Retrospective audit on time to surgery delays following the completion of neoadjuvant chemotherapy in breast cancer patients
- Authors:
- Published online on: March 17, 2022 https://doi.org/10.3892/wasj.2022.155
- Article Number: 20
-
Copyright: © Pedamallu et al. This is an open access article distributed under the terms of Creative Commons Attribution License.
Metrics: Total
Views: 0 (Spandidos Publications: | PMC Statistics: )
Total PDF Downloads: 0 (Spandidos Publications: | PMC Statistics: )
Abstract
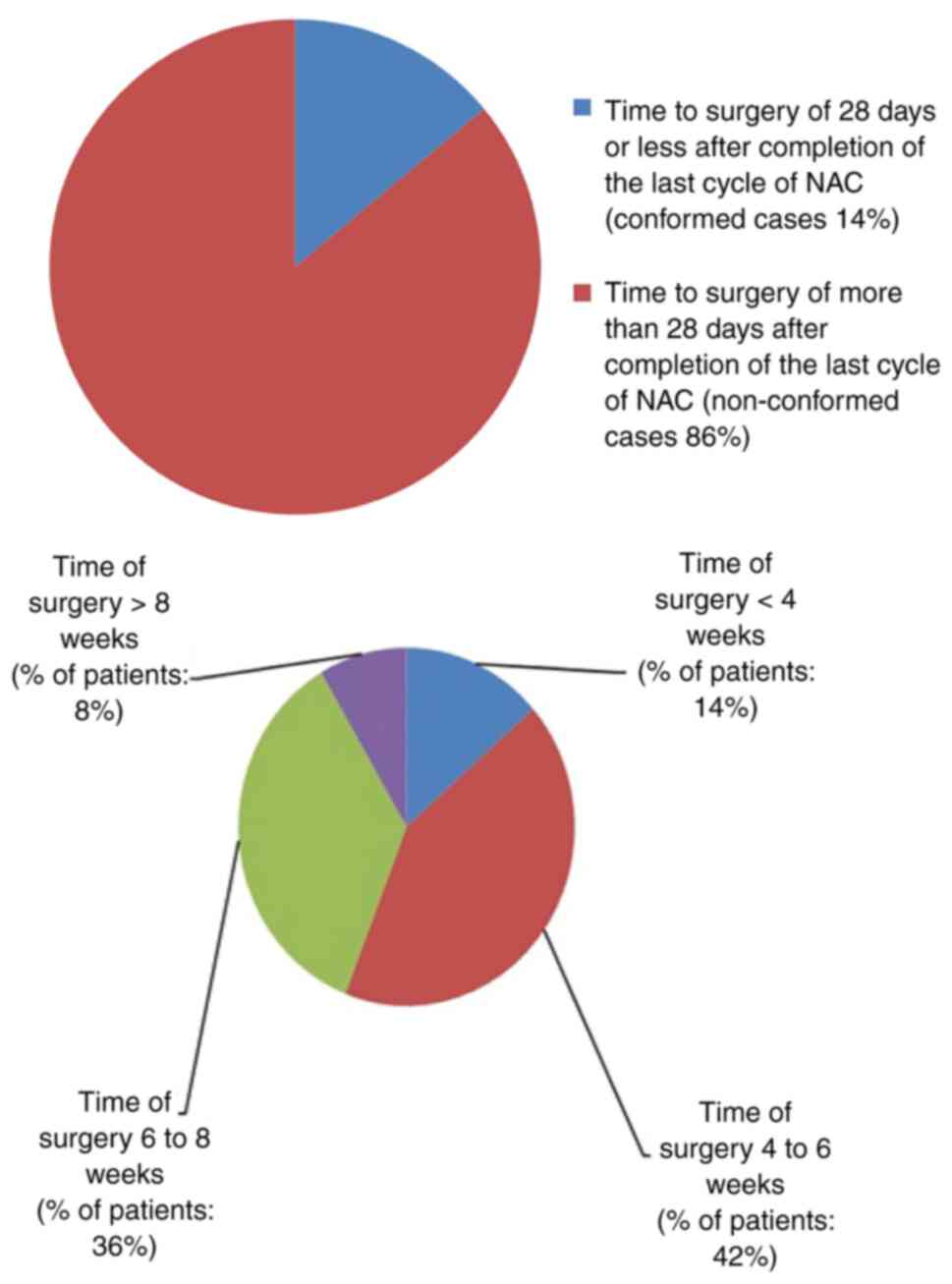
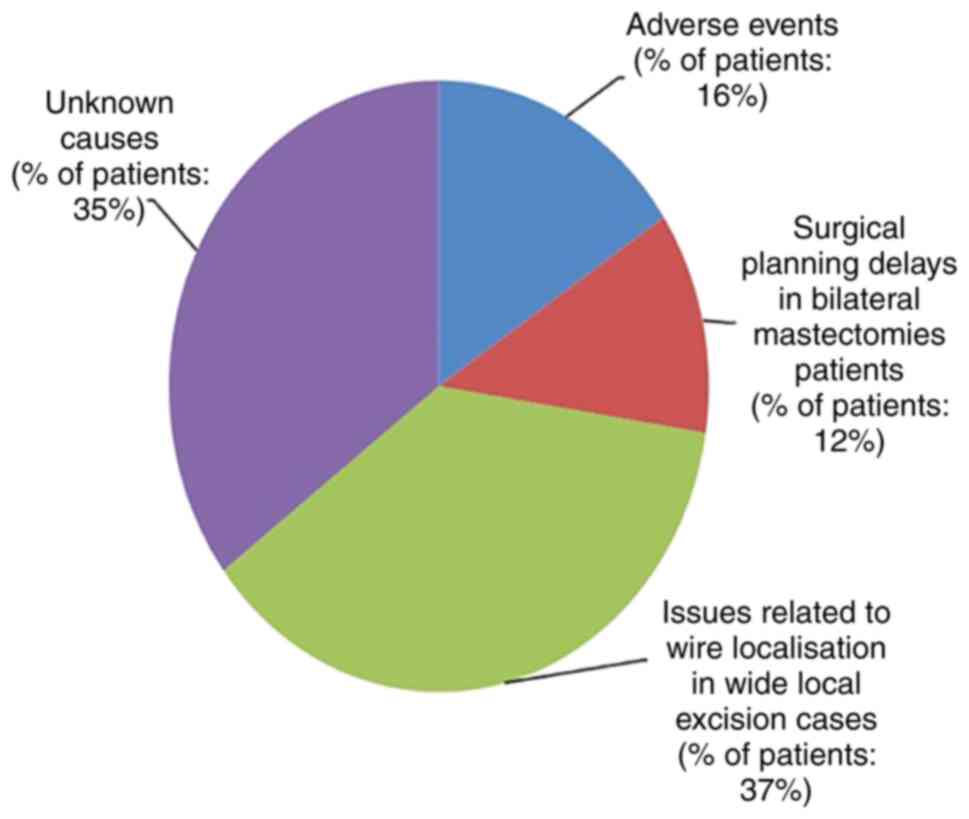
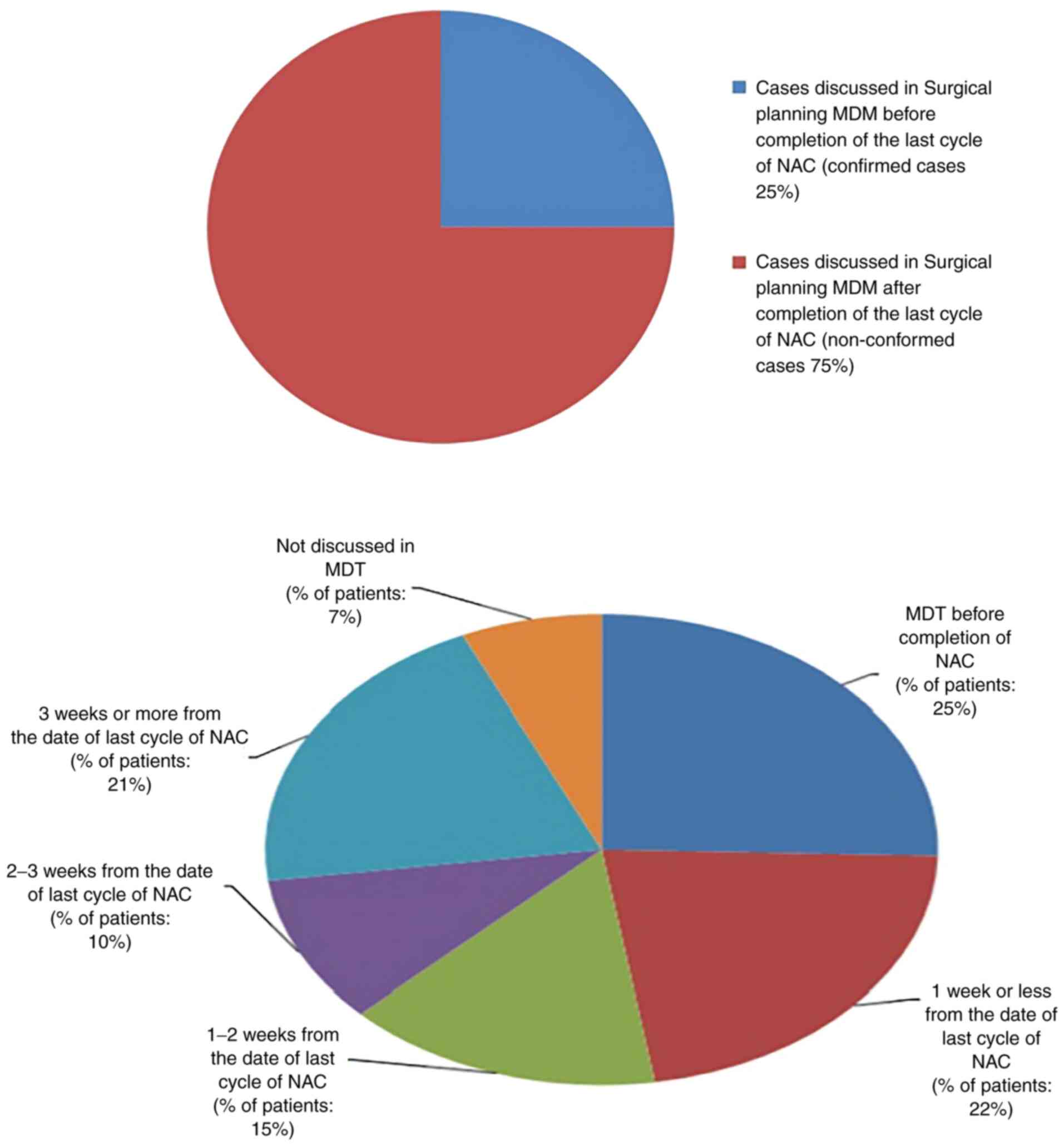
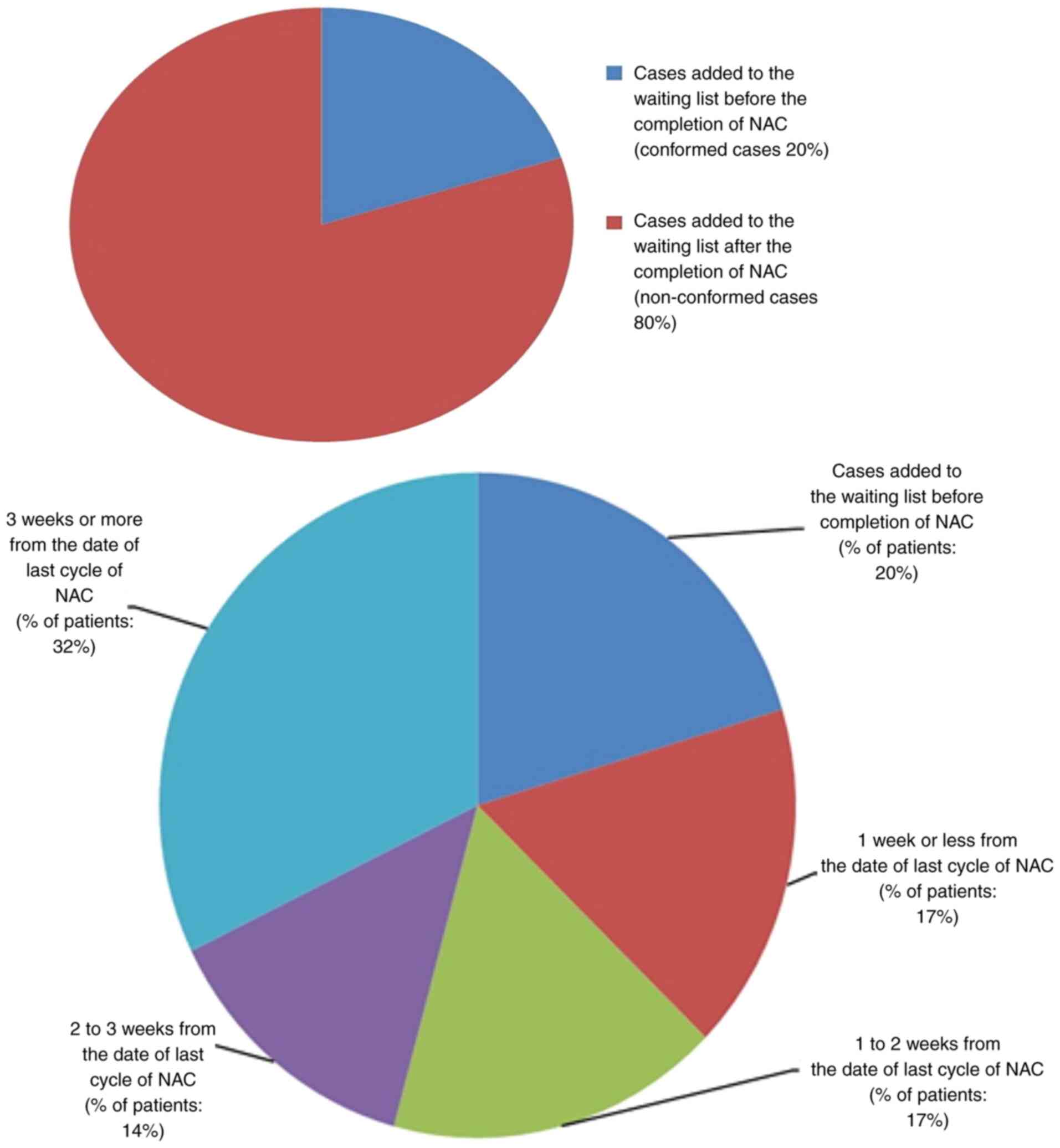
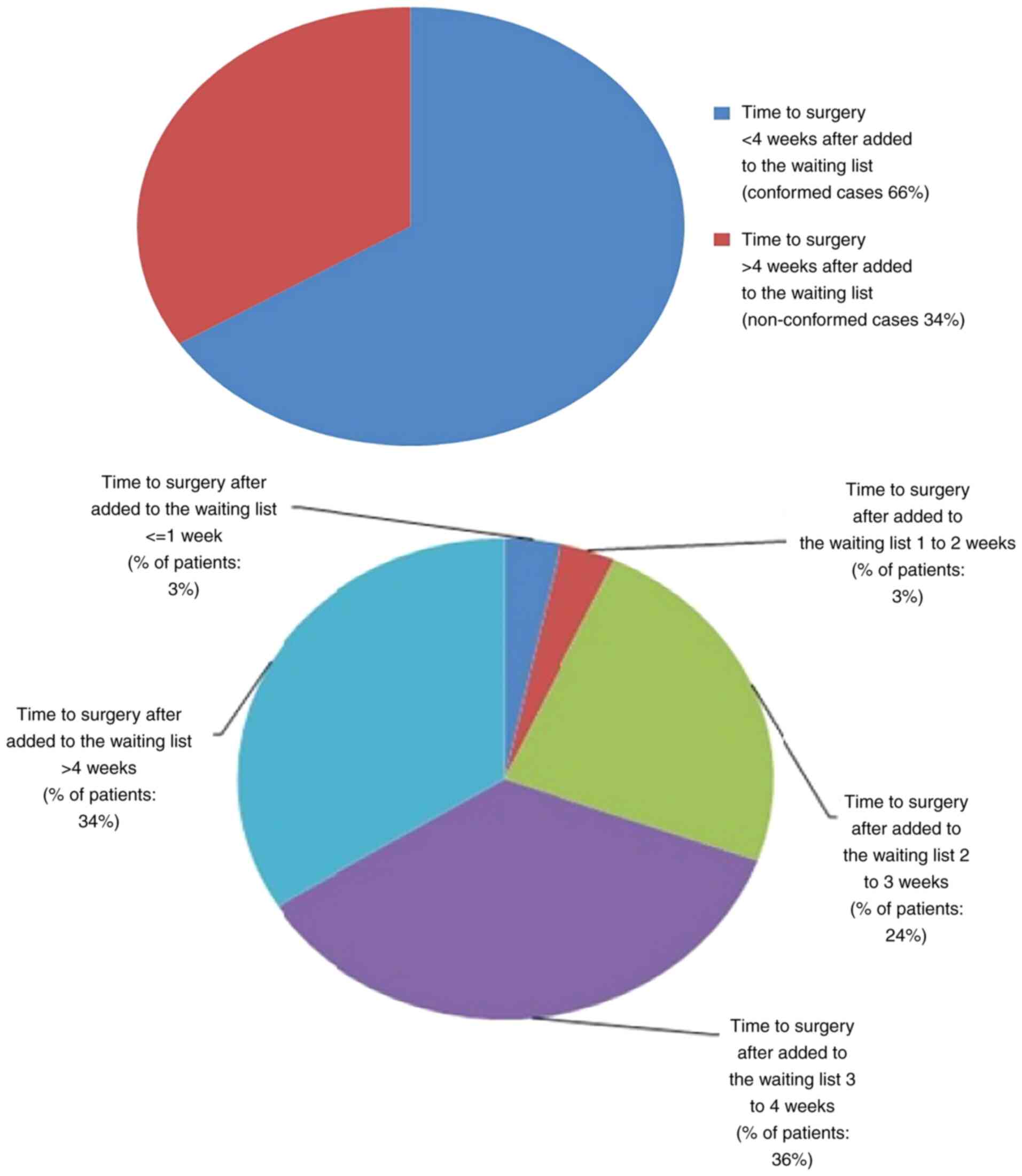
The ideal time to surgery interval in breast cancer patients following neoadjuvant chemotherapy according to published literature is as low as 21 days, 40 days and up to 8 weeks. The authors' hospital multidisciplinary team (MDT) accepted 28 days as a reasonable time interval to allow recovery from the side‑effects of neoadjuvant chemotherapy. The present retrospective clinical audit presents a review of all breast cancer cases that received neoadjuvant chemotherapy and examined the conformity to the set audit standards. The primary aim of the present audit was to investigate the time interval from the conclusion of neoadjuvant chemotherapy to surgery in breast cancer patients, and to determine the causes of potential delays and identifying means to improve the outcomes of the current process. The secondary objectives were to investigate the timing of tumour coil insertion, the timing of surgical planning multidisciplinary team meeting, the time to addition to the surgical waiting list and whether these patients had adequate tumour monitoring for effective surgical planning. A total of 59 breast cancer patients who had received neoadjuvant chemotherapy from November 1, 2011 to October 31, 2016 were identified. In total, one primary audit standard and six secondary audit standards were derived from the MDT consensus agreement and reasons for non‑conformity to these standards were audited. The primary standard was described as ‘surgery should be performed within 28 days of completion of last cycle of neoadjuvant chemotherapy’. The results revealed that conformity to this standard was 14%. The conformity of secondary standards was also poor, ranging from 20 to 98%. The results of the present audit were not very encouraging. Recommendations were drawn with a plan for a re‑audit following the implementation of changes agreed upon by the multidisciplinary team and a plan of action was prepared. It was concluded that achieving a time to surgery interval of <28 days following the completion of neoadjuvant chemotherapy is an achievable challenging task and only possible with an efficient multidisciplinary team work.