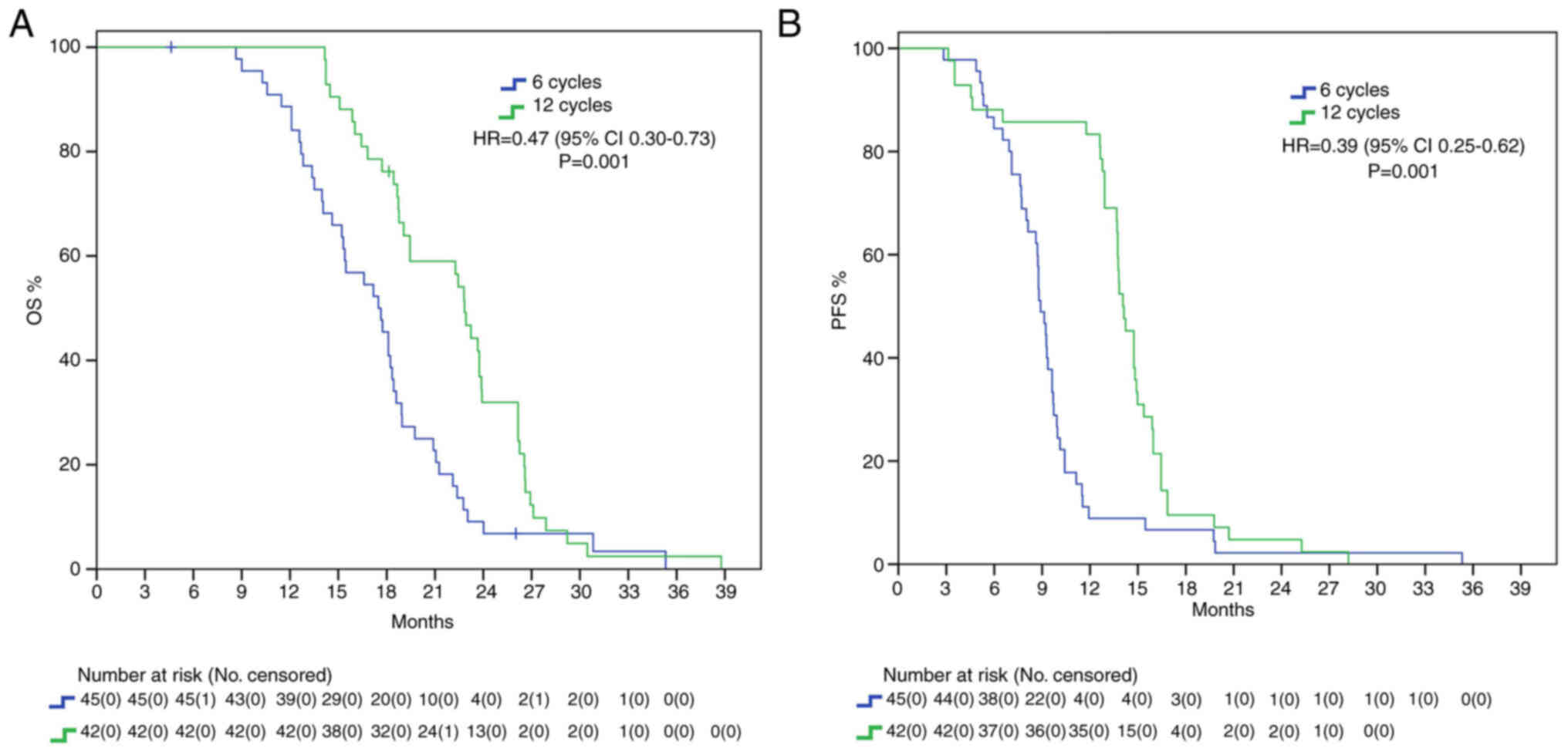
Patients whose adjuvant temozolomide therapy was stopped at 6 cycles had a mOS of 17.5 months, whereas those that received 12 cycles reached a mOS of 22.8 months, presenting with a statistically significant benefit (HR 0.47, 95% IC 0.30–0.73 P=0.001). Furthermore, mPFS difference was also statistically significant, with a delta of around 6 months between 12C group and 6C group (15.3 vs. 9 months, HR: 0.39, 95%IC 0.25–0.62, P=0.001) (Fig. 1).
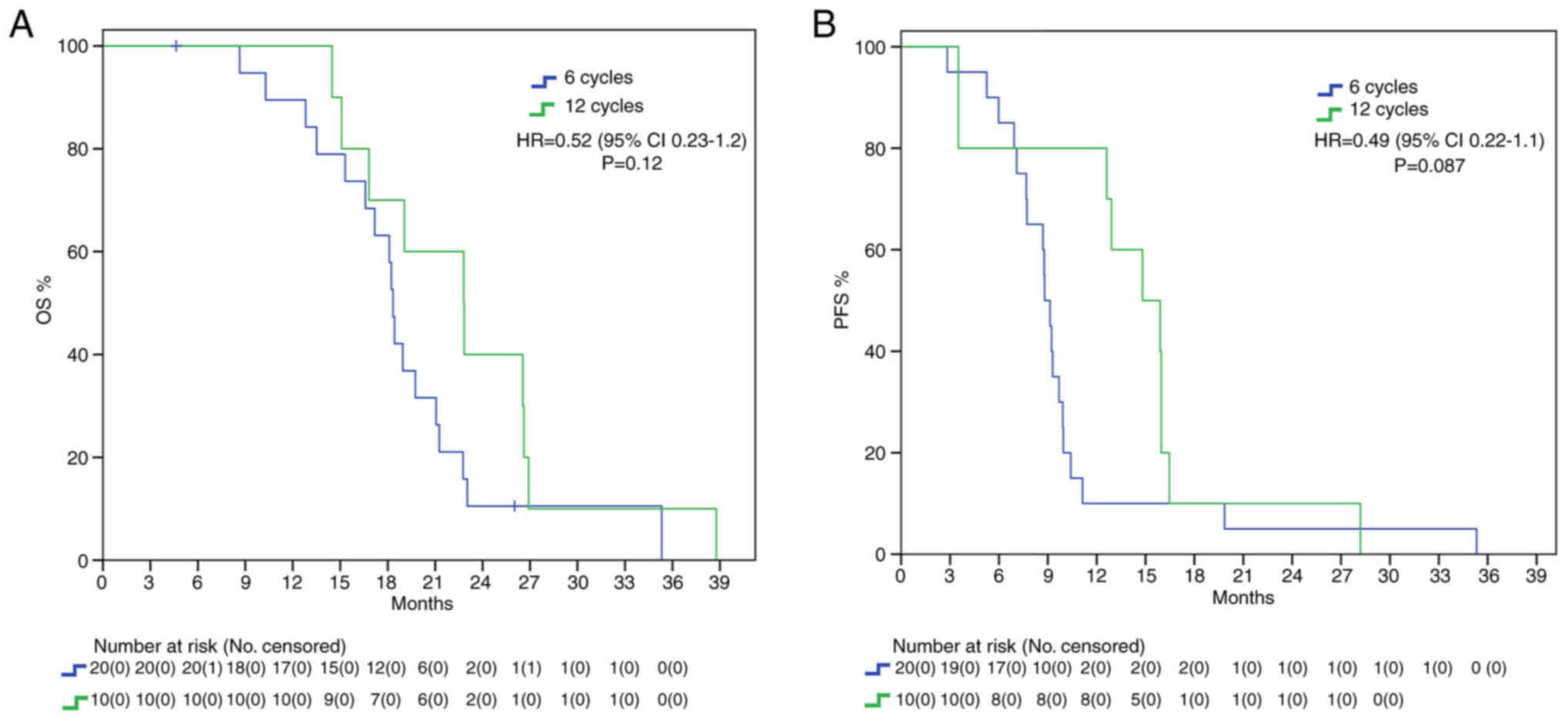
Endpoints were also evaluated in the different subgroups. In MGMT methylated patients, there was a mOS benefit trend in the 12C group (22.8 vs. 18.3 months, HR: 0.52, 95%IC 0.23–1.2, P=0.12) and there was also a positive trend for mPFS (14.8 vs. 8.8 months, HR: 0.49, 95%IC 0.22–1.1, P=0.087) without statistical significance (Fig. 2). Different findings resulted from the analysis in the MGMT unmethylated subgroup: we found a statistically significant benefit in mOS for the 12C group (22.4 vs. 15.4 months, HR: 0.18, 95%IC 0.063–0.52, P=0.002) but no statistical benefit in mPFS (12.9 vs. 8.8 months, HR: 0.42, 95%IC 0.17–1.02, P=0.056) (Fig. 3).
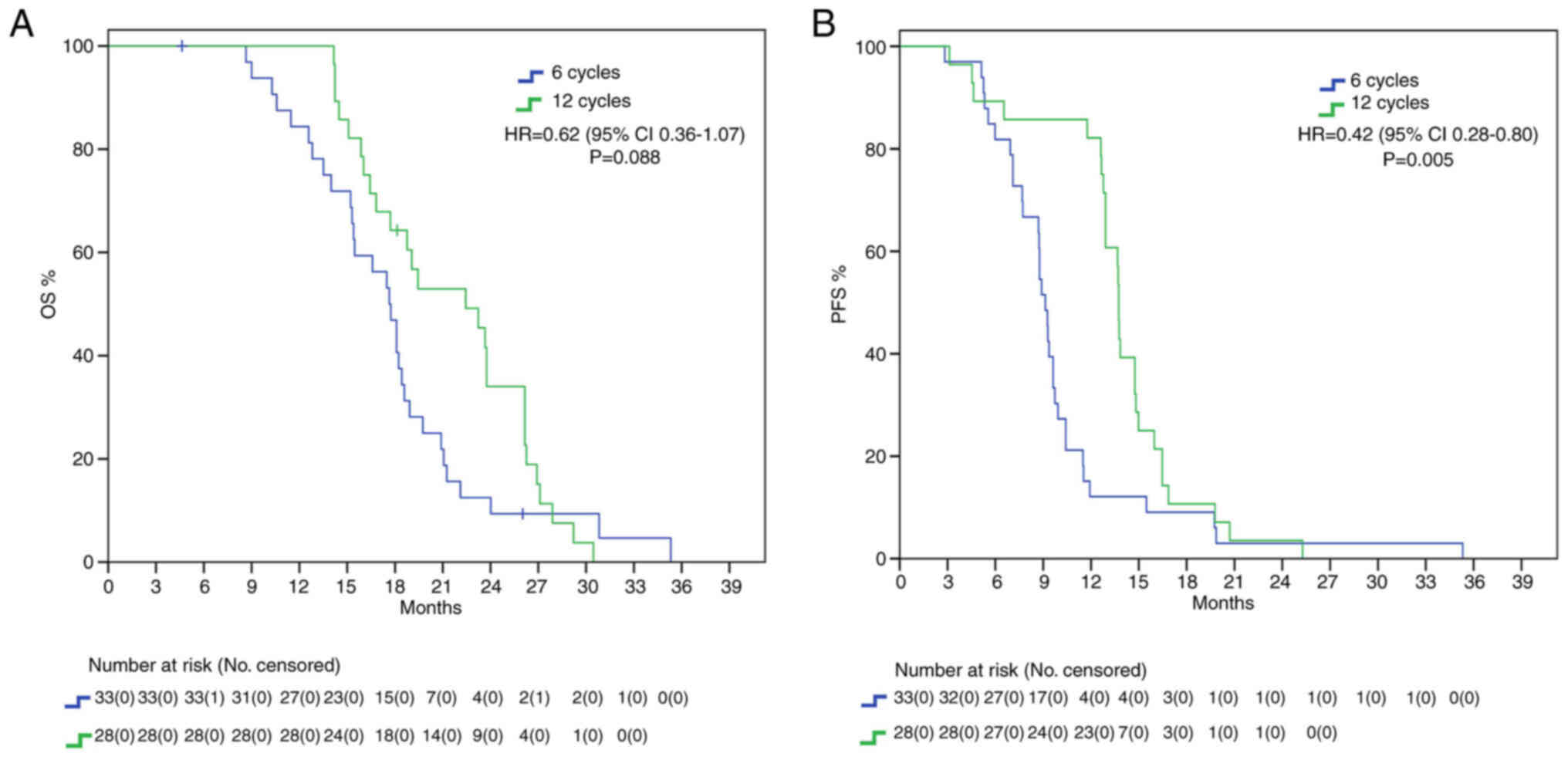
We repeated our analysis excluding IDH mutant patients, since according to the newest WHO CNS classification they cannot be diagnosed as glioblastomas (24). Nevertheless, here we found a positive trend for mOS (22.4 vs. 17.6 months, HR: 0.62, 95%IC 0.36–1.07, P=0.088) and a significant difference for mPFS (13.7 vs. 9.1 months, HR: 0.42, 95%IC 0.28–0.80, P=0.005) in favor of 12C group (Fig. 4).
Both treatments were generally well tolerated, with a toxicity profile consistent with literature data. Leukopenia was the most frequently observed treatment-related hematologic adverse event, while the most frequent non-hematologic adverse event was fatigue. Overall, in the group of patients treated with the extended schedule of TMZ, there was an increase of adverse events, however it was not necessary to report a statistically significant difference (Table II).
Discussion
The prognosis of patients with glioblastoma remains poor both because, despite after gross total resection there is a high chance of residual disease, and because of the poor efficacy of chemotherapy and radiotherapy treatments. Furthermore, the EORTC 26981/2981/NCIC CE.3 trial (17) allowed only 6 adjuvant cycles of temozolomide, but that was established as an arbitrary limit. For this reason, adjuvant temozolomide has been prolonged by many investigators, both in everyday practice and in clinical trials, generally up until 12 cycles. Easiness of oral administration and low toxicity profile have also favored prolonging treatment.
While many studies have investigated the benefits of extended adjuvant TMZ (Table III), no definitive indication has been implemented. NCCN guidelines (2023) advice against prolonging treatment (16); European Society for Medical Oncology (ESMO) guidelines, last updated in 2014, do not analyze the controversy; on the contrary, Associazione Italiana di Oncologia Medica (AIOM 2021) guidelines on brain tumor consider the possibility of continuing adjuvant treatment until 12 cycles (28). We found a statistically significant benefit in the 12C group both in mOS (22.8 vs. 17.5 months, HR 0.47) and in mPFS (15.3 vs. 9 months, HR 0.39) in the overall population, with around 5 months delta in both setting. It must be noted that our survival data are in range with expectations from known literature and thus do not classify as outlier. While mPFS curves seem to cross early on, casting benefit of extended adjuvant treatment in regard to mPFS, the curves tend to grow apart with time, showing its survival benefit. We further analyzed our results considering MGMT methylation status to consider the possibility of only MGMT methylated GBMs having increased survival from extended therapy. Our results show that this subgroup of patients presents with a trend in increased mOS and mPFS in the 12C group. This is in line with the well-known role of MGMT promoter methylation as a predictive factor of increased response to alkylating agents (29,30). It must be said that MGMT methylation determines better prognosis, and it has been speculated that increased survival allows patients to receive more extended treatment (31). Furthermore, mutational changes due to prolonged temozolomide, especially in patients with absence of MGMT-mediated DNA repair, may promote tumor resistance thanks to the acquisition of an alkylating agents-resistant phenotype (32,33). The phase II RESCUE trial on continuous dose-intense temozolomide in recurrent GBM demonstrated worse results in those patients that experienced progression while on extended treatment, while increased survival was found in those with at least a 2-month treatment free interval or experiencing progression on standard treatment (34). In contrast with these results, no mPFS benefit was found in the unmethylated cohort, with only a small trend in increased survival (12.9 vs. 8.8 months). Instead, mOS was found to be statistically significantly higher in the 12C group than in the 6C group (22.4 vs. 15.4 months). We speculated that the increase in OS may be due to second line treatment. About 99.7% of patients in 12C group and 87.5% in 6C group underwent second line therapy. However, no difference was found in PFS2 between the two group. IDH mutant gliomas are characterized by increased survival and tumor response (35). However, whereas the 2016 classification allowed for IDH wild type and IDH mutant GBMs, the newest 2021 WHO classification of CNS tumors classify GBM strictly as IDH wild type, IDH mutant being astrocytoma or oligodendroglioma according to 1p-19q codeletion status (24). We included in our analysis 6 patients who presented with mutation in IDH1 or IDH2 and 20 with unknown alterations. Nevertheless, we decided to include these patients due to the fact that, at the time of diagnosis, they were classified as GBM. We then repeated mOS and mPFS analysis excluding these 26 patients, limiting our scope accordingly to the newest definition of GBM. Even stratifying according to the newest definition, we confirmed a statistically significant difference in mOS (22.4 vs. 17.6 months) and mPFS (13.7 vs. 9.1 months) in the 12C arm compared to the 6C arm. IDH-based classification is a fundamental game-changer in CNS research. Many previous trials on GBMs often included IDH mutant GBMs, misclassified and now considered a different entity, thus limiting their interpretations. For example, Chen et al (36) study on extended temozolomide reported in a retrospective cohort an increased difference in mOS between the extended adjuvant cohort and the control group, around 9.3 months (29 vs. 16.7 months). However, looking at population characteristics, only 27.5% patients in the control group were IDH mutant against 43.4% in the extended adjuvant group, increasing the chance of a higher survival in the latter arm. Indeed, survival benefit was even higher in the IDH1 mutant subgroup (+20.5 months), while there was only a 7-months difference between the two arms in IDH1 wild type subgroup.
Our study is in line with previous analyses demonstrating increasing benefit from extended therapy (Table III) (19,22,36,37). Our results suggest, then, that extended adjuvant treatment may be a good therapeutic opportunity in fit patients to increase survival rates. Rigorous patient selection is of course needed, and while MGMT methylation may take the spotlight, several other factors may influence treatment choice. Keeping in mind the limitations of Chen study, they showed an increased mPFS in newly diagnosed GBMs with higher expression of Ki67 treated with extended adjuvant temozolomide, while no such difference was found in patients with lower Ki67 expression. No difference was found in mOS as both groups benefitted from extended adjuvant treatment (36). A retrospective analysis by Bocangel et al (38) evaluated the role of p53 status, since literature reported that p53 mutational and expression status was associated to GBM prognosis. Indeed, wild type p53 was found to inhibit MGMT expression, potentially increasing the response rate to alkylating agents. In Malkoun et al (20), p53 overexpression was associated with improved mPFS, even though contrasting results are available in literature (39–41). Furthermore, the study by Skardelly et al (42) published in 2017, while only demonstrating a benefit for prolonged temozolomide only for mPFS and not in mOS, found that MGMT status, extent of resection and age are significant covariates for survival analysis.
Reports on toxicity with extended treatment are contrasting. It is necessary to clearly whether prolonged therapy impacts on toxicity and consequently on the quality of life of patients. Quality of life still represents a primary objective to be pursued today since we cannot yet aim for cure. Only clinical data derived from a randomized study can disprove the common sensation that extended treatment is accompanied by greater toxicities, particularly hematological. In clinical practice only a limited percentage of patients manage to have prolonged treatment (43). The safety analysis of the Prolonged Adjuvant Temozolomide vs. ‘Stop & Go’ in Glioblastoma Patients (PATSGO) trial on 34 patients demonstrated that frequency of toxicity did not increase with number of cycles (44); instead, in the GEINO trial, lymphopenia, thrombocytopenia, nausea and vomiting were more frequent in the extended therapy group, although few patients experienced grade 3–4 adverse events of any kind and only three patients (3.7%) needed to discontinue treatment (23). However, there are other several reports of increased toxicity with prolonged temozolomide administration: increased cumulative doses of temozolomide have been associated with worse quality of life and fatigue (10,45), risk of myelosuppression and immunodepression (46), myelodysplasia and even leukemia (47).
As already mentioned before, while no consensus exists on the benefits of additional temozolomide, most physicians settle at a maximum of 12 cycles for maintenance therapy, trying to find balance between possible beneficial effects and toxicities. A single center study by Ohno et al (48) compared stopping treatment at 12 cycles or proceeding beyond 12 cycles. mPFS and mOS between the two groups demonstrated no difference (mPFS 11.3 vs. 9.2 months, mOS 25.7 vs. 30.2 months), with only Karnosfky performance status at 12 cycles having a significant association with increased survival (48).
Temozolamide treatment has also been associated with induced hypermutation. No data exists on the perfect treatment schedule or duration in order to reach the most benefit while reducing the risk of induced hypermutation and toxicity (49). While conferring resistance to temozolomide treatment (34), these changes may help identify new treatment strategies for recurrent/progressing GBM. Hypermutation seems to present with an increased sensitivity to DNA-damaging agents (50), with preclinical trial demonstrating improved sensitivity to lomustine in mismatch repair (MMR) deficient MGMT methylated GBM cells resistant to temozolamide (51). It has also been speculated that hypermutated cancer cells may be more responsive to immune checkpoint inhibition (52) but results from nivolumab trials both in newly diagnosed GBM and in recurrent GMB have demonstrated poor results. Pembrolizumab is now under investigation in patients with recurrent gliomas with hypermutator phenotype (NCT02658279) but a recent monocentric study by Lombardi et al (53) found no apparent benefit.
Of course, our study presents several limitations. It is a retrospective analysis, with only a modest sample size (87 patients), thus limiting extrapolation of its results. The study was of course not randomized and no information regarding treatment choice (6 vs. 12 cycles) is available, with the possibility of selection bias. MRIs at progression were not centrally reviewed, in line with the nature of the study, and recorded toxicity data was limited, not allowing for further study. OS data may also be influenced by second-line choices (mainly fotemustine and regorafenib). However, the number of patients enrolled and the results obtained in our study are substantially similar with that has already been published by Bhandari and colleagues (54).
In conclusion, our data suggests that extended adjuvant temozolomide (12 cycles) appears to be significantly more effective than standard treatment with only conventional six cycles. While literature data are quite heterogeneous and do not provide any strong evidence for stopping or continuing temozolomide, in the absence of larger phase III trials, continuing adjuvant temozolomide for more than six cycles may be an effective alternative.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
MF, FC and RA conceived the study. MF and RA described the methodology to be used. Investigation was done by MP, CCM, SF, AZ, VF, PV, IDG, CB, VC, DS, TS, LMC, PC, MC and RP. VDF, VF, PV, IDG, CB, VC, DS, TS, LMC, PC, MC and RP analyzed the data. Validation of data was performed by VDF, VF, PV, IDG, CB, VC, DS, TS, LMC, PC, MC and RP. The resources were collected by MF, VF, PV, IDG, CB, VC, DS, TS, LMC, PC, MC, RP, FC and RA. Data was curated by VDF, VF, MP and SF. The original draft was prepared by VDF, MP, CCM, SF and AZ. The final text was reviewed by MF, VDF and RA. Work was supervised by MF and RA. MF and RA confirm the authenticity of all the raw data. All authors have read and agreed to the published version of the manuscript.
Ethics approval and consent to participate
All subjects gave their written informed consent. The study was conducted in accordance with the Declaration of Helsinki. The retrospective study protocol was approved by the institutional review board of University of Campania Luigi Vanvitelli (Napoli, Italy; protocol no. 59;).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
|
Crocetti E, Trama A, Stiller C, Caldarella A, Soffietti R, Jaal J, Weber DC, Ricardi U, Slowinski J and Brandes A; RARECARE working group, : Epidemiology of glial and non-glial brain tumours in Europe. Eur J Cancer. 48:1532–1542. 2012. View Article : Google Scholar : PubMed/NCBI | |
|
Ostrom QT, Cioffi G, Gittleman H, Patil N, Waite K, Kruchko C and Barnholtz-Sloan JS: CBTRUS statistical report: Primary brain and other central nervous system tumors diagnosed in the United States in 2012–2016. Neuro Oncol. 21 (Suppl 5):v1–v100. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Davis FG, Smith TR, Gittleman HR, Ostrom QT, Kruchko C and Barnholtz-Sloan JS: Glioblastoma incidence rate trends in Canada and the United States compared with England, 1995–2015. Neuro Oncol. 22:301–302. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Philips A, Henshaw DL, Lamburn G and O'Carroll MJ: Brain tumours: Rise in glioblastoma multiforme incidence in England 1995–2015 suggests an adverse environmental or lifestyle factor. J Environ Public Health. 2018:79107542018. View Article : Google Scholar : PubMed/NCBI | |
|
Lee JH, Lee JE, Kahng JY, Kim SH, Park JS, Yoon SJ, Um JY, Kim WK, Lee JK, Park J, et al: Human glioblastoma arises from subventricular zone cells with low-level driver mutations. Nature. 560:243–247. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Lah TT, Novak M and Breznik B: Brain malignancies: Glioblastoma and brain metastases. Semin Cancer Biol. 60:262–273. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Sturm D, Witt H, Hovestadt V, Khuong-Quang DA, Jones DT, Konermann C, Pfaff E, Tönjes M, Sill M, Bender S, et al: Hotspot mutations in H3F3A and IDH1 define distinct epigenetic and biological subgroups of glioblastoma. Cancer Cell. 22:425–437. 2012. View Article : Google Scholar : PubMed/NCBI | |
|
Wang Q, Hu B, Hu X, Kim H, Squatrito M, Scarpace L, deCarvalho AC, Lyu S, Li P, Li Y, et al: Tumor evolution of glioma-intrinsic gene expression subtypes associates with immunological changes in the microenvironment. Cancer Cell. 32:42–56.e6. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Daniel M, Peek GW and Tollefsbol TO: Regulation of the human catalytic subunit of telomerase (hTERT). Gene. 498:135–146. 2012. View Article : Google Scholar : PubMed/NCBI | |
|
Gilbert MR, Wang M, Aldape KD, Stupp R, Hegi ME, Jaeckle KA, Armstrong TS, Wefel JS, Won M, Blumenthal DT, et al: Dose-dense temozolomide for newly diagnosed glioblastoma: A randomized phase III clinical trial. J Clin Oncol. 31:4085–4091. 2013. View Article : Google Scholar : PubMed/NCBI | |
|
Stupp R, Taillibert S, Kanner A, Read W, Steinberg D, Lhermitte B, Toms S, Idbaih A, Ahluwalia MS, Fink K, et al: Effect of tumor-treating fields plus maintenance temozolomide vs maintenance temozolomide alone on survival in patients with glioblastoma: a randomized clinical trial. JAMA. 318:2306–2316. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Liu G, Yuan X, Zeng Z, Tunici P, Ng H, Abdulkadir IR, Lu L, Irvin D, Black KL and Yu JS: Analysis of gene expression and chemoresistance of CD133+ cancer stem cells in glioblastoma. Mol Cancer. 5:672006. View Article : Google Scholar : PubMed/NCBI | |
|
Fountain DM, Bryant A, Barone DG, Waqar M, Hart MG, Bulbeck H, Kernohan A, Watts C and Jenkinson MD: Intraoperative imaging technology to maximise extent of resection for glioma: A network meta-analysis. Cochrane Database Syst Rev. 1:CD0136302021.PubMed/NCBI | |
|
Cahill DP, Levine KK, Betensky RA, Codd PJ, Romany CA, Reavie LB, Batchelor TT, Futreal PA, Stratton MR, Curry WT, et al: Loss of the mismatch repair protein MSH6 in human glioblastomas is associated with tumor progression during temozolomide treatment. Clin Cancer Res. 13:2038–2045. 2007. View Article : Google Scholar : PubMed/NCBI | |
|
Weller M: Where does O6-methylguanine DNA methyltransferase promoter methylation assessment place temozolomide in the future standards of care for glioblastoma? Cancer. 124:1316–1318. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Horbinski C, Nabors LB, Portnow J, Baehring J, Bhatia A, Bloch O, Brem S, Butowski N, Cannon DM, Chao S, et al: NCCN Guidelines® Insights: Central Nervous System Cancers, Version 2.2022: Featured Updates to the NCCN Guidelines. J Natl Comp Cancer Network. 21:12–20. 2023. View Article : Google Scholar | |
|
Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, et al: Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 352:987–996. 2005. View Article : Google Scholar : PubMed/NCBI | |
|
Stupp R, Hegi ME, Mason WP, van den Bent MJ, Taphoorn MJ, Janzer RC, Ludwin SK, Allgeier A, Fisher B, Belanger K, et al: Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-Year analysis of the EORTC-NCIC trial. Lancet Oncol. 10:459–466. 2009. View Article : Google Scholar : PubMed/NCBI | |
|
Roldán Urgoiti GB, Singh AD and Easaw JC: Extended adjuvant temozolomide for treatment of newly diagnosed glioblastoma multiforme. J Neurooncol. 108:173–177. 2012. View Article : Google Scholar : PubMed/NCBI | |
|
Malkoun N, Chargari C, Forest F, Fotso MJ, Cartier L, Auberdiac P, Thorin J, Pacaut C, Peoc'h M, Nuti C, et al: Prolonged temozolomide for treatment of glioblastoma: Preliminary clinical results and prognostic value of p53 overexpression. J Neurooncol. 106:127–133. 2012. View Article : Google Scholar : PubMed/NCBI | |
|
Hau P, Koch D, Hundsberger T, Marg E, Bauer B, Rudolph R, Rauch M, Brenner A, Rieckmann P, Schuth J, et al: Safety and feasibility of long-term temozolomide treatment in patients with high-grade glioma. Neurology. 68:688–690. 2007. View Article : Google Scholar : PubMed/NCBI | |
|
Refae AA, Ezzat A, Salem DA and Mahrous M: Protracted adjuvant temozolomide in glioblastoma multiforme. J Cancer Ther. 6:748–758. 2015. View Article : Google Scholar | |
|
Balana C, Vaz MA, Manuel Sepúlveda J, Mesia C, Del Barco S, Pineda E, Muñoz-Langa J, Estival A, de Las Peñas R, Fuster J, et al: A phase II randomized, multicenter, open-label trial of continuing adjuvant temozolomide beyond 6 cycles in patients with glioblastoma (GEINO 14-01). Neuro Oncol. 22:1851–1861. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Louis DN, Perry A, Wesseling P, Brat DJ, Cree IA, Figarella-Branger D, Hawkins C, Ng HK, Pfister SM, Reifenberger G, et al: The 2021 WHO classification of tumors of the central nervous system: A summary. Neuro Oncol. 23:1231–1251. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Bady P, Sciuscio D, Diserens AC, Bloch J, van den Bent MJ, Marosi C, Dietrich PY, Weller M, Mariani L, Heppner FL, et al: MGMT methylation analysis of glioblastoma on the Infinium methylation BeadChip identifies two distinct CpG regions associated with gene silencing and outcome, yielding a prediction model for comparisons across datasets, tumor grades, and CIMP-status. Acta Neuropathol. 124:547–560. 2012. View Article : Google Scholar : PubMed/NCBI | |
|
Vlassenbroeck I, Califice S, Diserens AC, Migliavacca E, Straub J, Di Stefano I, Moreau F, Hamou MF, Renard I, Delorenzi M, et al: Validation of real-time methylation-specific PCR to determine O6-methylguanine-DNA methyltransferase gene promoter methylation in glioma. J Mol Diagn. 10:332–337. 2008. View Article : Google Scholar : PubMed/NCBI | |
|
Capper D, Weissert S, Balss J, Habel A, Meyer J, Jäger D, Ackermann U, Tessmer C, Korshunov A, Zentgraf H, et al: Characterization of R132H mutation-specific IDH1 antibody binding in brain tumors. Brain Pathol. 20:245–254. 2010. View Article : Google Scholar : PubMed/NCBI | |
|
Franceschi E, Lombardi G, Balestrini D, Buglione M, Caranci F, Castellano A, Ferreri A, Franchino F, Giangaspero F and Mascarin M: Guidelines for brain neoplasies|AIOM. https://www.aiom.it/linee-guida-aiom-2021-neoplasie-cerebrali/June 23–2023 | |
|
Hegi ME, Diserens AC, Gorlia T, Hamou MF, de Tribolet N, Weller M, Kros JM, Hainfellner JA, Mason W, Mariani L, et al: MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 352:997–1003. 2005. View Article : Google Scholar : PubMed/NCBI | |
|
Weller M, van den Bent M, Preusser M, Le Rhun E, Tonn JC, Minniti G, Bendszus M, Balana C, Chinot O, Dirven L, et al: EANO guidelines on the diagnosis and treatment of diffuse gliomas of adulthood. Nat Rev Clin Oncol. 18:170–186. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Balañá C, Vaz MA, Lopez D, de la Peñas R, García-Bueno JM, Molina-Garrido MJ, Sepúlveda JM, Cano JM, Bugés C, Sanz SM, et al: Should we continue temozolomide beyond six cycles in the adjuvant treatment of glioblastoma without an evidence of clinical benefit? A cost analysis based on prescribing patterns in Spain. Clin Transl Oncol. 16:273–279. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
Attarian F, Taghizadeh-Hesary F, Fanipakdel A, Javadinia SA, Porouhan P, PeyroShabany B and Fazilat-Panah D: A systematic review and meta-analysis on the number of adjuvant temozolomide cycles in newly diagnosed glioblastoma. Front Oncol. 11:7794912021. View Article : Google Scholar : PubMed/NCBI | |
|
Blumenthal DT, Gorlia T, Gilbert MR, Kim MM, Burt Nabors L, Mason WP, Hegi ME, Zhang P, Golfinopoulos V, Perry JR, et al: Is more better? The impact of extended adjuvant temozolomide in newly diagnosed glioblastoma: A secondary analysis of EORTC and NRG oncology/RTOG. Neuro Oncol. 19:1119–1126. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Perry JR, Bélanger K, Mason WP, Fulton D, Kavan P, Easaw J, Shields C, Kirby S, Macdonald DR, Eisenstat DD, et al: Phase II trial of continuous dose-intense temozolomide in recurrent malignant glioma: RESCUE study. J Clin Oncol. 28:2051–2057. 2010. View Article : Google Scholar : PubMed/NCBI | |
|
van den Bent MJ, Dubbink HJ, Marie Y, Brandes AA, Taphoorn MJ, Wesseling P, Frenay M, Tijssen CC, Lacombe D, Idbaih A, et al: IDH1 and IDH2 mutations are prognostic but not predictive for outcome in anaplastic oligodendroglial tumors: A report of the European organization for research and treatment of cancer brain tumor group. Clin Cancer Res. 16:1597–1604. 2010. View Article : Google Scholar : PubMed/NCBI | |
|
Chen J, Wang T, Liu W, Qiu H, Zhang N, Chen X, Ding X and Zhang L: Extended adjuvant temozolomide in newly diagnosed glioblastoma: A single-center retrospective study. Front Oncol. 12:10005012022. View Article : Google Scholar : PubMed/NCBI | |
|
Darlix A, Baumann C, Lorgis V, Ghiringhelli F, Blonski M, Chauffert B, Zouaoui S, Pinelli C, Rech F, Beauchesne P and Taillandier L: Prolonged administration of adjuvant temozolomide improves survival in adult patients with glioblastoma. Anticancer Res. 33:3467–3474. 2013.PubMed/NCBI | |
|
Bocangel D, Sengupta S, Mitra S and Bhakat KK: p53-Mediated down-regulation of the human DNA repair gene O6-methylguanine-DNA methyltransferase (MGMT) via interaction with Sp1 transcription factor. Anticancer Res. 29:3741–3750. 2009.PubMed/NCBI | |
|
Weller M, Felsberg J, Hartmann C, Berger H, Steinbach JP, Schramm J, Westphal M, Schackert G, Simon M, Tonn JC, et al: Molecular predictors of progression-free and overall survival in patients with newly diagnosed glioblastoma: a prospective translational study of the German glioma network. J Clin Oncol. 27:5743–5750. 2009. View Article : Google Scholar : PubMed/NCBI | |
|
Pollack IF, Finkelstein SD, Burnham J, Holmes EJ, Hamilton RL, Yates AJ, Finlay JL and Sposto R; Children's Cancer Group, : Age and TP53 mutation frequency in childhood malignant gliomas: Results in a multi-institutional cohort. Cancer Res. 61:7404–7407. 2001.PubMed/NCBI | |
|
Miyagami M, Tazoe M and Nakamura S: Expression of vascular endothelial growth factor and p53 protein in association with neovascularization in human malignant gliomas. Brain Tumor Pathol. 15:95–100. 1998. View Article : Google Scholar : PubMed/NCBI | |
|
Skardelly M, Dangel E, Gohde J, Noell S, Behling F, Lepski G, Borchers C, Koch M, Schittenhelm J, Bisdas S, et al: Prolonged temozolomide maintenance therapy in newly diagnosed glioblastoma. Oncologist. 22:570–575. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Seiz M, Krafft U, Freyschlag CF, Weiss C, Schmieder K, Lohr F, Wenz F, Thomé C and Tuettenberg J: Long-term adjuvant administration of temozolomide in patients with glioblastoma multiforme: Experience of a single institution. J Cancer Res Clin Oncol. 136:1691–1695. 2010. View Article : Google Scholar : PubMed/NCBI | |
|
Hammouch F, Boterberg T, Clement P, Joosens E, Whenham N, Verschaeve V, Devriendt D, Renard L and Baurain JFR: 8744 POSTER extended use of adjuvant TMZ in newly diagnosed GBM patients is safe-results from the safety analysis of the PATSGO trial. Eur J Cancer. 47 (Suppl 1):S5872011. View Article : Google Scholar | |
|
Pouratian N, Gasco J, Sherman JH, Shaffrey ME and Schiff D: Toxicity and efficacy of protracted low dose temozolomide for the treatment of low grade gliomas. J Neurooncol. 82:281–288. 2007. View Article : Google Scholar : PubMed/NCBI | |
|
Su YB, Sohn S, Krown SE, Livingston PO, Wolchok JD, Quinn C, Williams L, Foster T, Sepkowitz KA and Chapman PB: Selective CD4+ lymphopenia in melanoma patients treated with temozolomide: A toxicity with therapeutic implications. J Clin Oncol. 22:610–616. 2004. View Article : Google Scholar : PubMed/NCBI | |
|
Momota H, Narita Y, Miyakita Y and Shibui S: Secondary hematological malignancies associated with temozolomide in patients with glioma. Neuro Oncol. 15:14452013. View Article : Google Scholar : PubMed/NCBI | |
|
Ohno M, Miyakita Y, Takahashi M, Yanagisawa S, Tamura Y and Narita Y: Continuing maintenance temozolomide therapy beyond 12 cycles confers no clinical benefit over discontinuation at 12 cycles in patients with IDH1/2-wildtype glioblastoma. Jpn J Clin Oncol. 52:1134–1142. 2022.PubMed/NCBI | |
|
Johnson BE, Mazor T, Hong C, Barnes M, Aihara K, McLean CY, Fouse SD, Yamamoto S, Ueda H, Tatsuno K, et al: Mutational analysis reveals the origin and therapy-driven evolution of recurrent glioma. Science. 343:189–193. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
Schlesner M and Eils R: Hypermutation takes the driver's seat. Genome Med. 7:312015. View Article : Google Scholar : PubMed/NCBI | |
|
Stritzelberger J, Distel L, Buslei R, Fietkau R and Putz F: Acquired temozolomide resistance in human glioblastoma cell line U251 is caused by mismatch repair deficiency and can be overcome by lomustine. Clin Transl Oncol. 20:508–516. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Snyder A, Makarov V, Merghoub T, Yuan J, Zaretsky JM, Desrichard A, Walsh LA, Postow MA, Wong P, Ho TS, et al: Genetic basis for clinical response to CTLA-4 blockade in melanoma. N Engl J Med. 371:2189–2199. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
Lombardi G, Barresi V, Indraccolo S, Simbolo M, Fassan M, Mandruzzato S, Simonelli M, Caccese M, Pizzi M, Fassina A, et al: Pembrolizumab activity in recurrent high-grade gliomas with partial or complete loss of mismatch repair protein expression: A monocentric, observational and prospective pilot study. Cancers (Basel). 12:22832020. View Article : Google Scholar : PubMed/NCBI | |
|
Bhandari M, Gandhi AK, Devnani B, Kumar P, Sharma DN and Julka PK: Comparative study of adjuvant temozolomide six cycles versus extended 12 cycles in newly diagnosed glioblastoma multiforme. J Clin Diagn Res. 11:XC04–XC08. 2017.PubMed/NCBI |