H19 lncRNA mediates 17β-estradiol-induced cell proliferation in MCF-7 breast cancer cells
- Authors:
- Published online on: April 3, 2015 https://doi.org/10.3892/or.2015.3899
- Pages: 3045-3052
Abstract
Introduction
Breast cancer is the most commonly diagnosed cancer and the second leading cause of cancer-related death in females worldwide (1). As estrogens acting via estrogen receptors (ERs) play a critical role in breast cancer development, growth and progression, and over 70% of breast cancers are ER-positive (2); ER is therefore a valuable target for breast cancer therapy (3–5). Although it is well documented that estrogens such as 17β-estradiol (E2), usually stimulate the proliferation of ER-positive breast cancer cells through the regulation of a large number of genes (6,7), it is a great challenge to determine which genes or gene networks underlie the estrogen proliferative response in breast cancer cells.
Recently, long non-coding RNAs (lncRNAs) have been emerged as an important target in cancer development and progression. Studies over the years have shown that lncRNAs, which are non-coding RNAs with a length larger than 200 bp, play a major role in embryogenesis and cell differentiation, and in the development and progression of various diseases including cancers (8–11). lncRNAs may function as either tumor suppressors or tumor promoters in such prevalent cancers as breast and prostate cancer (12). Among lncRNAs, Xist/Tsix, H19, HOTAIR and AIR are a few examples of lncRNAs whose functions have been investigated.
H19 is a well-known imprinted oncofetal gene, which is expressed on maternal alleles without a protein product (13), accumulates in the human placenta and several fetal tissues, and probably plays a pivotal role in embryogenesis and fetal growth and development (14). The H19 gene is localized at human chromosome 11p15.5 (15), in which a variety of disorders including cancer predisposition for both pediatric and adult tumors has been implicated (16). Aberrant expression of H19 is observed in numerous solid tumors, including hepatoma, bladder and breast cancer (16–18), and it has been suggested to be involved in oncogenesis and progression of various types of tumors (19).
Based on the previous observations that H19 expression is associated with the presence of estrogen and progesterone receptors (20), and estrogen regulates H19 expression in breast cancer cells (21), we hypothesized that estrogen-induced cell growth in ER-positive breast cancer cells is mediated through the upregulation of H19 gene expression. Our results indicate that H19 lncRNA is a critical factor in estrogen-stimulated cell growth in breast cancer cells, and blockade of H19 function is a potential strategy for the treatment of ER-positive breast cancer.
Materials and methods
Cell culture and hormone treatment
The MCF-7 and MDA-MB-231 breast cancer cells were obtained from the American Type Culture Collection (ATCC; Manassas, VA, USA). Cells were grown in RPMI-1640 culture medium (Gibco-BRL, Grand Island, NY, USA) supplemented with 10% fetal bovine serum (FBS; HyClone Laboratories, Inc., Logan, UT, USA), 20 mM L-glutamine, 100 U/ml of penicillin and 100 μg/ml of streptomycin. All cells were maintained in a humidified incubator with 5% CO2 at 37°C. Four days before drug treatment, cells were switched to phenol red-free RPMI-1640 medium supplemented with 5% charcoal-dextran-treated FBS (BI Technologies, Fullerton, CA, USA), 20 mM L-glutamine, 100 U/ml of penicillin and 100 μg/ml of streptomycin. Medium was changed every 2 days, and cells were treated with vehicle control [0.1% dimethyl sulfoxide (DMSO)], various concentrations of E2 and ICI 182780 alone or in combination for the times as indicated in each experiment. All chemicals and hormones were obtained from Sigma-Aldrich (St. Louis, MO, USA) except those indicated.
Breast cancer tissue samples
Forty-five patients with breast cancer who underwent surgery at Xiangya Hospital were enrolled in the present study. The resected tumor specimens were immediately frozen in liquid nitrogen and kept at −80°C until RNA extraction. The present study was approved by the Ethics Committee of Xiangya Hospital, China (project no. CTXY-140001-5) and a written informed consent was obtained from each subject.
RNA extraction and lncRNA screening
Total RNAs from harvested cells or tumor tissues were prepared using TRIzol reagent (Omega Bio-Tek, Inc., Norcross, GA, USA) according to the manufacturer’s instructions, and the concentration was determined using a NanoDrop 2000. The quality of the RNA samples was in assurance with an A260/A230 ratio >1.7 and an A260/A280 ratio between 1.8 and 2.0. One microgram of total RNA was subjected to RT-PCR and used for the screening of lncRNAs by the Disease-Related Human lncRNA Profiler kit following the manufacturer’s protocol (SBI, Mountain View, CA, USA). A change of log2(n) >2n was considered a meaningful change.
Quantitative real-time RT-PCR
Total RNA (500 ng) was reversely transcribed in a total volume of 20 μl with 200 units of reverse transcriptase, 50 pmol random hexamer and 1 mM deoxynucleotide triphosphates. The reaction products were then diluted to a total volume of 100 μl with distilled water. The real-time PCR reaction consisted of 2 μl of diluted reverse transcription product, 1X SYBR-Green Master Mix (Applied Biosystems, Foster City, CA, USA) and 50 nM forward and reverse primers. The reaction was carried out in a Roche LightCycler 480 Sequence Detection System for 40 cycles (95°C for 15 sec, 60°C for 1 min) after an initial 10-min incubation at 95°C. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as an internal control. The primers used were: H19, 5′-GTCCGGCCTTCCTGAACACCTT-3′ and 5′-GCTTCACCTTCCAGAGCCGAT-3′; ERα, 5′-CCACCAA CCAGTGCACCATT-3′ and 5′-GGTCTTTTCGTATCCCACCTTTC-3′; and GAPDH, 5′-TTGATTTTGGAGGGATCTCGCTC-3′ and 5′-GAGTCAACGGATTTGGTCGTATTG-3′. The level of RNA was expressed as a fold of the control calculated using the ΔΔCt method.
Knockdown of H19 lncRNA using siRNA and shRNA
Synthetic RNA oligonucleotides targeting ERα and H19 were obtained from RiboBio Co., Ltd. (Guangzhou, China). The siRNA sequences used for ERα were: 5′-CCAGUGCACCAUUGAUAAAdTdT-3′. H19 lncRNA was knocked down using a specific H19 siRNA and a hairpin siRNA vector (shRNAH19) as described by Tsang and Kwok (22). The sequences of H19 siRNA were: 5′-CCTGTAACCAAAAGTGACCG-3′; and the H19 hairpin siRNA sequences were: 5′-CATCAAAGACACCATCGGA-3′ that were subcloned into pSilencer 2.1-U6 neovector (shRNAH19) (RiboBio Co., Ltd.). A negative control siRNA and shRNA were purchased from RiboBio.
H19 expression vector construction
Total cellular RNAs from MCF-7 cells were subjected to reverse-transcription as described above. To construct the H19 expression vector, a 2-step strategy was used. First, the entire H19 RNA was amplified as two fragments by PCR using the following pairs of primers: 5′-AAAAGGATCCAGGGCCCTGCTCTGATTGG-3′ and 5′-AAAAAAGCTTCCTCGTCTCCAGCCCGAAC-3′ for the upstream fragment (1,430 bp); and 5′-GGGCGGG GCGGAGTGAATGAGC-3′ and 5′-AAAAAAGCTTTTGCT GTAACAGTGTTTATTGATGA-3′ for the downstream fragment (1,170 bp). These two PCR fragments were inserted into the pGEM-T easy vector and amplified following transformation to E. coli. Subsequently, the upstream and downstream H19 fragments in the pGEM-T easy vectors were digested with BamHI plus HindIII and BbvCI plus HindIII, respectively, ligated and inserted into the pcDNA3.1 vector as a full-length H19 cDNA. The sequences of the H19 expression vector were confirmed by DNA sequencing.
Transfection study
Cells were plated in phenol red-free medium containing 5% stripped FBS in 12-well plates at a density of ~70%. The transfections of various doses of siRNA or shRNA were performed using Lipofectamine RNAiMAX or Lipofectamine 2000 reagent (Invitrogen, Life Technologies, USA) following the manufacturer’s instructions. Twenty-four hours after transfection, the cells were treated with various hormones for different times and various analyses were performed as indicated in each experiment.
Western blot analysis
Western blotting was performed as previously described with minor modifications (23). Briefly, total cellular proteins were extracted from the harvested cells using a lysis buffer [62.5 mM Tris-HCl pH 6.8, 100 mM dithiothreitol (DTT), 2% SDS and 10% glycerol]. The protein concentrations were determined using the Bradford method with the Bio-Rad protein assay following the manufacturer’s instructions (Bio-Rad, Hercules, CA, USA). Cellular proteins were separated on sodium dodecyl sulfate polyacrylamide gels and transferred to nitrocellulose membranes. Blots were incubated in blocking buffer (5% non-fat dry milk in Tris-buffered saline with 0.5% Tween, TBS-T) at room temperature for 2 h. After washing with TBS-T, the nitrocellulose was incubated with a specific antibody against ERα (Santa Cruz Biotechnology, Santa Cruz, CA, USA) or β-actin (AC-15; Sigma Chemical Co., St. Louis, MO, USA) overnight at 4°C. Following the incubation with a horseradish peroxidase-conjugated secondary antibody, the signal was detected using an ECL Western Blotting system (Promega, Madison WI, USA) and visualized using the Bio-Rad ChemiDoc MP system.
Cell proliferation assay
For the determination of cell proliferation, MCF-7 cells were plated in 96-well plates at ~10,000 cells/well in phenol red-free RPMI-1640 media containing 5% charcoal-dextran-stripped FBS. Twenty-four hours after plating, the cells were treated with either vehicle control or various hormones as indicated in each experiment. For H19 knockdown and overexpression study, the cells were transfected with siRNA, shRNA or H19 expression vector for 24 h, and then treated with various hormones for 48 h. Cell proliferation was assayed using CellTiter 96 AQueous One Solution Cell Proliferation Assay as instructed by the manufacturer (Promega).
Statistical analysis
Each experiment was performed at least three times, and the data are presented as mean ± SEM. For parametric data, the Student’s t-test was used to determine the statistical significance between two groups, and one-way ANOVA following post-hoc Student-Newman-Keuls test was used to compare the difference among multiple groups using the SPSS software. A p-value <0.05 was considered to indicate a statistically significant result.
Results
Screening lncRNAs associated with estrogen action in breast cancer cells
To identify lncRNAs that are potentially associated with ER-positive breast cancer cells and with estrogen action, we employed the Disease-Related Human lncRNA Profiler kit to screen the expression levels of 83 disease-related lncRNAs in the breast cancer cells. As shown in Table I, out of the 83 lncRNAs screened, 18 had a higher expression and 7 had a lower expression as defined by a log2(n) >2n in the ER-positive MCF-7 cells compared to the ER-negative MDA-MB-231 cells. One of the highest differentially expressed lncRNAs was H19 lncRNA with ~3,300-fold difference between the MCF-7 and MDA-MB-231 cells, which was confirmed using quantitative RT-PCR analysis (1.00±0.04 in MDA-MB-231 cells vs. 3,335.48±965.37 in MCF-7 cells). Moreover, out of the 83 lncRNAs screened, only H19 was upregulated while 3 lncRNAs were downregulated when MCF-7 cells were treated with 100 nM E2 for 48 h as shown in Table II.
Table IIThe lncRNAs with a change in expression in MCF-7 cells following treatment with 17β-estradiol. |
H19 is upregulated by E2 in ER-positive MCF-7 cells
To further analyze the E2 regulation of H19 expression in breast cancer cells, MCF-7 cells were treated with various doses of E2 for 4–48 h. As shown in Fig. 1, E2 produced a dose (Fig. 1A) and time-dependent (Fig. 1B) induction of H19 expression. At 48 h of 1 nM E2 treatment, the level of H19 RNA was increased ~19-fold while there was no significant change observed at 4 h of treatment. At doses ranging from 0.05 to 1 nM, E2 caused a dose-dependent induction of H19 expression with a maximal effect observed at 1 nM (Fig. 1A). On the other hand, treatment of ER-negative MDA-MB-231 cells with E2 at doses up to 100 nM failed to induce H19 expression (data not shown).
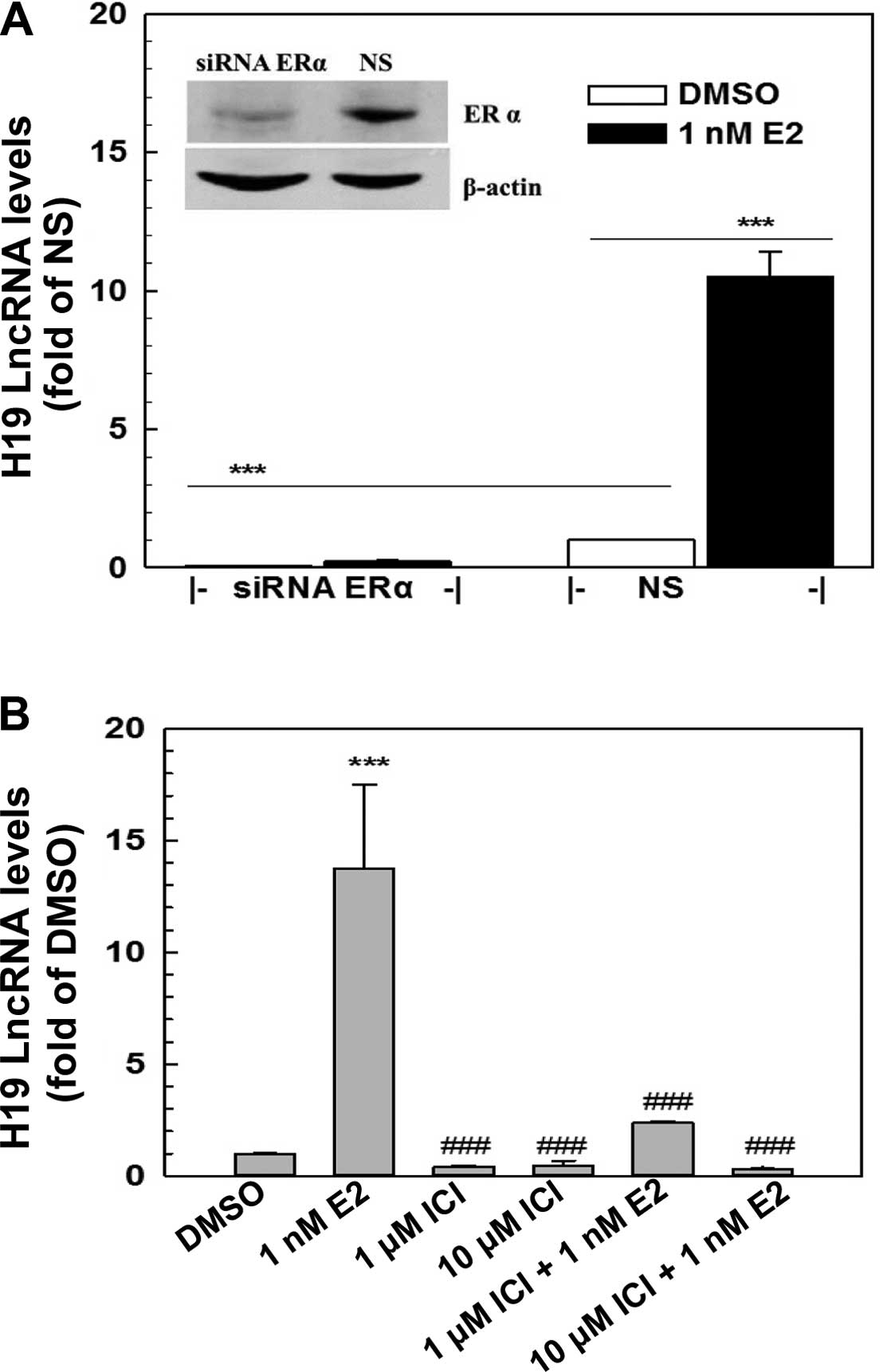
E2-induced H19 expression is mediated through ERα
The genomic action of estrogens is mainly mediated through estrogen receptors, ERα and/or ERβ. ERα yet not ERβ, was expressed in the MCF-7 cells as demonstrated by RT-PCR and western blot analysis (Fig. 2, and data not shown). To determine whether E2 induction of H19 expression is mediated via ERα, a specific ER antagonist, ICI 182780, and a specific ERα siRNA were employed to block ERα action and to knock down ERα expression in the MCF-7 cells, respectively. Transfection of a specific ERα siRNA (100 nM) into MCF-7 cells resulted in a knockdown of ERα of >70% as demonstrated by western blotting (Fig. 2A), leading to a complete elimination of E2-induced H19 expression (Fig. 2A). Furthermore, the E2-induced H19 expression was significantly blocked by ICI 182780, a specific ER antagonist, in a dose-dependent manner (Fig. 2B). The addition of 10 μM ICI 182780 in the medium completely blocked the E2 induction of H19 expression. These data revealed that the E2 induction of H19 expression in MCF-7 cells was mediated through ERα.
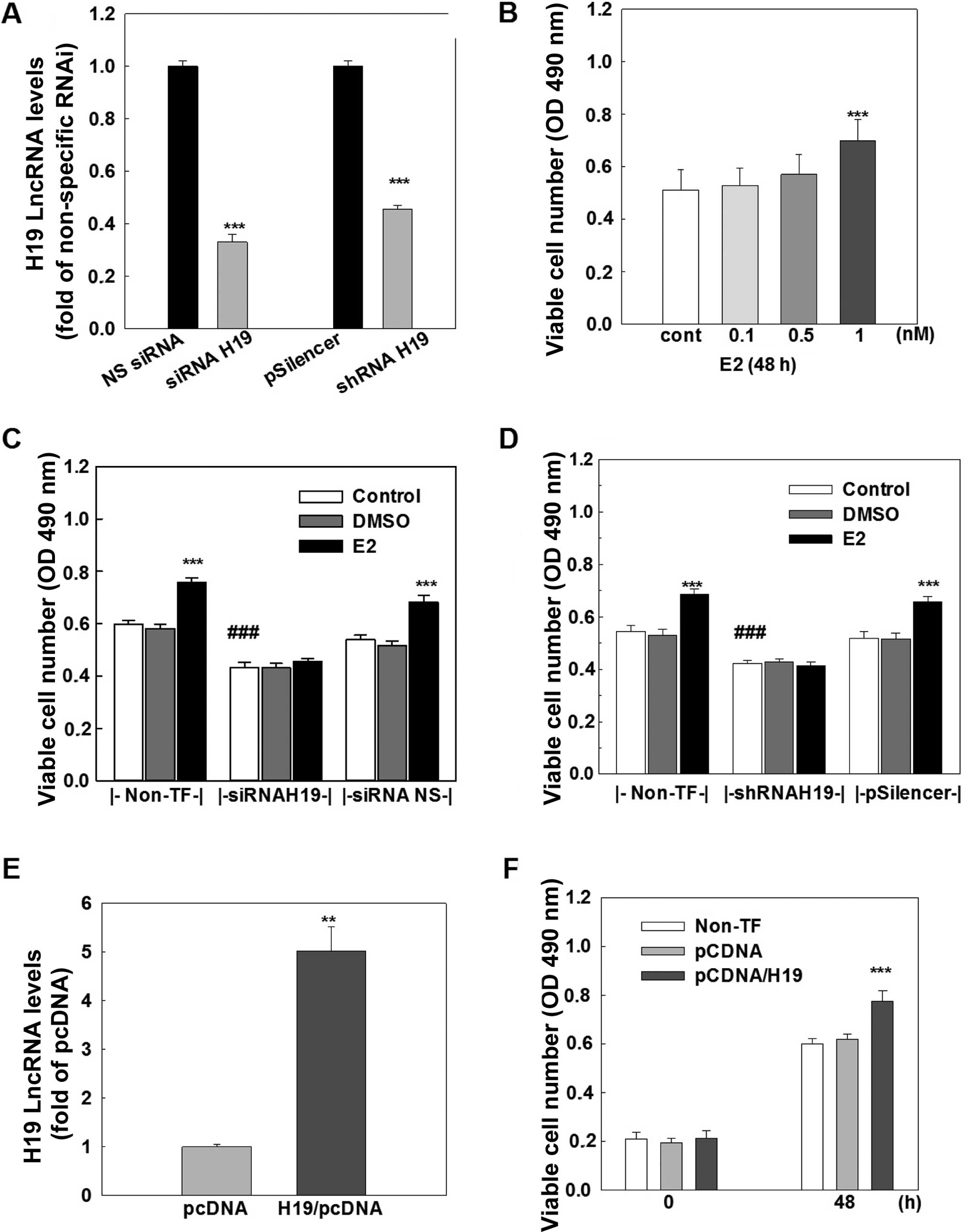
H19 lncRNA promotes cell growth in MCF-7 cells
The functional importance of H19 lncRNA was investigated by analyzing cell growth and cell cycle distribution in the MCF-7 cells. Treatment of MCF-7 cells with E2 produced a dose-dependent increase in viable cell number, and an ~30% induction of viable cells was observed at 1 nM E2 treatment (Fig. 3B). This E2-induced cell growth was completely blocked by transfection of either a specific siRNA (Fig. 3C) or a specific shRNA (Fig. 3D), which knocked down H19 lncRNA by 70 and 55%, respectively (Fig. 3A). In contrast, the transfection of either a non-specific siRNA or a non-specific shRNA that did not affect H19 expression had no effect on E2-induced cell growth. Notably, knockdown of H19 lncRNA caused a significant decrease (~30%) in viable cell number in the MCF-7 cells without E2 treatment (Fig. 3C and D).
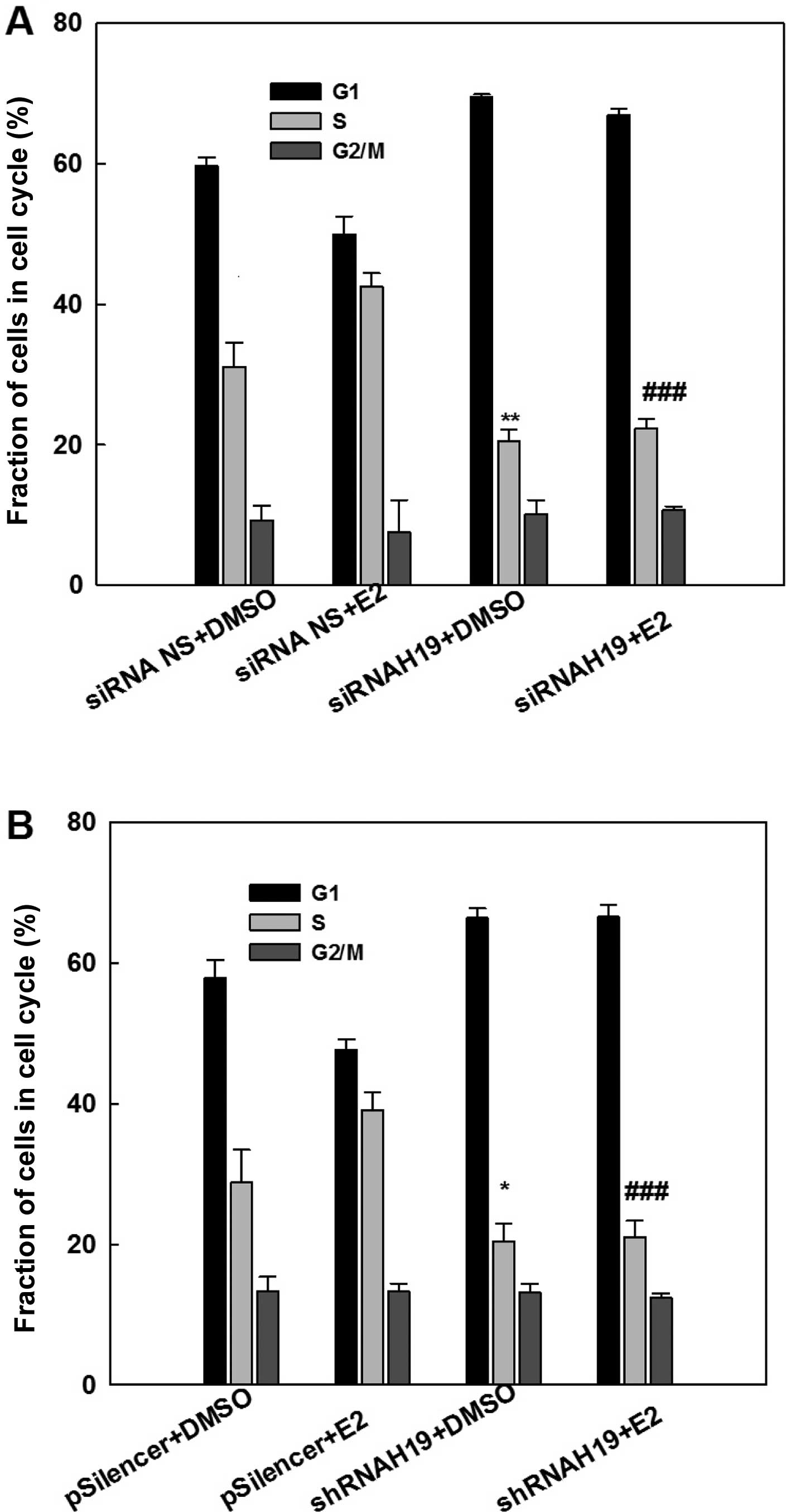
Consistent with the cell growth study, cell cycle analysis with flow cytometry demonstrated that E2 treatment increased the fraction of S-phase cells, an indication of an increase in DNA biosynthesis (Fig. 4). This E2-induced elevation in the S-phase cell fraction was eliminated by the knockdown of H19 lncRNA using either a specific siRNA (Fig. 4A) or a specific shRNA (Fig. 4B). Similarly, knockdown of H19 lncRNA decreased the S-phase fraction of cells in MCF-7 cells without E2 treatment. This analysis demonstrated that expression of H19 is important for the survival and maintenance of MCF-7 breast cancer cells.
To further determine the role of H19 lncRNA in cell growth, H19 lncRNA was overexpressed in the MCF-7 cells. The transfection of an H19 expression vector for 48 h resulted in a 4–5-fold increase in the H19 lncRNA level in the MCF-7 cells (Fig. 3E) and a significant increase in cell growth, which was not observed in cells transfected with a negative control vector (Fig. 3F). Taken together, these data suggest that H19 lncRNA possesses cell proliferative activity and the E2-induced cell growth is mediated through upregulation of H19 gene expression in MCF-7 breast cancer cells.
The level of H19 lncRNA is higher in the ER-positive than that in the ER-negative breast cancer tissues
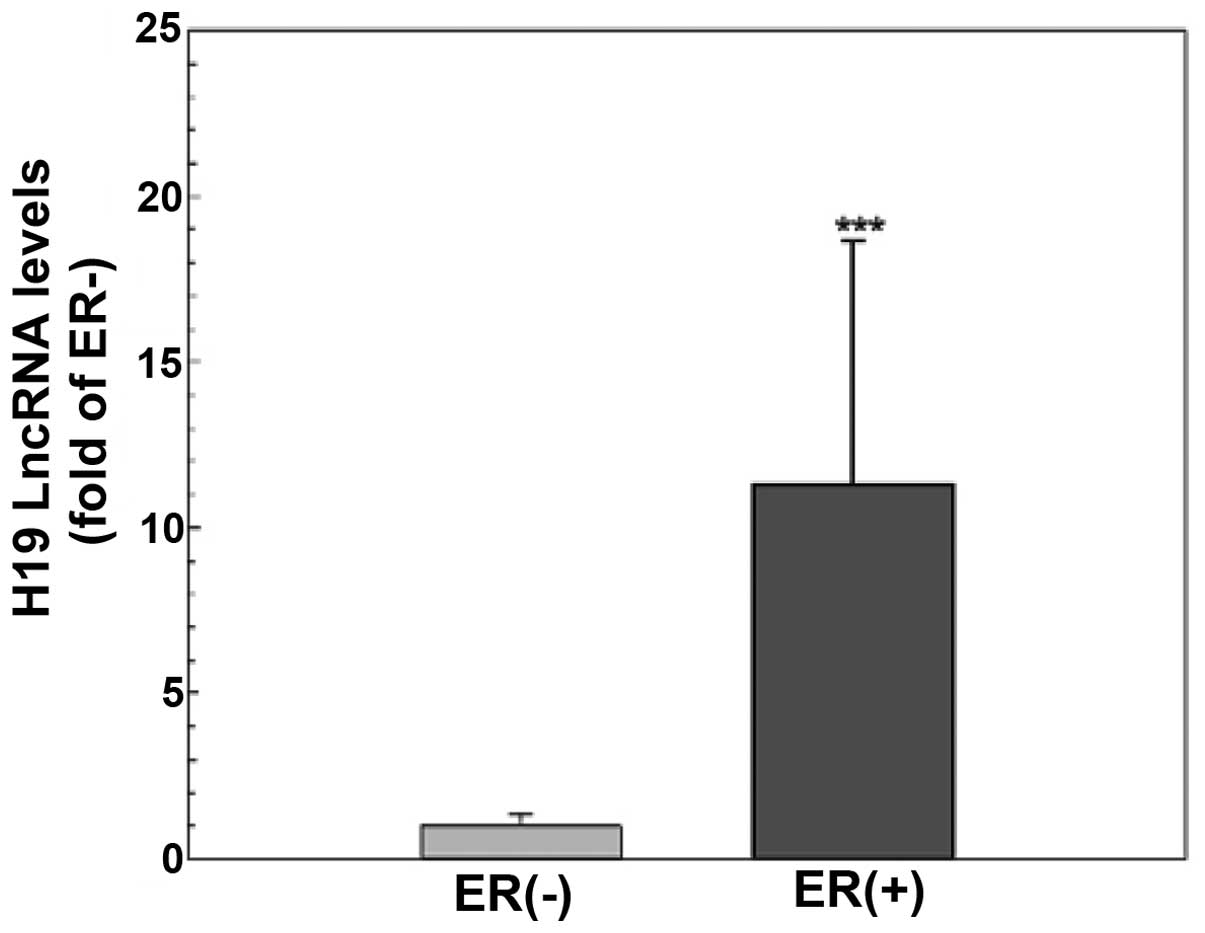
To explore the functional significance of H19 lncRNA and the estrogen inducibility of H19 expression in vivo, the levels of H19 lncRNA in primary tumors from breast cancer patients (n=45) were evaluated using quantitative RT-PCR. As shown in Fig. 5, tumor tissues from patients with ER-positive breast cancer (n=30) had a significantly higher H19 gene expression compared to those from ER-negative patients (n=15, p<0.001), suggesting that H19 expression is dependent on and/or induced by ER action in breast cancer cells.
Discussion
In the present study, we demonstrated that 17β-estradiol mediated through ERα produced a dose- and time-dependent induction of H19 lncRNA expression in the MCF-7 breast cancer cells. Most notably, knockdown of H19 lncRNA by RNAi in the MCF-7 cells resulted in a decrease in viable cell number and a blockade of estrogen-induced cell proliferation (Fig. 3). These results indicate that H19 lncRNA plays a significant role in cell survival and estrogen-induced cell proliferation in MCF-7 cells.
H19 RNA is originally identified as a transcribed, spliced and polyadenylated untranslatable RNA molecule (24), now termed as lncRNA. Functional analysis of H19 lncRNA has linked it to both oncogenic and tumor-suppressive activities (25). Although H19 lncRNA has been shown to cause growth retardation and morphological changes in tumor cells and abrogate the tumorigenicity of G401 tumor cells in nude mice following ectopic overexpression (26), growing evidence suggests that H19 lncRNA possesses oncogenic, proliferative and anti-apoptotic activity in various in vitro and in vivo systems (26). Barsyte-Lovejoy et al (27) reported that H19 lncRNA was directly induced by the c-Myc oncogene, resulting in an H19-associated promotion of clonogenicity and anchorage-independent growth in lung and breast cancer cells. Lottin et al showed that ectopic overexpression of H19 lncRNA in MDA-MB-231 breast cancer cells promoted tumor progression in a xenograft animal model (19). In the present study, we demonstrated that ectopic overexpression of H19 lncRNA in MCF-7 cells significantly increased cell growth while H19 lncRNA knockdown resulted in a decrease in cell survival (Figs. 3 and 4), further indicating the significance of H19 lncRNA in tumor cell proliferation and survival. Moreover, we observed that knockdown of estrogen-induced H19 lncRNA expression completely blocked estrogen-induced cell growth in MCF-7 cells (Figs. 3 and 4), suggesting that H19 lncRNA is a key factor meditating estrogen-induced cell growth in ER-positive breast cancer cells. Although the molecular mechanism of how H19 lncRNA stimulates cell proliferation remains to be investigated, a previous study demonstrated that H19 promoted the cell entry into S-phase in breast cancer cells through the E2F1 factor (20). Taken together, these data support the concept that H19 lncRNA plays a critical role in breast cancer development and progression, and it is therefore a valuable target for breast cancer therapy.
Previous studies have shown that H19 lncRNA is overexpressed in breast cancer cells (18,28,29). To determine whether H19 expression is associated with estrogen action, we compared the expression levels of 83 lncRNAs including H19 between an ER-positive and an ER-negative breast cancer cell line, and evaluated the inducibility of the 83 lncRNAs upon 17β-estradiol treatment in ER-positive MCF-7 cells. We revealed that the H19 lncRNA level was much higher in the ER-positive MCF-7 cells compared to the ER-negative MDA-MB-231 cells, and 17β-estradiol produced a dose- and time-dependent induction of H19 gene expression in the ER-positive MCF-7 yet not in the ER-negative MDA-MB-231 cells (Figs. 1 and 2, Tables I and II). The demonstration of estrogen induction of H19 expression is consistent with a previous report by Adriaenssens et al, in which an estrogen-associated induction of H19 expression was observed in mouse mammary gland and uterus as well as in MCF-7 cells (21). Using both chemical and genetic approaches, we further elucidated that the estrogen induction of H19 gene expression was mediated through ERα (Fig. 2). ICI 182780, a specific ER antagonist, inhibited 17β-estradiol-induced H19 expression in a dose-dependent manner; and knockdown of ERα by either a specific siRNA or an shRNA blocked this estrogen action. Furthermore, bioinformatic analysis of the H19 promoter region showed that multiple potential estrogen-response elements (EREs) are present in the promoter region of the H19 gene (Fig. 6) although the functional significance of these elements remains to be elucidated experimentally. Based on current and previous studies, we propose that similar to the estrogen regulation of protein-encoding genes, estrogen upregulation of lncRNA H19 expression is mediated through the traditional genomic pathway via the estrogen-ERα complex interacting with EREs in the H19 promoter region.
The biological significance of H19 lncRNA is further illustrated by quantification of H19 lncRNA levels in breast cancer tissues. Consistent with a previous observation of a steroid receptor dependency in H19 expression (18) and the screening result of a higher level of H19 lncRNA in ER-positive MCF-7 cells (Table I), we demonstrated that H19 lncRNA expression was >10-fold higher in the ER-positive tumor tissues compared to the ER-negative tissues (Fig. 5). These results not only provide evidence to support that H19 expression is dependent on estrogen-ER action, yet also indicate that H19 lncRNA is a valuable target for the diagnosis and treatment of ER-positive breast cancers.
In summary, lncRNAs have emerged as a critical field in cancer development and progression, and H19 lncRNA has been shown to be an oncogenic factor in breast cancer. The present study provides additional valuable evidence that H19 lncRNA plays a crucial role in cell survival and proliferation in ER-positive breast cancer cells. Our results indicate that H19 expression is ER-dependent and induced by estrogen through the traditional genomic pathway of estrogen-ER interaction with the gene. Since H19 is an ER-dependent gene, and mediates estrogen-induced cell proliferation, it may serve as a potential biomarker for breast cancer diagnosis and progression and as a valuable target for breast cancer therapy.
Acknowledgments
We are grateful to Dr A. Wakeling (Zeneca Pharmaceuticals, UK) who provided the ICI 182780. The present study was supported in part by grants from the National Natural Science Foundation of China (no. 81403021), the Natural Science Foundation of Fujian Province (no. 2013J01364), and the Central South University Special Talents Fund.
References
|
Jemal A, Bray F, Center MM, Ferlay J, Ward E and Forman D: Global cancer statistics. CA Cancer J Clin. 61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI | |
|
Harvey JM, Clark GM, Osborne CK and Allred DC: Estrogen receptor status by immunohistochemistry is superior to the ligand-binding assay for predicting response to adjuvant endocrine therapy in breast cancer. J Clin Oncol. 17:1474–1481. 1999.PubMed/NCBI | |
|
Stender JD, Frasor J, Komm B, Chang KC, Kraus WL and Katzenellenbogen BS: Estrogen-regulated gene networks in human breast cancer cells: Involvement of E2F1 in the regulation of cell proliferation. Mol Endocrinol. 21:2112–2123. 2007. View Article : Google Scholar : PubMed/NCBI | |
|
Osborne CK: Steroid hormone receptors in breast cancer management. Breast Cancer Res Treat. 51:227–238. 1998. View Article : Google Scholar | |
|
Katzenellenbogen BS, Montano MM, Ediger TR, et al: Estrogen receptors: Selective ligands, partners, and distinctive pharmacology. Recent Prog Horm Res. 55:163–195. 2000.PubMed/NCBI | |
|
Frasor J, Danes JM, Komm B, Chang KC, Lyttle CR and Katzenellenbogen BS: Profiling of estrogen up- and down-regulated gene expression in human breast cancer cells: Insights into gene networks and pathways underlying estrogenic control of proliferation and cell phenotype. Endocrinology. 144:4562–4574. 2003. View Article : Google Scholar : PubMed/NCBI | |
|
Stossi F, Barnett DH, Frasor J, Komm B, Lyttle CR and Katzenellenbogen BS: Transcriptional profiling of estrogen-regulated gene expression via estrogen receptor (ER) alpha or ERbeta in human osteosarcoma cells: Distinct and common target genes for these receptors. Endocrinology. 145:3473–3486. 2004. View Article : Google Scholar : PubMed/NCBI | |
|
Li CH and Chen Y: Targeting long non-coding RNAs in cancers: Progress and prospects. Int J Biochem Cell Biol. 45:1895–1910. 2013. View Article : Google Scholar : PubMed/NCBI | |
|
Pauli A, Rinn JL and Schier AF: Non-coding RNAs as regulators of embryogenesis. Nat Rev Genet. 12:136–149. 2011. View Article : Google Scholar : PubMed/NCBI | |
|
Ponting CP, Oliver PL and Reik W: Evolution and functions of long noncoding RNAs. Cell. 136:629–641. 2009. View Article : Google Scholar : PubMed/NCBI | |
|
Wapinski O and Chang HY: Long noncoding RNAs and human disease. Trends Cell Biol. 21:354–361. 2011. View Article : Google Scholar : PubMed/NCBI | |
|
Prensner JR and Chinnaiyan AM: The emergence of lncRNAs in cancer biology. Cancer Discov. 1:391–407. 2011. View Article : Google Scholar : PubMed/NCBI | |
|
Bartolomei MS, Zemel S and Tilghman SM: Parental imprinting of the mouse H19 gene. Nature. 351:153–155. 1991. View Article : Google Scholar : PubMed/NCBI | |
|
Ariel I, de Groot N and Hochberg A: Imprinted H19 gene expression in embryogenesis and human cancer: The oncofetal connection. Am J Med Genet. 91:46–50. 2000. View Article : Google Scholar : PubMed/NCBI | |
|
Glaser T, Housman D, Lewis WH, Gerhard D and Jones C: A fine-structure deletion map of human chromosome 11p: Analysis of J1 series hybrids. Somat Cell Mol Genet. 15:477–501. 1989. View Article : Google Scholar : PubMed/NCBI | |
|
Matouk IJ, DeGroot N, Mezan S, Ayesh S, Abu-lail R, Hochberg A and Galun E: The H19 non-coding RNA is essential for human tumor growth. PLoS One. 2:e8452007. View Article : Google Scholar : PubMed/NCBI | |
|
Luo M, Li Z, Wang W, Zeng Y, Liu Z and Qiu J: Long non-coding RNA H19 increases bladder cancer metastasis by associating with EZH2 and inhibiting E-cadherin expression. Cancer Lett. 333:213–221. 2013. View Article : Google Scholar : PubMed/NCBI | |
|
Adriaenssens E, Dumont L, Lottin S, Bolle D, Leprêtre A, Delobelle A, Bouali F, Dugimont T, Coll J and Curgy JJ: H19 overexpression in breast adenocarcinoma stromal cells is associated with tumor values and steroid receptor status but independent of p53 and Ki-67 expression. Am J Pathol. 153:1597–1607. 1998. View Article : Google Scholar : PubMed/NCBI | |
|
Lottin S, Adriaenssens E, Dupressoir T, Berteaux N, Montpellier C, Coll J, Dugimont T and Curgy JJ: Overexpression of an ectopic H19 gene enhances the tumorigenic properties of breast cancer cells. Carcinogenesis. 23:1885–1895. 2002. View Article : Google Scholar : PubMed/NCBI | |
|
Berteaux N, Lottin S, Monté D, Pinte S, Quatannens B, Coll J, Hondermarck H, Curgy JJ, Dugimont T and Adriaenssens E: H19 mRNA-like noncoding RNA promotes breast cancer cell proliferation through positive control by E2F1. J Biol Chem. 280:29625–29636. 2005. View Article : Google Scholar : PubMed/NCBI | |
|
Adriaenssens E, Lottin S, Dugimont T, Fauquette W, Coll J, Dupouy JP, Boilly B and Curgy JJ: Steroid hormones modulate H19 gene expression in both mammary gland and uterus. Oncogene. 18:4460–4473. 1999. View Article : Google Scholar : PubMed/NCBI | |
|
Tsang WP and Kwok TT: Riboregulator H19 induction of MDR1-associated drug resistance in human hepatocellular carcinoma cells. Oncogene. 26:4877–4881. 2007. View Article : Google Scholar : PubMed/NCBI | |
|
Tan C, Cai LQ, Wu W, Qiao Y, Imperato-McGinley J, Chen GQ and Zhu YS: NSC606985, a novel camptothecin analog, induces apoptosis and growth arrest in prostate tumor cells. Cancer Chemother Pharmacol. 63:303–312. 2009. View Article : Google Scholar | |
|
Brannan CI, Dees EC, Ingram RS and Tilghman SM: The product of the H19 gene may function as an RNA. Mol Cell Biol. 10:28–36. 1990.PubMed/NCBI | |
|
Gabory A, Jammes H and Dandolo L: The H19 locus: Role of an imprinted non-coding RNA in growth and development. BioEssays. 32:473–480. 2010. View Article : Google Scholar : PubMed/NCBI | |
|
Hao Y, Crenshaw T, Moulton T, Newcomb E and Tycko B: Tumour-suppressor activity of H19 RNA. Nature. 365:764–767. 1993. View Article : Google Scholar : PubMed/NCBI | |
|
Barsyte-Lovejoy D, Lau SK, Boutros PC, Khosravi F, Jurisica I, Andrulis IL, Tsao MS and Penn LZ: The c-Myc oncogene directly induces the H19 noncoding RNA by allele-specific binding to potentiate tumorigenesis. Cancer Res. 66:5330–5337. 2006. View Article : Google Scholar : PubMed/NCBI | |
|
Doucrasy S, Coll J, Barrois M, Joubel A, Prost S, Dozier C, Stehelin D and Riou G: Expression of the human fetal bac h19 gene in invasive cancers. Int J Oncol. 2:753–758. 1993.PubMed/NCBI | |
|
Dugimont T, Curgy JJ, Wernert N, Delobelle A, Raes MB, Joubel A, Stehelin D and Coll J: The H19 gene is expressed within both epithelial and stromal components of human invasive adenocarcinomas. Biol Cell. 85:117–124. 1995. View Article : Google Scholar : PubMed/NCBI |