Analysis of high‑risk factors for brain metastasis and prognosis after prophylactic cranial irradiation in limited‑stage small cell lung cancer
- Authors:
- Published online on: July 3, 2024 https://doi.org/10.3892/ol.2024.14555
- Article Number: 422
-
Copyright: © Yu et al. This is an open access article distributed under the terms of Creative Commons Attribution License.
Abstract
Introduction
Lung cancer remains a leading cause of cancer-related mortality globally, with small cell lung cancer (SCLC) representing ~13% of all cases (1). The United States Department of Veterans Affairs categorizes SCLC into two stages: Limited-stage (LS)-SCLC and extensive-stage (ES)-SCLC (2). In China, SCLC accounts for 13–15% of all lung cancer cases, with ~180,000 new cases reported annually (3).
SCLC is characterized by rapid proliferation and early onset of distant metastasis, with ~70% of patients diagnosed at the extensive stage (4). The brain is frequently affected by distant metastasis in SCLC, with 10–24% of patients exhibiting brain metastases (BM) at diagnosis and >50% developing them during the disease (5).
Recent advancements in comprehensive treatment have incrementally improved SCLC survival rates, subsequently increasing the incidence of BM. Within 2 years of achieving complete or partial remission, 67% of patients with LS-SCLC experience BM, with survival extending >2 years in 50–80% of cases (6). Prophylactic cranial irradiation (PCI) significantly reduces the risk of BM and enhances overall survival (OS), thus becoming the standard post-radiotherapy and chemotherapy treatment for LS-SCLC (7,8). Nevertheless, certain patients still develop BM post-PCI, underscoring the need for further refinement in selecting candidates for this intervention.
Advancements in therapeutic strategies, including enhanced chemoradiotherapy protocols and precise radiation techniques, have significantly improved the management of LS-SCLC (9,10). Nevertheless, the aggressive nature of SCLC, characterized by rapid cell division and early metastasis, remains challenging. Brain metastases are especially problematic due to the blood-brain barrier, which limits the effectiveness of many systemic therapies, thus necessitating the use of PCI as a preventive measure (11).
Identifying patients at higher risk for BM is crucial for optimizing treatment protocols and improving outcomes. Previous studies have emphasized the significance of factors such as tumor size and treatment response in predicting BM (12). Larger tumors and partial responses to treatment are associated with an increased risk of BM. Research into molecular and genetic markers, such as circulating tumor cells (CTCs) and specific gene mutations, holds promise for more accurately predicting BM risk (13). Integrating these biomarkers into clinical practice could lead to more personalized treatment approaches, thereby improving survival rates and quality of life for LS-SCLC patients (14).
In the present study, a retrospective analysis was performed of clinical data from 290 patients with LS-SCLC who achieved complete remission (CR)/partial remission (PR) following PCI at Chengde Central Hospital (Chengde, China) and Hebei Cangzhou Hospital of Integrated Traditional Chinese and Western Medicine (Cangzhou, China). The aim was to elucidate the clinical characteristics that influence the risk of developing BM and prognosis after PCI.
Patients and methods
Clinical data
The present study gathered clinical data from 290 patients diagnosed with LS-SCLC who received PCI after achieving CR or PR. The data collection spanned from January 2015 to December 2023 at Chengde Central Hospital and Hebei Cangzhou Hospital of Integrated Traditional Chinese and Western Medicine. The time of collecting the statistical data was the same for both hospitals. The present study is based entirely on previously recorded patient data. All patients had a confirmed diagnosis of SCLC, either pathologically or cytologically, and were free of secondary primary malignancies. Restaging was performed using the American Joint Committee on Cancer (AJCC) Lung Cancer 8th Edition tumor-node-metastasis (TNM) clinical staging criteria (15) and the Department of Veterans Affairs two-stage system (2). The factors analyzed in the present study included age, sex, performance status (PS) score, initial tumor maximum diameter and treatment modalities. The inclusion criteria were as follows: i) Histological or cytological confirmation of SCLC; ii) initial diagnosis of LS-SCLC staged according to the 8th edition of the AJCC Cancer Staging Manual and the Veterans Administration Lung Study Group two-tier system (2); and iii) initial treatment with curative intent chemoradiotherapy (CRT; concurrent or sequential), followed by PCI after achieving CR or PR. The exclusion criteria were as follows: i) Presence of a second primary malignancy or other histological types of cancer; ii) diagnosis of ES-SCLC; iii) loss to follow-up or incomplete clinical data; and iv) absence of brain magnetic imagining resonance (MRI) data prior to PCI to exclude BM; vi) those who had surgical interventions.
In the present study, levels of carcinoembryonic antigen (CEA) and neuron-specific enolase (NSE) were measured from blood samples collected from patients at the two medical centers. Assessments were performed using the Cobas® E 601 module analyzer (Roche Diagnostics) using the electrochemiluminescence method, with Elecsys® CEA and NSE assay kits (Roche Diagnostics, Elecsys® CEA Assay Kit: Cat. no. 11731629322; Elecsys® NSE Assay Kit: Cat. no. 04827021190). To maintain data integrity and accuracy, a dedicated Laboratory Data Collection Team was formed, which was responsible for the collection and verification of laboratory data from both centers, ensuring uniformity in reference ranges. Established reference ranges for CEA and NSE were set at 0–5 and 0–16 ng/ml, respectively.
Treatment
All patients underwent standard chemotherapy and PCI according to the Chinese Society of Clinical Oncology guidelines (12,16). The preferred modality was concurrent CRT, with sequential CRT used when the former was not tolerable; 54.5% received concurrent treatment. Those who had surgical interventions were excluded from the analysis. Chemotherapy comprised 4–6 cycles of etoposide combined with cisplatin or carboplatin, with concurrent and induction chemotherapy involving 2–3 cycles and 1–3 cycles, respectively (8).
Chemotherapy regimen
All patients underwent standard chemotherapy consisting of etoposide and platinum-based drugs (cisplatin or carboplatin). Etoposide was administered intravenously at a dose of 100 mg/m2 on days 1 to 3 of each cycle. Cisplatin was administered intravenously at a dose of 75 mg/m2 on day 1, or carboplatin was administered intravenously at an area under the curve (AUC) of 5 on day 1. Each chemotherapy cycle lasted 21 days, and patients typically received 4 to 6 cycles of chemotherapy. Thoracic radiotherapy was administered either as 45 Gy in 30 fractions twice daily or as 54–70 Gy in 28–30 fractions once daily. Response to CRT was evaluated using the Response Evaluation Criteria in Solid Tumours 1.1 criteria (17). Patients achieving a CR or PR proceeded with PCI. Brain MRI was performed prior to PCI in all cases to rule out metastases. PCI typically commenced 4–6 weeks post-CRT, delivered as 25 Gy in 5 weekly fractions over 2 weeks (18). Hippocampal delineation adhered to the RTOG0933 principles (19), ensuring a maximum dose to the hippocampus of <17 Gy and an average dose of <10 Gy (20). Dose constraints for high-risk organs were set as follows: Brainstem, ≤54 Gy; spinal cord, ≤45 Gy; temporal lobe, ≤65 Gy; optic chiasm and nerve, ≤54 Gy; pituitary, mean dose ≤45 Gy; eye, ≤50 Gy or mean dose, ≤35 Gy; lens, ≤9 Gy; mandible and temporomandibular joint, ≤70 Gy; parotid gland mean dose, ≤26 Gy, and V30, ≤50% (at least unilaterally) or D20cc, ≤20 Gy (bilaterally), with average doses kept at <10 Gy and maximum doses of <17 Gy.
Follow-up and efficacy evaluation
Efficacy evaluation was performed 1 month following the completion of CRT. Patients underwent follow-up assessments every 3 months for the first 2 years post-treatment, every 6 months until the fifth year, and annually thereafter. These assessments included chest and abdominal CT scans. In instances of headaches or neurological symptoms, an immediate brain MRI was administered. Follow-up methods comprised patient revisits, telephone consultations and reviews of registration data. Survival metrics, such as OS, were calculated from the onset of treatment to death or the last follow-up. The time to BM was measured from initiation of treatment to confirmation via imaging. As of January 2024, follow-up data was up-to-date, with a median duration of 55 months, ranging from 11–102 months.
Statistical analysis
Statistical analyses were performed using SPSS software, version 27.0 (IBM Corp.). Survival data were analyzed using the Kaplan-Meier method coupled with the log-rank test. Single-factor and multifactorial risk factors impacting BM and OS were assessed using Cox regression analysis. All tests performed were two-tailed and P<0.05 was considered to indicate a statistically significant difference.
Results
Analysis of clinical characteristics
At the time of follow-up, basic clinical data were collected from 290 patients involved in this study. The median age was 58 years, ranging from 42–74 years. A total of 44.5% of these patients (129 cases) presented with an initial tumor diameter of >5 cm at the onset of treatment. The clinical characteristic of the patients are presented in Table I. Representative brain MRI images are shown in Fig. 1. The MRI images presented in this study are representative images taken when brain metastases were first detected in the patients. These images provide a visual representation to help readers better understand the typical appearance and progression of brain metastases in patients with LS-SCLC.
Factors associated with BM
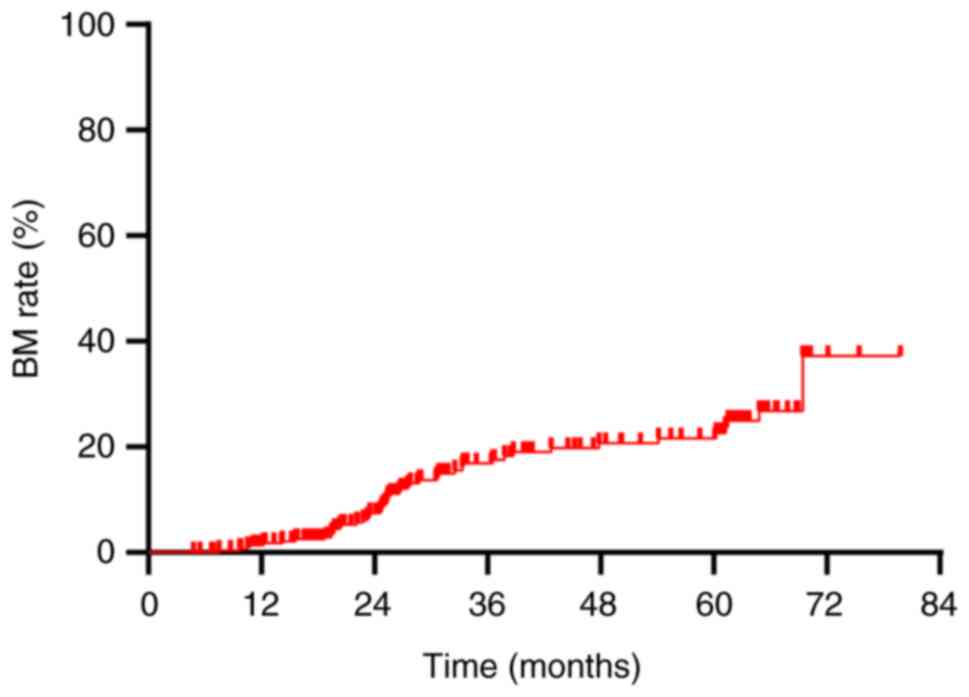
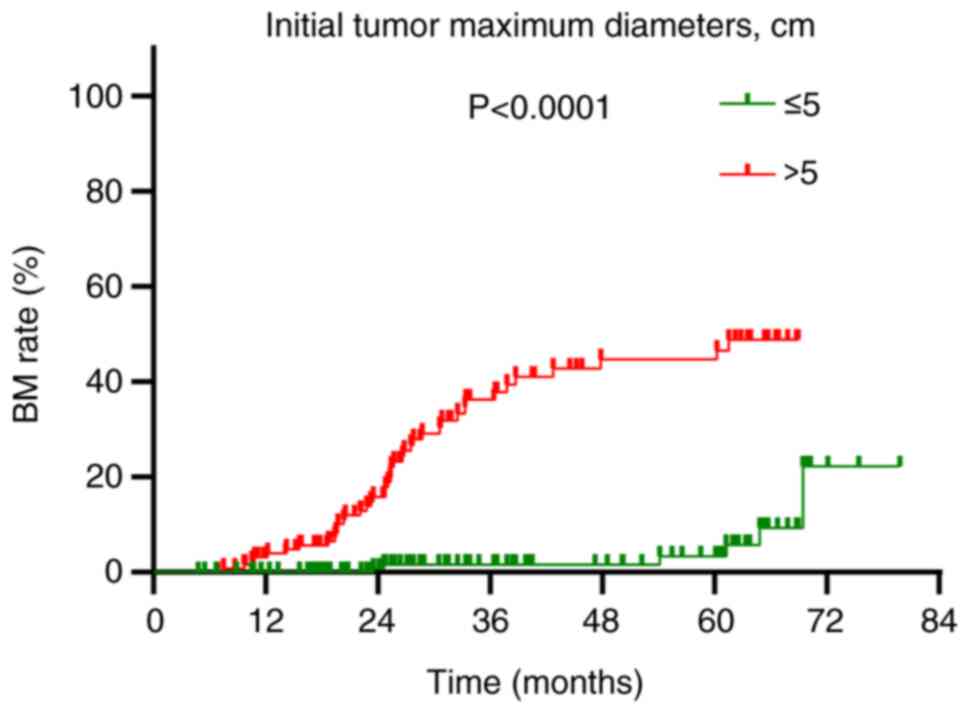
The overall BM rate was demonstrated to be 16.6% (48/290). Annual rates of BM at 1, 2 and 3 years post-diagnosis were 1.4, 6.6 and 12.8%, respectively (Fig. 2). This study established a tumor size of 5 cm as the initial standard, grounded in the AJCC staging criteria for lung cancer, where T3 is defined as a tumor greater than 5 cm. To validate this standard, statistical analyses were conducted using various tumor sizes as classification criteria, and the results were compared. The analyses revealed no statistically significant differences in BM and OS when 3,4,6 and 7 cm were used as classification criteria (P>0.05) (Table II). This finding further supports the statistical and clinical significance of using 5 cm as the grouping standard. A detailed analysis of factors influencing BM highlighted significant associations in univariate analysis: Notably, the maximum diameter of the initial tumor [hazard ratio (HR)=13.276; 95% confidence interval (CI): 5.248–33.586; P<0.001], type of treatment administered (HR=2.149; 95% CI: 1.199–3.851; P=0.010) and treatment response (HR=2.981; 95% CI: 1.231–7.223; P=0.016) were significantly associated with an increased risk of BM following Prophylactic cranial irradiation (PCI) (Table III). Multivariable Cox regression analysis identified that an initial tumor maximum diameter of >5 cm was an independent risk factor for BM post-PCI (HR=15.031; 95% CI: 5.610–40.270; P<0.001; Table III). Patients with tumors >5 cm in diameter experienced a BM rate of 32.6% (42/129), which was significantly higher than the 3.7% (6/161) observed in patients with tumors of ≤5 cm in diameter (P<0.001; Fig. 3).
Table III.Analysis of factors affecting brain metastasis in 290 patients with limited stage-small cell lung cancer. |
Factors associated with OS
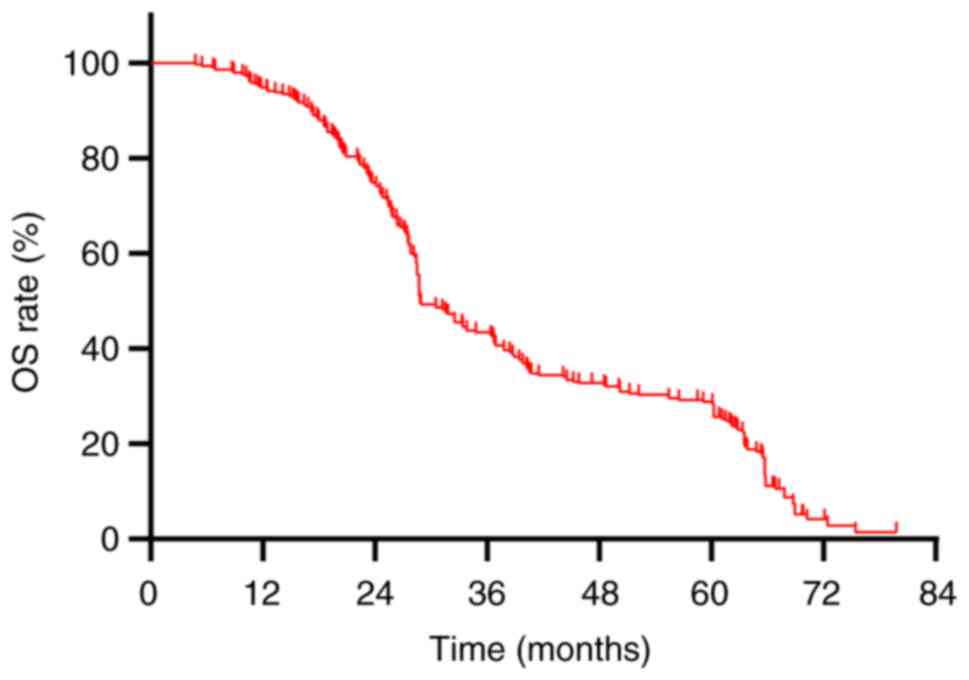
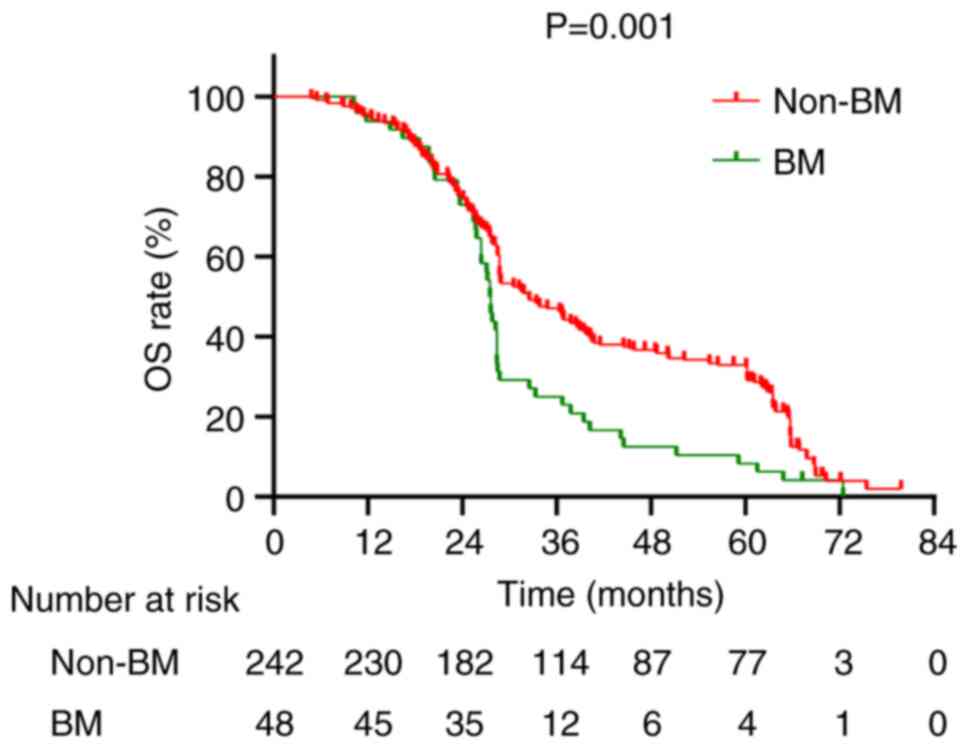
The median OS for the cohort of 290 patients was recorded at 28.8 months, accompanied by a 5-year OS rate of 27.9% (Fig. 4). A comparative analysis between patients with BM and those without revealed median OS values of 27.55 months and 32.5 months, respectively, with corresponding 5-year OS rates of 8.3 and 31.8%, respectively (P=0.001; Fig. 5). The median OS for stage I, II and III patients was 61.15, 48.5 and 28.4 months, respectively, with 5-year OS rates of 62.5, 47.1 and 21.6%, respectively (P<0.001; Fig. 6). Univariate analysis revealed several factors significantly associated with OS, including initial tumor maximum diameter (P=0.003), N staging (P<0.001), clinical staging (P<0.001), treatment modality (P=0.002), treatment response (P<0.001), and the presence of BM (P=0.001) (Table IV). Multivariate Cox regression analysis revealed that the presence of BM (HR=1.934; 95% CI: 1.358–2.764; P<0.001) and clinical staging (HR=1.741; 95% CI: 11.102–2.750; P=0.018) as significant independent risk factors for OS (Table IV).
Table IV.Cox proportional risk model analysis affecting overall survival in patients with limited stage-small cell lung cancer. |
Discussion
For patients with LS-SCLC who exhibit a favorable initial response to treatment, PCI is recommended as a class I intervention according to the National Comprehensive Cancer Network guidelines (21). In the modern MRI era, studies have reported that patients with LS-SCLC who did not receive PCI experienced a 1- and 3-year BM rate of 23.8 and 41.3%, respectively (22,23). Conversely, the 3-year BM rate among patients who underwent PCI was reported to be notably lower at 11.2% (24), and the 5-year progression-free rate for BM was 69% (25). The results of the present study revealed a 3-year BM rate of 12.8% post-PCI in patients with LS-SCLC, aligning closely with the outcomes observed in the aforementioned research.
The findings of the present study demonstrate that patients with an initial tumor maximum diameter of >5 cm at the time of initial diagnosis exhibit a substantially elevated risk of BM following PCI. This observation is consistent with prior research indicating that higher clinical stages, which consider local spread and tumor size, are associated with an increased risk of BM development. Levy et al (26) reported an association between the volume of the primary tumor in the thorax and the subsequent risk of BM in patients with LS-SCLC. Similarly, Chen et al (27) performed a retrospective analysis on 550 patients with LS-SCLC and reported that an initial tumor maximum diameter of >5 cm was a notable risk factor for BM. This increased risk may be attributed to larger tumors dispersing more malignant cells into the circulatory system, which then potentially seed metastases in distant organs (28).
In the present study, further analysis was performed on the factors affecting the prognosis of patients with SCLC following PCI. It was observed that patients developing BM post-PCI exhibited a significantly lower OS compared with those without BM, with a median OS of 27.55 months vs. 32.5 months, and five-year OS rates at 8.3 and 31.8%, respectively (P=0.001). Cen et al (29) reported that BM serve as an independent risk factor for the prognosis of patients with SCLC post-PCI. Moreover, the present study identified clinical staging as an independent risk factor influencing the OS of patients with LS-SCLC after PCI. Kim et al (30) noted that in patients aged ≥65 with stage II–III disease, PCI did not confer marked survival advantages. Similarly, Farooqi et al (31) reported no improvement in OS for individuals aged ≥70 with tumor diameters of ≥5 cm following PCI. Furthermore, the size of the tumor at initial diagnosis in the present study was not significantly associated with a worse OS. However, previous research suggests that larger tumor size may indicate a more aggressive phenotype, elevating the risk of metastasis, especially BM. Patients in advanced stages may exhibit higher rates of extracranial disease progression, potentially masking the survival benefits of PCI (27,31). This highlights the crucial role of utilizing TNM clinical staging more extensively for guiding clinical decisions (32).
The preventive role of PCI in reducing BM risk for patients with LS-SCLC achieving CR after CRT is well-established. Despite this, 16.6% of patients still developed BM post-PCI in the present study, suggesting that a fraction of patients with LS-SCLC who receive curative CRT may not benefit from PCI. Further research is necessary to delineate the characteristics of these patients. Given the limitations of traditional imaging methods such as CT and MRI in assessing early therapeutic effectiveness and prognostic outcomes, the exploration of molecular biomarkers for the early prediction of BM and evaluation of PCI efficacy represents a vital research direction. Slotman et al (33) examined the effectiveness of PCI in ES-SCLC, categorizing patients into a brain radiotherapy group and a control group, each consisting of 143 patients. The brain radiotherapy group received different dosages: 20 Gy in 5 fractions (n=89), 30 Gy in 10 fractions (n=23), 30 Gy in 12 fractions (n=9) and 25 Gy in 10 fractions (n=7). The results revealed symptomatic BM in 16.8% (n=24) of the radiotherapy group compared with 41.3% (n=59) in the control group (P<0.001). The cumulative risk of BM at 6 and 12 months for the radiotherapy group was 4.4 and 14.6%, respectively, compared with 32.0 and 40.4%, respectively, for the control group. Median disease-free survival was 14.7 weeks in the radiotherapy group and 12.0 weeks in the control group (P=0.02), with median OS at 6.7 and 5.4 months, respectively (P=0.003). The 1-year OS rate was 27.1% in the radiotherapy group and 13.3% in the control group. These findings underscore that PCI can enhance survival and reduce the incidence of subsequent BM in patients with ES-SCLC who respond well to systemic chemotherapy and thoracic radiotherapy.
Moreover, a Phase III randomized controlled trial performed in Japan by Takahashi et al (34) provided contrasting outcomes. This study included patients with ES-SCLC who had responded to platinum-based chemotherapy and exhibited no signs of BM on MRI. Participants were divided into two groups: A PCI group consisting of 113 patients and an observation group of 111 patients. The PCI group received a total of 25 Gy administered in 10 fractions. The findings revealed that the median OS was 11.6 months for the PCI group compared with 13.7 months for the observation group (P=0.094). The 1- and 2-year OS rates were 48.4 and 15.0% for the PCI group, respectively, compared with 53.6 and 18.8% for the observation group, respectively. Furthermore, the cumulative incidence of BM at 6, 12 and 18 months was markedly lower in the PCI group (15.0, 32.9 and 40.1%, respectively) compared with the observation group (46.2, 59.0 and 63.8%, respectively). Despite a significant reduction in the incidence of intracranial metastases (48 vs. 69%; P<0.0001), PCI did not provide a survival advantage.
PCI is implicated in the onset of delayed neurotoxicity, particularly when administered at doses of >3 Gy per fraction and/or in conjunction with CRT (31). Consequently, PCI is contraindicated for patients exhibiting a poor PS of 3–4 or compromised neurocognitive function (35). Additionally, a higher incidence of chronic neurotoxicity is observed in individuals of >60 years (36). The conflicting data from several clinical trials and the growing concerns regarding the use of PCI (33,37,38) led to the initiation of the SWOG S1827/MAVERICK trial in the United States (39). This randomized study evaluated the efficacy of exclusive brain MRI monitoring against the combination of brain MRI and PCI in managing both advanced and early-stage SCLC. Participants were randomly allocated to either the MRI-only group or the combined MRI and PCI group. The primary outcome measure was OS, with secondary outcomes including survival free from cognitive decline, survival free from BM, and rates of adverse events. Although the results are pending, this trial is expected to yield significant insight and data for the future management of SCLC (39). Moreover, the study by Chen et al (27) assessed this subject; however, the present study differs in several key aspects: The present study is based on data from a dual-center collaboration between the Hebei Province Cangzhou Hospital of Integrated Traditional and Western Medicine and the Chengde City Central Hospital, which offers more accurate and reliable statistical outcomes than single-center studies; the enrolled patients were re-staged using the 8th edition of the AJCC Cancer Staging Manual and the Veterans Administration Lung Study Group two-tier system, unlike the study by Chen et al (27), which used the 7th edition, potentially affecting the comparability of stage-related outcomes. The adoption of the widely applied 8th edition staging system enhances the credibility of the results of the present study; in addition to analyzing the incidence of BM post-PCI in LS-SCLC, the present study further assessed the high-risk factors and identified high-risk individuals, providing a clinical basis for tailored monitoring and treatment; and finally, the present study reclassified the nodal status and clinical staging into two groups for statistical analysis, differing from the grouping method of the study by Chen et al (27), thus ensuring data consistency and enhancing the reliability of the results of the present study.
In conclusion, retrospective analyses of patients with LS-SCLC indicate that an initial maximum tumor diameter of >5 cm serves as an independent risk factor for BM following PCI. Furthermore, both BM and clinical staging independently influence OS in these patients post-PCI. Presently, research into the risk factors for BM post-PCI remains sparse and predominantly retrospective. According to the Chinese Society of Clinical Oncology guidelines, concurrent CRT is the standard treatment for patients with LS-SCLC of stage >T1-2N0 (40). If patients cannot tolerate this regimen, sequential CRT is also an option (41). In the present study of 290 patients, 80% were Stage III (n=232), with 50.7% at N3 (n=147). Treatment plans were tailored for each patient using a multidisciplinary team approach, taking into account functional status, laboratory findings and imaging data. Considering the significant adverse reactions from concurrent CRT in Stage III (N3) patients, which many find intolerable, a portion opted for sequential CRT, resulting in a lower proportion of patients undergoing concurrent treatment (54.5%). Therefore, there is a compelling need for more prospective studies to further assess these associations.
Acknowledgements
Not applicable.
Funding
Funding was received from the Scientific Research Fund Program of Hebei Provincial Health and Wellness Commission (grant no. 20211577).
Availability of data and materials
The data generated in the present study may be requested from the corresponding author.
Authors' contributions
GY and JZ performed the data analysis and manuscript writing. RL was responsible for the research design and guided the revision of the manuscript. JD provided the enrolled cases and made substantial contributions to the conception or design of the work. All authors have read and approved the final manuscript. GY and RL confirm the authenticity of all the raw data.
Ethics approval and consent to participate
The current study was performed in accordance with the Declaration of Helsinki and approved by the local ethics committee of the Cangzhou Hospital of Integrated Traditional Chinese and Western Medicine-Hebei Province (Cangzhou, China; approval no. 2021-KY-062.1) and the Chengde Central Hospital (Chengde, China; approval no. CDCHLL2023-407). Each patient provided written informed consent for participation.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
|
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Micke P, Faldum A, Metz T, Beeh KM, Bittinger F, Hengstler JG and Buhl R: Staging small cell lung cancer: Veterans Administration Lung Study Group versus International Association for the Study of Lung Cancer-what limits limited disease? Lung Cancer Amst Neth. 37:271–276. 2002. View Article : Google Scholar : PubMed/NCBI | |
|
Wang B, Guo H, Xu H, Yu H, Chen Y and Zhao G: Research progress and challenges in the treatment of central nervous system metastasis of non-small cell lung cancer. Cells. 10:26202021. View Article : Google Scholar : PubMed/NCBI | |
|
Chauhan AF and Liu SV: Small cell lung cancer: Advances in diagnosis and management. Semin Respir Crit Care Med. 41:435–446. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Chu X, Li S, Xia B, Chu L, Yang X, Ni J, Zou L, Li Y, Xie C, Lin J and Zhu Z: Patterns of brain metastasis immediately before prophylactic cranial irradiation (PCI): implications for PCI optimization in limited-stage small cell lung cancer. Radiat Oncol Lond Engl. 14:1712019. View Article : Google Scholar : PubMed/NCBI | |
|
Wu Q and Chen M, Peng F, Zhang Q, Kong Y, Bao Y, Xu Y, Hu X and Chen M: A study of the prognosis of patients with limited-stage small cell lung cancer who did or did not receive prophylactic cranial irradiation after effective chemoradiotherapy. Front Oncol. 13:11183712023. View Article : Google Scholar : PubMed/NCBI | |
|
Tai P, Assouline A, Joseph K, Stitt L and Yu E: Prophylactic cranial irradiation for patients with limited-stage small-cell lung cancer with response to chemoradiation. Clin Lung Cancer. 14:40–44. 2013. View Article : Google Scholar : PubMed/NCBI | |
|
Aupérin A, Arriagada R, Pignon JP, Le Péchoux C, Gregor A, Stephens RJ, Kristjansen PE, Johnson BE, Ueoka H, Wagner H and Aisner J: Prophylactic cranial irradiation for patients with small-cell lung cancer in complete remission. Prophylactic Cranial Irradiation Overview Collaborative Group. N Engl J Med. 341:476–484. 1999. View Article : Google Scholar : PubMed/NCBI | |
|
Chun SG, Simone CB II, Amini A, Chetty IJ, Donington J, Edelman MJ, Higgins KA, Kestin LL, Movsas B, Rodrigues GB, et al: American Radium Society Appropriate Use Criteria: Radiation Therapy for Limited-Stage SCLC 2020. J Thorac Oncol. 16:66–75. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Couñago F, de la Pinta C, Gonzalo S, Fernández C, Almendros P, Calvo P, Taboada B, Gómez-Caamaño A, Guerra JLL, Chust M, et al: GOECP/SEOR radiotherapy guidelines for small-cell lung cancer. World J Clin Oncol. 12:115–143. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Moreno AC and Lin SH: The optimal treatment approaches for stage I small cell lung cancer. Transl Lung Cancer Res. 8:88–96. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Xu C, Li M, Cai X, Yuan S, Cao J, Zhu S, Chen M, Bi N, Hu X, Li J, et al: Practice patterns of treatment strategy of limited-stage small-cell lung cancer: Survey of Chinese Oncologists. Front Oncol. 12:8723242022. View Article : Google Scholar : PubMed/NCBI | |
|
Yuan M, Zhao Y, Arkenau HT, Lao T, Chu L and Xu Q: Signal pathways and precision therapy of small-cell lung cancer. Signal Transduct Target Ther. 7:1872022. View Article : Google Scholar : PubMed/NCBI | |
|
Patel SR and Das M: Small cell lung cancer: Emerging targets and strategies for precision therapy. Cancers (Basel). 15:40162023. View Article : Google Scholar : PubMed/NCBI | |
|
Rami-Porta R, Asamura H, Travis WD and Rusch VW: Lung cancer-major changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J Clin. 67:138–155. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Chen Y, Wang Y, Ren F, Huang Z, Tan B, Zhao Z, Yu X, Dong P, Yu J and Meng X: Prophylactic cranial irradiation (PCI) versus active surveillance in patients with limited-stage small cell lung cancer: A retrospective, multicentre study. Respir Res. 23:2742022. View Article : Google Scholar : PubMed/NCBI | |
|
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, et al: New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247. 2009. View Article : Google Scholar : PubMed/NCBI | |
|
Gondi V, Deshmukh S, Brown PD, Wefel JS, Armstrong TS, Tome WA, Gilbert MR, Konski A, Robinson CG, Bovi JA, et al: Sustained preservation of cognition and prevention of patient-reported symptoms with hippocampal avoidance during whole-brain radiation therapy for brain metastases: Final results of NRG oncology CC001. Int J Radiat Oncol Biol Phys. 117:571–580. 2023. View Article : Google Scholar : PubMed/NCBI | |
|
Gondi V, Tome WA, Marsh J, Struck A, Ghia A, Turian JV, Bentzen SM, Kuo JS, Khuntia D and Mehta MP: Estimated risk of perihippocampal disease progression after hippocampal avoidance during whole-brain radiotherapy: Safety profile for RTOG 0933. Radiother Oncol. 95:327–331. 2010. View Article : Google Scholar : PubMed/NCBI | |
|
Gondi V, Pugh SL, Tome WA, Caine C, Corn B, Kanner A, Rowley H, Kundapur V, DeNittis A, Greenspoon JN, et al: Preservation of memory with conformal avoidance of the hippocampal neural stem-cell compartment during whole-brain radiotherapy for brain metastases (RTOG 0933): A phase II multi-institutional trial. J Clin Oncol. 32:3810–3816. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
Bogart JA, Waqar SN and Mix MD: Radiation and systemic therapy for limited-stage small-cell lung cancer. J Clin Oncol. 40:661–670. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Pan L, Fan X, Wang L, Wang Y, Li Y, Cui Y, Zheng H, Yi Q and Wu K: Prophylactic cranial irradiation for limited-stage small-cell lung cancer in the magnetic resonance imaging era. Cancer Med. 12:2484–2492. 2023. View Article : Google Scholar : PubMed/NCBI | |
|
Held MK, Hansen O, Schytte T, Hansen KH, Bahij R, Nielsen M, Nielsen TB and Jeppesen SS: Outcomes of prophylactic cranial irradiation in patients with small cell lung cancer in the modern era of baseline magnetic resonance imaging of the brain. Acta Oncol. 61:185–192. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Pezzi TA, Fang P, Gjyshi O, Feng L, Liu S, Komaki R and Lin SH: Rates of overall survival and intracranial control in the magnetic resonance imaging era for patients with limited-stage small cell lung cancer with and without prophylactic cranial irradiation. JAMA Netw Open. 3:e2019292020. View Article : Google Scholar : PubMed/NCBI | |
|
Lim YJ, Song C and Kim HJ; Korean Association for Lung Cancer, Korea Central Cancer Registry, : Survival impact of prophylactic cranial irradiation in small-cell lung cancer in the modern era of magnetic resonance imaging staging. Radiat Oncol. 17:262022. View Article : Google Scholar : PubMed/NCBI | |
|
Levy A, Le Péchoux C, Mistry H, Martel-Lafay I, Bezjak A, Lerouge D, Padovani L, Taylor P and Faivre-Finn C: Prophylactic cranial irradiation for limited-stage small-cell lung cancer patients: Secondary findings from the prospective Randomized phase 3 CONVERT trial. J Thorac Oncol. 14:294–297. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Chen MY, Ji Y, Hu X and Chen M: Factors affecting the risk of brain metastasis in limited-stage small cell lung cancer after prophylactic cranial irradiation. Cancer Manag Res. 14:1807–1814. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Wang X, Ma K, Yang Z, Cui J, He H, Hoffman AR, Hu JF and Li W: Systematic correlation analyses of circulating tumor cells with clinical variables and tumor markers in lung cancer patients. J Cancer. 8:3099–3104. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Cen M, Jin J, Ji Y, Hu X and Chen M: Risk assessment of brain metastases after prophylactic brain irradiation in 550 patients with limited-stage small cell lung cancer who achieved remission through chemoradiotherapy. Chin J Radiat Oncol. 31:138–142. 2022. | |
|
Kim TG, Pyo H, Ahn YC, Noh JM and Oh D: Role of prophylactic cranial irradiation for elderly patients with limited-disease small-cell lung cancer: Inverse probability of treatment weighting using propensity score. J Radiat Res. 60:630–638. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Farooqi AS, Holliday EB, Allen PK, Wei X, Cox JD and Komaki R: Prophylactic cranial irradiation after definitive chemoradiotherapy for limited-stage small cell lung cancer: Do all patients benefit? Radiother Oncol. 122:307–312. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Wolfson AH, Bae K, Komaki R, Meyers C, Movsas B, Le Pechoux C, Werner-Wasik M, Videtic GM, Garces YI and Choy H: Primary analysis of a phase II randomized trial Radiation Therapy Oncology Group (RTOG) 0212: Impact of different total doses and schedules of prophylactic cranial irradiation on chronic neurotoxicity and quality of life for patients with limited-disease small-cell lung cancer. Int J Radiat Oncol Biol Phys. 81:77–84. 2011. View Article : Google Scholar : PubMed/NCBI | |
|
Slotman B, Faivre-Finn C, Kramer G, Rankin E, Snee M, Hatton M, Postmus P, Collette L, Musat E and Senan S; EORTC Radiation Oncology Group and Lung Cancer Group, : Prophylactic cranial irradiation in extensive small-cell lung cancer. N Engl J Med. 357:664–672. 2007. View Article : Google Scholar : PubMed/NCBI | |
|
Takahashi T, Yamanaka T, Seto T, Harada H, Nokihara H, Saka H, Nishio M, Kaneda H, Takayama K, Ishimoto O, et al: Prophylactic cranial irradiation versus observation in patients with extensive-disease small-cell lung cancer: A multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 18:663–671. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Choi M, Lee Y, Moon SH, Han JY, Kim HT and Lee JS: Effect of accurate staging using positron emission tomography on the outcomes of prophylactic cranial irradiation in patients with limited stage small-cell lung cancer. Clin Lung Cancer. 18:77–84. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Zeng H, Hendriks LEL, van Geffen WH, Witlox WJA, Eekers DBP and De Ruysscher DKM: Risk factors for neurocognitive decline in lung cancer patients treated with prophylactic cranial irradiation: A systematic review. Cancer Treat Rev. 88:1020252020. View Article : Google Scholar : PubMed/NCBI | |
|
Mehta MP: Models support prophylactic cranial irradiation. J Clin Oncol. 24:3524–3526. 2006. View Article : Google Scholar : PubMed/NCBI | |
|
Witlox WJA, Ramaekers BLT, Lacas B, Le Pechoux C, Pignon JP, Sun A, Wang SY, Hu C, Redman M, van der Noort V, et al: Individual patient data meta-analysis of prophylactic cranial irradiation in locally advanced non-small cell lung cancer. Radiother Oncol. 158:40–47. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Taylor JM, Rusthoven CG and Moghanaki D: Prophylactic cranial irradiation or MRI surveillance for extensive stage small cell lung cancer. J Thorac Dis. 12:6225–6233. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Zeng H, De Ruysscher DKM, Hu X, Zheng D, Yang L, Ricardi U, Kong FMS and Hendriks LEL: Radiotherapy for small cell lung cancer in current clinical practice guidelines. J Natl Cancer Cent. 2:113–125. 2022. View Article : Google Scholar | |
|
Takada M, Fukuoka M, Kawahara M, Sugiura T, Yokoyama A, Yokota S, Nishiwaki Y, Watanabe K, Noda K, Tamura T, et al: Phase III study of concurrent versus sequential thoracic radiotherapy in combination with cisplatin and etoposide for limited-stage small-cell lung cancer: Results of the Japan Clinical Oncology Group Study 9104. J Clin Oncol. 20:3054–3060. 2002. View Article : Google Scholar : PubMed/NCBI |