Important role and underlying mechanism of non‑SMC condensin I complex subunit G in tumours (Review)
- Authors:
- Published online on: April 15, 2024 https://doi.org/10.3892/or.2024.8736
- Article Number: 77
-
Copyright: © Li et al. This is an open access article distributed under the terms of Creative Commons Attribution License.
Abstract
Introduction
Non-SMC condensin I complex subunit G (NCAPG) was originally identified from nuclear extracts of HeLa cells (1). NCAPG has been shown to be involved in chromosomal organization and rearrangement via interaction with non-SMC condensin I complex subunit H (NCAPH) (2) and non-SMC condensin I complex subunit D2 (NCAPD2) (3) to form condensin complex I, which maintains the overall stability of chromosomes through participating in chromosome organization and rearrangement, and by promoting the correct segregation and accurate distribution of chromosomes during mitosis (4,5). Hara et al (2) found that the NCAPG-NCAPH subcomplex consists of the N-terminal domain of polypeptide human chromosome-associated polypeptide-G (hCAP-G) linked to its C-terminal domain and a fragment of polypeptide human chromosome-associated polypeptide-H (hCAP-H) containing motif IV. This subcomplex has the ability to interact with both double- and single-stranded DNA, contributing to the correct assembly and segregation of chromosomes. The homologue of the NCAPG subunit in Drosophila is the dCAP-G protein. The dCAP-G mutation results in delayed chromosome condensation at prometaphase, and failure of sister chromatid segregation at anaphase (6). Murphy and Sarge (7) found that the three potential phosphorylation sites on the hCAP-G subunit were Thr-308, Thr-332 and Thr-931, and mutation of these residues to alanine was demonstrated to affect the localization of NCAPG during mitosis.
However, it has been revealed that abnormal expression of NCAPG often affects the occurrence and development of a wide variety of tumours, including lung cancer (8,9), hepatocellular carcinoma (HCC) (10,11), colorectal cancer (12), pancreatic cancer (13,14), breast cancer (BC) (15), ovarian cancer (16), endometrial carcinoma (17), glioma (18), rhabdomyosarcoma (19) and melanoma (20). When NCAPG is highly expressed, the proliferation and invasion of tumours is promoted, and the expression level of NCAPG is negatively correlated with the prognosis of patients, suggesting that NCAPG is a factor that adversely affects the survival of patients with tumour. Surprisingly, NCAPG is expressed at low levels in basal cell carcinoma (BCC) (21), lymphoblastic acute myeloid leukemia (4) and multiple myeloma (22), and this may be associated with immune infiltration and reduced mitogenic gene expression, leading to cell proliferation arrest.
In HCC (11), colorectal cancer (12) and lung adenocarcinoma (LUAD) (23), NCAPG expression has been identified to be closely associated with the degree of lymph node metastasis, tumour clinical stage and tumour progression, and overexpression of NCAPG was associated with poor prognosis in these patients. In addition, high expression of NCAPG was associated with tumour infiltration of several immune cell types, including B cells and CD4 memory T cells in non-small cell lung cancer (NSCLC) (9) and neutrophils in HCC (24), suggesting accordingly that NCAPG fulfils an important role in regulating tumour immunity.
NCAPG has been reported to have pro-carcinogenic biological functions, including promoting tumour cell proliferation, cell cycle function, cell migration, in vivo tumour formation in mice and in vivo metastasis, and it has been proposed that this may be associated with cellular pathways, cell cycle, mismatch repair and cellular damage (25–28). Therefore, further study of the function and underlying mechanisms of NCAPG should enable improved understanding of the processes of tumourigenesis and development, in order to potentially provide novel targets and strategies for tumour therapy.
Role of NCAPG in tumourigenesis and development
Association between NCAPG and cell proliferation, apoptosis, migration and invasion
A large number of studies have demonstrated that NCAPG is able to regulate cell proliferation, apoptosis, migration and invasion. NCAPG has been shown to be overexpressed in HCC, and its level of expression correlates with clinicopathological features, such as recurrence, time to recurrence, metastasis, differentiation and tumour-node-metastasis staging (11). The long non-coding RNA (lncRNA) taurine-upregulated gene 1 (TUG1) was revealed to be overexpressed in HCC, where it mediates HCC cell growth, epithelial-mesenchymal transition (EMT) and metastasis (29). Li et al (30) found that TUG1 could target and regulate NCAPG, and the expression of NCAPG is negatively correlated with survival in HCC. It has been demonstrated that, upon knockdown of NCAPG, the expression levels of the cell cycle proteins A1 and CDK2, Bcl-2 and N-calmodulin are inhibited, causing the cell cycle to stall in the S-phase (11), resulting in a weakening of the ability of cells to migrate and invade (31), thereby inducing apoptosis. It has also been revealed that knockdown of NCAPG reduces cell viability, causes abnormal mitosis and mitochondrial fragmentation and promotes apoptosis (32). Ai et al (33) detected that the microRNA (miRNA) miR-181c was significantly downregulated in HCC and that the expression level of miR-181c was negatively correlated with the expression level of NCAPG. Knockdown of NCAPG was also found to result in decreased rates of cell proliferation, invasion and migration, a reduced level of EMT and the promotion of apoptosis.
A previously published study by Li et al (34) revealed that NCAPG is a key gene in castration-resistant prostate cancer (CRPC). In a previous study, Goto et al (35) showed that miR-145-3p was lowly expressed in CRPC tissues, where it acted as a negative regulator of NCAPG, thereby functioning as a tumour suppressor. Furthermore, the miRNA miR-99a-3p was found to significantly downregulate NCAPG expression in CRPC, suggesting that it may fulfil an important oncogenic function in CRPC (36).
Yu et al (37) reported that knockdown of NCAPG resulted in cell cycle blockade in the S and G2 phases of the cycle, which resulted in a marked decrease in the proliferative and invasive capabilities of ovarian cancer cells and in the induction of apoptosis.
Song et al (38) demonstrated that NCAPG is highly expressed in gastric cancer and is enriched in the cell cycle. NCAPG serves as a downstream target of miR-193b-3p, and it is negatively regulated by this miRNA; moreover, its overexpression was found to promote the proliferation of gastric cancer cells. Sun et al (39) found that the expression level of NCAPG in the tumour cells of patients with advanced gastric cancer was markedly increased compared with that in the early stage of the disease. Knockdown of NCAPG induced cell cycle arrest in G0/G1 phase, thereby inhibiting both the rates of cell proliferation, migration and invasion and EMT. Zhang et al (27) found that, in gastric cancer, silencing of NCAPG resulted in cell cycle arrest in G1 phase, downregulation of the expression of the cell cycle proteins cyclin D1, CDK4 and CDK6, an increase in the expression of cell cycle inhibitors (p21 and p27) and reduced cellular proliferation rates, whereas the opposite effects were observed with the overexpression of NCAPG. Taken together, these findings suggested that NCAPG affects cell proliferation via regulating the cell cycle, thereby providing a novel strategy for the treatment of gastric cancer with CDK4/6 inhibitors.
Clear cell renal cell carcinoma (ccRCC) is a common type of renal cancer (40). It has been revealed that the expression level of NCAPG is significantly upregulated in ccRCC (41). Li et al (42) showed that knocking down NCAPG resulted in a decrease in CDK1 expression, with the subsequent inhibition of cell proliferation, whereas overexpression of CDK1 partly reversed the reduction in the cell proliferation rate, suggesting that NCAPG is involved in the proliferation of ccRCC through its interaction with the CDK1 signalling pathway.
A previous study by Li et al (43) demonstrated that NCAPG is a key gene in LUAD, and a high expression level of NCAPG was shown to be strongly correlated with poor patient prognosis. Zhang et al (27) found that silencing NCAPG impeded the progression of NSCLC cells through inhibiting their proliferation, invasion and tumour growth, both in vitro and in vivo. Further investigation revealed that silencing NCAPG resulted in decreased expression levels of CDK4, CDK6 and cyclin D1, and an increased expression of p27 and p21, resulting in blockade of the cell cycle at G1 phase and the induction of apoptosis (27,44). Proline-rich protein 11 (PRR11) and spindle and kinetochore-associated 2 (SKA2) together form a classical head-to-head gene pair that serves an important role in tumour development (45). A previous study (46) demonstrated that, in NSCLC, NCAPG is able to interact with PRR11 and SKA2 to activate the Hedgehog (Hh) pathway, and the use of inhibitors of the Hh-regulated transcription factors GLI1 and GLI2 led to a marked reduction in the expression levels of PRR11, SKA2 and NCAPG. Taken together, these findings suggested that these three proteins are regulated by the Hh-GLI signalling pathway, thereby affecting the proliferation and migration rates of NSCLC cells.
Moura-Castro et al (47) reported that one of the mechanisms that may be associated with hyper-diploid acute lymphoblastic leukemia is an increased heterogeneity of the chromosome copy number due to the downregulation of NCAPG expression, which leads to the cohesion defect of sister chromosomes. It has been revealed that downregulation of NCAPG expression is also present in patients with multiple myeloma or acute myeloid leukemia, and that this may help to slow the proliferation rate and aggressiveness of cells associated with these types of cancer (22).
Association of NCAPG and cell stemness
Stemness, defined as the ability of a cell or tissue to self-renew and differentiate into multiple cell types, was originally identified in human embryonic stem cells, although subsequently it was found that pluripotent stem cells could be obtained from undifferentiated somatic cells by induction (48,49). In addition, certain normally differentiated cells have been shown to regain stem cell-like abilities in the event of loss of differentiation characteristics, and these are referred to as cancer stem cells (CSCs) (50,51). CSCs are capable of self-renewal and multidirectional differentiation, and exert an important role in promoting tumour progression, drug resistance and recurrence (52,53).
Through a biosignature study, Pan et al (54) demonstrated that NCAPG could be used as a biomarker for the characterization of bladder cancer stem cells. Subsequently, Li et al (55) found that, in brain low-grade gliomas (LGG), NCAPG was able to influence the E2F pathway and promote tumour recurrence through upregulating the expression level of the stemness indicator, aurora kinase A (AURKA). It has been found by Li et al (56) that expression of the circular RNA circNCAPG is higher in glioma stem cells compared with that in differentiated glioma cells, and that this is also associated with a worse prognosis. Ras response element binding protein 1 (RREB1) binds to circNCAPG and can regulate circNCAPG through the U2 nucleoprotein cofactor 65 kDa (U2AF65), thereby constituting a U2AF65/circNCAPG/RREB1 positive feedback loop. It was further found that RREB1 is able to promote the expression of proteins such as CD133, SRY-box transcription factor 2 (SOX2), Nanog and Oct4, whereas it had no direct regulatory effect on gene expression at the RNA level.
In addition, it has been identified that, in gastric cancer, high expression levels of NCAPG are associated with a higher stemness index and longer overall survival time compared with lower expression levels of NCAPG (57–59). Zhang et al (60) revealed that, in LUAD, NCAPG was positively correlated with the glycolysis marker genes HK2, PKM9 and LDHA. Upon knockdown of NCAPG, both the glycolytic level and the glycolytic capability of LUAD cells were found to be markedly reduced. Moreover, the expression levels of CD133, CD44 and Oct-4 were significantly increased when NCAPG was overexpressed, whereas the use of glycolysis inhibitors led to a reversal of the observed changes, suggesting that NCAPG promotes stemness of LUAD cells via activating the glycolytic pathway.
Association of NCAPG and immune infiltration
In tumour tissues, there are numerous other types of cells associated with the tumour microenvironment, such as normal stromal cells, immune cells and vascular endothelial cells (61,62). Interactions between tumour cells and the tumour microenvironment influence tumour development, and gaining an improved understanding of their roles should provide the key to unlocking a new era of tumour therapy (63,64).
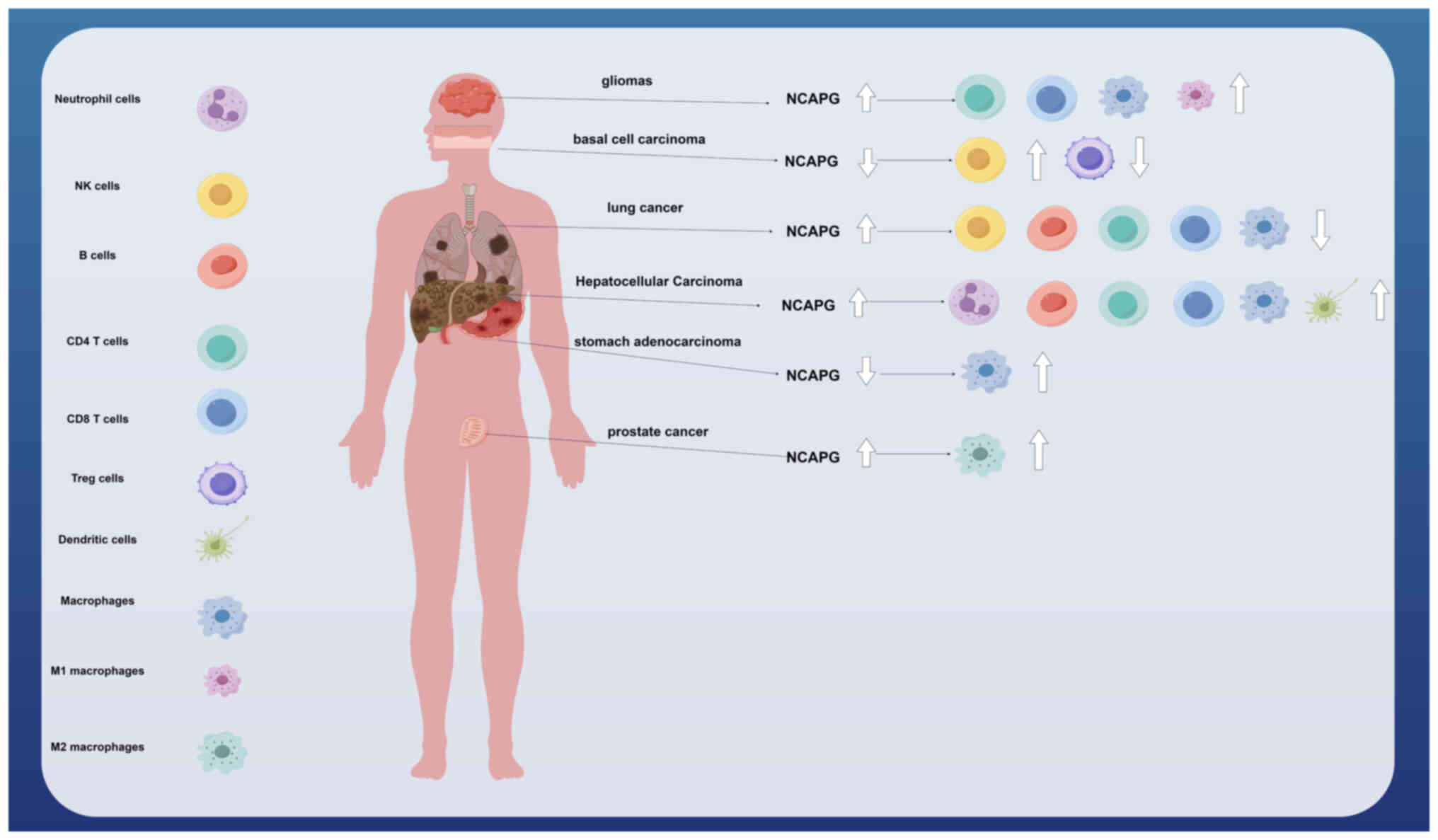
Xu et al (65) found that NCAPG is one of the pivotal genes associated with M2-tumour-associated macrophage infiltration in prostate cancer, and that patients with high NCAPG expression had a poor prognosis. In addition, Xiang et al (57) found that, in stomach adenocarcinoma (STAD), increased macrophage expression levels with low expression of NCAPG led to the promotion of tumour progression and poor prognosis. NCAPG was positively correlated with the expression of certain immune checkpoint genes, including CD80, CTLA4, IDO1 and CD274, suggesting that STAD may be treated with corresponding immune checkpoint inhibitors. Li et al (55) found that the expression of NCAPG was upregulated in LGG and correlated with poor prognosis and immune infiltration (including an increased expression of CD8 T-cells, CD4 memory resting T-cells, macrophages and M1 macrophages). In NSCLC, a high expression of NCAPG was found to be associated with immune infiltration in which the levels of B cells, CD4 memory T cells, CD8 memory T cells, macrophages and natural killer (NK) T cells were reduced, thereby affecting the prognosis of NSCLC (9). Furthermore, it was shown by Guo and Zhu (24) that a high expression of NCAPG in HCC tissues was positively correlated with both immune cell infiltration (B cells, CD4 memory T cells, CD8 memory T cells, macrophages, neutrophils and dendritic cells) and the expression of associated molecular markers (CD19, IRF5, ITGAM and ITGAX), leading to poor prognosis. Interestingly, Xie et al (21) found that NCAPG was lowly expressed in BCC, and that this was significantly negatively correlated with NK cells and positively correlated with T regulatory cells, suggesting that NCAPG may fulfil an oncogene role in BCC, thereby providing guidance for new treatment strategies (Fig. 1).
NCAPG is involved in the regulation of tumour chemotherapy resistance
The tyrosine kinase Src is a proto-oncogene that exerts a key role in regulating cell proliferation, differentiation and metastasis (66,67). A previous study (68) revealed that the expression level of NCAPG is positively correlated with Src-associated genes; moreover, the expression level of NCAPG is increased in HER2+/trastuzumab-resistant BC, and this is closely correlated with shorter patient survival times and recurrence. Overexpression of NCAPG led to a markedly increased level of Src phosphorylation, whereas inhibition of Src using either specific inhibitors or shRNA resulted in reduced rates of cell proliferation, suggesting the important influence of NCAPG-dependent trastuzumab resistance. NCAPG overexpression promotes BC cell proliferation and resistance to apoptosis, and confers resistance to trastuzumab, whereas knockdown of NCAPG causes trastuzumab resistance to be restored, suggesting that the maintenance of low NCAPG expression may be more sensitive to the application of chemotherapeutic agents.
lncRNAs have been shown to fulfil important roles in tumour drug resistance (69–71). A study by Bao et al (72) found that, in LUAD, the mutation frequency of EGFR was increased with high expression of NCAPG compared with low NCAPG expression, and the IC50 value (the half-maximal inhibitory concentration) was found to be higher with the EGFR-tyrosine kinase inhibitor (EGFR-TKI) erlotinib, suggesting an association with resistance to EGFR-TKIs. Higher expression levels of NCAPG were also found in resistant patients with LUAD who were treated with either erlotinib or gefitinib. Using Ensembl database (73), it was revealed that NCAPG is able to potentially regulate the lncRNA AC099850.3; therefore, understanding the mechanism of the lncRNA AC099850.3-NCAGP signalling axis, and how this is associated with resistance to EGFR-TKIs, may provide a novel approach for the treatment of LUAD.
Mechanisms associated with signalling pathways and NCAPG in regulating tumour cells
NCAPG and the PI3K/AKT pathway
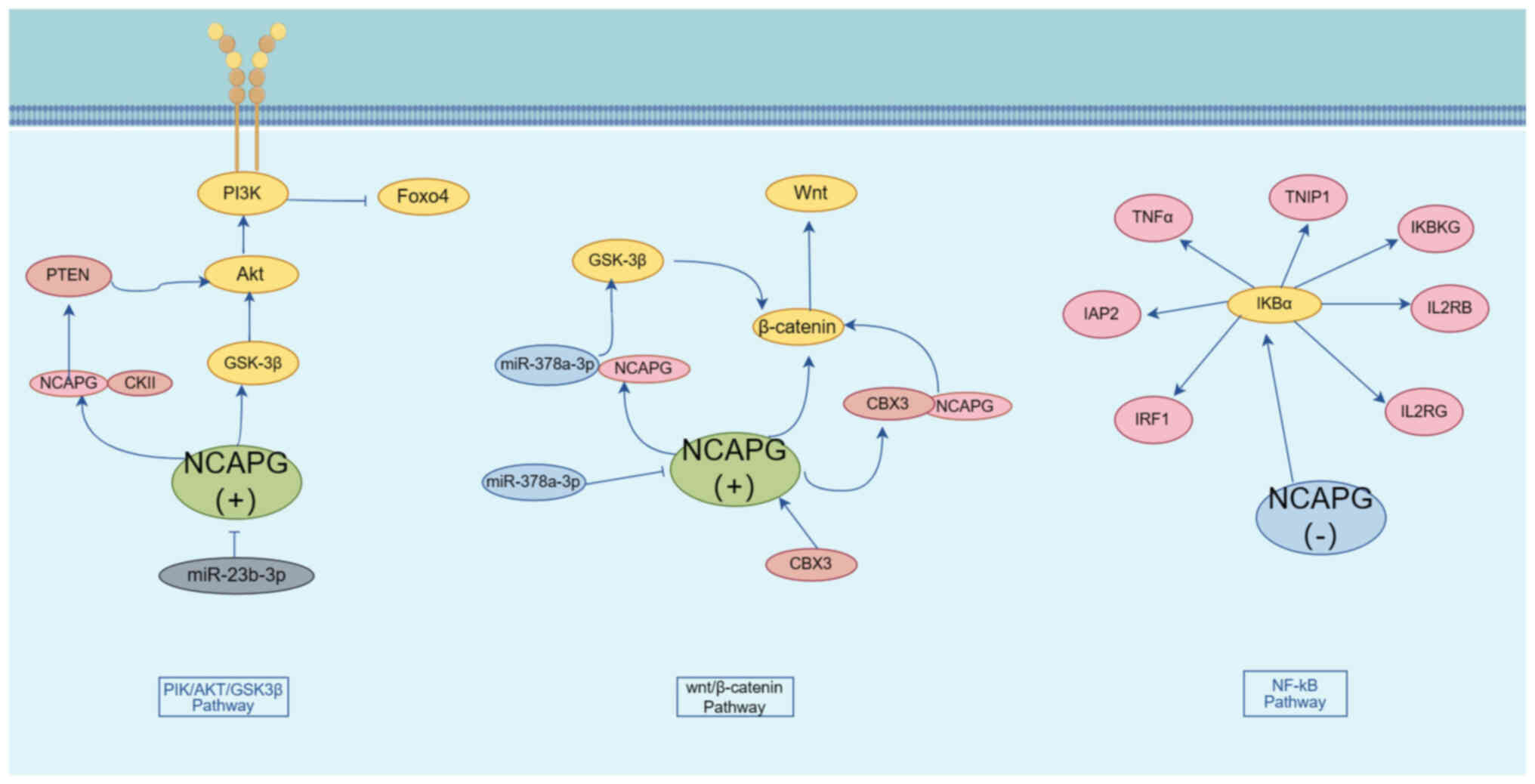
The PI3K/AKT pathway is an important cell signalling pathway that is involved in a variety of biological processes, including cell growth, proliferation, migration and invasion (74). A study by Gong et al (75) identified that NCAPG overexpression could both promote the phosphorylation and activation of PI3K and AKT and lead to the inhibition of FOXO4 phosphorylation, and that NCAPG was able to interact with PI3K, AKT and FOXO4 to activate PI3K/AKT/FOXO4 signalling, promote HCC cell proliferation and invasion and inhibit apoptosis; therefore, targeting and regulating NCAPG expression may provide a possible new avenue for the treatment of HCC. A study by Zhang et al (27) revealed that, in pancreatic adenocarcinoma, overexpression of NCAPG led to an increase in the phosphorylation levels of PI3K, AKT and GSK3β, whereas the use of AKT inhibitors caused a marked inhibition of the growth of lung cancer cells with high NCAPG expression.
miR-23b-3p is a cancer-associated biomarker that has been shown to be downregulated in colon cancer, which mediates tumour cell proliferation, migration and invasion (76,77). Li et al (78) demonstrated that miR-23b-3p was negatively correlated with NCAPG. Knockdown of NCAPG led to an inhibition of AKT phosphorylation and activation; moreover, NCAPG was found to interact with phosphorylated (p)-PI3K and p-AKT to negatively regulate apoptosis through influencing the miR-23b-3p/NCAPG/PI3K/AKT signalling pathway.
PTEN is a phosphatase that negatively regulates the PI3K/AKT pathway, and cancer may develop as a consequence of loss or mutation of the PTEN gene (79,80). Casein kinase 2 α1 (CKII) is a ubiquitous and highly conserved protein serine/threonine kinase that exerts an important role in cell cycle regulation and cell proliferation (81). Zhang et al (82) found that, in HCC, NCAPG is able to interact with CKII, thereby affecting PTEN expression. Upon overexpression of NCAPG, the levels of CKII, p-AKT and p-PTEN were found to be higher, resulting in the promotion of cell proliferation. Subsequently, the promotion of PTEN phosphorylation upon overexpression of NCAPG was found to be reversed with the use of CKII inhibitors, impairing cell proliferation. Taken together, these findings suggested that NCAPG inhibits PTEN expression through interaction with CKII, which in turn activates the PI3K/AKT pathway and promotes the proliferation of HCC cells.
NCAPG and the Wnt/β-catenin pathway
The Wnt pathway is an important signalling pathway that is involved in the regulation of cell proliferation, differentiation, apoptosis, stem cell self-renewal, tissue homeostasis and wound healing (83,84). Liu et al (85) showed that, in endometrial cancer, knockdown of NCAPG could inhibit tumour cell proliferation and promote cell apoptosis through inhibiting the expression of β-catenin. Zhang et al (86) demonstrated that, in pancreatic adenocarcinoma, overexpression of NCAPG led to an increase in the expression of vimentin, N-cadherin, Snail and Slug, and a decrease in the expression of E-cadherin, suggesting that overexpression of NCAPG may promote the EMT process in tumour cells. In addition, Shi et al (12) demonstrated that knockdown of NCAPG led to the inactivation of Wnt/β-catenin and EMT, which resulted in a marked inhibition in the migratory and invasion rates of colon cancer cells, thereby leading to the elimination of the accelerated cell migration and invasion rates that were caused by NCAPG overexpression. Moreover, it was revealed that NCAPG may be a downstream target of the Wnt/β-catenin signalling pathway, and that it is involved in cell proliferation, invasion, migration and EMT processes associated with colon cancer. Yang et al (87) reported that NCAPG interacts with chromobox protein homolog 3 (CBX3), which, in turn, regulates the expression of Wnt3a and β-linker proteins, whereas a deficiency of NCAPG led to an inhibition of cell invasion and the induction of apoptosis in colorectal cancer cells through its influence on the CBX3/NCAPG/Wnt pathway.
Li et al (88) revealed that NCAPG was highly expressed in oral cancer cells, and could directly bind to the oncogene miR-378a-3p; moreover, it was negatively regulated by miR-378a-3p. Knockdown of the NCAPG gene caused a marked inhibition of the GSK-3β/β-catenin pathway, suggesting that miR-378a-3p is able to regulate the GSK-3β/β-catenin pathway through affecting NCAPG expression.
NCAPG and the retinoblastoma (RB) tumour suppressor pathway
The RB pathway is a signalling pathway associated with cell cycle regulation, which serves an important role in cell proliferation and apoptosis (89,90). The core member of the RB pathway is the RB protein (pRb), a repressive transcription factor that inhibits cell cycle progression through binding to the transcription factor E2F (91,92). Xiao et al (4) showed that, in BC, the downregulation of NCAPG expression resulted in an increase in the level of poly(ADP-ribose) polymerase (PARP) protein, a decrease in the expression levels of pRb and cell cycle protein B1 and a marked inhibition of cell proliferation, suggesting that NCAPG may promote BC cell proliferation by regulating the RB pathway. In glioblastoma, Hou et al (26) revealed that NCAPG is able to interact with PARP1, a co-activator of E2F1, and that NCAPG is a downstream target gene of E2F1; moreover, a high expression of NCAPG positively regulates the E2F1 pathway.
NCAPG and the p53 pathway
p53 is an important oncogene that has significant roles in the regulation of cell cycle, senescence and apoptosis (93,94). Dong et al (95) identified a number of miRNAs (such as miR-101-3p, miR-195-5p, miR-214-3p and miR-944) that serve to reduce NCAPG expression to promote BC development, and these are enriched in the p53 signalling pathway. In addition, it was observed that knockdown of NCAPG gene expression resulted in a significant decrease in the expression level of CDC25C, which acts as a direct target of p53 transcription factor and cell cycle arrest in G2/M phase, further emphasizing its function as an oncogene. Taking all these findings into consideration, it has been proposed that NCAPG may influence the p53 signalling pathway via regulating the expression of CDC25C.
NCAPG and the NF-κB pathway
NF-κB represents a class of transcription factors that fulfill key roles in several biological processes, including inflammation, immune response and cell growth (96,97). A previous study by Swindell et al (98) identified NCAPG as a potential NF-κB target gene. In bladder cancer (99), knockdown of NCAPG was shown to result in a lower degradation rate of IκBα and inhibition of the NF-kB pathway in a dose-dependent manner. Knockdown of NCAPG also resulted in downregulation of the expression of NF-κB downstream genes, including TNFα, inhibitor of apoptosis 2 (IAP2), inhibitor of NF-κB kinase regulatory subunit γ (IKBKG), interleukin (IL)-2RB, IL-2RG, interferon regulatory factor 1 (IRF1) and TNFAIP3 interacting protein 1 (TNIP1), thereby attenuating cell proliferation. It has been suggested that NCAPG promotes bladder cancer progression through regulating the NF-κB signalling pathway (Fig. 2).
NCAPG and the signal transducer and activator of transcription 3 (STAT3) signalling pathway
STAT3 is a transcription factor that fulfils important roles in tumour cell proliferation, metastasis, invasion and immunosuppression (100,101). A previous study by Li et al (102) found that, in triple-negative BC, knockdown of NCAPG resulted in a significant decrease in the expression of p-EGFR, p-JAK1 and p-STAT3, although the inhibitory effect of knockdown of NCAPG on p-STAT3 could be reversed by using agonists of the EGFR and JAK/STAT3 signalling pathways, accompanied by an increase in cell proliferation, invasion and migration and a decrease in apoptosis. NCAPG was demonstrated to affect cell proliferation, invasion, migration and apoptosis through influencing the EGFR/JAK/STAT3 signalling pathway. Jiang et al (68) revealed that overexpression of NCAPG led to an increase in the transcriptional activity and phosphorylation level of STAT3, as well as increasing the expression level of the downstream factors of STAT3 signalling, cytosolic protein D1 and BCL2, whereas inhibition of NCAPG elicited the opposite results. Taken together, these results suggested that NCAPG may mediate BC cell proliferation and exert its anti-apoptotic effects through activation of the Src/STAT3 signalling pathway.
NCAPG and the transforming growth factor β (TGF-β) pathway
TGF-β is an important extracellular signalling molecule that has a key role in physiological processes, including cell growth, differentiation and migration, apoptosis, immunity and EMT (103,104). Wu et al (23) found that the expression of p-Smad2 and p-Smad3 in the TGF-β signalling pathway was increased upon overexpression of NCAPG, leading to the promotion of cell proliferation, invasion, migration and EMT. Subsequently, upon overexpression of NCAPG, the use of an inhibitor of the TGF-β signalling pathway effectively reversed the effects mediated on the EMT process and the proliferative, migratory and invasive capabilities of LUAD cells. Li et al (56) demonstrated that circNCAPG is highly expressed in glioma stem cells, where it interacts with the RNA-binding protein U2AF65. It was further found that circNCAPG binds to RREB1, promoting RREB1 entry into the nucleus and activating the TGF-β1 pathway. It was therefore suggested that RREB1 promotes glioma stem cell proliferation, invasion and maintenance of self-renewal through promoting the expression of U2AF65, enhancing the stability of circNCAPG and forming the U2AF65/circNCAPG/RREB1 positive feedback pathway.
Conclusions
Intracellularly, increased expression of NCAPG promotes cell proliferation by facilitating the transition from G1 to S and G2/M phases. NCAPG can also affect the proliferation, apoptosis and EMT of tumour cells through multiple signalling pathways, including PI3K/AKT, Wnt, RB, p53, STAT3 and TGF-β, promoting tumour development and progression. In addition, it is important to focus on the fact that NCAPG is able to promote the maintenance of stem cell properties by affecting the Wnt/β-catenin pathway. Extracellularly, high expression of NCAPG was associated with enhanced invasiveness and migration of tumour cells. Furthermore, NCAPG may help tumour cells evade the immune system by affecting immune cells in the tumour microenvironment. In conclusion, NCAPG, as a cell cycle regulatory protein, promotes cell proliferation not only inside the cell by affecting the cell cycle and cell stemness, but also outside the cell by affecting the tumour microenvironment to promote tumour invasion and metastasis. Continuing research and in-depth studies on the function and underlying molecular mechanisms of NCAPG should lay the foundation for the discovery of novel antitumour drug targets, and the realization of precise and personalized tumour therapies.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Affiliated Hospital of Zunyi Medical University [grant no. Institution (2015) 34], the Guizhou Provincial Department of Science and Technology [grant no. Qiankehe LH Zi (2014) 7573], the Affiliated Hospital of Zunyi Medical University [grant no. Institution (2016) 45] and the Collaborative Innovation Center of Chinese Ministry of Education (grant no. 2020-39).
Availability of data and materials
Not applicable.
Authors' contributions
RL and DW drafted the manuscript and contributed equally to the study. HY and LP participated in the literature search and analysis of the data to be included in the review. XL and FY were involved in the design of the study and assisted in the preparation of the figures. RZ edited and revised the manuscript. All authors have read and approved the final version of the manuscript. Data authentication is not applicable.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
|
Ono T, Losada A, Hirano M, Myers MP, Neuwald AF and Hirano T: Differential contributions of condensin I and condensin II to mitotic chromosome architecture in vertebrate cells. Cell. 115:109–121. 2003. View Article : Google Scholar | |
|
Hara K, Kinoshita K, Migita T, Murakami K, Shimizu K, Takeuchi K, Hirano T and Hashimoto H: Structural basis of HEAT-kleisin interactions in the human condensin I subcomplex. EMBO Rep. 20:e471832019. View Article : Google Scholar : PubMed/NCBI | |
|
Kinoshita K, Kobayashi TJ and Hirano T: Balancing acts of two HEAT subunits of condensin I support dynamic assembly of chromosome axes. Dev Cell. 33:94–106. 2015. View Article : Google Scholar | |
|
Xiao C, Gong J, Jie Y, Cao J, Chen Z, Li R, Chong Y, Hu B and Zhang Q: NCAPG is a promising therapeutic target across different tumor types. Front Pharmacol. 11:3872020. View Article : Google Scholar | |
|
Eberlein A, Takasuga A, Setoguchi K, Pfuhl R, Flisikowski K, Fries R, Klopp N, Fürbass R, Weikard R and Kühn C: Dissection of genetic factors modulating fetal growth in cattle indicates a substantial role of the non-SMC condensin I complex, subunit G (NCAPG) gene. Genetics. 183:951–964. 2009. View Article : Google Scholar : PubMed/NCBI | |
|
Dej KJ, Ahn C and Orr-Weaver TL: Mutations in the Drosophila condensin subunit dCAP-G: Defining the role of condensin for chromosome condensation in mitosis and gene expression in interphase. Genetics. 168:895–906. 2004. View Article : Google Scholar : PubMed/NCBI | |
|
Murphy LA and Sarge KD: Phosphorylation of CAP-G is required for its chromosomal DNA localization during mitosis. Biochem Biophys Res Commun. 377:1007–1011. 2008. View Article : Google Scholar : PubMed/NCBI | |
|
Sun H, Zhang H, Yan Y, Li Y, Che G, Zhou C, Nicot C and Ma H: Correction: NCAPG promotes the oncogenesis and progression of non-small cell lung cancer cells through upregulating LGALS1 expression. Mol Cancer. 21:2212022. View Article : Google Scholar : PubMed/NCBI | |
|
Yuan Y, Jiang X, Tang L, Wang J, Zhang D, Cho WC and Duan L: FOXM1/lncRNA TYMSOS/miR-214-3p-mediated high expression of NCAPG correlates with poor prognosis and cell proliferation in non-small cell lung carcinoma. Front Mol Biosci. 8:7857672022. View Article : Google Scholar | |
|
Fu Q, Yang F, Zhao J, Yang X, Xiang T, Huai G, Zhang J, Wei L, Deng S and Yang H: Bioinformatical identification of key pathways and genes in human hepatocellular carcinoma after CSN5 depletion. Cell Signal. 49:79–86. 2018. View Article : Google Scholar | |
|
Liu W, Liang B, Liu H, Huang Y, Yin X, Zhou F, Yu X, Feng Q, Li E, Zou Z and Wu L: Overexpression of non-SMC condensin I complex subunit G serves as a promising prognostic marker and therapeutic target for hepatocellular carcinoma. Int J Mol Med. 40:731–738. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Shi Y, Ge C, Fang D, Wei W, Li L, Wei Q and Yu H: NCAPG facilitates colorectal cancer cell proliferation, migration, invasion and epithelial-mesenchymal transition by activating the Wnt/β-catenin signaling pathway. Cancer Cell Int. 22:1192022. View Article : Google Scholar | |
|
Wu C, Huang ZH, Meng ZQ, Fan XT, Lu S, Tan YY, You LM, Huang JQ, Stalin A, Ye PZ, et al: A network pharmacology approach to reveal the pharmacological targets and biological mechanism of compound kushen injection for treating pancreatic cancer based on WGCNA and in vitro experiment validation. Chin Med. 16:1212021. View Article : Google Scholar : PubMed/NCBI | |
|
Zhang D, Cui F, Peng L, Wang M, Yang X, Xia C, Li K, Yin H, Zhang Y, Yu Q, et al: Establishing and validating an ADCP-related prognostic signature in pancreatic ductal adenocarcinoma. Aging (Albany NY). 14:6299–6315. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Hitti E, Bakheet T, Al-Souhibani N, Moghrabi W, Al-Yahya S, Al-Ghamdi M, Al-Saif M, Shoukri MM, Lánczky A, Grépin R, et al: Systematic analysis of AU-rich element expression in cancer reveals common functional clusters regulated by key RNA-binding proteins. Cancer Res. 76:4068–4080. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Xu T, Dong M, Wang Z, Li H and Li X: Elevated mRNA expression levels of NCAPG are associated with poor prognosis in ovarian cancer. Cancer Manag Res. 12:5773–5786. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Zhang W, Gao L, Wang C, Wang S, Sun D, Li X, Liu M, Qi Y, Liu J and Lin B: Combining bioinformatics and experiments to identify and verify key genes with prognostic values in endometrial carcinoma. J Cancer. 11:716–732. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Wang M, Cui Y, Cai Y, Jiang Y and Peng Y: Comprehensive bioinformatics analysis of mRNA expression profiles and identification of a miRNA-mRNA network associated with the pathogenesis of low-grade gliomas. Cancer Manag Res. 13:5135–5147. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Lu S, Sun C, Chen H, Zhang C, Li W, Wu L, Zhu J, Sun F, Huang J, Wang J, et al: Bioinformatics analysis and validation identify CDK1 and MAD2L1 as prognostic markers of rhabdomyosarcoma. Cancer Manag Res. 12:12123–12136. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Ryu B, Kim DS, Deluca AM and Alani RM: Comprehensive expression profiling of tumor cell lines identifies molecular signatures of melanoma progression. PLoS One. 2:e5942007. View Article : Google Scholar : PubMed/NCBI | |
|
Xie D, Chen X, Wu H, Ning D, Cao X and Wan C: Prediction of diagnostic gene biomarkers associated with immune infiltration for basal cell carcinoma. Clin Cosmet Investig Dermatol. 15:2657–2673. 2022. View Article : Google Scholar | |
|
Cohen Y, Gutwein O, Garach-Jehoshua O, Bar-Haim A and Kornberg A: The proliferation arrest of primary tumor cells out-of-niche is associated with widespread downregulation of mitotic and transcriptional genes. Hematology. 19:286–292. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
Wu Y, Lin Y, Pan J, Tu X, Xu Y, Li H and Chen Y: NCAPG promotes the progression of lung adenocarcinoma via the TGF-β signaling pathway. Cancer Cell Int. 21:4432021. View Article : Google Scholar | |
|
Guo ZY and Zhu ZT: NCAPG is a prognostic biomarker associated with vascular invasion in hepatocellular carcinoma. Eur Rev Med Pharmacol Sci. 25:7238–7251. 2021. | |
|
Sun DP, Wu CC, Chou CL, Cheng LC, Wang WC, Lin SS, Hung ST, Tian YF, Fang CL and Lin KY: NCAPG deregulation indicates poor patient survival and contributes to colorectal carcinogenesis. Pathol Res Pract. 241:1542382023. View Article : Google Scholar | |
|
Hou J, Huang P, Xu M, Wang H, Shao Y, Weng X, Liu Y, Chang H, Zhang L and Cui H: NCAPG promotes the progression of glioblastoma by facilitating PARP1-mediated E2F1 transactivation. Neuro Oncol. 25:2023. View Article : Google Scholar | |
|
Zhang X, Wang H, Han Y, Zhu M, Song Z, Zhan D and Jia J: NCAPG induces cell proliferation in cardia adenocarcinoma via PI3K/AKT signaling pathway. Onco Targets Ther. 13:11315–11326. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Guo M, Li X, Li J and Li B: Identification of the prognostic biomarkers and their correlations with immune infiltration in colorectal cancer through bioinformatics analysis and in vitro experiments. Heliyon. 9:e171012023. View Article : Google Scholar : PubMed/NCBI | |
|
Farzaneh M, Ghasemian M, Ghaedrahmati F, Poodineh J, Najafi S, Masoodi T, Kurniawan D, Uddin S and Azizidoost S: Functional roles of lncRNA-TUG1 in hepatocellular carcinoma. Life Sci. 308:1209742022. View Article : Google Scholar | |
|
Li L, Liu S, Peng L, Zhang Y, Zhang Y, Zeng H, Li G and Zhang C: The identification and preliminary study of lncRNA TUG1 and its related genes in hepatocellular carcinoma. Arch Med Sci. 18:1582–1595. 2019. | |
|
Liu K, Li Y, Yu B, Wang F, Mi T and Zhao Y: Silencing non-SMC chromosome-associated polypeptide G inhibits proliferation and induces apoptosis in hepatocellular carcinoma cells. Can J Physiol Pharmacol. 96:1246–1254. 2018. View Article : Google Scholar | |
|
Wang Y, Gao B, Tan PY, Handoko YA, Sekar K, Deivasigamani A, Seshachalam VP, OuYang HY, Shi M, Xie C, et al: Genome-wide CRISPR knockout screens identify NCAPG as an essential oncogene for hepatocellular carcinoma tumor growth. FASEB J. 33:8759–8770. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Ai J, Gong C, Wu J, Gao J, Liu W, Liao W and Wu L: MicroRNA-181c suppresses growth and metastasis of hepatocellular carcinoma by modulating NCAPG. Cancer Manag Res. 11:3455–3467. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Li Y, Shi H, Zhao Z and Xu M: Identification of castration-dependent and -independent driver genes and pathways in castration-resistant prostate cancer (CRPC). BMC Urol. 22:1622022. View Article : Google Scholar | |
|
Goto Y, Kurozumi A, Arai T, Nohata N, Kojima S, Okato A, Kato M, Yamazaki K, Ishida Y, Naya Y, et al: Impact of novel miR-145-3p regulatory networks on survival in patients with castration-resistant prostate cancer. Br J Cancer. 117:409–420. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Arai T, Okato A, Yamada Y, Sugawara S, Kurozumi A, Kojima S, Yamazaki K, Naya Y, Ichikawa T and Seki N: Regulation of NCAPG by miR-99a-3p (passenger strand) inhibits cancer cell aggressiveness and is involved in CRPC. Cancer Med. 7:1988–2002. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Yu H, Zou D, Ni N, Zhang S, Zhang Q and Yang L: Overexpression of NCAPG in ovarian cancer is associated with ovarian cancer proliferation and apoptosis via p38 MAPK signaling pathway. J Ovarian Res. 15:982022. View Article : Google Scholar : PubMed/NCBI | |
|
Song B, Du J, Song DF, Ren JC and Feng Y: Dysregulation of NCAPG, KNL1, miR-148a-3p, miR-193b-3p, and miR-1179 may contribute to the progression of gastric cancer. Biol Res. 51:442018. View Article : Google Scholar : PubMed/NCBI | |
|
Sun DP, Lin CC, Hung ST, Kuang YY, Hseu YC, Fang CL and Lin KY: Aberrant expression of NCAPG is associated with prognosis and progression of gastric cancer. Cancer Manag Res. 12:7837–7846. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Wolf MM, Kimryn Rathmell W and Beckermann KE: Modeling clear cell renal cell carcinoma and therapeutic implications. Oncogene. 39:3413–3426. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Liu B, Xiao Y, Li H, Zhang AL, Meng LB, Feng L, Zhao ZH, Ni XC, Fan B, Zhang XY, et al: Identification and verification of biomarker in clear cell renal cell carcinoma via bioinformatics and neural network model. Biomed Res Int. 2020:69547932020. | |
|
Li H, Zheng P, Li Z, Han Q, Zhou B, Wang X and Wang K: NCAPG promotes the proliferation of renal clear cell carcinoma via mediating with CDK1. Dis Markers. 2022:67585952022.PubMed/NCBI | |
|
Li S, Xuan Y, Gao B, Sun X, Miao S, Lu T, Wang Y and Jiao W: Identification of an eight-gene prognostic signature for lung adenocarcinoma. Cancer Manag Res. 10:3383–3392. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Wang X, Tian X, Sui X, Li X, Zhao X, Han K, Sun L and Dong Y: Increased expression of NCAPG (Non-SMC condensing I complex subunit G) is associated with progression and poor prognosis of lung adenocarcinoma. Bioengineered. 13:6113–6125. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Chen J, Yang HM, Zhou HC, Peng RR, Niu ZX and Kang CY: PRR11 and SKA2 promote the proliferation, migration and invasion of esophageal carcinoma cells. Oncol Lett. 20:639–646. 2020. View Article : Google Scholar | |
|
Sun Y, Xu D, Zhang C, Wang Y, Zhang L, Qiao D, Bu Y and Zhang Y: HEDGEHOG/GLI modulates the PRR11-SKA2 bidirectional transcription unit in lung squamous cell carcinomas. Genes (Basel). 12:1202021. View Article : Google Scholar : PubMed/NCBI | |
|
Moura-Castro LH, Peña-Martínez P, Castor A, Galeev R, Larsson J, Järås M, Yang M and Paulsson K: Sister chromatid cohesion defects are associated with chromosomal copy number heterogeneity in high hyperdiploid childhood acute lymphoblastic leukemia. Genes Chromosomes Cancer. 60:410–417. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, Nie J, Jonsdottir GA, Ruotti V, Stewart R, et al: Induced pluripotent stem cell lines derived from human somatic cells. Science. 318:1917–1920. 2007. View Article : Google Scholar : PubMed/NCBI | |
|
González F, Boué S and Izpisúa Belmonte JC: Methods for making induced pluripotent stem cells: Reprogramming à la carte. Nat Rev Genet. 12:231–242. 2011. View Article : Google Scholar | |
|
van Es JH, Sato T, van de Wetering M, Lyubimova A, Yee Nee AN, Gregorieff A, Sasaki N, Zeinstra L, van den Born M, Korving J, et al: Dll1+ secretory progenitor cells revert to stem cells upon crypt damage. Nat Cell Biol. 14:1099–1104. 2012. View Article : Google Scholar | |
|
Chaffer CL, Brueckmann I, Scheel C, Kaestli AJ, Wiggins PA, Rodrigues LO, Brooks M, Reinhardt F, Su Y, Polyak K, et al: Normal and neoplastic nonstem cells can spontaneously convert to a stem-like state. Proc Natl Acad Sci USA. 108:7950–7955. 2011. View Article : Google Scholar : PubMed/NCBI | |
|
Yang L, Shi P, Zhao G, Xu J, Peng W, Zhang J, Zhang G, Wang X, Dong Z, Chen F and Cui H: Targeting cancer stem cell pathways for cancer therapy. Signal Transduct Target Ther. 5:82020. View Article : Google Scholar : PubMed/NCBI | |
|
Huang T, Song X, Xu D, Tiek D, Goenka A, Wu B, Sastry N, Hu B and Cheng SY: Stem cell programs in cancer initiation, progression, and therapy resistance. Theranostics. 10:8721–8743. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Pan S, Zhan Y, Chen X, Wu B and Liu B: Identification of biomarkers for controlling cancer stem cell characteristics in bladder cancer by network analysis of transcriptome data stemness indices. Front Oncol. 9:6132019. View Article : Google Scholar | |
|
Li J, Zhou M, Huang D, Lin R, Cui X, Chen S, Yao Y, Xian S, Wang S, Fu Q, et al: The recurrent-specific regulation network of prognostic stemness-related signatures in low-grade glioma. Dis Markers. 2023:22439282023. View Article : Google Scholar : PubMed/NCBI | |
|
Li H, Jiang Y, Hu J, Xu J, Chen L, Zhang G, Zhao J, Zong S, Guo Z, Li X, et al: The U2AF65/circNCAPG/RREB1 feedback loop promotes malignant phenotypes of glioma stem cells through activating the TGF-β pathway. Cell Death Dis. 14:232023. View Article : Google Scholar : PubMed/NCBI | |
|
Xiang Z, Cha G, Wang Y, Gao J and Jia J: Characterizing the crosstalk of NCAPG with tumor microenvironment and tumor stemness in stomach adenocarcinoma. Stem Cells Int. 2022:18883582022. View Article : Google Scholar | |
|
Xia X and Li Y: Comprehensive analysis of transcriptome data stemness indices identifies key genes for controlling cancer stem cell characteristics in gastric cancer. Transl Cancer Res. 9:6050–6061. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Guo SH, Ma L and Chen J: Identification of prognostic markers and potential therapeutic targets in gastric adenocarcinoma by machine learning based on mRNAsi index. J Oncol. 2022:89261272022. View Article : Google Scholar | |
|
Zhang Z, Qi D, Liu X and Kang P: NCAPG stimulates lung adenocarcinoma cell stemness through aerobic glycolysis. Clin Respir J. 17:884–892. 2023. View Article : Google Scholar : PubMed/NCBI | |
|
Xiao Y and Yu D: Tumor microenvironment as a therapeutic target in cancer. Pharmacol Ther. 221:1077532021. View Article : Google Scholar : PubMed/NCBI | |
|
Hinshaw DC and Shevde LA: The tumor microenvironment innately modulates cancer progression. Cancer Res. 79:4557–4566. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Jin MZ and Jin WL: The updated landscape of tumor microenvironment and drug repurposing. Signal Transduct Target Ther. 5:1662020. View Article : Google Scholar : PubMed/NCBI | |
|
Wu T and Dai Y: Tumor microenvironment and therapeutic response. Cancer Lett. 387:61–68. 2017. View Article : Google Scholar | |
|
Xu N, Dong RN, Lin TT, Lin T, Lin YZ, Chen SH, Zhu JM, Ke ZB, Huang F, Chen YH and Xue XY: Development and validation of novel biomarkers related to M2 macrophages infiltration by weighted gene co-expression network analysis in prostate cancer. Front Oncol. 11:6340752021. View Article : Google Scholar | |
|
Aleshin A and Finn RS: SRC: A century of science brought to the clinic. Neoplasia. 12:599–607. 2010. View Article : Google Scholar | |
|
Roskoski R Jr: Src protein-tyrosine kinase structure and regulation. Biochem Biophys Res Commun. 324:1155–1164. 2004. View Article : Google Scholar : PubMed/NCBI | |
|
Jiang L, Ren L, Chen H, Pan J, Zhang Z, Kuang X, Chen X, Bao W, Lin C, Zhou Z, et al: NCAPG confers trastuzumab resistance via activating SRC/STAT3 signaling pathway in HER2-positive breast cancer. Cell Death Dis. 11:5472020. View Article : Google Scholar : PubMed/NCBI | |
|
Singh D, Assaraf YG and Gacche RN: Long non-coding RNA mediated drug resistance in breast cancer. Drug Resist Updat. 63:1008512022. View Article : Google Scholar | |
|
Wei L, Sun J, Zhang N, Zheng Y, Wang X, Lv L, Liu J, Xu Y, Shen Y and Yang M: Noncoding RNAs in gastric cancer: Implications for drug resistance. Mol Cancer. 19:622020. View Article : Google Scholar : PubMed/NCBI | |
|
Entezari M, Ghanbarirad M, Taheriazam A, Sadrkhanloo M, Zabolian A, Goharrizi MASB, Hushmandi K, Aref AR, Ashrafizadeh M, Zarrabi A, et al: Long non-coding RNAs and exosomal lncRNAs: Potential functions in lung cancer progression, drug resistance and tumor microenvironment remodeling. Biomed Pharmacother. 150:1129632022. View Article : Google Scholar : PubMed/NCBI | |
|
Bao J, Wu Y, Zhang K and Qi H: AC099850.3/NCAPG axis predicts poor prognosis and is associated with resistance to EGFR tyrosine-kinase inhibitors in lung Adenocarcinoma. Int J Gen Med. 15:6917–6930. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Cunningham F, Allen JE, Allen J, Alvarez-Jarreta J, Amode MR, Armean IM, Austine-Orimoloye O, Azov AG, Barnes I, Bennett R, et al: Ensembl 2022. Nucleic Acids Res. 50(D1): D988–D995. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
He Y, Sun MM, Zhang GG, Yang J, Chen KS, Xu WW and Li B: Targeting PI3K/Akt signal transduction for cancer therapy. Signal Transduct Target Ther. 6:4252021. View Article : Google Scholar : PubMed/NCBI | |
|
Gong C, Ai J, Fan Y, Gao J, Liu W, Feng Q, Liao W and Wu L: NCAPG promotes the proliferation of hepatocellular carcinoma through PI3K/AKT signaling. Onco Targets Ther. 12:8537–8552. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Grossi I, Salvi A, Baiocchi G, Portolani N and De Petro G: Functional role of microRNA-23b-3p in cancer biology. Microrna. 7:156–166. 2018. View Article : Google Scholar | |
|
Kou CH, Zhou T, Han XL, Zhuang HJ and Qian HX: Downregulation of mir-23b in plasma is associated with poor prognosis in patients with colorectal cancer. Oncol Lett. 12:4838–4844. 2016. View Article : Google Scholar | |
|
Li P, Wen J, Ren X, Zhou Y, Xue Y, Yan Z, Li S, Tian H, Tang XG and Zhang GJ: MicroRNA-23b-3p targets non-SMC condensing I complex subunit G to promote proliferation and inhibit apoptosis of colorectal cancer cells via regulation of the PI3K/AKT signaling pathway. Oncol Lett. 22:8122021. View Article : Google Scholar | |
|
Worby CA and Dixon JE: PTEN. Annu Rev Biochem. 83:641–669. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
Álvarez-Garcia V, Tawil Y, Wise HM and Leslie NR: Mechanisms of PTEN loss in cancer: It's all about diversity. Semin Cancer Biol. 59:66–79. 2019. View Article : Google Scholar | |
|
Oh NS, Yoon SH, Lee WK, Choi JY, Min do S and Bae YS: Phosphorylation of CKBBP2/CRIF1 by protein kinase CKII promotes cell proliferation. Gene. 386:147–153. 2007. View Article : Google Scholar : PubMed/NCBI | |
|
Zhang R, Ai J, Wang J, Sun C, Lu H, He A, Li M, Liao Y, Lei J, Zhou F, et al: NCAPG promotes the proliferation of hepatocellular carcinoma through the CKII-dependent regulation of PTEN. J Transl Med. 20:3252022. View Article : Google Scholar : PubMed/NCBI | |
|
Zhou Y, Xu J, Luo H, Meng X, Chen M and Zhu D: Wnt signaling pathway in cancer immunotherapy. Cancer Lett. 525:84–96. 2022. View Article : Google Scholar | |
|
Rim EY, Clevers H and Nusse R: The Wnt pathway: From signaling mechanisms to synthetic modulators. Annu Rev Biochem. 91:571–598. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Liu C, Yan Y, Di F, Li W, Yin X and Dong L: Inhibition of NCAPG expression inactivates the Wnt/β-catenin signal to suppresses endometrial cancer cell growth in vitro. Environ Toxicol. 36:2512–2520. 2021. View Article : Google Scholar | |
|
Zhang X, Zhu M, Wang H, Song Z, Zhan D, Cao W, Han Y and Jia J: Overexpression of NCAPG inhibits cardia adenocarcinoma apoptosis and promotes epithelial-mesenchymal transition through the Wnt/β-catenin signaling pathway. Gene. 766:1451632021. View Article : Google Scholar : PubMed/NCBI | |
|
Yang H, Pu L, Li R and Zhu R: NCAPG is transcriptionally regulated by CBX3 and activates the Wnt/β-catenin signaling pathway to promote proliferation and the cell cycle and inhibit apoptosis in colorectal cancer. J Gastrointest Oncol. 14:900–912. 2023. View Article : Google Scholar | |
|
Li J, Sun S, Li J, Zhao X, Li Z, Sha T and Cui Z: NCAPG, mediated by miR-378a-3p, regulates cell proliferation, cell cycle progression, and apoptosis of oral squamous cell carcinoma through the GSK-3β/β-catenin signaling. Neoplasma. 68:1201–1211. 2021. View Article : Google Scholar | |
|
Du W and Searle JS: The rb pathway and cancer therapeutics. Curr Drug Targets. 10:581–589. 2009. View Article : Google Scholar : PubMed/NCBI | |
|
Lin SC, Skapek SX and Lee EY: Genes in the RB pathway and their knockout in mice. Semin Cancer Biol. 7:279–289. 1996. View Article : Google Scholar | |
|
Nevins JR: The Rb/E2F pathway and cancer. Hum Mol Genet. 10:699–703. 2001. View Article : Google Scholar | |
|
Schaal C, Pillai S and Chellappan SP: The Rb-E2F transcriptional regulatory pathway in tumor angiogenesis and metastasis. Adv Cancer Res. 121:147–182. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
Liu J, Zhang C, Wang J, Hu W and Feng Z: The regulation of ferroptosis by tumor suppressor p53 and its pathway. Int J Mol Sci. 21:83872020. View Article : Google Scholar | |
|
Huang J: Current developments of targeting the p53 signaling pathway for cancer treatment. Pharmacol Ther. 220:1077202021. View Article : Google Scholar : PubMed/NCBI | |
|
Dong M, Xu T, Cui X, Li H, Li X and Xia W: NCAPG upregulation mediated by four microRNAs combined with activation of the p53 signaling pathway is a predictor of poor prognosis in patients with breast cancer. Oncol Lett. 21:3232021. View Article : Google Scholar | |
|
DiDonato JA, Mercurio F and Karin M: NF-κB and the link between inflammation and cancer. Immunol Rev. 246:379–400. 2012. View Article : Google Scholar : PubMed/NCBI | |
|
Oeckinghaus A, Hayden M S and Ghosh S: Crosstalk in NF-κB signaling pathways. Nat Immunol. 12:695–708. 2011. View Article : Google Scholar | |
|
Swindell WR, Bojanowski K and Chaudhuri RK: A novel fumarate, isosorbide di-(methyl fumarate) (IDMF), replicates astrocyte transcriptome responses to dimethyl fumarate (DMF) but specifically down-regulates genes linked to a reactive phenotype. Biochem Biophys Res Commun. 532:475–481. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Tang F, Yu H, Wang X, Shi J, Chen Z, Wang H, Wan Z, Fu Q, Hu X, Zuhaer Y, et al: NCAPG promotes tumorigenesis of bladder cancer through NF-κB signaling pathway. Biochem Biophys Res Commun. 622:101–107. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Yu H, Lee H, Herrmann A, Buettner R and Jove R: Revisiting STAT3 signalling in cancer: New and unexpected biological functions. Nat Rev Cancer. 14:736–746. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
Zou S, Tong Q, Liu B, Huang W, Tian Y and Fu X: Targeting STAT3 in cancer immunotherapy. Mol Cancer. 19:1452020. View Article : Google Scholar : PubMed/NCBI | |
|
Li J, Zheng J, Lin B, Sun H, Lu S, Wang D and Huo H: Knockdown of NCAPG promotes the apoptosis and inhibits the invasion and migration of triple-negative breast cancer MDA-MB-231 cells via regulation of EGFR/JAK/STAT3 signaling. Exp Ther Med. 25:1192023. View Article : Google Scholar : PubMed/NCBI | |
|
Peng D, Fu M, Wang M, Wei Y and Wei X: Targeting TGF-β signal transduction for fibrosis and cancer therapy. Mol Cancer. 21:1042022. View Article : Google Scholar : PubMed/NCBI | |
|
Derynck R, Turley SJ and Akhurst RJ: TGFβ biology in cancer progression and immunotherapy. Nat Rev Clin Oncol. 18:9–34. 2021. View Article : Google Scholar |