Rare primary intrapulmonary malignant peripheral nerve sheath tumor showing significant response to sintilimab: A case report and literature review
- Authors:
- Published online on: July 4, 2024 https://doi.org/10.3892/ol.2024.14556
- Article Number: 423
-
Copyright: © Chen et al. This is an open access article distributed under the terms of Creative Commons Attribution License.
Abstract
Introduction
Malignant peripheral nerve sheath tumor (MPNST) is a rare, biologically aggressive subtype of soft tissue sarcomas (STS), accounting for 5–10% of all STS (1). It is a high-grade spindle-cell tumor originating from the peripheral nerve sheaths (2), with high malignancy and poor prognosis. A retrospective review from the Mayo Clinic Arizona by Stucky et al (3) indicated that high tumor grade and tumor size ≥50 mm predict undesirable disease-specific survival for MPNST. The incidence of MPNST is low, only 0.001% in the general population, with no gender predilection. Neurofibromatosis type 1 (NF1) is the most important risk factor, with ~10% of patients with NF1 developing MPNST during their lifetime (4). Furthermore, patients with prior radiation exposure also have a higher incidence of MPNST than the general population (5), and MPNST induced by radiation accounts for ~5% of all MPNSTs (6). MPNSTs can grow throughout the whole body, but most commonly occur in the extremities, the proximal parts of the trunk, as well as the head and neck (7). The occurrence of intrapulmonary MPNST is exceedingly minimal (8–12).
At present, there is still no standard treatment for MPNST. The existing treatment options are mostly based on the treatment of STS. Although surgery is the preferred treatment for MPNST, it's difficult to achieve extended or complete resection due to its high aggressiveness. The role of radiation, chemotherapy and targeted therapy for MPNST is still limited and uncertain (13). Programmed death 1 (PD-1)/programmed death-ligand 1 (PD-L1)-related immune checkpoint inhibitors (ICIs) as an emerging and promising cure have been proven to be effective for diversified cancer. However, due to the rarity of MPNST, there are few large-scale randomized controlled trials on the effectiveness of immunotherapy in MPNST.
The present study reported a case of intrapulmonary MPNST in an elderly man who received sintilimab and achieved a remarkable response. Compared with previous case reports of MPNST, this case has several particularities. First, it is worth noting that the primary location of MPNST in the lung is something of a rarity. Furthermore, this patient had no pulmonary symptoms but a large space-occupying lesion in the right upper lung lobe, which was found due to dizziness and lower limb fatigue by coincidence. Of note, single-agent immunotherapy was greatly effective in this patient with intrapulmonary MPNST who had not received any anti-tumor therapy in the past.
Case report
A 63-year-old man visited the Neurology Department of Zhongshan Hospital of Traditional Chinese Medicine (Zhongshan, China) in March 2023 with complaints of dizziness and weakness. The patient had no family history of NF1 and any other cancer. The patient had not received any radiotherapy. Computed tomography (CT) scans of the brain, chest and abdomen were ordered as parts of the examinations. Unexpectedly, the chest and abdominal CT examination showed a giant mass in the right upper lung lobe invading the adjacent chest wall and the third and fourth ribs, and its size was 91×70 mm. The primary consideration was malignancy. Multiple metastases were also found in both lungs, mediastinal lymph nodes, liver and bilateral iliac bone. A circular low-density mass with a size of 54×47 mm in liver segment 8, with blurred boundaries, was observed. No primary tumors were found in any other areas, so the large mass in the right upper lung lobe was considered to be the primary lesion. Various tumor markers were within the normal range. After being seen by an oncologist, the patient was referred to the Oncology Department of Zhongshan Hospital of Traditional Chinese Medicine (Zhongshan, China) and underwent a percutaneous lung puncture biopsy one week after the initial presentation. Examination of the histopathological image stained with hematoxylin and eosin according to a standard protocol indicated the following: The puncture tissue of the right lung mass showed a large amount of necrosis under the microscope, and the local cells were fusiform and oval (Fig. 1A-D). Tumor tissue was stained according to a standard immunohistochemical protocol (14). The final immunohistochemical results showed that the tumor stained positive for Vimentin (anti-Vimentin antibody: Cat. no. Kit-0019; MXB; pre-diluted) (data not shown), SOX10 (anti-SOX10 antibody: Cat. no. RMA-0726; MXB; pre-diluted) (data not shown), Ki-67 (20%) (anti-Ki67 antibody: Cat. no. RMA-0542; MXB; pre-diluted) (Fig. 1F), S-100 protein (anti-S-100 protein antibody: Cat. no. Kit-0007; MXB; pre-diluted) (Fig. 1G), histone H3 lysine 27 trimethylation (H3K27Me3) (anti-H3K27Me3 antibody: Cat. no. RMA-0843; MXB; pre-diluted) (Fig. 1H) and the tumor proportion score of PD-L1 (anti-PD-L1 antibody: Cat. no. HY-13421; DAKO; 1:50 dilution) was 60% (Fig. 1E). Taking into account these factors, this patient was finally diagnosed with primary intrapulmonary MPNST.
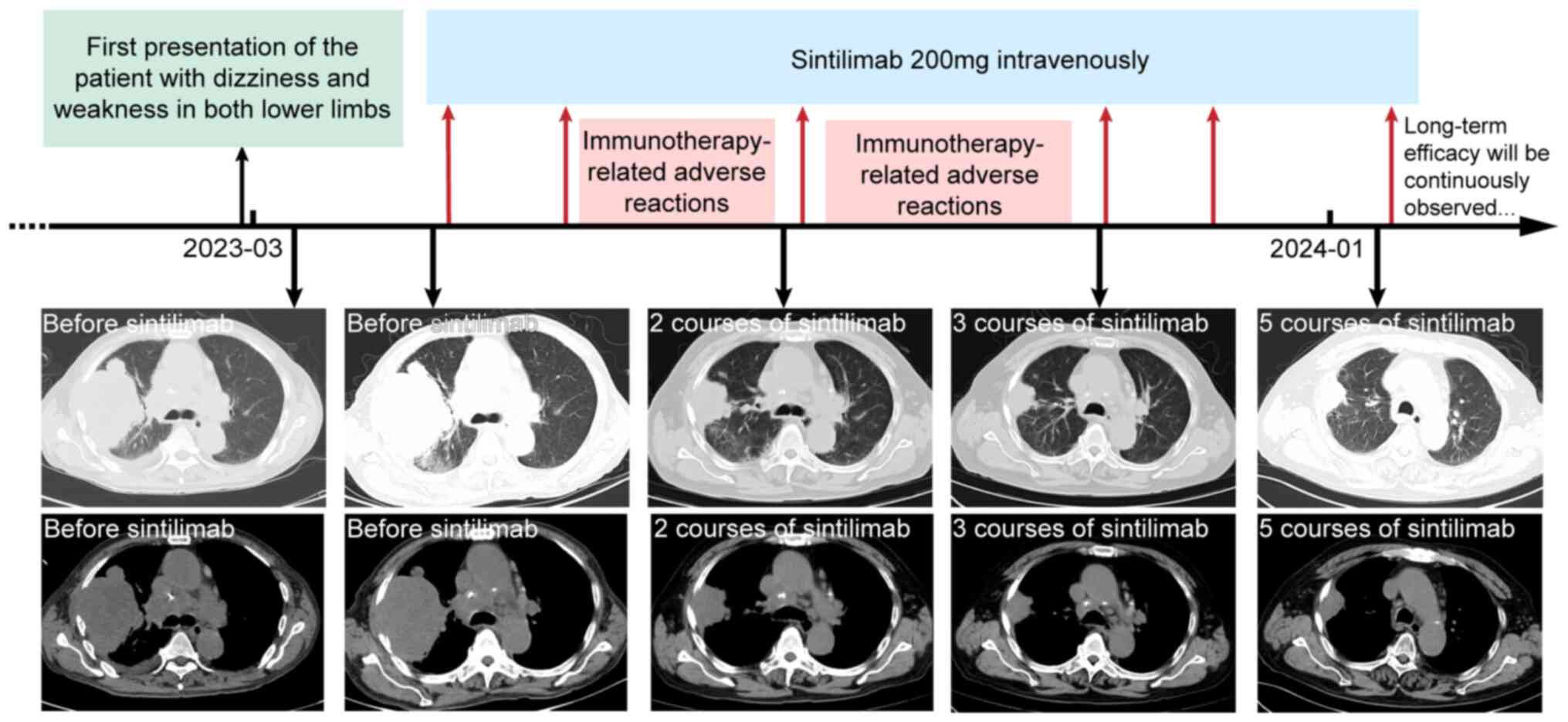
After the diagnosis, the patient refused to undergo surgery or chemotherapy. Considering that PD-L1 expression in 60% of tumor cells, it was decided to use pembrolizumab for treatment after reviewing relevant case reports. However, the patient refused to use pembrolizumab due to its high cost, and the more affordable sintilimab was started at a dose of 200 mg every 21 days in late April 2023. Initially, no immunotherapy-related adverse reactions (irAEs) occurred. After receiving the second course of sintilimab in late May 2023, the patient developed symptoms of generalized skin itching. Due to the irAEs, the patient did not proceed with the next course as scheduled. After symptomatic treatment, the patient stabilized and received the third course in August 2023. One week later, the patient developed symptoms of itching again and generalized erythema appeared. After treatment with antihistamines and glucocorticoids, the erythema gradually subsided. The patient was then treated with three further courses of sintilimab in October 2023, November 2023 and January 2024 without any grade 3 or higher irAEs. Sintilimab immunotherapy was scheduled to continue thereafter.
Throughout the immunotherapy period, the patient received a CT scan nearly every three months and each imaging review showed a significant clinical response. Specifically, the chest CT scan from July 2023 (i.e. after having received two courses of sintilimab) showed that the tumor in the right upper lung lobe and multiple metastases were significantly smaller than before. In October 2023 (i.e. after having received three courses of sintilimab), the patient's CT scan exhibited another regression of the tumor in the right upper lung lobe (40×30 mm). The latest chest CT scan in January 2024 (i.e. after having received five courses of sintilimab) revealed the tumor in the right upper lung lobe to be 34×24 mm and the mass in the right hepatic lobe had a diameter of 18 mm. A combination of CT images from all phases suggested marked partial remission of all measurable primary and metastatic lesions. CT manifestations of each metastatic lesion before and after immunotherapy are displayed in Fig. 2. The timeline of the complete treatment process and imaging of each stage are provided in Fig. 3. The long-term efficacy of sintilimab is still being observed in the patient by performing CT examinations every three months.
Discussion
MPNST is an uncommon and growth-delayed tumor with high occultation. Its clinical manifestations have no specificity, and accordingly, early diagnosis of this disease is difficult. Some patients may experience rapidly increasing masses. They may also have corresponding motor and paresthesia neurological symptoms, which are often caused by advanced tumor compression of the nerve (15). However, certain patients may be asymptomatic.
MPNST may occur throughout the whole body, with the extremities and trunk as the most common sites, followed by deep soft tissues, retroperitoneum and mediastinum. However, it is rarely observed in the lung. Certain patients with intrapulmonary MPNST may present with chest pain, cough, hemoptysis and dyspnea because of compression of the intercostal nerve or trachea (7). However, the patient of the current study did not present with any pulmonary symptoms and was diagnosed with intrapulmonary MPNST when the tumor had already reached a considerable size. Before this case, there were already seven reported cases of pulmonary MPNST (8–12). Details of these cases are presented in Table I. The surgical treatment of intrapulmonary MPNST has been highlighted in previous cases, whereas this article is the first to report remarkable efficacy of sintilimab in the treatment of this rare malignancy. This is undoubtedly a reflection of the innovativeness of immunotherapy in treating this disease.
The diagnosis of MPNST is one of the most difficult and elusive among STS. Its clinical manifestations, imaging features and histologic features are nonspecific, and thus, the clinical diagnosis relies on immunohistochemistry (15,16). The most studied immunohistochemical marker is S-100 protein. S-100 is usually weakly or patchily present in MPNST cases. S-100 expression may be present in 50–60% of MPNST tumor cells. Strong diffuse staining for S-100 nearly excludes a diagnosis of MPNST, except for epithelioid MPNST (17). At times, positive expression of SOX10, Ki-67, cytokeratin and glial fibrillary acidic protein may be found in MPNST tumor cells, but the diagnostic value of these immunohistochemical markers is limited (17–20). H3K27me3 is a new immunohistochemical marker for MPNST, which has better sensitivity and specificity than S-100. Approximately 80% of high-grade MPNSTs, 60% of intermediate-grade MPNSTs and 30% of low-grade MPNSTs showed loss of H3K27me3 expression (21). Several studies have assessed H3K27me3 in MPNST by immunohistochemistry and found that a subset of MPNST retained H3K27me3 expression (22–24). H3K27me3 loss is frequent in radiotherapy-related, NF1-related and sporadic MPNST, but it is less sensitive in low-grade and intermediate-grade tumors. Therefore, H3K27me3 loss, although more specific, is not a fully sensitive immunohistochemical marker.
At present, surgery remains the preferred treatment for MPNST. However, not all patients with MPNST can be treated with surgery (25). Whether MPNST can be resected or not mainly depends on the size of the tumor, the growth site of the tumor and the scope of nerve invasion of the tumor. Extensive local resection is more effective for MPNST involving distal extremities (26). However, for MPNST in the head, neck, chest and abdomen, it is difficult to achieve exact extensive resection because of tumors' proximity to vital organs, blood vessels and nerves. The local recurrence rate of MPNST following gross total resection is as high as 32–65% due to the limitations in the extent of resection and high aggressiveness of the tumor (13).
Radiotherapy is often used in conjunction with surgery to improve the local control rate of MPNST, but only has a minor effect on long-term survival and increases the risk of radiation-induced sarcoma (15,26). Chemotherapy regimens for MPNST are mostly based on STS. At present, the main first-line chemotherapeutic agents are doxorubicin and ifosfamide. When these two agents were used in combination to treat STS, the Response Evaluation Criteria in Solid Tumors (RECIST) response rate was ~25%; however, the RECIST response rate for MPNST was only 21% (27). Gemcitabine, docetaxel and etoposide can be used as second-line chemotherapeutic agents, but their efficacy is not optimal. There is insufficient data on the roles of radiotherapy and chemotherapy in MPNST management, and their roles remain controversial and uncertain. At present, radiotherapy and chemotherapy are still the main palliative treatments routinely used to alleviate local symptoms, due to the limited treatment options for MPNST.
With the deepening of the understanding of MPNST pathogenesis, certain clinical trials using targeted therapy blocking known signaling pathways that drive MPNST pathogenesis are underway (e.g. NCT05107037 and NCT02584647) or completed (e.g. NCT01661283 and NCT02008877). However, so far, existing research showed that the efficacy of targeted therapy for MPNST is also unsatisfactory (13).
PD-1 and PD-L1 can limit the killing effect of T cells on tumors and help avoid autoimmunity (28). Therefore, blocking PD-1/PD-L1 is an important method of tumor immunotherapy. PD-1/PD-L1-related ICIs are ideal tumor immunotherapy agents. Furthermore, PD-L1 expression by tumor cells has been identified as a predictive immunotherapy biomarker for the response to PD-1/PD-L1-related ICIs (29). Although vast information about the use of PD-1/PD-L1-related ICIs in treating common cancer has been published, limited data on the use of immunotherapy in MPNST and the expression of PD-L1 in MPNST are available. A study by Wang et al (30) described PD-1/PD-L1 axis-mediated immune escape mechanisms and revealed that PD-L1 is expressed in NF1- and NF2-associated tumors. A study by Farschtschi et al (31) showed that NF1 patients with MPNST had higher serum levels of PD-L1 compared with NF1 patients without MPNST and indicated that PD-L1 is upregulated in patients with MPNST. Another study by Liu et al (32) also proved PD-L1 expression in MPNST. Furthermore, prior to this case, there were four reports of patients with MPNST achieving significant remission after immunotherapy (33–36). Details of these cases are provided in Table II. Of these cases, three involved treatment with pembrolizumab, one after two courses combination of epirubicin, ifosfamide and mesna (33), one in combination with procarbazide (34), and one after surgical resection and radiation therapy (35). Furthermore, one case involved treatment with nivolumab plus radiation (36). Overall, significant remission was consistently seen in all five PD-L1-positive patients with MPNST treated with immunotherapy. These clinical studies and case reports supported the possibility of immunotherapy for MPNST and suggested immunotherapy as a promising treatment for MPNST that needs further exploration, particularly those ICIs aimed at inhibiting the PD-1/PD-L1 signaling axis.
Table II.List of case reports of malignant peripheral nerve sheath tumor treated with immunotherapy. |
Unlike the other four case reports, the patient with high PD-L1 expression in the present case had not received any prior anti-tumor therapy. The patient was treated with single-agent sintilimab without combining it with surgery, chemotherapy, radiotherapy or targeted therapy. For economic reasons, the patient chose the more affordable sintilimab instead of pembrolizumab. Sintilimab has been included in Chinese medical insurance in 2022, so it is more affordable than pembrolizumab, and the financial burden of patients is relatively small. Although previous case reports have reported on the use of pembrolizumab or nivolumab combined with chemotherapy or radiotherapy for MPNST, the efficacy of sintilimab alone was also excellent in this case. Pembrolizumab, nivolumab and sintilimab are humanized monoclonal IgG4 antibodies against PD-1. They can bind to PD-1 to block the connection of PD-1 with its ligands and impede inhibitory signals in T cells. While data from large-scale randomized clinical trials on the efficacy and safety immunotherapy for MPNST are scarce, several clinical trials on immunotherapy for MPNST are currently recruiting. Updated results of a phase II trial (NCT03611868) showed that alrizomadlin combined with pembrolizumab was well tolerated and demonstrated preliminary anti-tumor activity in an MPNST cohort with a 40% clinical benefit rate (37). A phase ІІ clinical trial (NCT02691026) is underway on the efficacy of pembrolizumab in patients with MPNST. There are also two ongoing clinical trials (NCT02834013 and NCT04465643) on the efficacy of nivolumab plus ipilimumab for MPNST (38,39). However, no clinical trial has been conducted on the efficacy and safety of sintilimab for MPNST, and it is necessary to perform this in the future.
The present study reported for the first time that sintilimab single-agent immunotherapy achieved a remarkable response of intrapulmonary MPNST. From this and previous cases, it may be speculated that single-agent immunotherapy may be a good choice of first-line treatment for MPNST in patients with high PD-L1 expression or in patients with an Eastern Cooperative Oncology Group Performance Status (ECOG PS) of 3–4 who cannot tolerate high-intensity chemotherapy. Immunotherapy in combination with chemotherapy may be a viable treatment option for MPNST in patients with low PD-L1 expression or in patients with an ECOG PS of 0–2. Rational combination of immunotherapy regimens may yield significant results. Additional prospective trials are still needed to confirm these preliminary results.
In conclusion, the case reported in the present study illustrates that PD-1/PD-L1-related ICIs may be an effective therapeutic method for patients with primary intrapulmonary MPNST with positive PD-L1 expression. Particularly for patients with high PD-L1 expression, a remarkable response may be achieved by using PD-1/PD-L1-related ICIs as first-line treatment. We are confident about the outlook of immunotherapy for MPNST and expect that the outcome of the ongoing clinical trials will contribute to the design of personalized immunotherapy.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study are included in the figures and/or tables of this article.
Authors' contributions
Manuscript writing, literature search and acquisition of data: YQC. Treatment and observation of the patient, study conception and design: TC. Manuscript drafting, aggregation of materials and analysis of data: WSZ, LZL and CTF. Manuscript revision, manuscript reviewing for intellectual content and interpretation of data: HTZ. All authors have read and approved the final manuscript. HTZ and YQC have confirm the authenticity of all the raw data.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Written informed consent was obtained from the patient to publish this report and any associated accompanying images.
Competing interests
The authors declare that they have no competing interests.
Glossary
Abbreviations
Abbreviations:
|
MPNST |
malignant peripheral nerve sheath tumor |
|
PD-1 |
programmed death 1 |
|
PD-L1 |
programmed death-ligand 1 |
|
STS |
soft tissue sarcomas |
|
NF1 |
neurofibromatosis type 1 |
|
ICI |
immune checkpoint inhibitor |
|
CT |
computed tomography |
|
H3K27Me3 |
histone H3 lysine 27 trimethylation |
|
irAEs |
immunotherapy-related adverse reactions |
|
RECIST |
Response Evaluation Criteria in Solid Tumors |
|
ECOG PS |
Eastern Cooperative Oncology Group Performance Status |
References
|
Fuchs B, Spinner RJ and Rock MG: Malignant peripheral nerve sheath tumors: An update. J Surg Orthop Adv. 14:168–174. 2005.PubMed/NCBI | |
|
Widemann BC: Current status of sporadic and neurofibromatosis type 1-associated malignant peripheral nerve sheath tumors. Curr Oncol Rep. 11:322–328. 2009. View Article : Google Scholar : PubMed/NCBI | |
|
Stucky CCH, Johnson KN, Gray RJ, Pockaj BA, Ocal IT, Rose PS and Wasif N: Malignant peripheral nerve sheath tumors (MPNST): The Mayo Clinic experience. Ann Surg Oncol. 19:878–885. 2012. View Article : Google Scholar : PubMed/NCBI | |
|
Goertz O, Langer S, Uthoff D, Ring A, Stricker I, Tannapfel A and Steinau HU: Diagnosis, treatment and survival of 65 patients with malignant peripheral nerve sheath tumors. Anticancer Res. 34:777–783. 2014.PubMed/NCBI | |
|
Yaga US, Shivakumar R, Kumar MA and Sathyaprakash: Malignant peripheral nerve sheath tumor: A rarity. Indian J Dent. 6:53–56. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Riad S, Biau D, Holt GE, Werier J, Turcotte RE, Ferguson PC, Griffin AM, Dickie CI, Chung PW, Catton CN, et al: The clinical and functional outcome for patients with radiation-induced soft tissue sarcoma. Cancer. 118:2682–2692. 2012. View Article : Google Scholar : PubMed/NCBI | |
|
Kolarov V, Stanić J, Eri Z, Zvezdin B, Kojičić M and Hromis S: Intrathoracic malignant peripheral nerve sheath tumor with poor outcome: A case report. Bosn J Basic Med Sci. 10:328–330. 2010. View Article : Google Scholar : PubMed/NCBI | |
|
Maane LA, Al Bouzidi A, Damou M and Ismaili N: Primary intrapulmonary malignant peripheral nerve sheath tumor: A rare case. Cancer Treat Res Commun. 25:1002432020. View Article : Google Scholar : PubMed/NCBI | |
|
Grzywa-Celińska A, Szmygin-Milanowska K, Emeryk-Maksymiuk J, Walczyna M, Palonka M and Siwiec J: Malignant peripheral nerve sheath tumor in a patient without neurofibromatosis 1 (NF1): A rare case of primary lung location. J Educ Health Sport. 8:11–17. 2018. | |
|
Inci I, Soltermann A, Schneiter D and Weder W: Pulmonary malignant peripheral nerve sheath tumour. Eur J Cardiothorac Surg. 46:331–332. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
La Mantia E, Franco R, Cantile M, Rocco R, De Chiara A, Martucci N and Rocco G: Primary intrapulmonary malignant peripheral nerve sheath tumor mimicking lung cancer. J Thorac Dis. 5:E155–E157. 2013.PubMed/NCBI | |
|
Desdiani D, Darifah S and Azali C: Giant intrapulmonary malignant peripheral nerve sheath tumour. Respirol Case Rep. 8:e005672020. View Article : Google Scholar : PubMed/NCBI | |
|
Bradford D and Kim A: Current treatment options for malignant peripheral nerve sheath tumors. Curr Treat Options Oncol. 16:3282015. View Article : Google Scholar : PubMed/NCBI | |
|
Committee of Consensus of Immunohistochemistry Test on Technology, . Consensus of immunohistochemistry test on technology. Zhonghua Bing Li Xue Za Zhi. 48:87–91. 2019.(In Chinese). PubMed/NCBI | |
|
Farid M, Demicco EG, Garcia R, Ahn L, Merola PR, Cioffi A and Maki RG: Malignant peripheral nerve sheath tumors. Oncologist. 19:193–201. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
Sharma S, Shah JS and Bali H: Malignant peripheral nerve sheath tumor: A rare malignancy. J Oral Maxillofac Pathol. 24 (Suppl 1):S86–S90. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Thway K and Fisher C: Malignant peripheral nerve sheath tumor: Pathology and genetics. Ann Diagn Pathol. 18:109–116. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
Pekmezci M, Reuss DE, Hirbe AC, Dahiya S, Gutmann DH, von Deimling A, Horvai AE and Perry A: Morphologic and immunohistochemical features of malignant peripheral nerve sheath tumors and cellular schwannomas. Mod Pathol. 28:187–200. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Rodriguez FJ, Folpe AL, Giannini C and Perry A: Pathology of peripheral nerve sheath tumors: Diagnostic overview and update on selected diagnostic problems. Acta Neuropathol. 123:295–319. 2012. View Article : Google Scholar : PubMed/NCBI | |
|
Olsen SH, Thomas DG and Lucas DR: Cluster analysis of immunohistochemical profiles in synovial sarcoma, malignant peripheral nerve sheath tumor, and Ewing sarcoma. Mod Pathol. 19:659–668. 2006. View Article : Google Scholar : PubMed/NCBI | |
|
Schaefer IM and Fletcher CDM: Recent advances in the diagnosis of soft tissue tumours. Pathology. 50:37–48. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Prieto-Granada CN, Wiesner T, Messina JL, Jungbluth AA, Chi P and Antonescu CR: Loss of H3K27me3 expression is a highly sensitive marker for sporadic and radiation-induced MPNST. Am J Surg Pathol. 40:479–489. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Schaefer IM, Fletcher CD and Hornick JL: Loss of H3K27 trimethylation distinguishes malignant peripheral nerve sheath tumors from histologic mimics. Mod Pathol. 29:4–13. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Cleven AH, Al Sannaa GA, Briaire-De Bruijn I, Ingram DR, van de Rijn M, Rubin BP, de Vries MW, Watson KL, Torres KE, Wang WL, et al: Loss of H3K27 tri-methylation is a diagnostic marker for malignant peripheral nerve sheath tumors and an indicator for an inferior survival. Mod Pathol. 29:582–590. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Grobmyer SR, Reith JD, Shahlaee A, Bush CH and Hochwald SN: Malignant peripheral nerve sheath tumor: Molecular pathogenesis and current management considerations. J Surg Oncol. 97:340–349. 2008. View Article : Google Scholar : PubMed/NCBI | |
|
Gupta G, Mammis A and Maniker A: Malignant peripheral nerve sheath tumors. Neurosurg Clin N Am. 19533–543. (v)2008. View Article : Google Scholar : PubMed/NCBI | |
|
Kroep JR, Ouali M, Gelderblom H, Le Cesne A, Dekker TJA, Van Glabbeke M, Hogendoorn PCW and Hohenberger P: First-line chemotherapy for malignant peripheral nerve sheath tumor (MPNST) versus other histological soft tissue sarcoma subtypes and as a prognostic factor for MPNST: An EORTC soft tissue and bone sarcoma group study. Ann Oncol. 22:207–214. 2011. View Article : Google Scholar : PubMed/NCBI | |
|
Chamoto K, Al-Habsi M and Honjo T: Role of PD-1 in immunity and diseases. Curr Top Microbiol Immunol. 410:75–97. 2017.PubMed/NCBI | |
|
Dong ZY, Wu SP, Liao RQ, Huang SM and Wu YL: Potential biomarker for checkpoint blockade immunotherapy and treatment strategy. Tumor Biol. 37:4251–4261. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Wang S, Liechty B, Patel S, Weber JS, Hollmann TJ, Snuderl M and Karajannis MA: Programmed death ligand 1 expression and tumor infiltrating lymphocytes in neurofibromatosis type 1 and 2 associated tumors. J Neurooncol. 138:183–190. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Farschtschi S, Kluwe L, Park SJ, Oh SJ, Mah N, Mautner VF and Kurtz A: Upregulated immuno-modulator PD-L1 in malignant peripheral nerve sheath tumors provides a potential biomarker and a therapeutic target. Cancer Immunol Immunother. 69:1307–1313. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Liu J, Li H, Wei C, Li Q and Wang Z: PD-L1 expression and tumor infiltrating lymphocytes in neurofibromatosis type 1-related benign tumors and malignant peripheral nerve sheath tumors: An implication for immune checkpoint inhibition therapy. Chin J Plast Reconstr Surg. 3:63–75. 2021. View Article : Google Scholar | |
|
Larson K, Russ A, Arif-Tiwari H, Mahadevan D, Elliott A, Bhattacharyya A and Babiker H: Pembrolizumab achieves a complete response in an NF-1 mutated, PD-L1 positive malignant peripheral nerve sheath tumor: A case report and review of the benchmarks. J Immunother. 45:222–226. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Payandeh M, Sadeghi M and Sadeghi E: Complete response to pembrolizumab in a patient with malignant peripheral nerve sheath tumor: The first case reported. J App Pharm Sci. 7:182–184. 2017. | |
|
Davis LE, Nicholls LA, Babiker HM, Liau J and Mahadevan D: PD-1 inhibition achieves a complete metabolic response in a patient with malignant peripheral nerve sheath tumor. Cancer Immunol Res. 7:1396–1400. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Özdemir BC, Bohanes P, Bisig B, Missiaglia E, Tsantoulis P, Coukos G, Montemurro M, Homicsko K and Michielin O: Deep response to anti-PD-1 therapy of metastatic neurofibromatosis type 1-associated malignant peripheral nerve sheath tumor with CD274/PD-L1 amplification. JCO Precis Oncol. 3:1–6. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Mckean M, Tolcher AW, Reeves JA, Chmielowsk B, Shaheen MF, Beck JT, Orloff MM, Somaiah N, Van Tine BA, Drabick JJ, et al: Newly updated activity results of alrizomadlin (APG-115), a novel MDM2/p53 inhibitor, plus pembrolizumab: Phase 2 study in adults and children with various solid tumors. J Clin Oncol. 40 (16 Suppl):S9517–2022. View Article : Google Scholar | |
|
González-Muñoz T, Kim A, Ratner N and Peinado H: The need for new treatments targeting MPNST: The potential of strategies combining MEK inhibitors with antiangiogenic agents. Clin Cancer Res. 28:3185–3195. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Paudel SN, Hutzen B and Cripe TP: The quest for effective immunotherapies against malignant peripheral nerve sheath tumors: Is there hope? Mol Ther Oncolytics. 30:227–237. 2023. View Article : Google Scholar : PubMed/NCBI |