Involvement of lncRNAs in the regulation of aerobic glycolysis in hepatocellular carcinoma: Main functions, regulatory mechanisms and potential therapeutic implications (Review)
- Authors:
- Published online on: April 25, 2024 https://doi.org/10.3892/or.2024.8743
- Article Number: 84
-
Copyright: © Huang et al. This is an open access article distributed under the terms of Creative Commons Attribution License.
Abstract
Introduction
Hepatocellular carcinoma (HCC) is one of the most common malignant tumors worldwide and the third most common cause of cancer-related mortality (1). The prognosis of patients with HCC remains unsatisfactory, despite the fact that various treatment strategies have been developed. HCC is a highly aggressive tumor. Frequent intrahepatic and distant metastases are the main reasons for the high recurrence rate and the low survival rate of patients with liver cancer following surgery (2). Therefore, in-depth studies on the developmental mechanisms of HCC are urgently required to identify safer and more effective therapeutic strategies for patients with HCC in order to prevent tumor recurrence and improve the survival rate of patients.
Normal cells rely on mitochondrial oxidative phosphorylation to provide energy for growth and differentiation under well-oxygenated conditions. In tumors and other proliferating or developing cells, even in the presence of oxygen and fully functioning mitochondria, the cells opt to undergo cellular metabolism and acquire an energy supply in the form of glycolysis, which leads to a marked increase in the rate of glucose uptake into the cells and promotes the production of large amounts of lactic acid. This phenomenon is known as the ‘Warburg’ effect or ‘aerobic glycolysis’ (3).
In glycolysis, when glucose is metabolized to lactate, only two ATPs are generated per glucose molecule, whereas the oxidative phosphorylation of a glucose molecule following complete oxidation can generate up to 36 ATPs for cellular energy supply, and cancer cells tend to favor glycolysis. This appears to be a ‘disadvantageous’ metabolic pattern, with the inefficient production of ATP. Indeed, the preference of tumors for glycolysis as their primary metabolic energy source represents an adaptive response to the environmental growth constraints commonly encountered in cancer development. Firstly, aerobic glycolysis provides the free energy or co-factors (for example ATP, NADPH and NADH) required for sustained cancer cell proliferation (4). Secondly, glycolysis metabolizes one molecule of glucose 10–100-fold more rapidly than the complete oxidation of one molecule of glucose in the mitochondria. These two different strategies of metabolizing glucose produce comparable amounts of ATP in any given time period (5). Therefore, a plausible explanation is that glycolytic metabolism occurs more rapidly than oxidative phosphorylation (OXPHOS) and ATP production is rapid enough to compensate for the insufficient amount of ATP produced (6,7). Thirdly, using glycolytic metabolism, tumor cells escape oxidative stress and are protected from reactive oxygen species-induced damage (8). In fact, the liver is one of the largest metabolic organs and a center for gluconeogenesis. Aerobic glycolysis affects the progression of HCC, including the maintenance of cell proliferation, the induction of immune escape, invasion and metastasis, and the promotion of angiogenesis and tumor resistance (9,10).
RNA sequencing techniques and transcriptional profiling have demonstrated that although the human genome is universally transcribed, only ~2% of RNAs code for proteins (11,12). Even if they do not code for proteins, long non-coding RNAs (lncRNAs) are not functionless ‘noise sequences’. lncRNAs can be involved in biological activities, including tumor growth, metastasis and metabolism (13–15).
In recent years, lncRNAs have been found to play a key role in the pathogenesis of cancers, including HCC. Understanding the novel regulatory roles of lncRNAs in glucose metabolism, particularly in HCC, could provide new insight into the underlying mechanisms of cancer onset and progression, which could ultimately lead to the identification of novel therapeutic targets. Therefore, in the present review, the major factors affecting the function of lncRNAs in HCC were briefly discussed and the mechanisms through which lncRNAs regulate HCC progression through glycolysis were summarized.
Overview of lncRNAs
High-throughput sequencing technologies and computing platforms have demonstrated that ~75% of the human genome can be transcribed into RNAs, of which 74% are encoded as non-protein coding RNAs (ncRNAs). Although not translated, ncRNAs play a regulatory role in the developmental and pathophysiological stages of human cells. According to the length of RNAs, ncRNAs can be mainly divided into small ncRNAs and lncRNAs. Among ncRNAs, those with >200 nucleotides are classified as lncRNAs. They are mostly transcribed by RNA polymerase II (pol II) and include various types of intergenic transcripts, enhancer RNAs and positive or antisense transcripts that overlap with other genes. lncRNAs can be classified according to four factors as follows: Genomic localization and influence on DNA sequence, functional mechanism and targeting mechanisms (16). lncRNAs have different subcellular localizations in cells, and the unique subcellular localization is closely related to the function of interacting molecules, post-transcriptional or co-transcriptional regulatory modifications and external stimuli.
Major factors affecting the function of lncRNAs in HCC
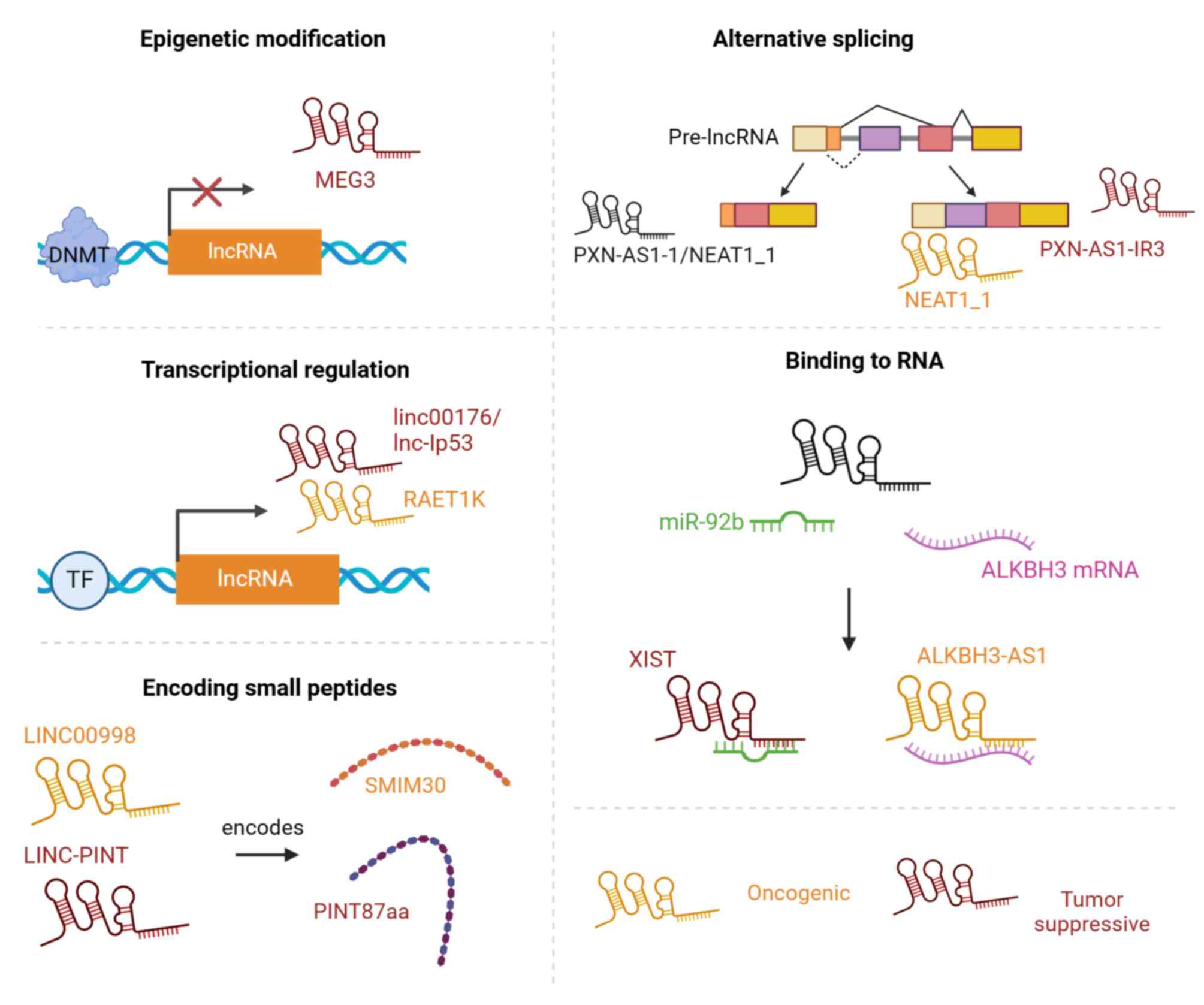
In HCC, lncRNAs can play a key role as oncogenes/tumor suppressor gene, and the main factors affecting the function of lncRNAs are the following: Epigenetic modification, selective splicing, transcriptional regulation, binding to RNAs and the encoding of small peptides (Fig. 1).
Epigenetic modification
Common epigenetic modifications in HCC progression include DNA methylation and histone modification (17). The regulation of epigenetic modification determines the function of lncRNAs. lncRNAs can recruit some chromatin remodeling complexes to mediate gene silencing and thus play a role in promoting or suppressing HCC. For example, linc00441 has been shown to recruit DNA methyltransferase (DNMT)3A for methylation, inducing the silencing of its neighboring gene, RB1, and thereby promoting the proliferation of HCC cells (18). In addition, DNMT1 and DNMT3 induce the hypermethylation of the MEG3 promoter of the tumor suppressor lncRNA, reducing MEG3 expression and leading to apoptotic resistance and tumor growth in HCC cells (19). Of note, Zhou et al (20) reported that lncRNA ID2-AS1 inhibited HCC tumor metastasis by blocking histone deacetylase 8.
Alternative splicing
Different forms of selective splicing of lncRNA precursors (pre-lncRNAs) generate different isoforms of lncRNAs, which may enable them to perform different functions in HCC. For example, lncRNA PXN-AS1 can be spliced into several different isoforms, among which the PXN-AS1-L isoform inhibits apoptosis in myeloid leukemia in a PXN-dependent manner (21). However, another isoform (PXN-AS1-IR3) promotes HCC metastasis by inducing transcriptional activation of MYC (22). In addition, the lncRNA NEAT1 can be alternatively spliced to produce two isoforms, NEAT1_1 and NEAT1_2. It has been found that the low expression of NEAT1_2 is significantly associated with the overall survival of patients with HCC. Follow-up data suggest that NEAT1_2, but not NEAT1_1, mediates mTORC1 signaling to control aerobic glycolysis in HCC cells, contributing to the Warburg effect and HCC development (23).
Transcriptional regulation
lncRNAs are subject to different transcriptional regulations, which markedly affect the cancer- or cancer-suppressive functions of lncRNAs. A number of tumor suppressor genes or oncogene transcription factors, including p53, hypoxia inducible factor-1α (HIF-1α) and myelocytomatosis (myc) have been shown to induce the transcription of lncRNA genes. For example, myc is a key proto-oncogene, and in HCC, lncRNA linc00176, which is transcribed by myc, has been found to play a role in promoting cell proliferation and survival by inhibiting cancer cell cycle arrest and cell necrosis (24). Another study demonstrated that HIF-1α binds to the promoter region of lncRNA RAET1K to activate its transcription, thereby enhancing its cancer-promoting effect in HCC (25). However, MEG3 has been identified to function as a tumor suppressor gene in hepatoma cells by interacting with tumor suppressor p53 protein to activate p53-mediated transcriptional activity and affect the expression of certain p53 target genes (26).
Binding to RNAs
The binding of lncRNAs to RNAs is another key factor in determining their role in cancer. ALKBH3-AS1 is a carcinogenic lncRNA, which directly binds ALKBH3 mRNA in HCC cells, upregulates the expression level of ALKBH3, and promotes the proliferation of cancer cells (27). In addition to directly regulating mRNAs, lncRNAs also affect the expression of their target genes by controlling microRNA (miRNA or miR) expression. For example, it has been demonstrated that lncRNA XIST binds miR-92b as a sponge in liver cancer tissues, preventing miR-92b from binding to its downstream target Smad7 mRNA, thereby inhibiting the proliferation and metastasis of HCC (28).
Encoding small peptides
Encoded small peptides play a crucial role in cancer development and determine the functions of lncRNAs in cancer. SMIM30, encoded by LINC00998 in HCC, has been found to be associated with a low survival rate of patients with HCC and is able to promote the development of HCC by inducing SRC/YES1 membrane anchoring and MAPK pathway activation (29). By contrast, LINC-PINT encodes a PINT87aa micropeptide that binds to the transcription factor, FOXM1, as a potential anticancer micropeptide, while promoting cancer cell senescence (30). A recent correlative study using ribosome analysis methods found that a 99-aa peptide termed KRASIM encoded by lncRNA NCBP2-AS2 bound to KRAS proteins to inhibit ERK signaling, thereby inhibiting the growth of HCC cells (31).
Interaction between lncRNAs, glycolysis and HCC progression
Aerobic glycolysis is strongly associated with the progression of HCC
Aerobic glycolysis in HCC cells has been reported to be closely related to their sustained proliferation, invasive metastasis, the induction of stem cell activity and the generation of therapeutic resistance. In the aerobic glycolytic metabolism of HCC cells, an increase in the glycolytic flux of the cancer cells, accompanied by an increase in intermediate metabolites, provides sufficient energy and metabolites for the biosynthetic molecules required for the sustained proliferation of the cancer cells. As previously demonstrated, in xenograft tumors, the growth rate of HCC tumors was reduced by ~50% by decreasing the aerobic glycolytic enzyme, hexokinase 2 (HK2) (32). The production of large amounts of lactate and H+ in aerobic glycolysis leads to the acidification of the extracellular environment, induces the transformation of cancer cell epithelial cells into mesenchymal cells, and promotes the invasion and metastasis of tumor cells. It has been found that the highly metastatic HCC cell lines, MHCC97H and LM3, exhibit higher levels of aerobic glycolysis compared with those with a lesser invasive ability (33). Increased lactate levels further enhance the stemness of tumor stem cells, and the elevated lactylation of H3 histone effectively promotes the oncogenicity of HCC stem cells (34). Furthermore, the substantial production of lactic acid and hydrogen ions (H+) during aerobic glycolysis leads to acidification of the tumor microenvironment, promoting immunosuppression and facilitating tumor immune evasion, as well as conferring resistance to immunotherapy (35). Therefore, targeting glycolytic metabolic pathways and controlling lactate levels may be an effective strategy with which to prevent or attenuate the progression of HCC.
lncRNAs involved in the regulation of glycolysis in HCC
In the tumor microenvironment, some lncRNAs can affect proliferation, metastasis and drug resistance in HCC by promoting or inhibiting aerobic glycolysis in tumor cells. For example, the knockdown of NPSR1-AS1 in HCC cells has been shown to reduce their glycolytic metabolism and abolish their tumorigenic potential. This suggests that NPSR1-AS1 has glycolysis-dependent tumorigenic activity in HCC (36). It has been demonstrated that overexpression of lncRNA RP11-620J15.3 in HCC suggests that it functions as a competitive endogenous RNA to upregulate glucose-6-phosphate isomerase via sponge-binding to miR-326 and promoting aerobic glycolysis, which in turn promotes HCC cell proliferation and metastasis (37). In addition to promoting tumor progression, targeting changes in cancer cell metabolism is currently providing new insight into tumor drug therapy. Tretinoin is a widely known compound capable of inhibiting the development of HCC. Zhang et al (38) demonstrated that lncRNA MBNL1-AS1 reduced the sensitivity of HCC cells to tretinoin by inhibiting miR-708-5p-mediated glycolysis. This finding revealed an effective therapeutic target for the treatment of HCC. The majority of the lncRNAs involved in glycolysis in HCC are summarized in Table I and are discussed below.
Glycolysis-promoting lncRNAs
Taurine upregulated gene 1 (TUG1)TUG1 has been found to function as an oncogenic lncRNA that is abnormally upregulated in the majority of cancers, including HCC tissues (64). Its overexpression has been found to be associated with the promotion of glycolysis and has been studied in HCC. Partially, TUG1 mediates its biological function by segregating with miRNAs. The overexpression of TUG1 has been shown to be significantly associated with HK2, and luciferase reporter gene-based assays have revealed that the TUG1/miR-455-3p/AMPKβ2 axis affects HCC cell growth, metastasis and glycolysis through the regulation of HK2 (40). SIX1 is a therapeutic target in HCC and directly regulates lactate levels in cancer cells, which in turn affects tumor cell proliferation, apoptosis and metastasis (65). Lu et al (39) found that the overexpression of TUG1 impaired the miR-524-5p mimic-mediated inhibition of SIX1 expression, and suppressed glucose uptake, LDHA activity, lactate levels and ATP levels. This suggests that the TCG1/miR-524-5p/SIX1 axis plays a key role in glycolysis, invasion and metastasis in HCC.
HOX transcriptional antisense intergenic RNA (HOTAIR)
HOTAIR is a new class of oncogenic lncRNA often involved in regulating chromatin remodeling and epigenetic changes (66). Previous research has indicated that HOTAIR is highly expressed in a variety of malignant tumors and is involved in cell proliferation, metastasis, DNA repair and metabolism (67). As previously demonstrated in HCC, HOTAIR promotes glycolysis through the upregulation of glucose transporter (GLUT)1 and the activation of the mTOR signaling pathway (42). In addition, has been revealed to promote glycolysis in HCC under hypoxic conditions by targeting and inhibiting miR-130a-3p (41).
Urothelial carcinoma associated 1 (UCA1)
The lncRNA UCA1 was originally identified in human bladder cancer. It has an aberrant expression in embryogenesis and in a wide range of cancerous tissues and cells, and plays a role in tumor growth and glycolysis (68). The expression of UCA1 has been found to be higher in HCC tissues than in paracancerous tissues. Upwardly mobile coding protein 1 (UPF1) is an evolutionarily conserved and ubiquitously expressed phosphoprotein that promotes cellular processes through G1/S (69). RIP experiments have revealed that UPF1 specifically binds to UCA1, and the downregulation of UPF1 significantly upregulates the expression level of UCA1 and effectively increases the rate of lactate production in HCC (70).
Metastasis-associated lung adenocarcinoma transcript 1 (MALAT1)
TCF7L2 has been identified to be an effector of the Wnt signaling pathway and directly binds to several genes which play a key role in the regulation of glucose metabolism. In addition, genome-wide association studies have identified single nucleotide polymorphisms in the TCF7L2 gene associated with diabetes mellitus (71). It has been previously indicated that MALAT1 plays a pivotal role in the regulation of HCC cell proliferation, migration, and metastasis (72). Furthermore, MALAT1 has been found to regulate the expression of glycolytic genes in HCC by increasing the translation of the transcription factor, TCF7L2, which increases lactate and glucose fluxes (43). In addition, gluconeogenesis, a major component of normal hepatocyte glucose metabolism, is negatively regulated by MALAT1.
Glycolysis-inhibiting lncRNAs
LINC00659Member 1 of 10 of the solute carrier family (SLC10A1) encodes Na+-taurine bile acid cotransporter peptide (73). The expression of SLC10A1 in tumor tissues has been shown to be lower than that in normal tissues, and it has been found to be associated with the poor survival of patients with HCC (74). SLC10A1 has also been demonstrated to inhibit glycolysis in HCC cells through rates of glucose utilization, lactic acid production and extracellular acidification (75). The transfection of pcDNA3.1LINC00659 can upregulate the expression of SLC10A1 in HCC cells. Similarly, seahorse experiments have revealed that an increase in the oxygen consumption rate and a decrease in the extracellular acidification rate of HepG2 and Huh7 cells induced by the overexpression of LINC00659 can be partially reversed by co-transfection with sh-SLC10A1. These results suggested that LINC00659 inhibits the aerobic glycolysis of HCC cells by modulating SLC10A1. Thus, LINC00659 is a potential therapeutic target in HCC (60).
LINC01554
LINC01554 is a tumor suppressor lncRNA and inhibits aerobic glycolysis in HCC (61). The primary target of LINC01554 is pyruvate kinase M2 (PKM2), which is the late rate-limiting enzyme of aerobic glycolysis. In vitro ubiquitination experiments demonstrated enhanced PKM2 degradation mediated by ubiquitination in LINC01554 cells compared with controls. In LINC01554-KO cells, the ubiquitination-mediated degradation of PKM2 by LINC01554 was significantly attenuated. This resulted in a reduced glucose consumption, lactate production and pyruvate production, and in increased ATP levels. LINC01554 has the characteristic of weakening the advantage of cancer cells in acquiring high glycolysis; therefore, LINC01554 functions as a tumor suppressor in HCC and may be used as a potential therapeutic target in patients with HCC.
lncRNAs affect glycolysis by regulating transporter proteins, enzymes and signaling pathways in HCC
Dysregulated lncRNAs in HCC are involved in an altered cancer metabolism and are considered to play a key role in regulating the dysregulation of transporter proteins, metabolic enzymes and related signaling pathways in tumors that are dependent on high rates of glycolysis (Fig. 2).
lncRNAs regulate glycosylation-related transporters and enzymes
GLUTOften cancer cells exhibit a higher glucose uptake, and GLUT proteins play a crucial role in the transmembrane transport of glucose. GLUT proteins can be classified into three isoforms. GLUT1, a facilitator of glucose transporter proteins, is highly expressed in a variety of human cancers, including lung cancer (76), colorectal cancer (77), prostate cancer (78) and HCC (89). GLUT1 has also been shown to be associated with a poor patient prognosis and plays a role in glycolysis in cancer. It has been demonstrated that lncRNAs can regulate glycolysis by affecting GLUT1 expression, thereby influencing the development of HCC. For example, a previous study found that SLC2A1-AS1-mediated the downregulation of GLUT1 and significantly inhibited glycolysis in HCC (52). Another study demonstrated that lncRNA FTO-IT1 promoted glycolysis and progression in HCC by regulating FTO-mediated N6-methyladenosine modification on GLUT1 (47). Furthermore, it has been revealed that lncRNA HOTAIR promotes glycolysis by upregulating GLUT1 and activating the mTOR signaling pathway (42). GLUT has emerged as a target for cancer therapy in recent years due to the dependence of tumor cell growth on extracellular glucose (80).
HK2
HK catalyzes the first step in glycolytic metabolism, catalyzing the formation of glucose-6-phosphate. It is considered to be a key regulator of cellular energy metabolism and contributes to the Warburg effect by promoting intracellular glucose uptake (81–83). HK2 has often been reported to be highly expressed in HCC cells and induces tumor development by promoting glycolysis. The lncRNA TUG1 has been shown to be significantly associated with HK2 overexpression and the poor prognosis of patients with HCC. It has also been demonstrated that TUG1 positively regulates HK2 expression by binding to miR-455-3p, which promotes glycolysis in tumor cells, and accelerates tumor growth and metastasis (40). In addition, MBNL1-AS1 and NR2F1-AS1 have been reported to positively regulate the expression level of HK2, which promotes aerobic glycolysis in HCC cells, and contributes to cancer cell proliferation, migration and resistance to therapeutic drugs (38,50). Therefore, an in-depth study of HK2 may provide insight into the tumorigenesis and progression of HCC, and may lead to the development of novel therapeutics.
Lactate dehydrogenase (LDH) A
LDHA is a member of the LDH family, which is the rate-limiting enzyme for the interconversion of pyruvate and lactate in the glycolytic pathway. LDHA is upregulated in a number of types of cancer and is associated with the clinicopathological features and prognosis of patients (84,85). The lncRNA RAET1K has been found to be positively associated with the expression of LDHA in cells and regulate its activity, contributing to increased levels of glycolysis, thereby promoting cell proliferation and invasion (25).
PKM2
PK functions as the final rate-limiting enzyme of glycolysis, converting phosphoenolpyruvate to pyruvate. PKM2 is one of the four isozymes of PK and plays a crucial role in cancer development (86). lncRNAs can participate in the regulation of aerobic glycolysis by affecting the expression of PKM2, thereby influencing HCC progression. For example, the lncRNA SOX2OT has been shown to promote PKM2-mediated glycolytic activation by targeting the binding to miR-122-5p, increasing its expression level and promoting the metastasis of HCC cells (48). In addition, the lncRNA SNHG1 has been revealed to function as a molecular sponge for miR-326, isolating the interaction of miR-326 with PKM2, and promoting PKM2 expression. The activation of PKM2 expression is one of the mechanisms through which SNHG1 promotes glycolysis and HCC cell proliferation (56). NONHSAT024276, a potential oncogene for HCC, directly binds to polypyrimidine bundle-binding protein 1 (PTBP1), increases the ratio of M1 to M2 isoforms of PKM1/PKM2 and blocks PTBP1/PKM-mediated glycolysis to inhibit cancer cell proliferation and migration (63).
6-Phosphofructo-2-kinase/fructose-2,6-biphosphatase4 (PFKFB4)
PFKFB4, a member of the PFKFB family, which has been identified to be a key regulator of glycolysis, controls the synthesis and degradation of fructose-2,6-bisphosphate (F-2,6-BP) (87). It has been recently revealed that lncRNAs are involved in the regulation of PFKFB4 expression in tumor tissues and that they play a key role in tumor glycolysis. The high expression of the lncRNA FIRRE has been shown to be associated with malignant clinical features and with the poor survival of patients with HCC. Mechanistically, FIRRE promotes HCC cell proliferation and glycolysis by facilitating PFKFB4 transcription and expression, mainly through cAMP-responsive element-binding protein (53). Another study confirmed that LINC01572 is aberrantly upregulated in HCC tissues, particularly in patients with type 2 diabetes. Mechanistically, LINC01572 increases the level of PFKFB4 by competitively inhibiting miR-195-5p, thereby enhancing glycolysis and triggering HCC development (54).
lncRNAs regulate glycolysis-related signaling pathways p53 signaling pathway
p53 (also known as TP53) is a well-known tumor suppressor gene. It has been demonstrated that lncRNAs, as functional components of the p53 pathway, may play a regulatory role in this pathway. lncRNA CERS6-AS1 can sponge miR-30b-3p to elevate MDM2, which promotes MDM2-mediated ubiquitin-dependent degradation of the p53 oncogene, and facilitates glycolysis in HCC cells (57). On the contrary, the interaction between p53 and lncRNA can promote cancer by influencing glycolytic enzymes. p53 forms a complex with the lncRNA CUDR, which binds to the promoter region of PKM2 to enhance PKM2 expression, phosphorylation and polymer formation, and, ultimately, p53 accelerates the growth of the HCC cell line, Hep3B, by lengthening telomeres through a cascade of reactions that promotes HCC development (88).
c-Myc signaling pathway
c-Myc is a transcription factor, mainly found in the nucleus, that has been implicated in the development and progression of cancer. c-Myc, as a major regulator of aerobic glycolysis, can regulate aerobic glycolysis by directly activating glycolytic enzymes (89). In addition to a large number of coding genes, lncRNAs function as downstream targets of c-Myc and participate in glycolysis in cancer cells. lncRNA FTO-IT1 has been shown to enhance glycolysis in HCC by inducing the stabilization of FTO mRNA, leading to the overexpression of c-Myc. Moreover, c-Myc has been found to regulate the expression of FTO-IT1 by binding to its promoter region under low glucose conditions, forming a positive feedback loop between c-Myc and FTO-IT1 (47). Wu et al (58) also demonstrated that MNX1-AS1, a c-Myc target gene, was upregulated in HCC, promoting aerobic glycolysis and tumorigenesis.
HIF-1α signaling pathway
When tumor cells continue to proliferate and expand to a certain limit, vascular hypoxia leads to the appearance of HIF, and HIF-1α plays a key role as a member of the aerobic glycolysis and lactate pathways (90). In hypoxic environments, NPSR1-AS1 induces the activation of the MAPK/ERK pathway in HCC cells, which promotes the proliferation and glycolysis of HCC cells (36). The overexpression of lncRNA UPK1A-AS1 has been reported to significantly increase the stability of the HIF-1α ubiquitin-modified expression of upregulated glycolysis-related genes, thereby promoting glycolysis levels in HCC cells (59).
Phosphatidylinositol 3-kinase (PI3K)/AKT/mTOR signaling pathway
The dysregulation of the PI3K/AKT/mTOR pathway is a prevalent occurrence in the majority of human cancers. In HCC, this signaling cascade plays a crucial role in facilitating glucose metabolism, tumor metastasis, and resistance to drugs (91–93). It has been demonstrated that lncRNA is a regulator of PI3K/AKT/mTOR signaling in a variety of cancer types, and can indirectly affect the expression of enzymes by regulating this pathway. In HCC tissues and cell lines, the upregulation of LINC01572 increases the expression of the glycolytic enzyme, PFKFB4, by activating PI3K/AKT signaling, thereby enhancing glycolysis and triggering HCC malignancy (54). mTOR is a serine/threonine kinase. It has been previously shown that the upregulation of lncRNA HOTAIR can induce the glycolysis of HCC cells by activating the mTOR signaling pathway (42). By contrast, LINC01554, a novel oncogene in HCC, has been identified to inhibit the AKT/mTOR signaling pathway and eliminate aerobic glycolysis in HCC cells, thereby suppressing tumor growth (61).
In summary, lncRNAs mediate changes in the expression of glycolysis-associated transporter proteins, enzymes and signaling pathways in HCCs, affecting the level of tumor aerobic glycolysis, and thus cancer formation and progression. Therefore, these glycolysis-associated lncRNAs have gradually become key targets for cancer research, and the inhibition of these lncRNAs is critical for controlling tumor development. Follow-up studies should continue to explore the mechanisms of the lncRNA regulation of glycolysis to provide new directions for cancer treatment.
Conclusions and future perspectives
Although there have been major breakthroughs in the study of malignant tumors, HCC remains a lethal disease. Due to the high aggressiveness of HCC, this type of cancer is very likely to metastasize, and the majority of cases are diagnosed in the middle and late stages; thus, the option of surgical treatment is only <40%, which is reflected in the low long-term survival rate of patients with HCC (94). Therefore, the influence of other molecular therapeutic targets on the progression and mechanism of HCC may have a key impact on the prevention and control of HCC and the long-term survival rate of patients.
Cancer cells are metabolically active, and they can alternate between glycolysis and mitochondrial OXPHOS in response to nutritional stress caused by environmental changes (95). Therefore, blocking the glycolytic pathway in tumor cells or inducing the transformation of cancer cells from aerobic glycolytic to mitochondrial OXPHOS may lead to novel approaches for the treatment of HCC. The present review focused on the effects of lncRNAs on HCC progression, and their specific functions and mechanisms in cancer metabolic pathways. In terms of the mechanisms, lncRNAs significantly affect the process of glucose metabolism mainly through glycolytic-related transporters, metabolic enzymes or related signaling pathways, and thus participate in the progression of HCC. lncRNAs are involved in the regulation of glucose metabolism in tumor cells, which suggests that lncRNAs, related glycolytic regulatory factors, may become novel targets for cancer therapy.
In the current stage of HCC research, clinical trials involving lncRNAs primarily focus on exosomal lncRNAs that can serve as detectable biomarkers (96). lncRNAs can participate in tumor cell metabolism through different mechanisms, which may have extensive therapeutic significance and may provide new insight into the treatment of HCC. Therefore, the role of lncRNAs in regulating aerobic glycolysis should be carefully considered when considering the development of future therapeutic drugs and methods. Further studies on lncRNAs inhibitors may provide strategies with which to block the progression of HCC. These may include: i) Targeting lncRNAs capable of regulating glycolytic enzymes; ii) targeting lncRNAs that regulate the glucose transporter GLUT to inhibit glucose uptake; and iii) lncRNAs that target glycolysis-related regulatory factors or signaling pathways. Therefore, the further in-depth exploration of the mechanisms of lncRNAs in HCC glycolysis may aid in the development of more effective therapeutic strategies with which to inhibit tumor progression.
Acknowledgements
Not applicable.
Funding
The present study was supported in part by the Key R & D program Natural Science Foundation of Guangxi (grant no. AB19110007). The present study was also supported in part by the Natural Science Foundation of Guangxi (grant no. 2017GXNSFAA198015) and the Innovation Project of Guangxi Graduate Education (grant no. YCSW2023215).
Availability of data and materials
Not applicable.
Authors' contributions
CO designed the present review. QioH wrote the manuscript. ZL and QiqH was involved in article revision. XL, JX, LH and LBH surveyed the literature and contributed to the revisions. All authors have read and approved the final manuscript. Data authentication is not applicable.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
|
Bertuccio P, Turati F, Carioli G, Rodriguez T, La Vecchia C, Malvezzi M and Negri E: Global trends and predictions in hepatocellular carcinoma mortality. J Hepatol. 67:302–309. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Yang JD, Hainaut P, Gores GJ, Amadou A, Plymoth A and Roberts LR: A global view of hepatocellular carcinoma: Trends, risk, prevention and management. Nat Rev Gastroenterol Hepatol. 16:589–604. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Vander Heiden MG, Cantley LC and Thompson CB: Understanding the Warburg effect: The metabolic requirements of cell proliferation. Science. 324:1029–1033. 2009. View Article : Google Scholar : PubMed/NCBI | |
|
Lunt SY and Vander Heiden MG: Aerobic glycolysis: meeting the metabolic requirements of cell proliferation. Annu Rev Cell Dev Biol. 27:441–464. 2011. View Article : Google Scholar : PubMed/NCBI | |
|
Shestov AA, Liu X, Ser Z, Cluntun AA, Hung YP, Huang L, Kim D, Le A, Yellen G, Albeck JG, et al: Quantitative determinants of aerobic glycolysis identify flux through the enzyme GAPDH as a limiting step. Elife. 3:e033422014. View Article : Google Scholar : PubMed/NCBI | |
|
Guido C, Whitaker-Menezes D, Capparelli C, Balliet R, Lin Z, Pestell RG, Howell A, Aquila S, Andò S, Martinez-Outschoorn U, et al: Metabolic reprogramming of cancer-associated fibroblasts by TGF-β drives tumor growth: Connecting TGF-β signaling with ‘Warburg-like’ cancer metabolism and L-lactate production. Cell Cycle. 11:3019–3035. 2012. View Article : Google Scholar : PubMed/NCBI | |
|
Liberti MV and Locasale JW: The Warburg effect: How does it benefit cancer cells? Trends Biochem Sci. 41:211–218. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Sattler UGA and Mueller-Klieser W: The anti-oxidant capacity of tumour glycolysis. Int J Radiat Biol. 85:963–971. 2009. View Article : Google Scholar : PubMed/NCBI | |
|
Beyoğlu D, Imbeaud S, Maurhofer O, Bioulac-Sage P, Zucman-Rossi J, Dufour JF and Idle JR: Tissue metabolomics of hepatocellular carcinoma: Tumor energy metabolism and the role of transcriptomic classification. Hepatology. 58:229–238. 2013. View Article : Google Scholar : PubMed/NCBI | |
|
Bustamante E and Pedersen PL: High aerobic glycolysis of rat hepatoma cells in culture: Role of mitochondrial hexokinase. Proc Natl Acad Sci USA. 74:3735–3739. 1977. View Article : Google Scholar : PubMed/NCBI | |
|
Djebali S, Davis CA, Merkel A, Dobin A, Lassmann T, Mortazavi A, Tanzer A, Lagarde J, Lin W, Schlesinger F, et al: Landscape of transcription in human cells. Nature. 489:101–108. 2012. View Article : Google Scholar : PubMed/NCBI | |
|
ENCODE Project Consortium, Birney E, Stamatoyannopoulos JA, Dutta A, Guigó R, Gingeras TR, Margulies EH, Weng Z, Snyder M, Dermitzakis ET, et al: Identification and analysis of functional elements in 1% of the human genome by the ENCODE pilot project. Nature. 447:799–816. 2007. View Article : Google Scholar : PubMed/NCBI | |
|
Deng H, Zhang J, Shi J, Guo Z, He C, Ding L, Tang JH and Hou Y: Role of long non-coding RNA in tumor drug resistance. Tumour Biol. 37:11623–11631. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Li J, Meng H, Bai Y and Wang K: Regulation of lncRNA and its role in cancer metastasis. Oncol Res. 23:205–217. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Shen XH, Qi P and Du X: Long non-coding RNAs in cancer invasion and metastasis. Mod Pathol. 28:4–13. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Statello L, Guo CJ, Chen LL and Huarte M: Gene regulation by long non-coding RNAs and its biological functions. Nat Rev Mol Cell Biol. 22:96–118. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Yuan SX, Zhang J, Xu QG, Yang Y and Zhou WP: Long noncoding RNA, the methylation of genomic elements and their emerging crosstalk in hepatocellular carcinoma. Cancer Lett. 379:239–244. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Tang J, Xie Y, Xu X, Yin Y, Jiang R, Deng L, Tan Z, Gangarapu V, Tang J and Sun B: Bidirectional transcription of Linc00441 and RB1 via H3K27 modification-dependent way promotes hepatocellular carcinoma. Cell Death Dis. 8:e26752017. View Article : Google Scholar : PubMed/NCBI | |
|
Braconi C, Kogure T, Valeri N, Huang N, Nuovo G, Costinean S, Negrini M, Miotto E, Croce CM and Patel T: microRNA-29 can regulate expression of the long non-coding RNA gene MEG3 in hepatocellular cancer. Oncogene. 30:4750–4756. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Zhou Y, Huan L, Wu Y, Bao C, Chen B, Wang L, Huang S, Liang L and He X: LncRNA ID2-AS1 suppresses tumor metastasis by activating the HDAC8/ID2 pathway in hepatocellular carcinoma. Cancer Lett. 469:399–409. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Yuan JH, Liu XN, Wang TT, Pan W, Tao QF, Zhou WP, Wang F and Sun SH: The MBNL3 splicing factor promotes hepatocellular carcinoma by increasing PXN expression through the alternative splicing of lncRNA-PXN-AS1. Nat Cell Biol. 19:820–832. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Zhou HZ, Li F, Cheng ST, Xu Y, Deng HJ, Gu DY, Wang J, Chen WX, Zhou YJ, Yang ML, et al: DDX17-regulated alternative splicing that produced an oncogenic isoform of PXN-AS1 to promote HCC metastasis. Hepatology. 75:847–865. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Zhang H, Su X, Burley SK and Zheng XFS: mTOR regulates aerobic glycolysis through NEAT1 and nuclear paraspeckle-mediated mechanism in hepatocellular carcinoma. Theranostics. 12:3518–3533. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Tran DDH, Kessler C, Niehus SE, Mahnkopf M, Koch A and Tamura T: Myc target gene, long intergenic noncoding RNA, Linc00176 in hepatocellular carcinoma regulates cell cycle and cell survival by titrating tumor suppressor microRNAs. Oncogene. 37:75–85. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Zhou Y, Huang Y, Hu K, Zhang Z, Yang J and Wang Z: HIF1A activates the transcription of lncRNA RAET1K to modulate hypoxia-induced glycolysis in hepatocellular carcinoma cells via miR-100-5p. Cell Death Dis. 11:1762020. View Article : Google Scholar : PubMed/NCBI | |
|
Zhu J, Liu S, Ye F, Shen Y, Tie Y, Zhu J, Wei L, Jin Y, Fu H, Wu Y and Zheng X: Long noncoding RNA MEG3 interacts with p53 protein and regulates partial p53 target genes in hepatoma cells. PLoS One. 10:e1397902015. | |
|
Lu Q, Wang H, Lei X, Ma Q, Zhao J, Sun W, Guo C, Huang D and Xu Q: LncRNA ALKBH3-AS1 enhances ALKBH3 mRNA stability to promote hepatocellular carcinoma cell proliferation and invasion. J Cell Mol Med. 26:5292–5302. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Zhuang LK, Yang YT, Ma X, Han B, Wang ZS, Zhao QY, Wu LQ and Qu ZQ: MicroRNA-92b promotes hepatocellular carcinoma progression by targeting Smad7 and is mediated by long non-coding RNA XIST. Cell Death Dis. 7:e22032016. View Article : Google Scholar : PubMed/NCBI | |
|
Pang Y, Liu Z, Han H, Wang B, Li W, Mao C and Liu S: Peptide SMIM30 promotes HCC development by inducing SRC/YES1 membrane anchoring and MAPK pathway activation. J Hepatol. 73:1155–1169. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Xiang X, Fu Y, Zhao K, Miao R, Zhang X, Ma X, Liu C, Zhang N and Qu K: Cellular senescence in hepatocellular carcinoma induced by a long non-coding RNA-encoded peptide PINT87aa by blocking FOXM1-mediated PHB2. Theranostics. 11:4929–4944. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Xu W, Deng B, Lin P, Liu C, Li B, Huang Q, Zhou H, Yang J and Qu L: Ribosome profiling analysis identified a KRAS-interacting microprotein that represses oncogenic signaling in hepatocellular carcinoma cells. Sci China Life Sci. 63:529–542. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Li S, Li J, Dai W, Zhang Q, Feng J, Wu L, Liu T, Yu Q, Xu S, Wang W, et al: Genistein suppresses aerobic glycolysis and induces hepatocellular carcinoma cell death. Br J Cancer. 117:1518–1528. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Li Y, Lu Z, Liang Z, Ji D, Zhang P, Liu Q, Zheng X and Yao Y: Metastasis-associated in colon cancer-1 is associated with poor prognosis in hepatocellular carcinoma, partly by promoting proliferation through enhanced glucose metabolism. Mol Med Rep. 12:426–434. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Pan L, Feng F, Wu J, Fan S, Han J, Wang S, Yang L, Liu W, Wang C and Xu K: Demethylzeylasteral targets lactate by inhibiting histone lactylation to suppress the tumorigenicity of liver cancer stem cells. Pharmacol Res. 181:1062702022. View Article : Google Scholar : PubMed/NCBI | |
|
Ganapathy-Kanniappan S: Linking tumor glycolysis and immune evasion in cancer: Emerging concepts and therapeutic opportunities. Biochim Biophys Acta Rev Cancer. 1868:212–220. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
He H, Chen T, Mo H, Chen S, Liu Q and Guo C: Hypoxia-inducible long noncoding RNA NPSR1-AS1 promotes the proliferation and glycolysis of hepatocellular carcinoma cells by regulating the MAPK/ERK pathway. Biochem Biophys Res Commun. 533:886–892. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Liu C, Xu K, Liu J, He C, Liu P, Fu Q, Zhang H and Qin T: LncRNA RP11-620J15.3 promotes HCC cell proliferation and metastasis by targeting miR-326/GPI to enhance glycolysis. Biol Direct. 18:152023. View Article : Google Scholar : PubMed/NCBI | |
|
Zhang H, Zhao L, Ren P and Sun X: LncRNA MBNL1-AS1 knockdown increases the sensitivity of hepatocellular carcinoma to tripterine by regulating miR-708-5p-mediated glycolysis. Biotechnol Genet Eng Rev. 1–18. 2023.(Epub ahead of print). View Article : Google Scholar : PubMed/NCBI | |
|
Lu L, Huang J, Mo J, Da X, Li Q, Fan M and Lu H: Exosomal lncRNA TUG1 from cancer-associated fibroblasts promotes liver cancer cell migration, invasion, and glycolysis by regulating the miR-524-5p/SIX1 axis. Cell Mol Biol Lett. 27:172022. View Article : Google Scholar : PubMed/NCBI | |
|
Lin YH, Wu MH, Huang YH, Yeh CT, Cheng ML, Chi HC, Tsai CY, Chung IH, Chen CY and Lin KH: Taurine up-regulated gene 1 functions as a master regulator to coordinate glycolysis and metastasis in hepatocellular carcinoma. Hepatology. 67:188–203. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Hu M, Fu Q, Jing C, Zhang X, Qin T and Pan Y: LncRNA HOTAIR knockdown inhibits glycolysis by regulating miR-130a-3p/HIF1A in hepatocellular carcinoma under hypoxia. Biomed Pharmacother. 125:1097032020. View Article : Google Scholar : PubMed/NCBI | |
|
Wei S, Fan Q, Yang L, Zhang X, Ma Y, Zong Z, Hua X, Su D, Sun H, Li H and Liu Z: Promotion of glycolysis by HOTAIR through GLUT1 upregulation via mTOR signaling. Oncol Rep. 38:1902–1908. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Malakar P, Stein I, Saragovi A, Winkler R, Stern-Ginossar N, Berger M, Pikarsky E and Karni R: Long Noncoding RNA MALAT1 regulates cancer glucose metabolism by enhancing mTOR-mediated translation of TCF7L2. Cancer Res. 79:2480–2493. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Xu M, Zhou C, Weng J, Chen Z, Zhou Q, Gao J, Shi G, Ke A, Ren N, Sun H and Shen Y: Tumor associated macrophages-derived exosomes facilitate hepatocellular carcinoma malignance by transferring lncMMPA to tumor cells and activating glycolysis pathway. J Exp Clin Cancer Res. 41:2532022. View Article : Google Scholar : PubMed/NCBI | |
|
Li X, Zhao Q, Qi J, Wang W, Zhang D, Li Z and Qin C: lncRNA Ftx promotes aerobic glycolysis and tumor progression through the PPARγ pathway in hepatocellular carcinoma. Int J Oncol. 53:551–566. 2018.PubMed/NCBI | |
|
Chen K, Wang X, Wei B, Sun R, Wu C and Yang HJ: LncRNA SNHG6 promotes glycolysis reprogramming in hepatocellular carcinoma by stabilizing the BOP1 protein. Anim Cells Syst (Seoul). 26:369–379. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Wang F, Hu Y, Wang H, Hu P, Xiong H, Zeng Z, Han S, Wang D, Wang J, Zhao Y, et al: LncRNA FTO-IT1 promotes glycolysis and progression of hepatocellular carcinoma through modulating FTO-mediated N6-methyladenosine modification on GLUT1 and PKM2. J Exp Clin Cancer Res. 42:2672023. View Article : Google Scholar : PubMed/NCBI | |
|
Liang Y, Zhang D, Zheng T, Yang G, Wang J, Meng F, Liu Y, Zhang G, Zhang L, Han J, et al: lncRNA-SOX2OT promotes hepatocellular carcinoma invasion and metastasis through miR-122-5p-mediated activation of PKM2. Oncogenesis. 9:542020. View Article : Google Scholar : PubMed/NCBI | |
|
Ye Y, Wang M, Wang G, Mai Z, Zhou B, Han Y, Zhuang J and Xia W: lncRNA miR4458HG modulates hepatocellular carcinoma progression by activating m6A-dependent glycolysis and promoting the polarization of tumor-associated macrophages. Cell Mol Life Sci. 80:992023. View Article : Google Scholar : PubMed/NCBI | |
|
Li X, Li Y, Bai S, Zhang J, Liu Z and Yang J: NR2F1-AS1/miR-140/HK2 axis regulates hypoxia-induced glycolysis and migration in hepatocellular carcinoma. Cancer Manag Res. 13:427–437. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Ma X, Mao Z, Zhu J, Liu H and Chen F: lncRNA PANTR1 upregulates BCL2A1 expression to promote tumorigenesis and warburg effect of hepatocellular carcinoma through restraining miR-587. J Immunol Res. 2021:17368192021. View Article : Google Scholar : PubMed/NCBI | |
|
Shang R, Wang M, Dai B, Du J, Wang J, Liu Z, Qu S, Yang X, Liu J, Xia C, et al: Long noncoding RNA SLC2A1-AS1 regulates aerobic glycolysis and progression in hepatocellular carcinoma via inhibiting the STAT3/FOXM1/GLUT1 pathway. Mol Oncol. 14:1381–1396. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Shen C, Ding L, Mo H, Liu R, Xu Q and Tu K: Long noncoding RNA FIRRE contributes to the proliferation and glycolysis of hepatocellular carcinoma cells by enhancing PFKFB4 expression. J Cancer. 12:4099–4108. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Lai S, Quan Z, Hao Y, Liu J, Wang Z, Dai L, Dai H, He S and Tang B: Long non-coding RNA LINC01572 promotes hepatocellular carcinoma progression via sponging miR-195-5p to enhance PFKFB4-mediated glycolysis and PI3K/AKT activation. Front Cell Dev Biol. 9:7830882021. View Article : Google Scholar : PubMed/NCBI | |
|
Ji W, Bai J and Ke Y: Exosomal ZFPM2-AS1 contributes to tumorigenesis, metastasis, stemness, macrophage polarization, and infiltration in hepatocellular carcinoma through PKM mediated glycolysis. Environ Toxicol. 38:1332–1346. 2023. View Article : Google Scholar : PubMed/NCBI | |
|
Wang Y, Yang F, Peng Q, Mei K, He H and Yang Q: Long non-coding RNA SNHG1 activates glycolysis to promote hepatocellular cancer progression through the miR-326/PKM2 axis. J Gene Med. 24:e34402022. View Article : Google Scholar : PubMed/NCBI | |
|
Xu B, Wei Y, Liu F, Li L, Zhou S, Peng Y and Li B: Long noncoding RNA CERS6-AS1 modulates glucose metabolism and tumor progression in hepatocellular carcinoma by promoting the MDM2/p53 signaling pathway. Cell Death Discov. 8:3482022. View Article : Google Scholar : PubMed/NCBI | |
|
Wu Y, Wang Y, Yao H, Li H, Meng F, Li Q, Lin X and Liu L: MNX1-AS1, a c-Myc induced lncRNA, promotes the Warburg effect by regulating PKM2 nuclear translocation. J Exp Clin Cancer Res. 41:3372022. View Article : Google Scholar : PubMed/NCBI | |
|
Zhang D, Zou X, Song Y and Wu D: Long non-coding RNA UPK1A-AS1 promotes glycolysis in hepatocellular carcinoma cells via stabilization of HIF-1α. Nan Fang Yi Ke Da Xue Xue Bao. 41:193–199. 2021.(In Chinese). PubMed/NCBI | |
|
Chen B, Xu X, Wu W, Zheng K and Yu Y: LINC00659 inhibits hepatocellular carcinoma malignant progression by blocking aerobic glycolysis through FUS recruitment and SLC10A1 modulation. Anal Cell Pathol (Amst). 2023:58529632023.PubMed/NCBI | |
|
Zheng YL, Li L, Jia YX, Zhang BZ, Li JC, Zhu YH, Li MQ, He JZ, Zeng TT, Ban XJ, et al: LINC01554-mediated glucose metabolism reprogramming suppresses tumorigenicity in hepatocellular carcinoma via downregulating PKM2 expression and inhibiting Akt/mTOR signaling pathway. Theranostics. 9:796–810. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Guan YF, Huang QL, Ai YL, Chen QT, Zhao WX, Wang XM, Wu Q and Chen HZ: Nur77-activated lncRNA WFDC21P attenuates hepatocarcinogenesis via modulating glycolysis. Oncogene. 39:2408–2423. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Li Y, Chen X, Huang H, Liao L, Chong H, Li G, Yuan T, Lu W, Deng S and Huang Q: A feedback loop between NONHSAT024276 and PTBP1 inhibits tumor progression and glycolysis in HCC by increasing the PKM1/PKM2 ratio. Cancer Sci. 114:1519–1540. 2023. View Article : Google Scholar : PubMed/NCBI | |
|
Huang MD, Chen WM, Qi FZ, Sun M, Xu TP, Ma P and Shu YQ: Long non-coding RNA TUG1 is up-regulated in hepatocellular carcinoma and promotes cell growth and apoptosis by epigenetically silencing of KLF2. Mol Cancer. 14:1652015. View Article : Google Scholar : PubMed/NCBI | |
|
Chen K, Wei H, Pan J, Chen Z, Pan D, Gao T, Huang J, Huang M, Ou M and Zhong W: Six1 is negatively correlated with poor prognosis and reduces 5-fluorouracil sensitivity via attenuating the stemness of hepatocellular carcinoma cells. Eur J Pharmacol. 861:1725992019. View Article : Google Scholar : PubMed/NCBI | |
|
Raju G, Pavitra E, Bandaru SS, Varaprasad GL, Nagaraju GP, Malla RR, Huh YS and Han YK: HOTAIR: A potential metastatic, drug-resistant and prognostic regulator of breast cancer. Mol Cancer. 22:652023. View Article : Google Scholar : PubMed/NCBI | |
|
Zhang J, Zhang P, Wang L, Piao HL and Ma L: Long non-coding RNA HOTAIR in carcinogenesis and metastasis. Acta Biochim Biophys Sin (Shanghai). 46:1–5. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
Li T, Sun X and Jiang X: UCA1 involved in the metformin-regulated bladder cancer cell proliferation and glycolysis. Tumour Biol. 39:10104283177108232017. View Article : Google Scholar : PubMed/NCBI | |
|
Lou CH, Shao A, Shum EY, Espinoza JL, Huang L, Karam R and Wilkinson MF: Posttranscriptional control of the stem cell and neurogenic programs by the nonsense-mediated RNA decay pathway. Cell Rep. 6:748–764. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
Zhou Y, Li Y, Wang N, Li X, Zheng J and Ge L: UPF1 inhibits the hepatocellular carcinoma progression by targeting long non-coding RNA UCA1. Sci Rep. 9:66522019. View Article : Google Scholar : PubMed/NCBI | |
|
Grant SFA, Thorleifsson G, Reynisdottir I, Benediktsson R, Manolescu A, Sainz J, Helgason A, Stefansson H, Emilsson V, Helgadottir A, et al: Variant of transcription factor 7-like 2 (TCF7L2) gene confers risk of type 2 diabetes. Nat Genet. 38:320–323. 2006. View Article : Google Scholar : PubMed/NCBI | |
|
Li GZ, Meng GX, Pan GQ, Zhang X, Yan LJ, Li RZ, Ding ZN, Tan SY, Wang DX, Tian BW, et al: MALAT1/mir-1-3p mediated BRF2 expression promotes HCC progression via inhibiting the LKB1/AMPK signaling pathway. Cancer Cell Int. 23:1882023. View Article : Google Scholar : PubMed/NCBI | |
|
Döring B, Lütteke T, Geyer J and Petzinger E: The SLC10 carrier family: Transport functions and molecular structure. Curr Top Membr. 70:105–168. 2012. View Article : Google Scholar : PubMed/NCBI | |
|
Tran QH, Nguyen VG, Tran CM and Nguyen MN: Down-regulation of solute carrier family 10 member 1 is associated with early recurrence and poorer prognosis of hepatocellular carcinoma. Heliyon. 7:e064632021. View Article : Google Scholar : PubMed/NCBI | |
|
Lu C, Fang S, Weng Q, Lv X, Meng M, Zhu J, Zheng L, Hu Y, Gao Y, Wu X, et al: Integrated analysis reveals critical glycolytic regulators in hepatocellular carcinoma. Cell Commun Signal. 18:972020. View Article : Google Scholar : PubMed/NCBI | |
|
Zhang B, Xie Z and Li B: The clinicopathologic impacts and prognostic significance of GLUT1 expression in patients with lung cancer: A meta-analysis. Gene. 689:76–83. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Kuai XY, Lei ZY, Liu XS and Shao XY: The interaction of GLUT1 and FOXM1 leads to a poor prognosis in colorectal cancer. Anticancer Agents Med Chem. 20:941–950. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Xiao H, Wang J, Yan W, Cui Y, Chen Z, Gao X, Wen X and Chen J: GLUT1 regulates cell glycolysis and proliferation in prostate cancer. Prostate. 78:86–94. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Sun HW, Yu XJ, Wu WC, Chen J, Shi M, Zheng L and Xu J: GLUT1 and ASCT2 as predictors for prognosis of hepatocellular carcinoma. PLoS One. 11:e01689072016. View Article : Google Scholar : PubMed/NCBI | |
|
Barbosa AM and Martel F: Targeting glucose transporters for breast cancer therapy: The effect of natural and synthetic compounds. Cancers (Basel). 12:1542020. View Article : Google Scholar : PubMed/NCBI | |
|
DeWaal D, Nogueira V, Terry AR, Patra KC, Jeon SM, Guzman G, Au J, Long CP, Antoniewicz MR and Hay N: Hexokinase-2 depletion inhibits glycolysis and induces oxidative phosphorylation in hepatocellular carcinoma and sensitizes to metformin. Nat Commun. 9:4462018. View Article : Google Scholar : PubMed/NCBI | |
|
Wu Q, Wang SP, Sun XX, Tao YF, Yuan XQ, Chen QM, Dai L, Li CL, Zhang JY and Yang AL: HuaChanSu suppresses tumor growth and interferes with glucose metabolism in hepatocellular carcinoma cells by restraining Hexokinase-2. Int J Biochem Cell Biol. 142:1061232022. View Article : Google Scholar : PubMed/NCBI | |
|
Ros S and Schulze A: Glycolysis back in the limelight: Systemic targeting of HK2 blocks tumor growth. Cancer Discov. 3:1105–1107. 2013. View Article : Google Scholar : PubMed/NCBI | |
|
Zhang R, Su J, Xue SL, Yang H, Ju LL, Ji Y, Wu KH, Zhang YW, Zhang YX, Hu JF and Yu M: HPV E6/p53 mediated down-regulation of miR-34a inhibits Warburg effect through targeting LDHA in cervical cancer. Am J Cancer Res. 6:312–320. 2016.PubMed/NCBI | |
|
Zhang K, Zhang T, Yang Y, Tu W, Huang H, Wang Y, Chen Y, Pan K and Chen Z: N6-methyladenosine-mediated LDHA induction potentiates chemoresistance of colorectal cancer cells through metabolic reprogramming. Theranostics. 12:4802–4817. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Christofk HR, Vander Heiden MG, Harris MH, Ramanathan A, Gerszten RE, Wei R, Fleming MD, Schreiber SL and Cantley LC: The M2 splice isoform of pyruvate kinase is important for cancer metabolism and tumour growth. Nature. 452:230–233. 2008. View Article : Google Scholar : PubMed/NCBI | |
|
Kotowski K, Rosik J, Machaj F, Supplitt S, Wiczew D, Jabłońska K, Wiechec E, Ghavami S and Dzięgiel P: Role of PFKFB3 and PFKFB4 in cancer: Genetic basis, impact on disease development/progression, and potential as therapeutic targets. Cancers (Basel). 13:9092021. View Article : Google Scholar : PubMed/NCBI | |
|
Wu M, An J, Zheng Q, Xin X, Lin Z, Li X, Li H and Lu D: Double mutant P53 (N340Q/L344R) promotes hepatocarcinogenesis through upregulation of Pim1 mediated by PKM2 and LncRNA CUDR. Oncotarget. 7:66525–66539. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Tang J, Yan T, Bao Y, Shen C, Yu C, Zhu X, Tian X, Guo F, Liang Q, Liu Q, et al: LncRNA GLCC1 promotes colorectal carcinogenesis and glucose metabolism by stabilizing c-Myc. Nat Commun. 10:34992019. View Article : Google Scholar : PubMed/NCBI | |
|
Yu Z, Wang Y, Deng J, Liu D, Zhang L, Shao H, Wang Z, Zhu W, Zhao C and Ke Q: Long non-coding RNA COL4A2-AS1 facilitates cell proliferation and glycolysis of colorectal cancer cells via miR-20b-5p/hypoxia inducible factor 1 alpha subunit axis. Bioengineered. 12:6251–6263. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Badoiu SC, Greabu M, Miricescu D, Stanescu-Spinu II, Ilinca R, Balan DG, Balcangiu-Stroescu AE, Mihai DA, Vacaroiu IA, Stefani C and Jinga V: PI3K/AKT/mTOR dysregulation and reprogramming metabolic pathways in renal cancer: Crosstalk with the VHL/HIF axis. Int J Mol Sci. 24:83912023. View Article : Google Scholar : PubMed/NCBI | |
|
Zhu YJ, Zheng B, Wang HY and Chen L: New knowledge of the mechanisms of sorafenib resistance in liver cancer. Acta Pharmacol Sin. 38:614–622. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Zhang H, Wang Q, Liu J and Cao H: Inhibition of the PI3K/Akt signaling pathway reverses sorafenib-derived chemo-resistance in hepatocellular carcinoma. Oncol Lett. 15:9377–9384. 2018.PubMed/NCBI | |
|
Li J, Xing J, Yang Y, Liu J, Wang W, Xia Y, Yan Z, Wang K, Wu D, Wu L, et al: Adjuvant 131I-metuximab for hepatocellular carcinoma after liver resection: A randomised, controlled, multicentre, open-label, phase 2 trial. Lancet Gastroenterol Hepatol. 5:548–560. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Elgendy M, Cirò M, Hosseini A, Weiszmann J, Mazzarella L, Ferrari E, Cazzoli R, Curigliano G, DeCensi A, Bonanni B, et al: Combination of hypoglycemia and metformin impairs tumor metabolic plasticity and growth by modulating the PP2A-GSK3β-MCL-1 axis. Cancer Cell. 35:798–815. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Sasaki R, Kanda T, Yokosuka O, Kato N, Matsuoka S and Moriyama M: Exosomes and hepatocellular carcinoma: From bench to bedside. Int J Mol Sci. 20:14062019. View Article : Google Scholar : PubMed/NCBI |