CAR T cell therapy: A new era for cancer treatment (Review)
- Authors:
- Rimjhim Mohanty
- Chitran Roy Chowdhury
- Solomon Arega
- Prakriti Sen
- Pooja Ganguly
- Niladri Ganguly
-
Affiliations: Cancer Biology Laboratory, School of Biotechnology, Kalinga Institute of Industrial Technology (KIIT) University, Bhubaneswar, Odisha 751024, India - Published online on: September 24, 2019 https://doi.org/10.3892/or.2019.7335
- Pages: 2183-2195
This article is mentioned in:
Abstract
 |
 |
 |
 |
|
Restifo NP, Dudley ME and Rosenberg SA: Adoptive immunotherapy for cancer: Harnessing the T cell response. Nat Rev Immunol. 12:269–281. 2012. View Article : Google Scholar : PubMed/NCBI | |
|
Galluzzi L and Martin P: CARs on a highway with roadblocks. Oncoimmunology. 6:e13884862017. View Article : Google Scholar : PubMed/NCBI | |
|
Perales MA, Kebriaei P, Kean LS and Sadelain M: Building a safer and faster CAR: Seatbelts, airbags, and CRISPR. Biol Blood Marrow Transplant. 24:27–31. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Sharpe M and Mount N: Genetically modified T cells in cancer therapy: Opportunities and challenges. Dis Model Mech. 8:337–350. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
McGuirk J, Waller EK, Qayed M, Abhyankar S, Ericson S, Holman P, Keir C and Myers GD: Building blocks for institutional preparation of CTL019 delivery. Cytotherapy. 19:1015–1024. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Prasad V: Immunotherapy: Tisagenlecleucel-the first approved CAR-T-cell therapy: Implications for payers and policy makers. Nat Rev Clin Oncol. 15:11–12. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Neelapu SS, Tummala S, Kebriaei P, Wierda W, Gutierrez C, Locke FL, Komanduri KV, Lin Y, Jain N, Daver N, et al: Chimeric antigen receptor T-cell therapy-assessment and management of toxicities. Nat Rev Clin Oncol. 15:47–62. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Miliotou AN and Papadopoulou LC: CAR T-cell therapy: A new era in cancer immunotherapy. Curr Pharm Biotechnol. 19:5–18. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Mirzaei HR, Jamali A, Jafarzadeh L, Masoumi E, Alishah K, Fallah Mehrjardi K, Emami SAH, Noorbakhsh F, Till BG and Hadjati J: Construction and functional characterization of a fully human anti-CD19 chimeric antigen receptor (huCAR)-expressing primary human T cells. J Cell Physiol. 234:9207–9215. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Vormittag P, Gunn R, Ghorashian S and Veraitch FS: A guide to manufacturing CAR T cell therapies. Curr Opin Biotechnol. 53:164–181. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
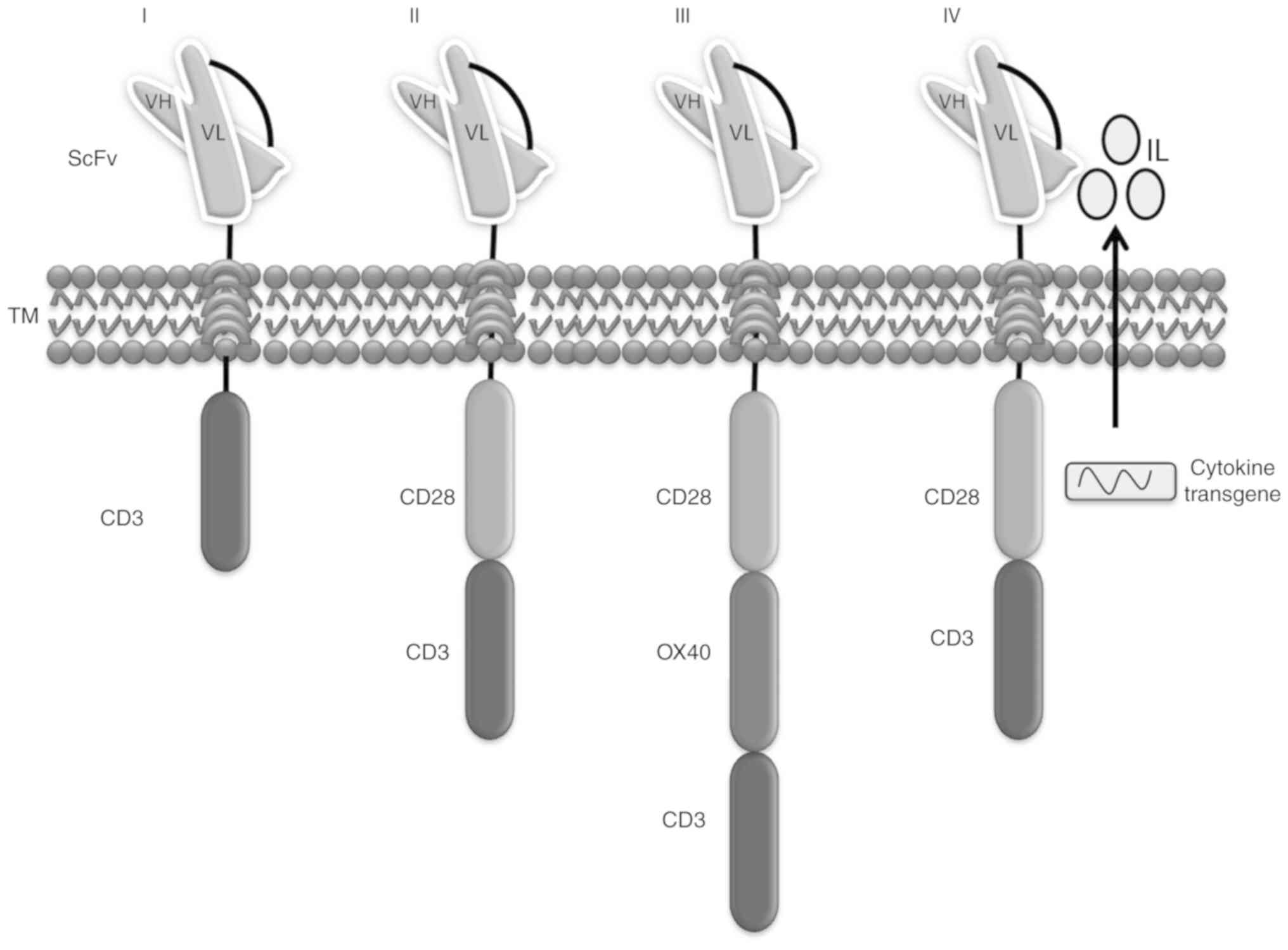
Abate-Daga D and Davila ML: CAR models: Next-generation CAR modifications for enhanced T-cell function. Mol Ther Oncolytics. 3:160142016. View Article : Google Scholar : PubMed/NCBI | |
|
Yang QY, Yang JD and Wang YS: Current strategies to improve the safety of chimeric antigen receptor (CAR) modified T cells. Immunol Lett. 190:201–205. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Mirzaei HR, Mirzaei H, Namdar A, Rahmati M, Till BG and Hadjati J: Predictive and therapeutic biomarkers in chimeric antigen receptor T-cell therapy: A clinical perspective. J Cell Physiol. 234:5827–5841. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Dai H, Wang Y, Lu X and Han W: Chimeric antigen receptors modified T-cells for cancer therapy. J Natl Cancer Inst. 108(pii): djv4392016.PubMed/NCBI | |
|
Mirzaei HR, Mirzaei H, Lee SY, Hadjati J and Till BG: Prospects for chimeric antigen receptor (CAR) γδ T cells: A potential game changer for adoptive T cell cancer immunotherapy. Cancer Lett. 380:413–423. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Morgan RA: Chapter 17-Adoptive T-cell therapy: Engineering T-cell receptors. Cancer Immunother (Sec Ed). 261–272. 2013. View Article : Google Scholar | |
|
Louis CU, Savoldo B, Dotti G, Pule M, Yvon E, Myers GD, Rossig C, Russell HV, Diouf O, Liu E, et al: Antitumor activity and long-term fate of chimeric antigen receptor-positive T cells in patients with neuroblastoma. Blood. 118:6050–6056. 2011. View Article : Google Scholar : PubMed/NCBI | |
|
Duong CP, Yong CS, Kershaw MH, Slaney CY and Darcy PK: Cancer immunotherapy utilizing gene-modified T cells: From the bench to the clinic. Mol Immunol. 67:46–57. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Sadelain M: CAR therapy: The CD19 paradigm. J Clin Invest. 125:3392–3400. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Eshhar Z: The T-body approach: Redirecting T cells with antibody specificity. Handb Exp Pharmacol. 329–342. 2008. View Article : Google Scholar : PubMed/NCBI | |
|
Brentjens R, Yeh R, Bernal Y, Riviere I and Sadelain M: Treatment of chronic lymphocytic leukemia with genetically targeted autologous T cells: Case report of an unforeseen adverse event in a phase I clinical trial. Mol Ther. 18:666–668. 2010. View Article : Google Scholar : PubMed/NCBI | |
|
Till BG, Jensen MC, Wang J, Qian X, Gopal AK, Maloney DG, Lindgren CG, Lin Y, Pagel JM, Budde LE, et al: CD20-specific adoptive immunotherapy for lymphoma using a chimeric antigen receptor with both CD28 and 4-1BB domains: Pilot clinical trial results. Blood. 119:3940–3950. 2012. View Article : Google Scholar : PubMed/NCBI | |
|
Park JH and Brentjens RJ: Are all chimeric antigen receptors created equal? J Clin Oncol. 33:651–653. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Qian L, Li D, Ma L, He T, Qi F, Shen J and Lu XA: The novel anti-CD19 chimeric antigen receptors with humanized scFv (single-chain variable fragment) trigger leukemia cell killing. Cell Immunol. 304-305:49–54. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Lock D, Mockel-Tenbrinck N, Drechsel K, Barth C, Mauer D, Schaser T, Kolbe C, Al Rawashdeh W, Brauner J, Hardt O, et al: Automated manufacturing of potent CD20-directed chimeric antigen receptor T cells for clinical use. Hum Gene Ther. 28:914–925. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Tang XY, Sun Y, Zhang A, Hu GL, Cao W, Wang DH, Zhang B and Chen H: Third-generation CD28/4-1BB chimeric antigen receptor T cells for chemotherapy relapsed or refractory acute lymphoblastic leukaemia: A non-randomised, open-label phase I trial protocol. BMJ Open. 6:e0139042016. View Article : Google Scholar : PubMed/NCBI | |
|
Yeku OO and Brentjens RJ: Armored CAR T-cells: Utilizing cytokines and pro-inflammatory ligands to enhance CAR T-cell anti-tumour efficacy. Biochem Soc Trans. 44:412–418. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Chmielewski M and Abken H: TRUCKs: The fourth generation of CARs. Expert Opin Biol Ther. 15:1145–1154. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Kueberuwa G, Kalaitsidou M, Cheadle E, Hawkins RE and Gilham DE: CD19 CAR T cells expressing IL-12 eradicate lymphoma in fully lymphoreplete mice through induction of host immunity. Mol Ther Oncolytics. 8:41–51. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Kerkar SP, Muranski P, Kaiser A, Boni A, Sanchez-Perez L, Yu Z, Palmer DC, Reger RN, Borman ZA, Zhang L, et al: Tumor-specific CD8+ T cells expressing interleukin-12 eradicate established cancers in lymphodepleted hosts. Cancer Res. 70:6725–6734. 2010. View Article : Google Scholar : PubMed/NCBI | |
|
Giacca M and Zacchigna S: Virus-mediated gene delivery for human gene therapy. J Control Release. 161:377–388. 2012. View Article : Google Scholar : PubMed/NCBI | |
|
Yi Y, Noh MJ and Lee KH: Current Advances in retroviral gene therapy. Curr Gene Ther. 11:218–228. 2011. View Article : Google Scholar : PubMed/NCBI | |
|
Kidd ME, Shin S and Shea LD: Fibrin hydrogels for lentiviral gene delivery in vitro and in vivo. J Control Release. 157:80–85. 2012. View Article : Google Scholar : PubMed/NCBI | |
|
Munñoz-López M and García-Perez JL: DNA transposons: Nature and applications in genomics. Curr Genomics. 11:115–128. 2010. View Article : Google Scholar : PubMed/NCBI | |
|
Morita D, Nishio N, Saito S, Tanaka M, Kawashima N, Okuno Y, Suzuki S, Matsuda K, Maeda Y, Wilson MH, et al: Enhanced expression of Anti-CD19 chimeric antigen receptor in piggyBac transposon-engineered T cells. Mol Ther Methods Clin Dev. 8:131–140. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Kebriaei P, Singh H, Huls MH, Figliola MJ, Bassett R, Olivares S, Jena B, Dawson MJ, Kumaresan PR, Su S, et al: Phase I trials using sleeping beauty to generate CD19-specific CAR T cells. J Clin Invest. 126:3363–3376. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Manuri PV, Wilson MH, Maiti SN, Mi T, Singh H, Olivares S, Dawson MJ, Huls H, Lee DA, Rao PH, et al: piggyBac Transposon/Transposase system to generate CD19-specific T cells for the treatment of B-lineage malignancies. Hum Gene Ther. 21:427–437. 2010. View Article : Google Scholar : PubMed/NCBI | |
|
Yarmush ML, Golberg A, Serša G, Kotnik T and Miklavčič D: Electroporation-based technologies for medicine: Principles, applications, and challenges. Annu Rev Biomed Eng. 16:295–320. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
Chicaybam L, Sodre AL, Curzio BA and Bonamino MH: An efficient low cost method for gene transfer to T lymphocytes. PLoS One. 8:e602982013. View Article : Google Scholar : PubMed/NCBI | |
|
Chicaybam L, Barcelos C, Peixoto B, Carneiro M, Limia CG, Redondo P, Lira C, Paraguassú-Braga F, Vasconcelos ZF, Barros L and Bonamino MH: An efficient electroporation protocol for the genetic modification of mammalian cells. Front Bioeng Biotechnol. 4:992017. View Article : Google Scholar : PubMed/NCBI | |
|
Kotnik T, Frey W, Sack M, Haberl Meglič S, Peterka M and Miklavčič D: Electroporation-based applications in biotechnology. Trends Biotechnol. 33:480–488. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Ramamoorth M and Narvekar A: Non viral vectors in gene therapy-an overview. J Clin Diagn Res. 9:GE01–GE06. 2015.PubMed/NCBI | |
|
Yin H, Kauffman KJ and Anderson DG: Delivery technologies for genome editing. Nat Rev Drug Discov. 16:387–399. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Smith TT, Stephan SB, Moffett HF, McKnight LE, Ji W, Reiman D, Bonagofski E, Wohlfahrt ME, Pillai SPS and Stephan MT: In situ programming of leukaemia-specific T cells using synthetic DNA nanocarriers. Nat Nanotechnol. 12:813–820. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Miller MA: Nanoparticles improve economic mileage for CARs. Sci Transl Med. 9(pii): eaan27842017. View Article : Google Scholar : PubMed/NCBI | |
|
Smith TT, Moffett HF, Stephan SB, Opel CF, Dumigan AG, Jiang X, Pillarisetty VG, Pillai SPS, Wittrup KD and Stephan MT: Biopolymers codelivering engineered T cells and STING agonists can eliminate heterogeneous tumors. J Clin Invest. 127:2176–2191. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Dowaidar M, Abdelhamid HN, Hällbrink M, Freimann K, Kurrikoff K, Zou X and Langel Ü: Magnetic nanoparticle assisted Self-assembly of cell penetrating peptides-oligonucleotides complexes for gene delivery. Sci Rep. 7:91592017. View Article : Google Scholar : PubMed/NCBI | |
|
Kebriaei P: CAR T-cell therapies: Overcoming the challenges and new strategies. Clin Lymphoma Myeloma Leuk. 17 (Suppl 2):S74–S78. 2017. View Article : Google Scholar | |
|
Bonifant CL, Jackson HJ, Brentjens RJ and Curran KJ: Toxicity and management in CAR T-cell therapy. Mol Ther Oncolytics. 3:160112016. View Article : Google Scholar : PubMed/NCBI | |
|
Brudno JN and Kochenderfer JN: Toxicities of chimeric antigen receptor T cells: Recognition and management. Blood. 127:3321–3330. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Neelapu SS, Locke FL, Bartlett NL, Lekakis LJ, Miklos DB, Jacobson CA, Braunschweig I, Oluwole OO, Siddiqi T, Lin Y, et al: Axicabtagene Ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N Engl J Med. 377:2531–2544. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Paietta E: Immunobiology of acute leukemia. In: Neoplastic diseases of the bloodSpringer; Cham: pp. 237–279. 2018 | |
|
Zah E, Lin MY, Jensen MC, Silva-Benedict A and Chen YY: Abstract IA12: Combating antigen escape with CD19/CD20 bispecific CAR-T cell therapy. Cancer Immunol Res 5 (3 Suppl). IA122017. | |
|
Majzner RG and Mackall CL: Tumor antigen escape from CAR T-cell therapy. Cancer Discov. 8:1219–1226. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Zhu M, Wu B, Brandl C, Johnson J, Wolf A, Chow A and Doshi S: Blinatumomab, a Bispecific T-cell Engager (BiTE(®)) for CD-19 targeted cancer immunotherapy: Clinical pharmacology and its implications. Clin Pharmacokinet. 55:1271–1288. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Martyniszyn A, Krahl AC, André MC, Hombach AA and Abken H: CD20-CD19 Bispecific CAR T cells for the treatment of B-cell malignancies. Hum Gene Ther. 28:1147–1157. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Zhu F, Shah N, Xu H, Schneider D, Orentas R, Dropulic B, Hari P and Keever-Taylor CA: Closed-system manufacturing of CD19 and dual-targeted CD20/19 chimeric antigen receptor T cells using the CliniMACS prodigy device at an academic medical center. Cytotherapy. 20:394–406. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Sun LL, Ellerman D, Mathieu M, Hristopoulos M, Chen X, Li Y, Yan X, Clark R, Reyes A, Stefanich E, et al: Anti-CD20/CD3 T cell-dependent bispecific antibody for the treatment of B cell malignancies. Sci Transl Med. 7:287ra702015. View Article : Google Scholar : PubMed/NCBI | |
|
Zhang W, Liu Y, Wang Y, Wang C, Yang QM, Zhu HL and Han WD: Long-term safety and efficacy of CART-20 cells in patients with refractory or relapsed B-cell non-Hodgkin lymphoma: 5-years follow-up results of the phase I and IIa trials. Signal Transduct Target Ther. 2:170542017. View Article : Google Scholar : PubMed/NCBI | |
|
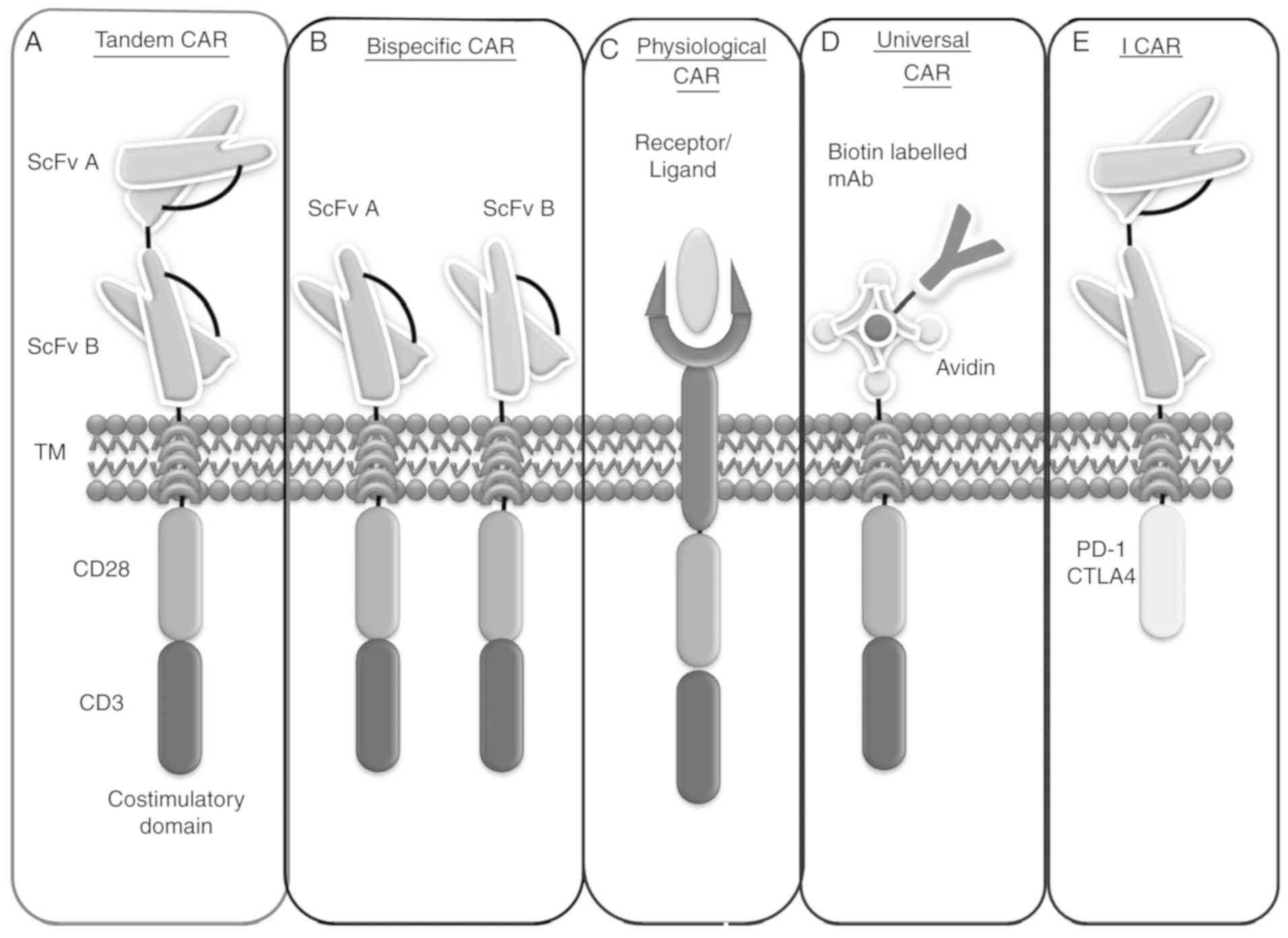
Hegde M, Mukherjee M, Grada Z, Pignata A, Landi D, Navai SA, Wakefield A, Fousek K, Bielamowicz K, Chow KK, et al: Tandem CAR T cells targeting HER2 and IL13Rα2 mitigate tumor antigen escape. J Clin Invest. 126:3036–3052. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Schneider D, Xiong Y, Wu D, Nӧlle V, Schmitz S, Haso W, Kaiser A, Dropulic B and Orentas RJ: A tandem CD19/CD20 CAR lentiviral vector drives on-target and off-target antigen modulation in leukemia cell lines. J Immunother Cancer. 5:422017. View Article : Google Scholar : PubMed/NCBI | |
|
Grada Z, Hegde M, Byrd T, Shaffer DR, Ghazi A, Brawley VS, Corder A, Schönfeld K, Koch J, Dotti G, et al: TanCAR: A novel bispecific chimeric antigen receptor for cancer immunotherapy. Mol Ther Nucleic Acids. 2:e1052013. View Article : Google Scholar : PubMed/NCBI | |
|
Ahmed N, Brawley V, Hegde M, Bielamowicz K, Kalra M, Landi D, Robertson C, Gray TL, Diouf O, Wakefield A, et al: HER2-specific chimeric antigen receptor-modified virus-specific T cells for progressive glioblastoma: A phase 1 dose-escalation trial. JAMA Oncol. 3:1094–1101. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Bielamowicz K, Fousek K, Byrd TT, Samaha H, Mukherjee M, Aware N, Wu MF, Orange JS, Sumazin P, Man TK, et al: Trivalent CAR T cells overcome interpatient antigenic variability in glioblastoma. Neuro Oncol. 20:506–518. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Ott PA, Hodi FS and Robert C: CTLA-4 and PD-1/PD-L1 blockade: New immunotherapeutic modalities with durable clinical benefit in melanoma patients. Clin Cancer Res. 19:5300–5309. 2013. View Article : Google Scholar : PubMed/NCBI | |
|
Curran MA, Montalvo W, Yagita H and Allison JP: PD-1 and CTLA-4 combination blockade expands infiltrating T cells and reduces regulatory T and myeloid cells within B16 melanoma tumors. Proc Natl Acad Sci USA. 107:4275–4280. 2010. View Article : Google Scholar : PubMed/NCBI | |
|
Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC, et al: Improved Survival with Ipilimumab in Patients with Metastatic Melanoma. N Engl J Med. 363:711–723. 2010. View Article : Google Scholar : PubMed/NCBI | |
|
Iwai Y, Ishida M, Tanaka Y, Okazaki T, Honjo T and Minato N: Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc Natl Acad Sci USA. 99:12293–12297. 2002. View Article : Google Scholar : PubMed/NCBI | |
|
Iwai Y, Hamanishi J, Chamoto K and Honjo T: Cancer immunotherapies targeting the PD-1 signaling pathway. J Biomed Sci. 24:262017. View Article : Google Scholar : PubMed/NCBI | |
|
Ansell SM, Lesokhin AM, Borrello I, Halwani A, Scott EC, Gutierrez M, Schuster SJ, Millenson MM, Cattry D, Freeman GJ, et al: PD-1 Blockade with Nivolumab in relapsed or refractory Hodgkin's lymphoma. N Engl J Med. 372:311–319. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Chen R, Zinzani PL, Fanale MA, Armand P, Johnson NA, Brice P, Radford J, Ribrag V, Molin D, Vassilakopoulos TP, et al: Phase II study of the efficacy and safety of pembrolizumab for relapsed/refractory classic Hodgkin lymphoma. J Clin Oncol. 35:2125–2132. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Barbee MS, Ogunniyi A, Horvat TZ and Dang TO: Current status and future directions of the immune checkpoint inhibitors ipilimumab, pembrolizumab, and nivolumab in oncology. Ann Pharmacother. 49:907–937. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Curran MA, Kim M, Montalvo W, Al-Shamkhani A and Allison JP: Combination CTLA-4 blockade and 4-1BB activation enhances tumor rejection by increasing T-cell infiltration, proliferation, and cytokine production. PLoS One. 6:e194992011. View Article : Google Scholar : PubMed/NCBI | |
|
Seidel JA, Otsuka A and Kabashima K: Anti-PD-1 and Anti-CTLA-4 therapies in cancer: Mechanisms of action, efficacy, and limitations. Front Oncol. 8:862018. View Article : Google Scholar : PubMed/NCBI | |
|
Ren J, Liu X, Fang C, Jiang S, June CH and Zhao Y: Multiplex genome editing to generate universal CAR T cells resistant to PD1 inhibition. Clin Cancer Res. 23:2255–2266. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Azijli K, Stelloo E, Peters GJ and Van Den Eertwegh AJ: New developments in the treatment of metastatic melanoma: Immune checkpoint inhibitors and targeted therapies. Anticancer Res. 34:1493–1505. 2014.PubMed/NCBI | |
|
Deng L, Liang H, Burnette B, Beckett M, Darga T, Weichselbaum RR and Fu YX: Irradiation and anti-PD-L1 treatment synergistically promote antitumor immunity in mice. J Clin Invest. 124:687–695. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
Shi H, Sun M, Liu L and Wang Z: Chimeric antigen receptor for adoptive immunotherapy of cancer: Latest research and future prospects. Mol Cancer. 13:2192014. View Article : Google Scholar : PubMed/NCBI | |
|
Liu L, Sun M and Wang Z: Adoptive T-cell therapy of B-cell malignancies: Conventional and physiological chimeric antigen receptors. Cancer Lett. 316:1–5. 2012. View Article : Google Scholar : PubMed/NCBI | |
|
Minutolo NG, Hollander EE and Powell DJ Jr: The emergence of universal immune receptor T cell therapy for cancer. Front Oncol. 9:1762019. View Article : Google Scholar : PubMed/NCBI | |
|
Zhao J, Lin Q, Song Y and Liu D: Universal CARs, universal T cells, and universal CAR T cells. J Hematol Oncol. 11:1322018. View Article : Google Scholar : PubMed/NCBI | |
|
Lohmueller JJ, Ham JD, Kvorjak M and Finn OJ: mSA2 affinity-enhanced biotin-binding CAR T cells for universal tumor targeting. Oncoimmunology. 7:e13686042018. View Article : Google Scholar | |
|
Qasim W, Zhan H, Samarasinghe S, Adams S, Amrolia P, Stafford S, Butler K, Rivat C, Wright G, Somana K, et al: Molecular remission of infant B-ALL after infusion of universal TALEN gene-edited CAR T cells. Sci Transl Med. 9:eaaj20132017. View Article : Google Scholar : PubMed/NCBI | |
|
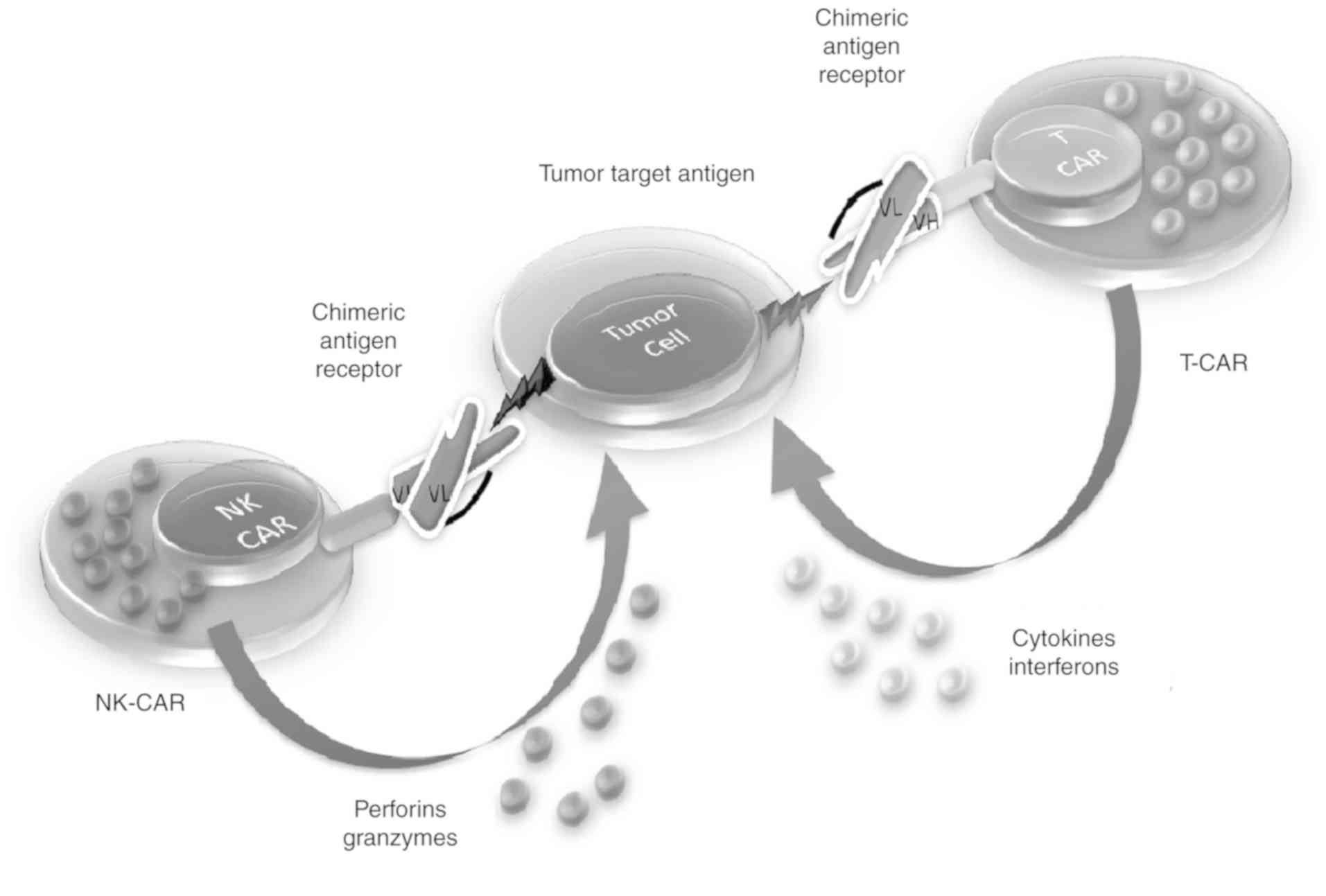
Glienke W, Esser R, Priesner C, Suerth JD, Schambach A, Wels WS, Grez M, Kloess S, Arseniev L and Koehl U: Advantages and applications of CAR-expressing natural killer cells. Front Pharmacol. 6:212015. View Article : Google Scholar : PubMed/NCBI | |
|
Klingemann H: Are natural killer cells superior CAR drivers? Oncoimmunology. 3:e281472014. View Article : Google Scholar : PubMed/NCBI | |
|
Jiang H, Zhang W, Shang P, Zhang H, Fu W, Ye F, Zeng T, Huang H, Zhang X, Sun W, et al: Transfection of chimeric anti-CD138 gene enhances natural killer cell activation and killing of multiple myeloma cells. Mol Oncol. 8:297–310. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
Boissel L, Betancur-Boissel M, Lu W, Krause DS, Van Etten RA, Wels WS and Klingemann H: Retargeting NK-92 cells by means of CD19- and CD20-specific chimeric antigen receptors compares favorably with antibody-dependent cellular cytotoxicity. Oncoimmunology. 2:e265272013. View Article : Google Scholar : PubMed/NCBI | |
|
Romanski A, Uherek C, Bug G, Seifried E, Klingemann H, Wels WS, Ottmann OG and Tonn T: CD19-CAR engineered NK-92 cells are sufficient to overcome NK cell resistance in B-cell malignancies. J Cell Mol Med. 20:1287–1294. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Hermanson DL and Kaufman DS: Utilizing chimeric antigen receptors to direct natural killer cell activity. Front Immunol. 6:1952015. View Article : Google Scholar : PubMed/NCBI | |
|
Tang X, Yang L, Li Z, Nalin AP, Dai H, Xu T, Yin J, You F, Zhu M, Shen W, et al: First-in-man clinical trial of CAR NK-92 cells: safety test of CD33-CAR NK-92 cells in patients with relapsed and refractory acute myeloid leukemia. Am J Cancer Res. 8:1083–1089. 2018.PubMed/NCBI | |
|
Hsu PD, Lander ES and Zhang F: Development and Applications of CRISPR-Cas9 for genome engineering. Cell. 157:1262–1278. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
Haurwitz RE, Jinek M, Wiedenheft B, Zhou K and Doudna JA: Sequence- and Structure-specific RNA processing by a CRISPR endonuclease. Science. 329:1355–1358. 2010. View Article : Google Scholar : PubMed/NCBI | |
|
Cox DB, Platt RJ and Zhang F: Therapeutic genome editing: Prospects and challenges. Nat Med. 21:121–131. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Shang W, Wang F, Fan G and Wang H: Key elements for designing and performing a CRISPR/Cas9-based genetic screen. J Genet Genomics. 44:439–449. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Mirzaei HR, Pourghadamyari H, Rahmati M, Mohammadi A, Nahand JS, Rezaei A and Hadjati J: Gene-knocked out chimeric antigen receptor (CAR) T cells: Tuning up for the next generation cancer immunotherapy. Cancer Lett. 423:95–104. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Eyquem J, Mansilla-Soto J, Giavridis T, van der Stegen SJ, Hamieh M, Cunanan KM, Odak A, Gönen M and Sadelain M: Targeting a CAR to the TRAC locus with CRISPR/Cas9 enhances tumour rejection. Nature. 543:113–117. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Mou H, Kennedy Z, Anderson DG, Yin H and Xue W: Precision cancer mouse models through genome editing with CRISPR-Cas9. Genome Med. 7:532015. View Article : Google Scholar : PubMed/NCBI | |
|
MacLeod DT, Antony J, Martin AJ, Moser RJ, Hekele A, Wetzel KJ, Brown AE, Triggiano MA, Hux JA, Pham CD, et al: Integration of a CD19 CAR into the TCR alpha chain locus streamlines production of allogeneic gene-edited CAR T cells. Mol Ther. 25:949–961. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Ren J, Zhang X, Liu X, Fang C, Jiang S, June CH and Zhao Y: A versatile system for rapid multiplex genome-edited CAR T cell generation. Oncotarget. 8:17002–17011. 2017.PubMed/NCBI | |
|
Grupp SA, Laetsch TW, Buechner J, Bittencourt H, Maude SL, Verneris MR, Myers GD, Boyer MW, Rives S, De Moerloose B, et al: Analysis of a global registration trial of the efficacy and safety of CTL019 in pediatric and young adults with relapsed/refractory acute lymphoblastic leukemia (ALL). Blood. 128:2212016. | |
|
Zhao Z, Chen Y, Francisco NM, Zhang Y and Wu M: The application of CAR-T cell therapy in hematological malignancies: Advantages and challenges. Acta Pharm Sin B. 8:539–551. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Beatty GL, Haas AR, Maus MV, Torigian DA, Soulen MC, Plesa G and Kalos M: Mesothelin-specific chimeric antigen receptor mRNA-engineered T cells induce antitumor activity in solid malignancies. Cancer Immunol Res. 2:112–120. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
Mirzaei HR, Rodriguez A, Shepphird J, Brown CE and Badie B: Chimeric antigen receptors T cell therapy in solid tumor: Challenges and clinical applications. Front Immunol. 8:18502017. View Article : Google Scholar : PubMed/NCBI | |
|
Boardman DA, Philippeos C, Fruhwirth GO, Ibrahim MA, Hannen RF, Cooper D, Marelli-Berg FM, Watt FM, Lechler RI, Maher J, et al: Expression of a chimeric antigen receptor specific for donor HLA class I enhances the potency of human regulatory T cells in preventing human skin transplant rejection. Am J Transplant. 17:931–943. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Shen CJ, Yang YX, Han EQ, Cao N, Wang YF, Wang Y, Zhao YY, Zhao LM, Cui J, Gupta P, et al: Chimeric antigen receptor containing ICOS signaling domain mediates specific and efficient antitumor effect of T cells against EGFRvIII expressing glioma. J Hematol Oncol. 6:332013. View Article : Google Scholar : PubMed/NCBI | |
|
Owens GL, Sheard VE, Kalaitsidou M, Blount D, Lad Y, Cheadle EJ, Edmondson RJ, Kooner G, Gilham DE and Harrop R: Preclinical assessment of CAR T-cell therapy targeting the tumor antigen 5T4 in ovarian cancer. J Immunother. 41:130–140. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Ellebrecht CT, Bhoj VG, Nace A, Choi EJ, Mao X, Cho MJ, Di Zenzo G, Lanzavecchia A, Seykora JT, Cotsarelis G, et al: Reengineering chimeric antigen receptor T cells for targeted therapy of autoimmune disease. Science. 353:179–184. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
O'Rourke DM, Nasrallah MP, Desai A, Melenhorst JJ, Mansfield K, Morrissette JJD, Martinez-Lage M, Brem S, Maloney E, Shen A, et al: A single dose of peripherally infused EGFRvIII-directed CAR T cells mediates antigen loss and induces adaptive resistance in patients with recurrent glioblastoma. Sci Transl Med. 9(pii): eaaa09842017. View Article : Google Scholar : PubMed/NCBI | |
|
Posey AD, Schwab RD, Boesteanu AC, Steentoft C, Mandel U, Engels B, Stone JD, Madsen TD, Schreiber K, Haines KM, et al: Engineered CAR T cells targeting the cancer-associated Tn-Glycoform of the membrane mucin MUC1 control adenocarcinoma. Immunity. 44:1444–1454. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Burga RA, Thorn M, Point GR, Guha P, Nguyen CT, Licata LA, DeMatteo RP, Ayala A, Joseph Espat N, Junghans RP and Katz SC: Liver myeloid-derived suppressor cells expand in response to liver metastases in mice and inhibit the anti-tumor efficacy of anti-CEA CAR-T. Cancer Immunol Immunother. 64:817–829. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Ahmed N, Brawley VS, Hegde M, Robertson C, Ghazi A, Gerken C, Liu E, Dakhova O, Ashoori A, Corder A, et al: Human epidermal growth factor receptor 2 (HER2)-specific chimeric antigen receptor-modified T cells for the immunotherapy of HER2-positive sarcoma. J Clin Oncol. 33:1688–1696. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Akce M, Zaidi MY, Waller EK, El-Rayes BF and Lesinski GB: The potential of CAR T cell therapy in pancreatic cancer. Front Immunol. 9:21662018. View Article : Google Scholar : PubMed/NCBI | |
|
Bach PB, Giralt SA and Saltz LB: FDA approval of tisagenlecleucel: Promise and complexities of a $475 000 cancer drug. JAMA. 318:1861–1862. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Fala L: Yescarta (Axicabtagene Ciloleucel) second CAR T-cell therapy approved for patients with certain types of large B-cell lymphoma. Am Health Drug Benefits. 11:109–111. 2018. | |
|
Hey SP and Kesselheim AS: The FDA, Juno therapeutics, and the ethical imperative of transparency. BMJ. 354:i44352016. View Article : Google Scholar : PubMed/NCBI | |
|
Rotolo A, Caputo V and Karadimitris A: The prospects and promise of chimeric antigen receptor immunotherapy in multiple myeloma. Br J Haematol. 173:350–364. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Almond LM, Charalampakis M, Ford SJ, Gourevitch D and Desai A: Myeloid sarcoma: Presentation, diagnosis, and treatment. Clin Lymphoma Myeloma Leuk. 17:263–267. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Locke FL, Neelapu SS, Bartlett NL, Siddiqi T, Chavez JC, Hosing CM, Ghobadi A, Budde LE, Bot A, Rossi JM, et al: Phase 1 results of ZUMA-1: A multicenter study of KTE-C19 Anti-CD19 CAR T cell therapy in refractory aggressive lymphoma. Mol Ther. 25:285–295. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Qasim W, Ciocarlie O, Adams S, Inglott S, Murphy C, Rivat C, Wright G, Lucchini G, Silva J, Rao K, et al: Preliminary results of UCART19, an allogeneic anti-CD19 CAR T-cell product in a first-in-human trial (PALL) in pediatric patients with CD19+ relapsed/refractory B-cell acute lymphoblastic leukemia. Blood. 130:12712017. | |
|
Cai T, Galetto R, Gouble A, Smith J, Cavazos A, Konoplev S, Lane AA, Guzman ML, Kantarjian HM, Pemmaraju N, et al: Pre-clinical studies of Anti-CD123 CAR-T cells for the treatment of blastic plasmacytoid dendritic cell neoplasm (BPDCN). Blood. 128:40392016. | |
|
Gouble A, Schiffer-Mannioui C, Thomas S, Gautron AS, Poirot L and Smith J: Abstract 3763: UCART22: Allogenic adoptive immunotherapy of leukemia by targeting CD22 with CAR T-cells. Cancer Res. 77:3763. 2017. | |
|
Galetto R, Chion-Sotinel I, Gouble A and Smith J: Allogenic TCRa/CS1 double knockout T-cells bearing an anti-CS1 chimeric antigen receptor: An improved immunotherapy approach for the treatment of multiple myeloma. Cancer Res. 76 (14 Suppl):Abstract nr 2289. 2016. | |
|
Drent E, Groen RW, Noort WA, Themeli M, Lammerts van Bueren JJ, Parren PW, Kuball J, Sebestyen Z, Yuan H, de Bruijn J, et al: Pre-clinical evaluation of CD38 chimeric antigen receptor engineered T cells for the treatment of multiple myeloma. Haematologica. 101:616–625. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Coulter I: Compositions. US Patent 2018/0228866A1. Filed August 12 2016; issued August 16 2018. |