Pain crisis management in a patient with sickle cell disease during SARS‑CoV‑2 infection: A case report and literature review
- Authors:
- Published online on: March 10, 2022 https://doi.org/10.3892/wasj.2022.149
- Article Number: 14
-
Copyright: © Campanella et al. This is an open access article distributed under the terms of Creative Commons Attribution License.
Abstract
Introduction
Severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2) may cause extra-pulmonary illnesses in addition to a broad spectrum of respiratory tract manifestations, ranging from asymptomatic infections to severe pneumonia (1). In terms of severity, patients with additional risk factors, including old age and chronic diseases, are more susceptible to severe coronavirus disease 2019 (COVID-19) infection, as compared to the general population (2).
Sickle cell disease (SCD) is an inherited hemoglobinopathy affecting millions of individuals, particularly among those of African descent. Patients with SCD may display several clinical manifestations, the most common symptoms being haemolytic anaemia, vaso-occlusive crisis (VOC), acute chest syndrome (ACS), and fever, frequently requiring hospitalization (3).
SCD can worsen the morbidity and mortality of SARS-CoV-2 infection (4). On the other hand, viral infections are significant contributors to morbidity and mortality in patients with SCD, and COVID-19, along with its clinical pattern (e.g., severe pneumonia with hypoxia), may exacerbate or trigger SCD manifestations, including ACS and VOC (5,6).
The SCD pathophysiology consists of endothelial dysfunction, chronic inflammation, chronic anaemia, immunocompromised status and hypercoagulability, all of which have been recognized as risk factors for poorer outcomes in patients with COVID-19. Furthermore, both patients with SCD (as well as those with sickle cell trait) and patients with COVID-19 present with a hypercoagulable state, being at higher risk of developing severe complications and organ dysfunctions (7).
There are only a limited number of reports concerning SARS-CoV-2 infection in patients with SCD. The present study describes the case of a 33-year-old female patient of African descent with SCD, who tested positive for COVID-19 infection and developed a severe painful crisis within 5 days after admission.
Case report
A 33-year-old female patient of Senegalese descent was admitted to the Emergency Department of ARNAS Garibaldi Hospital, Catania, Italy, with symptoms of 3 days of fever (up to a temperature of 38.5˚C), fatigue and cough. A nasopharyngeal swab resulted positive for SARS-COV-2 (both the antigenic (Panbio™-COVID-19 Ag test Abbott) and molecular test results (RT-qPCR targeting the E gene, RdRP gene and N gene of SARS-CoV-2).
Her past medical history included SCD, with a history of recurrent pain crisis (last crisis prior to admission was reported 6 months earlier) and previous treatment with hydroxyurea (HU), terminated 1 year prior. The patient did not take any medication at the time of admission and had received a two-dose vaccination against SARS-CoV-2 (BNT162b2 vaccine; BioNTech Pfizer, Inc.). Upon admission, the patient was febrile (temperature, 38˚C); blood pressure was measured at 130/70 mmHg, the pulse rate at 90 bpm, saturation at 97% in room air and the respiratory rate at 16 breaths/min.
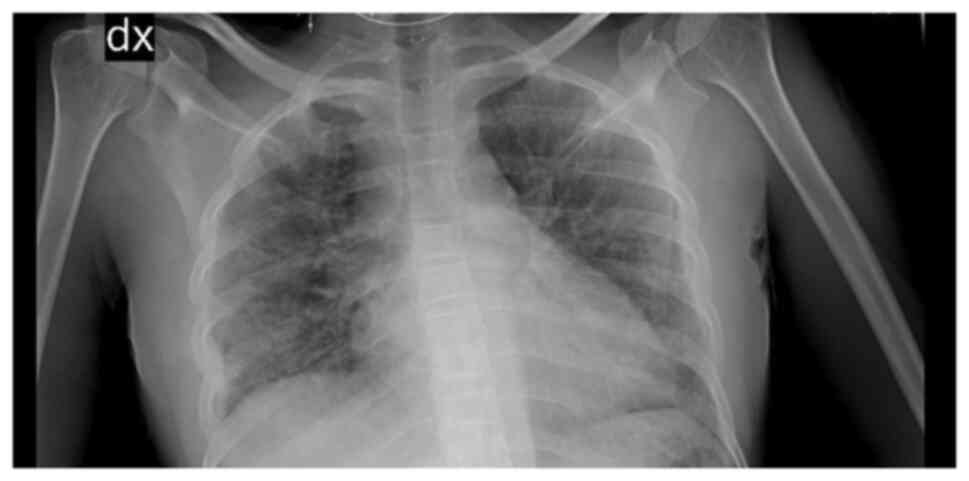
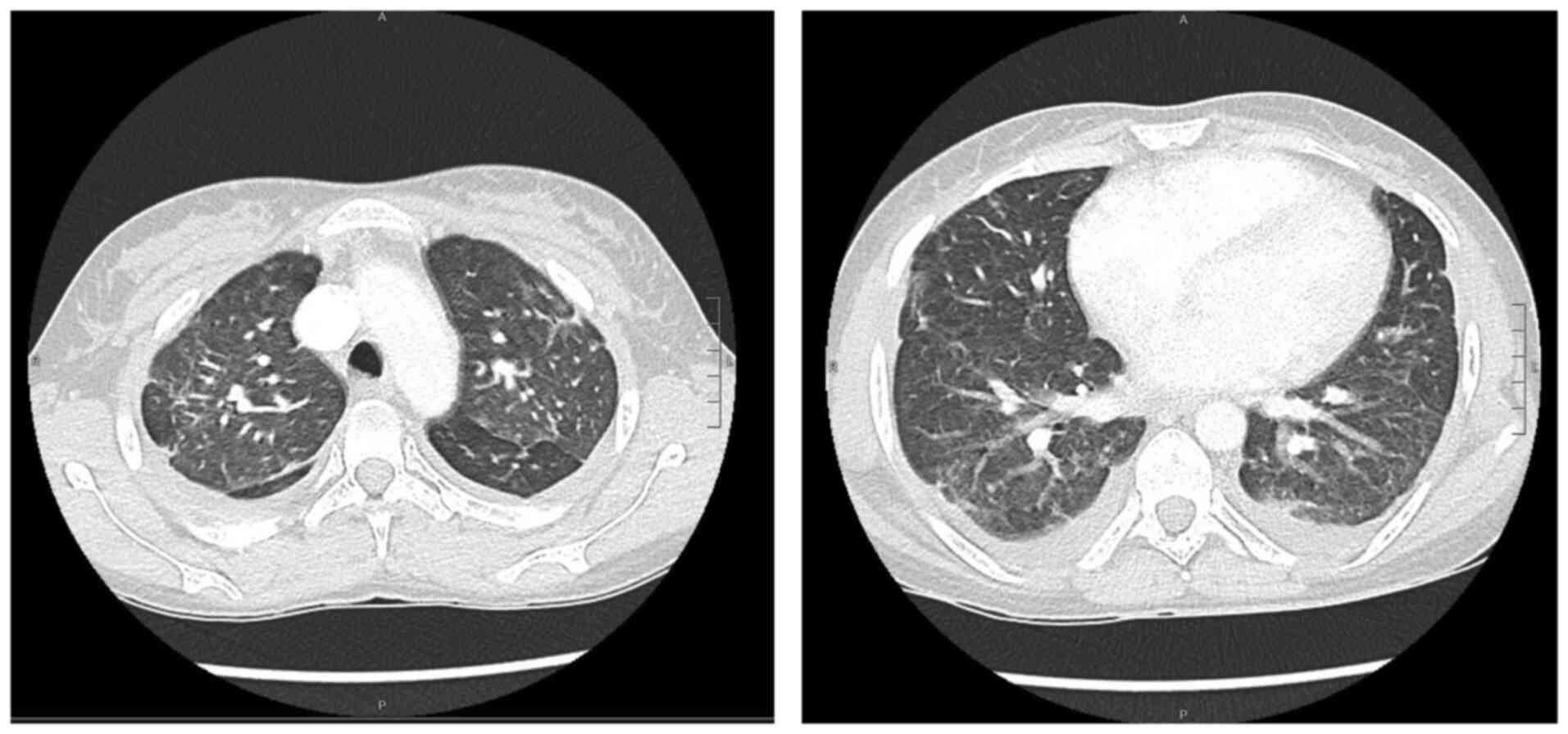
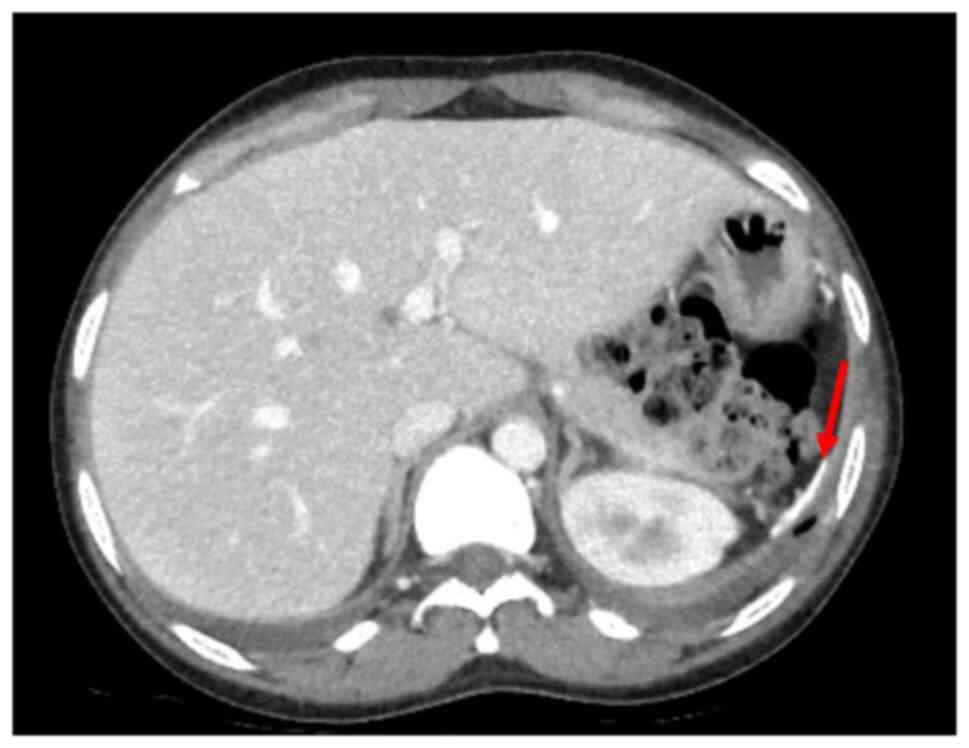
Blood tests revealed mild anaemia with a reduced red blood cell (RBC) count [haemoglobin (Hb), 9.8 g/dl; RBC, 3.34x106/mm3, normal white blood cell count (WBC), increased C-reactive protein (CRP) levels (2.38 mg/dl) along with high erythrocyte sedimentation rate levels (104 mm/h), increased total bilirubin levels (2.85 mg/dl), aspartate aminotransferase at 83 U/l and alanine aminotransferase at 56 U/l. Creatinine levels and liver function marker (coagulation and albumin) levels were normal (creatinine level, 0.56 mg/dl; international normalized ratio, 0.98; and albumin, 3.8 g/dl). Hepatitis B virus (HBV) and hepatitis C virus (HBC) along with human immunodeficiency virus (HIV) tests were negative. A chest X-ray revealed right medio-basal lung consolidation with a perivascular interstitial thickening (Fig. 1). Thorax computed tomography (CT) scan images (acquired before and after contrast administration, using a SOMATOM® Definition Flash scanner; Xenetix, 350 mg/ml; Siemens AG) revealed bilateral ground glass opacities with interlobular septal thickening, lower lobes consolidations and bilateral pleural effusion (Fig. 2), whereas an abdomen CT scan revealed homogeneous hepatomegaly, and a 3 cm densely calcified spleen (Fig. 3).
Treatment with subcutaneous prophylactic enoxaparin 6,000 U/day, intravenous fluids (sodium chloride, 0.5%), and amoxicillin-clavulanate (Fidia Farmaceutici S.p.A.; 1 g, three times per day) was commenced. The fever dissipated in 48 h and the clinical conditions ameliorated.
On the fifth day, the patient became febrile again (temperature, 38.5˚C) and began to complain of intense pain involving the chest, abdominal area and lower limbs [visual analogue scale (VAS) score of 8)] (8); the response to non-steroidal anti-inflammatory drugs or tramadol administration was poor. The laboratory tests revealed lower Hb levels (8.8 g/dl) with increasing WBC (19.800/mm3 75% neutrophils), higher inflammatory markers levels (CRP, 17.46 mg/dl), procalcitonin at 2 ng/ml, higher lactate dehydrogenase (1,116 mU/ml) and bilirubin levels (3.5 mg/dl) along with low haptoglobin levels (9 mg/dl). Haemoglobin S (HbS) levels (high-performance liquid chromatography technique) were 60.7%. In addition, two blood cultures and a urine culture demonstrated no bacterial growth.
Due to the elevations in the levels of inflammatory markers along with procalcitonin levels, in order to rule out bacterial superinfection, antibiotic therapy was empirically switched to meropenem (1 g, three times daily) plus teicoplanin (400 mg/day, after a loading dose of 400 mg two times daily for three administrations). In addition, an analgesic therapeutics cocktail, including diclofenac, tramadol and metoclopramide was administered in continuous infusion. Additionally, with the aid of a haematologist consultant, suspecting a VOC, manual red cell exchange transfusion was performed (venesection of 500 ml of blood and transfusion of two units of packed red blood cells). Blood exchange procedure was performed without adverse events, achieving rapid pain relief.
Antibiotics therapy was terminated after 7 days, due to the resolution of fever along with blood test normalization (CRP, procalcitonin and WBC) and the patient was discharged after having been tested negative to SARS-CoV-2 and addressed to the haematological unit for follow-up.
Discussion
Several studies have highlighted that patients with chronic comorbidities, such as diabetes, chronic obstructive pulmonary disease and cardiovascular diseases are at an increased risk of developing severe COVID-19 infection (9-11). SCD is a common inherited blood cell disorder, caused by a valine replacement of a single glutamic acid in the sixth residue of the β-globin subunit, resulting in HbS. HbS is an abnormal haemoglobin which, in specific circumstances, including hypoxia and infections, may polymerize, shifting red blood cells into sickle-shaped cells. Sickle cells cause microcirculatory occlusion, leading to tissue ischemia with acute painful episodes and crises, and several long-term complications (3).
Lee et al (5) reviewed the outcomes of patients with COVID-19 with haemoglobinopathy, demonstrating that these patients were more susceptible to severe SARS-CoV-2 infection and disease compared to the general population, highlighting the need for greater caution.
A higher fatality rate in patients with SCD with COVID-19 has also been reported by Minniti et al (12); however, they revealed that the risk of adverse outcomes in these patients may be reduced by anticoagulant therapy. As a matter of fact, SARS-CoV-2 infection, due to the resulting cytokine storm along with the hypercoagulable state, culminates in an increased risk of thromboembolic events in patients with SCD (13-15).
In addition, patients with SCD, due to hyposplenism and vascular compromise, have an impaired immunity, particularly against encapsulated bacteria (including Streptococcus pneumoniae and Neisseria meningitidis), resulting in worse outcomes and often requiring further antibiotic prophylaxis (3). It has been highlighted in previous studies that patients with SCD have a higher prevalence for severe bacterial infections, in comparison with patients without SCD (16). In addition, infections may trigger SCD complications, including VOC (6).
VOC pathophysiology is a multifactorial process, mainly characterized by high plasma viscosity due to a combination of erythrocytes sickling, thrombosis, haemolysis, tissue damage and inflammation. Those features may lead to blood vessel occlusion, causing painful tissue ischemic injuries (6). All VOC characteristics have been also observed in COVID-19 pathophysiology, resulting in ‘synergistic’ damage.
The association between SCD morbidity and respiratory infections had been highlighted during the 2009 H1N1 pandemic (17), when those patients faced both SCD and flu complications, leading to worse outcomes compared to the general population.
The case series study conducted by Chakravorty et al (18) explained the benefits of early hospitalization for patients with SCD with SARS-CoV-2 infection, even in asymptomatic or mild cases, since acute complications appear to occur within 1 week following infection.
There are several different strategies, including pharmacological and non-pharmacological, for the treatment of VOC in patients with SCD, depending on pain intensity, duration and location (18,19). According to Italian guidelines (20), in case of severe pain (VAS score of 9-11) it is recommended to administer an initial analgesic approach, consisting of a continuous intravenous infusion of weak opioid (including codeine or tramadol) along with nonsteroidal anti-inflammatory drugs (e.g., ketorolac) plus adjuvants (e.g., metoclopramide).
For patients with SCD, blood transfusion aims to restore Hb levels in patients with acute anaemia (e.g., transient aplastic anaemia or acute splenic sequestration) and to the rapid reduction of the percentage of HbS, in order to prevent VOCs deterioration, due to further sickle cell formation (19).
Transfusion therapy management during acute events requires the monitoring of both total Hb levels and the percentage of HbS. One of the goals of blood transfusion therapy is to mitigate anaemia symptoms by avoiding hyperviscosity, without exceeding post transfusion a Hb value of 10-11 g/dl. Another goal is to treat or prevent acute events, including VOCs, decreasing the percentage of HbS <40% (19). Exchange transfusions can be performed manually or may be automated according to Hb levels and possible complications are represented by hyperviscosity and iron overload (19).
In the present case report study, the patient developed acute painful VOC after 5 days of admission, probably triggered by SARS-CoV-2 infection, despite being twice vaccinated, demonstrating anaemia, leukocytosis and increasing levels of anti-inflammatory markers and procalcitonin, along with high HbS percentages. As suggested by guidelines and literature evidence (19,20), an analgesic cocktail was administered, along with RBC exchange to achieve prompt pain control. Moreover, due to the presence of fever along with increased CRP and procalcitonin levels, antibiotic therapy was administered, and cultural tests were performed to rule out and treat bacterial superinfections, whereas viral coinfections, including HIV, HBV and HCV, were already excluded according to protocols (21-27). Previous research has highlighted the risk of delaying appropriate management during VOCs in patients with SCD (12).
HU is an oral medication with disease-modifying benefits for the treatment of SCD, preventing the onset and reducing the frequency of SCD-related complications, reducing the need for blood transfusions (28). However, since it requires months to be effective, HU cannot be used during acute episodes (29). Indeed, it has been reported by Mucalo et al (4) that the use of HU by patients with SCD may decrease the risk of pain occurrence during COVID-19 infection. Therefore, the use of HU as a prophylaxis of SCD complications may be a strategy with which to avoid analgesic medication and blood transfusion in patients with SCD with SARS-CoV-2 infection; however, further studies and additional solid evidence are required to be collected.
In conclusion, case described in the present study highlighted the association between SCD-related complications and SARS-CoV-2 infection, which had already been pointed out by other authors (17). Patients with SCD and COVID-19 infection need to be critically evaluated by clinicians, as a result of the worse outcomes predicted for this group. Under the suspicion of the VOC, it is suggested that patients with SCD be managed with analgesic therapy and to be evaluated using RBC exchange therapy.
Acknowledgements
The authors would like to thank Dr Pietro Leanza, University of Catania, Catania, Italy for assisting with the language revisions.
Funding
Funding: No funding was received.
Availability of data and materials
Data sharing is not applicable to this article, as no datasets were generated or analyzed during the current study.
Authors' contributions
EC and AM contributed to the conceptualization and design of the study. MC and MG acquired the patient data. FC, VM and CM analysed the data. GN, BMC and BC critically interpreted the data. EC and AM confirm the authenticity of all the raw data. All authors have read and approved the final manuscript.
Ethics approval and consent to participate
The patient signed a consent for the publication of personal data and radiological imagines.
Patient consent for publication
Written informed consent was obtained from the patient for the publication of this case report.
Competing interests
The authors declare that they have no competing interests.
References
|
Esakandari H, Nabi-Afjadi M, Fakkari-Afjadi J, Farahmandian N, Miresmaeili SM and Bahreini E: A comprehensive review of COVID-19 characteristics. Biol Proced Online. 22(19)2020.PubMed/NCBI View Article : Google Scholar | |
|
Li J, Huang DQ, Zou B, Yang H, Hui WZ, Rui F, Yee NTS, Liu C, Nerurkar SN, Kai JCY, et al: Epidemiology of COVID-19: A systematic review and meta-analysis of clinical characteristics, risk factors, and outcomes. J Med Virol. 93:1449–1458. 2021.PubMed/NCBI View Article : Google Scholar | |
|
Kato GJ, Piel FB, Reid CD, Gaston MH, Ohene-Frempong K, Krishnamurti L, Smith WR, Panepinto JA, Weatherall DJ, Costa FF and Vichinsky EP: Sickle cell disease. Nat Rev Dis Primers. 14(18010)2018.PubMed/NCBI View Article : Google Scholar | |
|
Mucalo L, Brandow AM, Dasgupta M, Mason SF, Simpson PM, Singh A, Taylor BW, Woods KJ, Yusuf FI and Panepinto JA: Comorbidities are risk factors for hospitalization and serious COVID-19 illness in children and adults with sickle cell disease. Blood Adv. 5:2717–2724. 2021.PubMed/NCBI View Article : Google Scholar | |
|
Lee JX, Chieng WK, Lau SCD and Tan CE: COVID-19 and Hemoglobinopathies: A systematic review of clinical presentations, investigations, and outcomes. Front Med (Lausanne). 8(1848)2021.PubMed/NCBI View Article : Google Scholar | |
|
Booth C, Inusa B and Obaro SK: Infection in sickle cell disease: A review. Int J Infect Dis. 14:e2–e12. 2010.PubMed/NCBI View Article : Google Scholar | |
|
Hoogenboom WS, Alamuri TT, McMahon DM, Balanchivadze N, Dabak V, Mitchell WB, Morrone KB, Manwani D and Duong TQ: Clinical outcomes of COVID-19 in patients with sickle cell disease and sickle cell trait: A critical appraisal of the literature. Blood Rev: Nov 20, 2021 (Epub ahead of print). | |
|
McCormack HM, Horne DJ and Sheather S: Clinical applications of visual analogue scales: A critical review. Psychol Med. 18:1007–1019. 1988.PubMed/NCBI View Article : Google Scholar | |
|
Sanyaolu A, Okorie C, Marinkovic A, Patidar R, Younis K, Desai P, Hosein Z, Padda I, Mangat J and Altaf M: Comorbidity and its impact on patients with COVID-19. SN Compr Clin Med. 2:1069–1076. 2020.PubMed/NCBI View Article : Google Scholar | |
|
Alyammahi SK, Abdin SM, Alhamad DW, Elgendy SM, Altell AT and Omar HA: The dynamic association between COVID-19 and chronic disorders: An updated insight into prevalence, mechanisms and therapeutic modalities. Infect Genet Evol. 87(104647)2021.PubMed/NCBI View Article : Google Scholar | |
|
Wang B, Li R, Lu Z and Huang Y: Does comorbidity increase the risk of patients with COVID-19: Evidence from meta-analysis. Aging (Albany NY). 12:6049–6057. 2020.PubMed/NCBI View Article : Google Scholar | |
|
Minniti CP, Zaidi AU, Nouraie M, Manwani D, Crouch GD, Crouch AS, Callaghan MU, Carpenter S, Jacobs C, Han J, et al: Clinical predictors of poor outcomes in patients with sickle cell disease and COVID-19 infection. Blood Adv. 5:207–215. 2021.PubMed/NCBI View Article : Google Scholar | |
|
Menapace LA and Thein SL: COVID-19 and sickle cell disease. Haematologica. 105:2501–2504. 2020.PubMed/NCBI View Article : Google Scholar | |
|
Ceccarelli M, Marino A, Cosentino F, Moscatt V, Celesia BM, Gussio M, Bruno R, Rullo EV, Nunnari G and Cacopardo BS: Post-infectious ST elevation myocardial infarction following a COVID-19 infection: A case report. Biomed Rep. 16(10)2022.PubMed/NCBI View Article : Google Scholar | |
|
Marino A, Pampaloni A, Scuderi D, Cosentino F, Moscatt V, Ceccarelli M, Gussio M, Celesia BM, Bruno R, Borraccino S, et al: High-flow nasal cannula oxygenation and tocilizumab administration in patients critically ill with COVID-19: A report of three cases and a literature review. World Acad Sci J. 2(23)2020. | |
|
Cannas G, Merazga S and Virot E: Sickle cell disease and infections in high- and low-income countries. Mediterr J Hematol Infect Dis. 11(e2019042)2019.PubMed/NCBI View Article : Google Scholar | |
|
Strouse JJ, Reller ME, Bundy DG, Amoako M, Cancio M, Han RN, Valsamakis A and Casella JF: Severe pandemic H1N1 and seasonal influenza in children and young adults with sickle cell disease. Blood. 116:3431–3434. 2010.PubMed/NCBI View Article : Google Scholar | |
|
Chakravorty S, Padmore-Payne G, Ike F, Tshibangu V, Graham C, Rees D and Stuart-Smith S: COVID-19 in patients with sickle cell disease-A case series from a UK tertiary hospital. Haematologica. 105:2691–2693. 2020.PubMed/NCBI View Article : Google Scholar | |
|
Brandow AM, Carroll CP, Creary S, Edwards-Elliott R, Glassberg J, Hurley RW, Kutlar A, Seisa M, Stinson J, Strouse JJ, et al: American society of hematology 2020 guidelines for sickle cell disease: Management of acute and chronic pain. Blood Adv. 4:2656–2701. 2020.PubMed/NCBI View Article : Google Scholar | |
|
Russo G, De Franceschi L, Colombatti R, Rigano P, Perrotta S, Voi V, Palazzi G, Fidone C, Quota A, Graziadei G, et al: Current challenges in the management of patients with sickle cell disease-A report of the Italian experience. Orphanet J Rare Dis. 14(120)2019.PubMed/NCBI View Article : Google Scholar | |
|
El-Sokkary R, Uysal S, Erdem H, Kullar R, Pekok AU, Amer F, Grgić S, Carevic B, El-Kholy A, Liskova A, et al: Profiles of multidrug-resistant organisms among patients with bacteremia in intensive care units: An international ID-IRI survey. Eur J Clin Microbiol Infect Dis. 40:2323–2334. 2021.PubMed/NCBI View Article : Google Scholar | |
|
Erdem H, Hargreaves S, Ankarali H, Caskurlu H, Ceviker SA, Bahar-Kacmaz A, Meric-Koc M, Altindis M, Yildiz-Kirazaldi Y, Kizilates F, et al: Managing adult patients with infectious diseases in emergency departments: International ID-IRI study. J Chemother. 33:302–318. 2021.PubMed/NCBI View Article : Google Scholar | |
|
Marino A, Zafarana G, Ceccarelli M, Cosentino F, Moscatt V, Bruno G, Bruno R, Benanti F, Cacopardo B and Celesia BM: Immunological and clinical impact of DAA-Mediated HCV Eradication in a Cohort of HIV/HCV Coinfected Patients: Monocentric Italian Experience. Diagnostics (Basel). 11(2336)2021.PubMed/NCBI View Article : Google Scholar | |
|
Celesia BM, Marino A, Borracino S, Arcadipane AF, Pantò G, Gussio M, Coniglio S, Pennisi A, Cacopardo B and Panarello G: Successful extracorporeal membrane oxygenation treatment in an acquired immune deficiency syndrome (AIDS) patient with acute respiratory distress syndrome (ARDS) complicating pneumocystis jirovecii pneumonia: A challenging case. Am J Case Rep. 21(e919570)2020.PubMed/NCBI View Article : Google Scholar | |
|
Ceccarelli M, Venanzi Rullo E, Marino MA, D'Aleo F, Pellicanò GF, D'Andrea F, Marino A, Cacopardo B, Celesia BM, La Rocca G, et al: Non-AIDS defining cancers: A comprehensive update on diagnosis and management. Eur Rev Med Pharmacol Sci. 24:3849–3875. 2020.PubMed/NCBI View Article : Google Scholar | |
|
Celesia BM, Marino A, Del Vecchio RF, Bruno R, Palermo F, Gussio M, Nunnari G and Cacopardo B: Is it safe and cost saving to defer the CD4+ cell count monitoring in stable patients on art with more than 350 or 500 cells/µl? Mediterr J Hematol Infect Dis. 11(e2019063)2019.PubMed/NCBI View Article : Google Scholar | |
|
Marino A, Cosentino F, Ceccarelli M, Moscatt V, Pampaloni A, Scuderi D, D'Andrea F, Rullo EV, Nunnari G, Benanti F, et al: Entecavir resistance in a patient with treatment-naïve HBV: A case report. Mol Clin Oncol. 14(113)2021.PubMed/NCBI View Article : Google Scholar | |
|
McGann PT and Ware RE: Hydroxyurea therapy for sickle cell anemia. Expert Opin Drug Saf. 14:1749–1758. 2015.PubMed/NCBI View Article : Google Scholar | |
|
Yasara N, Premawardhena A and Mettananda S: A comprehensive review of hydroxyurea for β-haemoglobinopathies: The role revisited during COVID-19 pandemic Orphanet J Rare. Dis. 16(114)2021.PubMed/NCBI View Article : Google Scholar |