Regulation of Drp1 and enhancement of mitochondrial fission by the deubiquitinating enzyme PSMD14 facilitates the proliferation of bladder cancer cells
- Authors:
- Published online on: November 16, 2023 https://doi.org/10.3892/or.2023.8665
- Article Number: 6
-
Copyright: © Song et al. This is an open access article distributed under the terms of Creative Commons Attribution License.
Abstract
Introduction
Mitochondria provides energy for almost all cellular activities, thereby maintaining the cell's physiological functions (1). In addition, mitochondria play a vital role in cell physiological processes such as cell growth, cell division, innate immunity, calcium homeostasis, stem cell reprogramming, energy metabolism, and apoptosis (1,2). Mitochondria can adjust their tubular network through morphological changes, and fission and fusion affect the size and number of mitochondria, which is termed mitochondrial dynamics (3).
Mitochondrial fission is primarily driven by Dynein-related protein 1 (Drp1) (4), which is located in the cytoplasm under physiological conditions. The activated Drp1 translocates to the outer mitochondrial membrane, where it binds to its mitochondrial adaptors (including FIS1 and MFF) to promote fission events (5). In certain cases, cancer cells exhibit high levels of fission mitochondrial fragmentation and enhanced function, which leads to tumor growth and the development of chemoresistance (6). The activation and upregulation of Drp1 have been observed in various types of cancer, such as lung cancer, breast cancer, glioblastoma, colorectal cancer, pancreatic cancer, thyroid tumor, nasopharyngeal carcinoma, and melanoma (7–13). Previous studies have found that the loss of Drp1 leads to prolongation of the mitochondrial network, inhibition of cell proliferation, and induction of spontaneous apoptosis in a variety of cancer cells (6,14). However, the role of Drp1 in bladder cancer remains unclear.
The ubiquitin-proteasome pathway is the primary mechanism of protein catabolism in the cytoplasm and nucleus of mammals and is involved in the degradation of >80% of the proteins in the cell (15). The deubiquitinating enzyme (DUB) catalyzes the process of removing ubiquitin from ubiquitinated substrates, which is termed deubiquitylation (16). Due to the wide range of substrates, deubiquitylation plays an important role in a variety of cellular functions. Previous studies have reported that deubiquitylation dysregulation may the induction of various diseases, including cancer (17,18). As a subunit of the 19S regulatory particle in the 26S proteasome, the 26S proteasome non-ATPase regulatory subunit 14 (PSMD14) belongs to the JAB1/MPN+/MOV34 (JAMM) domain protease family of DUB proteins and has been reported to be involved in the occurrence and development of various types of cancer (19). Downregulation of PSMD14 can induce G0/G1 phase arrest, and apoptosis, and reduce the proliferation and epithelial-mesenchymal transition (EMT) of lung adenocarcinoma, prostate cancer, and breast cancer cells (20–24). A previous study found that PSMD14 exerts carcinogenic effects in head and neck squamous cell carcinoma and glioma by inhibiting the ubiquitination and degradation of cancer-related transcription factor E2F1 (25,26). PSMD14 also promotes EMT in esophageal squamous cell carcinoma by targeting SNAIL for deubiquitylation and stabilization (27). Therefore, PSMD14 acts as an oncogene, deubiquitinating a variety of protein substrates. However, the function and mechanism of PSMD14 in bladder cancer remain to be explored.
In the present study, it was found that PSMD14 functions upstream of Drp1 and is upregulated in patients with bladder cancer, which indicated a potential mechanism of PSMD14 and mitochondrial fission in cancer. The results of the present study revealed the importance of PSMD14 in tumorigenesis and a novel treatment strategy to attenuate mitochondrial fission by inhibiting the PSMD14-Drp1 pathway.
Materials and methods
Clinical tissues
This study was approved by the Institutional Ethics Committee of the Hunan Provincial People's Hospital and in accordance with the Ethical Management Guidelines of Hunan Provincial People's Hospital (approval no. 210139). A total of 32 bladder cancer tissue specimens and matched normal specimens were collected from Hunan Provincial People's Hospital. Informed written consent was obtained from all the participants.
Cell culture
T24 and 5637 cells (American Type Culture Collection) were cultured in RPMI-1640 medium (Thermo Fisher Scientific, Inc.) supplemented with 10% FBS, 100 mg/ml streptomycin, and 100 units of penicillin. All the cells were cultured in a humidified incubator at 37°C, supplied with 5% CO2, 21% O2, and 74% N2.
siRNA transfections
Cells were first seeded in six-well plates and cultured until they reached 30–50% confluence. siRNAs were introduced to the cells through transfection. Cells were transfected with Lipofectamine® 3000 Transfection Reagent (Beyotime Institute of Biotechnology). For the present study, six groups of cells were used: i) Lipofectamine® 3000 Transfection Reagent alone; ii) Lipofectamine® 3000 Transfection Reagent combined with 50 nM/µl NC siRNA; iii) Lipofectamine® 3000 Transfection Reagent combined with 50 nM/µl si-PSMD14 (5′-AAGGTTGTTATTGATGCCTTCAGAT-3′); iv) Lipofectamine® 3000 Transfection Reagent combined with 50 nM/µl si-NC for PSMD14 (5′-AAGTTGTGTTACGTACTTCATGGAT-3′); v) Lipofectamine® 3000 Transfection Reagent combined with 50 nM/µl si-Drp1 (5′-AAGTGGTGACTTGTCTTCTTCGTAA-3′); vi) Lipofectamine® 3000 Transfection Reagent combined with or 50 nM/µl si-NC for DRP1 (5′-AAGAGTGGTTCTTCTCTTCGGTTAA-3′). Cells were transfected for 24 h before subsequent experiments. Drp1 agonist Mdivi-1 (50 mg/ml; Beyotime Institute of Biotechnology) was also used to manipulate Drp expression. The manufacturer's instructions were followed during the transfection process, and the efficiency of the transfection was confirmed using qPCR.
Immunohistochemical staining
The bladder tissue sections were treated first by removing the paraffin using xylene and ethanol, and then performing antigen retrieval. Following this, tissues were blocked using 3% BSA and then stained using two different types of antibodies anti-PSMD14 (cat. no. 4197S; Cell Signaling Technology, Inc.; 1:100) or anti-Drp1 (cat. no. 8570S; Cell Signaling Technology, Inc.; 1:100) at 4°C overnight. To ensure the reliability of the results, a control was also included by using mouse serum at the same protein concentration as the antibody solution. Under a light microscope at an ×100 magnification, the positively stained tissue sections could be seen as appearing brown or tawny-brown in color. Images of the tissue were taken using an optical microscope, and the integrated optical density (IOD) values of the tissue sections were then measured using Image-Pro Plus version 6.0 (Media Cybernetics, Inc.). To ensure accuracy, five different fields of view were selected from each tissue section, and three tissue sections were used for each group to determine the positive IOD values. The mean IOD values were then used to calculate the relative expressions of PSMD14 and Drp1.
Immunofluorescent staining
Cells that had been grown on slides were fixed with 4% paraformaldehyde solution for 15 min at room temperature, and then permeabilized with 0.1% Triton X-100 at room temperature for 15 min. The samples were washed three times with PBS and then sealed with a 5% BSA solution at room temperature for 45 min. Next, the primary antibody was incubated with the sample overnight at 4°C. The primary antibodies used were: Anti-PSMD14 (cat. no. 4197S; 1:200; Cell Signaling Technology, Inc.), anti-Drp1 (cat. no. 8570S; 1:200; Cell Signaling Technology, Inc.), or anti-TOM20 (cat. no. 42406S; 1:200; Cell Signaling Technology, Inc.). DAPI (cat. no. 4083S; Cell Signaling Technology, Inc. 1:200) was used to counterstain the nuclei. The secondary antibodies included Alexa FluoR 555 goat anti-rabbit (cat. no. A27017; 1:400; Invitrogen; Thermo Fisher Scientific, Inc.). A total of five different fields of view were selected and randomly scored using a fluorescence microscope (magnification, ×40, Olympus Corporation).
Western blotting
Cells were collected and lysed using RIPA buffer (Beyotime Institute of Biotechnology) for 30 min at 4°C. The nuclear protein was collected using cytoplasmic and nuclear extraction kit (cat. no. A37487; Invitrogen; Thermo Fisher Scientific, Inc.). The protein lysates were quantified by BCA Protein Assay Kit (Beyotime Institute of Biotechnology). SDS gels (8–12%) were used for SDS-PAGE. The resolved proteins were then transferred to PVDF. To block the membranes, a 10% BSA solution was dissolved in TBS buffer containing 0.05% Tween 20 and added to the membranes at room temperature for 1 h. The primary antibody was then added to TBS containing 0.05% Tween 20 and incubated for at least 10 h at 4°C. The primary antibodies used were anti-PSMD14 (cat. no. 4197S; Cell Signaling Technology, Inc.; 1:1,000) and anti-Drp1 (cat. no. 8570S; Cell Signaling Technology, Inc.; 1:1,000). To ensure accuracy, the protein content was normalized using GAPDH (cat. no. 5174S; Cell Signaling Technology, Inc.; 1:8,000) as the internal control. After washing the membrane three times, the anti-rabbit second antibody (cat. no. 7074S; Cell Signaling Technology, Inc.; 1:8,000) and anti-mouse mice secondary antibody (cat. no. 7076S; Cell Signaling Technology, Inc.; 1:8,000) in TBS containing 0.05% Tween 20 were used at room temperature for 1 h. After three additional washes, the bands were detected by enhanced chemiluminescence and autoradiography. The experiments were repeated three times, and the results were evaluated using Image Lab 6.0 (Bio-Rad Laboratories, Inc.). Drp1 agonist Mdivi-1 (50 mg/ml; Beyotime Institute of Biotechnology) was also used to manipulate the expression of Drp1. The blots were grouped and cropped from the same gel.
RNA extraction and qPCR
Total RNA was extracted using TRIzol® reagent (Invitrogen; Thermo Fisher Scientific, Inc.) and RNA purity was assessed using a spectrophotometer. First-strand cDNA was synthesized using a cDNA synthesis kit (Promega Corporation) at 65°C for 5 min according to the manufacturer's protocol. Subsequently, cDNA was amplified by qPCR using an Applied Biosystems SYBR Green mix kit and the ABI 7900 Real-Time PCR system (both Applied Biosystems; Thermo Fisher Scientific, Inc.) following the manufacturer's instructions. qPCR was performed as follows: Denaturation at 94°C for 4 min; followed by 40 cycles of amplification at 94°C for 30 sec, hybridization at 56°C for 30 sec, and extension at 72°C for 30 sec. Quantification of mRNA was automatically conducted using SDS version 1.3 software (Applied Biosystems; Thermo Fisher Scientific, Inc.), and the 2−∆∆Cq method was employed to calculate mRNA expression (28,29). The sequences of the primers used were: PSMD14 forward, 5′-GTCAGTGTGGAGGCAGTTGATC-3′, and reverse, 5′-CCACACCAGAAAGCCAACAACC-3′; DRP1 forward, 5′-TCACCCGGAGACCTCTCATTC-3′ and reverse, 5′-GGTTCAGGGCTTACTCCCTTAT-3′, and β-actin forward, 5′-CGAGCGCGGCTACAGCTT-3′ and reverse, 5′-TCCTTAATGTCACGCACGATTT-3′.
Cell viability assay
T24 and 5637 cells in the cell culture flask were digested with 0.25% trypsin. Then these cells were seeded in a 96-well culture plate at a density of 1×104 cells/ml for 24 h. After 24 h of incubation, 10 µl CCK-8 reagent was added to each well for 2 h. The optical density was measured at a wavelength of 450 nm using a Bio-Rad 680 microplate reader. Each experiment was performed in triplicates.
In vitro ubiquitination assay
Cells were transfected with si-PSMD14. After transfection for 48 h, the cells were treated with MG132 (15 µm; Beyotime Institute of Biotechnology) overnight and then lysed with RIPA lysis buffer, follow by passing 5 times through a 21-gauge needle. Whole-cell extract (1 mg) was used for immunoprecipitation with 1 µg anti-PSMD14 (cat. no. 4197S; Cell Signaling Technology, Inc.) or anti-Drp1 (cat. no. 8570S; Cell Signaling Technology, Inc.). The proteins that had been pulled down were subjected to SDS/PAGE for immunoblot analysis.
Statistical analysis
Each experiment was repeated at least three times. The categorical data were assessed using a Fisher's exact test, and the quantitative data are presented as the mean ± SEM. All statistical analyses were performed using SPSS version 19.0 (IBM Corp.). The differences between groups were compared using a Student's t-test or ANOVA followed by a post hoc Dunnett's test or Tukey's test. P<0.05 was considered to indicate a statistically significant difference.
Results
Drp1 and PSMD14 expression are upregulated in bladder cancer
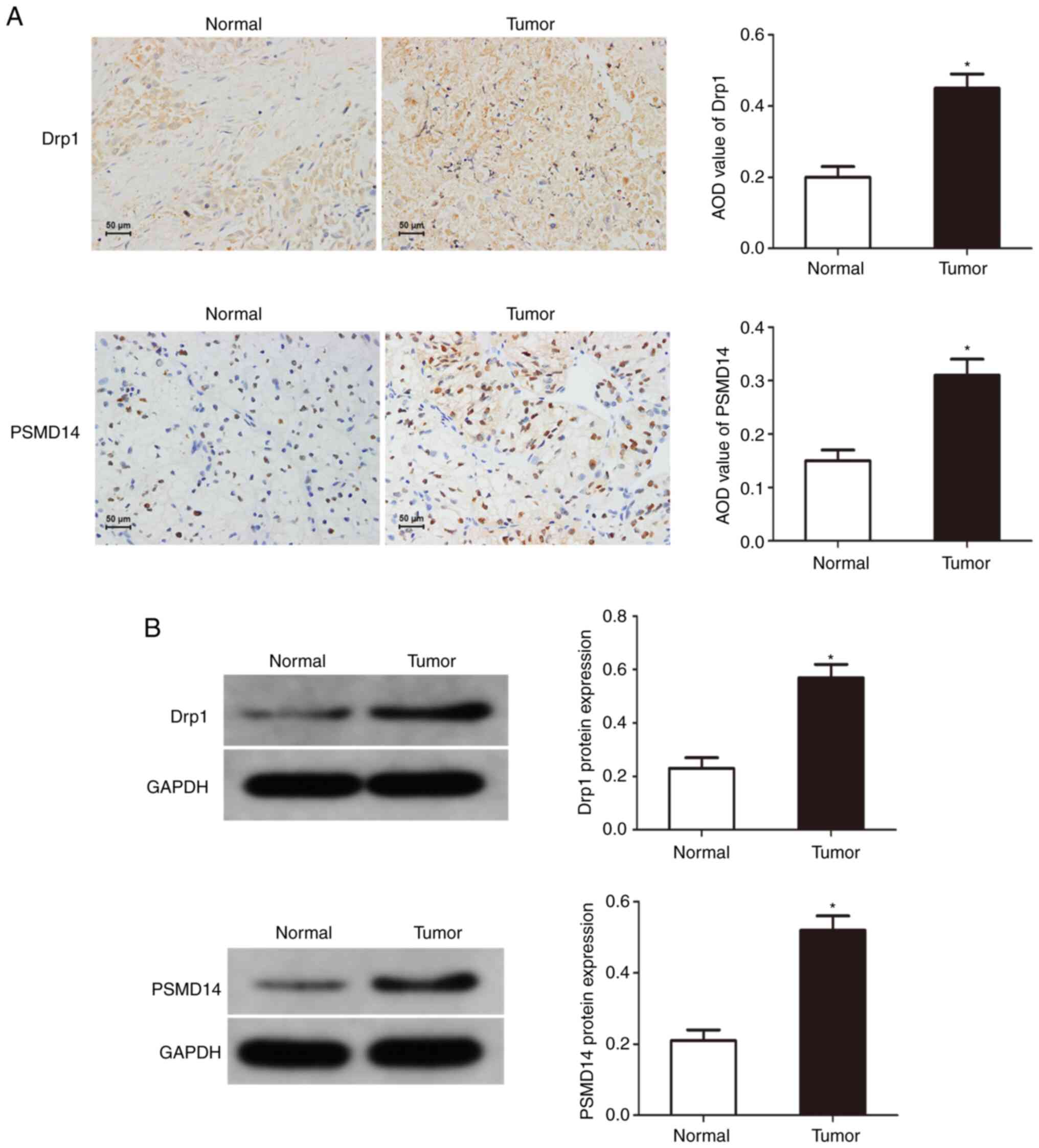
To reveal the potential function of PSMD14 in cancer, immunohistochemical staining and western blotting were used to determine the expression of Drp1 and PSMD14 in bladder cancer tissues, and it was found that compared with normal tissues, Drp1 and PSMD14 expression were abnormally upregulated in bladder cancer tissues (Fig. 1). These results suggest that Drp1 and PSMD14 may play important roles in the occurrence and development of bladder cancer.
Knockdown of PSMD14 inhibits the growth of bladder cancer cells
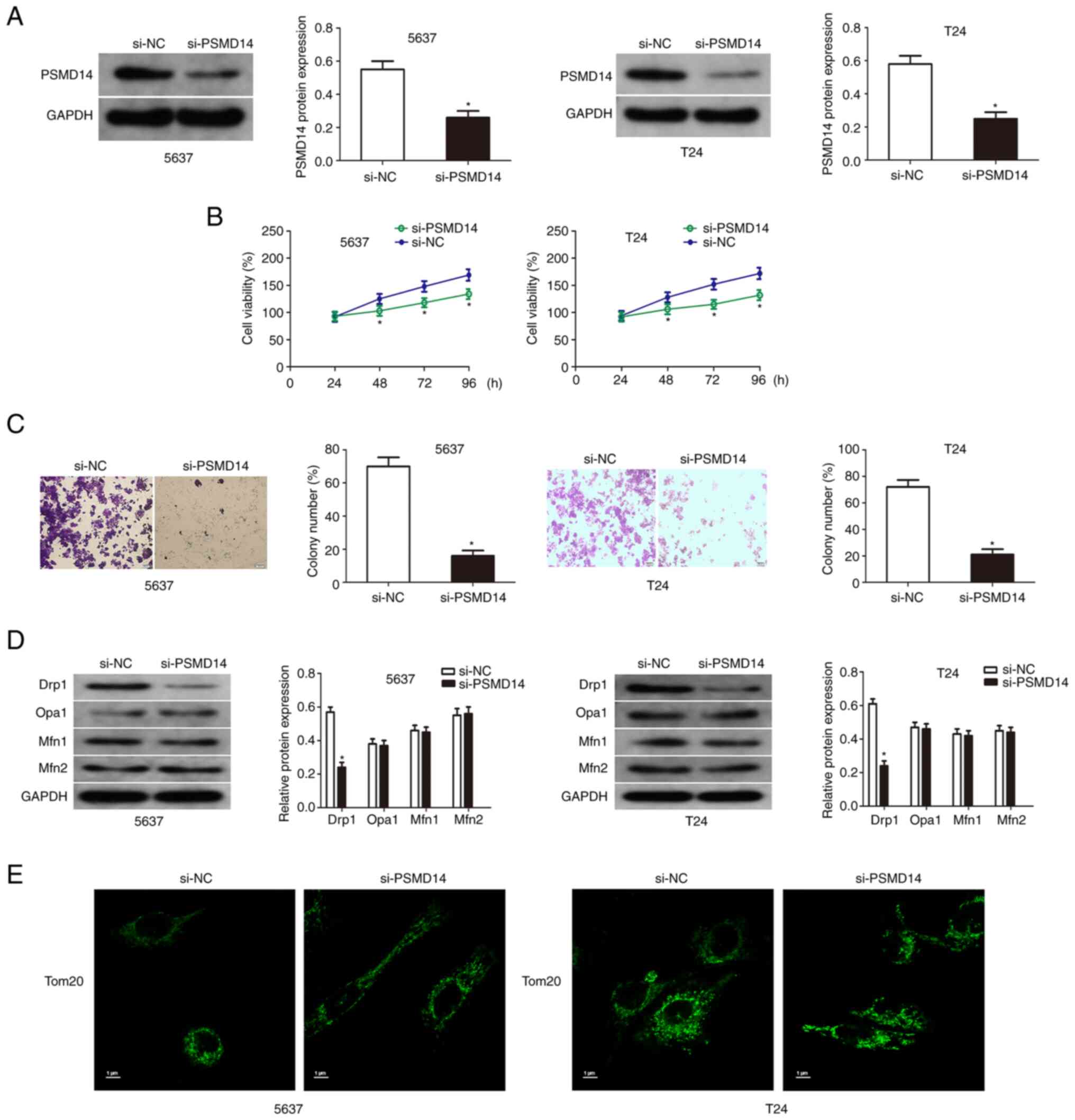
The expression of PSMD14 of T24 and 5637 cells transfected with PSMD14 siRNA was detected by western blotting (Fig. 2A). Then cell viability assay and colony formation assays were used to detect the proliferation and colony formation of the transfected bladder cancer cells compared with the respective control cells. The results showed that the knockdown of PSMD14 significantly reduced the growth and proliferation capacity of T24 and 5637 cells (Fig. 2B and C). Mitochondrial fusion in the bladder cancer cells was assessed to explore the relationship between PSMD14 and mitochondrial dynamics. PSMD14 knockdown did not affect the expression of mitochondrial fusion factors (OPA1, Mfn1, and Mfn2) by immunofluorescent staining, and the levels of mitochondrial factor-Drp1 were downregulated by PSMD14 knockdown (Fig. 2D). Drp1 induced mitochondria to produce several fragments, and PSMD14 knockdown blocked this process (Fig. 2E).
The interaction mode between PSMD14 and Drp1
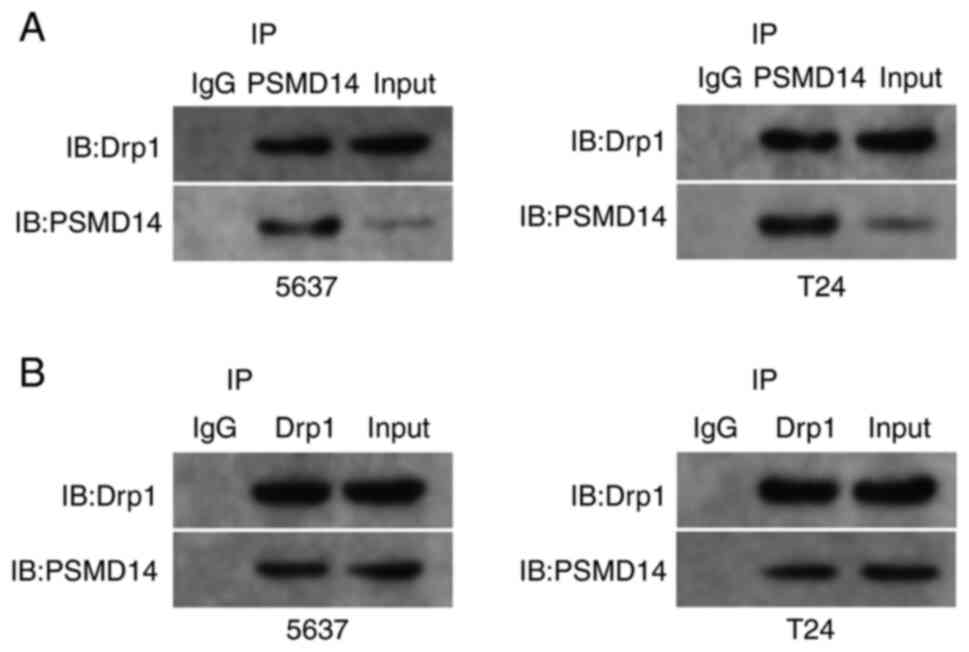
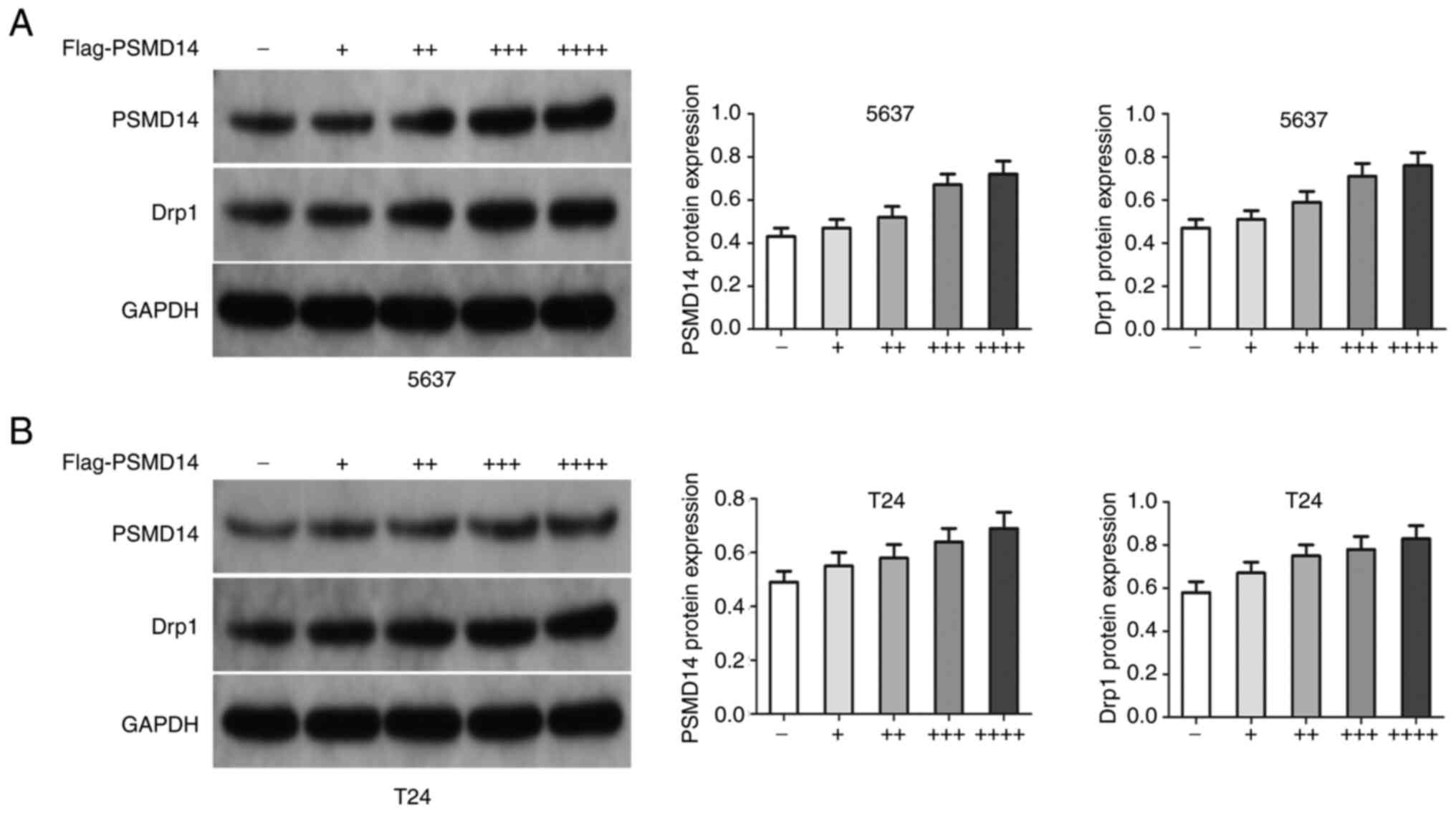
Next, whether endogenous Drp1 interacted with endogenous PSMD14 was assessed. Endogenous Drp1 and PSMD14 proteins were co-immunoprecipitated (Fig. 3A), and this finding was confirmed through reciprocal co-IP test (Fig. 3B). Then, si-PSMD14 transfected T24 and 5637 cells were treated with exogenous PSMD14. The results showed that the PSMD14 resulted in an increase in Drp1 in T24 and 5637 cells (Fig. 4A and B).
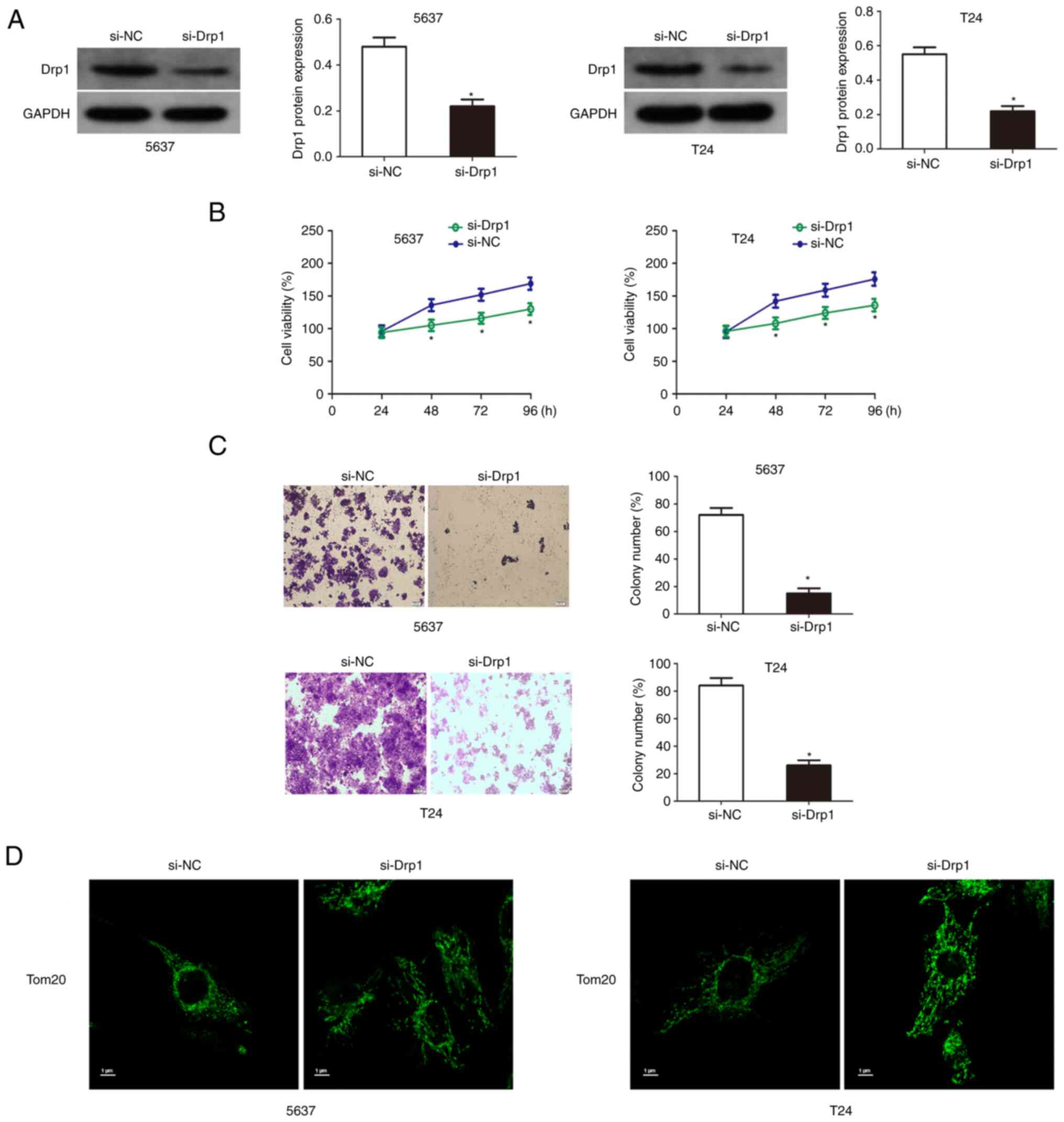
Knockdown of Drp1 inhibits the growth of bladder cancer cells
The results of western blotting results showed that Drp1 was significantly downregulated in T24 and 5637 cells transfected with si-Drp1 (Fig. 5A). Then cell viability and colony formation assays were used to detect the proliferation and colony formation ability of Drp1 knockdown bladder cancer cells. The results showed that the knockdown of Drp1 significantly reduced the growth and proliferation capacity of T24 and 5637 cells (Fig. 5B and C). Additionally, Drp1 knockdown significantly increased mitochondrial length and inhibited mitochondrial fission (Fig. 5D).
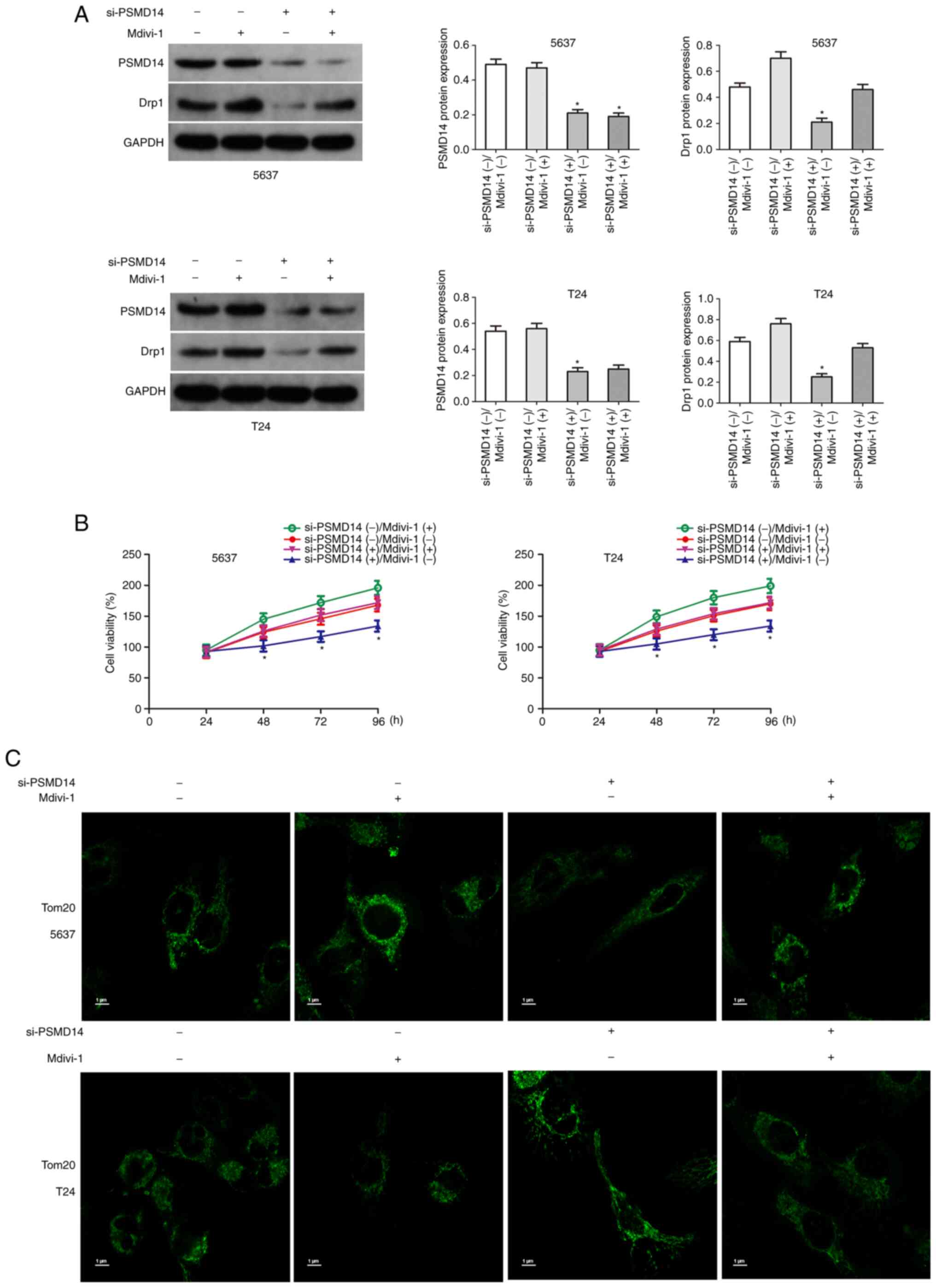
Upregulation of Drp1 partially restores the carcinogenic effects of PSMD14
The above results confirmed that Drp1 plays an important role in PSMD14-mediated bladder cancer. Next, whether the upregulation of Drp1 reversed the anticancer effects mediated by PSMD14 knockdown was assessed. Drp1 agonist Mdivi-1 (50 mg/ml) was used to upregulate Drp1 in bladder cancer cells (Fig. 6A). Then the proliferation of si-PSMD14 transfected bladder cancer cells treated with Mdivi-1 was determined. The results of the cell viability assays showed that Mdivi-1 significantly increased the growth of T24 and 5637 cells transfected with si-PSMD14 (Fig. 6B). Mdivi-1 treatment significantly reduced mitochondrial length and promoted mitochondrial fission (Fig. 6C).
Discussion
The ubiquitin-proteasome system is the primary mechanism of protein catabolism in the mammalian cytoplasm and nucleus, and it participates in the degradation of most proteins in the cell (30). The ubiquitin-proteasome system is modified by ubiquitination and deubiquitylation to maintain the dynamic balance of intracellular proteins and is one of the important balance systems for maintaining the stability of the intracellular environment. Additional evidence has shown that the changes in expression levels of deubiquitinating enzymes are closely related to human diseases, such as autoimmune diseases and tumors (31–34). PSMD14 plays an important role under normal physiological conditions, including the repair process of DNA damage, embryonic cell development, cell differentiation, and cell apoptosis (33–35). The abnormal expression of PSMD14 disrupts the dynamic balance of proteins in cells, leading to a variety of diseases including tumors (36,37). The results confirmed that the progression of bladder cancer is significantly associated with upregulated expression of PSMD14. PSMD14 can affect the malignant biological behavior of tumor cells, such as apoptosis resistance, proliferation, chemotherapy resistance, invasion, and metastasis by inhibiting the ubiquitination and degradation of specific proteins. A previous study showed that high expression of PSMD14 increased the resistance of colon cancer cells to chemotherapeutic drugs (38). Zhu et al (36) found that PSMD14 induced the invasion and metastasis of esophageal cancer by regulating the ubiquitination and degradation of the EMT transcription factor Snail. PSMD14 reversed the ubiquitination of GRB2 and promoted cell growth and metastasis of hepatocellular carcinoma (20). PSMD14 can also regulate the stability of ALK2 to make colorectal cancer cells more resistant to chemotherapy (37). PSMD14 can also determine the fate of its substrate protein. PSMD14 promotes substrate transfer and proteolysis by removing the ubiquitin label and induces the release of substrate proteins from the proteasome to avoid degradation (36,39). Therefore, an increasing number of studies are suggesting that PSMD14 may be used as a marker in the ubiquitin-proteasome system to determine the fate of substrates. Here, it was found that PSMD14 knockdown can inhibit cell growth of bladder cancer, suggesting that PSMD14 plays an important role in the progression of bladder cancer.
Mitochondria are the central hub of cellular energy metabolism, biosynthesis, and signal transduction, and can help cells sense stress and adapt to the environment; thus, they play a special role in the occurrence and development of tumors (40). Disturbances in mitochondrial dynamics have been observed in different tumors. Mitochondrial fusion and fission proteins of tumor cells are often abnormally expressed, and mitochondrial morphology has also been shown to be altered (41–43). The expression levels of the mitochondrial division protein Drp1 were upregulated in liver cancer, breast cancer, and lung cancer (44–46). The increased mitochondrial fission in tumor cells suggests the changes in mitochondrial dynamics in tumors, and the intervention of disordered mitochondrial fusion or fission may be a potential tumor therapeutic target for tumors (47). The present study found that Drp1 expression is upregulated, and mitochondrial fission increased significantly in bladder cancer cells. Furthermore, PSMD14 could deubiquitinate and stabilize Drp1.
Mitochondrial dynamics also play a vital role in the regulation of the cell cycle. Mitra et al (48) revealed that the cycle of mitochondrial division and fusion is highly coupled with cell cycle progression. In the process of cell division, the mitochondria assigned to each daughter cell are divided from those in the parent cells. In the G1 and G2 phases, a network is formed between the mitochondria. However, in the S phase and mitotic phase, mitochondria are present in a highly fragmented state, which may be due to the increased expression of Drp1 during the cell cycle, which promotes the division of mitochondria (6). Mitra et al (48) also found that inhibiting the expression of Drp1 in cells can induce cell cycle arrest in the G1 phase. Previous studies have confirmed that overexpression of Drp1 promotes the proliferation of cancer cells by accelerating the transition of cells in the G1/S phase (6,49). Kitamura et al (50) found that Drp1 knockdown can arrest cell cycle progression of skin squamous cell carcinoma at the G2/M phase. Studies have found that overexpression of Drp1 can promote the proliferation of breast cancer, lung cancer, and glioma cells; however, the underlying mechanism has not been fully elucidated (51–53). The present study confirmed that the upregulation of Drp1 could reverse the anticancer effects mediated by PSMD14 knockdown. These results have shown that the overexpression of Drp1 may be related to tumor cell proliferation. The primary limitation of the present study is the lack of in vivo experiments, and thus, this will be taken into consideration in our future studies.
In conclusion, at present, the role of PSMD14 in the occurrence and development of cancer has not been fully elucidated. The results indicated that PSMD14 is closely related to the occurrence and development of bladder cancer. In addition, the present study proposes for the first time that PSMD14 mediates the mitochondrial fission of bladder cancer cells by deubiquitinating and stabilizing Drp1. PSMD14 as an important oncogenic protein of bladder cancer, may thus serve as a novel anti-cancer target.
Acknowledgements
Not available.
Funding
This project is supported by the Hunan Provincial Health Commission (grant no. 20200053), Changsha Municipal Natural Science Foundation (grant no. kq2014096), and the Natural Science Foundation of Hunan Province (grant no. 2022JJ70099).
Availability of data and materials
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.
Authors' contributions
WX contributed to the conception and design of the study. WS performed the experiments. ZL and MX analyzed data and wrote this manuscript. WX revised and reviewed the manuscript. WS, MX, ZL and WX confirm the authenticity of all the raw data.
Ethics approval and consent to participate
This study was approved by the Institutional Ethics Committee of the Hunan Provincial People's Hospital and in accordance with the Ethical Management Guidelines of Hunan Provincial People's Hospital (approval no. 210139). Written informed consent was obtained from all participants.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
|
Zong WX, Rabinowitz JD and White E: Mitochondria and cancer. Mol Cell. 61:667–676. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Rangaraju V, Lewis TJ Jr, Hirabayashi Y, Bergami M, Motori E, Cartoni R, Kwon SK and Courchet J: Pleiotropic mitochondria: The influence of mitochondria on neuronal development and disease. J Neurosci. 39:8200–8208. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Tilokani L, Nagashima S, Paupe V and Prudent J: Mitochondrial dynamics: Overview of molecular mechanisms. Essays Biochem. 62:341–360. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Schmitt K, Grimm A, Dallmann R, Oettinghaus B, Restelli LM, Witzig M, Ishihara N, Mihara K, Ripperger JA, Albrecht U, et al: Circadian control of DRP1 activity regulates mitochondrial dynamics and bioenergetics. Cell Metab. 27:657–666.e5. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Zhou BH, Wei SS, Jia LS, Zhang Y, Miao CY and Wang HW: Drp1/Mff signaling pathway is involved in fluoride-induced abnormal fission of hepatocyte mitochondria in mice. Sci Total Environ. 725:1381922020. View Article : Google Scholar : PubMed/NCBI | |
|
Ma Y, Wang L and Jia R: The role of mitochondrial dynamics in human cancers. Am J Cancer Res. 10:1278–1293. 2020.PubMed/NCBI | |
|
Chauhan SS, Toth RK, Jensen CC, Casillas AL, Kashatus DF and Warfel NA: PIM kinases alter mitochondrial dynamics and chemosensitivity in lung cancer. Oncogene. 39:2597–2611. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Zhou D, Ren K, Wang M, Wang J, Li E, Hou C, Su Y, Jin Y, Zou Q, Zhou P and Liu X: Long non-coding RNA RACGAP1P promotes breast cancer invasion and metastasis via miR-345-5p/RACGAP1-mediated mitochondrial fission. Mol Oncol. 15:543–559. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Kamradt ML, Jung JU, Pflug KM, Lee DW, Fanniel V and Sitcheran R: NIK promotes metabolic adaptation of glioblastoma cells to bioenergetic stress. Cell Death Dis. 12:2712021. View Article : Google Scholar : PubMed/NCBI | |
|
Zhang BY, Zhang L, Chen YM, Qiao X, Zhao SL, Li P, Liu JF, Wen X and Yang J: Corosolic acid inhibits colorectal cancer cells growth as a novel HER2/HER3 heterodimerization inhibitor. Br J Pharmacol. 178:1475–1491. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Courtois S, de Luxán-Delgado B, Penin-Peyta L, Royo-García A, Parejo-Alonso B, Jagust P, Alcalá S, Rubiolo JA, Sánchez L, Sainz B Jr, et al: Inhibition of mitochondrial dynamics preferentially targets pancreatic cancer cells with enhanced tumorigenic and invasive potential. Cancers (Basel). 13:6982021. View Article : Google Scholar : PubMed/NCBI | |
|
Zhou TJ, Zhang SL, He CY, Zhuang QY, Han PY, Jiang SW, Yao H, Huang YJ, Ling WH, Lin YC and Lin ZN: Downregulation of mitochondrial cyclooxygenase-2 inhibits the stemness of nasopharyngeal carcinoma by decreasing the activity of dynamin-related protein 1. Theranostics. 7:1389–1406. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Ferraz LS, Costa RTD, Costa CAD, Ribeiro CAJ, Arruda DC, Maria-Engler SS and Rodrigues T: Targeting mitochondria in melanoma: Interplay between MAPK signaling pathway and mitochondrial dynamics. Biochem Pharmacol. 178:1141042020. View Article : Google Scholar : PubMed/NCBI | |
|
Qian W, Wang J and Van Houten B: The role of dynamin-related protein 1 in cancer growth: A promising therapeutic target? Expert Opin Ther Targets. 17:997–1001. 2013. View Article : Google Scholar : PubMed/NCBI | |
|
Luza S, Opazo CM, Bousman CA, Pantelis C, Bush AI and Everall IP: The ubiquitin proteasome system and schizophrenia. Lancet Psychiatry. 7:528–537. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Zhu D, Xu R, Huang X, Tang Z, Tian Y, Zhang J and Zheng X: Deubiquitinating enzyme OTUB1 promotes cancer cell immunosuppression via preventing ER-associated degradation of immune checkpoint protein PD-L1. Cell Death Differ. 28:1773–1789. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Park HB, Kim JW and Baek KH: Regulation of Wnt signaling through ubiquitination and deubiquitination in cancers. Int J Mol Sci. 21:39042020. View Article : Google Scholar : PubMed/NCBI | |
|
Sun T, Liu Z and Yang Q: The role of ubiquitination and deubiquitination in cancer metabolism. Mol Cancer. 19:1462020. View Article : Google Scholar : PubMed/NCBI | |
|
Sukenik S, Braunstein I and Stanhill A: An arsenite relay between PSMD14 and AIRAP enables revival of proteasomal DUB activity. Biomolecules. 11:13172021. View Article : Google Scholar : PubMed/NCBI | |
|
Lv J, Zhang S, Wu H, Lu J, Lu Y, Wang F, Zhao W, Zhan P, Lu J, Fang Q, et al: Deubiquitinase PSMD14 enhances hepatocellular carcinoma growth and metastasis by stabilizing GRB2. Cancer Lett. 469:22–34. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Luo G, Hu N, Xia X, Zhou J and Ye C: RPN11 deubiquitinase promotes proliferation and migration of breast cancer cells. Mol Med Rep. 16:331–338. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Yu W, Li J, Wang Q, Wang B, Zhang L, Liu Y, Tang M, Xu G, Yang Z, Wang X, et al: Targeting POH1 inhibits prostate cancer cell growth and enhances the suppressive efficacy of androgen deprivation and docetaxel. Prostate. 79:1304–1315. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Zhang L, Xu H, Ma C, Zhang J, Zhao Y, Yang X, Wang S and Li D: Upregulation of deubiquitinase PSMD14 in lung adenocarcinoma (LUAD) and its prognostic significance. J Cancer. 11:2962–2971. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Byrne A, McLaren RP, Mason P, Chai L, Dufault MR, Huang Y, Liang B, Gans JD, Zhang M, Carter K, et al: Knockdown of human deubiquitinase PSMD14 induces cell cycle arrest and senescence. Exp Cell Res. 316:258–271. 2010. View Article : Google Scholar : PubMed/NCBI | |
|
Jing C, Duan Y, Zhou M, Yue K, Zhuo S, Li X, Liu D, Ye B, Lai Q, Li L, et al: Blockade of deubiquitinating enzyme PSMD14 overcomes chemoresistance in head and neck squamous cell carcinoma by antagonizing E2F1/Akt/SOX2-mediated stemness. Theranostics. 11:2655–2669. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Zhi T, Jiang K, Xu X, Yu T, Zhou F, Wang Y, Liu N and Zhang J: ECT2/PSMD14/PTTG1 axis promotes the proliferation of glioma through stabilizing E2F1. Neuro Oncol. 21:462–473. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Jing C, Li X, Zhou M, Zhang S, Lai Q, Liu D, Ye B, Li L, Wu Y, Li H, et al: The PSMD14 inhibitor Thiolutin as a novel therapeutic approach for esophageal squamous cell carcinoma through facilitating SNAIL degradation. Theranostics. 11:5847–5862. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Rao X, Huang X, Zhou Z and Lin X: An improvement of the 2ˆ(−delta delta CT) method for quantitative real-time polymerase chain reaction data analysis. Biostat Bioinforma Biomath. 3:71–85. 2013.PubMed/NCBI | |
|
Livak KJ and Schmittgen TD: Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001. View Article : Google Scholar : PubMed/NCBI | |
|
Asmamaw MD, Liu Y, Zheng YC, Shi XJ and Liu HM: Skp2 in the ubiquitin-proteasome system: A comprehensive review. Med Res Rev. 40:1920–1949. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Wang J and Maldonado MA: The ubiquitin-proteasome system and its role in inflammatory and autoimmune diseases. Cell Mol Immunol. 3:255–261. 2006.PubMed/NCBI | |
|
Singh SR, Meyer-Jens M, Alizoti E, Bacon WC, Davis G, Osinska H, Gulick J, Reischmann-Düsener S, Orthey E, McLendon PM, et al: A high-throughput screening identifies ZNF418 as a novel regulator of the ubiquitin-proteasome system and autophagy-lysosomal pathway. Autophagy. 17:3124–3139. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Narayanan S, Cai CY, Assaraf YG, Guo HQ, Cui Q, Wei L, Huang JJ, Ashby CR Jr and Chen ZS: Targeting the ubiquitin-proteasome pathway to overcome anti-cancer drug resistance. Drug Resist Updat. 48:1006632020. View Article : Google Scholar : PubMed/NCBI | |
|
Yerlikaya A, Kanbur E, Stanley BA and Tümer E: The ubiquitin-proteasome pathway and epigenetic modifications in cancer. Anticancer Agents Med Chem. 21:20–32. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Sakai W, Yuasa-Sunagawa M, Kusakabe M, Kishimoto A, Matsui T, Kaneko Y, Akagi JI, Huyghe N, Ikura M, Ikura T, et al: Functional impacts of the ubiquitin-proteasome system on DNA damage recognition in global genome nucleotide excision repair. Sci Rep. 10:197042020. View Article : Google Scholar : PubMed/NCBI | |
|
Zhu R, Liu Y, Zhou H, Li L, Li Y, Ding F, Cao X and Liu Z: Deubiquitinating enzyme PSMD14 promotes tumor metastasis through stabilizing SNAIL in human esophageal squamous cell carcinoma. Cancer Lett. 418:125–134. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Seo D, Jung SM, Park JS, Lee J, Ha J, Kim M and Park SH: The deubiquitinating enzyme PSMD14 facilitates tumor growth and chemoresistance through stabilizing the ALK2 receptor in the initiation of BMP6 signaling pathway. EbioMedicine. 49:55–71. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Fire A, Xu S, Montgomery MK, Kostas SA, Driver SE and Mello CC: Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature. 391:806–811. 1998. View Article : Google Scholar : PubMed/NCBI | |
|
Yao T and Cohen RE: A cryptic protease couples deubiquitination and degradation by the proteasome. Nature. 419:403–407. 2002. View Article : Google Scholar : PubMed/NCBI | |
|
Vyas S, Zaganjor E and Haigis MC: Mitochondria and cancer. Cell. 166:555–566. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Dai W and Jiang L: Dysregulated mitochondrial dynamics and metabolism in obesity, diabetes, and cancer. Front Endocrinol (Lausanne). 10:5702019. View Article : Google Scholar : PubMed/NCBI | |
|
Chan DC: Mitochondrial dynamics and its involvement in disease. Annu Rev Pathol. 15:235–259. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Rodrigues T and Ferraz LS: Therapeutic potential of targeting mitochondrial dynamics in cancer. Biochem Pharmacol. 182:1142822020. View Article : Google Scholar : PubMed/NCBI | |
|
Deng X, Liu J, Liu L, Sun X, Huang J and Dong J: Drp1-mediated mitochondrial fission contributes to baicalein-induced apoptosis and autophagy in lung cancer via activation of AMPK signaling pathway. Int J Biol Sci. 16:1403–1416. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Moghaddam FD, Mortazavi P, Hamedi S, Nabiuni M and Roodbari NH: Apoptotic effects of melittin on 4T1 breast cancer cell line is associated with up regulation of Mfn1 and Drp1 mRNA expression. Anticancer Agents Med Chem. 20:790–799. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Zhan L, Cao H, Wang G, Lyu Y, Sun X, An J, Wu Z, Huang Q, Liu B and Xing J: Drp1-mediated mitochondrial fission promotes cell proliferation through crosstalk of p53 and NF-κB pathways in hepatocellular carcinoma. Oncotarget. 7:65001–65011. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Hu J, Zhang Y, Jiang X, Zhang H, Gao Z, Li Y, Fu R, Li L, Li J, Cui H and Gao N: ROS-mediated activation and mitochondrial translocation of CaMKII contributes to Drp1-dependent mitochondrial fission and apoptosis in triple-negative breast cancer cells by isorhamnetin and chloroquine. J Exp Clin Cancer Res. 38:2252019. View Article : Google Scholar : PubMed/NCBI | |
|
Mitra K, Wunder C, Roysam B, Lin G and Lippincott-Schwartz J: A hyperfused mitochondrial state achieved at G1-S regulates cyclin E buildup and entry into S phase. Proc Natl Acad Sci USA. 106:11960–11965. 2009. View Article : Google Scholar : PubMed/NCBI | |
|
Liang J, Yang Y, Bai L, Li F and Li E: DRP1 upregulation promotes pancreatic cancer growth and metastasis through increased aerobic glycolysis. J Gastroenterol Hepatol. 35:885–895. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Kitamura S, Yanagi T, Imafuku K, Hata H, Abe R and Shimizu H: Drp1 regulates mitochondrial morphology and cell proliferation in cutaneous squamous cell carcinoma. J Dermatol Sci. 88:298–307. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Xie Q, Wu Q, Horbinski CM, Flavahan WA, Yang K, Zhou W, Dombrowski SM, Huang Z, Fang X, Shi Y, et al: Mitochondrial control by DRP1 in brain tumor initiating cells. Nat Neurosci. 18:501–510. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Zhao J, Zhang J, Yu M, Xie Y, Huang Y, Wolff DW, Abel PW and Tu Y: Mitochondrial dynamics regulates migration and invasion of breast cancer cells. Oncogene. 32:4814–4824. 2013. View Article : Google Scholar : PubMed/NCBI | |
|
Rehman J, Zhang HJ, Toth PT, Zhang Y, Marsboom G, Hong Z, Salgia R, Husain AN, Wietholt C and Archer SL: Inhibition of mitochondrial fission prevents cell cycle progression in lung cancer. FASEB J. 26:2175–2186. 2012. View Article : Google Scholar : PubMed/NCBI |